Maitotoxin Is a Potential Selective Activator of the Endogenous Transient Receptor Potential Canonical Type 1 Channel in Xenopus laevis Oocytes
Abstract
:1. Introduction
2. Results
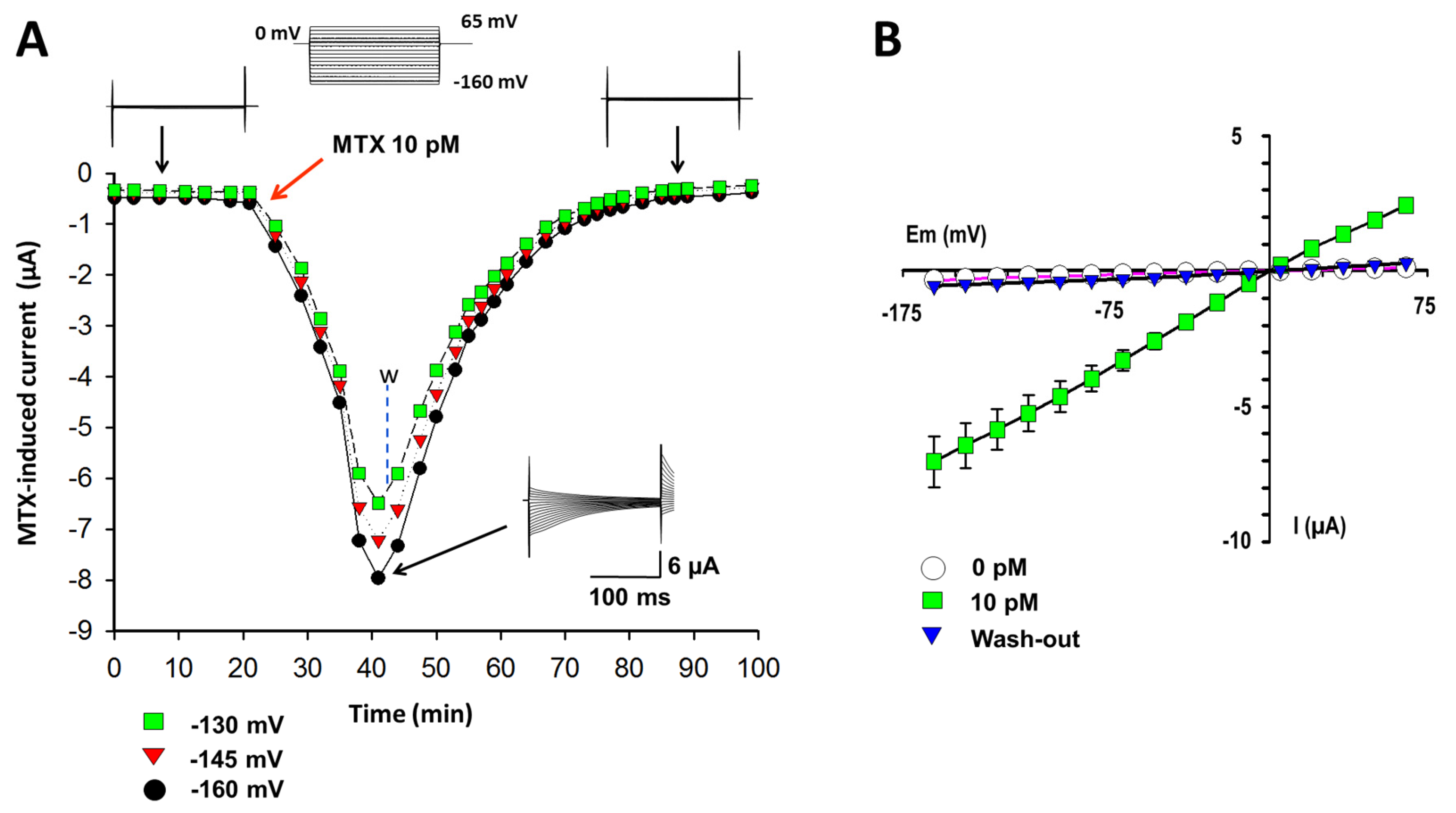
2.1. Currents Induced by MTX in X. laevis Oocytes
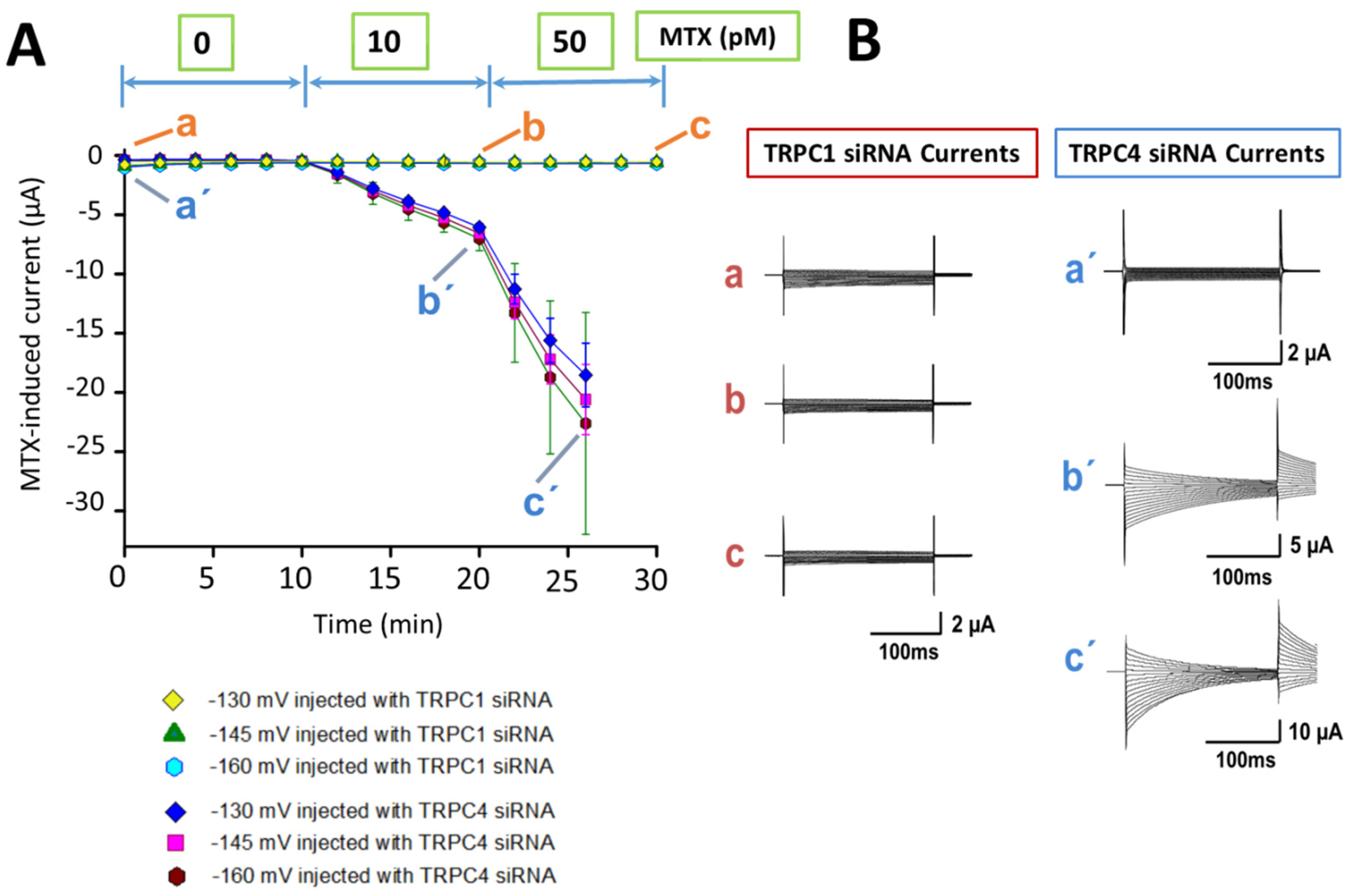
2.2. Identification of the MTX Current
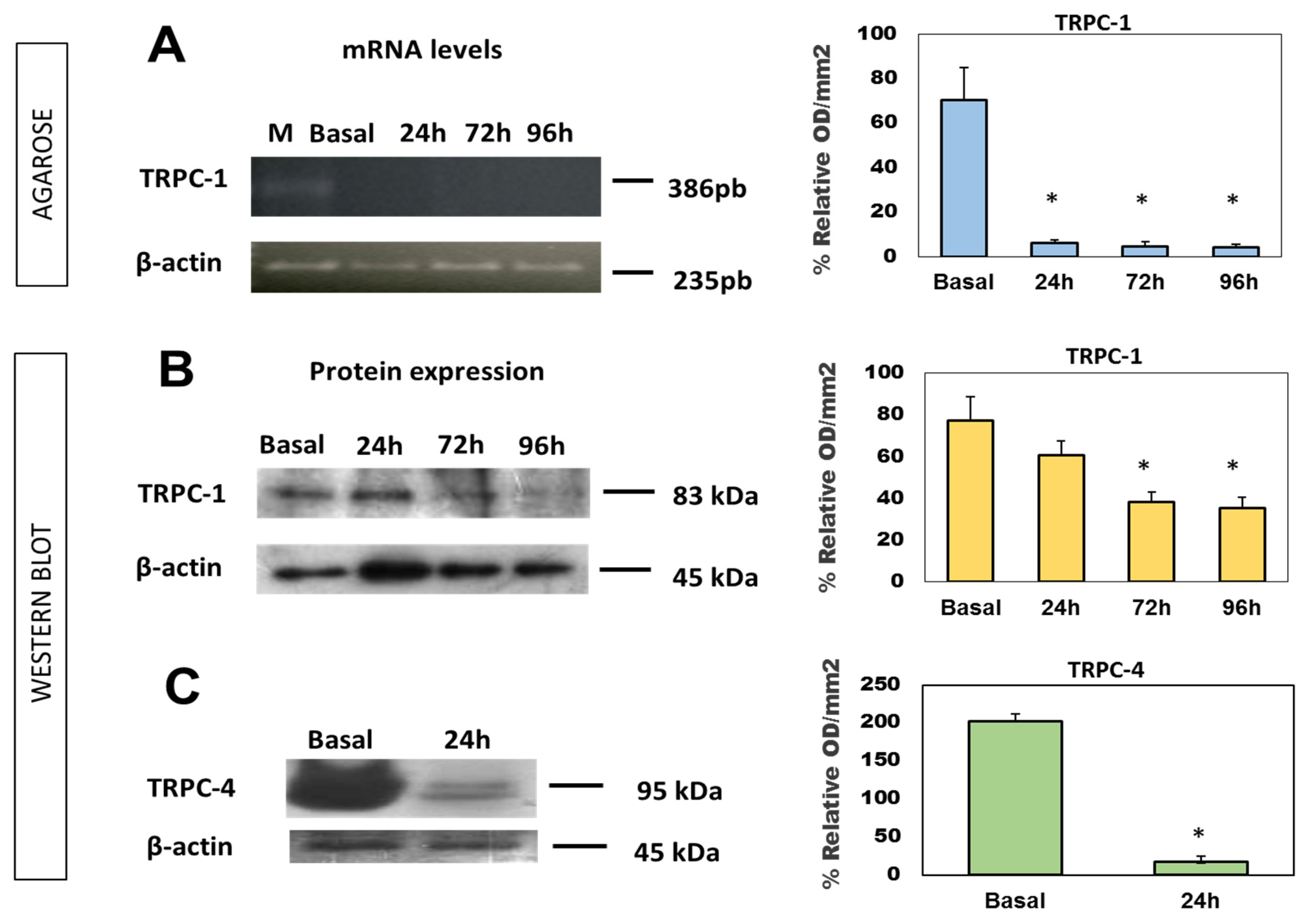
2.3. Expression of TRPC mRNA and Protein
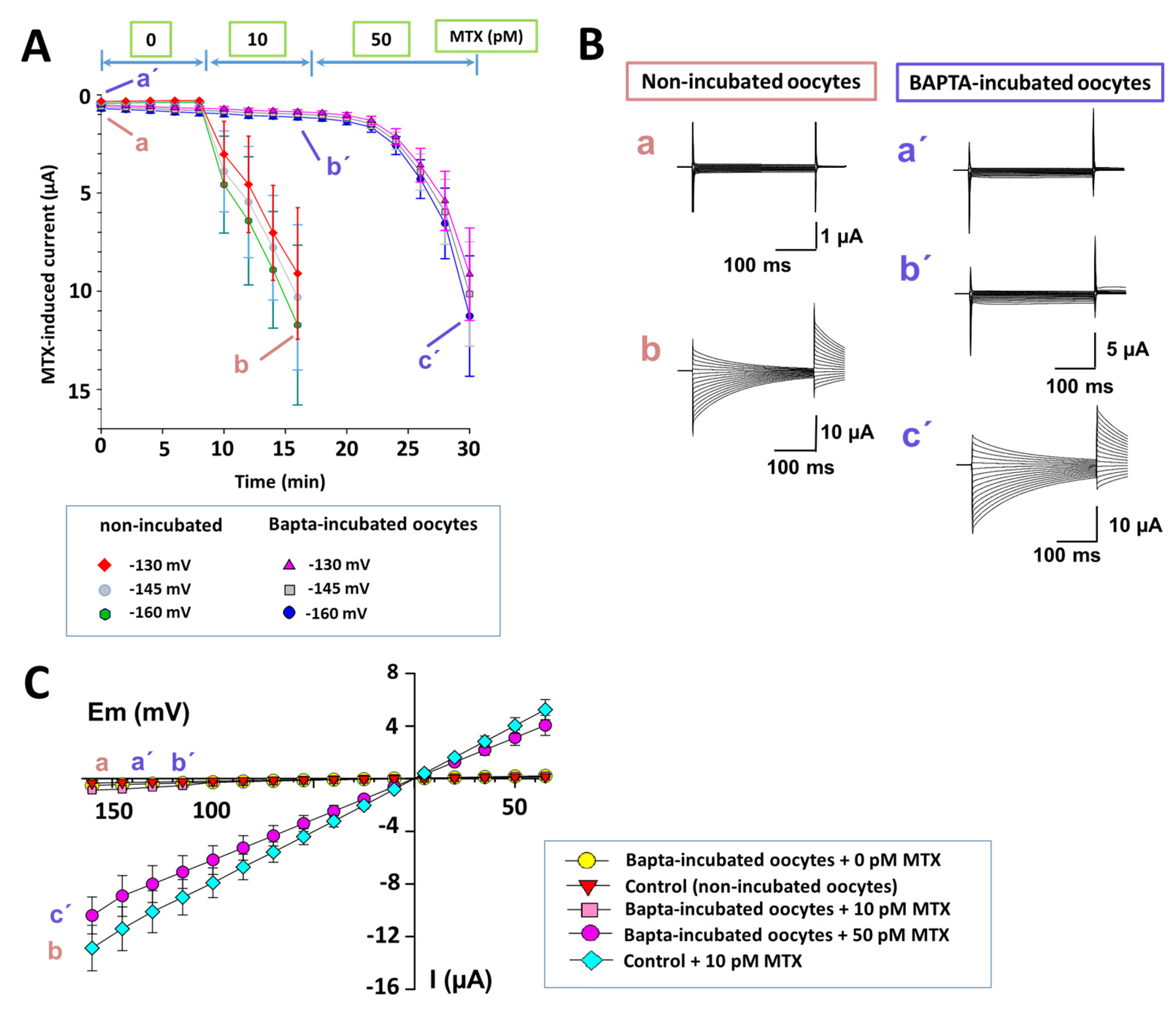
2.4. Intracellular Calcium Participation in the MTX Effects
3. Discussion
4. Material and Methods
4.1. Animals and Ethical Statement
4.2. Oocytes
4.3. Electrophysiology
4.4. Small Interference RNA (siRNA) Assay
4.5. RT-PCR
4.6. Western Blotting
4.7. Statistical Analyses
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Holmes, M.J.; Lewis, R.J. Purification and characterisation of large and small maitotoxins from cultured Gambierdiscus toxicus. Nat. Toxins 1994, 2, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, K.C.; Frederick, M.O.; Aversa, R.J. The Continuing Saga of the Marine Polyether Biotoxins. Angew. Chem. Int. Ed. Engl. 2008, 47, 7182–7225. [Google Scholar] [CrossRef] [PubMed]
- Legrand, A.M.; Galonnier, M.; Baqnis, R. Studies on the mode of action of ciguateric toxins. Toxicon 1982, 20, 311–315. [Google Scholar] [CrossRef]
- Terao, K.; Ito, E.; Sakamaki, Y.; Igarashi, K.; Yokoyama, A.; Yasumoto, T. Histopathological studies of experimental marine toxin poisoning. II. The acute effects of maitotoxin on the stomach, heart and lymphoid tissues in mice and rats. Toxicon 1988, 26, 395–402. [Google Scholar] [CrossRef]
- Terao, K.; Ito, E.; Kakinuma, Y.; Igarashi, K.; Kobayashi, M.; Ohizumi, Y.; Yasumoto, T. Histopathological studies of experimental marine toxin poisoning. 4. Pathogenesis of experimental maitotoxin poisoning. Toxicon 1989, 27, 979–988. [Google Scholar] [CrossRef]
- Friedman, M.A.; Fernandez, M.; Backer, L.C.; Dickey, R.W.; Bernstein, J.; Schrank, K.; Kibler, S.; Stephan, W.; Gribble, M.O.; Bienfang, P.; et al. An Updated Review of Ciguatera Fish Poisoning: Clinical, Epidemiological, Environmental, and Public Health Management. Mar. Drugs 2017, 15, 72. [Google Scholar] [CrossRef] [PubMed]
- Llewellyn, L.E. Revisiting the association between sea surface temperature and the epidemiology of fish poisoning in the South Pacific: Reassessing the link between ciguatera and climate change. Toxicon 2010, 56, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Litaker, R.W.; Vandersea, M.W.; Faust, M.A.; Kibler, S.R.; Nau, A.W.; Holland, W.C.; Chinain, M.; Holmes, M.J.; Tester, P.A. Global distribution of ciguatera causing dinoflagellates in the genus Gambierdiscus. Toxicon 2010, 56, 711–730. [Google Scholar] [CrossRef] [PubMed]
- Kohli, G.S.; Murray, S.A.; Neilan, B.A.; Rhodes, L.L.; Harwood, D.T.; Smith, K.F.; Meyer, L.; Capper, A.; Brett, S.; Hallegraeff, G.M. High abundance of the potentially maitotoxic dinoflagellate Gambierdiscus carpenteri in temperate waters of New South Wales, Australia. Harmful Algae 2014, 39, 134–145. [Google Scholar] [CrossRef]
- Berdalet, E.; Fleming, L.E.; Gowen, R.; Davidson, K.; Hess, P.; Backer, L.C.; Moore, S.K.; Hoagland, P.; Enevoldsen, H. Marine harmful algal blooms, human health and wellbeing: Challenges and opportunities in the 21st century. J. Mar. Biol. Assoc. U. K. 2016, 96, 61–91. [Google Scholar] [CrossRef] [PubMed]
- Ajani, P.; Harwood, D.T.; Murray, S.A. Recent Trends in Marine Phycotoxins from Australian Coastal Waters. Mar. Drugs. 2017, 15, 33. [Google Scholar] [CrossRef]
- Grattan, L.M.; Holobaugh, S.; Morris, J.G., Jr. Harmful Algal Blooms and Public Health. Harmful Algae 2016, 57, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Roeder, K.; Erler, K.; Kibler, S.; Tester, P.; Van The, H.; Nguyen-Ngoc, L.; Gerdts, G.; Luckas, B. Characteristic profiles of Ciguatera toxins in different strains of Gambierdiscus spp. Toxicon 2010, 56, 731–781. [Google Scholar] [CrossRef] [PubMed]
- Gusovsky, F.; Daly, J. Maitotoxin: A unique pharmacological tool for research on calcium-dependent mechanism. Biochem. Pharmacol. 1990, 39, 1633–1639. [Google Scholar] [CrossRef]
- Bielfeld-Ackermann, A.; Range, C.; Korbmacher, C. Maitotoxin (MTX) activates a nonselective cation channel in Xenopus laevis oocytes. Plügers Arch. 1998, 436, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Escobar, L.; Salvador, C.; Martínez, M.; Vaca, L. Maitotoxin: A cationic channel activator. Neurobiology 1998, 6, 59–74. [Google Scholar] [PubMed]
- Brereton, H.M.; Chen, J.; Rychkov, G.; Harland, M.L.; Barritt, G.J. Maitotoxin activates an endogenous non-selective cation channel and is an effective initiator of the activation of the heterologously expressed hTRPC-1 (transient receptor potential) non-selective cation channel in H4-IIE liver cells. Biochim. Biophys. Acta 2001, 1540, 107–126. [Google Scholar] [CrossRef]
- Egido, W.; Castrejón, V.; Antón, B.; Martínez, M. Maitotoxin induces two dose-dependent conductances in Xenopus oocytes. Comparison with nystatin effects as a pore inductor. Toxicon 2008, 5, 797–812. [Google Scholar] [CrossRef] [PubMed]
- Montell, C. Drosophila TRP channels. Pflugers Arch. 2005, 451, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Flockerzi, V. An introduction on TRP channels. Handb. Exp. Pharmacol. 2007, 179, 1–19. [Google Scholar]
- Kaneko, Y.; Szallasi, A. Transient receptor potential (TRP) channels: A clinical perspective. Br. J. Pharmacol. 2014, 171, 2474–2507. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Chu, P.B.; Peyton, M.; Birnbaumer, L. Molecular cloning of a widely expressed human homologue for the Drosophila trp gene. FEBS Lett. 1995, 373, 193–198. [Google Scholar] [CrossRef]
- Bobanovic, L.K.; Laine, M.; Petersen, C.C.; Bennett, D.L.; Berridge, M.J.; Lipp, P.; Ripley, S.J.; Bootman, M.D. Molecular cloning and immunolocalization of a novel vertebrate trp homologue from Xenopus. Biochem. J. 1999, 340, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Maroto, R.; Raso, A.; Wood, T.G.; Kurosky, A.; Martinac, B.; Hamill, O.P. TRPC1 forms the stretch-activated cation channel in vertebrate cells. Nat. Cell Biol. 2005, 7, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Wes, P.D.; Chevesich, J.; Jeromin, A.; Rosenberg, C.; Stetten, G.; Montell, C. TRPC1, a human homolog of a Drosophila store operated channel. Proc. Natl. Acad. Sci. USA 1995, 92, 9652–9656. [Google Scholar] [CrossRef] [PubMed]
- Brereton, H.M.; Harland, M.L.; Auld, A.M.; Barritt, G.J. Evidence that the TRP-1 protein is unlikely to account for store-operated Ca2+ inflow in Xenopus laevis oocytes. Mol. Cell. Biochem. 2000, 214, 63–74. [Google Scholar] [CrossRef] [PubMed]
- de la Rosa, L.A.; Alfonso, A.; Vilarino, N.; Vieytes, M.R.; Yasumoto, T.; Botana, L.M. Maitotoxin-induced calcium entry in human lymphocytes: Modulation by yessotoxin, Ca(2+) channel blockers and kinases. Cell Signal. 2001, 13, 711–716. [Google Scholar] [CrossRef]
- Reyes, J.G.; Osses, N.; Knox, M.; Darszon, A.; Treviño, C.L. Glucose and lactate regulate maitotoxin-activated Ca2+ entry in spermatogenic cells: The role of intracellular [Ca2+]. FEBS Lett. 2010, 584, 3111–3115. [Google Scholar] [CrossRef] [PubMed]
- Trevino, C.L.; De la Vega-Beltran, J.L.; Nishigaki, T.; Felix, R.; Darszon, A. Maitotoxin potently promotes Ca2+ influx in mouse spermatogenic cells and sperm and induces the acrosome reaction. J. Cell. Physiol. 2006, 206, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Xi, D.; Kurtz, D.T.; Ramsdell, J.S. Maitotoxin-elevated cytosolic free calcium in GH4C1 rat pituitary cells nimodipine-sensitive and insensitive mechanisms. Biochem. Pharmacol. 1996, 51, 759–769. [Google Scholar] [CrossRef]
- Chen, J.; Barritt, G.J. Evidence that TRPC1 (transient receptor potential canonical 1) forms a Ca(2+)-permeable channel linked to the regulation of cell volume in liver cells obtained using small interfering RNA targeted against TRPC1. Biochem. J. 2003, 373, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Nesin, V.; Tsiokas, L. TRPC1. Handb. Exp. Pharmacol. 2014, 222, 15–51. [Google Scholar] [PubMed]
- Kukkonen, J.P. A menage a trois made in heaven: G-protein-coupled receptors, lipids and TRP channels. Cell Calcium. 2011, 50, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Bernard, V.; Laurent, A.; Derancourt, J.; Clement-Durand, M.; Picard, A.; Le Peuch, C.; Berta, P.; Doree, M. Maitotoxin triggers the cortical reaction and phosphatidylinositol-4,5-biphosphate breakdown in amphibian oocytes. Eur. J. Biochem. 1988, 174, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Strubing, C.; Krapivinsky, G.; Krapivinsky, L.; Clapham, D.E. TRPC1 and TRPC5 form a novel cation channel in mammalian brain. Neuron 2001, 29, 645–655. [Google Scholar] [CrossRef]
- Hofmann, T.; Schaeffer, M.; Schultz, G.; Gudermann, T. Subunit composition of mammalian transient receptor potential channels in living cells. Proc. Natl. Acad. Sci. USA 2002, 99, 7461–7466. [Google Scholar] [CrossRef] [PubMed]
- Strubing, C.; Krapivinsky, G.; Krapivinsky, L.; Clapham, D.E. Formation of novel TRPC channels by complex subunit interactions in embryonic brain. J. Biol. Chem. 2003, 278, 39014–39019. [Google Scholar] [CrossRef] [PubMed]
- Storch, U.; Forst, A.L.; Philipp, M.; Gudermann, T.; Mederosy Schnitzler, M. Transient receptor potential channel 1 (TRPC1) reduces calcium permeability in heteromeric channel complexes. J. Biol. Chem. 2012, 287, 3530–3540. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, A.; Fahlbusch, M.; Gudermann, T. Classical Transient Receptor Potential 1 (TRPC1): Channel or Channel Regulator? Cells 2014, 3, 939–962. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Legislation on the Protection of Animals Used for Scientific Purposes. European Commission Web Site. Available online: http://ec.europa.eu/environment/chemicals/lab_animals/legislation_en.htm (accessed on 28 May 2017).
- Martínez, M.; Salvador, C.; Farias, J.M.; Vaca, L.; Escobar, L.I. Modulation of a calcium-activated chloride current by Maitotoxin. Toxicon 1999, 37, 359–370. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores, P.L.; Rodríguez, E.; Zapata, E.; Carbó, R.; Farías, J.M.; Martínez, M. Maitotoxin Is a Potential Selective Activator of the Endogenous Transient Receptor Potential Canonical Type 1 Channel in Xenopus laevis Oocytes. Mar. Drugs 2017, 15, 198. https://doi.org/10.3390/md15070198
Flores PL, Rodríguez E, Zapata E, Carbó R, Farías JM, Martínez M. Maitotoxin Is a Potential Selective Activator of the Endogenous Transient Receptor Potential Canonical Type 1 Channel in Xenopus laevis Oocytes. Marine Drugs. 2017; 15(7):198. https://doi.org/10.3390/md15070198
Chicago/Turabian StyleFlores, Pedro L., Emma Rodríguez, Estrella Zapata, Roxana Carbó, José María Farías, and Martín Martínez. 2017. "Maitotoxin Is a Potential Selective Activator of the Endogenous Transient Receptor Potential Canonical Type 1 Channel in Xenopus laevis Oocytes" Marine Drugs 15, no. 7: 198. https://doi.org/10.3390/md15070198
APA StyleFlores, P. L., Rodríguez, E., Zapata, E., Carbó, R., Farías, J. M., & Martínez, M. (2017). Maitotoxin Is a Potential Selective Activator of the Endogenous Transient Receptor Potential Canonical Type 1 Channel in Xenopus laevis Oocytes. Marine Drugs, 15(7), 198. https://doi.org/10.3390/md15070198






