The Structure and Nephroprotective Activity of Oligo-Porphyran on Glycerol-Induced Acute Renal Failure in Rats
Abstract
:1. Introduction
2. Results
2.1. The Structure Study of Oligo-Porphran
2.1.1. Preparation, Fraction, and Analysis of Oligo-Porphyran (OP)
2.1.2. Structure of Oligo-Porphyran (OP)
2.2. The Nephroprotective Activity of Oligo-Porphyran on Glycerol-Induced Acute Renal Failure in Rats
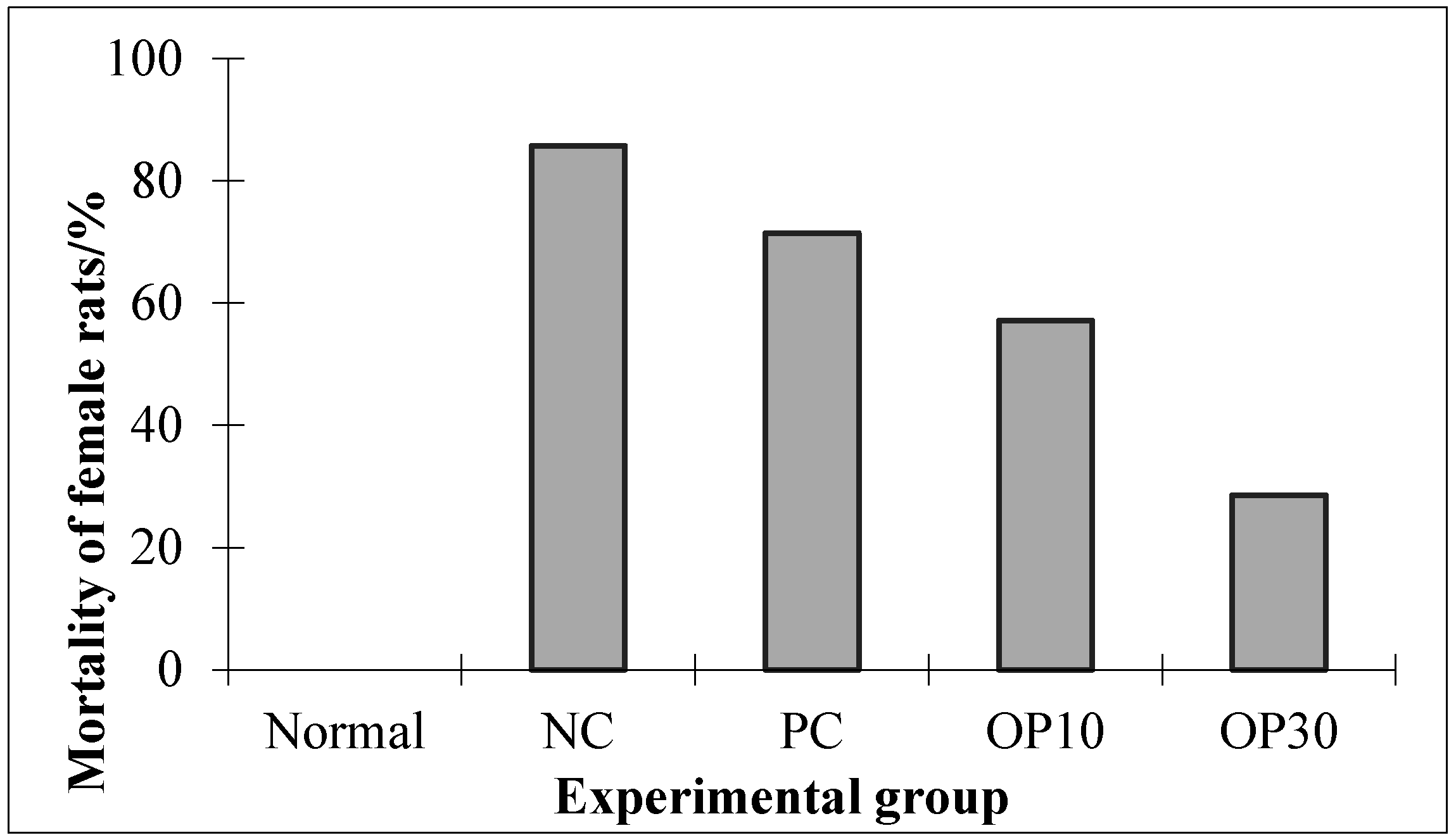
2.2.1. The Mortality Effect of Oligo-Porphyran (OP) in ARF Rats
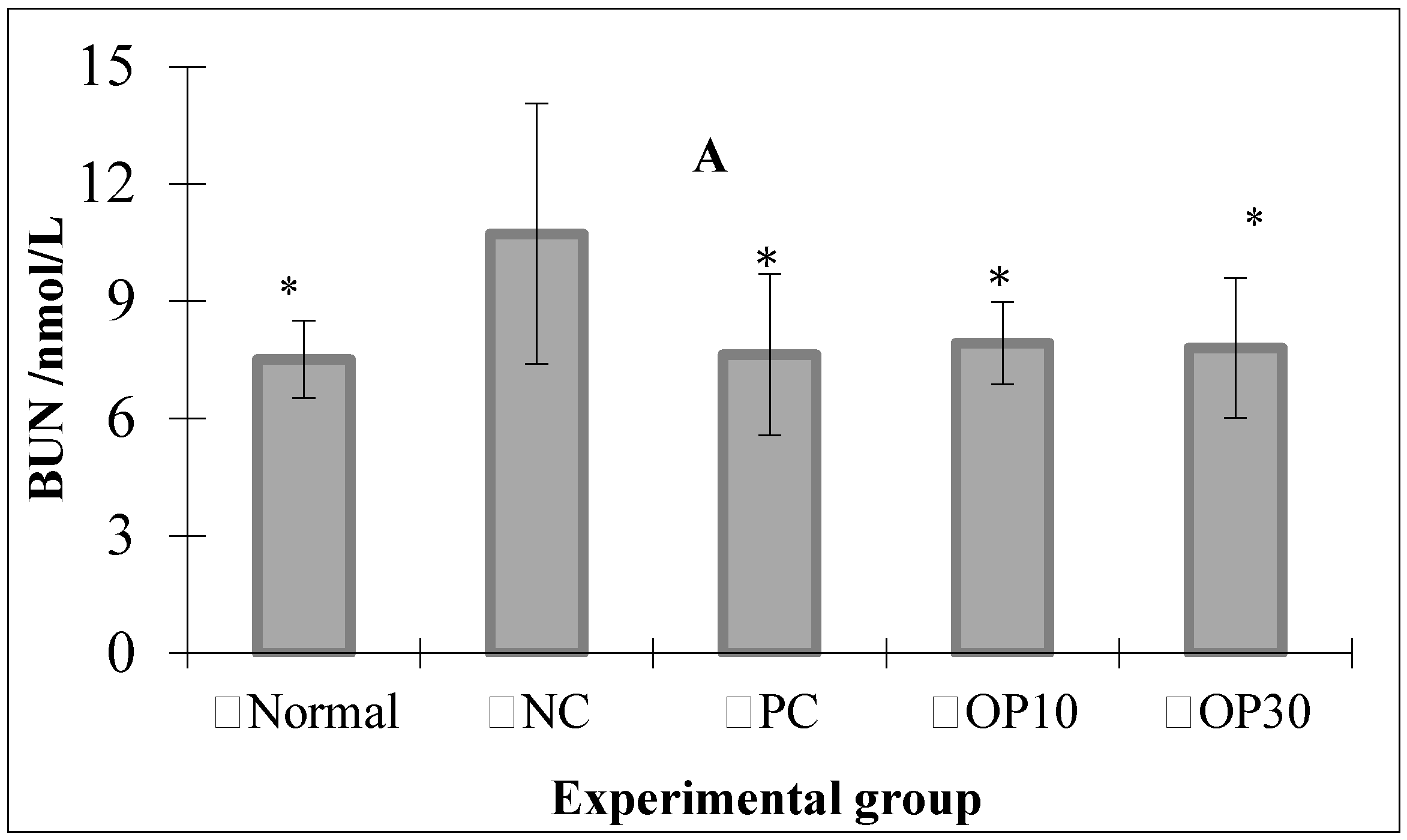
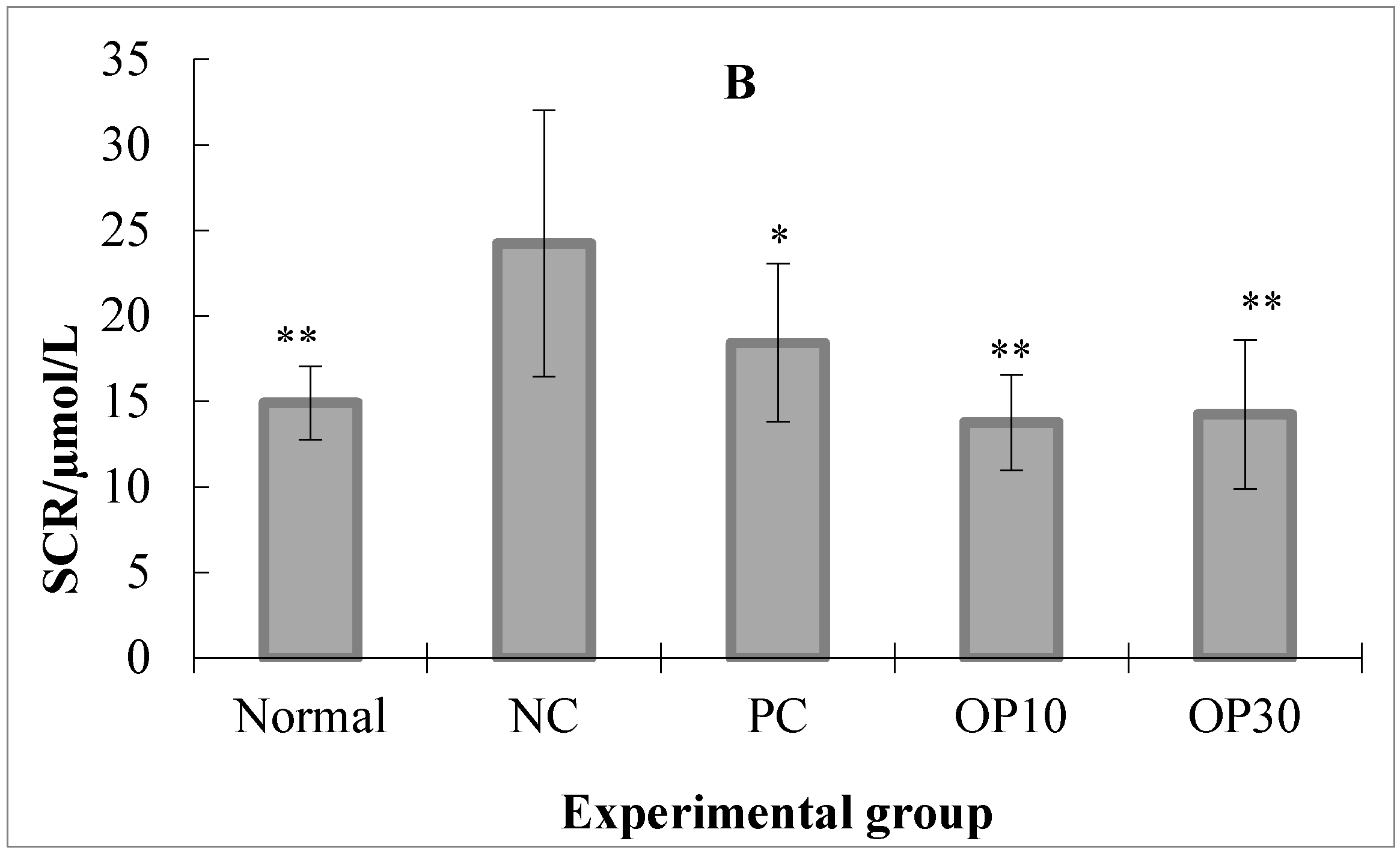
2.2.2. The BUN and SCR Level in ARF Rats
2.2.3. The Ions and Protein Levels in the Urine and Plasma of ARF Rats
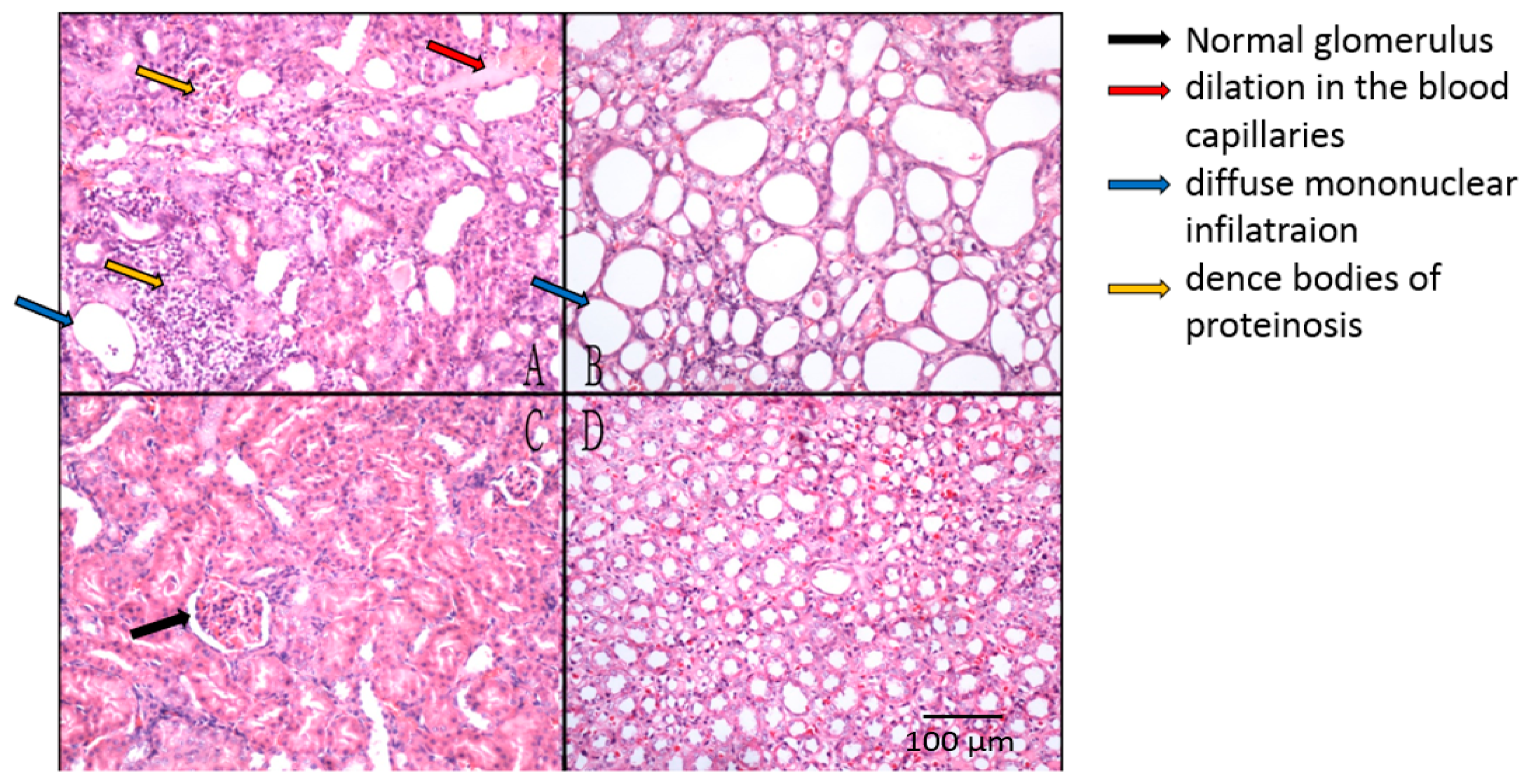
2.2.4. Histopathological Studies on Renal Tissues of ARF Rats
3. Discussion
4. Materials and Methods
4.1. Preparation and Analysis of Oligo-Porphyran
4.2. Fraction of Oligo-Porphyran OP
4.3. The Structural Analysis of Oligo-Porphyran
4.4. Experimental Animals and Treatments
4.5. Biological Measurement
4.6. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Koza, Y. Acute kidney injury: Current concepts and new insights. J. Inj. Violence Res. 2016, 8, 58–62. [Google Scholar] [PubMed]
- Jim, S.; Oken, D.E.; Arce, M.L.; Wilson, D.R. Glycerol-induced hemoglobinuric acute renal failure in the rat. I. Micropuncture study of the development of oliguria. J. Clin. Investig. 1966, 45, 724–735. [Google Scholar]
- Thiel, G.; Wilson, D.R.; Arce, M.L.; Oken, D.E. Glycerol-induced hemoglobinuric acute renal failure in the rat. Nephron 1967, 4, 276–297. [Google Scholar] [CrossRef] [PubMed]
- Ferenbach, D.A.; Bonventre, J.V. Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD. Nat. Rev. Nephrol. 2015, 11, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Basile, D.P.; Anderson, M.D.; Sutton, T.A. Pathophysiology of acute kidney injury. Compr. Physiol. 2012, 2, 1303–1353. [Google Scholar] [PubMed]
- Shusterman, N.; Strom, B.L.; Murray, T.G.; Morrison, G.; West, S.L.; Maislin, G. Risk factors and outcome of hospital-acqusiology of acute renal failure. Am. J. Med. 1987, 83, 65–71. [Google Scholar] [CrossRef]
- Bell, S.; Dekker, F.W.; Vadiveloo, T.; Marwick, C.; Deshmukh, H.; Donnan, P.T.; Diepen, M.V. Risk of postoperative acute kidney injury in patients undergoing orthopaedic surgery—Development and validation of a risk score and effect of acute kidney injury on survival: observational cohort study. BMJ 2015, 351, 5639–5642. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Qi, H.; Zhao, T.; Deslandes, E.; Ismaeli, N.M.; Molloy, F.; Critchley, A.T. Chemical characteristics of a polysaccharide from Porphyra capensis (Rhodophyta). Carbohydr. Res. 2005, 340, 2447–2450. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Sharma, K.; Sharma, A.; Nagpal, K.; Bera, T. Anti-inflammatory, Analgesic and Antiulcer properties of Porphyra vietnamensis. Avicenna J. Phytomed. 2015, 5, 69–77. [Google Scholar] [PubMed]
- Isaka, S.; Cho, K.; Nakazono, S.; Abu, R.; Ueno, M.; Kim, D.; Oda, T. Antioxidant and anti-inflammatory activities of porphyran isolated from discolored nori (Porphyra yezoensis). Int. J. Biol. Macromol. 2015, 74, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, N.; Zhao, T.; Qi, H.; Xu, Z.; Li, Z. Fucoidan inhibits the development of proteinuria in active Heymann nephritis. Phytother. Res. 2005, 19, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Q.; Jin, W.; Niu, X.; Zhang, H. Effects and mechanism of low molecular weight fucoidan in mitigating the peroxidative and renal damage induced by adenine. Carbohydr. Polym. 2011, 84, 417–423. [Google Scholar] [CrossRef]
- Ustundag, S.; Sen, S.; Yalcin, O.; Ciftci, S.; Demirkan, B.; Ture, M. L-Carnitine Ameliorates Glycerol-Induced Myoglobinuric Acute Renal Failure in Rats. Ren. Fail. 2009, 31, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Ricksten, S.E.; Bragadottir, G.; Redfors, B.; Nordquist, L. Renal oxygenation and hemodynamics in acute kidney injury and chronic kidney disease. Clin. Exp. Pharmacol. Physiol. 2013, 40, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Ratliff, B.B.; Abdulmahdi, W.; Pawar, R.; Wolin, M.S. Oxidant Mechanisms in Renal Injury and Disease. Antioxi. Redox Signal. 2016, 25, 119–146. [Google Scholar] [CrossRef] [PubMed]
- Aydogdu, N.; Atmaca, G.; Yalcin, O.; Taskiran, R.; Tastekin, E.; Kaymak, K. Protective effects of 1-caenitine on myoglobinuric acute renal failure in rats. Clin. Exp. Pharmacol. Physiol. 2006, 33, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Zhang, Q.B.; Qi, H.M.; Zhang, H.; Niu, X.; Xu, Z.; Li, Z. Degradation of porphyran from Porphyra haitanensis and the antioxidant activities of the degraded porphyrans with different molecular weight. Int. J. Biol. Macromol. 2006, 38, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Zager, R.A. Rhabdomyolysis and myohemoglobinuric acute renal failure. Kidney Int. 1996, 49, 314–326. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Tanaka, T.; Cho, E.J.; Park, J.C.; Shibahara, N.; Yokozawa, T. Glycerol-Induced Renal Damage Improved by 7-O-Galloyl-d-sedoheptulose Treatment through Attenuating Oxidative Stress. Biol. Pharm. Bull. 2012, 35, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Müller, V.; Losonczy, G.; Heemann, U.; Vannay, Á.; Fekete, A.; Reusz, G.; Tulassay, T.; Szabó, A.J. Sexual dimorphism in renal ischemia-reperfusion injury in rats: Possible role of endothelin. Kidney Int. 2002, 62, 1364–1371. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Wang, M.H.; Dong, Z. Differential Gender Differences in Ischemic and Nephrotoxic Acute Renal Failure. J. Am. Soc. Nephrol. 2005, 25, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yu, P.; Li, Z.; Zhang, H.; Xu, Z.; Li, P. Antioxidant activities of sulfated polysaccharide fractions from Porphyra haitanesis. J. Appl. Phycol. 2003, 15, 305–310. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, J.; Simerly, T.; Jin, W.; Zhang, H.; Zhang, Q. Hydrogen peroxide released from Pyropia yezoensis induced by oligo-porphyrans: Mechanisms and effect. J. Appl. Phycol. 2015, 27, 1639–1649. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Q.B.; Wang, J.; Shi, X.L.; Zhang, Z. Analysis of the monosaccharide composition of fucoidan by precolumn derivation HPLC. Chin. J. Oceanol. Limnol. 2009, 27, 578–582. [Google Scholar] [CrossRef]
- Yaphe, W.; Arsenault, G.P. Improved resorcinol reagent for the determination of fructose, and of 3,6-anhydrogalactose in polysaccharides. Anal. Biochem. 1965, 13, 143–148. [Google Scholar] [CrossRef]
- Kawai, Y.; Seno, N.; Anno, K. A modified method for chondrosulfatase assay. Anal. Biochem. 1969, 32, 314–321. [Google Scholar] [CrossRef]
- Guo, Z.; Lei, A.; Zhang, Y.; Xu, Q.; Xue, X.; Zhang, F.; Liang, X. “Click saccharides”: Novel separation materials for hydrophilic interaction liquid chromatography. Chem. Commun. 2007, 24, 2491–2493. [Google Scholar] [CrossRef] [PubMed]
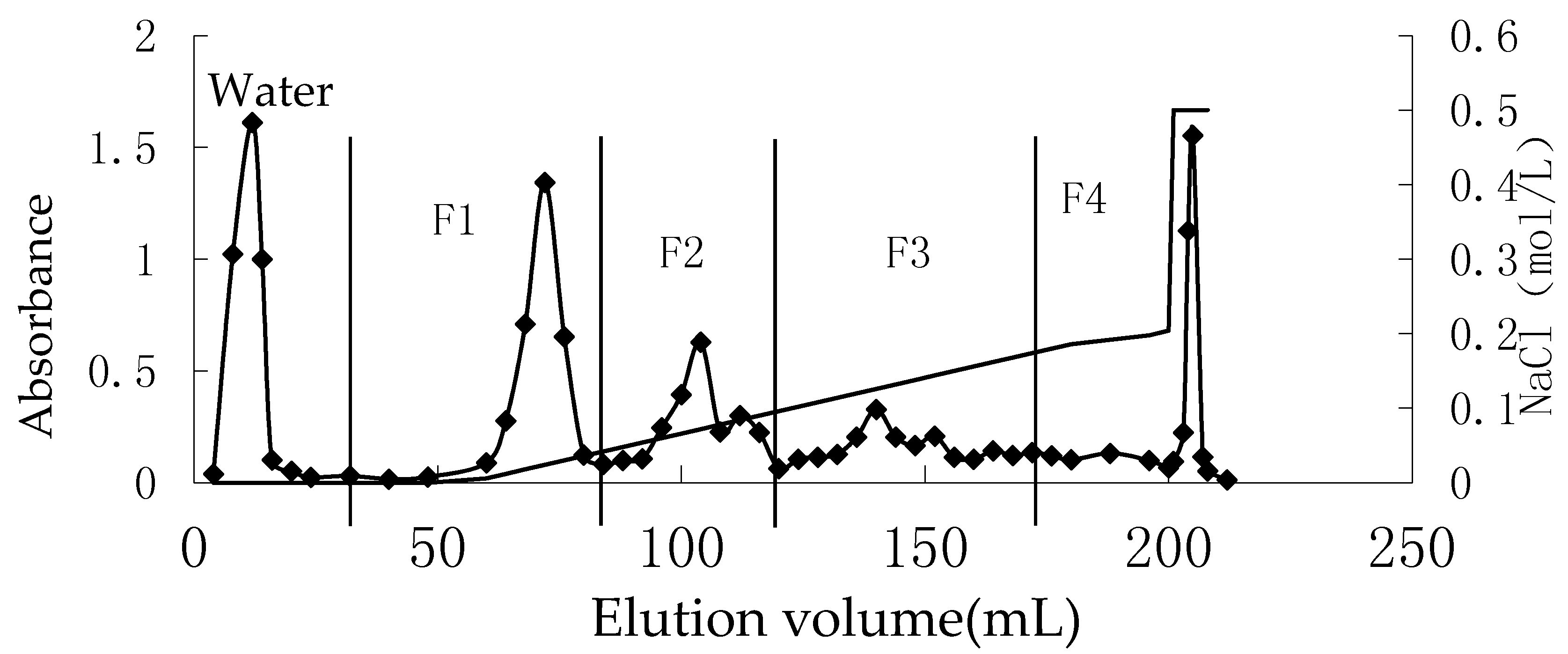
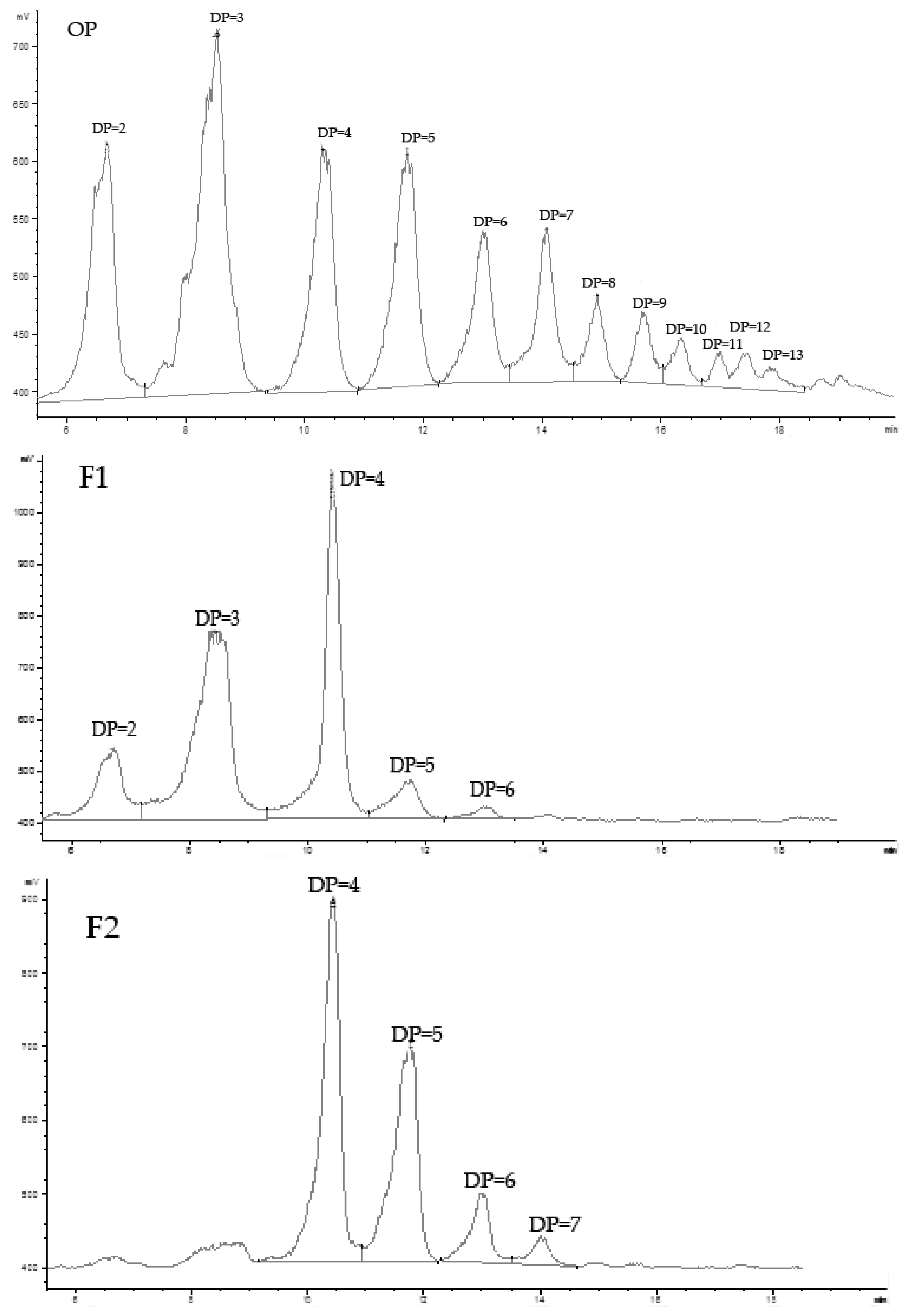
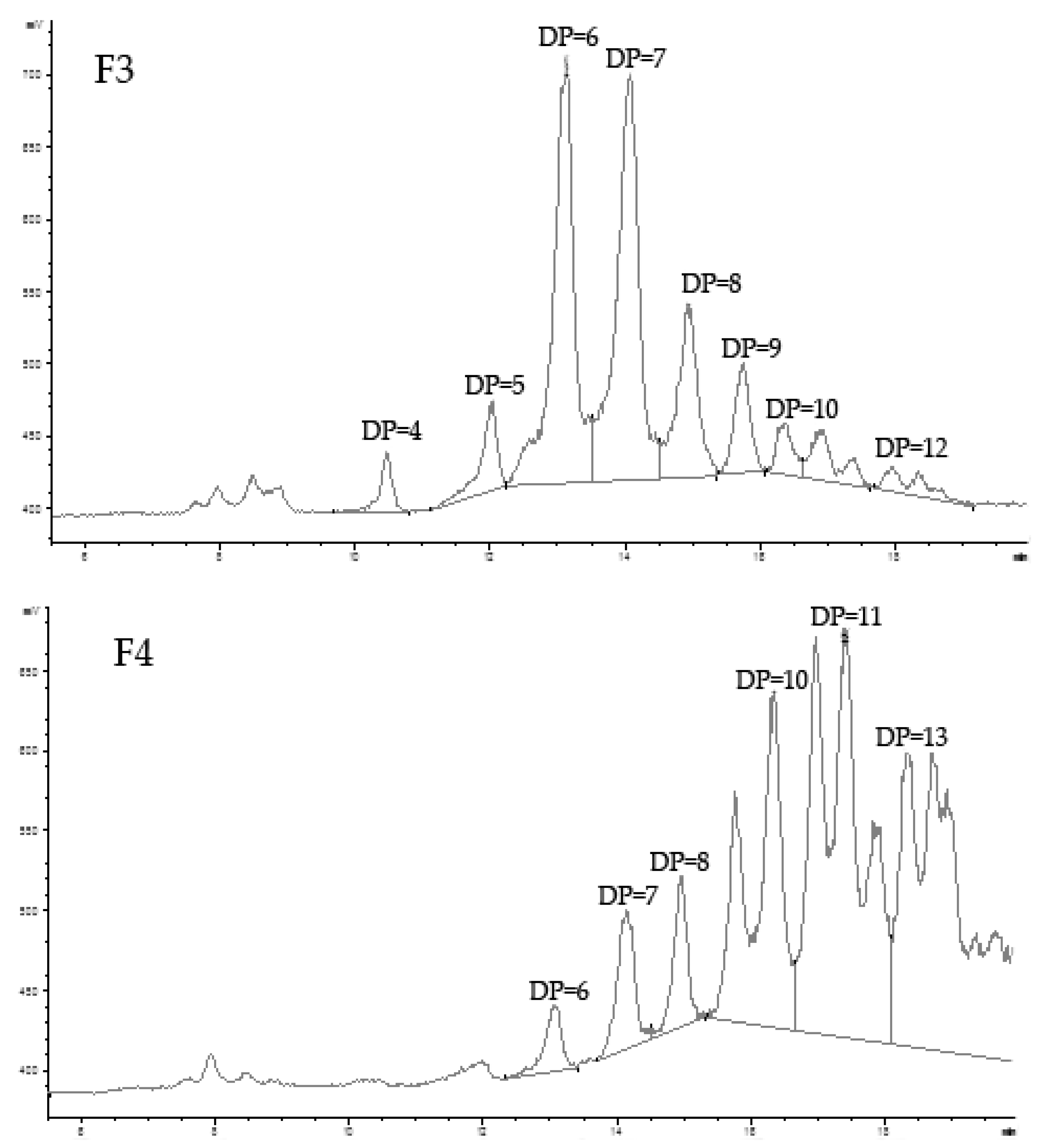
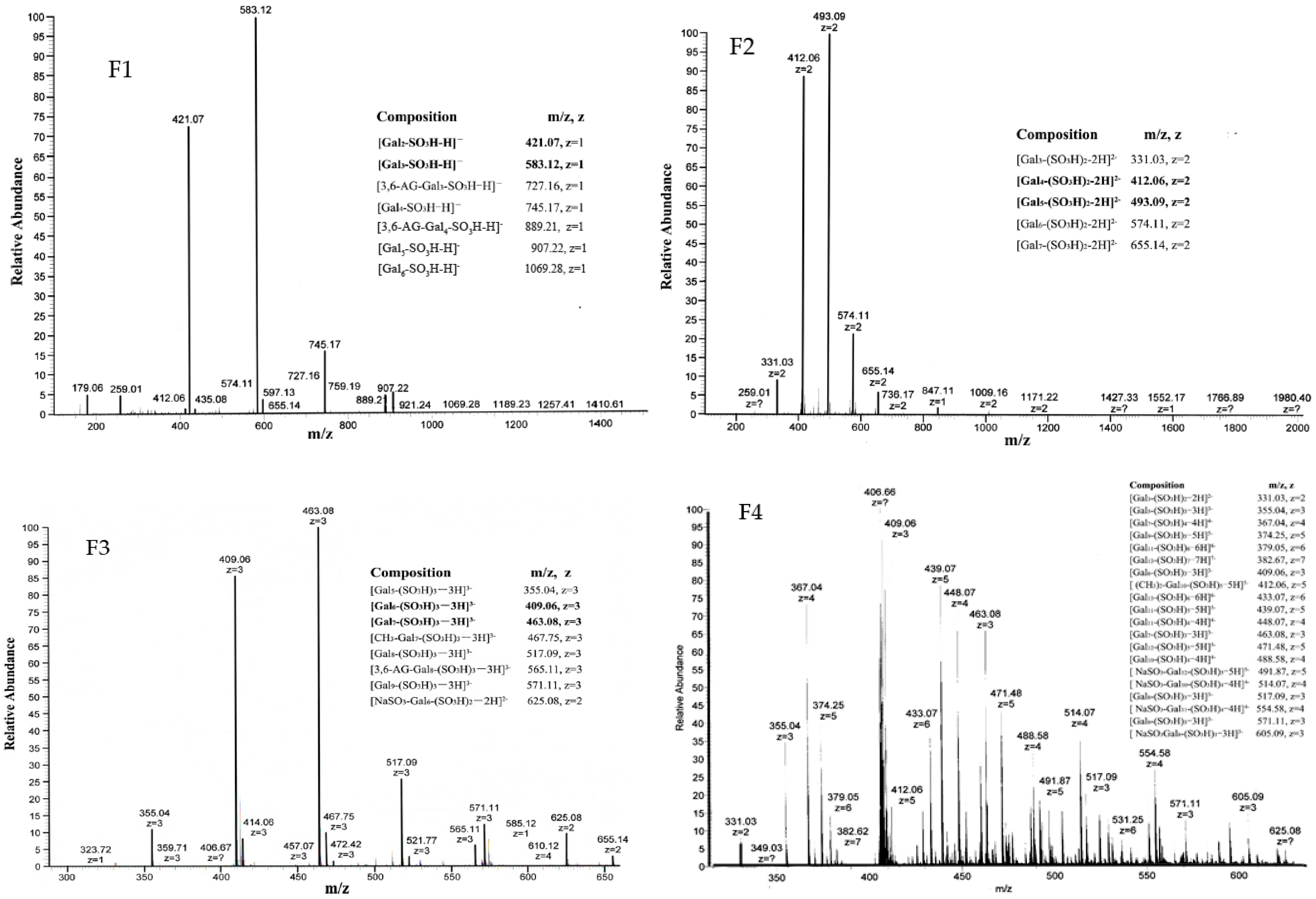
| Sample | Mw (Kda) | Sulfate Group (%) | Galactose (%) | 3,6-anhydrogalactose (%) |
|---|---|---|---|---|
| OP | 1.43 | 16.90 ± 0.51 | 59.93 ± 1.44 | 6.45 ± 0.17 |
| Gender | Index | Normal | NC | PC | OP10 | OP30 |
|---|---|---|---|---|---|---|
| Male | BUN (nmol/L) | 7.73 ± 0.27 | 11.48 ± 1.23 ** | 12.27 ± 0.74 ** | 11.96 ± 1.08 ** | 12.32 ± 2.11 ** |
| SCR (μmol/L) | 15.32 ± 0.74 | 26.72 ± 3.18 ** | 24.48 ± 1.64 ** | 27.11 ± 2.54 ** | 26.22 ± 1.71 ** | |
| Female | BUN (nmol/L) | 6.91 ± 0.54 (n = 7) | 13.44 ± 0.88 ** (n =4) | 13.18 ± 0.68 ** (n =4) | 12.64 ± 0.67 ** (n = 5) | 14.97 ± 1.29 ** (n = 5) |
| SCR (μmol/L) | 13.94 ± 1.05 (n = 7) | 25.64 ± 1.94 ** (n =4) | 24.99 ± 0.96 ** (n =4) | 26.70 ± 1.27 ** (n = 5) | 26.02 ± 1.13 ** (n = 5) |
| A | |||||
| Group | Albumin (g/L) | K+ (mmol/L) | Na+ (mmol/L) | Cl− (mmol/L) | Ca2+ (mmol/L) |
| Normal | 32.95 ± 2.27 | 5.48 ± 0.63 | 148.27 ± 0.81 ** | 106.43 ± 2.00 ** | 2.52 ± 0.13 |
| NC | 33.19 ± 1.34 | 6.27 ± 2.22 | 144.53 ± 2.04 | 99.41 ± 3.84 | 2.54 ± 0.23 |
| PC | 36.96 ± 1.79 | 7.03 ± 3.04 | 145.68 ± 1.98 | 97.04 ± 3.66 | 2.66 ± 0.19 |
| OP10 | 32.84 ± 1.55 | 5.46 ± 0.49 | 144.24 ± 1.66 | 103.70 ± 1.99 * | 2.49 ± 0.10 |
| OP30 | 32.82 ± 1.53 | 5.12 ± 0.24 | 143.63 ± 1.35 | 103.55 ± 0.54 * | 2.56 ± 0.07 |
| B | |||||
| Group | Albumin (g/L) | K+ (mmol/L) | Na+ (mmol/L) | Cl− (mmol/L) | Ca2+ (mmol/L) |
| Normal | 0.41 ± 0.19 | 64.56 ± 10.27 * | 228.48 ± 27.75 ** | 338.58 ± 68.46 ** | 3.67 ± 2.06 |
| NC | 0.29 ± 0.22 | 47.13 ± 10.60 | 74.07 ± 33.67 | 113.93 ± 54.31 | 5.00 ± 2.58 |
| PC | 0.62 ± 0.57 | 42.88 ± 17.50 | 43.33 ± 31.89 * | 71.49 ± 66.48 | 2.16 ± 2.10 * |
| OP10 | 0.25 ± 0.12 | 62.19 ± 5.22 ** | 185.21 ± 29.06 ** | 278.41 ± 60.09 ** | 3.78 ± 2.40 |
| OP30 | 0.29 ± 0.12 | 67.45 ± 4.19 ** | 201.38 ± 61.62 ** | 282.80 ± 054 ** | 4.09 ± 1.01 |
| Time (min) | Acetonitrile (%) | Buffer (%) |
|---|---|---|
| 0 | 80 | 20 |
| 30 | 40 | 60 |
| 40 | 10 | 90 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Hou, Y.; Duan, D.; Zhang, Q. The Structure and Nephroprotective Activity of Oligo-Porphyran on Glycerol-Induced Acute Renal Failure in Rats. Mar. Drugs 2017, 15, 135. https://doi.org/10.3390/md15050135
Wang J, Hou Y, Duan D, Zhang Q. The Structure and Nephroprotective Activity of Oligo-Porphyran on Glycerol-Induced Acute Renal Failure in Rats. Marine Drugs. 2017; 15(5):135. https://doi.org/10.3390/md15050135
Chicago/Turabian StyleWang, Jing, Yun Hou, Delin Duan, and Quanbin Zhang. 2017. "The Structure and Nephroprotective Activity of Oligo-Porphyran on Glycerol-Induced Acute Renal Failure in Rats" Marine Drugs 15, no. 5: 135. https://doi.org/10.3390/md15050135
APA StyleWang, J., Hou, Y., Duan, D., & Zhang, Q. (2017). The Structure and Nephroprotective Activity of Oligo-Porphyran on Glycerol-Induced Acute Renal Failure in Rats. Marine Drugs, 15(5), 135. https://doi.org/10.3390/md15050135





