Abstract
The long-lasting interest in bioactive molecules (namely toxins) produced by (microalga) dinoflagellates has risen in recent years. Exhibiting wide diversity and complexity, said compounds are well-recognized for their biological features, with great potential for use as pharmaceutical therapies and biological research probes. Unfortunately, provision of those compounds is still far from sufficient, especially in view of an increasing demand for preclinical testing. Despite the difficulties to establish dinoflagellate cultures and obtain reasonable productivities of such compounds, intensive research has permitted a number of advances in the field. This paper accordingly reviews the characteristics of some of the most important biotoxins (and other bioactive substances) produced by dinoflagellates. It also presents and discusses (to some length) the main advances pertaining to dinoflagellate production, from bench to large scale—with an emphasis on material published since the latest review available on the subject. Such advances encompass improvements in nutrient formulation and light supply as major operational conditions; they have permitted adaptation of classical designs, and aided the development of novel configurations for dinoflagellate growth—even though shearing-related issues remain a major challenge.
1. Introduction
During the latest few years, demand for new biocompounds, and its derivatives with biotechnological and pharmacological potential have experienced a remarkable increase [1]. This trend—to create/find innovative and competitive products through win-win approaches—has placed a considerable emphasis upon research on marine organisms, including microalgae and macroalgae [2]. Dinoflagellates, in particular, are a unique group of the former; they are unicellular planktonic microalgae, and a source of biotoxins affecting seafood safety—yet bearing a potential for human health at large. A recent boom has indeed been noticed, due to their unexpected applications as pharmacological drugs, and potential uses in the biology, biomedical and toxicological fields [3].
Commonly found in all types of ecosystems (marine, freshwater, benthic and even sea ice), this complex taxon is estimated to include 2000+ living species [4]. Half of them are photoautotrophs [5], whereas the remainder rely on mixotrophy or heterotrophy, as well as parasitic or symbiotic behaviors [3,6]. Ecologically speaking, dinoflagellates possessing photosynthetic pigments play a major role as primary producers in freshwater and marine habitats [7]. They can also be found in association with other marine organisms, e.g., sea anemones, protozoa, tissues of certain invertebrates, and stony corals [8].
Dinoflagellates have been reported as potent natural biotoxin producers, as many of these compounds are effective at far lower dosages than conventional chemical agents [9]. Their ability to synthesize toxic compounds, accompanied by sudden and booming growth in marine environments make them the major cause of harmful algal blooms (HABs). HABs cause discoloration on the sea surface, and are thus called ‘red tides’; 75–80% of toxic phytoplankton species therein are in fact dinoflagellates [10]. ‘Red tides’ may vary in color from common red to brown, yellow, green or blue, depending on the dominant species, concentration and depth [11]. Such high densities of dinoflagellates have been associated to aquatic faunal mortalities worldwide, since they can kill fish and/or shellfish—either directly via toxin production, or because their large numbers block animal gills and deplete available oxygen [12]. In addition, toxins accumulated in these organisms can be transferred to higher trophic levels through the food chain [9]. The negative impact on coastal areas is particularly notorious; it can not only disrupt the marine environment, but also disturb human economic activities (e.g., tourism, aquaculture, fisheries), and ultimately affect human health. How and why these natural phenomena occur remain to be fully understood, but weather and hydrographic conditions probably play a role [13].
According to the Taxonomic Reference List of Toxic Plankton Algae of Intergovernmental Oceanographic Commission (IOC), there are at least 95 dinoflagellates known to produce toxins (called phycotoxins), among 179 species of marine microalgae [14]. For some toxins, doses at the microgram per kilogram level are more than sufficient to kill [15]. It is not clear why some microalgal species produce biotoxins. Quilliam [16] claimed some of these second metabolites to be allellochemical agents against other species, aids in competing for a specific niche, defense against predation, and enzyme regulation or sexual response induction (feromones).
Several biotoxins produced by marine microalgae can traditionally be organized on the basis on their effects upon humans: paralytic shellfish poisoning (PSP), neurotoxic shellfish poisoning (NSP), diarrheic shellfish poisoning (DSP) and amnesic shellfish poisoning (ASP)—released by diatoms, azaspiracid poisoning (AZP), and ciguatera fish poisoning (CFP). Most such poisonings are caused by neurotoxins, which exhibit highly specific effects upon the nervous system in birds, animals or even humans upon ingestion of contaminated shellfish [9]. Human clinical effects/symptoms have been revised elsewhere [9,17].
Despite the above unfavorable aspects, the unique structure and diverse functionality of dinoflagellate biotoxins make them valuable and quite interesting compounds. Several toxicological and biological studies, entailing mixed and axenic cultures, have unfolded the potential of dinoflagellate-derived compounds (including biotoxins) as promising pharmacological effectors and/or biological investigation probes.
This review will focus on the pharmacological and biotechnological potential specifically of dinoflagellate-originated biotoxins, but will also briefly cover other important bioactive compounds produced thereby for the sake of completeness. It will also provide a brief overview of efforts (in terms of bioreactors and operational conditions) to improve their production, and discuss a few issues that still need improvement in attempts to attain higher dinoflagellate biomass and toxin productivities.
2. Characterization of Main Dinoflagellate Bioactive (Potential) Applications
Dinoflagellates are able to produce bioactive compounds with distinctive chemical structures, and a wide range of functional groups and toxicological and biological features; macrolides, cyclic polyethers, spirolides and purine alkaloids are but examples of such categories [18]. Due to their disparate functional structures, said biocompounds form a heterogeneous group that may strongly affect a variety of biological receptors and metabolic processes [10]. Hence, they may find application in human or veterinary medicine—in view of their wide range of potential pharmacological activities, i.e., in analgesic, antitumor, anticholesterol, cytotoxic, anti-infective, immuno-suppressive and/or neurological disease therapeutics [3]. Furthermore, other interesting dinoflagellate-derived compounds, such as pigments (e.g., peridinin), fatty acids (e.g., PUFAs) or polysaccharides, have shown noteworthy evidence for extra health benefits as nutraceuticals, prevention of development and anti-proliferation of tumor cells, and anti-inflammatory and antiviral activities [2,19,20].
Due to their biological potential—and despite several difficulties in getting the minimum amounts of biotoxins for testing, several studies and patents encompassing applications of dinoflagellates, directly associated to biotoxins, have been published or filed (Table 1).

Table 1.
Selected patents related to biotoxins produced by dinoflagellates and potential therapeutic uses.
The next sub-sections entail an overview of the most important biotoxins, complemented later by a brief reference to selected bioactives (i.e., gambieric acid, goniodomin, ampidinolide and ampidinol)—regarding their mode of action and biological potential, toward pharmaceutical and biotechnological applications.
2.1. Saxitoxin (and Analogues)
Saxitoxin (STX) and ca. six dozen naturally occurring analogues (such as gonyautoxins and neosaxitoxin) are produced mainly by marine dinoflagellates belonging to genera Alexandrium (e.g., A. minutum, A. tamarense, A. catenella), Gymnodinium (G. catenatum) and Pyrodinium (P. bahamense). However, other sources of STX-group toxins were identified—as is the case of cyanobacteria, including Anabaena, Cylindrospermopsis, Aphanizomenon, Planktothrix and Lyngbya genera [21].
STX is an alkaloid belonging to a group of marine natural products containing guanidine groups as main structural components. STX is composed of a 3,4-propinoperhydropurine tricyclic system (Figure 1), and the presence of two guanidine groups makes this molecule highly polar [21,22,23]. According to the specific functional group, there are carbamonyl (e.g., STX, neosaxitoxin, gonyautoxin 1 and 4 GTX1 and GTX4), decarbamoyl (e.g., GTX2 and GTX3) and N-sulfocarbamoyl (i.e., GTX5 and GTX6) saxitoxins. Another group comprises hydroxylated saxitoxins [24]. The corresponding chemical substituent in the main structure is a clue to its toxic potency (carbamoyl > decarbamoyl > N-sulfocarbamoyl) in model organisms (i.e., mouse) [24,25].
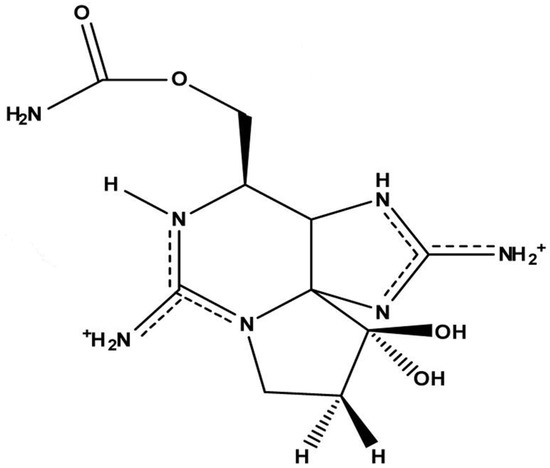
Figure 1.
Chemical structure of saxitoxin (STX) (adapted from [21]).
This group of biotoxins act as highly selective sodium channel blockers, thus preventing the influx flow of Na+ ions and compromising generation of action potentials [21,26]. Since STXs and their analogues make neurons and muscle cells lose their ability to transmit electrical impulses [27], they have a therapeutic potential as anesthetic agents. They may indeed reduce, or even block pain sensation, decrease muscle spasm, induce muscle relaxation and reduce wrinkles. Despite its potential applications, human clinical trials pose an obstacle—since toxicity often persists [21]. Several studies suggest that interaction with binding site 1 of voltage-gated Na+ channels (VGSCs) can induce prolonged anesthetic effects when STX is combined with other drugs [28,29]. For instance, some liposomal formulations of STX (either alone or conjugated) were tested, and able to provide extended sciatic nerve block within rats, along with marginal systemic and local toxicities [28]. Apart from their application as therapeutic agents, STXs and their analogues may behave as markers to locate sodium channels, and constitute a research tool in the study of those channels. This could be an asset for sodium channel related-diseases, including diagnosis and treatment of patients suffering from those disorders.
Additionally, STXs have been reported as possessing antimicrobial activity (namely antibacterial, antifungal, antialgal and antiprotozoal). However, most such studies have resorted only to in vitro assays [30].
Some important analogues of STXs, e.g., gonyautoxins (GTXs), are produced by Amphydinium dinoflagellates, and exhibit a similar mode of action. They are paralytic toxins as well, and bind to VGSCs thus blocking the synaptic function. However, those biotoxins have proven a safe therapeutic approach against acute or chronic anal fissures. GTXs aid in sphincter relaxation, and thus function as pain killer [31]. GTX2 and GTX3 have also been used to treat chronic tension-type headache [32].
2.2. Tetrodotoxin
Tetrodotoxin (TTX) is traditionally known as the chief toxin in pufferfish, even though it is also produced by other marine animals (e.g., octopuses, gobies, sea stars) [17]. TTX-bacteria producing species were also identified in Actinomyces, Aeromonas, Alteromonas, Bacillus and Pseudomonas genera [33]; the only reference to a microalga producer is (dinoflagellate) A. tamarense [34]. There are at least 30 structural analogues of TTX reported to date, and their toxicity degree can differ according to the chemical structure [35]. TTX possesses a highly unusual chemical structure, containing a positively charged guanidinium moiety attached to a highly oxygenated carbon backbone. The carbon backbone of TTX consists of a 2,4-dioxyadamantane structure, decorated with 5 hydroxyl groups (Figure 2) [36]. This potent neurotoxin is of particular interest owing to its resemblance to saxitoxins (and analogues) in terms of effects. In fact, TTX has high affinity to VGSCs, thus blocking the propagating nerve and muscle action potentials [33]. Although TTX is extremely sensitive to Nav1.1, Nav1.2, Nav1.3 and Nav1.7, it can bind to other VGSCs subtypes to a lesser extent [37,38].
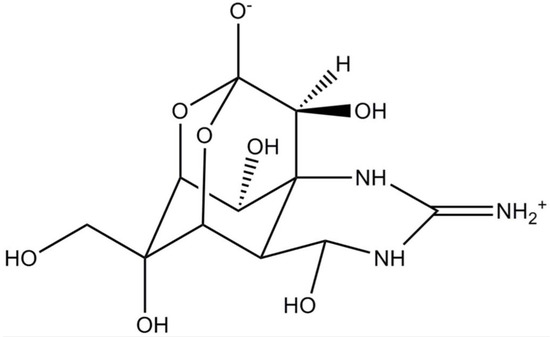
Figure 2.
Chemical structure of tetrodotoxin (TTX) (adapted from [39]).
Besides VGSC key role in pain, TTX-sensitive subtypes have been implicated in normal and pathological pain [39]; TTX is indeed a powerful and selective drug, with an analgesic/anesthetic effect associated to its sodium channel-blocking properties. Several studies have demonstrated its effectiveness in many types of pain management protocols [40,41,42]. For instance, a powerful drug from this potent neurotoxin—Tectin® by Wex Pharmaceuticals in Canada (http://www.wexpharma.com), is currently undergoing phase III clinical trials, with great success as pain controller in cancer patients [43]. Phase II clinical trials are also ongoing, aimed at assessing the efficacy of TTX against neuropathic pain produced by chemotherapy-induced peripheral neuropathy. In addition, a formulation—TocudinTM, is under investigation for local and topical anesthesia, and preclinical testing will start soon [39]. The aforementioned TTX is currently obtained from pufferfish, since the production directly by dinoflagellates has proven unfeasible to date [3]. Additionally, TTX had been applied as moderator for acute heroin withdrawal symptoms (headache), with minor side effects [44].
2.3. Okadaic Acid and Dinophysistoxin
Okadaic acid (OA) and its derivatives, including dinophysistoxins (DTX)-1, 2 and 3, are polyether marine biotoxins found in various species of shellfish, and produced by several dinoflagellates [45]. They were first isolated from benthic dinoflagellates of Prorocentrum genus (e.g., P. lima, P. concavum, P. belizeanum, P. maculosum) and Dinophysis genus (e.g., D. acuta, D. acuminate, D. fortii) [46]; said biotoxins are potent protein phosphatase inhibitors, specifically serine and threonine phosphatases [10]. They are organized into long chain compounds (Figure 3), containing transfused or spiro-linked cyclic polyether rings with hydroxyl and carboxyl functions and methyl groups differing in number or position [16,47].
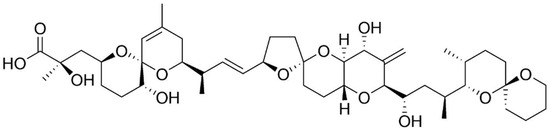
Figure 3.
General structure of okadaic acid (adapted from [65]).
OA and DTXs are highly selective inhibitors of protein phosphatase types 1 (PP1) and 2A (PP2A); these enzymes have been implicated in a wide spectrum of reaction cascades. In fact, they were associated with metabolism, gene expression, cell proliferation, morphogenesis, ion regulation, neurotransmission, membrane transport, and cell cycle progression or secretion [48]. Blocking protein phosphatase activity results in hyperphosphorylation of many cell proteins, which in turn leads to dramatic effects upon normal regulatory pathways [49]. Therefore, OA and its analogues are extremely useful research tools for investigating cellular regulation processes, especially those related to reversible phosphorylation of proteins—such as signal transduction, cell division and memory [50].
Several studies, either in vitro or in vivo, have demonstrated the value of OA in medical/pharmacological research [51]. OA is a potent promoter of tumorgenesis [52], apart from having cytotoxic effects (apoptosis and cell growth inhibition) in many cell types—including intestinal cells, blood cells, neuronal cells, lung cells and hepatic cells. Its cytotoxic effects extend to embryonic development, immune and nervous system [51]. Due to inhibition of protein PP2A, OA has been used as an emerging tool for research on Alzheimer’s and other neurodegenerative disorders associated to memory-impairment [53,54,55,56,57]. Studies on diabetes, AIDS and cancer have also resorted to OA as biotoxin-model to elucidate several mechanisms associated thereto [58,59,60]. Furthermore, OA seems to have immunoregulatory potential, since it induces down-regulation of T-cell receptor expression—thus compromising T-cell responsiveness, and consequently immune response [61]. It has also the ability to stimulate inflammatory response via a considerable increase of interleukin 8 (IL-8) in HL-60 human cells [62]. Being a powerful tumor promoter, OA has also been claimed as angiogenic inducer in human endothelial cells, via the increasing activity of hypoxia-inducible factor-1 (HIF-1)—closely related to vascular endothelial growth factor [63].
Finally, OA from Prorocentrum has been shown to possess fungicidal activity—namely ability to inhibit growth of Candida albicans [64].
2.4. Yessotoxin
Yessotoxin (YTX) is a marine sulphated polyether, produced by Protoceratium reticulatum, Lingulodinium polyedra [66] and Gonyaulax spinifera [67] dinoflagellate species. Data have been generated pertaining to more than 90 natural analogues of YTX recovered from cultures of P. reticulatum; the chemical structure of this toxin has already been elucidated, yet the structures of most of its analogues remain to be resolved [68]. This toxin is composed by a distinctive ladder-shape formed by several ether rings of different sizes, and a terminal acyclic unsaturated side chain consisting of 9 carbons and 2 sulfate ethers (Figure 4). The core structure is liposoluble, but the two sulfate groups convey amphoteric features to the molecule. Owing to this characteristic, such a compound is considered one of the most polar among the otherwise lipophilic toxins [69,70].
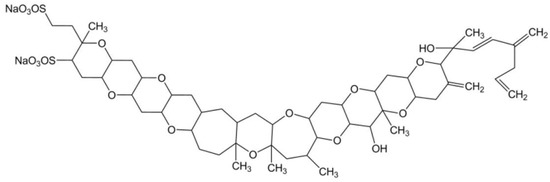
Figure 4.
Chemical structure of yessotoxin (adapted from [89]).
YTX and its analogues are particularly interesting tools for probing biological and pharmacological mechanisms [71]; they indeed can interfere with several biological apoptotic pathways in a variety of cellular systems, including tumor cells [72]. YTX can also induce non-apoptotic cell death in BC3H1 myoblast cells, primary cortical neurons and glioma cells [71,73]. Some studies have pointed at YTX as a potent phospodiesterase (PDE) activator [74,75], although the exact mode of action remains uncertain [17]. PDEs play a key role as regulators of signal transduction, mediated by such second messenger molecules as cyclic adenosine monophosphate (cAMP) [76]. Moderate modulation of intracellular calcium and cAMP levels [75,77], promotion of caspase protein activation [78], permeability transition through mitochondria [79], alteration of cytoskeleton (viz. selective disruption of F-actin microfilaments) [80,81], and fragmentation of adhesion proteins (specifically E-cadherin) [82], are among the reported YTX effects—dependent on cell line used and treatment duration [83]. Recently, YTX was found to induce mitotic catastrophe and genetic alterations—which may be of interest for control of tumor progression [84]. Additionally, Tobío et al. [85] have claimed regression of melanoma tumor cells in mouse cells in vivo, along with negligible toxicity. YTX may also pay a minor role as anti-allergenic and -asthmatic drug, even though the mechanism underlying these therapies remains poorly understood [85]. YTX seems to interfere with the immune function, since it reduces phagocytic activity on J774 cell line and increases expression of cytokines in J774 phagocyte mammalian cells [80]. Moreover, it appears to regulate the immune-effect on T-lymphocyte EL-4 cells via reversible down-regulation of the T-cell receptor complex [49,61]. Regarding other pharmacological effects, YTX and its analogues may be employed as therapy for Alzheimer’s disease [73]. These compounds have improved the levels of t- and β-amyloid—both insoluble structures that appear in the brain and are responsible for triggering said disease [86]. Furthermore, YTX may aid in treatment/prevention of lipid and glucose metabolism-associated diseases [73]. Early studies unfolded fatty degeneration, with alterations in pancreas and liver [87]; significant transcriptional alterations in lipid and glucose metabolism were in fact described in glioma cells [88].
A markedly increased activity against fungi and yeasts was reported when YTX had been chemically desulfated, with reduced toxicity toward mouse species. Therefore, expectations remain high with regard to YTX produced by dinoflagellates as promising candidates for novel and potent antifungals [64].
2.5. Pectenotoxin
Pectenotoxin (PTX), together with its analogues, are polyether macrolide compounds produced exclusively by Dinophysis species (e.g., D. fortii, D. acuta, D. tripus, D. acuminate, D. caudate, D. rotundata, D. norvegica) [65]. More than 20 analogues have been isolated to date [90], with disparate toxicological potency. Their common structural features include a spiroketal group, three oxolanes, a bicyclic ketal and a six-membered cyclic hemiketal (Figure 5) [91].
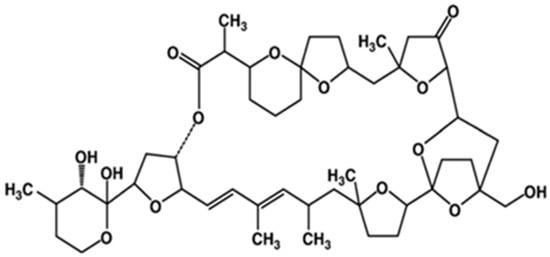
Figure 5.
General structure of pectenotoxin (adapted from [97]).
In general, they exhibit strong toxicity against hepatocytes; their action mechanism in vitro and in vivo encompasses actin filament depolymerization, leading to notorious effects upon cytoskeleton arrangement [92,93]. As a result, PTX causes cell cycle arrest and apoptosis [94]—being particularly effective against tumor cells, rather than normal cells of the same tissue [92]. For instance, PTX-2 has demonstrated antitumor activity against human lung, colon and breast cancer cells [95]; and was claimed as potent chemotherapeutic agent against p53-deficient tumors [96].
2.6. Ciguatoxin
Ciguatoxin (CTX) belongs to the group of marine polycyclic ether biotoxines implicated in ciguatera fish poisoning outbreaks. This fat-soluble substance is produced by certain strains of benthic Gambierdiscus toxicus, and may arise in fish from a biotransformation of gambiertoxins (e.g., maitotoxins) as precursors [98]. It accumulates throughout the food chain up to higher predators, and may ultimately reach human consumers—thus causing neurological, gastrointestinal and cardiovascular disorders [99,100,101,102]. More than 20 analogues have been found in the Pacific area, and multiple forms of CTX with minor molecular differences and toxicities were found in Caribbean waters [100] and the Indian Ocean [103]. This group of lipid-soluble polyethers are composed by a distinct and long semi-rigid ladder-like structure comprising several trans/syn ether rings at different sizes (Figure 6) [104,105].
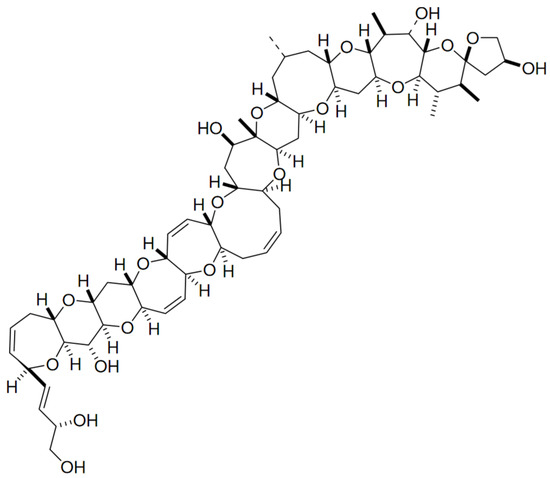
Figure 6.
General structure of ciguatoxin (adapted from [97]).
CTX is a potent modulator of site 5 on VGSCs of a wide variety of cells, following a mechanism similar to BTX; however, it is one hundred-fold more potent than BTX in eliciting repetitive neuron firing [105,106,107]. This compound is able to shift the potential activation (hyperpolarization), and change gating properties by activating VGSCs in a persistent way (from nM- to pM-concentration range), thus resulting in an enhanced Na+ inward current directly into excitable cells accompanied by an efflux of K+ [108]. The plasma membrane is unable to maintain the internal conditions leading to modification of bioenergetics mechanisms, bleb formation and cell and mitochondrial swelling [100,109]. They were found to significantly slow nerve conduction rate, and reduce amplitude in human nerves—consistent with abnormal and extended Na+ channel opening in nerve membranes in vivo [110,111]. In addition, some normal cellular mechanisms counteract this effect when Na+ ions move into the cytosol—while evoking Ca2+ capture and increase of its level inside the cells. This calcium acts as a second messenger, thus disrupting important ion-exchange systems. Hence, elevated muscle contraction, especially in cardiac tissue, and high fluid secretion by gastrointestinal cells are observed [100].
In neuromuscular junctions—and apart from elicitation of repetitive action potentials, said biotoxin may cause a dramatic increase in asynchronous acetylcholine release, and impair synaptic vesicles [112]. Other effects of CTX encompass catecholamine secretion from neuroendocrine cells [113]. The diversity of human symptoms associated to ciguatera may arise from the different affinity of CTX for the various VGSCs (Nav) subtypes. The fact that CTX is reported as discriminatory of several Nav channel subtypes—particularly Nav 1.2 and 1.3 (brain), Nav 1.4 (skeletal muscle), Nav 1.5 (heart), Nav 1.6 (motor neuron, smooth muscle), Nav 1.7 (peripheral nervous system) and Nav 1.8 (peripheral nervous system) [114,115,116], make this biotoxin a resourceful tool to investigate the biological function and structure of said ion channels in further depth [107]. Note that these types of channels underlie several human diseases and channelopathies (e.g., chronic pain, cardiac arrhythmias, epilepsy, and even cancer) [116,117].
2.7. Maitotoxin
A unique polyketide-derived polycyclic compound, maitotoxin (MTX), has been recognized for its potential to aid in research in chemistry and biology [118]. It has indeed been reported as the largest and most potent secondary metabolite ever isolated; its acute toxicity against mice far exceeds that of tetrodotoxin [118,119]. Gambierdiscus (i.e., G. toxicus, G. australes, G. pacificus) is the only genus found so far that produces the three existing forms of MTX (i.e., MTX-1, -2 and -3) [120,121,122]. Recently, a new analogue—MTX-4, was found that is by Gambierdiscus excentricus (from Canary Islands) [123]. In addition, some MTX precursors are produced by Amphidinium carterae, Prorocentrum sp., Ostreopsis sp., Thecadinium sp. and Coolia monotis. MTX is water-soluble, and entais a complex ladder-shaped polycyclic molecule composed by several hydroxyl and sulphate groups (Figure 7) [9,119]. This toxin is believed to cause ciguatera, but with symptoms different from those caused by ciguatoxins—due to an apparently distinct mode of action [124]. In early reports, direct involvement with calcium voltage gated-channels was claimed for MTX. Nonetheless, other observations [125] indicate that MTX binds to the cell membrane (lipophilic domain), thus inducing non-selective influx of ions into the cells—which, in turn, activates the voltage-sensitive calcium channels [125]. Unfortunately, the specific target of this compound remains unknown [118]. MTX is believed to be a powerful disruptor of Ca2+ homeostasis, with a multiplicity of pharmacological effects upon several cell lines [119]. Its ability to trigger intracellular cascades of events—e.g., membrane depolarization in excitable cells [126], insulin [127] and neurotransmitter secretion [128,129], phosphounisitide breakdown (important in cell lipids and cell signaling) [130], programmed cell death [131], and fertilization [132,133], justify why this compound is a powerful tool for research in cell biology, namely when attempting to elucidate Ca2+-dependent cellular processes [119]. MTX has also been suggested to play a role in innate immune responses and inflammation in vivo [119,134]. Its toxic effect seems to trigger a mediated inflammation response via secretion of pro-inflammatory cytokines IL-1β; this may be viewed as an interesting tool for studying specific components of innate immune response and/or the physiology of inflammatory effector cells [119]. More recently, MTX was claimed as selective activator of a specific transient receptor potential (TRP) in Xenopus laevis Oocytes; TRP channels are apparently involved in the regulation of non-selective cation channels. MTX may be of potential use for further studies in these type of biological channels [135].
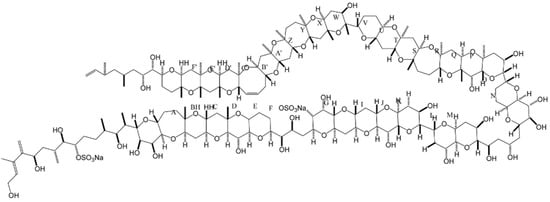
Figure 7.
Chemical structure of maitotoxin (adapted from [9]).
2.8. Palytoxin and Ostreocin
Palytoxin (PLTX) is a large and complex polyether compound, with a remarkable biological activity [9]—including a wide spectrum of pharmacological effects [136]. Originally isolated from the zoantharians of Palythoa genus [137], PLTX is also found in a number of marine organisms, including all species of Ostreopsis dinoflagellates (e.g., O. siamensis, O. mascarenensis, O. lenticularis, O. ovata, O. fattorussoi) [138]. This complex polyhydroxylated marine-derived molecule has both lipophilic and hydrophilic regions, and is composed by the longest continuous carbon atom chain of any known natural product (next to maitotoxin). Along its backbone, it possesses several hydroxyl groups, two diene motifs, and two hydrophobic hydrocarbon chains, among other structural features (Figure 8) [137,139].
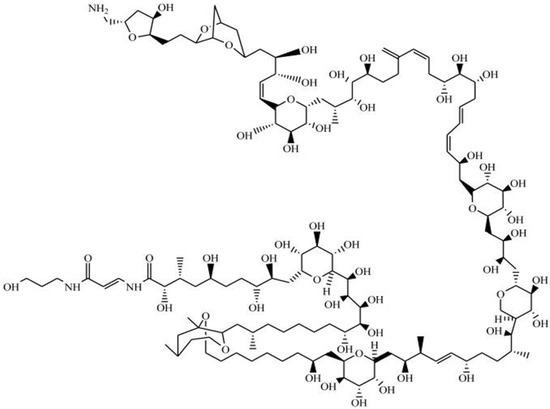
Figure 8.
Chemical structure of palytoxin (adapted from [139]).
PLTX-like compounds produced by dinoflagellates are commonly known as ostreocins. They are quite toxic against mammals; PLTX and its analogues may actually be the most lethal marine toxins known at present [136]. These compounds affect cellular function via inhibition of ATPase Na+/K+ pump—a transmembrane enzyme, essential to maintain ion homeostasis in excitable and non-excitable tissues [140]. PLTX accordingly restrains active transport of ions, and blocks the electrochemical gradient generated across the cell membrane—thus transforming the pump into a non-specific, permanently open ion channel. This leads to membrane depolarization and massive influx of calcium into the cytosol, thus compromising several cellular functions [139]. A number of studies have also indicated a wide variety of secondary pharmacological actions—including hemolysis, modulation of some neurotransmitters (norepinephrine and/or acetylcholine), and activation of pro-inflammatory signaling cascades (i.e., release of histamine and prostaglandin-E2) [141]. PLTX and ostreocin-D are apparently also involved in actin cytoskeleton distortion and dynamics, as proven via different cellular models (e.g., intestinal and neuroblastoma cells) [139,142]. The data so far available suggest that PLTX and ostreocin-D can modulate the unassembled actin pool, by activating signal transduction pathways not related to Ca2+ influx [143]. Despite these two compounds sharing the same molecular target, a few small structural differences can significantly reduce cytotoxicity and hemolytic potency in the case of ostreocin-D [144].
PLTX has also been claimed as powerful tumor promoter, and accounts for several effects, e.g., stimulation of arachidonic acid metabolism, modulation of epidermal growth factor (EGF) receptor, production of prostaglandins, and activation of mitogen-activated protein (MAP) kinase cascades [140]. In addition, patent EP3087172 claims that a pharmaceutical formulation with PLTX (sourced from Palythoa clavata polyps, comprising Symbiodinium dinoflagellate) is suitable for therapeutic use against lymphoblastic or myelogenous leukemia (Table 1).
Discovery of novel properties of PLTX and PLTX like-compounds, from marine dinoflagellates, may constitute a potential pathway for biotechnological characterization of living systems (e.g., focused on pump mechanism) [145,146]; and it may set the basis for a promising form of anti-tumor therapeutics.
2.9. Gambierol
Produced by Gambierdiscus toxicus dinoflagellate, gambierol is part of the group of polycyclic ethers; it is believed to be one of the components involved in ciguatera fish poisoning [147]. Its chemical structure resembles those of cigatoxins and brevetoxins, with potent neurotoxicity [105,148]; and is characterized by eight ether rings with two pyranyl rings—with methyl groups in a 1,3-diaxial orientation (Figure 9) [149,150]. As happens with other marine polyether metabolites, its scarcity from natural sources has hampered further biological studies. Chemical synthesis has been attempted to overcome these difficulties, and assure a higher availability of this substance for tests in vitro and in vivo [151]. Unlike such other marine polycyclic toxins as ciaguatoxins, gambierol does not envisage VGSCs as main targets [152]; it instead exerts a powerful modulatory action upon voltage-gated K+ channels (Kv) [153,154]. It acts as an intermembrane anchor, by binding specifically to Kv3.1 channels that, in turn, block Kv channels. As a consequence, the channels remain closed, thus lowering K+ ion currents [155]. Furthermore, it is able to evoke cytosolic calcium oscillations in cerebrocortical neurons, as an outcome of channel Kv inhibition [156,157,158]. Cao and co-workers [156] demonstrated that gambierol also induces outgrowth of neurites in a bidirectional manner; this may be promising for victims of neural injury.
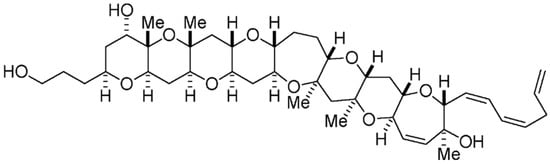
Figure 9.
Chemical structure of gambierol (adapted from [162]).
Despite its toxicity, this compound and its (less toxic) synthetic analogues have been suggested as new drugs for immunotherapy [159,160]. In fact, Kv3.1 channels play a key role on modulation of Ca2+ signaling, which in turn induces T-cell proliferation, immune activation and cytokine production. Said channels are believed to be therapeutic targets of T-cell mediated autoimmune diseases [159,161]. Given its particular capacity to block Kv3.1 channels, gambierol is an interesting compound for application as immunosupressor in dysfunctional immune system diseases, such as multiple sclerosis, diabetes mellitus type 1 and rheumatoid arthritis [159,161]. Gambierol and two of its analogues (tetra and heptacyclic forms) are promising molecules for modulation of Alzheimer’s disease hallmarks in primary cortical neurons. It was shown that β-amyloid and/or tau hyperphosphorylation overexpression can be reduced by gambierol, both in vitro and in vivo (Table 1).
2.10. Brevetoxin
Brevetoxin (BTX) is a ladder-like polycyclic ether, recognized for its powerful neurotoxic and ichthyotoxic features [9]. BTX originates from unarmoured dinoflagellate Karenia brevis (formerly known as Gymnodinium breve or Ptychodiscus brevis) [163]. BTX has nine analogues, classified based on its backbone structure: A-type or B-type [47,164]. More recently, a few other analogues were found in fish-killing species that belong to the class of raphidophytes [165,166,167]. The brevetoxin type-A is a decacyclic molecule, consisting in 10 transfused rings; and breveotxin type-B is an undecacyclic molecule, with 11 transfused rings—both with a functional lactone in one of the terminal rings, denominated “head”. They have also a strictly rigid region in the terminal four rings, a spacer region that separates the rigid region from the A-ring lactone, and a side chain allowing modest modification at the molecule terminus, or “tail” (Figure 10) [168]. Alterations in any type of such regions may induce modification in their activity, or induce significant loss in binding activities [169]. BTX binds to the α-unit of VGSCs, specifically site 5 [107,170,171,172].
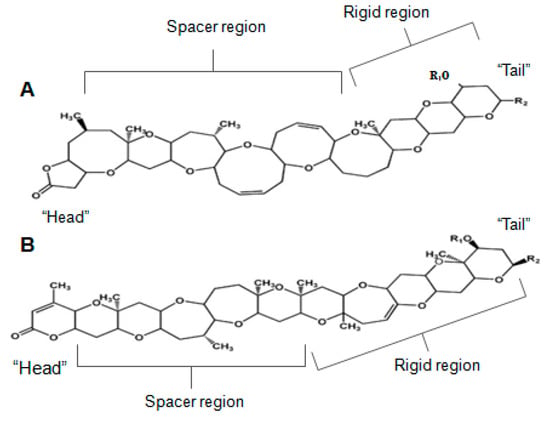
Figure 10.
Chemical structures of (A) brevetoxin type-A and (B) brevetoxin type-B (adapted from [185]).
Several BTX analogues and derivatives possess distinct toxicity efficacies, depending on binding affinity to VGSCs on site 5 [168,173]. Instead of blocking the channel, BTX action produces persistent activation of VGSCs, and their extended opening leads to prolonged Na+ entry into the cells [174,175,176]. This causes membrane depolarization at the resting membrane potentials—which triggers repetitive firing or excitatory cellular responses, and leads to such other physiological disturbances as Ca2+ influx [116,173,177,178]. Mattei and co-workers [109] also identified water movement across the membranes in myelinated nerve fibers; however, the regulatory mechanisms involved could not be elucidated. Another effect is enhanced release of neurotransmitters from autonomic nerve endings, acetylcholine in particular—which lead to smooth tracheal contraction [179]. In fact, during exposure to this substance, such symptoms as respiratory irritation (cough, nose irritability, congestion), bronchoconstriction and/or asthma attacks were observed in healthy individuals, but were more serious in airway-disease sensitive persons [180]. Therefore, it appears to yield an immune response, and play a major role in allergic inflammation in pulmonary tissue [181,182]. Sas and Baatz [182] suggested a primary inflammatory response in alveolar macrophage cells, mediated by the increase of cytokines (such as TNF- and IL-2) involved in immune cell activation and phagocytosis promotion. Another study [181], involving mouse bone marrow-derived mast cells, has shown that BTX can directly activate mouse mast cells—thus leading to degranulation, as well as inflammatory cytokine production involving Ca2+ signaling. Conversely, other authors claimed that exposure to BTX impairs the immune function, thus leading to reduced phagocytosis activity, decreased plaque-forming ability and/or decreased lymphocyte proliferation [183]. This biotoxin has also been described [178,183] to affect cell proliferation in a dose-dependent manner, cause cell death through an apoptotic mechanism, and possess genotoxic features.
Based on its neuro-activation properties, BTX-2 has been found to behave as neuronal stimulator, able to increase neuronal plasticity. It might thus support advances in pharmacological treatment aimed at recovering brain function after stroke or other traumatic brain injury [184]. Furthermore, a therapeutic formulation based on BTX derivatives has been designed to regulate such diseases as cystic fibrosis and mucociliary dysfunction related to increased mucus transport (Table 1).
2.11. Azaspiracid
Azaspiracid (AZA) is a recently discovered polyether phycotoxin, quite toxic for mammal systems [10]. AZA is responsible for azaspiracid poisoning, and is produced by dinoflagellate Azadinium genus (e.g., A. spinosum, A. poporum, A. dexteroporum) [186,187,188]. Among its increasing number of derivatives (over 30 so far) [189], Azaspiracid-1 (AZA1)—the first compound to be isolated and the one with major toxicity in humans, appears to be the most important, followed by AZA2 and AZA3 [190]. AZA toxin consists in a highly hydroxylated linear carbon chain with a tri-spiro ring assembly, together with a unique cyclic amine (or aza group) and a carboxylix acid group (Figure 11) [188,191]. The unique cyclic amine is a structural feature that differentiates AZA from the other dinoflagellate toxins [9]. Its mechanism of development of toxic effects is not fully understood [17], yet AZA is expected to possess a strong biotechnological significance. Toxicological studies in vivo and in vitro have unfolded several aspects of cell biology that can be affected thereby [192]. AZA presents indeed cytotoxicity against several human cell types [193], as well as teratogenicity to finfish [194]. It has also the ability to induce alterations on cell morphology and cytoskeleton structure, particularly on the E-cadherin system [65,192]. Furthermore, it was reported to be a potent activator of c-Jun-N-terminal kinase (JNK) and caspases—implicated in stress-signaling pathways, such as cell damage, apoptosis, and cytoskeleton regulation; and as an effective modulator of intracellular cAMP and calcium levels [195,196,197]. Other known effects relate to altered gene expression patterns in cells, and inhibition of cell cholesterol levels (particularly in T-lymphocyte cells) [198,199]. Finally, AZA apparently affects potassium ion channels [200].
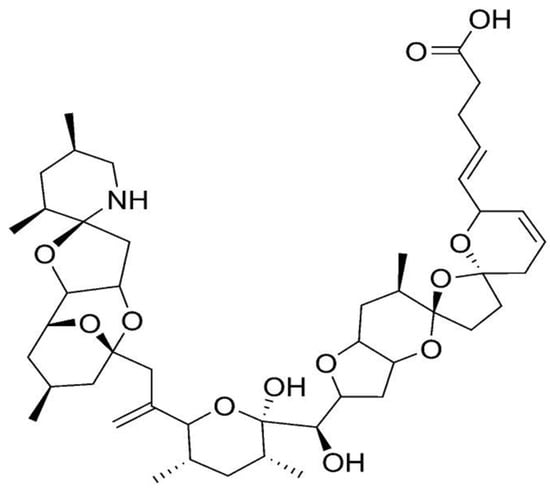
Figure 11.
Chemical structure of azaspiracid-1 (AZA1) (adapted from [65]).
2.12. Gymnocin
Gymnocin-A (GYMA) is a rare and complex polyether toxin, isolated from the red tide dinoflagellate Gymnodinium mikimotoi. This toxin is composed by fourteen contiguous polyether rings, with 2-methyl-2-butenal side-chains (Figure 12) [201]. It is weakly toxic upon fish, but quite cytotoxic against P388 mouse leukemia cells. Several other forms of GYMA have meanwhile been isolated, including Gymnocin-B bearing even higher cytotoxicity [202].
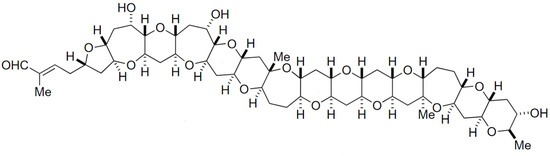
Figure 12.
Chemical structure of gymnocin-A (GYMA) (adapted from [202]).
2.13. Karlotoxin
Karlotoxin (KmTx) is a linear polyketide toxin [203], synthesized exclusively by Karlodinium genus; K. veneficum sp. is indeed considered as the main source of this biotoxin [204]. Different strains of this dinoflagellate, collected across distinct geographic locations produce several forms of KmTx with differing physicochemical properties [205,206]. Its chemical structure has recently been elucidated, and three groups of KmTxs accordingly emerged—differing mostly in length of lipophilic arm, a structural feature that apparently modulates haemolytic activity [207,208,209,210,211]. In KmTx 1, the side chain has 18 carbons in length, whereas KmTx 2 is two carbons shorter and KmTx 3 differs from KmTx 1 in having one less methylene group in the saturated portion of its lipophilic arm (Figure 13) [210,211]. Surprisingly, KmTxs have remarkable structural similarities with amphidinols—bioactives produced by dinoflagellate of genus Amphidinium sp. [212], characterized by long carbon chains with multiple hydroxyl groups and polyolefins. KmTx chemical structure consists of a complex hairpin-like structure, with three distinct regions: a polyol arm bearing variable hydroxylation and methylation; a hinge region containing two pyran rings; and a lipophilic arm with a terminal diene [213].
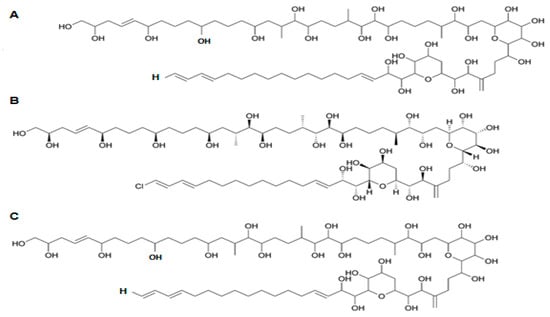
Figure 13.
Chemical structure of (A) kartoxin-1 (KmTx-1); (B) karlotoxin-2 (KmTx-2) and (C) karlotoxin-3 (KmTx-3) (adapted from [218]).
KmTx holds a range of activities, such as haemolytic, cytotoxic, ichthyotoxic and antifungal [206,207,214,215]. Its similarity to amphidinols (produced by Amphidnium genus) suggests a similar mode of action, based on membrane cell permeabilization [203,206,216]. KmTx acts on cell membranes via pore formation, which disrupts cell osmotic balance and eventually leads to cell death. A study on Km-Tx2 revealed that lysis is preceded by permeabilization of the plasma membrane to various cations, including Ca2+, K+ and Na+ [207,215]. The bioactivity of these compounds is dependent on the sterol composition of the target cell [204,205]. During pore formation, KmTxs selectively bind to 4-desmethyl sterols (e.g., cholesterol or ergosterol), whereas cells containing 4α-methyl sterols (e.g., gymnodinosterol and brevesterol) are immune to said biotoxin. This might explain why KmTx is capable of lysing animal cells, fungi and/or protists, but leaves K. veneficum cell membranes intact; dominance of 4α-methyl sterols in the latter may be the key to this lack of autotoxicity [205,217]. The KmTx properties to trigger formation of pores in cholesterol-containing cell membranes convey a noteworthy potential to treat several human health conditions, including coronary heart disease (CHD). Furthermore, KmTx may be formulated as a new chemotherapeutic agent for cancer control. Cholesterol acts as both adhesive and spacer unit between the sphingolipids that hold the lipid raft in cell membranes together—being critical for biological competence. In some solid tumor lines, e.g., breast and prostate cancer cells (two of the most widespread cancer forms worldwide), much more lipid rafts are present than in their healthy counterparts—so they are more sensitive to cholesterol depletion-induced cell death [204,207].
2.14. Cyclic Imine Toxins (Spirolide and Gymonodimine)
Spirolide (SPX) and gymnodimine (GYM) are biotoxins belonging to the cyclic imine group, known to be “fast action toxins”—i.e., able to produce potent and rapid death in rodents [10]. SPX is synthesized by Alexandrium ostenfeldii/peruvianum [219,220], and also by Karenia selliformes [221]; and 16 isoforms have been identified to date [222]. This toxin is a macrolide characterized by a cyclic imine group; the most common analogue found is 13-des-methyl-C-spirolide (see Figure 14A) [89]. The latter is included in the first of the four groups of SPXs, defined as per functional group present. For instance, the first group includes ten forms having a characteristic 6,5,5-spiroketal ring system (i.e., SPX-A, SPX-B, SPX-C, SPX-D, and 13-des-methyl-C-spirolide, among others). The second group of spirolides (SPX-E and -F) chemically resembles SPX-A and SPX-B, except for lack of toxicity; instead of the cyclic imine group, they have other structural component (acyclic aminoketone) [223,224,225]. The third and fourth groups (i.e., SPX-G or SPX-I) are represented by analogues resembling SPX-C and -D, except that some compounds have a 6,5- instead of the 6,5,5-spiroketal ring system [225,226]. Generally speaking, SPX toxins have confirmed their major activity upon muscarinic and nicotinic acetylcholine receptors, along with damage to astrocytes and neurons that negatively disturb the central nervous system [227].
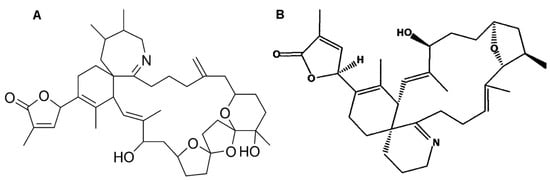
Figure 14.
Chemical structure of (A) 13-desmethyl spirolide C (adapted from [65]) and (B) gymnodimines A (GYMA) (adapted from [235]).
GYM (including Gymnodimine-A, and its two analogues GYM-B and -C) [221] are produced by gymnodinoid dinoflagellates, specifically Karenia selliformis (formerly named Gymnodinium selliforme) [221,228]. A fourth analogue of GYM—12-methylgymnodimine—was isolated from Alexandrium ostenfeldii [229]; GYM-D was recently found as new analogue [230]. GYM molecules present typically a six-membered cyclic imine, with no methyl substituents in spiroimine ring system, and with such typical fragments as tetrahydrofuran ring and unsaturated lactone (Figure 14B) [230].
Both SPXs and GYMs contain a unique cyclic imine ring, hypothesized to be their pharmacophore moiety [223]; it might be responsible for activation of L-type calcium channels of brain receptors [228]. Nevertheless, recent studies have demonstrated that these compounds can target neuronal and muscular nicotinic acetylcholine receptors with high affinity [231,232]. Such dinoflagellate toxins are accordingly proposed as additional tools to elucidate structural domains on various acetylcholine receptors (AChR); and to advance understanding of interactions between antagonists, and nicotinic and muscular AChR [232]. SPX and GYM mechanisms of action appear to be similar, yet they remain largely undisclosed [10]. Some reports have shown that GYM (combined with OA) can be used therapeutically to enhance the anti-cancer effects of chemotherapeutic agents—many of which work partially via toxicity against tumor cells. It was demonstrated that GYM can sensitize cells to apoptotic stimuli Neuro2a neuroblastoma cell line [233]. GYM has also been claimed to cause a reduction of β-amyloid levels and tau phosphorylation, which could potentially contribute for treatment of degenerative diseases [234].
2.15. Gambieric Acid (Bioactive)
Gambieric acid (GA) belongs to a family of marine polyether natural products, originated in dinoflagellate Gambierdiscus toxicus; gambieric acids A, B, C and D have been isolated from their culture broth [236]. Such compounds are composed by nine trans-fused ether rings of six, seven and nine members, and one isolated tetrahydrofuran ring (Figure 15) [237]. They are potent antifungal agents, displaying a remarkable activity against filamentous fungi, while being ineffective against bacteria or yeasts. A study involving GA-A and GA-B has confirmed their potency against fungus Aspergillus niger—more than 2000-fold that of amphotericin B, a common antifungal drug [238]. On the other hand, GA does not show substantial toxicity against cultured mammalian cells, or even in vivo. Mice subjected to doses of 1 mg per kg of body weight, via intraperitoneal injection, did not develop abnormal reactions or considerable toxicity—despite GA sharing structural features with polycyclic ethers, e.g., ciguatoxins, brevetoxins, gambierol and maitotoxin [239]. GA-A is able to displace binding of tritiated brevetoxin B to voltage-gated sodium channels in excitable membranes—even though its binding affinity is significantly lower than those of brevetoxins and ciguatoxins [240].
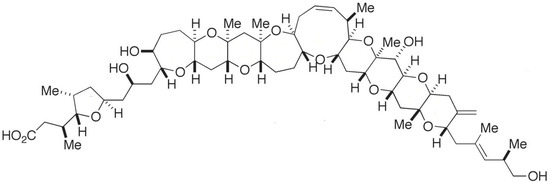
Figure 15.
Chemical structure of gambieric acid (GA-A) (adapted from [148]).
2.16. Goniodomin A (Bioactive)
Goniodomin A (GDA) is an antifungal polyether macrolide, produced by Alexandrium genus, namely A. hiranoi [241], A. monilatum [242] and A. pseudogonyaulax [243]. Pharmacological studies have indicated its strong effect upon cytoskeleton reorganization [242]. Structurally similar to pectenotoxin, this compounds is composed by a macrolide lactone ring, with a spirocetal ring and a hemiacetal ring attached thereto (Figure 16) [244]. This compound inhibits angiogenesis (regeneration of vessels) by inhibiting endothelial cell migration and basic fibroblast growth factor (bFGF)-induced tube formation—in part via inhibition of actin reorganization. Angiogenesis is also inhibited by GDA in vivo [245]. Mizuno et al. [246] reported that GDA affects the actin state in astrocytoma cells, causing cell morphological changes by increasing filamentous actin. GDA was shown to induce increase of filamentous actin content in Clone 9 rat hepatocytes, as well as cytotoxicity against human neuroblastoma cells. Goniodomin B, an analogue of GDA, seems to have effects similar to GDA but less potent [247].
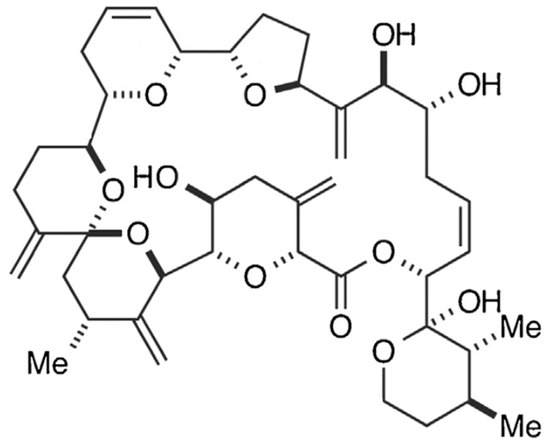
Figure 16.
Chemical structure of goniodomin-A (GDA) (adapted from [247]).
GDA can be a useful tool for analysis of the relationship between structure and function of actin. On the other hand, this substance has shown antifungal activity against Candida albicans and Mortierella ramannianus [241].
2.17. Amphidinolide (Bioactive)
Amphidinolide (AMP) constitutes a group of citotoxic macrolides, produced by symbiotic dinoflagellates of Amphidinium genus [248]; more than 40 AMPs were identified to date [249]. Such compounds exhibit great variation in size of macrocyclic lactone rings, from twelve- to twenty seven-membered systems (Figure 17) [250,251], with other unique structural features that make them quite complex molecules. In general, they exhibit potent cytotoxicity against murine lymphoma L1210, and human epidermoid carcinoma KB cells in vitro [248]. Among all AMPs, the noteworthy anti-tumor capacity of AMP-N and AMP-H is likely related to distinct inhibitory patterns. While AMP-N seems to have more affinity for mitochondria of malignant cells, AMP-H apparently targets actin cytoskeleton [252]. These compounds are expected to lead to new anticancer drugs—but again their limited availability has hampered more detailed studies [248,251]. Other related compound, caribenolide-I, was reported to possess strong cytotoxic activity against human colon tumor cell line and murine tumor P388 in vivo [97].
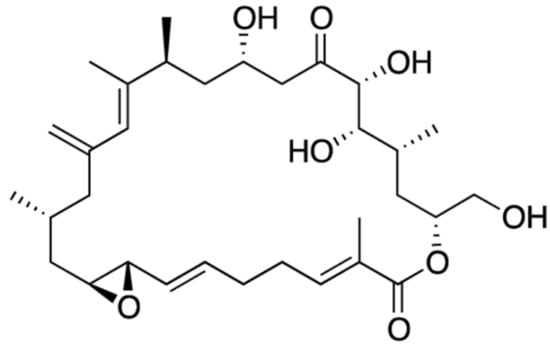
Figure 17.
Example of chemical structure of amphidinolide-H (adapated from [249]).
2.18. Amphidinol (Bioactive)
Amphidinol (AM) belongs to a unique group of polyhydroxy polyene compounds, produced by Amphidinium species (e.g., A. klebsii, A. carterae); it possesses antifungal and haemolytic properties [253]. At least 23 AMs (including 7 analogues) [254,255,256] have been reported, ever since amphidinol 1 (AM1) was first isolated from A. klebsii in 1991 [257]. AMs belong to a large family of long-chain linear polyethers, presenting noteworthy potent biological activities—including antifungal, cytotoxic, and haemolytic properties [255]. Amphidinol-3 (AM3) (Figure 18)—the first of this series of compounds to be fully elucidated [212,258], notably exceeds other derivatives in terms of both activities. It was initially believed that AM permeabilizes phospholipid membranes, by interacting directly with such bilipidic layer and forming ion-permeable pores across the membrane (toroidal pore model) [203,259]. A recent study revealed, however, that AM3 is able to perforate membranes by specific molecular recognition, but without apparent disruption of the membrane itself. A novel action mechanism based on a barrel-stave pore model has been proposed for the interaction of AM with sterol membranes [260]. Cholesterol and ergosterol enhance AM permeabilization, thus bringing along potent cytotoxic and antifungal capacities. In addition, AM3 showed higher affinity to ergosterol membrane; this suggests formation of a more stable complex, which may provide insights for a new antifungal drug [216].
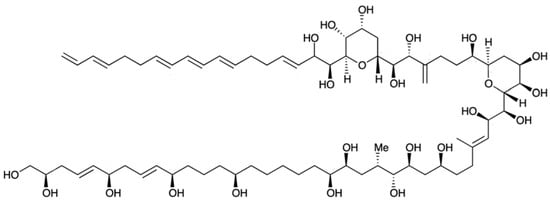
Figure 18.
Chemical strucuture of Amphidinol-3 (AM3) (adapted from [264]).
Another structural class of AM related-compounds isolated from benthic Amphidinium species—amphirionin-5, seems to potentiate proliferation of murine bone marrow stromal ST-2 cells and murine osteblastic MC3T3-E1 cells [261]. Interestingly, another study encompassing amphirionin-4 [262] reported promotion of high intensive proliferation only in murine bone marrow stromal ST-2 cells at low concentrations, but not in NIH3T3 and MC3T3-E1 cells. It was suggested that ST-2 cells, treated with amphitionin-4, experience an increase in actin and tubulin—so enhancement of assembly of proteinaceous cytoskeleton may be involved. The proliferation-promoting ability in ST-2 cells by amphitionin-4 unfolds a great potential for bone and cartilage regeneration—as well as other organs obtained from mesenchymal stem cells, derived in turn from multipotent marrow stromal cells. Since ST-2 cells are relevant toward development of lymphocytes from bone marrow cells, this compound may improve the immune system in its ability to detect infection [262].
Another AM related-compound, also isolated from Amphidinium benthic species, is iriomoteolide; it revealed cytotoxic activity against human cervix adenocarcinoma HeLa cells [263].
3. Biotoxin and Bioactive Production
3.1. Dinoflagellate Bioactive Supply
Owing to a wide diversity and compound production complexity, microalgae at large—and dinoflagellates in particular, are attractive as natural sources of bioactive molecules. Remember that natural product screening continues to seek and explore a variety of chemical structures, for eventual use as structural models for new drug development by the pharmaceutical industry [265].
Despite its potentially wide applicability, unavailability of dinoflagellate-generated material to sufficient amounts has raised systematic challenges in attempts to further biochemical investigation and clinical testing—thus compromising eventual development into commercial products [266]. Cripthecodinium chonii is the only nontoxic dinoflagellate grown to commercial level as of now; it indeed produces docosahexaenoic acid (DHA) to great levels. DHA is used for enrichment of infant formulae—and its production is via heterotrophic culture in conventional fermenters [267]. Only scarce quantities of bioactives from photoautotrophic dinoflagellates are indeed commercially available (Table 2).

Table 2.
Commercially available biotoxins—with corresponding suppliers, sources and price range (per mg).
Such compounds derive from a limited number of dinoflagellates (e.g., Gambierdiscus toxicus, Prorocentrum concavum, Karenia brevis, Protogonyalaux sp.) [3]—and prices can range from 1000 up to 500,000 €/mg, depending on purity and source. Furthermore, these substances are often discontinued without previous notice, and the effective purity and quantity claimed by companies is sometimes doubtful [16]. Pure compounds are needed for use as analytical standards in seafood safety screening programs, as well as research purposes (e.g., study of mechanism of action and pharmacology); lack, or unsuitable amounts of material will obviously hamper regular development of those studies [268].
Dinoflagellates in general grow slowly, and are quite shear-sensitive. It might seem paradoxal that they can cause algal blooms in Nature—yet the latter are observed only during periods of calm (and warm) waters; in fact, turbulent waves disrupt such a possibility, due to the associated high shearing. Furthermore, the rate of shearing in classical bioreactors is much higher than that prevailing in relatively still open waters. Therefore, massive production of biotoxins and other products of interest from dinoflagellate culture has proven extremely difficult [97,269]. Unlike culture of microalgae at large, the maximum biomass concentration attainable in photosynthetic cultures of a dinoflagellate remains well below 1 gram per liter [3,270]; regarding toxin production per cell, it is of the order of picogram [266]. Improvements in cultivation techniques still lag far behind practical requirements: around 150 g of a pure bioactive compound is typically needed for preclinical studies and clinical trials, so current cultivation methods will require broth medium volumes in excess of 100,000 m3—i.e., far above that needed by a typical antibiotic [3]. Genetic and metabolic engineering have also been attempted [271] to circumvent low productivities and enhance concentrations of target compound; however, the efforts involved are absolutely not straightforward [3]. These approaches are normally available for nondinoflagellate microalgae, and target chiefly biofuel production [272,273]. The difficulties arise from the astonishingly large and complex dinoflagellate genomes—with great amounts of introns, bearing redundant repetitive noncoding sequences [274]. Their massive genome is organized into a permanent liquid crystalline form, with a high proportion of unusual bases [275]. Furthermore, dinoflagellate genes lack recognizable promoter features, as well as common eukaryotic transcription factor binding sites [276]; and their biotoxin production capabilities apparently result from multiple independent evolutionary origins [277]—which make the identification of toxin-related genes particularly complex. Despite said difficulties, high-throughput omics technologies have been moving toward exploring toxin genes and proteins related to dinoflagellate toxin production, and are expected to provide some insights about their biosynthesis in the near future [278].
Chemical synthesis of dinoflagellate-derived toxins had also been tested, with more than 100 steps reported in some cases. Hence, de novo synthesis is considered as exceedingly complex—and economically unfeasible at present (except in the case of okadaic acid). Nevertheless, this process has proven useful for elucidation of structure and biological mode of action in the case of several complex bioactives of dinoflagellates (e.g., brevetoxin B [279], brevetoxin A [280], gambierol [281], gymnocin A [282], azaspiracid-1 [283], and gambieric acid A [284]). Moreover, some chemical routes from biotoxin fragments have been proposed as more efficient for some compounds [3]—such as maitotoxin [118] or yessotoxin [285]; however, more practical synthetic ways remain a challenge, unlikely to succeed in the short run.
In view of the above arguments, cultivation of dinoflagellates and extraction and purification of biotoxins (and other metabolites of interest) from closed photobioreactor seems to be the best approach to obtain significant amounts of those compounds [286]. Researchers have been investing a great deal of effort into developing dinoflagellate bioreactor controlled cultures [268,287,288,289,290], and understand what mechanisms underlie their low biomass and biotoxin/bioactive productivities [3,269,270]. A deeper know-how on dinoflagellate metabolism, cultivation and production processes is essential to rationally develop reproducible and economical systems with improved productivities—in order to permit these microalgae acquire a distinctive biotechnological role in value-added biotoxin production [291], for eventual pharmaceutical and biomedical purposes.
3.2. Culture of Dinoflagellates and Biotoxin Production
While culturing nondinoflagellate microalgae in large volume photobioreactor cultures is a routine practice [292,293,294], dinoflagellate culturing usually poses a number of difficulties [97]. Besides their quite low rates of growth, dinoflagellates exhibit an intricate metabolism and low biomass yields—thus resulting in low biotoxin production. Said fastidious growth may be explained by a complex nucleus, with cumulative acquisition of several prokaryotic genes throughout evolution—coupled to an inefficient Rubisco enzyme to distinguish CO2 from O2 [295].
Dinoflagellate cells exert some exceptional features compared to eukaryotic cells though. They possess a distinctive nucleus with lack of nucleosomes and histones, and chromosomes remain permanently condensed, even during mitosis [295,296]. These marine microorganisms are known to have complex circadian systems that control behavior in vivo, as they establish a vertical migration pattern according to daylight and nutrient level [297]. In general, photosynthetic dinoflagellate cells divide at the end of the dark period, and grow during the light phase (corresponding to the G1 phase of the cell cycle), precisely when production of many toxins occur. Apparently, coupling progression of cell cycle to cell growth enables them to make best use of available resources [295]. Therefore, one promptly realizes that dinoflagellate cells are extremely complex, and have singular metabolic requirements that can hardly be provided by conventional (closed) photobioreactor configurations and operating conditions. In fact, classical photobioreactors comprise simplistic modes of light supply (e.g., continuous external illumination)—which, combined with the typical uniform levels of nutrients, may break down natural rhythms and cause metabolic behavior to deviate from the original one. Continuous supply of CO2 is also a sine qua non for photosynthesis, owing to its low solubility in water—which calls for turbulence to minimize resistance to mass transfer. However, dinoflagellates are extremely sensitive to turbulence, since it leads to high shear stress or cell damage [3]. Even though dinoflagellate microalgae may grow well in natural environment (i.e., HABs), agitation and turbulent conditions in water columns in vitro have proven unsuccessful. It has been suggested that hydrodynamic forces may reduce time-integrated light exposure of individual cells, and promote physical dispersion; another explanation claims that mechano-external stimuli impact directly on cell physiology [298].
Nutritional requirements, illumination (i.e., cycle, intensity, irradiance) and specific patterns of agitation (i.e., laminar flow) are thus essential issues to circumvent their low rates of growth, and accordingly attain higher levels of metabolite synthesis. Parameters such as optimal temperature, pH, oxygen tolerance and ionic strength are also important, because they can influence production of some toxins. For instance, temperature and salinity were shown to induce variations in saxitoxin content in Alexandrium catenella [299,300]; and temperature and light have been shown to affect palytoxin-like compounds content in Ostreopsis ovata [301], as well as amount of okadaic acid (OA) produced by Prorocentrum belizeanum [302]. Elucidation of what triggers synthesis of biotoxins and other bioactive compounds, and mitigation of shear-stress through reactor engineering are central issues to be addressed regarding mass cultivation of dinoflagellates and bioactive production thereby.
3.2.1. Nutritional Requirements
The composition of the nutrient mixture in growth media can strongly influence dinoflagellate survival (i.e., cell division) and biotoxin production. The L1 is the most frequently used formulation to culture dinoflagellates [303], despite the fact that it was originally developed to grow marine diatoms. Other common cited media are f/2 and K, initially designed to grow diatoms [304,305] and oligotrophic oceanic phytoplankters [306], respectively. Media formulation typically derives from enrichments of natural seawater, and is almost exclusively used in dinoflagellate culture.
In some dinoflagellate cultures, the effect of macronutrients is essential for maintenance of cellular processes; for instance, nitrates and phosphates seem to trigger different responses regarding biotoxin synthesis. Gallardo-Rodríguez et al. [307] have shown unsatisfactory phosphorous concentrations in L1 basal medium to achieve high growth rates and biomass yields of Protoceratium reticulatum cultures. In addition, cell-specific production of yessotoxin bioactive was not influenced by concentration of phosphate, but by higher nitrate concentrations. On the other hand, studies with Alexandrium spp. and Karenia brevis have demonstrated that limited-phosphorous concentrations induce higher biotoxin content [308,309]. Other reports suggest an increase in toxin content when N and P are severely depleted, thus suggesting a synergistic effect of their availability [310]; while studies using Ostreopsis ovata suggest an opposite effect, with biotoxin production limited by N and P depletion [311].
Trace elements and vitamins have also proven to be of great importance. For instance, P. reticulatum was found to exhibit higher growth rates when selenium and iron were added to cultures, and yessotoxin production was significantly improved with selenium addition [312]. Field and culture-based studies with HAB dinoflagellates support the idea that exogenous B vitamins (i.e., B1, B7, B12) have the potential to broadly influence marine biomass productivity and associated composition [313]. Tests with P. reticulatum and K. veneficum, using artificial neural networks as predictive tool for nonlinear interactions among all nutrients in culture media, suggest that micronutrients and vitamins (even to lower concentrations) are relatively more significant than macronutrients toward growth of both microalgae [314,315].
Since medium formulations comprise many components, virtually hundreds of combinations are possible to improve biotoxin production—but quite difficult to test in practice. For that reason, the process to improve nutritional requirements of dinoflagellates may to advantage resort to a genetic-algorithm (GA). This tool is superior in performance to conventional statistical experimental designs, and has been commonly employed to develop microbial culture media [316]. GA-based stochastic search is able to explore a large experimental space, and has been successfully applied in medium formulation for P. reticulatum and K. veneficum. The new media developed have allowed 40% and 190%-enhancement of biotoxin titer, and 60% and 120%-enhancement in final cell concentration relative to basal L1 medium, respectively [317,318].
The effect of nutritional requirements upon overall growth and total amount of biotoxin synthesized seem to strongly depend on dinoflagellate species. Hence, efforts to find better medium formulations, via efficient methods of search and optimization, remain a priority.
3.2.2. Culture Light Provision
Light is the basic energy source, and a critical parameter for dinoflagellate autotrophic growth—and thus for achieving higher productivities. Both natural and artificial light have been reported to bring about growth of dinoflagellates of interest [270,289,302]. Artificial light provides better control of the light spectrum, irradiance or photosynthetic photon flux density (PPFD), as well as photoperiod (light/dark cycle) in closed photobioreactors. Several culture studies have employed conventional cool fluorescent lamp (Table 3), but light-emitting diodes (LEDs) are gaining importance in dinoflagellate culturing [319]. LED performance is very similar to fluorescent light, but they require less energy and narrower wavelength bands are possible. They are also less damaging to dinoflagellate cells, as they do not generate excessive heat; and can be easily designed to convey predefined levels of light delivery to photobioreactors. Until now, K. veneficium and A. tamarense were the only dinoflagellate species of interest to be cultured with success using LED technology, at pilot-scale [319,320]. For instance, A. tamarense growth was stimulated under blue LED, but suppressed under yellow and red LEDs to below 70 µmolphoton.m−2.s−1. In fact, high growth efficiencies under blue wavelength have been reported for several dinoflagellates [320], but a correlation between cellular toxin levels and wavelengths remains to be established [321].

Table 3.
Major results of studies on bioreactor type, culture mode and other operational conditions for dinoflagellate growth and/or biotoxin synthesis optimization.
The light intensity (irradiance) and utilization efficiency are crucial in dinoflagellate cultures, and consequently in toxin bioproduction. Light energy should be delivered evenly over the photobioreactor; in order to prevent growth-limiting, photoxidation and/or photoinibition, an adequate PPFD must indeed be provided to cells [322]. Conventional green microalgae can stand elevated irradiance levels (e.g., 3000 µmolphoton.m−2.s−1) [302], but those levels are detrimental for dinoflagellates. Several intensities have been reported for different dinoflagellate cultures [302,323,324]. Maximal and optimum intensity thresholds in dinoflagellate cultures seem to be species-dependent [325]. While some dinoflagellates grow better under low light intensities (e.g., 10–40 µmolphoton.m−2.s−1), others can effectively grow between 50–500 µmolphoton.m−2.s−1 or even more [47,268,324,325]. On the other hand, the light time exposure or photoperiod are also of interest for growth and biosynthesis of toxins. The most commonly used light/dark cycles are 12 h/12 h, 14 h/10 h and 16 h/8 h (Table 3). Optimal cellular DTX-1 and OA concentrations and good growth performance of P. lima were reported under 12 h/12 h photoperiod, thus emphasizing the importance of photosynthesis and dark respiration in such toxin biosyntheses [321]. Early studies suggests that biotoxin production of microalgal cells is controlled by light and dependent on cell cycle [326,327]. The biosynthesis of SPX in A. ostenfeldii is in fact governed by light-dependent mechanisms; toxin concentration per cell quota increases in the beginning of the dark period, probably corresponding to the G1 or S phase cell cycle [328].
Understanding the photosynthetic apparatus and light requirements at large-scale will pose great challenges in attempts to obtain successful amounts of dinoflagellate biomass and biotoxins. Several aspects regarding light have thus to be considered for closed bioreactor systems.
3.2.3. Bioreactor Culture and Design
The dropping productivities associated with dinoflagellate growth in photobioreactors are often related to the shear-sensitivity of their cells—as made apparent by the negative effects of turbulence. The success of biotoxin production for pharmaceutical and/or investigational purposes implies attainment of adequate amounts of biomass in a safe way—which challenges conventional photobioreactor engineering design [286,336]. At present, cultivation in open ponds is not a viable solution for safety reasons and environmental contamination [3]. The majority of reports on dinoflagellate cultivation are indeed limited to flask or bottle cultures at laboratory scale—yet complementary studies have been developed aiming at larger scale operation [344]. Based on different technologies employed, volumes ranging from 2 L to 700 L have been tested (Table 3). The culture systems at stake include a variety of configurations—encompassing from carboys, chemostats or stirred-tanks, to typical airlift, bubble column, tubular reactor and flat-plate PBRs (photobioreactors). The technology Twin-Layer PBR, at laboratory scale, introduced by Benstein and coworkers [341] for growth of Symbiodinium voratum and production of peridinin pigment in an immobilized support, suggested a new configuration approach that could also be suitable for biotoxin production.
Culture operation modes can greatly influence the efficacy of biomass and toxin productivities. Beuzenberg and coworkers [268] have demonstrated that K. selliformis, A. ostenfeldii and K. brevisulata continous cultures in column PBRs led to substantial improvements in productivity. Biotoxin yields increased 2–3 fold relative to batch mode, except for K. brevisulata. Fuentes and co-workers [289] reported successful production of biomass by A. caratarea in a semi-batch mode, either indoors or outdoors. Wang and coworkers [333,334] observed lower growth of A. tamarense under semi-batch mode—possibly due to inadequate dilution cycles and excess disturbance on cultures; however, C2 toxin yields were considerably higher in batch mode. Special attention should also be given to aeration regime applied to dinoflagellate cultures. Despite turbulent environments triggering a few negative effects upon those cultures—e.g., reactive oxygen species accumulation, lipoperoxide formation, changes in cell membrane fluidity, or/and calcium mobilization [3,269], aeration seems to improve biomass productivities and biotoxin concentrations if provided and carefully controlled [336]. Air flow helps stripe dissolved oxygen, hence minimizing harmful effects upon microalga cells [270]. Hu and co-workers [332] reported on a two-step batch culture method, first favoring growth in static conditions, and then applying aeration in a subsequent step to improve saxitoxin yields by A. tamarense. Wang and co-workers [333,334] have reached higher contents of C2 toxin Alexandrium permeabilization following a similar strategy; Gallardo-Rodríguez and co-workers [269,286] also observed increasing levels of yessotoxin in cultures of P. reticulatum.
Consequently, bioreactor scale-up remains largely undeveloped, so researchers are to explore several economical and viable options regarding bioreactor design and culture strategies. Successful scale-up will ultimately dictate industrial feasibility of any process based on dinoflagellate biotoxins.
4. Final Considerations
Dinoflagellates have proven to be a rich biotechnological source of biotoxins, with interesting biological activities that are potentially useful in a wide spectrum of pharmacological and medical fields, besides being promising tools for chemical biology. Despite such recognized value, scarcity of such biotoxins for preclinical testing (and later for commercial exploitation) remains a major issue. As chemical synthesis and genetic engineering are extremely difficult to achieve, a lot of effort and resources have been directed to improve modes of culturing dinoflagellates in photobioreactors, aimed at obtaining larger biotoxin concentrations. Nutritional and operation conditions, such as light and aeration/agitation patterns, have to take into account that dinoflagellates obey specific circadian rhythms, and are extremely sensitive to shearing when cultivated in a reactor. Conventional engineering and bioreactor design methods have thus to be overcome, so as to circumvent the fastidious growth and shear-sensitivity of dinoflagellate cells.
Acknowledgments
This work was financially supported by: Project DINOSSAUR—PTDC/BBB-EBB/1374/2014-POCI-01-0145-FEDER-016640, funded by FEDER funds through COMPETE2020—Programa Operacional Competitividade e Internacionalização (POCI), and by national funds through FCT—Fundação para a Ciência e a Tecnologia, I.P. A postdoctoral fellowship (ref. SFRH/BPD/72777/2010) for author A.C.G., supervised by author F.X.M., was granted by Fundação para a Ciência e Tecnologia (FCT, Portugal, Lisbon), under the auspices of ESF and Portuguese funds (MEC). This research was also partially supported by POCI-01-0145-FEDER-006939 (Laboratory for Process Engineering, Environment, Biotechnology and Energy—UID/EQU/00511/2013), funded by the European Regional Development Fund (ERDF), through COMPETE2020—Programa Operacional Competitividade e Internacionalização (POCI) and by national funds via FCT, through project NORTE-01-0145-FEDER-000005—LEPABE-2-ECO-INNOVATION, supported by North Portugal Regional Operational Programme (NORTE 2020), under the Portugal 2020 Partnership Agreement, through the European Regional Development Fund (ERDF).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Glaser, K.B.; Mayer, A.M.S. A renaissance in marine pharmacology: From preclinical curiosity to clinical reality. Biochem. Pharmacol. 2009, 78, 440–448. [Google Scholar] [CrossRef] [PubMed]
- De Jesus Raposo, M.F.; De Morais, A.M.B.; De Morais, R.M.S.C. Marine polysaccharides from algae with potential biomedical applications. Mar. Drugs 2015, 13, 2967–3028. [Google Scholar] [CrossRef] [PubMed]
- Gallardo-Rodríguez, J.J.; Sánchez-Mirón, A.; García-Camacho, F.; López-rosales, L.; Chisti, Y.; Molina-Grima, E. Bioactives from microalgal dinoflagellates. Biotechnol. Adv. 2012, 30, 1673–1684. [Google Scholar] [CrossRef] [PubMed]
- Taylor, F.J.R. Dinoflagellates: An introduction. In The Biology of Dinoflagellates; Taylor, F.J.R., Ed.; Blackwell Scientific Publications: Oxford, UK, 1987; pp. 1–13. [Google Scholar]
- Gaines, G.; Elbrachter, M. Heterotrophic nutrition. In The Biology of Dinoflagellates; Taylor, F.J.R., Ed.; Blackwell Scientific Publications: Oxford, UK, 1987; pp. 224–247. [Google Scholar]
- Bralewska, J.M.; Witek, Z. Heterotrophic dinoflagellates in the ecosystem of the Gulf of Gdansk. Mar. Ecol. Prog. Ser. 1995, 117, 241–248. [Google Scholar] [CrossRef]
- Hickman, C.P.; Roberts, L.S.; Larson, A.L. Integrated Principles of Zoology; McGraw-Hill International Edition: New York, NY, USA, 2008; pp. 213–222. [Google Scholar]
- Coffroth, M.; Santos, S. Genetic Diversity of Symbiotic Dinoflagellates in the Genus Symbiodinium. Protist 2005, 156, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-Z. Neurotoxins from Marine Dinoflagellates: A Brief Review. Mar. Drugs 2008, 6, 349–371. [Google Scholar] [CrossRef] [PubMed]
- Cembella, A.D. Chemical ecology of eukaryotic microalgae in marine ecosystems. Phycologia 2003, 42, 420–447. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Marine Biotoxins. In FAO Food and Nutritrion; Food and Agriculture Organization of the United Nations: Rome, Italy, 2004; p. 278. [Google Scholar]
- Smayda, T.J. What is a bloom? A commentary. Limnol. Oceanogr. 1997, 42, 1132–1136. [Google Scholar] [CrossRef]
- Bower, D.J.; Hart, R.J.; Matthews, P.A.; Howden, M.E.H. Nonprotein Neurotoxins. Clin. Toxicol. 1981, 18, 813–863. [Google Scholar] [CrossRef] [PubMed]
- Moestrup, Ø.; Akselmann, R.; Fraga, S.; Hoppenrath, M.; Iwataki, M.; Komárek, J.; Larsen, J.; Lundholm, N.; Zingone, A. IOC-UNESCO Taxonomic Reference List of Harmful Micro Algae (HABs). Available online: http://www.marinespecies.org/hab/ (accessed on 28 October 2017).
- Ciminiello, P.; Fattorusso, E. Bivalve Molluscs as Vectors of Marine Biotoxins Involved in Seafood Poisoning. In Molluscs: Progress in Molecular and Subcellular Biology; Cimino, G., Gavagnin, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 53–82. [Google Scholar]
- Quilliam, M.A. Chemical methods for lipophilic shellfish toxins. In Manual on Harmful Marine Microalgae; Hallegraeff, G., Anderson, D., Cembella, A., Eds.; Intergovernmental Oceanographic Comission (UNESCO): Paris, France, 2003; pp. 211–246. ISBN 92-3-103948-2. [Google Scholar]
- FAO (Food and Agriculture Organization)/WHO (World Health Organization). Toxicity Equivalency Factors for Marine Biotoxins Associated with Bivalve Molluscs; Food and Agriculture Organization of the United Nations: Rome, Italy, 2016; ISBN 978-92-5-109345-0. [Google Scholar]
- Kellmann, R.; Stüken, A.; Orr, R.J.S.; Svendsen, H.M.; Jakobsen, K.S. Biosynthesis and molecular genetics of polyketides in marine dinoflagellates. Mar. Drugs 2010, 8, 1011–1048. [Google Scholar] [CrossRef] [PubMed]
- Senanayake, S.P.J.N.; Ahmed, N.; Fichtali, J. Nutraceuticals and Bioactives from Marine Algae. In Handbook of Seafood Quality, Safety and Health Applications; Wiley-Blackwell: Oxford, UK, 2010; pp. 455–463. ISBN 9781444325546. [Google Scholar]
- Sugawara, T.; Yamashita, K.; Sakai, S.; Asai, A.; Nagao, A.; Shiraishi, T.; Imai, I.; Hirata, T. Induction of Apoptosis in DLD-1 Human Colon Cancer Cells by Peridinin Isolated from the Dinoflagellate, Heterocapsa triquetra. Biosci. Biotechnol. Biochem. 2007, 71, 1069–1072. [Google Scholar] [CrossRef] [PubMed]
- Wiese, M.; D’Agostino, P.M.; Mihali, T.K.; Moffitt, M.C.; Neilan, B.A. Neurotoxic alkaloids: Saxitoxin and its analogs. Mar. Drugs 2010, 8, 2185–2211. [Google Scholar] [CrossRef] [PubMed]
- Berlinck, R.G.S. Natural guanidine derivatives. Nat. Prod. Rep. 2002, 19, 617–649. [Google Scholar] [CrossRef] [PubMed]
- Cusick, K.D.; Sayler, G.S. An overview on the marine neurotoxin, saxitoxin: Genetics, molecular targets, methods of detection and ecological functions. Mar. Drugs 2013, 11, 991–1018. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Marine biotoxins in shellfish Marine biotoxins in shellfish—Saxitoxin group. Scientific Opinion of the Panel on Contaminants in the Food chain. EFSA J. 2009, 1109, 1–47. [Google Scholar] [CrossRef]
- Durán-Riveroll, L.M.; Cembella, A.D.; Band-Schmidt, C.J.; Bustillos-Guzmán, J.J.; Correa-Basurto, J. Docking Simulation of the Binding Interactions of Saxitoxin Analogs Produced by the Marine Dinoflagellate Gymnodinium catenatum to the Voltage-Gated Sodium Channel Nav1.4. Toxins 2016, 8, 129. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, K.; Musgrave, I.F.; Humpage, A. Low dose extended exposure to saxitoxin and its potential neurodevelopmental effects: A review. Environ. Toxicol. Pharmacol. 2016, 48, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Munday, R.; Thomas, K.; Gibbs, R.; Murphy, C.; Quilliam, M.A. Acute toxicities of saxitoxin, neosaxitoxin, decarbamoyl saxitoxin and gonyautoxins 1&4 and 2&3 to mice by various routes of administration. Toxicon 2013, 76, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Epstein-Barash, H.; Shichor, I.; Kwon, A.H.; Hall, S.; Lawlor, M.W.; Langer, R.; Kohane, D.S. Prolonged duration local anesthesia with minimal toxicity. Proc. Natl. Acad. Sci. USA 2009, 106, 7125–7130. [Google Scholar] [CrossRef] [PubMed]
- Kohane, D.S.; Lu, N.T.; Gökgöl-Kline, A.C.; Shubina, M.; Kuang, Y.; Hall, S.; Strichartz, G.R.; Berde, C.B. The local anesthetic properties and toxicity of saxitonin homologues for rat sciatic nerve block in vivo. Reg. Anesth. Pain Med. 2000, 25, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Borowitzka, M.A. Microalgae as sources of pharmaceuticals and other biologically active compounds. J. Appl. Phycol. 1995, 7, 3–15. [Google Scholar] [CrossRef]
- Garrido, R.; Lagos, N.; Lattes, K.; Abedrapo, M.; Bocic, G.; Cuneo, A.; Chiong, H.; Jensen, C.; Azolas, R.; Henriquez, A.; et al. Gonyautoxin: New treatment for healing acute and chronic anal fissures. Dis. Colon Rectum 2005, 48, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Lattes, K.; Venegas, P.; Lagos, N.; Lagos, M.; Pedraza, L.; Rodriguez-Navarro, A.; García, C. Local infiltration of gonyautoxin is safe and effective in treatment of chronic tension-type headache. Neurol. Res. 2009, 31, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Moczydlowski, E.G. The molecular mystique of tetrodotoxin. Toxicon 2013, 63, 165–183. [Google Scholar] [CrossRef] [PubMed]
- Kodama, M.; Sato, S.; Sakamoto, S.; Ogata, T. Occurrence of tetrodotoxin in Alexandrium tamarense, a causative dinoflagellate of paralytic shellfish poisoning. Toxicon 1996, 34, 1101–1105. [Google Scholar] [CrossRef]
- Bane, V.; Lehane, M.; Dikshit, M.; O’Riordan, A.; Furey, A. Tetrodotoxin: Chemistry, Toxicity, Source, Distribution and Detection. Toxins 2014, 6, 693–755. [Google Scholar] [CrossRef] [PubMed]
- Chau, R.; Kalaitzis, J.A.; Neilan, B.A. On the origins and biosynthesis of tetrodotoxin. Aquat. Toxicol. 2011, 104, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.R.; Novick, P.A.; Parsons, W.H.; Mcgregor, M.; Zablocki, J.; Pande, V.S.; Bois, J.D. Marked difference in saxitoxin and tetrodoxin affinity for the human nociceptive voltage-gated sodium. Proc. Natl. Acad. Sci. USA 2012, 109, 18102–18107. [Google Scholar] [CrossRef] [PubMed]
- Chong, H.L.; Ruben, P.C. Interaction between voltage-gated sodium channels and the neurotoxin, tetrodotoxin. Channels 2008, 2, 407–412. [Google Scholar] [CrossRef]
- Lago, J.; Rodriguez, L.P.; Blanco, L.; Vieites, J.M.; Cabado, A.G. Tetrodotoxin, an extremely potent marine neurotoxin: Distribution, toxicity, origin and therapeutical uses. Mar. Drugs 2015, 13, 6384–6406. [Google Scholar] [CrossRef] [PubMed]
- Nieto, F.R.; Cobos, E.J.; Tejada, M.Á.; Sánchez-Fernández, C.; González-Cano, R.; Cendán, C.M. Tetrodotoxin (TTX) as a therapeutic agent for pain. Mar. Drugs 2012, 10, 281–305. [Google Scholar] [CrossRef] [PubMed]
- Hagen, N.A.; Fisher, K.M.; Lapointe, B.; Souich, P.; Chary, S.; Moulin, D. An Open-Label, Multi-Dose Efficacy and Safety Study of Intramuscular Tetrodotoxin in Patients with Severe. J. Pain Symptom Manag. 2007, 34, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Hagen, N.A.; Souich, P.; Lapointe, B.; Ong-lam, M.; Dubuc, B.; Walde, D. Tetrodotoxin for Moderate to Severe Cancer Pain: A Randomized, Double Blind, Parallel Design Multicenter Study. J. Pain Symptom Manag. 2008, 35, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Berde, C.B.; Athiraman, U.; Yahalom, B.; Zurakowski, D.; Corfas, G.; Bognet, C. Tetrodotoxin-bupivacaine-epinephrine combinations for prolonged local anesthesia. Mar. Drugs 2011, 9, 2717–2728. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Li, J.; Lu, C.L.; Kang, L.; Xie, L.; Zhang, Y.Y.; Zhou, X.B.; Zhong, S. Tetrodotoxin alleviates acute heroin withdrawal syndrome: A multicentre, randomized, double-blind, placebo-controlled study. Clin. Exp. Pharmacol. Physiol. 2011, 38, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.; Benford, D.; Cockburn, A.; Dogliotti, E.; Di Domenico, A.; Fernández-Cruz, M.L.; Fürst, P.; Galli, C.; Grandjean, P.; Gzyl, J.; et al. Scientific Opinion of the Panel on Contaminants in the Food chain on a request from the European Commission on marine biotoxins in shellfish—Okadaic acid and analogues. EFSA J. 2008, 589, 1–62. [Google Scholar] [CrossRef]
- Cetinkaya, F.; Mus, T.E. Shellfish Poisoning and Toxins. J. Biol. Environ. Sci. 2012, 6, 115–119. [Google Scholar]
- Hallegraeff, G.F. Harmful algal blooms: A global overview. In Manual of Harmful Marine Microalgae; Hallegraeff, G.F., Anderson, D.M., Cembella, A.D., Eds.; UNESCO Publishing: Paris, France, 2003; pp. 25–45. ISBN 9231039482. [Google Scholar]
- Wera, S.; Hemmingst, B.A. Serine/threonine protein phosphatases. Biochem. J. 1995, 311, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Martín-López, A.; Gallardo-Rodríguez, J.J.; Sánchez-Mirón, A.; García-Camacho, F.; Molina-Grima, E. Cytotoxicity of yessotoxin and okadaic acid in mouse T lymphocyte cell line EL-4. Toxicon 2012, 60, 1049–1056. [Google Scholar] [CrossRef] [PubMed]
- Cruz, P.G.; Norte, M.; Creus, A.H.; Fernández, J.J.; Daranas, A.H. Self-association of okadaic acid: Structural and pharmacological significance. Mar. Drugs 2013, 11, 1866–1877. [Google Scholar] [CrossRef] [PubMed]
- Valdiglesias, V.; Prego-Faraldo, M.V.; Paśaro, E.; Meńdez, J.; Laffon, B. Okadaic Acid: More than a diarrheic toxin. Mar. Drugs 2013, 11, 4328–4349. [Google Scholar] [CrossRef] [PubMed]
- Thévenin, C.; Kim, S.J.; Kehrl, J.H. Inhibition of protein phosphatases by okadaic acid induces AP1 in human T cells. J. Biol. Chem. 1991, 266, 9363–9366. [Google Scholar] [PubMed]
- Kamat, P.K.; Rai, S.; Nath, C. Okadaic acid induced neurotoxicity: An emerging tool to study Alzheimer’s disease pathology. Neurotoxicology 2013, 37, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Kamat, P.K.; Tota, S.; Rai, S.; Swarnkar, S.; Shukla, R.; Nath, C. A study on neuroinflammatory marker in brain areas of okadaic acid (ICV) induced memory impaired rats. Life Sci. 2012, 90, 713–720. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Yang, Y.; Xu, H.; Zhang, X.; Li, X.-M. Olanzapine attenuates the okadaic acid-induced spatial memory impairment and hippocampal cell death in rats. Neuropsychopharmacology 2005, 30, 1511–1520. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.; Götz, J. A local insult of okadaic acid in wild-type mice induces tau phosphorylation and protein aggregation in anatomically distinct brain regions. Acta Neuropathol. Commun. 2016, 4, 32. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, S.; Rajasekar, N.; Hanif, K.; Nath, C.; Shukla, R. Sulforaphane Ameliorates Okadaic Acid-Induced Memory Impairment in Rats by Activating the Nrf2/HO-1 Antioxidant Pathway. Mol. Neurobiol. 2016, 53, 5310–5323. [Google Scholar] [CrossRef] [PubMed]
- Re, F.; Braaten, D.; Franke, E.K.; Luban, J. Human immunodeficiency virus type 1 Vpr arrests the cell cycle in G2 by inhibiting the activation of p34cdc2-cyclin B. J. Virol. 1995, 69, 6859–6864. [Google Scholar] [PubMed]
- Liu, J.; Sidell, N. Anti-estrogenic effects of conjugated linoleic acid through modulation of estrogen receptor phosphorylation. Breast Cancer Res. Treat. 2005, 94, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Obanda, D.N.; Ribnicky, D.; Yu, Y.; Stephens, J.; Cefalu, W.T. An extract of Urtica dioica L. mitigates obesity induced insulin resistance in mice skeletal muscle via protein phosphatase 2A (PP2A). Sci. Rep. 2016, 6, 22222. [Google Scholar] [CrossRef] [PubMed]
- López, A.M.; Gallardo-Rodríguez, J.J.; Sánchez-Mirón, A.; García-Camacho, F.; Molina-Grima, E. Immunoregulatory potential of marine algal toxins yessotoxin and okadaic acid in mouse T lymphocyte cell line EL-4. Toxicol. Lett. 2011, 207, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Sonoda, Y.; Kasahara, T.; Yamaguehi, Y.; Kuno, K.; Matsushima, K.; Mukaida, N. Stimulation of interleukin-8 production by okadaic acid and vanadate in a human promyelocyte cell line, an HL-60 subline: Possible role of mitogen-activated protein kinase on the okadaic acid-induced NF-κB activation. J. Biol. Chem. 1997, 272, 15366–15372. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Ahn, K.; Kim, S.; Jeong, J. Okadaic acid promotes angiogenesis via activation of hypoxia-inducible factor-1. Cancer Lett. 2009, 276, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Nagai, H.; Satake, M.; Yasumoto, T. Antimicrobial activities of polyether compounds of dinoflagellate origins. J. Appl. Phycol. 1990, 2, 305–308. [Google Scholar] [CrossRef]
- Gerssen, A.; Pol-Hofstad, I.E.; Poelman, M.; Mulder, P.P.J.; van den Top, H.J.; Dde Boer, J. Marine toxins: Chemistry, toxicity, occurrence and detection, with special reference to the dutch situation. Toxins 2010, 2, 878–904. [Google Scholar] [CrossRef] [PubMed]
- Paz, B.; Riobó, P.; Luisa Fernández, M.; Fraga, S.; Franco, J.M. Production and release of yessotoxins by the dinoflagellates Protoceratium reticulatum and Lingulodinium polyedrum in culture. Toxicon 2004, 44, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, L.; McNabb, P.; de Salas, M.; Briggs, L.; Beuzenberg, V.; Gladstone, M. Yessotoxin production by Gonyaulax spinifera. Harmful Algae 2006, 5, 148–155. [Google Scholar] [CrossRef]
- Miles, C.O.; Samdal, I.A.; Aasen, J.A.G.; Jensen, D.J.; Quilliam, M.A.; Petersen, D.; Briggs, L.M.; Wilkins, A.L.; Rise, F.; Cooney, J.M.; et al. Evidence for numerous analogs of yessotoxin in Protoceratium reticulatum. Harmful Algae 2005, 4, 1075–1091. [Google Scholar] [CrossRef]
- Murata, M.; Kumagai, M.; Lee, J.S.; Yasumoto, T. Isolation and structure of yessotoxin, a novel polyether compound implicated in diarrhetic shellfish poisoning. Tetrahedron Lett. 1987, 28, 5869–5872. [Google Scholar] [CrossRef]
- Paz, B.; Daranas, A.H.; Norte, M.; Riobó, P.; Franco, J.M.; Fernández, J.J. Yessotoxins, a group of marine polyether toxins: An overview. Mar. Drugs 2008, 6, 73–102. [Google Scholar] [CrossRef] [PubMed]
- Korsnes, M.S.; Røed, S.S.; Tranulis, M.A.; Espenes, A.; Christophersen, B. Yessotoxin triggers ribotoxic stress. Toxicol. In Vitro 2014, 28, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Suárez Korsnes, M.; Espenes, A. Yessotoxin as an apoptotic inducer. Toxicon 2011, 57, 947–958. [Google Scholar] [CrossRef] [PubMed]
- Alfonso, A.; Vieytes, M.; Botana, L. Yessotoxin, a Promising Therapeutic Tool. Mar. Drugs 2016, 14, 30. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Araujo, A.; Alfonso, A.; Vieytes, M.R.; Botana, L.M. Key role of phosphodiesterase 4A (PDE4A) in autophagy triggered by yessotoxin. Toxicology 2015, 329, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Alfonso, A.; de la Rosa, L.; Vieytes, M.R.; Yasumoto, T.; Botana, L.M. Yessotoxin, a novel phycotoxin, activates phosphodiesterase activity: Effect of yessotoxin on cAMP levels in human lymphocytes. Biochem. Pharmacol. 2003, 65, 193–208. [Google Scholar] [CrossRef]
- Jeon, Y.H.; Heo, Y.-S.; Kim, C.M.; Hyun, Y.-L.; Lee, T.G.; Ro, S.; Cho, J.M. Phosphodiesterase: Overview of protein structures, potential therapeutic applications and recent progress in drug development. Cell. Mol. Life Sci. 2005, 62, 1198–1220. [Google Scholar] [CrossRef] [PubMed]
- De la Rosa, L.A.; Alfonso, A.; Vilariño, N.; Vieytes, M.R.; Botana, L.M. Modulation of cytosolic calcium levels of human lymphocytes by yessotoxin, a novel marine phycotoxin. Biochem. Pharmacol. 2001, 61, 827–833. [Google Scholar] [CrossRef]
- Korsnes, M.S.; Hetland, D.L.; Espenes, A.; Tranulis, M.A.; Aune, T. Apoptotic events induced by yessotoxin in myoblast cell lines from rat and mouse. Toxicol. In Vitro 2006, 20, 1077–1087. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, C.; Fato, R.; Angelin, A.; Trombetti, F.; Ventrella, V.; Borgatti, A.R.; Fattorusso, E.; Ciminiello, P.; Bernardi, P.; Lenaz, G.; et al. Yessotoxin, a shellfish biotoxin, is a potent inducer of the permeability transition in isolated mitochondria and intact cells. Biochim. Biophys. Acta Bioenerg. 2004, 1656, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Orsi, C.F.; Colombari, B.; Callegari, F.; Todaro, A.M.; Ardizzoni, A.; Rossini, G.P.; Blasi, E.; Peppoloni, S. Yessotoxin inhibits phagocytic activity of macrophages. Toxicon 2010, 55, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Korsnes, M.S.; Hetland, D.L.; Espenes, A.; Aune, T. Cleavage of tensin during cytoskeleton disruption in YTX-induced apoptosis. Toxicol. In Vitro 2007, 21, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Stemmler, M.P. Cadherins in development and cancer. Mol. Biosyst. 2008, 4, 835–850. [Google Scholar] [CrossRef] [PubMed]
- Tubaro, A.; Dell’Ovo, V.; Sosa, S.; Florio, C. Yessotoxins: A toxicological overview. Toxicon 2010, 56, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Korsnes, M.S.; Korsnes, R. Mitotic Catastrophe in BC3H1 Cells following Yessotoxin Exposure. Front. Cell Dev. Biol. 2017, 5, 30. [Google Scholar] [CrossRef] [PubMed]
- Tobío, A.; Alfonso, A.; Madera-Salcedo, I.; Botana, L.M.; Blank, U. Yessotoxin, a Marine Toxin, Exhibits Anti-Allergic and Anti-Tumoural Activities Inhibiting Melanoma Tumour Growth in a Preclinical Model. PLoS ONE 2016, 11, e0167572. [Google Scholar] [CrossRef] [PubMed]
- Bloom, G.S. Amyloid-β and Tau The Trigger and Bullet in Alzheimer Disease Pathogenesis. JAMA Neurol. 2014, 71, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Terao, K.; Ito, E.; Oarada, M.; Murata, M.; Yasumoto, T. Histopathological studies on experimental marine toxin poisoning—5. The effects in mice of yessotoxin isolated from Patinopecten yessoensis and of a desulfated derivative. Toxicon 1990, 28, 1095–1104. [Google Scholar] [CrossRef]
- Rubiolo, J.A.; López-Alonso, H.; Martínez, P.; Millán, A.; Cagide, E.; Vieytes, M.R.; Vega, F.V.; Botana, L.M. Yessotoxin induces ER-stress followed by autophagic cell death in glioma cells mediated by mTOR and BNIP3. Cell. Signal. 2014, 26, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, L.; González, V.; Martínez, A.; Paz, B.; Lago, J.; Cordeiro, V.; Blanco, L.; Vieites, J.; Cabado, A. Occurrence of Lipophilic Marine Toxins in Shellfish from Galicia (NW of Spain) and Synergies among Them. Mar. Drugs 2015, 13, 1666–1687. [Google Scholar] [CrossRef] [PubMed]
- Espiña, B.; Louzao, M.C.; Ares, I.R.; Fonfría, E.S.; Vilariño, N.; Vieytes, M.R.; Yasumoto, T.; Botana, L.M. Impact of the pectenotoxin C-43 oxidation degree on its cytotoxic effect on rat hepatocytes. Chem. Res. Toxicol. 2010, 23, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Allingham, J.S.; Miles, C.O.; Rayment, I. A Structural Basis for Regulation of Actin Polymerization by Pectenotoxins. J. Mol. Biol. 2007, 371, 959–970. [Google Scholar] [CrossRef] [PubMed]
- Espiña, B.; Rubiolo, J.A. Marine toxins and the cytoskeleton: Pectenotoxins, unusual macrolides that disrupt actin. FEBS J. 2008, 275, 6082–6088. [Google Scholar] [CrossRef] [PubMed]
- Ares, I.R.; Louzao, M.C.; Espiña, B.; Vieytes, M.R.; Miles, C.O.; Yasumoto, T.; Botana, L.M. Lactone ring of pectenotoxins: A key factor for their activity on cytoskeletal dynamics. Cell. Physiol. Biochem. 2007, 19, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Moon, D.-O.; Kim, M.-O.; Nam, T.-J.; Kim, S.-K.; Choi, Y.H.; Kim, G.-Y. Esculetin inhibits cell proliferation through the Ras/ERK1/2 pathway in human colon cancer cells. Oncol. Rep. 2011, 25, 223–230. [Google Scholar] [CrossRef]
- Jung, J.H.; Sim, C.J.; Lee, C.O. Cytotoxic compounds from a two-sponge association. J. Nat. Prod. 1995, 58, 1722–1726. [Google Scholar] [CrossRef] [PubMed]
- Chae, H.-D.; Choi, T.-S.; Kim, B.-M.; Jung, J.H.; Bang, Y.-J.; Shin, D.Y. Oocyte-based screening of cytokinesis inhibitors and identification of pectenotoxin-2 that induces Bim/Bax-mediated apoptosis in p53-deficient tumors. Oncogene 2005, 24, 4813–4819. [Google Scholar] [CrossRef] [PubMed]
- García-Camacho, F.; Gallardo-Rodríguez, J.J.; Sánchez-Mirón, A.; Cerón-García, M.C.; Belarbi, E.H.; Chisti, Y.; Grima-Molina, E. Biotechnological significance of toxic marine dinoflagellates. Biotechnol. Adv. 2007, 25, 176–194. [Google Scholar] [CrossRef] [PubMed]
- Holmes, M. The origin of ciguatera—An update. Ciguatera Inf. Bull. 1992, 2, 8–9. [Google Scholar]
- Friedman, M.A.; Fernandez, M.; Backer, L.C.; Dickey, R.W.; Bernstein, J.; Schrank, K.; Kibler, S.; Stephan, W.; Gribble, M.O.; Bienfang, P.; et al. An Updated Review of Ciguatera Fish Poisoning: Clinical, Epidemiological, Environmental, and Public Health Management. Mar. Drugs 2017, 15, 72. [Google Scholar] [CrossRef] [PubMed]
- Lehane, L.; Lewis, R.J. Ciguatera: Recent advances but the risk remains. Int. J. Food Microbiol. 2000, 61, 91–125. [Google Scholar] [CrossRef]
- Crump, J.A.; McLay, C.L.; Chambers, S.T. Ciguatera fish poisoning. Postgrad. Med. J. 1999, 75, 678–679. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.J.; Holmes, M.J. Origin and transfer of toxins involved in ciguatera. Comp. Biochem. Physiol. C 1993, 106, 615–628. [Google Scholar] [CrossRef]
- Hamilton, B.; Hurbungs, M.; Jones, A.; Lewis, R.J. Multiple ciguatoxins present in Indian Ocean reef fish. Toxicon 2002, 40, 1347–1353. [Google Scholar] [CrossRef]
- Scheuer, P.J.; Takahashi, W.; Tsutsumi, J.; Yoshida, T. Ciguatoxin: Isolation and chemical nature. Science 1967, 155, 1267–1268. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, G.M.; Lewis, R.J. Ciguatoxins: Cyclic polyether modulators of voltage-gated ion channel function. Mar. Drugs 2006, 4, 82–118. [Google Scholar] [CrossRef]
- Hogg, R.C.; Lewis, R.J.; Adams, D.J. Ciguatoxin-induced oscillations in membrane potential and action potential firing in rat parasympathetic neurons. Eur. J. Neurosci. 2002, 16, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Lombet, A.; Bidard, J.N.; Lazdunski, M. Ciguatoxin and brevetoxins share a common receptor site on the neuronal voltage-dependent Na+ channel. FEBS Lett. 1987, 219, 355–359. [Google Scholar] [CrossRef]
- Mattei, C.; Molgó, J.; Benoit, E. Involvement of both sodium influx and potassium efflux in ciguatoxin-induced nodal swelling of frog myelinated axons. Neuropharmacology 2014, 85, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Mattei, C.; Dechraoui, M.Y.; Molgó, J.; Meunier, F.; Legrand, A.M.; Benoit, E. Neurotoxins targeting receptor site 5 of voltage-dependent sodium channels increase the nodal volume of myelinated axons. J. Neurosci. Res. 1999, 55, 666–673. [Google Scholar] [CrossRef]
- Cameron, J.; Flowers, A.E.; Capra, M.F. Effects of ciguatoxin on nerve excitability in rats (Part I). J. Neurol. Sci. 1991, 101, 87–92. [Google Scholar] [CrossRef]
- Cameron, J.; Flowers, A.E.; Capra, M.F. Electrophysiological studies on ciguatera poisoning in man (Part II). J. Neurol. Sci. 1991, 101, 93–97. [Google Scholar] [CrossRef]
- Molgó, J.; Comella, J.X.; Legrand, A.M. Ciguatoxin enhances quantal transmitter release from frog motor nerve terminals. Br. J. Pharmacol. 1990, 99, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Mattei, C.; Wen, P.J.; Nguyen-Huu, T.D.; Alvarez, M.; Benoit, E.; Bourdelais, A.J.; Lewis, R.J.; Baden, D.G.; Molgó, J.; Meunier, F.A. Brevenal inhibits pacific ciguatoxin-1B-induced neurosecretion from bovine chromaffin cells. PLoS ONE 2008, 3, e3448. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka, K.; Inoue, M.; Miyazaki, K.; Hirama, M.; Kondo, C.; Kinoshita, E.; Miyoshi, H.; Seyama, I. Synthetic ciguatoxins selectively activate Nav1.8-derived chimeric sodium channels expressed in HEK293 cells. J. Biol. Chem. 2009, 284, 7597–7605. [Google Scholar] [CrossRef] [PubMed]
- Vetter, I.; Touska, F.; Hess, A.; Hinsbey, R.; Sattler, S.; Lampert, A.; Sergejeva, M.; Sharov, A.; Collins, L.S.; Eberhardt, M.; et al. Ciguatoxins activate specific cold pain pathways to elicit burning pain from cooling. EMBO J. 2012, 31, 3795–3808. [Google Scholar] [CrossRef] [PubMed]
- Mattei, C.; Legros, C. The voltage-gated sodium channel: A major target of marine neurotoxins. Toxicon 2014, 91, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Eijkelkamp, N.; Linley, J.E.; Baker, M.D.; Minett, M.S.; Cregg, R.; Werdehausen, R.; Rugiero, F.; Wood, J.N. Neurological perspectives on voltage-gated sodium channels. Brain 2012, 135, 2585–2612. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, K.C.; Aversa, R.J. Maitotoxin: An inspiration for synthesis. Isr. J. Chem. 2011, 51, 359–377. [Google Scholar] [CrossRef] [PubMed]
- Reyes, J.G.; Sánchez-cárdenas, C.; Acevedo-Castillo, W.; Leyton, P.; López-gonzález, I.; Felix, R.; Gandini, M.A.; Treviño, M.B.; Treviño, C.L. Maitotoxin: An Enigmatic Toxic Molecule with Useful Applications in the Biomedical Sciences. In Seafood and Freshwater Toxins Pharmacology, Physiology, and Detection; Botana, L.M., Ed.; CRC Press: London, UK, 2014; pp. 677–694. ISBN 978-1-4665-0515-5. [Google Scholar]
- Holmes, M.J.; Lewis, R.J. Purification and characterisation of large and small maitotoxins from cultured Gambierdiscus toxicus. Nat. Toxins 1994, 2, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, A.; Murata, M.; Oshima, Y.; Iwashita, T.; Yasumoto, T. Some chemical properties of maitotoxin, a putative calcium channel agonist isolated from a marine dinoflagellate. J. Biochem. 1988, 104, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, L.; Harwood, T.; Smith, K.; Argyle, P.; Munday, R. Production of ciguatoxin and maitotoxin by strains of Gambierdiscus australes, G. pacificus and G. polynesiensis (Dinophyceae) isolated from Rarotonga, Cook Islands. Harmful Algae 2014, 39, 185–190. [Google Scholar] [CrossRef]
- Pisapia, F.; Sibat, M.; Herrenknecht, C.; Lhaute, K.; Gaiani, G.; Ferron, P.-J.; Fessard, V.; Fraga, S.; Nascimento, S.M.; Litaker, R.W.; et al. Maitotoxin-4, a Novel MTX Analog Produced by Gambierdiscus excentricus. Mar. Drugs 2017, 15, 220. [Google Scholar] [CrossRef] [PubMed]
- Hambright, K.D.; Zamor, R.M.; Easton, J.D.; Allison, B. Algae. In Encyclopedia of Toxicology; Wexler, P., Ed.; Academic Press: Oxford, UK, 2014; pp. 130–141. [Google Scholar]
- Konoki, K.; Hashimoto, M.; Murata, M.; Tachibana, K. Maitotoxin-induced calcium influx in erythrocyte ghosts and rat glioma C6 cells, and blockade by gangliosides and other membrane lipids. Chem. Res. Toxicol. 1999, 12, 993–1001. [Google Scholar] [CrossRef] [PubMed]
- Xi, D.; Van Dolah, F.M.; Ramsdell, J.S. Maitotoxin induces a calcium-dependent membrane depolarization in GH4C1 pituitary cells via activation of type L voltage-dependent calcium channels. J. Biol. Chem. 1992, 267, 25025–25031. [Google Scholar] [PubMed]
- Soergel, D.G.; Gusovsky, F.; Yasumoto, T.; Daly, J.W. Stimulatory effects of maitotoxin on insulin release in insulinoma HIT cells: Role of calcium uptake and phosphoinositide breakdown. J. Pharmacol. Exp. Ther. 1990, 255, 1360–1365. [Google Scholar] [PubMed]
- Taglialatela, M.; Amoroso, S.; Yasumoto, T.; Di Renzo, G.; Annunziato, L. Maitotoxin and Bay-K-8644: Two putative calcium channel activators with different effects on endogenous dopamine release from tuberoinfundibular neurons. Brain Res. 1986, 381, 356–358. [Google Scholar] [CrossRef]
- Takahashi, M.; Tatsumi, M.; Ohizumi, Y.; Yasumoto, T. Ca2+ channel activating function of maitotoxin, the most potent marine toxin known, in clonal rat pheochromocytoma cells. J. Biol. Chem. 1983, 258, 10944–10949. [Google Scholar] [PubMed]
- Gusovsky, F.; Daly, J.W.; Yasumoto, T.; Rojas, E. Differential effects of maitotoxin on ATP secretion and on phosphoinositide breakdown in rat pheochromocytoma cells. FEBS Lett. 1988, 233, 139–142. [Google Scholar] [CrossRef]
- Estacion, M.; Schilling, W.P. Maitotoxin-induced membrane blebbing and cell death in bovine aortic endothelial cells. BMC Physiol. 2001, 1, 2. [Google Scholar] [CrossRef]
- Chávez, J.C.; de Blas, G.A.; de la Vega-Beltrán, J.L.; Nishigaki, T.; Chirinos, M.; González-González, M.E.; Larrea, F.; Solís, A.; Darszon, A.; Treviño, C.L. The opening of maitotoxin-sensitive calcium channels induces the acrosome reaction in human spermatozoa: Differences from the zona pellucida. Asian J. Androl. 2011, 13, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Treviño, C.L.; de la Vega-Beltrán, J.L.; Nishigaki, T.; Felix, R.; Darszon, A. Maitotoxin potently promotes Ca2+ influx in mouse spermatogenic cells and sperm, and induces the acrosome reaction. J. Cell. Physiol. 2006, 206, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Verhoef, P.A.; Kertesy, S.B.; Estacion, M.; Schilling, W.P.; Dubyak, G.R. Maitotoxin induces biphasic interleukin-1beta secretion and membrane blebbing in murine macrophages. Mol. Pharmacol. 2004, 66, 909–920. [Google Scholar] [CrossRef] [PubMed]
- Flores, P.; Rodríguez, E.; Zapata, E.; Carbó, R.; Farías, J.; Martínez, M. Maitotoxin Is a Potential Selective Activator of the Endogenous Transient Receptor Potential Canonical Type 1 Channel in Xenopus laevis Oocytes. Mar. Drugs 2017, 15, 198. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.H. Pharmacological Actions of Palytoxin. In Toxins and Biologically Active Compounds from Microalgae, Volume 2: Biological Effects and Risk Management; Rossini, P., Ed.; CRC Press/Taylor and Francis: Boca Raton, FL, USA, 2014; p. 663. [Google Scholar]
- Moore, R.E.; Scheuer, P.J. Palytoxin: A new marine toxin from a coelenterate. Science 1971, 172, 495–498. [Google Scholar] [CrossRef] [PubMed]
- Franchini, A.; Malagoli, D.; Ottaviani, E. Targets and effects of yessotoxin, okadaic acid and palytoxin: A differential review. Mar. Drugs 2010, 8, 658–677. [Google Scholar] [CrossRef] [PubMed]
- Ramos, V.; Vasconcelos, V. Palytoxin and analogs: Biological and ecological effects. Mar. Drugs 2010, 8, 2021–2037. [Google Scholar] [CrossRef] [PubMed]
- Patocka, J.; Gupta, R.C.; Wu, Q.H.; Kuca, K. Toxic potential of palytoxin. J. Huazhong Univ. Sci. Technol.—Med. Sci. 2015, 35, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Pelin, M.; Florio, C.; Ponti, C.; Lucafò, M.; Gibellini, D.; Tubaro, A.; Sosa, S. Pro-inflammatory effects of palytoxin: An in vitro study on human keratinocytes and inflammatory cells. Toxicol. Res. 2016, 5, 1172–1181. [Google Scholar] [CrossRef]
- Louzao, M.C.; Ares, I.R.; Cagide, E. Marine toxins and the cytoskeleton: A new view of palytoxin toxicity. FEBS J. 2008, 275, 6067–6074. [Google Scholar] [CrossRef] [PubMed]
- Ares, I.R.; Cagide, E.; Louzao, M.C.; Espiña, B.; Vieytes, M.R.; Yasumoto, T.; Botana, L.M. Ostreocin-d Impact on Globular Actin of Intact Cells. Chem. Res. Toxicol. 2009, 22, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Carmen Louzao, M.; Fraga, M.; Vilariño, N. Pharmacology of palytoxins and ostreocins. In Phycotoxins; Botana, L.M., Amparo, A., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2015; pp. 113–135. ISBN 9781118500354. [Google Scholar]
- Bigiani, A. Palytoxin action on the Na+, K+-ATPase and the disruption of ion equilibria in biological systems. Toxicon 2011, 57, 429–439. [Google Scholar] [CrossRef]
- Wattenberg, E.V. Palytoxin: Exploiting a novel skin tumor promoter to explore signal transduction and carcinogenesis. Am. J. Physiol. Cell Physiol. 2007, 292, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Satake, M.; Murata, M.; Yasumoto, T. Gambierol: A new toxic polyether compound isolated from the marine dinoflagellate Gambierdiscus toxicus. J. Am. Chem. Soc. 1993, 115, 361–362. [Google Scholar] [CrossRef]
- Fuwa, H.; Fukazawa, R.; Sasaki, M. Concise synthesis of the A/BCD-ring fragment of gambieric acid A. Front. Chem. 2015, 2, 116. [Google Scholar] [CrossRef] [PubMed]
- Fuwa, H.; Kainuma, N.; Satake, M.; Sasaki, M. Synthesis and biological evaluation of gambierol analogues. Bioorg. Med. Chem. Lett. 2003, 13, 2519–2522. [Google Scholar] [CrossRef]
- Ito, E.; Suzuki-Toyota, F.; Toshimori, K.; Fuwa, H.; Tachibana, K.; Satake, M.; Sasaki, M. Pathological effects on mice by gambierol, possibly one of the ciguatera toxins. Toxicon 2003, 42, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Alonso, E.; Rubiolo, J.A. Therapeutics of marine toxins. In Phycotoxins: Chemistry and Biochemistry; Botana, L.M., Alfonso, A., Eds.; John Wiley & Sons Ltd.: New York, NY, USA, 2015; pp. 181–195. ISBN 9781118500347. [Google Scholar]
- Cao, Z.; George, J.; Gerwick, W.H.; Baden, D.G.; Rainier, J.D.; Murray, T.F. Influence of lipid-soluble gating modifier toxins on sodium influx in neocortical neurons. J. Pharmacol. Exp. Ther. 2008, 326, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Cuypers, E.; Abdel-Mottaleb, Y.; Kopljar, I.; Rainier, J.D.; Raes, A.L.; Snyders, D.J.; Tytgat, J. Gambierol, a toxin produced by the dinoflagellate Gambierdiscus toxicus, is a potent blocker of voltage-gated potassium channels. Toxicon 2008, 51, 974–983. [Google Scholar] [CrossRef] [PubMed]
- Ghiaroni, V.; Sasaki, M.; Fuwa, H.; Rossini, G.P.; Scalera, G.; Yasumoto, T.; Pietra, P.; Bigiani, A. Inhibition of voltage-gated potassium currents by gambierol in mouse taste cells. Toxicol. Sci. 2005, 85, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Kopljar, I.; Labro, A.J.; de Block, T.; Rainier, J.D.; Tytgat, J.; Snyders, D.J. The ladder-shaped polyether toxin gambierol anchors the gating machinery of Kv3.1 channels in the resting state. J. Gen. Physiol. 2013, 141, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Cui, Y.; Busse, E.; Mehrotra, S.; Rainier, J.D.; Murray, T.F. Gambierol inhibition of voltage-gated potassium channels augments spontaneous Ca2+ oscillations in cerebrocortical neurons. J. Pharmacol. Exp. Ther. 2014, 350, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Pérez, S.; Vale, C.; Alonso, E.; Fuwa, H.; Sasaki, M.; Konno, Y.; Goto, T.; Suga, Y.; Vieytes, M.R.; Botana, L.M. Effect of Gambierol and Its Tetracyclic and Heptacyclic Analogues in Cultured Cerebellar Neurons: A Structure–Activity Relationships Study. Chem. Res. Toxicol. 2012, 25, 1929–1937. [Google Scholar] [CrossRef] [PubMed]
- Alonso, E.; Vale, C.; Sasaki, M.; Fuwa, H.; Konno, Y.; Perez, S.; Vieytes, M.R.; Botana, L.M. Calcium oscillations induced by gambierol in cerebellar granule cells. J. Cell. Biochem. 2010, 110, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Huang, J.; Yuan, X.; Peng, B.; Liu, W.; Han, S.; He, X. Toxins Targeting the Kv1.3 Channel: Potential Immunomodulators for Autoimmune Diseases. Toxins 2015, 7, 1749–1764. [Google Scholar] [CrossRef] [PubMed]
- Rubiolo, J.A.; Vale, C.; Martín, V.; Fuwa, H.; Sasaki, M.; Botana, L.M. Potassium currents inhibition by gambierol analogs prevents human T lymphocyte activation. Arch. Toxicol. 2015, 89, 1119–1134. [Google Scholar] [CrossRef] [PubMed]
- Beeton, C.; Wulff, H.; Standifer, N.E.; Azam, P.; Mullen, K.M.; Pennington, M.W.; Kolski-Andreaco, A.; Wei, E.; Grino, A.; Counts, D.R.; et al. Kv1.3 channels are a therapeutic target for T cell-mediated autoimmune diseases. Proc. Natl. Acad. Sci. USA 2006, 103, 17414–17419. [Google Scholar] [CrossRef] [PubMed]
- LePage, K.T.; Rainier, J.D.; Johnson, H.W.B.; Baden, D.G.; Murray, T.F. Gambierol acts as a functional antagonist of neurotoxin site 5 on voltage-gated sodium channels in cerebellar granule neurons. J. Pharmacol. Exp. Ther. 2007, 323, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Baden, D.G. Brevetoxins: Unique polyether dinoflagellate toxins. FASEB J. 1989, 3, 1807–1817. [Google Scholar] [PubMed]
- Plakas, S.M.; Dickey, R.W. Advances in monitoring and toxicity assessment of brevetoxins in molluscan shellfish. Toxicon 2010, 56, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Xu, J.; Tsang, T.Y.; Au, D.W.T. Toxicity comparison between Chattonella marina and Karenia brevis using marine medaka (Oryzias melastigma): Evidence against the suspected ichthyotoxins of Chattonella marina. Chemosphere 2010, 80, 585–591. [Google Scholar] [CrossRef] [PubMed]
- De Boer, M.K.; Tyl, M.R.; Fu, M.; Kulk, G.; Liebezeit, G.; Tomas, C.R.; Lenzi, A.; Naar, J.; Vrieling, E.G.; van Rijssel, M. Haemolytic activity within the species Fibrocapsa japonica (Raphidophyceae). Harmful Algae 2009, 8, 699–705. [Google Scholar] [CrossRef]
- Khan, S.; Arakawa, O.; Onoue, Y. Neurotoxins in a toxic red tide of Heterosigma akashiwo (Raphidophyceae) in Kagoshima Bay, Japan. Aquac. Res. 1997, 28, 9–14. [Google Scholar] [CrossRef]
- Baden, D.G.; Bourdelais, A.J.; Jacocks, H.; Michelliza, S.; Naar, J. Natural and derivative brevetoxins: Historical background, multiplicity, and effects. Environ. Health Perspect. 2005, 113, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Michelliza, S.; Abraham, W.M.; Jacocks, H.M.; Schuster, T.; Baden, D.G. Synthesis, modeling, and biological evaluation of analogues of the semisynthetic brevetoxin antagonist beta-naphthoyl-brevetoxin. Chembiochem 2007, 8, 2233–2239. [Google Scholar] [CrossRef] [PubMed]
- Benson, J.; Hahn, F.; March, T.; McDonald, J.; Sopori, M.; Seagrave, J.; Gomez, A.; Bourdelais, A.; Naar, J.; Zaias, J.; et al. Inhalation toxicity of brevetoxin 3 in rats exposed for 5 days. J. Toxicol. Environ. Health A 2004, 67, 1443–1456. [Google Scholar] [CrossRef] [PubMed]
- Poli, M.A.; Mende, T.J.; Baden, D.G. Brevetoxins, unique activators of voltage-sensitive sodium channels, bind to specific sites in rat brain synaptosomes. Mol. Pharmacol. 1986, 30, 129–135. [Google Scholar] [PubMed]
- Catterall, W.A.; Gainer, M. Interaction of brevetoxin A with a new receptor site on the sodium channel. Toxicon 1985, 23, 497–504. [Google Scholar] [CrossRef]
- LePage, K.T.; Baden, D.G.; Murray, T.F. Brevetoxin derivatives act as partial agonists at neurotoxin site 5 on the voltage-gated Na+ channel. Brain Res. 2003, 959, 120–127. [Google Scholar] [CrossRef]
- Cestèle, S.; Catterall, W.A. Molecular mechanisms of neurotoxin action on voltage-gated sodium channels. Biochimie 2000, 82, 883–892. [Google Scholar] [CrossRef]
- Jeglitsch, G.; Rein, K.; Baden, D.G.; Adams, D.J. Brevetoxin-3 (PbTx-3) and its derivatives modulate single tetrodotoxin-sensitive sodium channels in rat sensory neurons. J. Pharmacol. Exp. Ther. 1998, 284, 516–525. [Google Scholar] [PubMed]
- Gawley, R.E.; Rein, K.S.; Jeglitsch, G.; Adams, D.J.; Theodorakis, E.A.; Tiebes, J.; Nicolaou, K.C.; Baden, D.G. The relationship of brevetoxin “length” and A-ring functionality to binding and activity in neuronal sodium channels. Chem. Biol. 1995, 2, 533–541. [Google Scholar] [CrossRef]
- Joseph, A. Oceans: Abode of Nutraceuticals, Pharmaceuticals, and Biotoxins. In Investigating Seafloors and Oceans; Joseph, A., Ed.; Candice Janco: Goa, India, 2016; pp. 493–554. ISBN 9780128093573. [Google Scholar]
- Murrell, R.N.; Gibson, J.E. Brevetoxins 2, 3, 6, and 9 show variability in potency and cause significant induction of DNA damage and apoptosis in Jurkat E6-1 cells. Arch. Toxicol. 2009, 83, 1009–1019. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, T.; Krzanowski, J.; Nelson, R.; Martin, D.F.; Polson, J.; Duncan, R.; Lockey, R. In vitro red tide toxin effects on human bronchial smooth muscle. J. Allergy Clin. Immunol. 1988, 81, 1187–1191. [Google Scholar] [CrossRef]
- Abraham, W.M.; Bourdelais, A.J.; Sabater, J.R.; Ahmed, A.; Lee, T.A.; Serebriakov, I.; Baden, D.G. Airway responses to aerosolized brevetoxins in an animal model of asthma. Am. J. Respir. Crit. Care Med. 2005, 171, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Hilderbrand, S.C.; Murrell, R.N.; Gibson, J.E.; Brown, J.M. Marine brevetoxin induces ige-independent mast cell activation. Arch. Toxicol. 2011, 85, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Sas, K.M.; Baatz, J.E. Brevetoxin-2 induces an inflammatory response in an alveolar macrophage cell line. Int. J. Hyg. Environ. Health 2010, 213, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C.J.; Leggett, S.R.; Strohbehn, K.; Pierce, R.H.; Sleasman, J.W. Effects of in vitro brevetoxin exposure on apoptosis and cellular metabolism in a leukemic T cell line (Jurkat). Mar. Drugs 2008, 6, 291–307. [Google Scholar] [CrossRef] [PubMed]
- George, J.; Baden, D.G.; Gerwick, W.H.; Murray, T.F. Bidirectional in fl uence of sodium channel activation on NMDA receptor–dependent cerebrocortical neuron structural plasticity. Proc. Natl. Acad. Sci. USA 2012, 109, 19840–19845. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Xu, X.; Li, T.; Liu, Z. Shellfish Toxins Targeting Voltage-Gated Sodium Channels. Mar. Drugs 2013, 11, 4698–4723. [Google Scholar] [CrossRef] [PubMed]
- Tillmann, U.; Elbrächter, M.; Krock, B.; John, U.; Cembella, A. Azadinium spinosum gen. et sp. nov. (Dinophyceae) identified as a primary producer of azaspiracid toxins. Eur. J. Phycol. 2009, 44, 63–79. [Google Scholar] [CrossRef]
- Percopo, I.; Siano, R.; Rossi, R.; Soprano, V.; Sarno, D.; Zingone, A. A new potentially toxic Azadinium species (Dinophyceae) from the Mediterranean Sea, A. dexteroporum sp. nov. J. Phycol. 2013, 49, 950–966. [Google Scholar] [CrossRef] [PubMed]
- Krock, B.; Tillmann, U.; Potvin, É.; Jeong, H.J.; Drebing, W.; Kilcoyne, J.; Al-Jorani, A.; Twiner, M.J.; Gothel, Q.; Kock, M. Structure elucidation and in vitro toxicity of new azaspiracids isolated from the marine dinoflagellate Azadinium poporum. Mar. Drugs 2015, 13, 6687–6702. [Google Scholar] [CrossRef] [PubMed]
- Hess, P.; Mccarron, P.; Krock, B.; Miles, C.O. Azaspiracids: Chemistry, Biosynthesis, Metabolism, and Detection. In Seafood and Freshwater Toxins; Botana, L.M., Ed.; CRC Press: London, UK, 2014; pp. 799–822. [Google Scholar]
- Busch, J.A.; Andree, K.B.; Diogène, J.; Fernández-Tejedor, M.; Toebe, K.; John, U.; Krock, B.; Tillmann, U.; Cembella, A.D. Toxigenic algae and associated phycotoxins in two coastal embayments in the Ebro Delta (NW Mediterranean). Harmful Algae 2016, 55, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Satake, M.; Ofuji, K.; Naoki, H.; James, K.J.; Furey, A.; McMahon, T.; Silke, J.; Yasumoto, T. Azaspiracid, a New Marine Toxin Having Unique Spiro Ring Assemblies, Isolated from Irish Mussels, Mytilus edulis. J. Am. Chem. Soc. 1998, 120, 9967–9968. [Google Scholar] [CrossRef]
- Vilariño, N. Marine toxins and the cytoskeleton: Azaspiracids. FEBS J. 2008, 275, 6075–6081. [Google Scholar] [CrossRef] [PubMed]
- Twiner, M.J.; Hess, P.; Bottein Dechraoui, M.Y.; McMahon, T.; Samons, M.S.; Satake, M.; Yasumoto, T.; Ramsdell, J.S.; Doucette, G.J. Cytotoxic and cytoskeletal effects of azaspiracid-1 on mammalian cell lines. Toxicon 2005, 45, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Colman, J.R.; Twiner, M.J.; Hess, P.; McMahon, T.; Satake, M.; Yasumoto, T.; Doucette, G.J.; Ramsdell, J.S. Teratogenic effects of azaspiracid-1 identified by microinjection of Japanese medaka (Oryzias latipes) embryos. Toxicon 2005, 45, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; LePage, K.T.; Frederick, M.O.; Nicolaou, K.C.; Murray, T.F. Involvement of Caspase Activation in Azaspiracid-Induced Neurotoxicity in Neocortical Neurons. Toxicol. Sci. 2010, 114, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Vale, C.; Nicolaou, K.C.; Frederick, M.O.; Vieytes, M.R.; Botana, L.M. Cell volume decrease as a link between azaspiracid-induced cytotoxicity and c-Jun-.-terminal kinase activation in cultured neurons. Toxicol. Sci. 2009, 113, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Román, Y.; Alfonso, A.; Vieytes, M.R.; Ofuji, K.; Satake, M.; Yasumoto, T.; Botana, L.M. Effects of Azaspiracids 2 and 3 on Intracellular cAMP, [Ca2+], and pH. Chem. Res. Toxicol. 2004, 17, 1338–1349. [Google Scholar] [CrossRef] [PubMed]
- Twiner, M.J.; Hanagriff, J.C.; Butler, S.; Madhkoor, A.K.; Doucette, G.J. Induction of Apoptosis Pathways in Several Cell Lines following Exposure to the Marine Algal Toxin Azaspiracid. Chem. Res. Toxicol. 2012, 25, 1493–1501. [Google Scholar] [CrossRef] [PubMed]
- Twiner, M.J.; Ryan, J.C.; Morey, J.S.; Smith, K.J.; Hammad, S.M.; Van Dolah, F.M.; Hess, P.; McMahon, T.; Satake, M.; Yasumoto, T.; et al. Transcriptional profiling and inhibition of cholesterol biosynthesis in human T lymphocyte cells by the marine toxin azaspiracid. Genomics 2008, 91, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Twiner, M.J.; Doucette, G.J.; Rasky, A.; Huang, X.-P.; Roth, B.L.; Sanguinetti, M.C. Marine Algal Toxin Azaspiracid Is an Open-State Blocker of hERG Potassium Channels. Chem. Res. Toxicol. 2012, 25, 1975–1984. [Google Scholar] [CrossRef] [PubMed]
- Satake, M.; Shoji, M.; Oshima, Y.; Naoki, H.; Fujita, T.; Yasumoto, T. Gymnocin-A, a cytotoxic polyether from the notorious red tide dinoflagellate, Gymnodinium mikimotoi. Tetrahedron Lett. 2002, 43, 5829–5832. [Google Scholar] [CrossRef]
- Tsukano, C.; Sasaki, M. Structure-activity relationship studies of gymnocin-A. Tetrahedron Lett. 2006, 47, 6803–6807. [Google Scholar] [CrossRef]
- Houdai, T.; Matsuoka, S.; Matsumori, N.; Murata, M. Membrane-permeabilizing activities of amphidinol 3, polyene-polyhydroxy antifungal from a marine dinoflagellate. Biochim. Biophys. Acta-Biomembr. 2004, 1667, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Waters, A.L.; Hill, R.T.; Place, A.R.; Hamann, M.T. The expanding role of marine microbes in pharmaceutical. Curr. Opin. Biotechnol. 2011, 21, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Adolf, J.E.; Bachvaroff, T.R.; Deeds, J.R.; Place, A.R. Ichthyotoxic Karlodinium veneficum (Ballantine) J Larsen in the Upper Swan River Estuary (Western Australia): Ecological conditions leading to a fish kill. Harmful Algae 2015, 48, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Bachvaroff, T.R.; Adolf, J.E.; Squier, A.H.; Harvey, H.R.; Place, A.R. Characterization and quantification of karlotoxins by liquid chromatography-mass spectrometry. Harmful Algae 2008, 7, 473–484. [Google Scholar] [CrossRef]
- Waters, A.L.; Oh, J.; Place, A.R.; Hamann, M.T. Stereochemical Studies of the Karlotoxin Class Using NMR Spectroscopy and DP4 Chemical-Shift Analysis: Insights into their Mechanism of Action. Angew. Chem. Int. Ed. 2015, 54, 15705–15710. [Google Scholar] [CrossRef] [PubMed]
- Van Wagoner, R.M.; Deeds, J.R.; Tatters, A.O.; Place, A.R.; Tomas, C.R.; Wright, J.L.C. Structure and relative potency of several karlotoxins from Karlodinium veneficum. J. Nat. Prod. 2010, 73, 1360–1365. [Google Scholar] [CrossRef] [PubMed]
- Cai, P.; He, S.; Zhou, C.; Place, A.R.; Haq, S.; Ding, L.; Chen, H.; Jiang, Y.; Guo, C.; Xu, Y.; et al. Two new karlotoxins found in Karlodinium veneficum (strain GM2) from the East China Sea. Harmful Algae 2016, 58, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Place, A.R.; Yoshida, W.; Anklin, C.; Hamann, M.T. Structure and absolute configuration of Karlotoxin-2, an ichthyotoxin from the marine dinoflagellate Karlodinium veneficum. J. Am. Chem. Soc. 2010, 132, 3277–3279. [Google Scholar] [CrossRef] [PubMed]
- Van Wagoner, R.M.; Deeds, J.R.; Satake, M.; Ribeiro, A.A.; Place, A.R.; Wright, J.L.C. Isolation and characterization of karlotoxin 1, a new amphipathic toxin from Karlodinium veneficum. Tetrahedron Lett. 2008, 49, 6457–6461. [Google Scholar] [CrossRef] [PubMed]
- Murata, M.; Matsuoka, S.; Matsumori, N.; Paul, G.K.; Tachibana, K. Absolute configuration of amphidinol 3, the first complete structure determination from amphidinol homologues: Application of a new configuration analysis based on carbon-hydrogen spin-coupling constants. J. Am. Chem. Soc. 1999, 121, 870–871. [Google Scholar] [CrossRef]
- Place, A.R.; Bowers, H.A.; Bachvaroff, T.R.; Adolf, J.E.; Deeds, J.R.; Sheng, J. Karlodinium veneficum—The little dinoflagellate with a big bite. Harmful Algae 2012, 14, 179–195. [Google Scholar] [CrossRef]
- Deeds, J.R.; Reimschuessel, R.; Place, A.R. Histopathological Effects in Fish Exposed to the Toxins from Karlodinium micrum. J. Aquat. Anim. Health 2006, 18, 136–148. [Google Scholar] [CrossRef]
- Deeds, J.R.; Hoesch, R.E.; Place, A.R.; Kao, J.P.Y. The cytotoxic mechanism of karlotoxin 2 (KmTx 2) from Karlodinium veneficum (Dinophyceae). Aquat. Toxicol. 2014, 159, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Swasono, R.T.; Mouri, R.; Morsy, N.; Matsumori, N.; Oishi, T.; Murata, M. Sterol effect on interaction between amphidinol 3 and liposomal membrane as evidenced by surface plasmon resonance. Bioorg. Med. Chem. Lett. 2010, 20, 2215–2218. [Google Scholar] [CrossRef] [PubMed]
- Deeds, J.; Place, A. Sterol-specific membrane interactions with the toxins from Karlodinium micrum (Dinophyceae)—A strategy for self-protection? Afr. J. Mar. Sci. 2006, 28, 421–425. [Google Scholar] [CrossRef]
- Sieg, R.D.; Poulson-Ellestad, K.L.; Kubanek, J. Chemical ecology of the marine plankton. Nat. Prod. Rep. 2011, 28, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Cembella, A.D.; Lewis, N.I.; Quilliam, M.A. The marine dinoflagellate Alexandrium ostenfeldii (Dinophyceae) as the causative organism of spirolide shellfish toxins. Phycologia 2000, 39, 67–74. [Google Scholar] [CrossRef]
- Touzet, N.; Franco, J.M.; Raine, R. Morphogenetic diversity and biotoxin composition of Alexandrium (Dinophyceae) in Irish coastal waters. Harmful Algae 2008, 7, 782–797. [Google Scholar] [CrossRef]
- Miles, C.O.; Wilkins, A.L.; Stirling, D.J.; MacKenzie, A.L. Gymnodimine C, an Isomer of Gymnodimine B, from Karenia selliformis. J. Agric. Food Chem. 2003, 51, 4838–4840. [Google Scholar] [CrossRef] [PubMed]
- Davidson, K.; Baker, C.; Higgins, C.; Higman, W.; Swan, S.; Veszelovszki, A.; Turner, A.D. Potential threats posed by new or emerging marine biotoxins in UK waters and examination of detection methodologies used for their Control: Cyclic imines. Mar. Drugs 2015, 13, 7087–7112. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Curtis, J.M.; Walter, J.A.; Wright, J.L.C. Characterization of biologically inactive spirolides E and F: Identification of the spirolide pharmacophore. Tetrahedron Lett. 1996, 37, 7671–7674. [Google Scholar] [CrossRef]
- Guéret, S.M.; Brimble, M.A. Spiroimine shellfish poisoning (SSP) and the spirolide family of shellfish toxins: Isolation, structure, biological activity and synthesis. Nat. Prod. Rep. 2010, 27, 1350–1366. [Google Scholar] [CrossRef] [PubMed]
- Couesnon, A.; Aráoz, R.; Iorga, B.I.; Benoit, E.; Reynaud, M.; Servent, D.; Molgó, J. The Dinoflagellate Toxin 20-Methyl Spirolide-G Potently Blocks Skeletal Muscle and Neuronal Nicotinic Acetylcholine Receptors. Toxins 2016, 8, 249. [Google Scholar] [CrossRef] [PubMed]
- Roach, J.S.; LeBlanc, P.; Lewis, N.I.; Munday, R.; Quilliam, M.A.; MacKinnon, S.L. Characterization of a Dispiroketal Spirolide Subclass from Alexandrium ostenfeldii. J. Nat. Prod. 2009, 72, 1237–1240. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.; Murphy, M.; Clausen, J.; Richard, D.; Quilliam, M.; MacKinnon, S.; LaBlanc, P.; Mueller, R.; Pulido, O. Neural injury biomarkers of novel shellfish toxins, spirolides: A pilot study using immunochemical and transcriptional analysis. Neurotoxicology 2003, 24, 593–604. [Google Scholar] [CrossRef]
- Seki, T.; Satake, M.; Mackenzie, L.; Kaspar, H.F.; Yasumoto, T. Gymnodimine, a new marine toxin of unprecedented structure isolated from New Zealand oysters and the dinoflagellate, Gymnodinium sp. Tetrahedron Lett. 1995, 36, 7093–7096. [Google Scholar] [CrossRef]
- Van Wagoner, R.M.; Misner, I.; Tomas, C.R.; Wright, J.L.C. Occurrence of 12-methylgymnodimine in a spirolide-producing dinoflagellate Alexandrium peruvianum and the biogenetic implications. Tetrahedron Lett. 2011, 52, 4243–4246. [Google Scholar] [CrossRef]
- Harju, K.; Koskela, H.; Kremp, A.; Suikkanen, S.; De La Iglesia, P.; Miles, C.O.; Krock, B.; Vanninen, P. Identification of gymnodimine D and presence of gymnodimine variants in the dinoflagellate Alexandrium ostenfeldii from the Baltic Sea. Toxicon 2016, 112, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Kharrat, R.; Servent, D.; Girard, E.; Ouanounou, G.; Amar, M.; Marrouchi, R.; Benoit, E.; Molgó, J. The marine phycotoxin gymnodimine targets muscular and neuronal nicotinic acetylcholine receptor subtypes with high affinity. J. Neurochem. 2008, 107, 952–963. [Google Scholar] [CrossRef] [PubMed]
- Hauser, T.A.; Hepler, C.D.; Kombo, D.C.; Grinevich, V.P.; Kiser, M.N.; Hooker, D.N.; Zhang, J.; Mountfort, D.; Selwood, A.; Akireddy, S.R.; et al. Comparison of acetylcholine receptor interactions of the marine toxins, 13-desmethylspirolide C and gymnodimine. Neuropharmacology 2012, 62, 2238–2249. [Google Scholar] [CrossRef] [PubMed]
- Dragunow, M.; Trzoss, M.; Brimble, M.A.; Cameron, R.; Beuzenberg, V.; Holland, P.; Mountfort, D. Investigations into the cellular actions of the shellfish toxin gymnodimine and analogues. Environ. Toxicol. Pharmacol. 2005, 20, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Alonso, E.; Vale, C.; Vieytes, M.R.; Laferla, F.M.; Giménez-Llort, L.; Botana, L.M. The Cholinergic Antagonist Gymnodimine Improves Aβ and Tau Neuropathology in an in Vitro Model of Alzheimer Disease. Cell. Physiol. Biochem. 2011, 27, 783–794. [Google Scholar] [CrossRef] [PubMed]
- Naila, I.B.; Hamza, A.; Gdoura, R.; Diogène, J.; de la Iglesia, P. Prevalence and persistence of gymnodimines in clams from the Gulf of Gabes (Tunisia) studied by mouse bioassay and LC–MS/MS. Harmful Algae 2012, 18, 56–64. [Google Scholar] [CrossRef]
- Nagai, H.; Murata, M.; Torigoe, K.; Satake, M.; Yasumoto, T. Gambieric acids, new potent antifungal substances with unprecedented polyether structures from a marine dinoflagellate Gambierdiscus toxicus. J. Org. Chem. 1992, 57, 5448–5453. [Google Scholar] [CrossRef]
- Morohashi, A.; Satake, M.; Nagai, H.; Oshima, Y.; Yasumoto, T. The Absolute Configuration of Gambieric Acids A–D, Potent Antifungal Polyethers, Isolated from the Marine Dinoflagellate Gambierdiscus toxicus. Tetrahedron 2000, 56, 8995–9001. [Google Scholar] [CrossRef]
- Nagai, H.; Mirakami, Y.; Yazawa, K.; Gonoi, T.; Yasumoto, T. Biological activities of novel polyether antifungals, gamberic acids A and B from a marine dinogflagellate Gambierdiscus toxicus. J. Antibiot. 1993, 46, 520–522. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Fuwa, H. Total synthesis and complete structural assignment of gambieric acid A, a large polycyclic ether marine natural product. Chem. Rec. 2014, 14, 678–703. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Hirama, M.; Satake, M.; Sugiyama, K.; Yasumoto, T. Inhibition of brevetoxin binding to the voltage-gated sodium channel by gambierol and gambieric acid-A. Toxicon 2003, 41, 469–474. [Google Scholar] [CrossRef]
- Murakami, M.; Makabe, K.; Yamaguchi, K.; Konosu, S.; Wälchli, M.R. Goniodomin a, a novel polyether macrolide from the dinoflagellate goniodoma pseudogoniaulax. Tetrahedron Lett. 1988, 29, 1149–1152. [Google Scholar] [CrossRef]
- Hsia, M.H.; Morton, S.L.; Smith, L.L.; Beauchesne, K.R.; Huncik, K.M.; Moeller, P.D.R. Production of goniodomin A by the planktonic, chain-forming dinoflagellate Alexandrium monilatum (Howell) Balech isolated from the Gulf Coast of the United States. Harmful Algae 2006, 5, 290–299. [Google Scholar] [CrossRef]
- Triki, H.Z.; Laabir, M.; Moeller, P.; Chomerat, N.; Daly-Yahia, K.O. First report of goniodomin A production by the dinoflagellate Alexandrium pseudogonyaulax developing in southern Mediterranean (Bizerte Lagoon, Tunisia). Toxicon 2016, 111, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Takeda, Y.; Shi, J.; Oikawa, M.; Sasaki, M. Assignment of the Absolute Configuration of Goniodomin A by NMR Spectroscopy and Synthesis of Model Compounds. Org. Lett. 2008, 10, 1013–1016. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Inoue, D.; Matsunaga, K.; Ohizumi, Y.; Ueda, H.; Asano, T.; Murakami, M.; Sato, Y. Goniodomin A, an antifungal polyether macrolide, exhibits antiangiogenic activities via inhibition of actin reorganization in endothelial cells. J. Cell. Physiol. 2002, 190, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, K.; Nakahata, N.; Ito, E.; Murakami, M.; Yamaguchi, K.; Ohizumi, Y. Goniodomin A, an antifungal polyether macrolide, increases the filamentous actin content of 1321N1 human astrocytoma cells. J. Pharm. Pharmacol. 1998, 50, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Espiña, B.; Cagide, E.; Louzao, M.C.; Vilariño, N.; Vieytes, M.R.; Takeda, Y.; Sasaki, M.; Botana, L.M. Cytotoxicity of goniodomin A and B in non contractile cells. Toxicol. Lett. 2016, 250–251, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, J.; Tsuda, M. Amphidinolides, bioactive macrolides from symbiotic marine dinoflagellates. Nat. Prod. Rep. 2004, 21, 77–93. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, J. Search for New Bioactive Marine Natural Products. Chem. Pharm. Bull. 2016, 64, 1079–1083. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, T.K.; Das, S. Chemistry of Potent Anti-Cancer Compounds, Amphidinolides. Curr. Med. Chem. Anticancer Agents 2001, 1, 131–149. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, J. Amphidinolides and its related macrolides from marine dinoflagellates. J. Antibiot. 2008, 61, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Usui, T.; Kazami, S.; Dohmae, N.; Mashimo, Y.; Kondo, H.; Tsuda, M.; Terasaki, A.G.; Ohashi, K.; Kobayashi, J.; Osada, H. Amphidinolide H, a Potent Cytotoxic Macrolide, Covalently Binds on Actin Subdomain 4 and Stabilizes Actin Filament. Chem. Biol. 2004, 11, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Echigoya, R.; Rhodes, L.; Oshima, Y.; Satake, M. The structures of five new antifungal and hemolytic amphidinol analogs from Amphidinium carterae collected in New Zealand. Harmful Algae 2005, 4, 383–389. [Google Scholar] [CrossRef]
- Nuzzo, G.; Cutignano, A.; Sardo, A.; Fontana, A. Antifungal amphidinol 18 and its 7-sulfate derivative from the marine dinoflagellate Amphidinium carterae. J. Nat. Prod. 2014, 77, 1524–1527. [Google Scholar] [CrossRef] [PubMed]
- Cutignano, A.; Nuzzo, G.; Sardo, A.; Fontana, A. The missing piece in biosynthesis of amphidinols: First evidence of glycolate as a starter unit in New polyketides from Amphidinium carterae. Mar. Drugs 2017, 15, 157. [Google Scholar] [CrossRef] [PubMed]
- Satake, M.; Cornelio, K.; Hanashima, S.; Malabed, R.; Murata, M.; Matsumori, N.; Zhang, H.; Hayashi, F.; Mori, S.; Kim, J.S.; et al. Structures of the Largest Amphidinol Homologues from the Dinoflagellate Amphidinium carterae and Structure–Activity Relationships. J. Nat. Prod. 2017, 80, 2883–2888. [Google Scholar] [CrossRef] [PubMed]
- Satake, M.; Murata, M.; Yasumoto, T.; Fujita, T.; Naoki, H. Amphidinol, a polyhydroxy-polyene antifungal agent with an unprecedented structure, from a marine dinoflagellate, Amphidinium klebsii. J. Am. Chem. Soc. 1991, 113, 9859–9861. [Google Scholar] [CrossRef]
- Manabe, Y.; Ebine, M.; Matsumori, N.; Murata, M.; Oishi, T. Confirmation of the Absolute Configuration at C45 of Amphidinol 3. J. Nat. Prod. 2012, 75, 2003–2006. [Google Scholar] [CrossRef] [PubMed]
- Houdai, T.; Matsuoka, S.; Morsy, N.; Matsumori, N.; Satake, M.; Murata, M. Hairpin conformation of amphidinols possibly accounting for potent membrane permeabilizing activities. Tetrahedron 2005, 61, 2795–2802. [Google Scholar] [CrossRef]
- Espiritu, R.A.; Matsumori, N.; Tsuda, M.; Murata, M. Direct and stereospecific interaction of amphidinol 3 with sterol in lipid bilayers. Biochemistry 2014, 53, 3287–3293. [Google Scholar] [CrossRef] [PubMed]
- Akakabe, M.; Kumagai, K.; Tsuda, M.; Konishi, Y.; Tominaga, A.; Tsuda, M.; Fukushi, E.; Kawabata, J. Amphirionin-5, a novel linear polyketide from a cultured marine dinoflagellate Amphidinium species with a potent cell proliferation-promoting activity. Tetrahedron Lett. 2014, 55, 3491–3494. [Google Scholar] [CrossRef]
- Minamida, M.; Kumagai, K.; Ulanova, D.; Akakabe, M.; Konishi, Y.; Tominaga, A.; Tanaka, H.; Tsuda, M.; Fukushi, E.; Kawabata, J.; et al. Amphirionin-4 with potent proliferation-promoting activity on bone marrow stromal cells from a marine dinoflagellate Amphidinium species. Org. Lett. 2014, 16, 4858–4861. [Google Scholar] [CrossRef] [PubMed]
- Akakabe, M.; Kumagai, K.; Konishi, Y.; Tominaga, A.; Tsuda, M.; Fukushi, E.; Kawabata, J.; Tsuda, M. Iriomoteolide-13a, a cytotoxic 22-membered macrolide from a marine dinoflagellate Amphidinium species. Tetrahedron 2014, 70, 2962–2965. [Google Scholar] [CrossRef]
- Bensoussan, C.; Rival, N.; Hanquet, G.; Colobert, F.; Reymond, S.; Cossy, J. Isolation, structural determination and synthetic approaches toward amphidinol 3. Nat. Prod. Rep. 2014, 31, 468. [Google Scholar] [CrossRef] [PubMed]
- Lahlou, M. The Success of Natural Products in Drug Discovery. Pharmacol. Pharm. 2013, 4, 17–31. [Google Scholar] [CrossRef]
- Shimizu, Y. Microalgae as a Drug Source. In Drugs from the Sea; Fusetani, N., Ed.; Karger Medical and Scientific Publishers: Basel, Switzerland, 2000; pp. 30–45. ISBN 978-3-318-00599-8. [Google Scholar]
- Mendes, A.; Reis, A.; Vasconcelos, R.; Guerra, P.; Lopes Da Silva, T. Crypthecodinium cohnii with emphasis on DHA production: A review. J. Appl. Phycol. 2009, 21, 199–214. [Google Scholar] [CrossRef]
- Beuzenberg, V.; Mountfort, D.; Holland, P.; Shi, F.; Mackenzie, L. Optimization of growth and production of toxins by three dinoflagellates in photobioreactor cultures. J. Appl. Phycol. 2012, 24, 1023–1033. [Google Scholar] [CrossRef]
- Gallardo-Rodríguez, J.J.; Sánchez-Mirón, A.; García-Camacho, F.; Cerón-García, M.C.; Belarbi, E.H.; Chisti, Y.; Molina-Grima, E. Causes of shear sensitivity of the toxic dinoflagellate Protoceratium reticulatum. Biotechnol. Prog. 2009, 25, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Gallardo-Rodríguez, J.J.; Sánchez-Mirón, A.; García-Camacho, F.; Cerón-García, M.; Belarbi, E.H.; Molina-Grima, E. Culture of dinoflagellates in a fed-batch and continuous stirred-tank photobioreactors: Growth, oxidative stress and toxin production. Process Biochem. 2010, 45, 660–666. [Google Scholar] [CrossRef]
- Ten Lohuis, M.R.; Miller, D.J. Genetic transformation of dinoflagellates (Amphidinium and Symbiodinium): Expression of GUS in microalgae using heterologous promoter constructs. Plant J. 1998, 13, 427–435. [Google Scholar] [CrossRef]
- Radakovits, R.; Jinkerson, R.E.; Darzins, A.; Posewitz, M.C. Genetic engineering of algae for enhanced biofuel production. Eukaryot. Cell 2010, 9, 486–501. [Google Scholar] [CrossRef] [PubMed]
- Beer, L.L.; Boyd, E.S.; Peters, J.W.; Posewitz, M.C. Engineering algae for biohydrogen and biofuel production. Curr. Opin. Biotechnol. 2009, 20, 264–271. [Google Scholar] [CrossRef] [PubMed]
- McEwan, M.; Humayun, R.; Slamovotis, C.H.; Keeling, P.J. Nuclear Genome Sequence Survey of the Dinoflagellate Heterocapsa triquetra. J. Eukaryot. Microbiol. 2008, 55, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Wisecaver, J.H.; Hackett, J.D. Dinoflagellate Genome Evolution. Annu. Rev. Microbiol. 2011, 65, 369–387. [Google Scholar] [CrossRef] [PubMed]
- Jaeckisch, N.; Yang, I.; Wohlrab, S.; Glöckner, G.; Kroymann, J.; Vogel, H.; Cembella, A.; John, U. Comparative Genomic and Transcriptomic Characterization of the Toxigenic Marine Dinoflagellate Alexandrium ostenfeldii. PLoS ONE 2011, 6, e28012. [Google Scholar] [CrossRef] [PubMed]
- Lin, S. Genomic understanding of dinoflagellates. Res. Microbiol. 2011, 162, 551–569. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Z.; Zhang, S.F.; Zhang, Y.; Lin, L. Paralytic shellfish toxin biosynthesis in cyanobacteria and dinoflagellates: A molecular overview. J. Proteom. 2016, 135, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, K.C.; Rutjes, F.P.J.T.; Theodorakis, E.A.; Tiebes, J.; Sato, M.; Untersteller, E. Total Synthesis of Brevetoxin B. 3. Final Strategy and Completion. J. Am. Chem. Soc. 1995, 117, 10252–10263. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Gunzner, J.L.; Shi, G.; Agrios, K.A.; Gärtner, P.; Yang, Z. Total Synthesis of Brevetoxin A: Part 4: Final Stages and Completion. Chem. Eur. J. 1999, 5, 646–658. [Google Scholar] [CrossRef]
- Fuwa, H.; Kainuma, N.; Tachibana, K.; Sasaki, M. Total Synthesis of (−)-Gambierol. J. Am. Chem. Soc. 2002, 124, 14983–14992. [Google Scholar] [CrossRef] [PubMed]
- Tsukano, C.; Ebine, M.; Sasaki, M. Convergent Total Synthesis of Gymnocin-A and Evaluation of Synthetic Analogues. J. Am. Chem. Soc. 2005, 127, 4326–4335. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, K.C.; Koftis, T.V.; Vyskocil, S.; Petrovic, G.; Tang, W.; Frederick, M.O.; Chen, D.Y.; Li, Y.; Ling, T.; Yamada, Y.M. Total Synthesis and Structural Elucidation of Azaspiracid-1. Final Assignment and Total Synthesis of the Correct Structure of Azaspiracid-1. J. Am. Chem. Soc. 2006, 128, 2244–2257. [Google Scholar] [CrossRef] [PubMed]
- Fuwa, H.; Ishigai, K.; Hashizume, K.; Sasaki, M. Total Synthesis and Complete Stereostructure of Gambieric Acid A. J. Am. Chem. Soc. 2012, 134, 11984–11987. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Rainier, J.D. Synthesis of the ABCDEF and FGHI ring system of yessotoxin and adriatoxin. J. Antibiot. 2016, 69, 259–272. [Google Scholar] [CrossRef] [PubMed]
- García-Camacho, F.; Gallardo-Rodríguez, J.J.; Sánchez-Mirón, A.; Cerón-García, M.C.; Belarbi, E.H.; Molina-Grima, E. Determination of shear stress thresholds in toxic dinoflagellates cultured in shaken flasks. Implications in bioprocess engineering. Process Biochem. 2007, 42, 1506–1515. [Google Scholar] [CrossRef]
- López-Rosales, L.; García-Camacho, F.; Sánchez-Mirón, A.; Beato, E.M.; Chisti, Y. Pilot-scale bubble column photobioreactor culture of a marine dinoflagellate microalga illuminated with light emission diodes. Bioresour. Technol. 2016, 216, 845–855. [Google Scholar] [CrossRef] [PubMed]
- López-Rosales, L.; Sánchez-Mirón, A.; Contreras-Gomes, A.; García-Camacho, F.; Molina-Grima, E. An optimisation approach for culturing shear-sensitive dinoflagellate microalgae in bench-scale bubble column photobioreactors. Bioresour. Technol. 2015, 197, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Grünewald, C.; Bayliss, C.; Fonlut, F.; Chapuli, E. Long-term dinoflagellate culture performance in a commercial photobioreactor: Amphidinium carterae case. Bioresour. Technol. 2016, 218, 533–540. [Google Scholar] [CrossRef] [PubMed]
- García-Camacho, F.; Gallardo-Rodríguez, J.J.; Sánchez-Mirón, A.; Belarbi, E.H.; Chisti, Y.; Molina-Grima, E. Photobioreactor scale-up for a shear-sensitive dinoflagellate microalga. Process Biochem. 2011, 46, 936–944. [Google Scholar] [CrossRef]
- Hyka, P.; Lickova, S.; Přibyl, P.; Melzoch, K.; Kovar, K. Flow cytometry for the development of biotechnological processes with microalgae. Biotechnol. Adv. 2013, 31, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Grima, E.M.; Acie, F.G.; Medina, A.R.; Chisti, Y. Recovery of microalgal biomass and metabolites: Process options and economics. Biotechnol. Adv. 2003, 20, 491–515. [Google Scholar] [CrossRef]
- Grima, E.; Belarbi, E.-H.; Acién Fernández, F.; Chisti, Y. Tubular photobioreactor design for algal cultures. J. Biotechnol. 2001, 92, 113–131. [Google Scholar]
- Acién, F.F.G.; Fernández, S.J.M.; Sánchez, P.J.A.; Molina, G.E.; Chisti, Y. Airlift-driven external-loop tubular photobioreactors for outdoor production of microalgae: Assessment of design and performance. Chem. Eng. Sci. 2001, 56, 2721–2732. [Google Scholar] [CrossRef]
- Wong, J.T.Y.; Kwok, A.C.M. Proliferation of dinoflagellates: Blooming or bleaching. BioEssays 2005, 27, 730–740. [Google Scholar] [CrossRef] [PubMed]
- Fensome, R.A.; MacRae, R.A.; Williams, G.L. Dinoflagellate evolution and diversity through time. Mar. Micropaleontol. 1995, 4, 1–12. [Google Scholar] [CrossRef]
- Doblin, M.A.; Thompson, P.A.; Revill, A.T.; Butler, E.C.V.; Blackburn, S.I.; Hallegraeff, G.M. Vertical migration of the toxic dinoflagellate Gymnodinium catenatum under different concentrations of nutrients and humic substances in culture. Harmful Algae 2006, 5, 665–677. [Google Scholar] [CrossRef]
- Juhl, A.R.; Trainer, V.L.; Latz, M.I. Effect of fluid shear and irradiance on population growth and cellular toxin content of the dinoflagellate Alexandrium fundyense. Limnol. Oceanogr. 2001, 46, 758–764. [Google Scholar] [CrossRef]
- Laabir, M.; Collos, Y.; Masseret, E.; Grzebyk, D.; Abadie, E.; Savart, V.; Sibat, M.; Amzil, Z. Influence of environmental factors on the paralytic shellfish toxin content and profile of Alexandrium catenella (Dinophyceae) isolated from the Mediterranean Sea. Mar. Drugs 2013, 11, 1583–1601. [Google Scholar] [CrossRef] [PubMed]
- Navarro, J.M.; Muñoz, M.G.; Contreras, A.M. Temperature as a factor regulating growth and toxin content in the dinoflagellate Alexandrium catenella. Harmful Algae 2006, 5, 762–769. [Google Scholar] [CrossRef]
- Scalco, E.; Brunet, C.; Marino, F.; Rossi, R.; Soprano, V.; Zingone, A.; Montresor, M. Growth and toxicity responses of Mediterranean Ostreopsis cf. ovata to seasonal irradiance and temperature conditions. Harmful Algae 2012, 17, 25–34. [Google Scholar] [CrossRef]
- López-Rosales, L.; Gallardo-Rodríguez, J.J.; Sánchez-Mirón, A.; Cerón-García, M.d.C.; Belarbi, E.H.; García-Camacho, F.; Molina-Grima, E. Simultaneous effect of temperature and irradiance on growth and okadaic acid production from the marine dinoflagellate Prorocentrum belizeanum. Toxins 2013, 6, 229–253. [Google Scholar] [CrossRef] [PubMed]
- Guillard, R.R.L.; Hargraves, P.E. Stichochrysis immobilis is a diatom, not a chrysophyte. Phycologia 1993, 32, 234–236. [Google Scholar] [CrossRef]
- Guillard, R.R.L. Culture of Phytoplankton for Feeding Marine Invertebrates. In Culture of Marine Invertebrate Animals; Chanley, M.H., Smith, W.L., Eds.; Springer: Boston, MA, USA, 1975; pp. 29–60. ISBN 0306308045. [Google Scholar]
- Guillard, R.R.; Ryther, J.H. Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt, and Detonula confervacea (cleve) Gran. Can. J. Microbiol. 1962, 8, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.D.; Selvin, R.C.; Claus, W.; Guillard, R.R.L. Media for the culture of oceanic ultraphytoplankton. J. Phycol. 1987, 23, 633–638. [Google Scholar] [CrossRef]
- Gallardo-Rodríguez, J.J.; Sánchez-Mirón, A.; Cerón-García, M.C.; Belarbi, E.H.; García-Camacho, F.; Chisti, Y.; Molina-Grima, E. Macronutrients requirements of the dinoflagellate Protoceratium reticulatum. Harmful Algae 2009, 8, 239–246. [Google Scholar] [CrossRef]
- Hardison, D.R.; Sunda, W.G.; Shea, D.; Litaker, R.W. Increased toxicity of Karenia brevis during phosphate limited growth: Ecological and evolutionary implications. PLoS ONE 2013, 8, e58545. [Google Scholar] [CrossRef] [PubMed]
- Frangópulos, M.; Guisande, C.; deBlas, E.; Maneiro, I. Toxin production and competitive abilities under phosphorus limitation of Alexandrium species. Harmful Algae 2004, 3, 131–139. [Google Scholar] [CrossRef]
- John, E.H.; Flynn, K.J. Growth dynamics and toxicity of Alexandrium fundyense (Dinophyceae): The effect of changing N:P supply ratios on internal toxin and nutrient levels. Eur. J. Phycol. 2000, 35, 11–23. [Google Scholar] [CrossRef]
- Vanucci, S.; Pezzolesi, L.; Pistocchi, R.; Ciminiello, P.; Dell’Aversano, C.; Iacovo, E.D.; Fattorusso, E.; Tartaglione, L.; Guerrini, F. Nitrogen and phosphorus limitation effects on cell growth, biovolume, and toxin production in Ostreopsis cf. ovata. Harmful Algae 2012, 15, 78–90. [Google Scholar] [CrossRef]
- Mitrovic, S.M.; Fernández Amandi, M.; McKenzie, L.; Furey, A.; James, K.J. Effects of selenium, iron and cobalt addition to growth and yessotoxin production of the toxic marine dinoflagellate Protoceratium reticulatum in culture. J. Exp. Mar. Biol. Ecol. 2004, 313, 337–351. [Google Scholar] [CrossRef]
- Cruz-López, R.; Maske, H. The vitamin B1 and B12 required by the marine dinoflagellate Lingulodinium polyedrum can be provided by its associated bacterial community in culture. Front. Microbiol. 2016, 7, 560. [Google Scholar] [CrossRef] [PubMed]
- García-Camacho, F.; López-Rosales, L.; Sánchez-Mirón, A.; Belarbi, E.H.; Chisti, Y.; Molina-Grima, E. Artificial neural network modeling for predicting the growth of the microalga Karlodinium veneficum. Algal Res. 2016, 14, 58–64. [Google Scholar] [CrossRef]
- López-Rosales, L.; Gallardo-Rodríguez, J.J.; Sánchez-Mirón, A.; Contreras-Gómez, A.; García-Camacho, F.; Molina-Grima, E. Modelling of multi-nutrient interactions in growth of the dinoflagellate microalga Protoceratium reticulatum using artificial neural networks. Bioresour. Technol. 2013, 146, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Weuster-Botz, D. Experimental design for fermentation media development: Statistical design or global random search? J. Biosci. Bioeng. 2000, 90, 473–483. [Google Scholar] [CrossRef]
- García-Camacho, F.; Gallardo-Rodríguez, J.J.; Sánchez-Mirón, A.; Chisti, Y.; Molina-Grima, E. Genetic algorithm-based medium optimization for a toxic dinoflagellate microalga. Harmful Algae 2011, 10, 697–701. [Google Scholar] [CrossRef]
- López-Rosales, L.; García-Camacho, F.; Sánchez-Mirón, A.; Chisti, Y. An optimal culture medium for growing Karlodinium veneficum: Progress towards a microalgal dinoflagellate-based bioprocess. Algal Res. 2015, 10, 177–182. [Google Scholar] [CrossRef]
- Schulze, P.S.C.; Barreira, L.A.; Pereira, H.G.C.; Perales, J.A.; Varela, J.C.S. Light emitting diodes (LEDs) applied to microalgal production. Trends Biotechnol. 2014, 32, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.K.; Oh, S.J.; Yang, H.-S.; Kim, D.-M.; Kang, I.J.; Oshima, Y. Laboratory Study for the Phytoremediation of Eutrophic Coastal Sediment Using Benthic Microalgae and Light Emitting Diode (LED). J. Fac. Agric. Kyushu Univ. 2013, 58, 417–425. [Google Scholar]
- Wang, S.; Chen, J.; Li, Z.; Wang, Y.; Fu, B.; Han, X.; Zheng, L. Cultivation of the benthic microalga Prorocentrum lima for the production of diarrhetic shellfish poisoning toxins in a vertical flat photobioreactor. Bioresour. Technol. 2015, 179, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Abu-Ghosh, S.; Fixler, D.; Dubinsky, Z.; Iluz, D. Flashing light in microalgae biotechnology. Bioresour. Technol. 2016, 203, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, H.; Tomori, Y.; Tanimoto, Y.; Oku, O.; Adachi, M. Evaluation of the effects of light intensity on growth of the benthic dinoflagellate Ostreopsis sp. 1 using a newly developed photoirradiation-culture system and a novel regression analytical method. Harmful Algae 2014, 39, 48–54. [Google Scholar] [CrossRef]
- Etheridge, S.M.; Roesler, C.S. Effects of temperature, irradiance, and salinity on photosynthesis, growth rates, total toxicity, and toxin composition for Alexandrium fundyense isolates from the Gulf of Maine and Bay of Fundy. Deep Sea Res. Part II Top. Stud. Oceanogr. 2005, 52, 2491–2500. [Google Scholar] [CrossRef]
- Lee, T.C.H.; Fong, F.L.Y.; Ho, K.C.; Lee, F.W.F. The mechanism of diarrhetic shellfish poisoning toxin production in Prorocentrum spp.: Physiological and molecular perspectives. Toxins 2016, 8, 272. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Cembella, A.D.; Quilliam, M.A. Cell cycle and toxin production in the benthic dinoflagellate Prorocentrum lima. Mar. Biol. 1999, 134, 541–549. [Google Scholar] [CrossRef]
- Taroncher-Oldenburg, G.; Kulis, D.M.; Anderson, D.M. Toxin variability during the cell cycle of the dinoflagellate Alexandrium fundyense. Limnol. Oceanogr. 1997, 42, 1178–1188. [Google Scholar] [CrossRef]
- John, U.; Quilliam, M.A.; Medlin, L.; Cembella, A.D. Spirolide production and photoperiod-dependent growth of the marine dinoflagellate Alexandrium ostenfeldii. In Harmful Algal Blooms 2000; Hallegraeff, G.M., Blackburn, S.I., Bolch, C.J., Lewis, R.J., Eds.; International Oceanographic Commission (UNESCO): Paris, France, 2001; pp. 299–302. [Google Scholar]
- Loader, J.I.; Hawkes, A.D.; Beuzenberg, V.; Jensen, D.J.; Cooney, J.M.; Wilkins, A.L.; Fitzgerald, J.M.; Briggs, L.R.; Miles, C.O. Convenient Large-Scale Purification of Yessotoxin from Protoceratium reticulatum Culture and Isolation of a Novel Furanoyessotoxin. J. Agric. Food Chem. 2007, 55, 11093–11100. [Google Scholar] [CrossRef] [PubMed]
- Jauffrais, T.; Sechet, V.; Herrenknecht, C.; Krock, B.; Amzil, Z.; Hess, P. Growth and toxin production of Azadinium spinosum in batch and continuous culture. In Proceedings of the 14th International Conference on Harmful Algae, Hersonissos, Crete, Greece, 1–5 November 2010; Volume 3, pp. 18–20. [Google Scholar]
- Jauffrais, T.; Kilcoyne, J.; Séchet, V.; Herrenknecht, C.; Truquet, P.; Hervé, F.; Bérard, J.B.; Nulty, C.; Taylor, S.; Tillmann, U.; et al. Production and isolation of azaspiracid-1 and -2 from Azadinium spinosum culture in pilot scale photobioreactors. Mar. Drugs 2012, 10, 1360–1382. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Shi, Y.; Cong, W. Improvement in growth and toxin production of Alexandrium tamarense by two-step culture method. J. Appl. Phycol. 2006, 18, 119–126. [Google Scholar] [CrossRef]
- Wang, D.Z.; Ho, A.Y.T.; Hsieh, D.P.H. Production of C2 toxin by Alexandrium tamarense CI01 using different culture methods. J. Appl. Phycol. 2002, 14, 461–468. [Google Scholar] [CrossRef]
- Wang, D.; Hsieh, D.P.H. Dynamics of C2 toxin and chlorophyll-a formation in the dinoflagellate Alexandrium tamarense during large scale cultivation. Toxicon 2001, 39, 1533–1536. [Google Scholar] [CrossRef]
- Gallardo-Rodríguez, J.J.; García-Cerón, M.C.; García-Camacho, F.; Sánchez-Mirón, A.; Belarbi, E.H.; Molina-Grima, E. New culture approaches for yessotoxin production from the dinoflagellate Protoceratium reticulatum. Biotechnol. Prog. 2007, 23, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Parker, N.S.; Negri, A.P.; Frampton, D.M.F.; Rodolfi, L.; Tredici, M.R.; Blackburn, S.I. Growth of the toxic dinoflagellate Alexandrium minutum (Dinophyceae) using high biomass culture systems. J. Appl. Phycol. 2002, 14, 313–324. [Google Scholar] [CrossRef]
- Medhioub, W.; Sechet, V.; Truquet, P.; Bardouil, M.; Amzil, Z.; Lassus, P.; Soudant, P. Alexandrium ostenfeldii growth and spirolide production in batch culture and photobioreactor. Harmful Algae 2011, 10, 794–803. [Google Scholar] [CrossRef]
- Han, M.; Lee, H.; Anderson, D.M.; Kim, B. Paralytic shellfish toxin production by the dinoflagellate Alexandrium pacificum (Chinhae Bay, Korea) in axenic, nutrient-limited chemostat cultures and nutrient-enriched batch cultures. Mar. Pollut. Bull. 2016, 104, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Samarakoon, K.W.; Ko, J.Y.; Shah, M.M.R.; Lee, J.H.; Kang, M.C.; O-Nam, K.; Lee, J.B.; Jeon, Y.J. In vitro studies of anti-inflammatory and anticancer activities of organic solvent extracts from cultured marine microalgae. Algae 2013, 28, 111–119. [Google Scholar] [CrossRef]
- Fuentes-Grünewald, C.; Garcés, E.; Alacid, E.; Rossi, S.; Camp, J. Biomass and Lipid Production of Dinoflagellates and Raphidophytes in Indoor and Outdoor Photobioreactors. Mar. Biotechnol. 2013, 15, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Benstein, R.M.; Çebi, Z.; Podola, B.; Melkonian, M. Immobilized Growth of the Peridinin-Producing Marine Dinoflagellate Symbiodinium in a Simple Biofilm Photobioreactor. Mar. Biotechnol. 2014, 16, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Rahman Sha, M.M.; Samarakoon, K.W.; An, S.-J.; Jeon, Y.-J.; Lee, J.-B. Growth Characteristics of Three Benthic Dinoflagellates in Mass Culture and Their Antioxidant Properties. J. Fish. Aquat. Sci. 2016, 11, 268–277. [Google Scholar] [CrossRef]
- Tilney, C.L.; Hoadley, K.D.; Warner, M.E. Comparing the diel vertical migration of Karlodinium veneficum (dinophyceae) and Chattonella subsalsa (Raphidophyceae): PSII photochemistry, circadian control, and carbon assimilation. J. Photochem. Photobiol. B Biol. 2015, 143, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Zittelli, G.C.; Rodolfi, L.; Bassi, N.; Biondi, N.; Tredici, M.R. Photobioreactors for Microalgal Biofuel Production. In Algae for Biofuels and Energy; Borowitzka, M.A., Moheimani, N., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 115–131. [Google Scholar]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).