The Anti-Inflammatory Effect and Structure of EPCP1-2 from Crypthecodinium cohnii via Modulation of TLR4-NF-κB Pathways in LPS-Induced RAW 264.7 Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation, Extraction and Purification of Polysaccrides
2.3. Characterizations Analysis of EPCP1-2
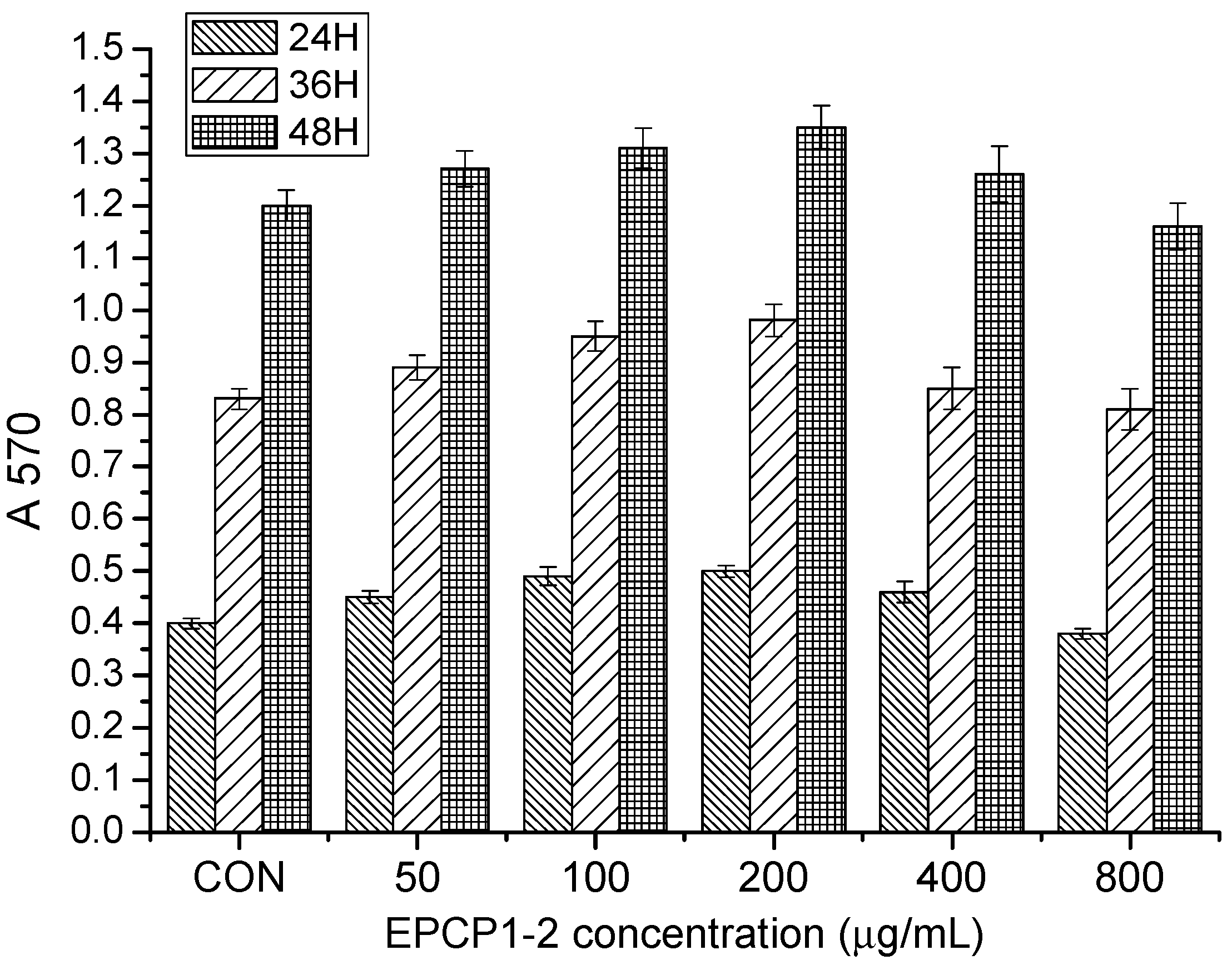
2.4. Cell Culture and Cell Viability Assay
2.5. NO Assay
2.6. ELISA Assay of Cytokines
2.7. Protein Extraction and Western Blotting
2.8. Statistical Analysis
3. Result
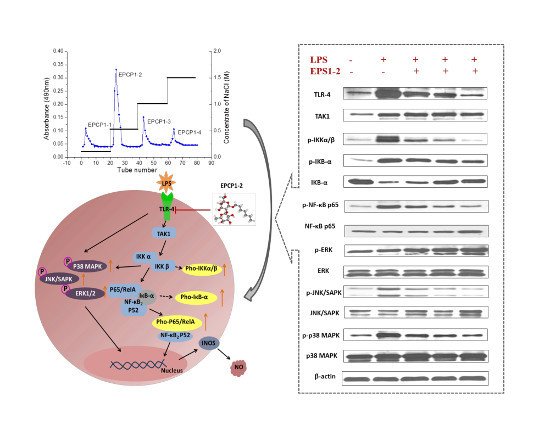
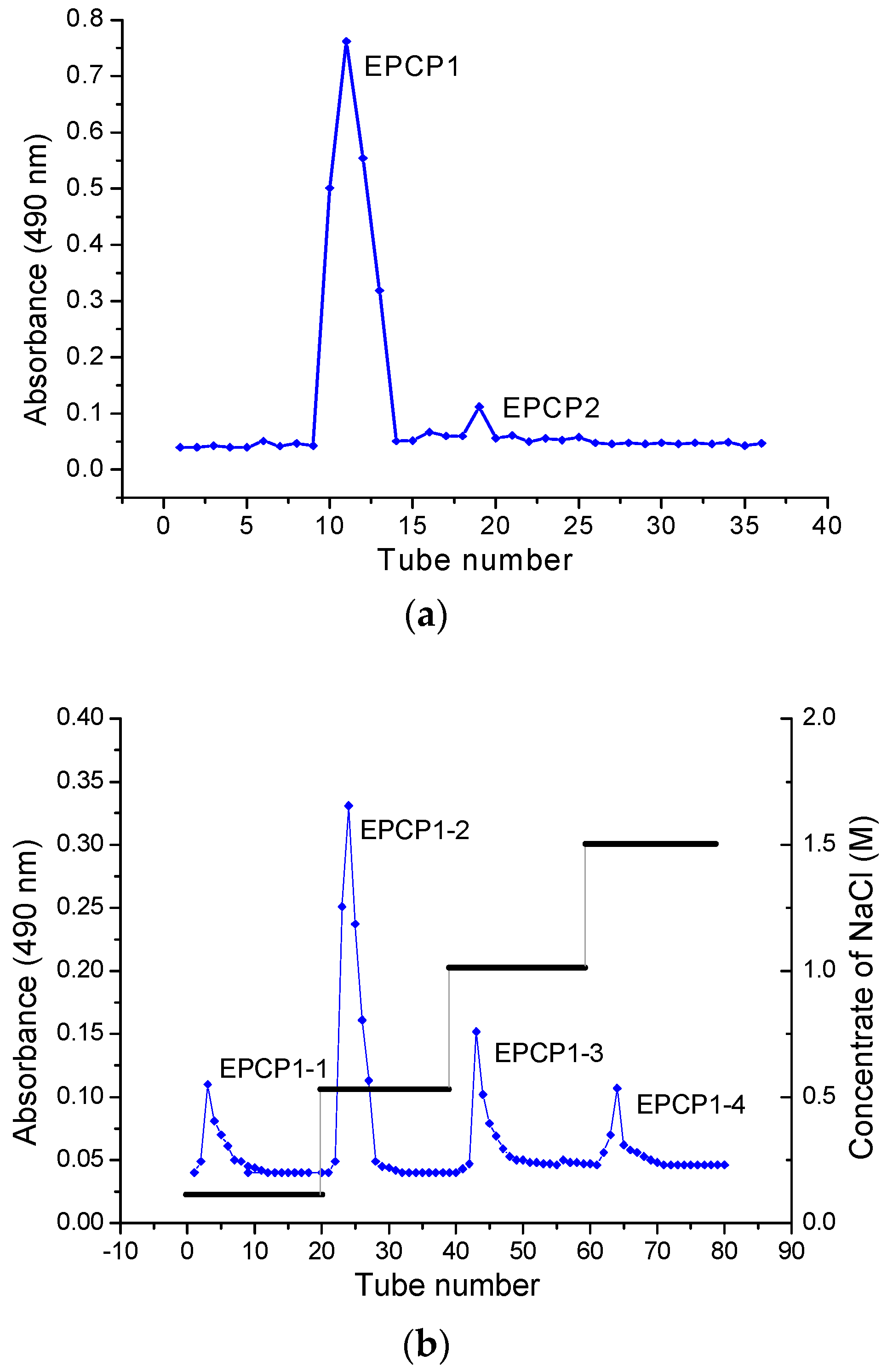
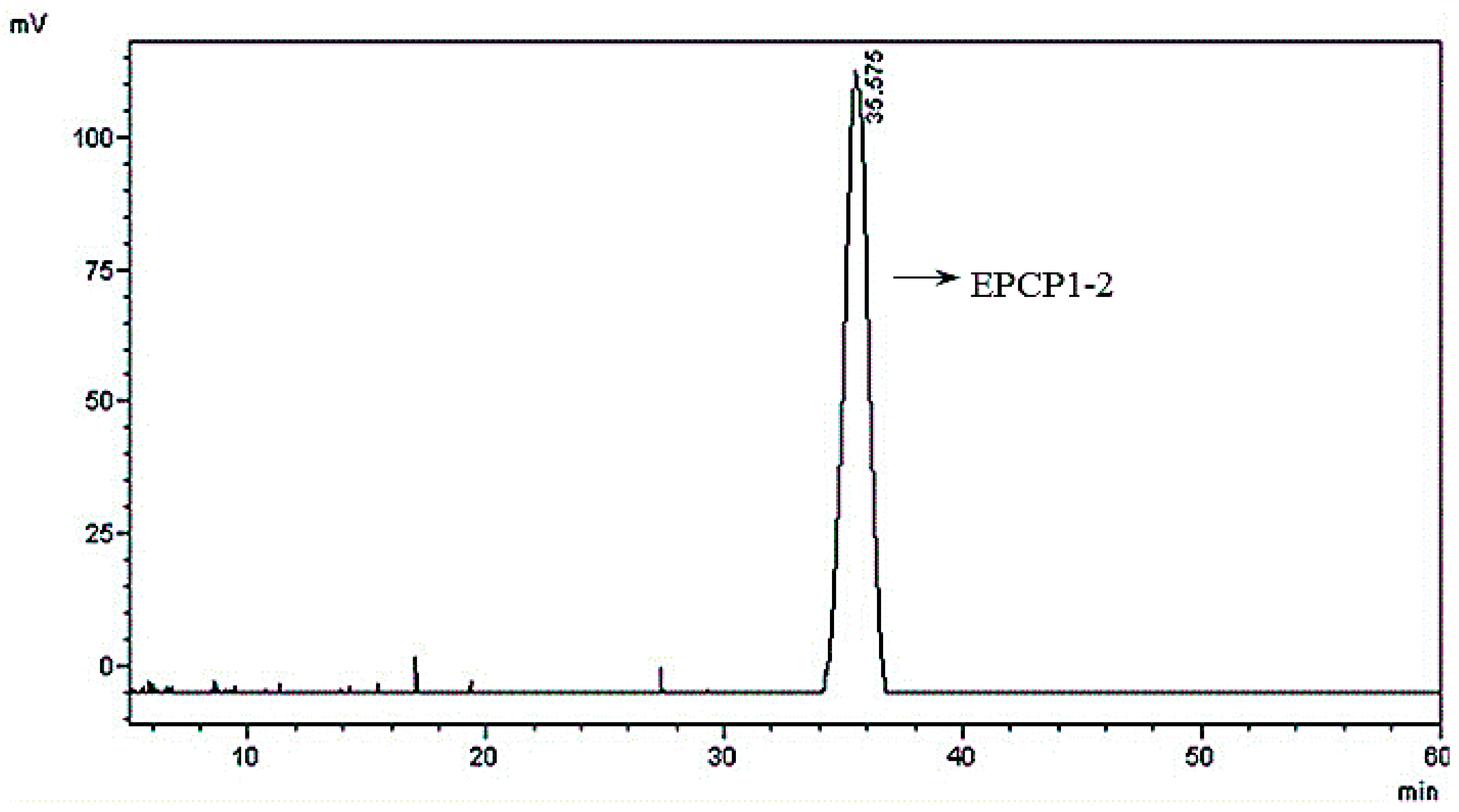
3.1. Purification of Hydrolysates of Exopolysaccharide of Crypthecodinium Cohnii (EPCP)
3.2. Basic Properties of EPCP1-2
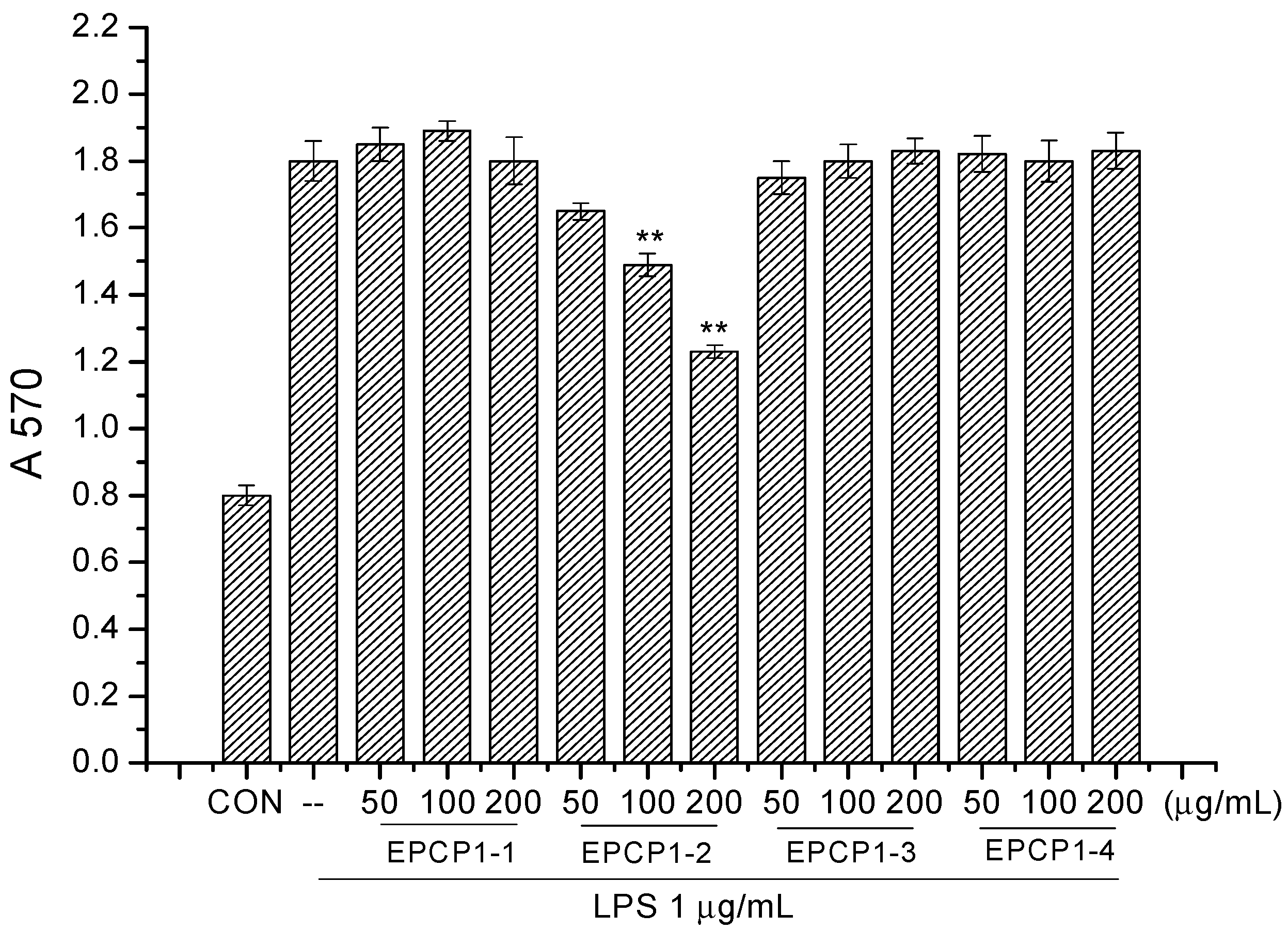
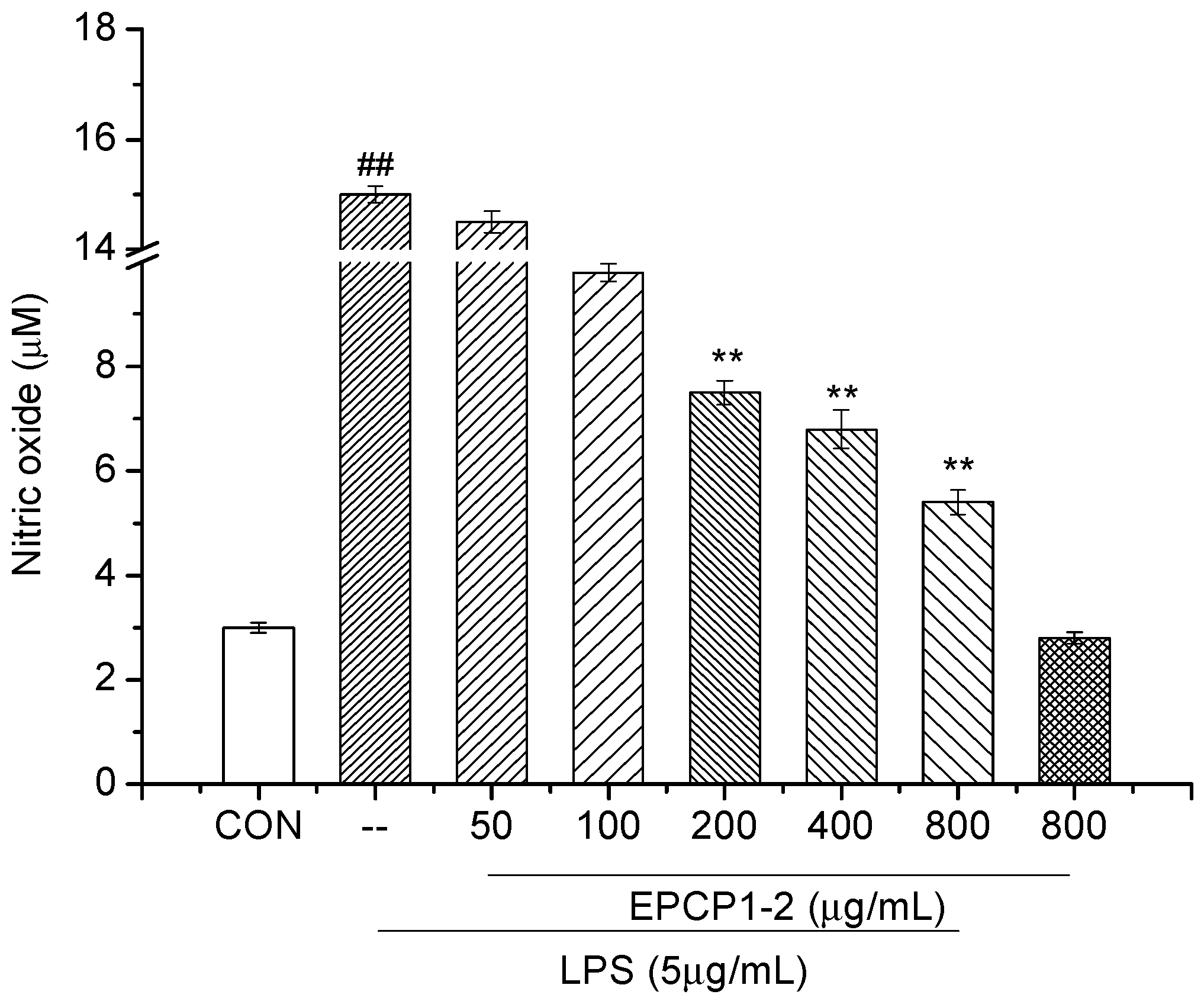
3.3. Effect of EPCP1-2 on LPS Induced NO Production from RAW 264.7
3.4. Effect of EPCP1-2 on LPS Induced Cytokines Production from RAW 264.7
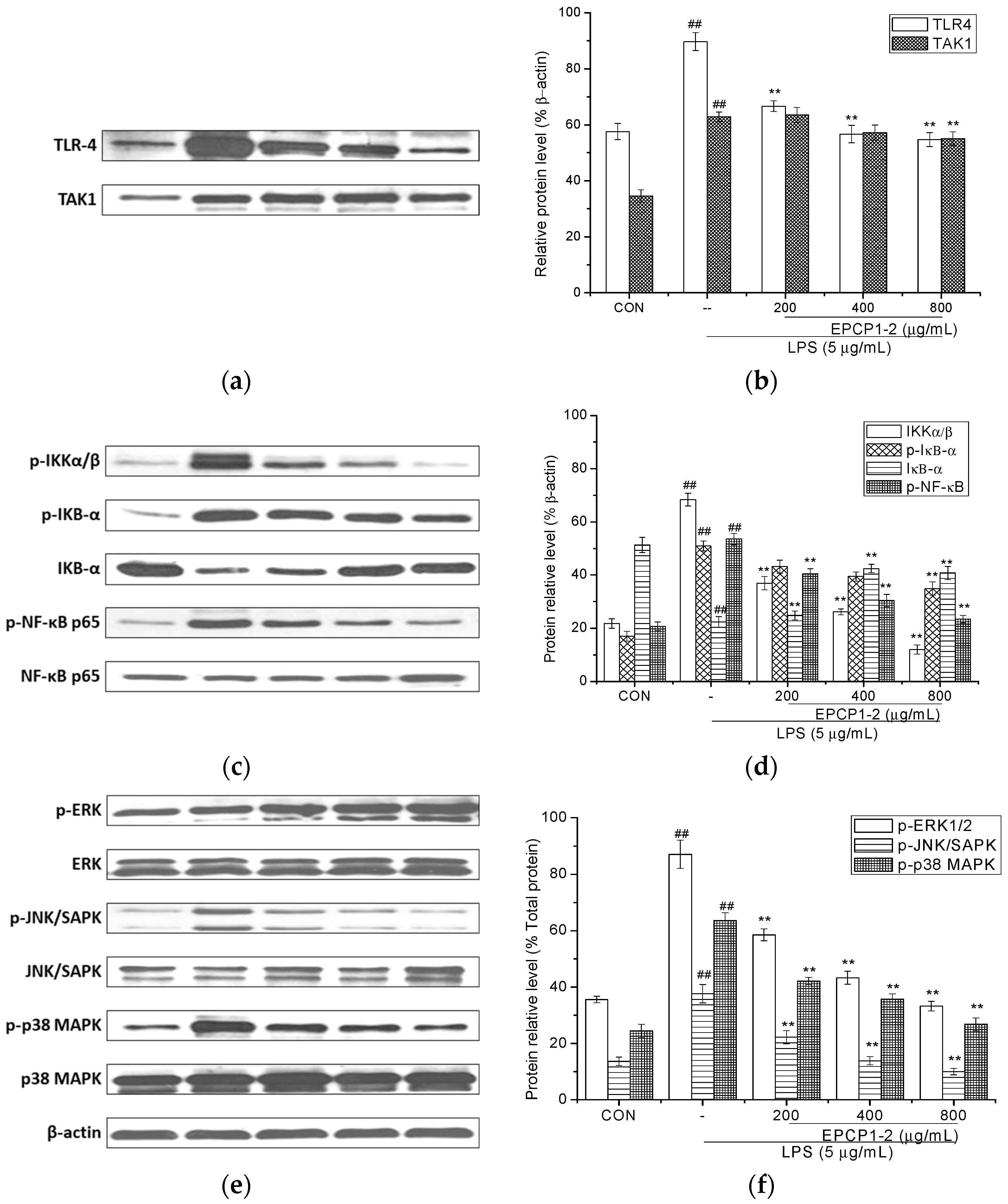
3.5. Effect of EPCP1-2 on Expression of Phosphorylation and Total TLR4
3.6. Effect of EPCP1-2 on Expression of Phosphorylation and Total NF-κB
3.7. Effect of EPCP1-2 on Expression of MAPKS Phosphoorylation and JNK/SAPK Phosphoorylation Induced by LPS
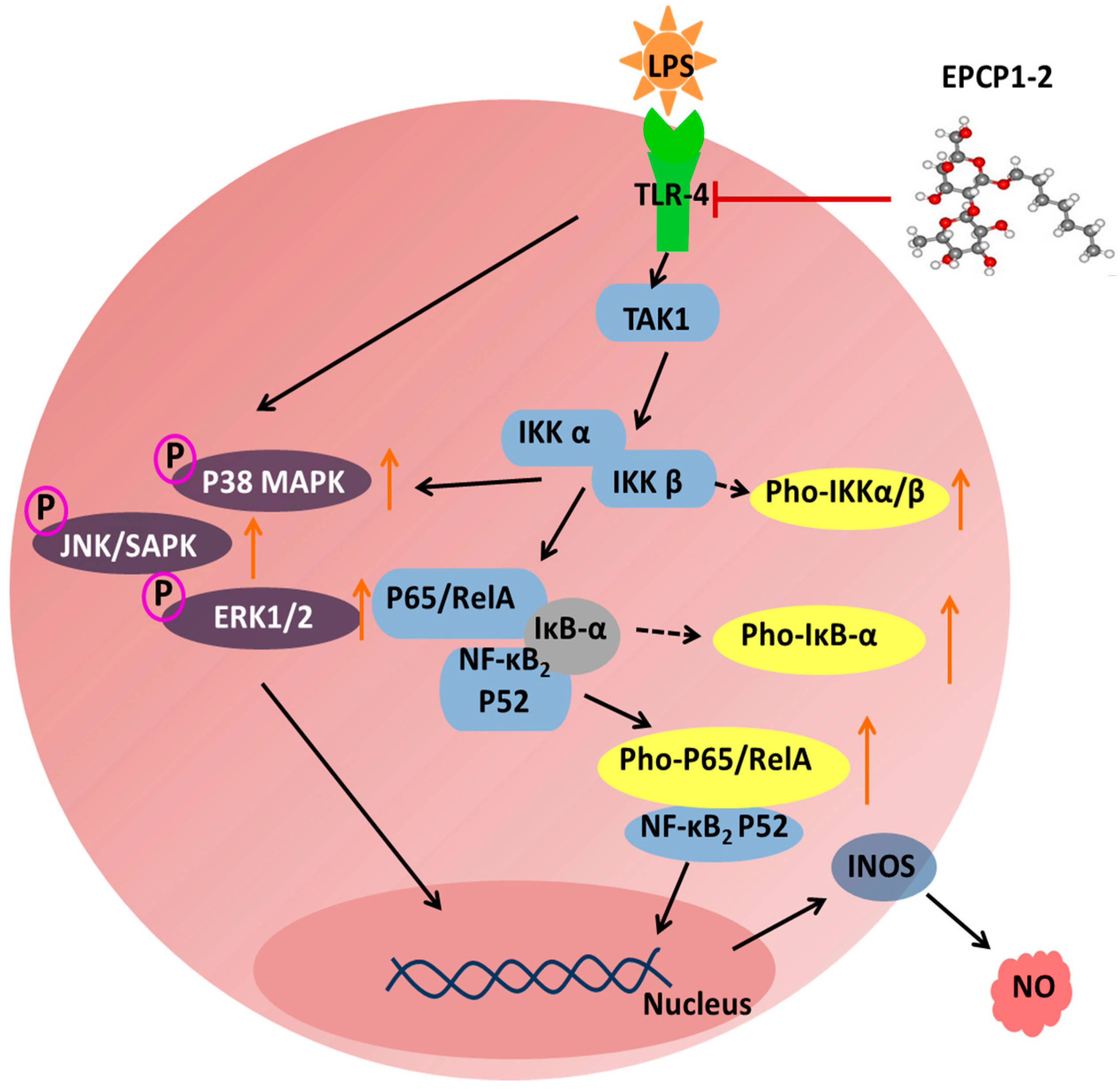
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Henderson, B.; Poole, S.; Wilson, M. Bacterial modulins: A novel class of virulence factors which cause host tissue pathology by including cytokine synthesis. Microbiol. Rev. 1996, 60, 316–341. [Google Scholar] [PubMed]
- Hersh, D.; Weiss, J.; Zychlinsky, A. How bacteria initiate inflammation: Aspects of the emerging story. Curr. Opin. Microbiol. 1998, 1, 43–48. [Google Scholar] [CrossRef]
- Ulevitch, R.J.; Tobias, P.S. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu. Rev. Immunol. 1995, 13, 437–457. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Proinflammatory cytokines. Chest 2000, 188, 503–508. [Google Scholar] [CrossRef]
- Turcanu, V.; Williams, N.A. Cell identification and isolation on the basis of cytokine secretion: A novel tool for investigating immune responses. Nat. Med. 2001, 7, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Moncada, S.; Palmer, R.M.; Higgs, E.A. Nitric oxide: Physiology, pathophysiology. Pharmacol. Rev. 1992, 43, 109–142. [Google Scholar]
- MacMiking, J.; Xie, Q.W.; Nathan, C. Nitric oxide and macrophage function. Annu. Rev. Immunol. 1997, 15, 323–350. [Google Scholar] [CrossRef] [PubMed]
- Nijkamp, F.P.; Parnham, M.J. Principles of Immunopharacology, 2nd ed.; Birkhauser: Basel, Switzerland, 2005; p. 188. [Google Scholar]
- Ma, X.; Meng, M.; Han, L.; Cheng, D.; Cao, X.; Wang, C. Structural characterization and immunomodulatory activity of Grifola frondosa polysaccharide via toll-like receptor 4-mitogen-activated protein-kinases-nuclear factor κB pathways. Food Funct. 2016, 7, 2763–2772. [Google Scholar] [CrossRef] [PubMed]
- Chevolot, L.; Foucault, A.; Chaubet, F.; Kervarec, N.; Sinquin, C.; Fisher, A.M. Further data on the structure of brown seaweed fucans: Relationships with anticoagulant activity. Carbohydr. Res. 1999, 319, 154–165. [Google Scholar] [CrossRef]
- Vieira, R.P.; Mourao, P.A. Occurrence of a unique fucose-branched chondroitin sulfate in the body wall of a sea cucumber. J. Biol. Chem. 1988, 263, 18176–18183. [Google Scholar] [PubMed]
- Bilan, M.I.; Grachev, A.A.; Ustuzhanina, N.E.; Shashkov, A.S.; Nifantiev, N.E.; Usov, A.I. Structure of a fucoidan from the brown seaweed Fucus evanescens C.Ag. Carbohydr. Res. 2002, 337, 719–730. [Google Scholar] [CrossRef]
- Chizhov, A.O.; Dell, A.; Morris, H.R.; Haslam, S.M.; McDowell, A. A study of fucoidan from the brown seaweed Chorda filum. Carbohydr. Res. 1999, 320, 108–119. [Google Scholar] [CrossRef]
- Partankar, M.S.; Oehninger, S.; Barnett, T.; Williams, R.L.; Clark, G.F. A revised structure for fucoidan may explain some of its biological activities. J. Biol. Chem. 1993, 268, 21770–21776. [Google Scholar]
- Xu, J.; Xu, L.; Zhou, Q.; Hao, S.; Zhou, T.; Xie, H. Isolation, purification, and antioxidant activities of degraded polysaccharides from Enteromorpha prolifera. Int. J. Biol. Macromol. 2015, 81, 1026–1030. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, T.L.; Reis, A. The use of multi-parameter flow cytometry to study the impact of n-dodecane additions to marine dinoflagellate microalga Crypthecodinium cohnii batch fermentations and DHA production. J. Ind. Microbiol. Biotechnol. 2008, 35, 875–887. [Google Scholar] [CrossRef] [PubMed]
- De Swaaf, M.E.; de Rijk, T.C.; Eggink, G.; Sijtsma, L. Optimisation of docosahexaenoic acid production in batch cultivations by Crypthecodinium cohnii. J. Biotechnol. 1999, 70, 185–192. [Google Scholar] [CrossRef]
- Seung-Hong, L.; Chang-Ik, K.; Youngheun, J.; Yoonhwa, J.; Misook, K.; Jin-Soo, K.; You-Jin, J. Anti-inflammatory effect of fucoidan extracted from Ecklonia cava in zebrafish model. Carbohydr. Polym. 2013, 92, 84–89. [Google Scholar]
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef] [PubMed]
- Xiao-Lei, M.; Meng, M.; Li-Rong, H.; Zheng, L.; Xiao-Hong, C.; Chun-Ling, W. Immunomodulatory activity of macromolecular polysaccharide isolated from Grifola frondosa. Chin. J. Nat. Med. 2015, 13, 906–914. [Google Scholar]
- Liu, B.; Sun, Z.; Ma, X.; Yang, B.; Jiang, Y.; Wei, D.; Chen, F. Mutation breeding of extracellular polysacchride-producing microalga Crypthecodinium cohnii by novel mutagenesis with atmospheric and room temperature plasma. Int. J. Mol. Sci. 2015, 16, 8201–8212. [Google Scholar] [CrossRef] [PubMed]
- Qiao, D.; Hu, B.; Gan, D.; Sun, Y.; Ye, H.; Zeng, X. Extraction optimized by using response surface methodology, purification and preliminary characterization of polysaccharides from Hyriopsis cumingii. Carbonhydr. Polym. 2009, 76, 422–429. [Google Scholar] [CrossRef]
- Staub, A.M. Removal of protein: Sevag method. Methods Carbohydr. Chem. 1965, 5, 5–7. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 78, 248–254. [Google Scholar] [CrossRef]
- Dodgson, K.S.; Price, R.G. A note on the determination of theester sulphate content of sulphated polysaccharides. Biochem. J. 1962, 84, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Blumencrantz, N.; Asboe-Hansen, G. New methods for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Cheong, J.Y.; Jung, W.T.; Park, W.B. Characterization of an alkaliextracted peptidoglycan from Korean Ganoderma lucidum. Arch. Pharm. Res. 1999, 22, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhou, F.; Chen, Y.; Zhang, Y.; Hou, L.; Cao, X.; Wang, C. A polysaccharide from Grifola frondosa relieves insulin resistance of HepG2 cell by Akt-GSK-3 pathway. Glycoconj. J. 2014, 31, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Beutler, B. Inferences, questions and possibilities in Toll-like receptor signalling. Nature 2004, 430, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Wua, M.; Yuan, Y.; Wang, Z.Z.; Jiang, H.; Chen, T. Priming of Toll-like receptor 4 pathway in mesenchymal stem cells increases expression of B cell activating factor. Biochem. Biophys. Res. Commun. 2014, 448, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Gloire, G.; Legrand-Poels, S.; Piette, J. NF-κB activation by reactive oxygen species: Fifteen years later. Biochem. Pharmacol. 2006, 72, 1493–1505. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.M.; Zhang, X.T.; Meng, J.; Wang, Z.Y. An anticancer agent icaritin induces sustained activation of the extracellular signal-regulated kinase (ERK) pathway and inhibits growth of breast cancer cells. Eur. J. Pharmacol. 2011, 658, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Coskun, M.; Olsen, J.; Seidelin, J.B.; Nielsen, O.H. MAP kinases in inflammatory bowel disease. Clin. Chim. Acta 2011, 412, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Herrero, C.; Sebastian, C.; Marques, L.; Comalada, M.; Xaus, J.; Valledor, A.F.; Lloberas, J.; Celada, A. Immunosenescence of macrophages: Reduced MHC class II gene expression. Exp. Gerontol. 2002, 37, 389–394. [Google Scholar] [CrossRef]
- Dziarski, R.; Gupta, D. Role of MD-2 in TLR2- and TLR4-mediated recognition of Gram-negative and Gram-positive bacteria and activation of chemokine genes. J. Endotoxin Res. 2000, 6, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Jiang, W.; Zhang, Z.; Qian, M.; Du, B. Nitidine chloride inhibits LPS-induced inflammatory cytokines production via MAPK and NF-kappaB pathway in RAW 264.7 cells. J. Ethnopharmacol. 2012, 144, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, W. TLR4-mediated activation of macrophages by the polysaccharide fraction from Polyporus umbellatus (pers.) Fries. J. Ethnopharmacol. 2011, 135, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Janeway, C.A.; Medzhitov, R. Innate immune recognition. Annu. Rev. Immunol. 2002, 20, 197–216. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H.; Hokari, R.; Miura, S.; Shigematsu, T.; Hirokawa, M.; Akiba, Y.; Kurose, I.; Higuchi, H.; Fujimori, H.; Tsuzuki, Y.; et al. Increased expression of an inducible isoform of nitric oxide synthase and the formation of peroxynitrite in colonic mucosa of patients with active ulcerative colitis. Gut 1998, 42, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Hang, L.; Slack, J.H.; Amundson, C.; Izui, S.; Theofilopoulos, A.N.; Dixon, F.J. Induction of murine autoimmune disease by chronic polyclonal B cell activation. J. Exp. Med. 1983, 157, 874–883. [Google Scholar] [CrossRef] [PubMed]
- Summers, S.A.; Hoi, A.; Steinmetz, O.M.; O’Sullivan, K.M.; Ooi, J.D.; Odobasic, D.; Akira, S.; Kitching, A.R.; Holdsworth, S.R. TLR9 and TLR4 are required for the development of autoimmunity and lupus nephritis in pristane nephropathy. J. Autoimmun. 2010, 35, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Lartigue, A.; Colliou, N.; Calbo, S.; Francois, A.; Jacquot, S.; Arnoult, C.; Tron, F.; Gilbert, D.; Musette, P. Critical role of TLR2 and TLR4 in autoantibody production and glomerulonephritis in lpr mutation-induced mouse lupus. J. Immunol. 2009, 183, 6207–6216. [Google Scholar] [CrossRef] [PubMed]
- Andreucci, M.; Lucisano, G.; Faga, T.; Bertucci, B.; Tamburrini, O.; Pisani, A.; Sabbatini, M.; Salzano, S.; Vitale, M.; Fuiano, G.; et al. Differential activation of signaling pathways involved in cell death, survival and inflammation by radiocontrast media in human renal proximal tubular cells. Toxicol. Sci. 2011, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Yuan, W.; Wang, L.; Shang, M.; Peng, Y. Parathyroid hormonepotentiated connective tissue growth factor expression in human renal proximal tubular cells through activating the MAPK and NF-kappaB signalling pathways. Nephrol. Dial. Transplant. 2011, 26, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Synytsya, A.; Kim, H.B.; Choi, D.J.; Lee, S.; Lee, J.; Kim, W.J.; Jang, S.; Park, Y.I. Purification, characterization and immunomodulating activity of a pectic polysaccharide isolated from Korean mulberry fruit Oddi (Morus alba L.). Int. Immunopharmacol. 2013, 17, 858–866. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Du, Q. Structure characterization and antitumor activity of an α β-glucan polysaccharide from Auricularia polytricha. Food Res. Int. 2012, 45, 381–387. [Google Scholar] [CrossRef]
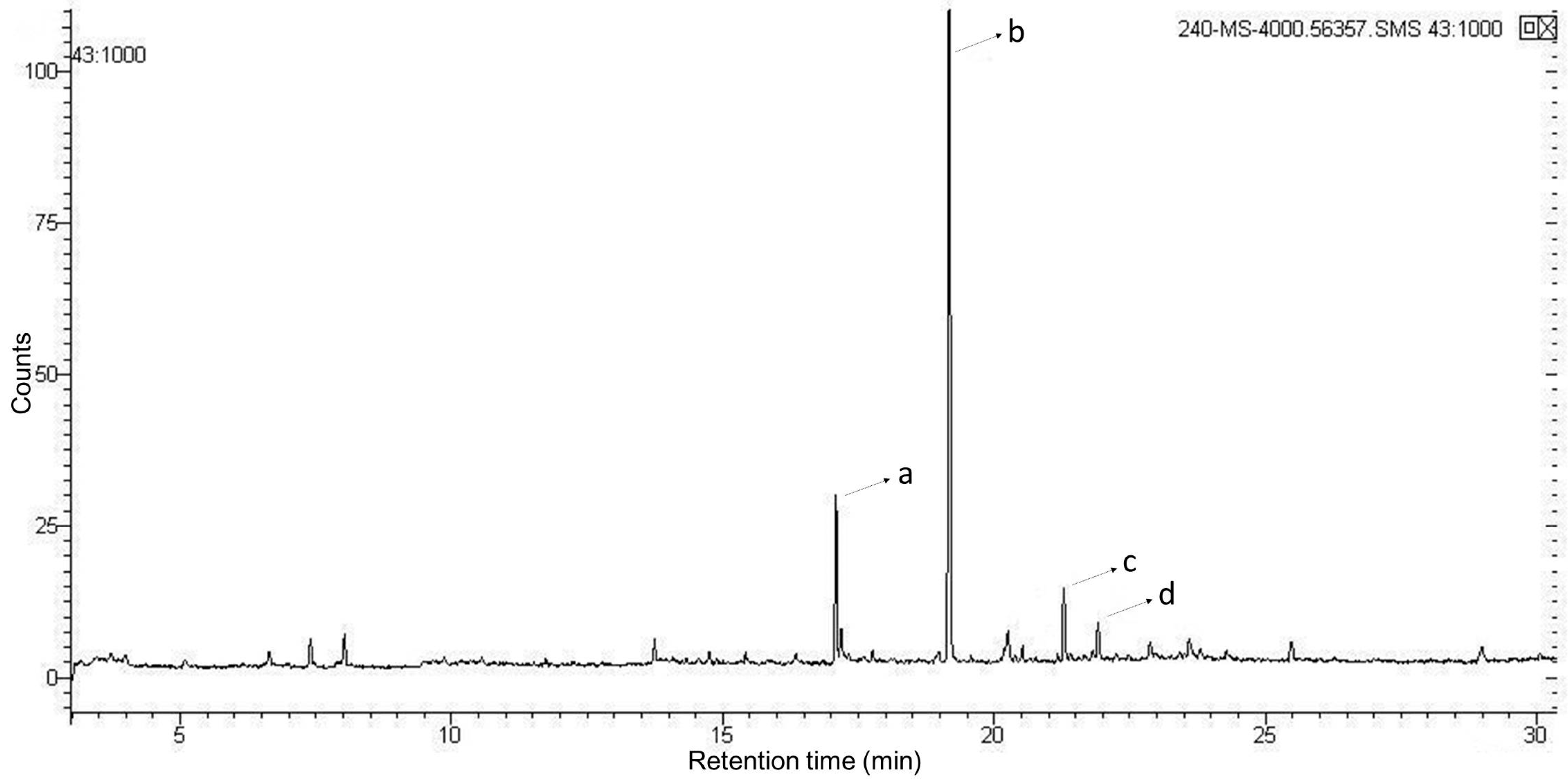
| Peak No. | Methylated Sugar Residue | Retention Time (min) | Linkage Type |
|---|---|---|---|
| a | 2,4-Me2-Gala | 17.085 | 1,3-6-d-Galacp |
| b | 2,3,4-Me3-Man | 19.175 | 1,6-d-Manp |
| c | 2,3,4-Me3-Glc | 21.294 | 1,6-d-Glcp |
| d | 2,3,4,6-Me3-Rha | 21.911 | t-l-Rhap |
| Group | EPCP1-2 (μg/mL) | LPS (μg/mL) | IL-1β (ng/L) | IFN-γ (ng/L) | TNF-α (ng/L) |
|---|---|---|---|---|---|
| Control | - | - | 175.5 ± 5.8 | 200.3 ± 5.1 | 187.8 ± 3.5 |
| Positive control | - | 5 | 365.3 ± 9.1 ## | 445.6 ± 3.5 ## | 315.3 ± 6.4 ## |
| Treatment group | 200 | 5 | 320.3 ± 5.3 ** | 395.4 ± 5.6 ** | 300.5 ± 9.2 ** |
| 400 | 5 | 284.7 ± 6.2 ** | 318.6 ± 8.1 ** | 281.3 ± 7.4 ** | |
| 800 | 5 | 214.1 ± 4.4 | 245.4 ± 6.7 | 226.4 ± 7.3 | |
| Negative control | 800 | - | 180.5 ± 7.5 | 204.9 ± 4.3 | 195.2 ± 6.3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Xie, B.; Du, J.; Zhang, A.; Hao, J.; Wang, S.; Wang, J.; Cao, J. The Anti-Inflammatory Effect and Structure of EPCP1-2 from Crypthecodinium cohnii via Modulation of TLR4-NF-κB Pathways in LPS-Induced RAW 264.7 Cells. Mar. Drugs 2017, 15, 376. https://doi.org/10.3390/md15120376
Ma X, Xie B, Du J, Zhang A, Hao J, Wang S, Wang J, Cao J. The Anti-Inflammatory Effect and Structure of EPCP1-2 from Crypthecodinium cohnii via Modulation of TLR4-NF-κB Pathways in LPS-Induced RAW 264.7 Cells. Marine Drugs. 2017; 15(12):376. https://doi.org/10.3390/md15120376
Chicago/Turabian StyleMa, Xiaolei, Baolong Xie, Jin Du, Aijun Zhang, Jianan Hao, Shuxun Wang, Jing Wang, and Junrui Cao. 2017. "The Anti-Inflammatory Effect and Structure of EPCP1-2 from Crypthecodinium cohnii via Modulation of TLR4-NF-κB Pathways in LPS-Induced RAW 264.7 Cells" Marine Drugs 15, no. 12: 376. https://doi.org/10.3390/md15120376
APA StyleMa, X., Xie, B., Du, J., Zhang, A., Hao, J., Wang, S., Wang, J., & Cao, J. (2017). The Anti-Inflammatory Effect and Structure of EPCP1-2 from Crypthecodinium cohnii via Modulation of TLR4-NF-κB Pathways in LPS-Induced RAW 264.7 Cells. Marine Drugs, 15(12), 376. https://doi.org/10.3390/md15120376