The Brown Alga Stypopodium zonale (Dictyotaceae): A Potential Source of Anti-Leishmania Drugs
Abstract
:1. Introduction
2. Results
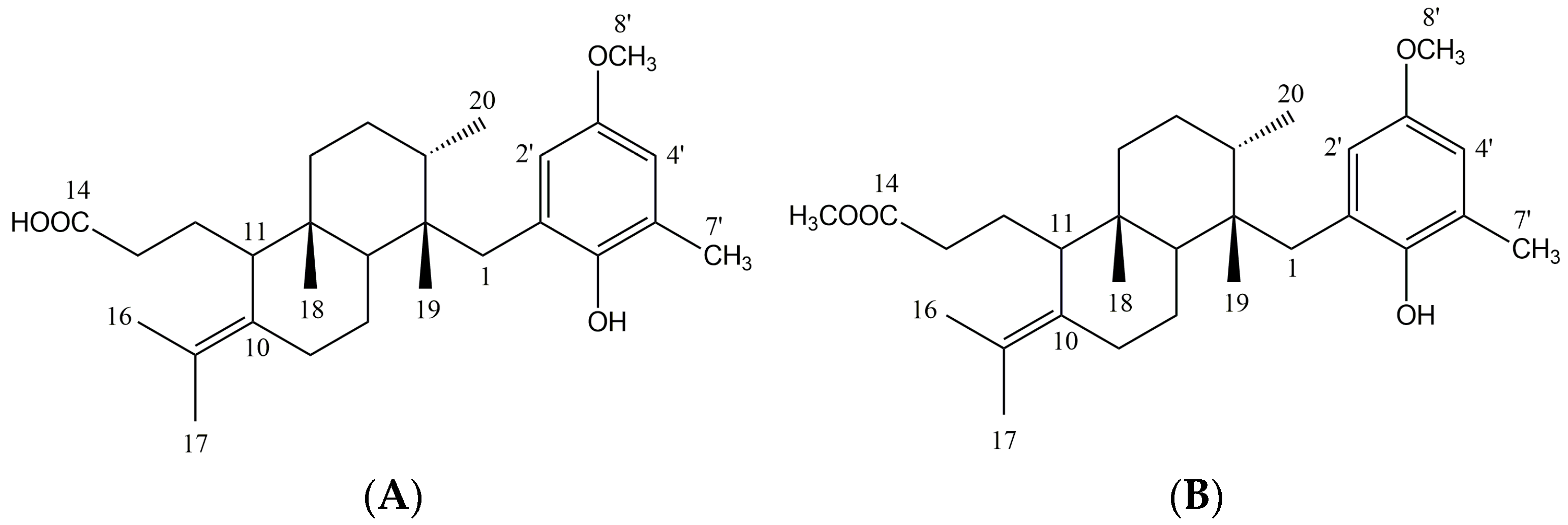
2.1. Crude Extract Analysis and Structural Elucidation of Pure Compounds
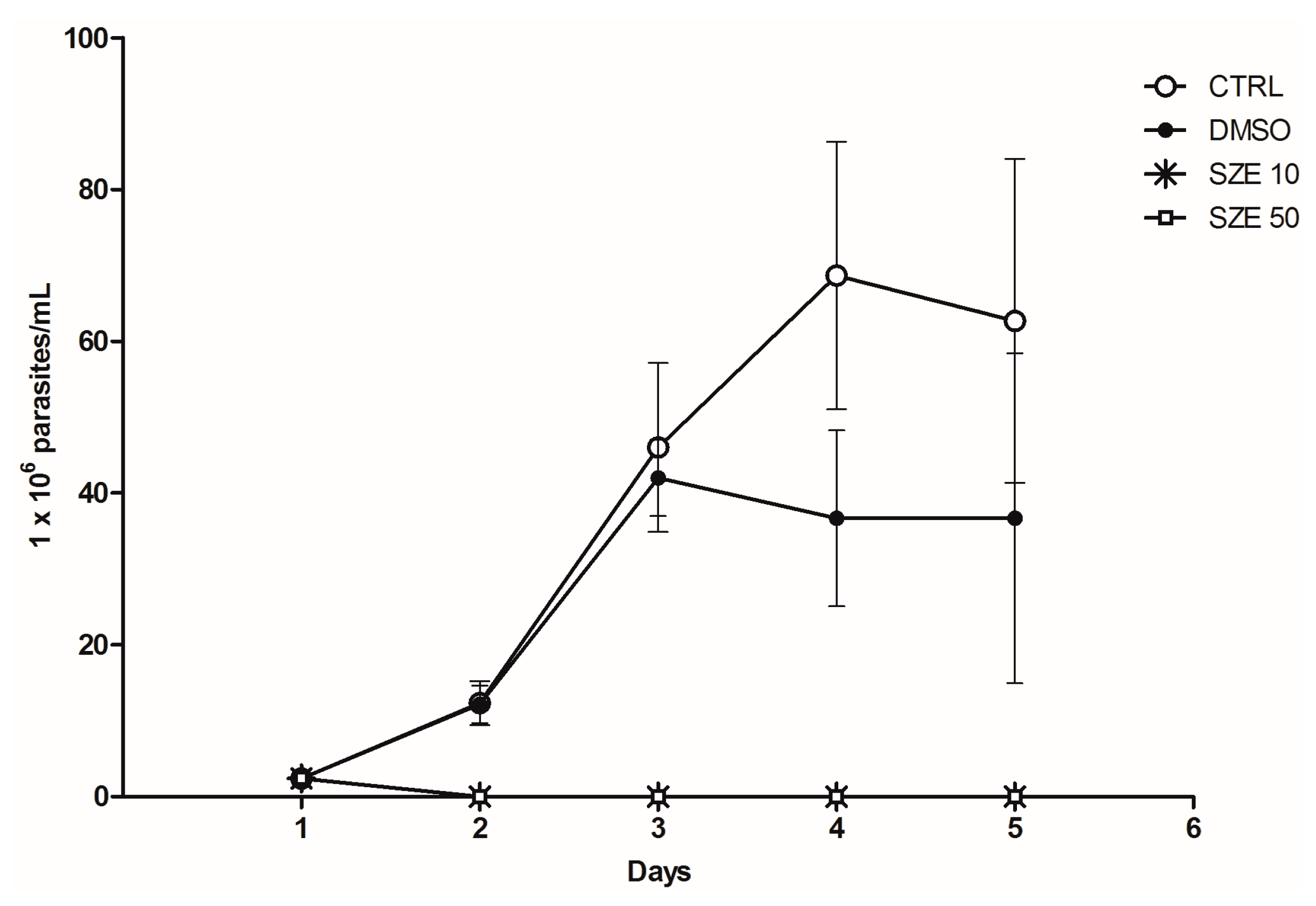
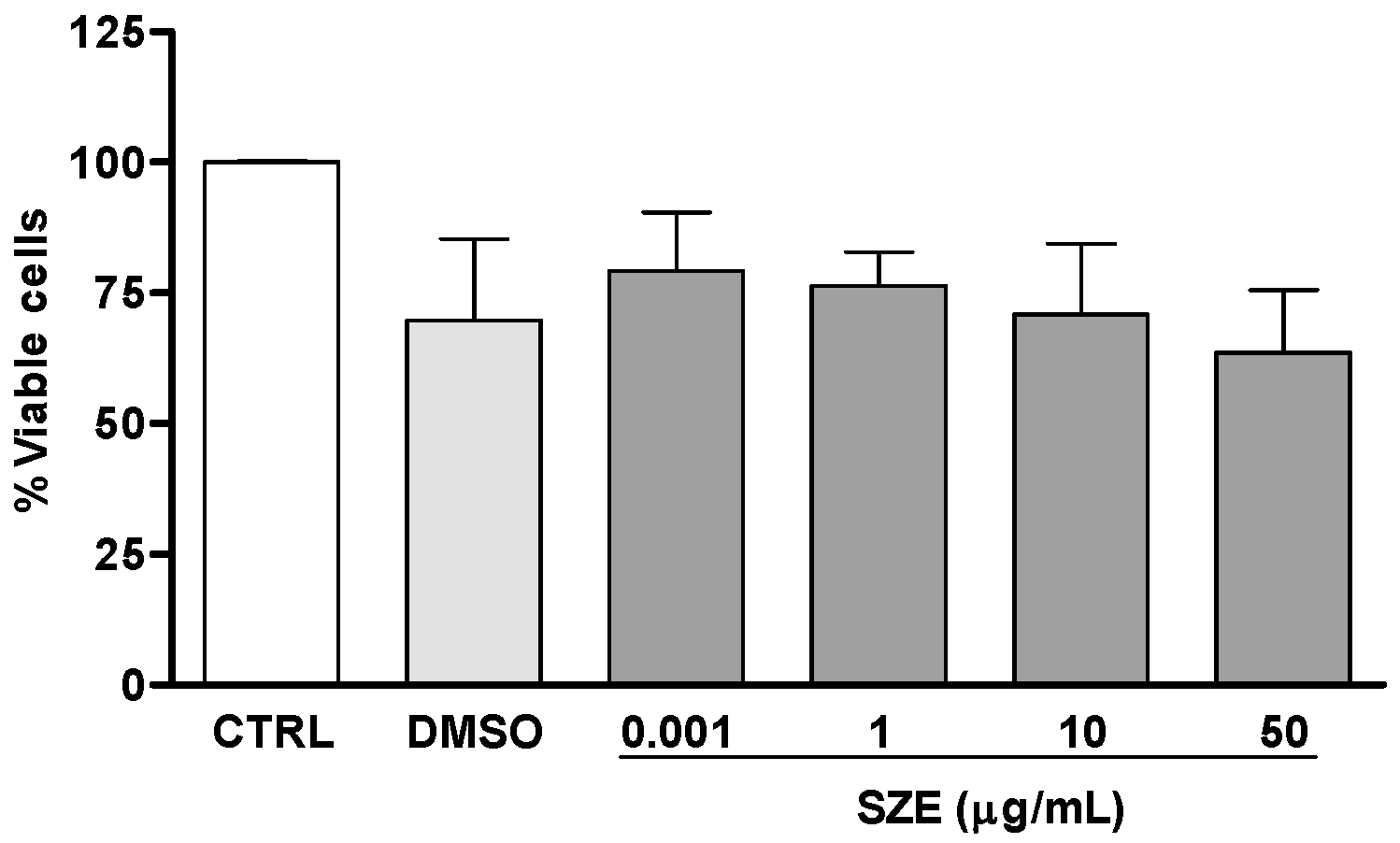
2.2. Anti-Leishmania Activity of Stypopodium zonale Extract
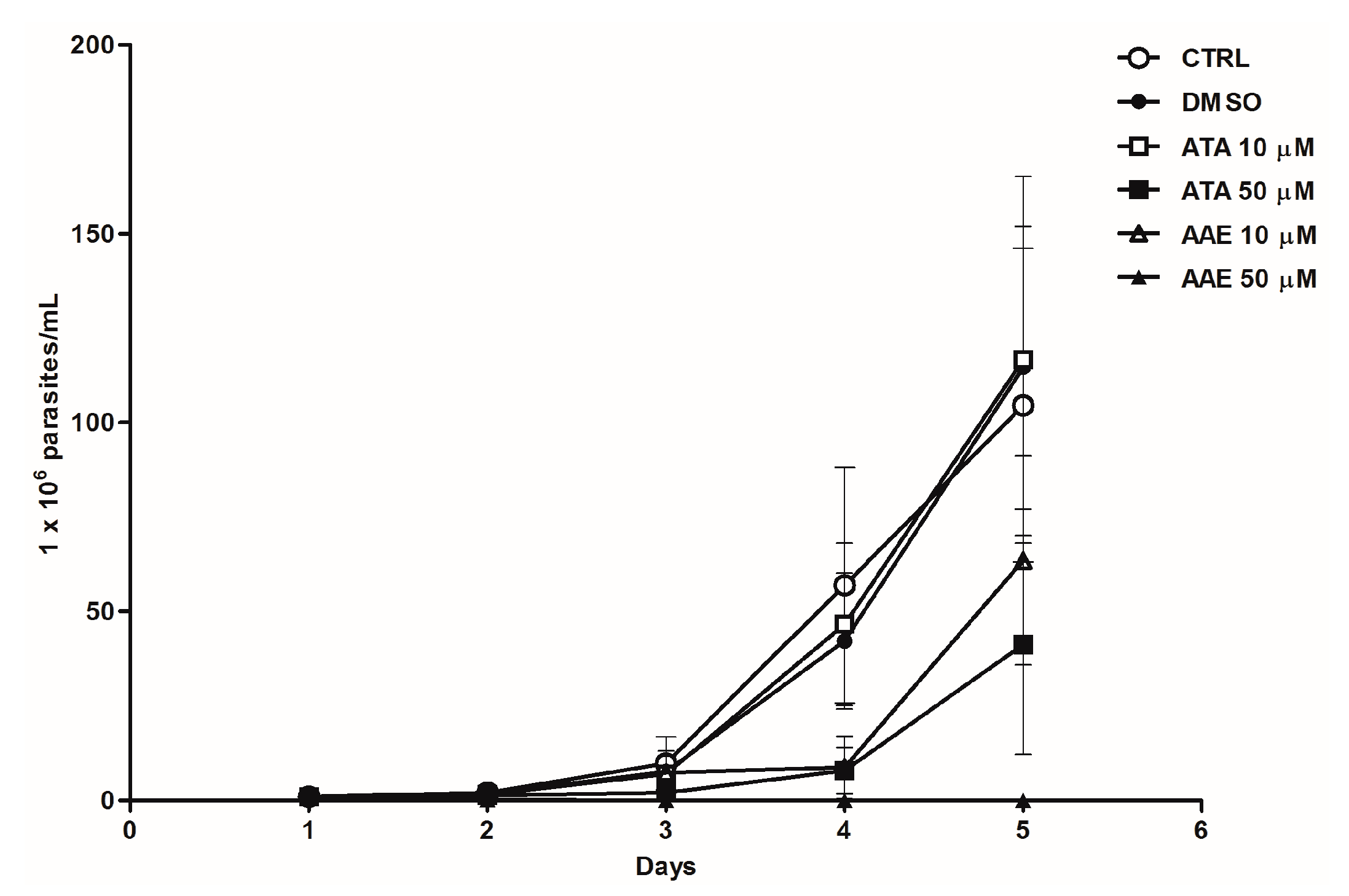
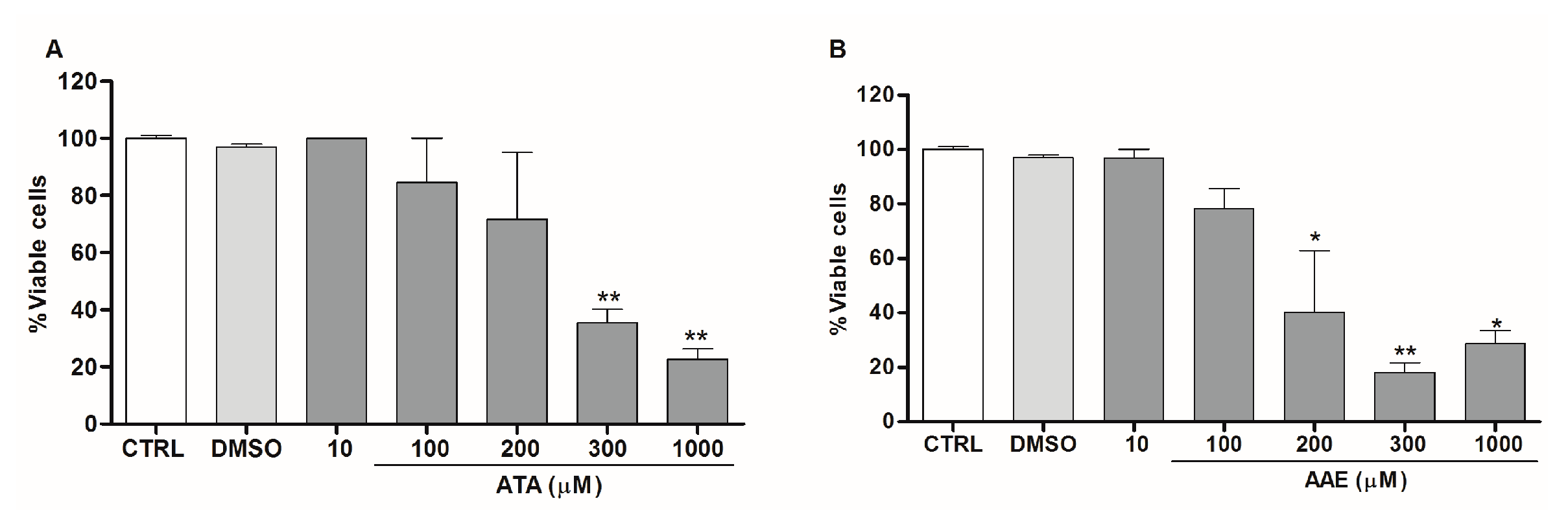
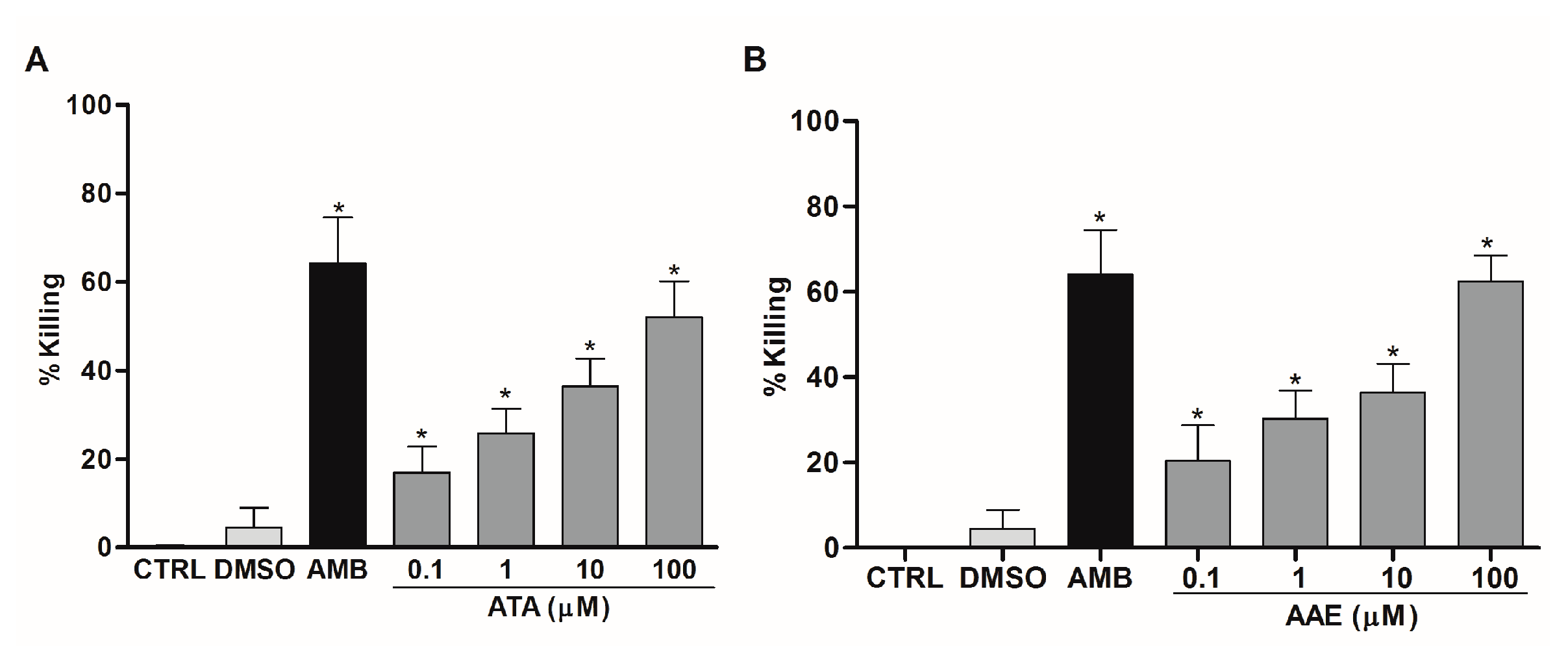
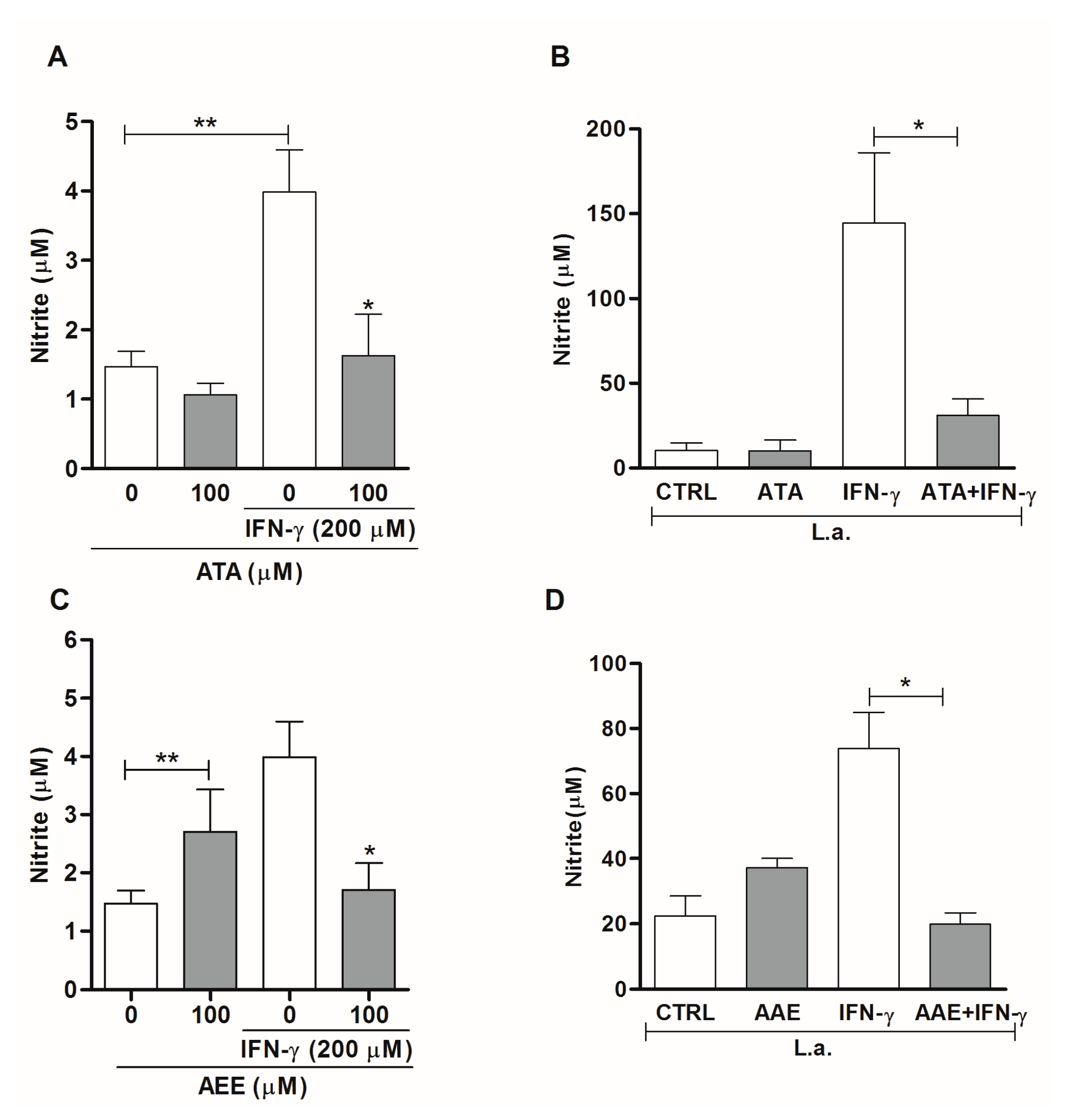
2.3. Leishmanicidal Activity of Atomaric Acid (ATA) and Its Methyl Ester Derivative (AAE)
3. Discussion
4. Experimental Section
4.1. Seaweed Sampling
4.2. Extract Preparation and Procedures for Obtaining Meroditerpene
4.3. Preparation of the Methyl Ester of Atomaric Acid (AAE)
4.4. Structural Elucidation
4.5. Ethics Statement
4.6. Parasite Culture
4.7. Anti-Promastigote Activity
4.8. Anti-Amastigote Activity
4.9. Cytotoxicity for Host Macrophages
4.10. Nitric Oxide Production
4.11. Detection of Reactive Oxygen Species (ROS)
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Alvar, J.; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; den Boer, M.; The WHO Leishmaniasis Control Team. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef] [PubMed]
- Barrett, M.P.; Croft, S.L. Management of trypanosomiasis and leishmaniasis. Br. Med. Bull. 2012, 104, 175–196. [Google Scholar] [CrossRef] [PubMed]
- Ouellette, M.; Drummelsmith, J.; Papadopoulou, B. Leishmaniasis: Drugs in the clinic, resistance and new developments. Drug Resist. Updat. 2004, 7, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.M.S.; Glaser, K.B.; Cuevas, C.; Jacobs, R.S.; Kem, W.; Little, R.D.; McIntosh, J.M.; Newman, D.J.; Potts, B.C.; Shuster, D.E. The odyssey of marine pharmaceuticals: A current pipeline perspective. Trends Pharmacol. Sci. 2010, 31, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Molinski, T.F.; Dalisay, D.S.; Lievens, S.L.; Saludes, J.P. Drug development from marine natural products. Nat. Rev. 2015, 8, 69–85. [Google Scholar] [CrossRef] [PubMed]
- Orhan, I.; Sener, B.; Atici, T.; Brun, R.; Perozzo, R.; Tasdemir, D. Turkish freshwater and marine macrophyte extracts show in vitro antiprotozoal activity and inhibit FabI, a key enzyme of Plasmodium falciparum fatty acid biosynthesis. Phytomedicine 2006, 13, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Freile-Pelegrin, Y.; Robledo, D.; Chan-Bacab, M.J.; Ortega-Morales, B.O. Antileishmanial properties of tropical marine algae extracts. Fitoterapia 2008, 79, 374–377. [Google Scholar] [CrossRef] [PubMed]
- Genovese, G.; Tedone, L.; Hamann, M.T.; Morabito, M. The Mediterranean red alga Asparagopsis: A source of compounds against Leishmania. Mar. Drugs 2009, 7, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Allmendinger, A.; Spavieri, J.; Kaiser, M.; Casey, R.; Hingley-Wilson, S.; Lalvani, A.; Guiry, M.; Blunden, G.; Tasdemir, D. Antiprotozoal, antimycobacterial and cytotoxic potential of twenty-three British and Irish red algae. Phytother. Res. 2010, 24, 1099–1103. [Google Scholar] [CrossRef] [PubMed]
- De Felício, R.; De Albuquerque, S.; Young, M.C.; Yokoya, N.S.; Debonsi, H.M. Trypanocidal, leishmanicidal and antifungal potential from marine red alga Bostrychia tenella, J. Agardh (Rhodomelaceae, Ceramiales). J. Pharm. Biomed. Anal. 2010, 52, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, A.O.; Veiga-Santos, P.; Ueda-Nakamura, T.; Filho, B.P.; Sudatti, D.B.; Bianco, É.M.; Pereira, R.C.; Nakamura, C.V. Effect of elatol, isolated from red seaweed Laurencia dendroidea, on Leishmania amazonensis. Mar. Drugs 2010, 8, 2733–2743. [Google Scholar] [CrossRef] [PubMed]
- Spavieri, J.; Kaiser, M.; Casey, R.; Hingley-Wilson, S.; Lalvani, A.; Blunden, G.; Tasdemir, D. Antiprotozoal, antimycobacterial and cytotoxic potential of some British green algae. Phytother. Res. 2010, 24, 1095–1098. [Google Scholar] [CrossRef] [PubMed]
- Spavieri, J.; Allmendinger, A.; Kaiser, M.; Casey, R.; Hingley-Wilson, S.; Lalvani, A.; Guiry, M.D.; Blunden, G.; Tasdemir, D. Antimycobacterial, antiprotozoal and cytotoxic potential of twenty-one brown algae (Phaeophyceae) from British and Irish waters. Phytother. Res. 2010, 24, 1724–1729. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Machado, F.L.; Pacienza-Lima, W.; Rossi-Bergmann, B.; de Souza Gestinari, L.M.; Fujii, M.T.; Campos de Paula, J.; Costa, S.S.; Lopes, N.P.; Kaiser, C.R.; Soares, A.R. Antileishmanial sesquiterpenes from the Brazilian red alga Laurencia dendroidea. Planta Med. 2011, 77, 733–735. [Google Scholar] [CrossRef] [PubMed]
- Süzgeç-Selçuk, S.; Meriçli, A.H.; Güven, K.C.; Kaiser, M.; Casey, R.; Hingley-Wilson, S.; Lalvani, A.; Tasdemir, D. Evaluation of Turkish seaweeds for antiprotozoal, antimycobacterial and cytotoxic activities. Phytother. Res. 2011, 25, 778–783. [Google Scholar] [CrossRef] [PubMed]
- Soares, D.C.; Calegari-Silva, T.C.; Lopes, U.G.; Teixeira, V.L.; de Palmer Paixão, I.C.N.; Cirne-Santos, C.; Bou-Habib, D.C.; Saraiva, E.M. Dolabelladienetriol, a compound from Dictyota pfaffii algae, inhibits the infection by Leishmania amazonensis. PLoS Negl. Trop. Dis. 2012, 6, e1787. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.R.; Duarte, H.M.; Tinnoco, L.W.; Pereira, R.C.; Teixeira, V.L. Intraspecific variation of meroditerpenoids in the brown alga Stypopodium zonale guiding the isolation of new compounds. Rev. Bras. Farmacogn. 2015, 25, 627–633. [Google Scholar] [CrossRef]
- Wessels, M.; König, G.M.; Wright, A.D. A new tyrosine kinase inhibitor from the marine brown alga Stypopodium zonale. J. Nat. Prod. 1999, 62, 927–930. [Google Scholar] [CrossRef] [PubMed]
- Dorta, E.; Diaz-Marrero, A.R.; Cueto, M.; Darias, J. On the relative stereochemistry of atomaric acid and related compounds. Tetrahedron 2003, 59, 2059–2062. [Google Scholar] [CrossRef]
- Sabry, O.M.M.; Andrews, S.; McPhail, K.L.; Goeger, D.E.; Yokochi, A.; LePage, K.T.; Murray, T.F.; Gerwick, W.H. Neurotoxic Meroditerpenoids from the Tropical Marine Brown Alga Stypopodium flabelliforme. J. Nat. Prod. 2005, 68, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- Vallim, A.; De Paula, J.C.; Pereira, R.C.; Teixeira, V.L. The diterpenes from Dictyotaceaen marine Brown algae in the Tropical Atlantic American region. Biochem. Syst. Ecol. 2005, 33, 1–16. [Google Scholar] [CrossRef]
- Areche, C.; San-Martín, A.; Rovirosa, J.; Muñoz, M.A.; Hernández-Barragán, A.; Bucio, M.A.; Joseph-Nathan, P. Stereostructure Reassignment and Absolute Configuration of Isoepitaondiol, a Meroditerpenoid from Stypopodium flabelliforme. J. Nat. Prod. 2010, 73, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.R.; Abrantes, J.L.; Lopes Souza, T.M.; Leite Fontes, C.F.; Pereira, R.C.; de Palmer Paixão Frugulhetti, I.C.; Teixeira, V.L. In vitro antiviral effect of meroditerpenes isolated from the Brazilian seaweed Stypopodium zonale (Dictyotales). Planta Med. 2007, 73, 1221–1224. [Google Scholar] [CrossRef] [PubMed]
- Dorta, E.; Cueto, M.; Brito, I.; Darias, J. New terpenoids from the brown alga Stypopodium zonale. J. Nat. Prod. 2002, 65, 1727–1730. [Google Scholar] [CrossRef] [PubMed]
- Rovirosa, J.; Sepulveda, M.; Quezada, E.; San-Martin, A. Isoepitaondiol, a diterpenoid of Stypopodium flabelliforme and the insecticidal activity of stypotriol, epitaondiol and derivatives. Phytochemistry 1992, 31, 2679–2681. [Google Scholar] [CrossRef]
- Mendes, G.; Soares, A.R.; Sigiliano, L.; Machado, F.; Kaiser, C.; Romeiro, N.; Gestinari, L.; Santos, N.; Romanos, M.T.V. In vitro anti-HMPV activity of meroditerpenoids from marine alga Stypopodium zonale (Dictyotales). Molecules 2011, 16, 8437–8450. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.C.; Soares, A.R.; Teixeira, V.L.; Villaça, R.; da Gama, B.A.P. Variation in chemical defenses against herbivory in southwestern Atlantic Stypopodium zonale (Phaeophyta). Bot. Mar. 2004, 47, 202–208. [Google Scholar] [CrossRef]
- Fouladvand, M.; Barazesh, A.; Farokhzad, F.; Malekizadeh, H.; Sartavi, K. Evaluation of in vitro anti-Leishmanial activity of some brown, green and red algae from the Persian Gulf. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 597–600. [Google Scholar] [PubMed]
- Soares, A.R.; Teixeira, V.L.; Pereira, R.C.; Villaça, R. Variation on diterpene production by the Brazilian alga Stypopodium zonale (Dictyotales, Phaeophyta). Biochem. Syst. Ecol. 2003, 31, 1347–1350. [Google Scholar] [CrossRef]
- Soares, A.R.; da Gama, B.A.P.; Cunha, A.P.; Teixeira, V.L.; Pereira, R.C. Induction of Attachment of the Mussel Perna perna by Natural Products from the Brown Seaweed Stypopodium zonale. Mar. Biotechnol. 2008, 10, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Kar, S.; Sharma, G.; Das, P.K. Fucoidan cures infection with both antimony-susceptible and -resistant strains of Leishmania donovani through Th1 response and macrophage-derived oxidants. J. Antimicrob. Chemother. 2011, 66, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Roehm, N.W.; Rodgers, G.H.; Hatfield, S.M.; Glasebrook, A.L. An improved colorimetric assay for cell proliferation and viability utilizing the tetrazolium salt XTT. J. Immun. Methods 1991, 142, 257–265. [Google Scholar] [CrossRef]
- Green, S.J.; Meltzer, M.S.; Hibbs, J.B., Jr.; Nacy, C.A. Activated macrophages destroy intracellular Leishmania major amastigotes by an l-arginine-dependent killing mechanism. J. Immunol. 1990, 144, 278–283. [Google Scholar] [PubMed]


| Compound | CC50 | IC50 | SI |
|---|---|---|---|
| ATA | 169.5 μM (75 μg/mL) | 20.2 μM (9 μg/mL) | 8.4 (8.3) |
| AAE | 262.5 μM (209 μg/mL) | 22.9 μM (10 μg/mL) | 11.5 (21) |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soares, D.C.; Szlachta, M.M.; Teixeira, V.L.; Soares, A.R.; Saraiva, E.M. The Brown Alga Stypopodium zonale (Dictyotaceae): A Potential Source of Anti-Leishmania Drugs. Mar. Drugs 2016, 14, 163. https://doi.org/10.3390/md14090163
Soares DC, Szlachta MM, Teixeira VL, Soares AR, Saraiva EM. The Brown Alga Stypopodium zonale (Dictyotaceae): A Potential Source of Anti-Leishmania Drugs. Marine Drugs. 2016; 14(9):163. https://doi.org/10.3390/md14090163
Chicago/Turabian StyleSoares, Deivid Costa, Marcella Macedo Szlachta, Valéria Laneuville Teixeira, Angelica Ribeiro Soares, and Elvira Maria Saraiva. 2016. "The Brown Alga Stypopodium zonale (Dictyotaceae): A Potential Source of Anti-Leishmania Drugs" Marine Drugs 14, no. 9: 163. https://doi.org/10.3390/md14090163
APA StyleSoares, D. C., Szlachta, M. M., Teixeira, V. L., Soares, A. R., & Saraiva, E. M. (2016). The Brown Alga Stypopodium zonale (Dictyotaceae): A Potential Source of Anti-Leishmania Drugs. Marine Drugs, 14(9), 163. https://doi.org/10.3390/md14090163






