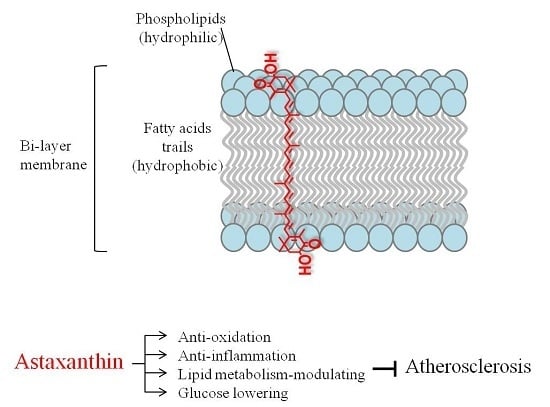
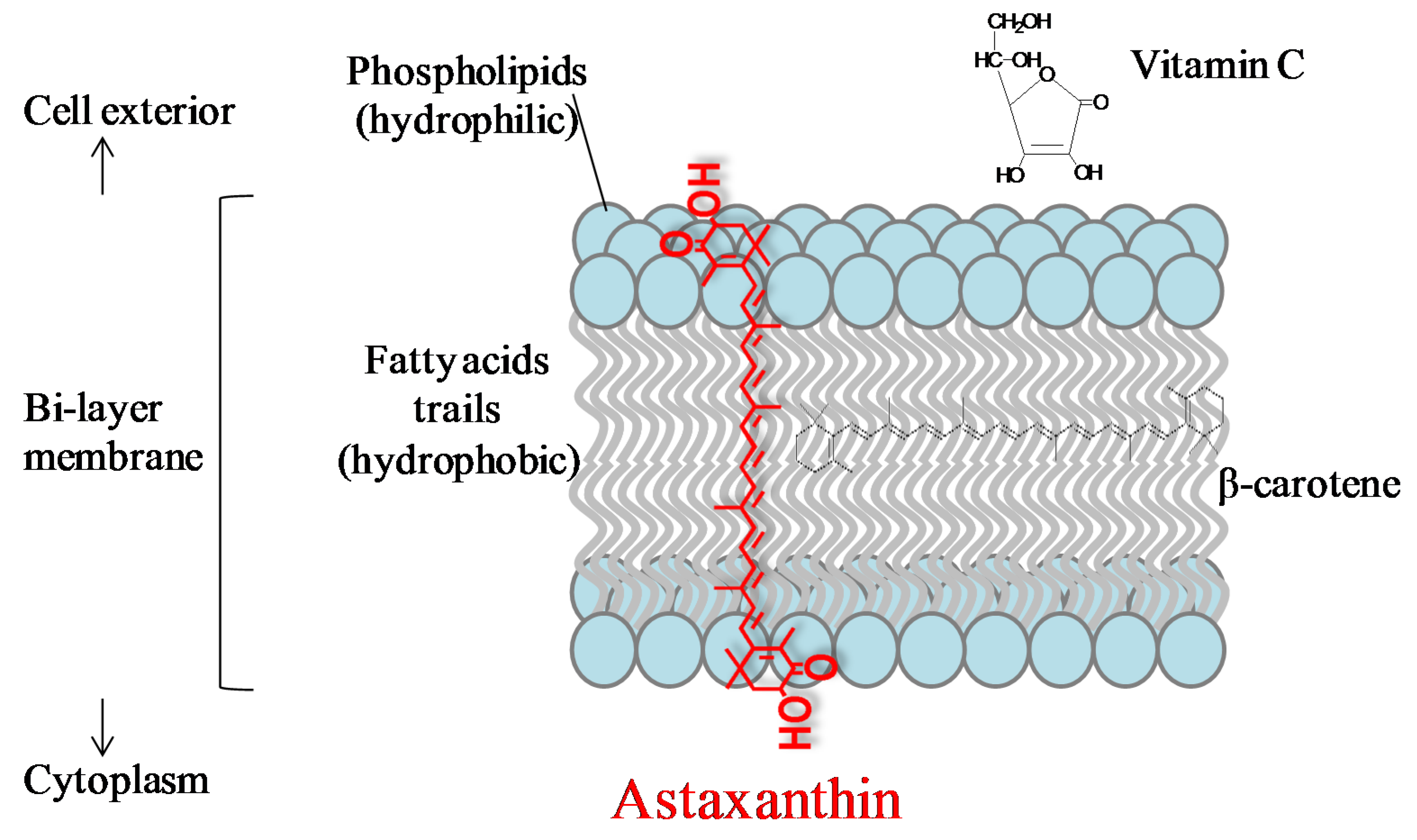
Potential Anti-Atherosclerotic Properties of Astaxanthin
Abstract
:1. Introduction
2. Food Sources and Bioavailability of Astaxanthin
3. Multiple Anti-Atherosclerotic Effects of Astaxanthin
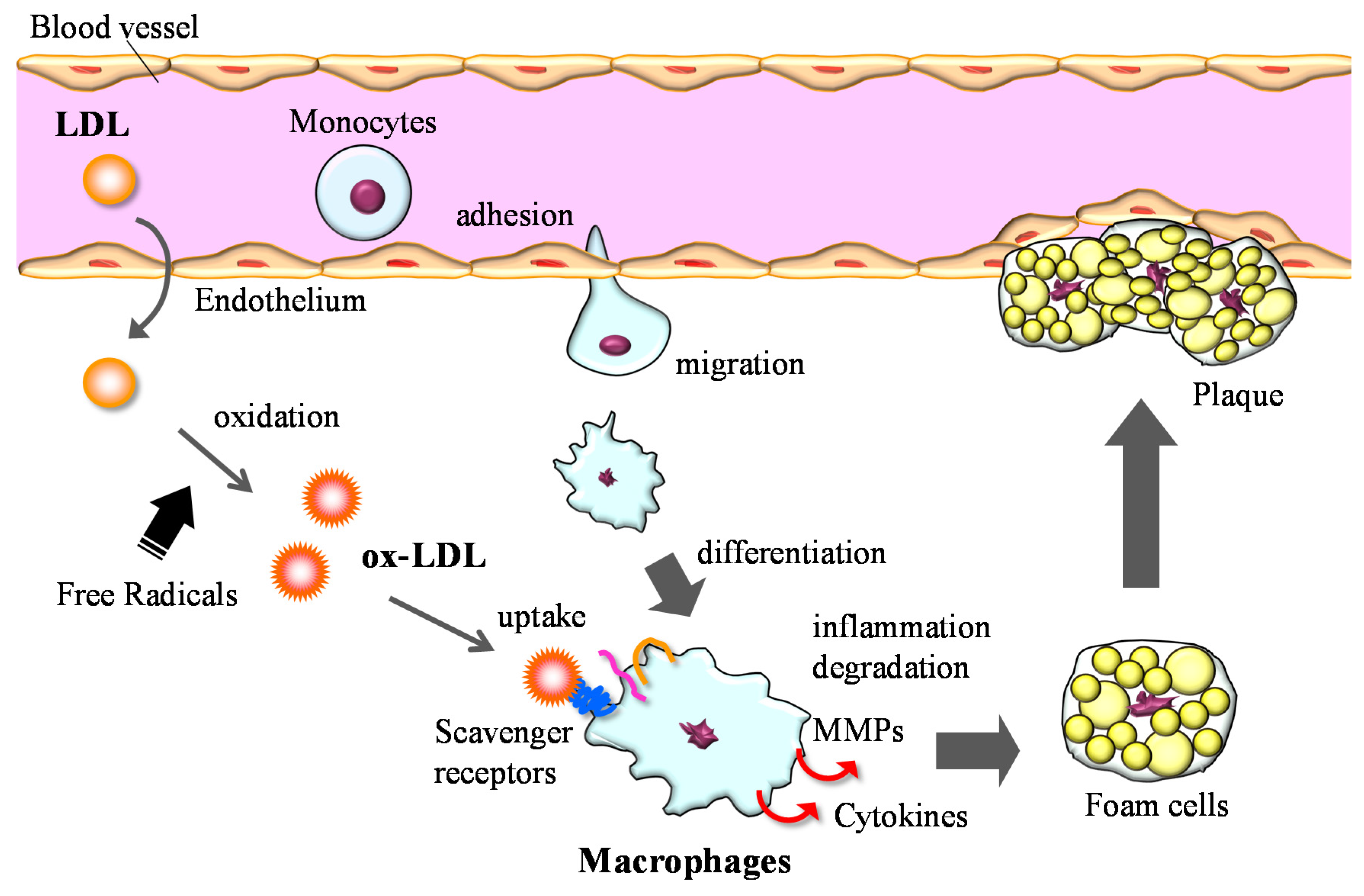
3.1. Anti-Oxidation
3.2. Anti-Inflammation
3.3. Lipid Metabolism
3.4. Glucose Metabolism and Blood Pressure Control
4. Conclusions
| Anti-Oxidation | |||
| Iwamoto et al. (2000) [16] | Healthy volunteers (n = 24) | Open labeled; 2 weeks; 1.8, 3.6, 14.4 or 21.6 mg/day | ↓ LDL oxidation |
| Nakagawa et al. (2011) [17] | Middle-aged and senior subjects (n = 30) | Randomized, double-blind, placebo controlled; 12 weeks; 6 or 12 mg/day | ↓ phospholipid peroxidation in erythrocytes |
| Karppi et al. (2007) [18] | Healthy non-smoking males (n = 40) | Randomized, double-blind, placebo controlled; 12 weeks; 8 mg/day | ↓ plasma 12- and 15-hydroxy fatty acids |
| Choi et al. (2011) [19] | Obese and overweight adults (n = 23) | Randomized, double-blind; 3 weeks; 5 or 20 mg/day | ↓ plasma MDA, isoprastane ↑ SOD, TAC |
| Anti-Inflammation | |||
| Park et al. (2010) [47] | Healthy female college students (n = 42) | Randomized, double-blind, placebo controlled; 8 weeks; 0, 2 or 8 mg/day | ↓ plasma 8-hydroxy-2′-deoxyguanosine, CRP |
| Lipid Metabolism-Modulating | |||
| Yoshida et al. (2010) [57] | Non-obese subjects with mild hypertriglycemia (n = 61) | Randomized, placebo-controlled study; 12 weeks; 0, 6, 12 or 18 mg/day | ↓ serum TG, ↑ HDL-C, adiponectin |
| Ursoniu et al. (2015) [64] | Meta-analysis of seven randomized controlled studies | No significant effect on plasma lipid profile (LDL-C, HDL-C, TG) | |
| Glucose Lowering | |||
| Ursoniu et al. (2015) [64] | Meta-analysis of seven randomized controlled studies | Slight lowering effect on plasma glucose | |
Acknowledgments
Conflicts of Interest
References
- Ambati, R.R.; Phang, S.M.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications–A review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [PubMed]
- Lorenz, R.T.; Cysewski, G.R. Commercial potential for haematococcus microalgae as a natural source of astaxanthin. Trends Biotechnol. 2000, 18, 160–167. [Google Scholar] [CrossRef]
- Yuan, J.P.; Peng, J.; Yin, K.; Wang, J.H. Potential health-promoting effects of astaxanthin: A high-value carotenoid mostly from microalgae. Mol. Nutr. Food Res. 2011, 55, 150–165. [Google Scholar] [CrossRef] [PubMed]
- Hussein, G.; Sankawa, U.; Goto, H.; Matsumoto, K.; Watanabe, H. Astaxanthin, a carotenoid with potential in human health and nutrition. J. Nat. Prod. 2006, 69, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Fassett, R.G.; Coombes, J.S. Astaxanthin in cardiovascular health and disease. Molecules 2012, 17, 2030–2048. [Google Scholar] [CrossRef] [PubMed]
- Guerin, M.; Huntley, M.E.; Olaizola, M. Haematococcus astaxanthin: Applications for human health and nutrition. Trends Biotechnol. 2003, 21, 210–216. [Google Scholar] [CrossRef]
- Coral-Hinostroza, G.N.; Ytrestoyl, T.; Ruyter, B.; Bjerkeng, B. Plasma appearance of unesterified astaxanthin geometrical E/Z and optical R/S isomers in men given single doses of a mixture of optical 3 and 3′R/S isomers of astaxanthin fatty acyl diesters. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2004, 139, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Okada, Y.; Ishikura, M.; Maoka, T. Bioavailability of astaxanthin in haematococcus algal extract: The effects of timing of diet and smoking habits. Biosci. Biotechnol. Biochem. 2009, 73, 1928–1932. [Google Scholar] [CrossRef] [PubMed]
- Pashkow, F.J.; Watumull, D.G.; Campbell, C.L. Astaxanthin: A novel potential treatment for oxidative stress and inflammation in cardiovascular disease. Am. J. Cardiol. 2008, 101, 58D–68D. [Google Scholar] [CrossRef] [PubMed]
- Goto, S.; Kogure, K.; Abe, K.; Kimata, Y.; Kitahama, K.; Yamashita, E.; Terada, H. Efficient radical trapping at the surface and inside the phospholipid membrane is responsible for highly potent antiperoxidative activity of the carotenoid astaxanthin. Biochim. Biophys. Acta 2001, 1512, 251–258. [Google Scholar] [CrossRef]
- Terao, J. Antioxidant activity of beta-carotene-related carotenoids in solution. Lipids 1989, 24, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Lim, B.P.; Nagao, A.; Terao, J.; Tanaka, K.; Suzuki, T.; Takama, K. Antioxidant activity of xanthophylls on peroxyl radical-mediated phospholipid peroxidation. Biochim. Biophys. Acta 1992, 1126, 178–184. [Google Scholar] [PubMed]
- Palozza, P.; Krinsky, N.I. Astaxanthin and canthaxanthin are potent antioxidants in a membrane model. Arch. Biochem. Biophys. 1992, 297, 291–295. [Google Scholar] [CrossRef]
- Ciccone, M.M.; Cortese, F.; Gesualdo, M.; Carbonara, S.; Zito, A.; Ricci, G.; De Pascalis, F.; Scicchitano, P.; Riccioni, G. Dietary intake of carotenoids and their antioxidant and anti-inflammatory effects in cardiovascular care. Mediators Inflamm. 2013, 2013. [Google Scholar] [CrossRef]
- Martin, H.D.; Ruck, C.; Schmidt, M.; Sell, S.; Beutner, S.; Mayer, B.; Walsh, R. Chemistry of carotenoid oxidation and free radical reactions. Pure Appl. Chem. 1999, 71, 2253–2262. [Google Scholar] [CrossRef]
- Iwamoto, T.; Hosoda, K.; Hirano, R.; Kurata, H.; Matsumoto, A.; Miki, W.; Kamiyama, M.; Itakura, H.; Yamamoto, S.; Kondo, K. Inhibition of low-density lipoprotein oxidation by astaxanthin. J. Atheroscler. Thromb. 2000, 7, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, K.; Kiko, T.; Miyazawa, T.; Carpentero Burdeos, G.; Kimura, F.; Satoh, A.; Miyazawa, T. Antioxidant effect of astaxanthin on phospholipid peroxidation in human erythrocytes. Br. J. Nutr. 2011, 105, 1563–1571. [Google Scholar] [CrossRef] [PubMed]
- Karppi, J.; Rissanen, T.H.; Nyyssonen, K.; Kaikkonen, J.; Olsson, A.G.; Voutilainen, S.; Salonen, J.T. Effects of astaxanthin supplementation on lipid peroxidation. Int. J. Vitam. Nutr. Res. 2007, 77, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.D.; Kim, J.H.; Chang, M.J.; Kyu-Youn, Y.; Shin, W.G. Effects of astaxanthin on oxidative stress in overweight and obese adults. Phytother. Res. 2011, 25, 1813–1818. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Chiba, T.; Takahashi, S.; Ishii, T.; Igarashi, K.; Katoh, Y.; Oyake, T.; Hayashi, N.; Satoh, K.; Hatayama, I.; et al. An NRF2/small maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 1997, 236, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, W.; Su, Z.Y.; Kong, A.T. The complexity of the NRF2 pathway: Beyond the antioxidant response. J. Nutr. Biochem. 2015, 26, 1401–1413. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, D.N.; Jena, G.B. Astaxanthin intervention ameliorates cyclophosphamide-induced oxidative stress, DNA damage and early hepatocarcinogenesis in rat: Role of Nrf2, p53, p38 and phase-II enzymes. Mutat. Res. 2010, 696, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Saw, C.L.; Yang, A.Y.; Guo, Y.; Kong, A.N. Astaxanthin and omega-3 fatty acids individually and in combination protect against oxidative stress via the Nrf2-are pathway. Food Chem. Toxicol. 2013, 62, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Aviram, M. Introduction to the serial review on paraoxonases, oxidative stress, and cardiovascular diseases. Free Radic. Biol. Med. 2004, 37, 1301–1303. [Google Scholar] [CrossRef] [PubMed]
- Augusti, P.R.; Quatrin, A.; Somacal, S.; Conterato, G.M.; Sobieski, R.; Ruviaro, A.R.; Maurer, L.H.; Duarte, M.M.; Roehrs, M.; Emanuelli, T. Astaxanthin prevents changes in the activities of thioredoxin reductase and paraoxonase in hypercholesterolemic rabbits. J. Clin. Biochem. Nutr. 2012, 51, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Senti, M.; Tomas, M.; Anglada, R.; Elosua, R.; Marrugat, J.; Covas, M.I.; Fito, M. Interrelationship of smoking, paraoxonase activity, and leisure time physical activity: A population-based study. Eur. J. Intern. Med. 2003, 14, 178–184. [Google Scholar] [CrossRef]
- Baralic, I.; Djordjevic, B.; Dikic, N.; Kotur-Stevuljevic, J.; Spasic, S.; Jelic-Ivanovic, Z.; Radivojevic, N.; Andjelkovic, M.; Pejic, S. Effect of astaxanthin supplementation on paraoxonase 1 activities and oxidative stress status in young soccer players. Phytother. Res. 2013, 27, 1536–1542. [Google Scholar] [CrossRef] [PubMed]
- Djordjevic, B.; Baralic, I.; Kotur-Stevuljevic, J.; Stefanovic, A.; Ivanisevic, J.; Radivojevic, N.; Andjelkovic, M.; Dikic, N. Effect of astaxanthin supplementation on muscle damage and oxidative stress markers in elite young soccer players. J. Sports Med. Phys. Fit. 2012, 52, 382–392. [Google Scholar] [PubMed]
- Miller, Y.I.; Chang, M.K.; Binder, C.J.; Shaw, P.X.; Witztum, J.L. Oxidized low density lipoprotein and innate immune receptors. Curr. Opin. Lipidol. 2003, 14, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Kunjathoor, V.V.; Febbraio, M.; Podrez, E.A.; Moore, K.J.; Andersson, L.; Koehn, S.; Rhee, J.S.; Silverstein, R.; Hoff, H.F.; Freeman, M.W. Scavenger receptors class A-I/II and cd36 are the principal receptors responsible for the uptake of modified low density lipoprotein leading to lipid loading in macrophages. J. Biol. Chem. 2002, 277, 49982–49988. [Google Scholar] [CrossRef] [PubMed]
- Ross, R. Atherosclerosis is an inflammatory disease. Am. Heart J. 1999, 138, S419–S420. [Google Scholar] [CrossRef]
- Chakraborti, S.; Mandal, M.; Das, S.; Mandal, A.; Chakraborti, T. Regulation of matrix metalloproteinases: An overview. Mol. Cell. Biochem. 2003, 253, 269–285. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, N.; Ikeda, U. Matrix metalloproteinases and atherosclerosis. Curr. Atheroscler. Rep. 2004, 6, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Eriksson, P.; Hansson, G.K.; Herzfeld, I.; Klein, M.; Hansson, L.O.; Valen, G. Expression of matrix metalloproteinase 9 and its regulators in the unstable coronary atherosclerotic plaque. Int. J. Mol. Med. 2005, 15, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Zeng, B.; Prasan, A.; Fung, K.C.; Solanki, V.; Bruce, D.; Freedman, S.B.; Brieger, D. Elevated circulating levels of matrix metalloproteinase-9 and -2 in patients with symptomatic coronary artery disease. Intern. Med. J. 2005, 35, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, Y.; Tani, M.; Uto-Kondo, H.; Iizuka, M.; Saita, E.; Sone, H.; Kurata, H.; Kondo, K. Astaxanthin suppresses scavenger receptor expression and matrix metalloproteinase activity in macrophages. Eur. J. Nutr. 2010, 49, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Bai, S.K.; Lee, K.S.; Namkoong, S.; Na, H.J.; Ha, K.S.; Han, J.A.; Yim, S.V.; Chang, K.; Kwon, Y.G.; et al. Astaxanthin inhibits nitric oxide production and inflammatory gene expression by suppressing I-kappaB kinase-dependent NF-kappaB activation. Mol. Cells 2003, 16, 97–105. [Google Scholar] [PubMed]
- Macedo, R.C.; Bolin, A.P.; Marin, D.P.; Otton, R. Astaxanthin addition improves human neutrophils function: In vitro study. Eur. J. Nutr. 2010, 49, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Speranza, L.; Pesce, M.; Patruno, A.; Franceschelli, S.; de Lutiis, M.A.; Grilli, A.; Felaco, M. Astaxanthin treatment reduced oxidative induced pro-inflammatory cytokines secretion in U937: SHP-1 as a novel biological target. Mar. Drugs 2012, 10, 890–899. [Google Scholar] [CrossRef] [PubMed]
- Tsui, H.W.; Hasselblatt, K.; Martin, A.; Mok, S.C.; Tsui, F.W. Molecular mechanisms underlying SHP-1 gene expression. Eur. J. Biochem. 2002, 269, 3057–3064. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, T.H.; Reynolds, C.A.; Kumar, R.; Przyklenk, K.; Huttemann, M. Molecular mechanisms of ischemia-reperfusion injury in brain: Pivotal role of the mitochondrial membrane potential in reactive oxygen species generation. Mol. Neurobiol. 2013, 47, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Curek, G.D.; Cort, A.; Yucel, G.; Demir, N.; Ozturk, S.; Elpek, G.O.; Savas, B.; Aslan, M. Effect of astaxanthin on hepatocellular injury following ischemia/reperfusion. Toxicology 2010, 267, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, F.; Xia, Y.; Dai, W.; Chen, K.; Li, S.; Liu, T.; Zheng, Y.; Wang, J.; Lu, W.; et al. Astaxanthin pretreatment attenuates hepatic ischemia reperfusion-induced apoptosis and autophagy via the ROS/MAPK pathway in mice. Mar. Drugs 2015, 13, 3368–3387. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.P.; Liu, S.Y.; Sun, H.; Wu, X.M.; Li, J.J.; Zhu, L. Neuroprotective effect of astaxanthin on H2O2-induced neurotoxicity in vitro and on focal cerebral ischemia in vivo. Brain Res. 2010, 1360, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Kuo, C.C.; Chou, J.; Delvolve, A.; Jackson, S.N.; Post, J.; Woods, A.S.; Hoffer, B.J.; Wang, Y.; Harvey, B.K. Astaxanthin reduces ischemic brain injury in adult rats. FASEB J. 2009, 23, 1958–1968. [Google Scholar] [CrossRef] [PubMed]
- Lauver, D.A.; Lockwood, S.F.; Lucchesi, B.R. Disodium disuccinate astaxanthin (cardax) attenuates complement activation and reduces myocardial injury following ischemia/reperfusion. J. Pharmacol. Exp. Ther. 2005, 314, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Chyun, J.H.; Kim, Y.K.; Line, L.L.; Chew, B.P. Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Nutr. Metab. (Lond.) 2010, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- Chew, B.P.; Mathison, B.D.; Hayek, M.G.; Massimino, S.; Reinhart, G.A.; Park, J.S. Dietary astaxanthin enhances immune response in dogs. Vet. Immunol. Immunopathol. 2011, 140, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Chew, B.P.; Park, J.S.; Wong, T.S.; Kim, H.W.; Weng, B.B.; Byrne, K.M.; Hayek, M.G.; Reinhart, G.A. Dietary beta-carotene stimulates cell-mediated and humoral immune response in dogs. J. Nutr. 2000, 130, 1910–1913. [Google Scholar] [PubMed]
- Kim, H.W.; Chew, B.P.; Wong, T.S.; Park, J.S.; Weng, B.B.; Byrne, K.M.; Hayek, M.G.; Reinhart, G.A. Dietary lutein stimulates immune response in the canine. Vet. Immunol. Immunopathol. 2000, 74, 315–327. [Google Scholar] [CrossRef]
- Hussein, G.; Nakagawa, T.; Goto, H.; Shimada, Y.; Matsumoto, K.; Sankawa, U.; Watanabe, H. Astaxanthin ameliorates features of metabolic syndrome in SHR/NDmcr-cp. Life Sci. 2007, 80, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Ikeuchi, M.; Koyama, T.; Takahashi, J.; Yazawa, K. Effects of astaxanthin in obese mice fed a high-fat diet. Biosci. Biotechnol. Biochem. 2007, 71, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Seo, J.M.; Nguyen, A.; Pham, T.X.; Park, H.J.; Park, Y.; Kim, B.; Bruno, R.S.; Lee, J. Astaxanthin-rich extract from the green alga haematococcus pluvialis lowers plasma lipid concentrations and enhances antioxidant defense in apolipoprotein E knockout mice. J. Nutr. 2011, 141, 1611–1617. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, M.; Ayaori, M.; Uto-Kondo, H.; Yakushiji, E.; Takiguchi, S.; Nakaya, K.; Hisada, T.; Sasaki, M.; Komatsu, T.; Yogo, M.; et al. Astaxanthin enhances ATP-binding cassette transporter A1/G1 expressions and cholesterol efflux from macrophages. J. Nutr. Sci. Vitaminol. (Tokyo) 2012, 58, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Pham, T.X.; Wegner, C.J.; Kim, B.; Ku, C.S.; Park, Y.K.; Lee, J.Y. Astaxanthin lowers plasma tag concentrations and increases hepatic antioxidant gene expression in diet-induced obesity mice. Br. J. Nutr. 2014, 112, 1797–1804. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Hellsten, A.; Jacobsson, L.S.; Blomqvist, H.M.; Olsson, A.G.; Yuan, X.M. Alpha-tocopherol and astaxanthin decrease macrophage infiltration, apoptosis and vulnerability in atheroma of hyperlipidaemic rabbits. J. Mol. Cell. Cardiol. 2004, 37, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Yanai, H.; Ito, K.; Tomono, Y.; Koikeda, T.; Tsukahara, H.; Tada, N. Administration of natural astaxanthin increases serum HDl-cholesterol and adiponectin in subjects with mild hyperlipidemia. Atherosclerosis 2010, 209, 520–523. [Google Scholar] [CrossRef] [PubMed]
- Siri-Tarino, P.W. Effects of diet on high-density lipoprotein cholesterol. Curr. Atheroscler. Rep. 2011, 13, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Cnop, M.; Havel, P.J.; Utzschneider, K.M.; Carr, D.B.; Sinha, M.K.; Boyko, E.J.; Retzlaff, B.M.; Knopp, R.H.; Brunzell, J.D.; Kahn, S.E. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: Evidence for independent roles of age and sex. Diabetologia 2003, 46, 459–469. [Google Scholar] [PubMed]
- Ng, T.W.; Watts, G.F.; Farvid, M.S.; Chan, D.C.; Barrett, P.H. Adipocytokines and VLDL metabolism: Independent regulatory effects of adiponectin, insulin resistance, and fat compartments on VLDL apolipoprotein B-100 kinetics? Diabetes 2005, 54, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.C.; Barrett, P.H.; Ooi, E.M.; Ji, J.; Chan, D.T.; Watts, G.F. Very low density lipoprotein metabolism and plasma adiponectin as predictors of high-density lipoprotein apolipoprotein AI kinetics in obese and nonobese men. J. Clin. Endocrinol. Metab. 2009, 94, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.G.; von Eynatten, M.; Schiekofer, S.; Nawroth, P.P.; Dugi, K.A. Low plasma adiponectin levels are associated with increased hepatic lipase activity in vivo. Diabetes Care 2005, 28, 2181–2186. [Google Scholar] [PubMed]
- Tsubakio-Yamamoto, K.; Matsuura, F.; Koseki, M.; Oku, H.; Sandoval, J.C.; Inagaki, M.; Nakatani, K.; Nakaoka, H.; Kawase, R.; Yuasa-Kawase, M.; et al. Adiponectin prevents atherosclerosis by increasing cholesterol efflux from macrophages. Biochem. Biophys. Res. Commun. 2008, 375, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Ursoniu, S.; Sahebkar, A.; Serban, M.C.; Banach, M. Lipid profile and glucose changes after supplementation with astaxanthin: A systematic review and meta-analysis of randomized controlled trials. Arch. Med. Sci. 2015, 11, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.K.; King, T.J.; Fujioka, K.; Pattison, J.; Pashkow, F.J.; Tsimikas, S. Effect of an oral astaxanthin prodrug (CDX-085) on lipoprotein levels and progression of atherosclerosis in IDIR (−/−) and ApoE (−/−) mice. Atherosclerosis 2012, 222, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.K.; Malinski, T.; Mason, R.P.; Kubant, R.; Jacob, R.F.; Fujioka, K.; Denstaedt, S.J.; King, T.J.; Jackson, H.L.; Hieber, A.D.; et al. Novel astaxanthin prodrug (CDX-085) attenuates thrombosis in a mouse model. Thromb. Res. 2010, 126, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Nishizawa, H.; Funahashi, T.; Shimomura, I.; Shimabukuro, M. Systemic oxidative stress is associated with visceral fat accumulation and the metabolic syndrome. Circ. J. 2006, 70, 1437–1442. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, M.; Rivabene, R.; Avella, M.; Amicone, L.; Tripodi, M.; Botham, K.M.; Bravo, E. Oxidation affects the regulation of hepatic lipid synthesis by chylomicron remnants. Free Radic. Biol. Med. 2001, 30, 506–515. [Google Scholar] [CrossRef]
- Aoi, W.; Naito, Y.; Takanami, Y.; Ishii, T.; Kawai, Y.; Akagiri, S.; Kato, Y.; Osawa, T.; Yoshikawa, T. Astaxanthin improves muscle lipid metabolism in exercise via inhibitory effect of oxidative CPT I modification. Biochem. Biophys. Res. Commun. 2008, 366, 892–897. [Google Scholar] [CrossRef] [PubMed]
- The DECODE study group on behalf of the Europe an Diabetes Epidemiology Group. Glucose tolerance and mortality: comparison of WHO and American Diabetic Association diagnostic criteria. Lancet 1999, 354, 617–621. [Google Scholar]
- Saydah, S.H.; Loria, C.M.; Eberhardt, M.S.; Brancati, F.L. Subclinical states of glucose intolerance and risk of death in the U.S. Diabetes Care 2001, 24, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Ning, F.; Tuomilehto, J.; Pyorala, K.; Onat, A.; Soderberg, S.; Qiao, Q.; Group, D.S. Cardiovascular disease mortality in europeans in relation to fasting and 2-h plasma glucose levels within a normoglycemic range. Diabetes Care 2010, 33, 2211–2216. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, K.; Naito, Y.; Hasegawa, G.; Nakamura, N.; Takahashi, J.; Yoshikawa, T. Astaxanthin protects beta-cells against glucose toxicity in diabetic db/db mice. Redox. Rep. 2002, 7, 290–293. [Google Scholar] [CrossRef] [PubMed]
- Arunkumar, E.; Bhuvaneswari, S.; Anuradha, C.V. An intervention study in obese mice with astaxanthin, a marine carotenoid--effects on insulin signaling and pro-inflammatory cytokines. Food Funct. 2012, 3, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Bhuvaneswari, S.; Anuradha, C.V. Astaxanthin prevents loss of insulin signaling and improves glucose metabolism in liver of insulin resistant mice. Can. J. Physiol. Pharmacol. 2012, 90, 1544–1552. [Google Scholar] [CrossRef] [PubMed]
- Gammone, M.A.; D’Orazio, N. Anti-obesity activity of the marine carotenoid fucoxanthin. Mar. Drugs 2015, 13, 2196–2214. [Google Scholar] [CrossRef] [PubMed]
- Abidov, M.; Ramazanov, Z.; Seifulla, R.; Grachev, S. The effects of Xanthigen in the weight management of obese premenopausal women with non-alcoholic fatty liver disease and normal liver fat. Diabetes Obes. Metab. 2010, 12, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Boden, G.; Duan, X.; Homko, C.; Molina, E.J.; Song, W.; Perez, O.; Cheung, P.; Merali, S. Increase in endoplasmic reticulum stress-related proteins and genes in adipose tissue of obese, insulin-resistant individuals. Diabetes 2008, 57, 2438–2444. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, A.; Hotamisligil, G.S. Impact of ER protein homeostasis on metabolism. Diabetes 2013, 62, 691–693. [Google Scholar] [CrossRef] [PubMed]
- Bhuvaneswari, S.; Yogalakshmi, B.; Sreeja, S.; Anuradha, C.V. Astaxanthin reduces hepatic endoplasmic reticulum stress and nuclear factor-kappab-mediated inflammation in high fructose and high fat diet-fed mice. Cell. Stress Chaperones 2014, 19, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Naito, Y.; Uchiyama, K.; Aoi, W.; Hasegawa, G.; Nakamura, N.; Yoshida, N.; Maoka, T.; Takahashi, J.; Yoshikawa, T. Prevention of diabetic nephropathy by treatment with astaxanthin in diabetic db/db mice. Biofactors 2004, 20, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Preuss, H.G.; Echard, B.; Yamashita, E.; Perricone, N.V. High dose astaxanthin lowers blood pressure and increases insulin sensitivity in rats: Are these effects interdependent? Int. J. Med. Sci. 2011, 8, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Hussein, G.; Nakamura, M.; Zhao, Q.; Iguchi, T.; Goto, H.; Sankawa, U.; Watanabe, H. Antihypertensive and neuroprotective effects of astaxanthin in experimental animals. Biol. Pharm. Bull. 2005, 28, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Yanai, H.; Ito, K.; Yoshida, H.; Tada, N. Antihypertensive effects of astaxanthin. Integr. Blood Press. Control 2008, 1, 1–3. [Google Scholar] [PubMed]
- Xu, L.; Zhu, J.; Yin, W.; Ding, X. Astaxanthin improves cognitive deficits from oxidative stress, nitric oxide synthase and inflammation through upregulation of PI3K/Akt in diabetes rat. Int. J. Clin. Exp. Pathol. 2015, 8, 6083–6094. [Google Scholar] [PubMed]
- Katagiri, M.; Satoh, A.; Tsuji, S.; Shirasawa, T. Effects of astaxanthin-rich haematococcus pluvialis extract on cognitive function: A randomised, double-blind, placebo-controlled study. J. Clin. Biochem. Nutr. 2012, 51, 102–107. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kishimoto, Y.; Yoshida, H.; Kondo, K. Potential Anti-Atherosclerotic Properties of Astaxanthin. Mar. Drugs 2016, 14, 35. https://doi.org/10.3390/md14020035
Kishimoto Y, Yoshida H, Kondo K. Potential Anti-Atherosclerotic Properties of Astaxanthin. Marine Drugs. 2016; 14(2):35. https://doi.org/10.3390/md14020035
Chicago/Turabian StyleKishimoto, Yoshimi, Hiroshi Yoshida, and Kazuo Kondo. 2016. "Potential Anti-Atherosclerotic Properties of Astaxanthin" Marine Drugs 14, no. 2: 35. https://doi.org/10.3390/md14020035
APA StyleKishimoto, Y., Yoshida, H., & Kondo, K. (2016). Potential Anti-Atherosclerotic Properties of Astaxanthin. Marine Drugs, 14(2), 35. https://doi.org/10.3390/md14020035