Abstract
Fucoxanthin is a carotenoid present in the chloroplasts of brown seaweeds. When ingested, it is metabolized mainly to fucoxanthinol by digestive enzymes of the gastrointestinal tract. These compounds have been shown to have many beneficial health effects, including anti-mutagenic, anti-diabetic, anti-obesity, anti-inflammatory and anti-neoplastic actions. In every cancer tested, modulatory actions of fucoxanthinol on viability, cell-cycle arrest, apoptosis and members of the NF-κB pathway were more pronounced than that of fucoxanthin. Anti-proliferative and cancer preventing influences of fucoxanthin and fucoxanthinol are mediated through different signalling pathways, including the caspases, Bcl-2 proteins, MAPK, PI3K/Akt, JAK/STAT, AP-1, GADD45, and several other molecules that are involved in cell cycle arrest, apoptosis, anti-angiogenesis or inhibition of metastasis. In this review, we address the mechanisms of action of fucoxanthin and fucoxanthinol according to different types of cancers. Current findings suggest that these compounds could be effective for treatment and/or prevention of cancer development and aggressiveness.
1. Introduction
Fucoxanthin (Fx) is a naturally occurring brown- or orange-coloured pigment that belongs to the class of non-provitamin A carotenoids present in the chloroplasts of brown seaweeds. It is the most abundant of all carotenoids, accounting for more than 10% of the estimated total natural production of carotenoids [1]. It forms a complex with chlorophyll–protein and plays an important role in light harvesting and photoprotection for effective light use and up-regulation of photosynthesis. Under experimental conditions in mice, oral administration of Fx does not exhibit toxicity and mutagenicity [2,3].
Sources and Metabolites of Fucoxanthin
Fx (Figure 1) is a predominant carotenoid found in edible brown algae, such as wakame (Undaria pinnatifida), kombu (Laminaria japonica), hijiki (Hijikia fusiformis), arame (Eisena bicyclis) and Sargassum fulvellum [4,5]. Carotenoids and Fx, being hydrophobic, are absorbed at the intestinal level through the same path as dietary fats. Ingested Fx is metabolized mainly to fucoxanthinol (Fxol), which is further converted to amarouciaxanthin A in the liver [3,6]. Dietary Fx is hydrolysed to Fxol in the gastrointestinal tract by digestive enzymes, such as lipase and cholesterol esterase, and then absorbed into intestinal cells [7]. Thus, the bioactive forms of Fx in vivo are Fxol and/or amarouciaxanthin A. However, limited research covers the actions of amarouciaxanthin A in cancer cells. Nonetheless, Fx and Fxol have many beneficial health effects, including anti-mutagenic [8], anti-diabetic [9], anti-obesity [10], anti-inflammatory [11,12] and preventive actions on liver, breast, prostate, colon and lung cancers [8,13,14,15,16,17]. In this review, cancer prevention using Fx and its metabolite Fxol, as well as their possible mechanisms of action, will be discussed according to different types of cancers.
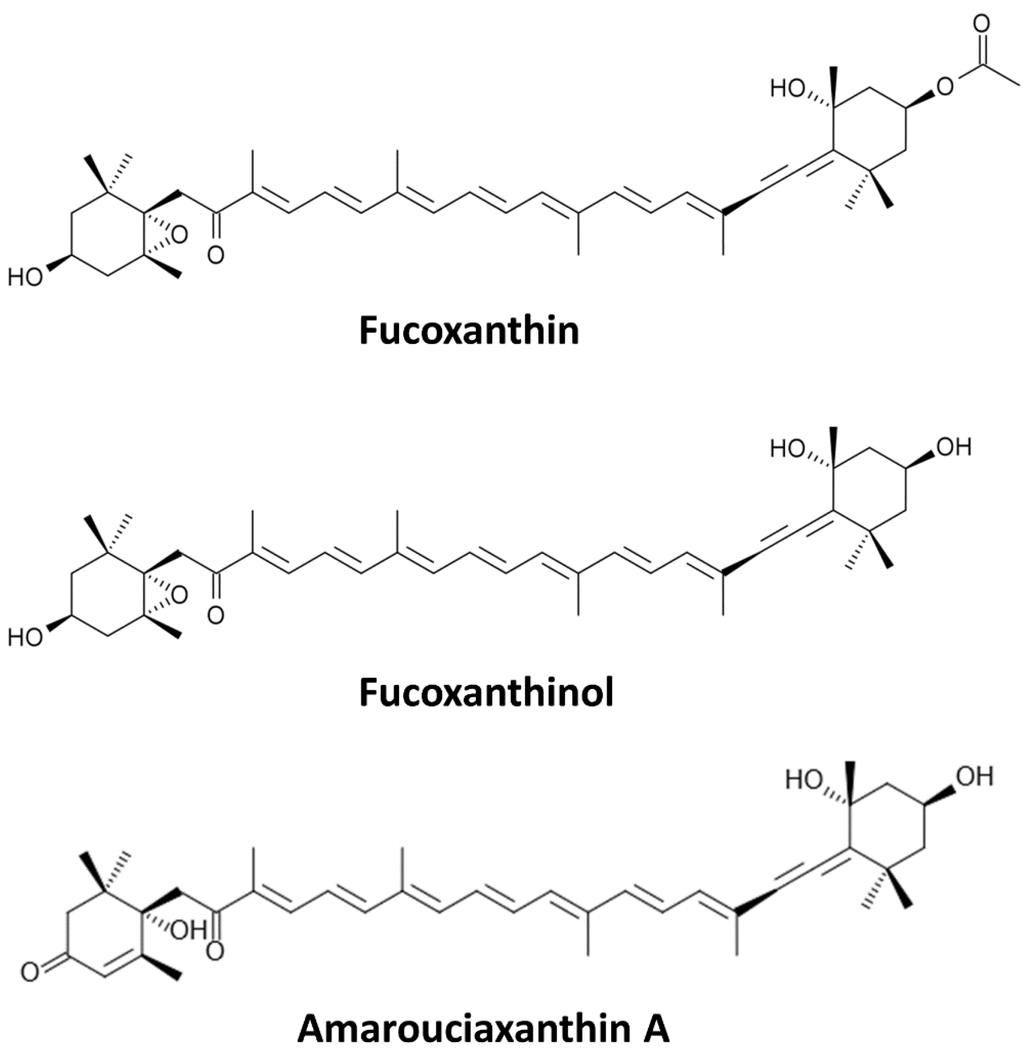
Figure 1.
Chemical structures of Fucoxanthin (Fx) and of its metabolites Fucoxanthinol (Fxol) and Amarouciaxanthin A.
Figure 1.
Chemical structures of Fucoxanthin (Fx) and of its metabolites Fucoxanthinol (Fxol) and Amarouciaxanthin A.
2. Cancer Prevention
Fx and Fxol exert their anti-proliferative and cancer preventive influences via different molecules and pathways involved in either cell cycle arrest, apoptosis, or metastasis (reviewed in [18]). In addition, Fx has been shown to have anti-angiogenic potential using human umbilical vein endothelial (HUVEC) cells [19], thus, contributing to cancer prevention. Indeed, Fx suppresses the mRNA expression of pro-angiogenic fibroblast growth factor 2 (FGF-2) and its receptor (FGFR-1) as well as their trans-activation factor, early growth response protein 1 (EGR-1), known to activate FGF-2 transcription [20]. In addition, Fx also resulted in down-regulation of the phosphorylation of FGF-2-mediated by intracellular signalling proteins, such as extracellular signal-regulated kinases (ERK1/2) and protein kinase B (Akt) [19], leading to reduced migration of endothelial cells. Fx has also been shown to inhibit cancer cells proliferation by increasing gap junction intercellular communication in human cancer cells [21], possibly leading to increased intracellular signalling promoting cell cycle arrest and apoptosis.
Having low toxicity for normal cells, Fx and its metabolites show great promises as chemopreventive and/or chemotherapeutic agents in cancer. In the next sections, the anticancer effects of Fx and Fxol will be reported according to cancer types.
2.1. Osteosarcoma
Chances of survival of osteosarcoma patients depend on prevention and treatments of metastasis. Anti-osteosarcoma properties of several carotenoids have been evaluated in cell lines. Among them, Fx and, to a greater extent, Fxol inhibited cell viabilities of Saos-2, LM8, MNNG and 143B cells [22]. Specifically, Fxol induced cell cycle arrest by reducing the expression of cyclin-dependent kinase 4 (CDK4), cyclin-dependent kinase 6 (CDK6) and cyclin E, and increased apoptosis by reducing the expressions of important anti-apoptotic mediators such as survivin, XIAP, Bcl-2 and Bcl-xL and increasing activation of caspases-3, -8 and -9 [22]. Fx treatments also inhibited the expressions of proliferative mediators, such as survivin, vascular endothelial growth factor (VEGF) and epidermal growth factor receptor (EGFR) [23].
Cell growth and survival of osteosarcoma cells is also dependent on the activity of the phosphatidylinositol 3-kinase (PI3K)-Akt pathway [24]. Akt prevents apoptosis through phosphorylation of caspase-9 and the cell cycle regulator glycogen synthase kinase-3β (GSK3β), as well as activation of NF-κB transcription factors [25,26]. Interestingly, Fxol resulted in inhibition of phosphoinositide-dependent kinase 1 (PDPK1) phosphorylation, leading to reduced phosphorylations of Akt and of its target GSK3β [22]. Being a downstream target of GSK3β, the proto-oncogene β-Catenin was also downregulated by Fxol in Saos-2 cells [22]. β-Catenin is known to regulate cell-to-cell adhesion and its mutations or overexpression are associated with the development of many types of cancers, including osteosarcoma [27,28].
Cell migration and invasiveness of osteosarcoma cells is known to be promoted by the upregulation of the expression of matrix metalloproteinase-1 (MMP-1) through activation of activator protein-1 (AP-1) transcription factors [29]. MMP-1 expression was inhibited by Fxol through reduction of AP-1 signalling in Saos-2 cells [22]. Thus, Fxol showed promising anti-metastatic activities on osteosarcoma cells by blocking AP-1, resulting in inhibition of MMP-1.
Normally, signal transducers and activators of transcription (STAT)-3/5 transcription factors contribute to tumourigenesis when deregulated [30,31]. In sarcoma cells, high concentrations of Fx (50–150 µM) has been shown to inhibit STAT3 expression and phosphorylation [23]. Anti-tumour effects of Fx have also been investigated on xenografted sarcoma 180 in mice, resulting in significant growth inhibition with increased apoptosis as a result of epidermal growth factor receptor (EGFR)/Janus kinase (JAK)/STAT signalling disruption [23]. Therefore, Fx, and possibly Fxol, may prevent sarcoma development through modulation of numerous signalling pathways involved in tumourigenesis and metastasis.
2.2. Leukemia and Lymphoma
Hematologic cancers are the most common malignancies of childhood. Leukemia is characterized by an increased cell division of white blood cells, leading to impairment in the maintenance of red blood cells population. Unlike leukemia, lymphoma specifically affects the lymph nodes. Fx and Fxol have promising inhibitory effects on proliferation of these cancers. Indeed, these compounds have been shown to trigger apoptosis through induced procaspase-3, -7, and poly (ADP-ribose) polymerase (PARP) cleavages and through regulation of Bcl expression (Bcl-2 and Bcl-xL) in human leukemia (HL-60) cancer cells [32,33]. However, others have shown that Fx treatment of these cells increased cleavages of procaspase-3 and PARP without any effect on the protein levels of anti-apoptotic Bcl-2, Bcl-xL, or pro-apoptotic Bax [34].
In primary effusion lymphoma cells, a rare and highly aggressive non-Hodgkin’s lymphoma caused by human herpesvirus 8, Fxol-induced suppression of cell viability was more pronounced than that of Fx [35]. Fx and Fxol also induced cell cycle arrest in G0/G1 phase, increased caspase-dependent apoptosis, inhibited the activation of NF-κB, AP-1 and Akt, and down-regulated anti-apoptotic proteins (Bcl-xL and XIAP) in these cells [35]. Consistent with these results, Fx has also been shown to inhibit the nuclear translocation of p50 and p65 proteins in lipopolysaccharide (LPS)-stimulated RAW 264.7 macrophages, resulting in lower levels of nuclear transactivation by NF-κB transcription factors [11,36].
Adult T-cell leukemia (ATL) is a fatal malignancy of T lymphocytes caused by human T-cell leukemia virus type 1 (HTLV-1) infection and remains incurable. Interestingly, Fx and Fxol inhibited cell viability of HTLV-1-infected T-cell lines and ATL cells, with a higher potency of Fxol compared to Fx [37]. Both carotenoids induced cell cycle arrest during G0/G1 phase by reducing the expression of cyclin D1, cyclin D2, CDK4 and CDK6, and inducing the expression of Growth Arrest and DNA Damage 45 alpha (GADD45α) [37]. Being involved in DNA repair, cell cycle control, senescence, genotoxic stress and having pro-apoptotic activities, GADD45α is known to have multiple inhibitory effects on tumourigenesis [38]. Fx and Fxol also induced apoptosis of ATL cells by reducing the expressions of anti-apoptotic Bcl-2, XIAP, cIAP2 and survivin and activation of procaspase-3, -8 and -9 [37]. Fx and Fxol suppressed IκBα phosphorylation and JunD expression, resulting in inactivations of NF-κB and AP-1 [37]. Confirming these results, mice with severe combined immunodeficiency and harboring tumours induced by inoculation of HTLV-1-infected T cells responded to treatment with Fxol by suppression of tumour growth [37], thus suggesting that Fx and Fxol could be useful therapeutic agents for patients with ATL.
Effects of Fx and Fxol on B-cell malignancies, such as Burkitt’s lymphoma, Hodgkin’s lymphoma and Epstein-Barr virus-immortalized B cells have been investigated. Fx and, with a higher efficiency, Fxol, reduced viability of these malignant B cells in a dose-dependent manner with increased cell cycle arrest during G0/G1 phase and caspase-dependent apoptosis [39]. In these cells, Fxol inhibited NF-κB activity, leading to downregulation of NF-κB-dependent anti-apoptotic and cell cycle regulator genes, such as Bcl-2, cIAP-2, XIAP, cyclin D1 and cyclin D2 [39]. Accordingly, Fx and Fxol could be considered as anti-proliferative or preventive agents against leukemia and lymphomas.
2.3. Lung Cancer
Lung cancer is one of the most common cancers worldwide and is still a leading cause of cancer death in North America. Although carotenoid levels are well known to be associated with a lower risk of lung cancer death [40], inhibitory actions of Fx and Fxol on this type of cancer have been barely investigated. However, in experimental lung metastasis in vivo assays, Fx resulted in reduced tumour nodules formation [41]. In addition, extracts from New Zealand seaweed Undaria pinnatifida containing low levels of Fx were found to have inhibitory effects on growth of human lung carcinoma cell lines A549 and NCI-H522 [42]. Thus, more studies using Fx and/or Fxol as preventive agents against lung cancer metastasis may be promising to lower the important death rate associated with such complications.
2.4. Prostate Cancer
With the increased life expectancy, it is anticipated that the diagnosis of prostate cancer will increase by 55% between 2010 and 2030 [43]. Fx has been shown to reduce viability and induce apoptosis in human prostate cancer cells (PC-3, DU 145 and LNCaP) [44]. As in HepG2 liver cancer cells, Fx was also shown to induce cell cycle arrest in G1 and increase GADD45 gene expression in DU145 and LNCaP cells, possibly through activation of c-Jun N-terminal kinase (SAPK/JNK) [45,46,47]. This subfamily member of the mitogen-activated protein kinases (MAPKs) is known to be an upstream activator of GADD45 expression [48]. In addition, Fx has been shown to induce procaspase-3 and PARP cleavages, reduce the expression of anti-apoptotic Blc-2 and, surprisingly, of pro-apoptotic Bax proteins, leading to increased apoptosis of human prostate cancer PC-3 cells [17]. Interestingly, Fx was converted to Fxol within treated PC-3 cells [17]. Compared to Fx, Fxol had a stronger anti-proliferative effect on PC-3 cells [6]. Hence, effects of Fx on prostate cancer cells apoptosis may rather be attributed to Fxol and such conversion of Fx to Fxol is normally limited to the gastrointestinal tract.
2.5. Gastrointestinal Cancer
With recent changes in nutritional habits of Asians, gastrointestinal cancer incidence is increasing at an alarming rate in this population [49]. As in other cancers, Fx has been shown to suppress the level of Bcl-2 protein and induce apoptosis in human colon cancer cells (Caco-2, HT-29 and DLD-1) [50]. In addition, Fxol also had a stronger anti-proliferative effect than Fx on Caco-2 human colon cancer cells [33].
Cell proliferation may be associated with retinoblastoma protein (Rp) phosphorylation, releasing E2F transcription factors and promoting G1 to S phase transition [51,52]. In WiDr human colon adenocarcinoma cells, Fx may induce cell cycle arrest during the G0/G1 phase and apoptosis through inhibition of Rb phosphorylation and increased the expression of a CDK and proliferating cell nuclear antigen (PCNA) inhibitory protein, p21WAF1/Cip1 [16]. As in sarcoma, high concentrations of Fx (50–150 µM) also inhibited STAT3 expression and phosphorylation, resulting in downregulation of cyclin B1 in gastric adenocarcinoma cells [53].
Interestingly, Fx has been shown to reverse multidrug (such as 5-FU, vinblastine and etoposide) resistance of Caco-2 cells by interfering with ATP-binding cassette (ABC) transporters [54], an important strategy to overcome multidrug resistance in cancer pharmacotherapy [55]. Thus, Fx may be used to sensitize colon cancer cells and possibly other types of cancers to chemotherapeutic drugs.
2.6. Liver Cancer
Liver cancer is one of the most serious types of cancer with a five year survival rate of 14% after diagnosis [56]. Fx has been shown to significantly inhibit proliferation of human hepatoma HepG2 cells by 28% after 48 h of incubation with a 10 μM dose [57]. Growth inhibition of HepG2 cells by Fx was triggered by induction of cell cycle arrest in the G0/G1 phase, down-regulation of cyclin D/CDK4 complex and decreased phosphorylation of the retinoblastoma protein (Rb) [58]. In addition, others have also shown that Fx treatments resulted in inhibition of p38 MAPK and increased GADD45α expression [45]. Interestingly, Fx also inhibited proliferation of SK-Hep-1 human hepatoma cells through G1 cell cycle arrest and increased apoptosis, whereas murine embryonic hepatic (BNL CL.2) cells growth was enhanced [21]. Thus, antiproliferative actions of Fx were selective to liver cancer cells compared to normal embryonic hepatic cells.
These results may be explained, in part, by the influence of Fx on cell-to-cell communication. Indeed, Fx enhanced gap junctional intercellular communication of SK-Hep-1 cells by increasing connexin43 and connexin32 expressions, but not of mouse liver BNL CL.2 cells [21]. Increased cell-to-cell communication may result in modulation of cytosolic calcium levels, contributing to cell cycle arrest and apoptosis.
Drug resistance is a major inconvenient lowering the efficacy of chemotherapy. The Pregnane X receptor (PXR) is known to regulate the expression of drug-metabolizing enzymes, such as the cytochrome P450 3A (CYP3A) family, and transporters, like multiple drug resistance 1 (MDR1), thus promoting drug resistance [59]. In addition, PXR activation was found to increase tumour aggressiveness of primary human colon cancer tissue xenografted into immunodeficient mice [60]. Interestingly, Fx has been shown to attenuate rifampicin-induced CYP3A4 and MDR1 gene expressions through inhibition of PXR interaction with the steroid receptor coactivator-1 (SRC-1) in HepG2 cells [61]. Moreover, when combined with the anticancer drug cisplatin, Fx further decreased HepG2 cells proliferation [57]. To do this, Fx inhibited cisplatin-induced expressions of NF-κB, excision repair cross complementation 1 (ERCC1) and thymidine phosphorylase (TP) [57], leading to improvement of chemotherapeutic efficacy of cisplatin by activation of apoptosis, inhibition of DNA repair and promoting tumour regression. As in gastrointestinal cancer, Fx may also be used to sensitize liver cancer, and possibly other types of cancers, to chemotherapeutic treatments.
Although numerous effects of Fx on liver cancer have been reported, it is important to recall that dietary Fx is hydrolysed into Fxol in the gastrointestinal tract before absorption in the intestine. Then, hepatocytes and HepG2 are able to convert Fxol into amarouciaxanthin A [6], suggesting that the latter compound may be responsible, in part, for the anti-proliferative effects of Fx in liver cancer.
2.7. Bladder Cancer
Urinary bladder cancer development, influenced by genetic and environmental factors, often results into multiple tumours appearing at different times and different locations within the bladder. Fx reduced viability and triggered apoptosis of human bladder cancer EJ-1 cells as a result of caspase-3 activation [62]. Fx also inhibited human T24 bladder cancer cells proliferation in a dose- and time-dependent manner through growth arrest at G0/G1 phase of cell cycle, which is mediated by the up-regulation of p21, a cyclin-dependent kinase (CDK)-inhibitory protein and the down-regulation of CDK-2, CDK-4, cyclin D1, and cyclin E [63]. In addition, high levels of Fx induced apoptosis of T24 cells by the abrogation of mitochondrial mortalin-p53 complex, translocation and transcriptional activation of p53 [63]. Mortalin is a stress regulator and a member of heat shock protein 70 known to induce cell differentiation. Thus, these fragmentary results suggest that Fx, and possibly Fxol, may act as chemopreventive agents against bladder cancer development.
2.8. Skin Cancer
Since 1970, skin cancer had the second highest increase in mortality rate in Canada [64]. Fx has been shown to inhibit metastatic potential of melanoma cancer cells [41]. Metastasis is a major determinant of mortality in malignant cancers. Fx inhibited the expression and secretion of matrix metalloproteinase-9 (MMP-9), linked to tumour invasion and migration, and also suppressed invasion of highly metastatic B16-F10 melanoma cells [41]. In addition, Fx also reduced the expressions of cell surface glycoprotein CD44 and CXC chemokine receptor-4 (CXCR4), known to be involved in migration, invasion and cancer-endothelial cell adhesion [41]. Hence, the inhibitory effects of Fx on melanoma cells’ adhesion to endothelial cells strongly support its anti-metastatic potential.
In addition, Fx caused cell cycle arrest of B16-F10 cells through decreased protein expressions of phosphorylated-Rb, cyclin D (1 and 2) and CDK4 as well as up-regulation of protein levels for p15(INK4B) and p27(Kip1), which are important cyclin-dependent kinase inhibitors involved in repression of tumourigenesis [65]. Fx also induced apoptosis of these cells through down-regulation of Bcl-xL, resulting in activations of caspase-9, caspase-3, and PARP [65].
In melanoma and UVB-induced skin pigmentation, Fx inhibited tyrosinase activity and melanogenesis, whereas topical applications suppressed cyclooxygenase (COX)-2, endothelin receptor A, p75 neurotrophin receptor (NTR), prostaglandin E receptor 1 (EP1), melanocortin 1 receptor (MC1R) and tyrosinase-related protein 1 (Tyrp1) mRNA expressions [66]. These are all stimulants of melanogenesis of epithelial cells. Such inhibitory effects of Fx on melanogenesis may be attributed to suppression of prostaglandin E2 (PGE2) synthesis and of melanocyte-stimulating hormone receptor (MC1R), resulting in reduced phospholipase C and PKA signalling and leading to suppression of Tyrp1 expression and melanin formation. Altogether, these results suggest that Fx may also be used as an anti-pigmentary ingredient for cosmetics.
2.9. Cervical Cancer
Cervical cancer arises from malignant cells originating from the cervix and is among the most prevalent cancers in women. The enzyme phosphatidylinositol 3-kinase (PI3K) is known to be overexpressed in cervical cancer, leading to phosphorylation of Akt and activation of mTOR signalling. Interestingly, Fx has been shown to induce apoptosis in human cervical cancer HeLa cells through inhibition of the PI3K/Akt/mTOR pathway [67,68]. Fx also increased expressions of important mediators of autophagy, LC3 II and Beclin 1 [67], potentially leading to non-apoptotic cell death. Accordingly, Fx and possibly Fxol could be used as a preventive agent against cervical cancer in highly predisposed women.
2.10. Breast Cancer
Breast cancer is the most common cancer diagnosed in women worldwide. Several studies have linked diets rich in carotenoids with reduced risk of chronic diseases and cancers, including breast cancer [69,70,71]. Indeed, Fx has been shown to be highly cytotoxic to MCF-7 cells [72]. In addition, our research group have shown that 10–20 µM Fx and Fxol have inhibitory effects on cell viability and apoptosis-inducing effects on two human breast cancer cell lines, MCF-7 and MDA-MB-231 [73,74]. Fxol induced apoptosis of breast cancer cells MDA-MB-231 and MCF-7 by increased cleavages of procaspase-3 and PARP [33,73].
In our research, we looked at possible differences in the inhibitory actions of Fx and Fxol on components of the NF-κB pathway between estrogen sensitive MCF-7 and estrogen resistant MDA-MB-231 breast cancer cell lines. Estrogen receptors play an important role in breast cancer; women with ER positive tumours have an overall better prognosis and are more likely to have their tumours respond to therapy. However, in up to 25% of cases, ER positive tumours are non-responsive to therapy as a result of acquired resistance, possibly linked to constitutive NF-κB leading to estrogen-independent growth [75,76,77]. Indeed, constitutive nuclear localization of p50, p52, c-Rel, and overexpression of p100/p52 in breast cancer have been reported [78,79]. Among NF-κB members, p65 and p50 are constitutively active and overexpressed in breast cancer cells [80], resulting in increased transcription of anti-apoptotic genes [81]. Major differences were observed in the inhibitory mechanisms between Fx and Fxol on members of the NF-κB pathway in breast cancer cell lines. Interestingly, the apoptosis-inducing activities of Fxol were more potent than that of Fx and were correlated, for hormone independent MDA-MB-231 cells, to inhibitory actions on members of the NF-κB pathway p65, p50, RelB, and p52/p100 [73,74]. Thus, enhanced sensitivity of breast tumour cells to apoptosis in response to Fx/Fxol may be associated in part with inhibitory actions on the NF-κB pathway [82,83]. Fxol treatment may contribute to reduce viability of aggressive estrogen-independent tumour growth and may involve inhibitions of nuclear translocation and transcriptional activity of members of the NF-κB pathway. However, more research will be required to clearly establish a regulatory action of Fxol on members of NF-κB.
Being overexpressed and regulated by NF-κB in different types of cancers [84,85,86], SOX9 expression is highly correlated to invasiveness and poor clinical outcome of breast cancer. Indeed, cytoplasmic accumulation of SOX9 has been shown to increase in invasive ductal carcinoma and metastatic breast cancer [87]. The transcription factor SOX9 have been identified as a downstream target of different signalling pathways contributing to breast cancer aggressiveness [88,89,90,91,92,93]. In MDA-MB-231, SOX9 was decreased at the nuclear level by Fx and Fxol [73]. Hence, SOX9 may be involved in MDA-MB-231 cells proliferation and downregulation of its expression and/or phosphorylation may contribute to the inhibitory effects of Fx and Fxol on viability of estrogen resistant breast cancers.
3. Conclusions
As summarized in Figure 2, anticancer effects of Fx and Fxol are mediated through several mechanisms including inhibition of cell proliferation, induction of apoptosis, cell cycle arrest and anti-angiogenesis. In addition, combined treatments of Fx or Fxol with anticancer drugs may lead to important new therapeutic strategies having limited multi-drug resistance against many types of cancers. According to current results, Fx and Fxol can induce growth inhibition and apoptosis in different cancer cell types; however, the effective concentrations differ among cancers, and the detailed molecular mechanisms remain to be elucidated. Most dietary Fx may be converted to Fxol, and the latter may exert a suppressive effect on cancer cells more efficiently than Fx in vivo. Taken together, current findings suggest that Fxol and Fx could be potentially effective for the treatment and/or prevention of different types of cancers.
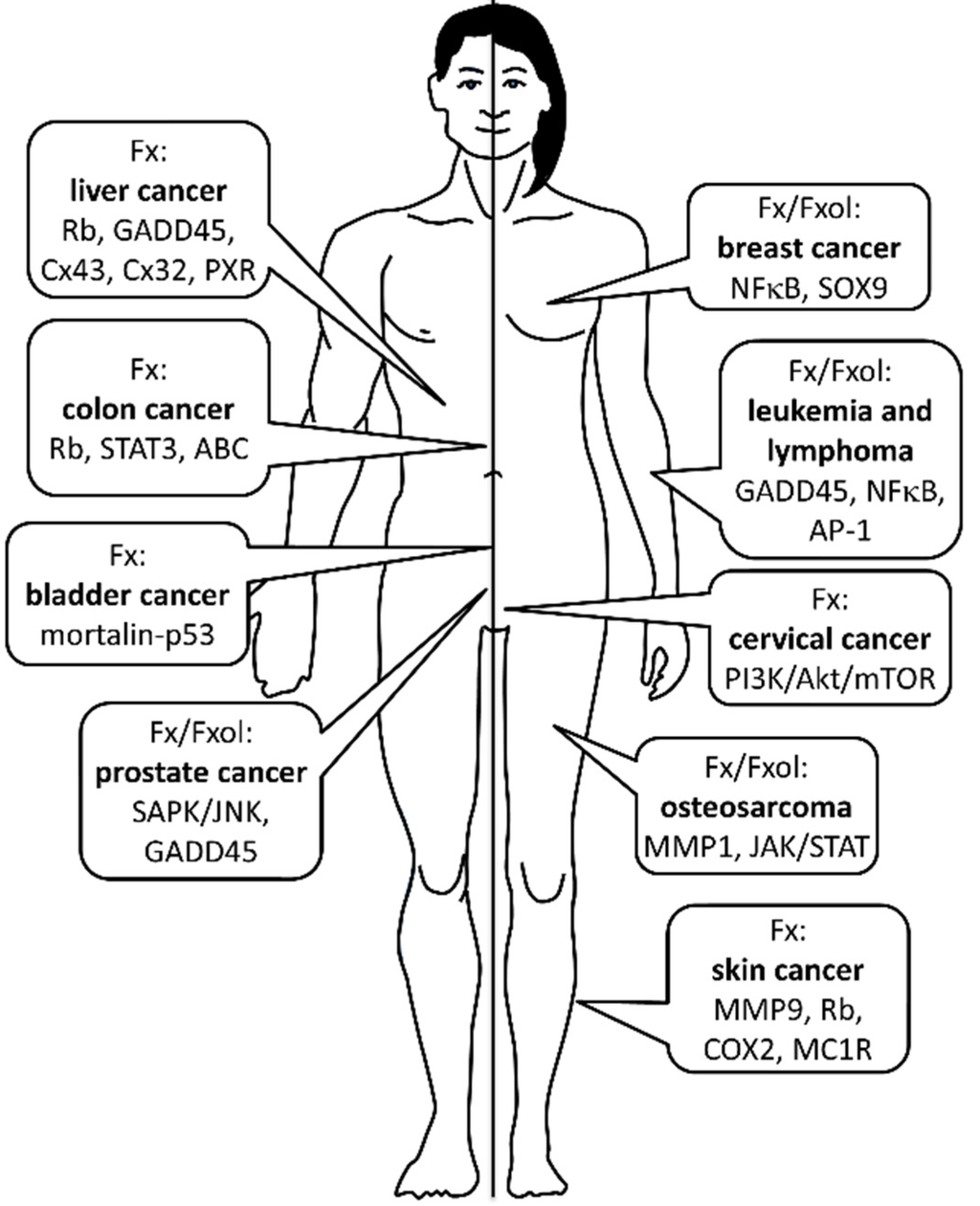
Figure 2.
Anticancer effects of Fucoxanthin (Fx) and Fucoxanthinol (Fxol) according to different types of cancers. Most important molecular pathways involved in Fx/Fxol’s actions are also depicted. No sex differences have been made regarding the efficiency Fx/Fxol against any types of cancers. See text for more details.
Figure 2.
Anticancer effects of Fucoxanthin (Fx) and Fucoxanthinol (Fxol) according to different types of cancers. Most important molecular pathways involved in Fx/Fxol’s actions are also depicted. No sex differences have been made regarding the efficiency Fx/Fxol against any types of cancers. See text for more details.
Acknowledgments
Current work was funded by the Canadian Breast Cancer Foundation (CBCF) and the New Brunswick Health Research Foundation (NBHRF), Canada.
Conflicts of Interest
The author declares no conflict of interest.
References
- Matsuno, T. Aquatic animal carotenoids. Fish. Sci. 2001, 67, 771–783. [Google Scholar] [CrossRef]
- Beppu, F.; Niwano, Y.; Tsukui, T.; Hosokawa, M.; Miyashita, K. Single and repeated oral dose toxicity study of fucoxanthin (FX), a marine carotenoid, in mice. J. Toxicol. Sci. 2009, 34, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Beppu, F.; Niwano, Y.; Sato, E.; Kohno, M.; Tsukui, T.; Hosokawa, M.; Miyashita, K. In vitro and in vivo evaluation of mutagenicity of fucoxanthin (FX) and its metabolite fucoxanthinol (FXOH). J. Toxicol. Sci. 2009, 34, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Airanthi, M.K.W.A.; Hosokawa, M.; Miyashita, K. Comparative antioxidant activity of edible Japanese brown seaweeds. J. Food Sci. 2011, 76, C104–C111. [Google Scholar] [CrossRef] [PubMed]
- D’Orazio, N.; Gemello, E.; Gammone, M.A.; de Girolamo, M.; Ficoneri, C.; Riccioni, G. Fucoxantin: A treasure from the sea. Mar. Drugs 2012, 10, 604–616. [Google Scholar] [CrossRef] [PubMed]
- Asai, A.; Sugawara, T.; Ono, H.; Nagao, A. Biotransformation of fucoxanthinol into amarouciaxanthin A in mice and HepG2 cells: Formation and cytotoxicity of fucoxanthin metabolites. Drug Metab. Dispos. 2004, 32, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, T.; Baskaran, V.; Tsuzuki, W.; Nagao, A. Brown algae fucoxanthin is hydrolyzed to fucoxanthinol during absorption by Caco-2 human intestinal cells and mice. J. Nutr. 2002, 132, 946–951. [Google Scholar] [PubMed]
- Nishino, H.; Murakoshi, M.; Tokuda, H.; Satomi, Y. Cancer prevention by carotenoids. Arch. Biochem. Biophys. 2009, 483, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, S.; Hosokawa, M.; Miyashita, K. Fucoxanthin promotes translocation and induction of glucose transporter 4 in skeletal muscles of diabetic/obese KK-Ay mice. Phytomedicine 2012, 19, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Funayama, K.; Miyashita, K. Fucoxanthin from edible seaweed, Undaria pinnatifida, shows antiobesity effect through UCP1 expression in white adipose tissues. Biochem. Biophys. Res. Commun. 2005, 332, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.N.; Heo, S.J.; Yoon, W.J.; Kang, S.M.; Ahn, G.; Yi, T.H.; Jeon, Y.J. Fucoxanthin inhibits the inflammatory response by suppressing the activation of NF-κB and MAPKs in lipopolysaccharide-induced RAW 264.7 macrophages. Eur. J. Pharmacol. 2010, 649, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Shiratori, K.; Ohgami, K.; Ilieva, I.; Jin, X.H.; Koyama, Y.; Miyashita, K.; Yoshida, K.; Kase, S.; Ohno, S. Effects of fucoxanthin on lipopolysaccharide-induced inflammation in vitro and in vivo. Exp. Eye Res. 2005, 81, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Hunter, D.J.; Forman, M.R.; Rosner, B.A.; Speizer, F.E.; Colditz, G.A.; Manson, J.E.; Hankinson, S.E.; Willett, W.C. Dietary carotenoids and vitamins A, C, and E and risk of breast cancer. J. Natl. Cancer Inst. 1999, 91, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Slattery, M.L.; Benson, J.; Curtin, K.; Ma, K.N.; Schaeffer, D.; Potter, J.D. Carotenoids and colon cancer. Am. J. Clin. Nutr. 2000, 71, 575–582. [Google Scholar] [PubMed]
- Le Marchand, L.; Hankin, J.H.; Kolonel, L.N.; Beecher, G.R.; Wilkens, L.R.; Zhao, L.P. Intake of specific carotenoids and lung cancer risk. Cancer Epidemiol. Biomark. Prev. 1993, 2, 183–187. [Google Scholar]
- Das, S.K.; Hashimoto, T.; Shimizu, K.; Yoshida, T.; Sakai, T.; Sowa, Y.; Komoto, A.; Kanazawa, K. Fucoxanthin induces cell cycle arrest at G0/G1 phase in human colon carcinoma cells through up-regulation of p21WAF1/Cip1. Biochim. Biophys. Acta 2005, 1726, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Kotake-Nara, E.; Asai, A.; Nagao, A. Neoxanthin and fucoxanthin induce apoptosis in PC-3 human prostate cancer cells. Cancer Lett. 2005, 220, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.R.; Hosokawa, M.; Miyashita, K. Fucoxanthin: A marine carotenoid exerting anti-cancer effects by affecting multiple mechanisms. Mar. Drugs 2013, 11, 5130–5147. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, P.; Matsubara, K.; Sugawara, T.; Hirata, T. Marine algal carotenoids inhibit angiogenesis by down-regulating FGF-2-mediated intracellular signals in vascular endothelial cells. Mol. Cell. Biochem. 2013, 380. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Mayo, M.W.; Baldwin, A.S. Basic fibroblast growth factor transcriptional autoregulation requires EGR-1. Oncogene 1997, 14, 2291–2299. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.L.; Huang, Y.S.; Hosokawa, M.; Miyashita, K.; Hu, M.L. Inhibition of proliferation of a hepatoma cell line by fucoxanthin in relation to cell cycle arrest and enhanced gap junctional intercellular communication. Chem. Biol. Interact. 2009, 182, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Rokkaku, T.; Kimura, R.; Ishikawa, C.; Yasumoto, T.; Senba, M.; Kanaya, F.; Mori, N. Anticancer effects of marine carotenoids, fucoxanthin and its deacetylated product, fucoxanthinol, on osteosarcoma. Int. J. Oncol. 2013, 43, 1176–1186. [Google Scholar] [PubMed]
- Wang, J.; Chen, S.; Xu, S.; Yu, X.; Ma, D.; Hu, X.; Cao, X. In vivo induction of apoptosis by fucoxanthin, a marine carotenoid, associated with down-regulating STAT3/EGFR signaling in sarcoma 180 (S180) xenografts-bearing mice. Mar. Drugs 2012, 10, 2055–2068. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Pang, R.P.; Shen, J.N.; Huang, G.; Wang, J.; Zhou, J.G. Grifolin induces apoptosis via inhibition of PI3K/AKT signalling pathway in human osteosarcoma cells. Apoptosis 2007, 12, 1317–1326. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, E.; Oki, E.; Egashira, A.; Sadanaga, N.; Morita, M.; Kakeji, Y.; Maehara, Y. Deregulation of the Akt pathway in human cancer. Curr. Cancer Drug Targets 2008, 8, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Romashkova, J.A.; Makarov, S.S. NF-κB is a target of AKT in anti-apoptotic PDGF signalling. Nature 1999, 401, 86–90. [Google Scholar] [PubMed]
- Morin, P.J. β-Catenin signaling and cancer. BioEssays 1999, 21, 1021–1030. [Google Scholar] [CrossRef]
- Behrens, J. Control of β-Catenin signaling in tumor development. Ann. N. Y. Acad. Sci. 2000, 910, 21–33; discussion 33–35. [Google Scholar] [CrossRef] [PubMed]
- Kimura, R.; Ishikawa, C.; Rokkaku, T.; Janknecht, R.; Mori, N. Phosphorylated c-Jun and Fra-1 induce matrix metalloproteinase-1 and thereby regulate invasion activity of 143B osteosarcoma cells. Biochim. Biophys. Acta 2011, 1813, 1543–1553. [Google Scholar] [CrossRef] [PubMed]
- Desrivières, S.; Kunz, C.; Barash, I.; Vafaizadeh, V.; Borghouts, C.; Groner, B. The biological functions of the versatile transcription factors STAT3 and STAT5 and new strategies for their targeted inhibition. J. Mammary Gland Biol. Neoplasia 2006, 11, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Kisseleva, T.; Bhattacharya, S.; Braunstein, J.; Schindler, C.W. Signaling through the JAK/STAT pathway, recent advances and future challenges. Gene 2002, 285, 1–24. [Google Scholar] [CrossRef]
- Kim, K.N.; Heo, S.J.; Kang, S.M.; Ahn, G.; Jeon, Y.J. Fucoxanthin induces apoptosis in human leukemia HL-60 cells through a ROS-mediated Bcl-xL pathway. Toxicol. Vitro 2010, 24, 1648–1654. [Google Scholar] [CrossRef] [PubMed]
- Konishi, I.; Hosokawa, M.; Sashima, T.; Kobayashi, H.; Miyashita, K. Halocynthiaxanthin and fucoxanthinol isolated from Halocynthia roretzi induce apoptosis in human leukemia, breast and colon cancer cells. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2006, 142, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Kotake-Nara, E.; Terasaki, M.; Nagao, A. Characterization of apoptosis induced by fucoxanthin in human promyelocytic leukemia cells. Biosci. Biotechnol. Biochem. 2005, 69, 224–227. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Ishikawa, C.; Katano, H.; Yasumoto, T.; Mori, N. Fucoxanthin and its deacetylated product, fucoxanthinol, induce apoptosis of primary effusion lymphomas. Cancer Lett. 2011, 300, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Lee, M.S.; Choi, H.J.; Choi, J.W.; Shin, T.; Woo, H.C.; Kim, J.I.; Kim, H.R. Hexane fraction from Laminaria japonica exerts anti-inflammatory effects on lipopolysaccharide-stimulated RAW 264.7 macrophages via inhibiting NF-κB pathway. Eur. J. Nutr. 2013, 52, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, C.; Tafuku, S.; Kadekaru, T.; Sawada, S.; Tomita, M.; Okudaira, T.; Nakazato, T.; Toda, T.; Uchihara, J.N.; Taira, N.; et al. Anti-adult T-cell leukemia effects of brown algae fucoxanthin and its deacetylated product, fucoxanthinol. Int. J. Cancer 2008, 123, 2702–2712. [Google Scholar] [CrossRef] [PubMed]
- Tamura, R.E.; de Vasconcellos, J.F.; Sarkar, D.; Libermann, T.A.; Fisher, P.B.; Zerbini, L.F. GADD45 proteins: Central players in tumorigenesis. Curr. Mol. Med. 2012, 12, 634–651. [Google Scholar] [CrossRef] [PubMed]
- Tafuku, S.; Ishikawa, C.; Yasumoto, T.; Mori, N. Anti-neoplastic effects of fucoxanthin and its deacetylated product, fucoxanthinol, on Burkitt’s and Hodgkin’s lymphoma cells. Oncol. Rep. 2012, 28, 1512–1518. [Google Scholar] [PubMed]
- Min, K.B.; Min, J.Y. Serum carotenoid levels and risk of lung cancer death in US adults. Cancer Sci. 2014, 105, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Chung, T.W.; Choi, H.J.; Lee, J.Y.; Jeong, H.S.; Kim, C.H.; Joo, M.; Choi, J.Y.; Han, C.W.; Kim, S.Y.; Choi, J.S.; et al. Marine algal fucoxanthin inhibits the metastatic potential of cancer cells. Biochem. Biophys. Res. Commun. 2013, 439, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.K.; Li, Y.; White, W.L.; Lu, J. Extracts from New Zealand Undaria pinnatifida Containing Fucoxanthin as Potential Functional Biomaterials against Cancer in Vitro. J. Funct. Biomater. 2014, 5, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.D.; Smith, G.L.; Hurria, A.; Hortobagyi, G.N.; Buchholz, T.A. Future of cancer incidence in the United States: Burdens upon an aging, changing nation. J. Clin. Oncol. 2009, 27, 2758–2765. [Google Scholar] [CrossRef] [PubMed]
- Kotake-Nara, E.; Kushiro, M.; Zhang, H.; Sugawara, T.; Miyashita, K.; Nagao, A. Carotenoids affect proliferation of human prostate cancer cells. J. Nutr. 2001, 131, 3303–3306. [Google Scholar] [PubMed]
- Satomi, Y.; Nishino, H. Implication of mitogen-activated protein kinase in the induction of G1 cell cycle arrest and gadd45 expression by the carotenoid fucoxanthin in human cancer cells. Biochim. Biophys. Acta 2009, 1790, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Satomi, Y. Fucoxanthin induces GADD45A expression and G1 arrest with SAPK/JNK activation in LNCap human prostate cancer cells. Anticancer Res. 2012, 32, 807–813. [Google Scholar] [PubMed]
- Yoshiko, S.; Hoyoku, N. Fucoxanthin, a natural carotenoid, induces G1 arrest and GADD45 gene expression in human cancer cells. In Vivo 2007, 21, 305–309. [Google Scholar] [PubMed]
- Yin, F.; Bruemmer, D.; Blaschke, F.; Hsueh, W.A.; Law, R.E.; Herle, A.J.V. Signaling pathways involved in induction of GADD45 gene expression and apoptosis by troglitazone in human MCF-7 breast carcinoma cells. Oncogene 2004, 23, 4614–4623. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Dhakal, I.B.; Zhao, Z.; Li, L. Trends in mortality from cancers of the breast, colon, prostate, esophagus, and stomach in East Asia: Role of nutrition transition. Eur. J. Cancer Prev. 2012, 21, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, M.; Kudo, M.; Maeda, H.; Kohno, H.; Tanaka, T.; Miyashita, K. Fucoxanthin induces apoptosis and enhances the antiproliferative effect of the PPARgamma ligand, troglitazone, on colon cancer cells. Biochim. Biophys. Acta 2004, 1675, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Duronio, R.J.; Xiong, Y. Signaling pathways that control cell proliferation. Cold Spring Harb. Perspect. Biol. 2013, 5, a008904. [Google Scholar] [CrossRef] [PubMed]
- Dick, F.A.; Rubin, S.M. Molecular mechanisms underlying RB protein function. Nat. Rev. Mol. Cell Biol. 2013, 14, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.X.; Hu, X.M.; Xu, S.Q.; Jiang, Z.J.; Yang, W. Effects of fucoxanthin on proliferation and apoptosis in human gastric adenocarcinoma MGC-803 cells via JAK/STAT signal pathway. Eur. J. Pharmacol. 2011, 657, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Eid, S.Y.; El-Readi, M.Z.; Wink, M. Carotenoids reverse multidrug resistance in cancer cells by interfering with ABC-transporters. Phytomedicine 2012, 19, 977–987. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Yu, A.M. ABC transporters in multidrug resistance and pharmacokinetics, and strategies for drug development. Curr. Pharm. Des. 2014, 20, 793–807. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics, 2012. CA Cancer J. Clin. 2012, 62, 10–29. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.L.; Lim, Y.P.; Hu, M.L. Fucoxanthin enhances cisplatin-induced cytotoxicity via NFκB-mediated pathway and downregulates DNA repair gene expression in human hepatoma HepG2 cells. Mar. Drugs 2013, 11, 50–66. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Hashimoto, T.; Kanazawa, K. Growth inhibition of human hepatic carcinoma HepG2 cells by fucoxanthin is associated with down-regulation of cyclin D. Biochim. Biophys. Acta 2008, 1780, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Pondugula, S.R.; Mani, S. Pregnane xenobiotic receptor in cancer pathogenesis and therapeutic response. Cancer Lett. 2013, 328, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Venkatesh, M.; Li, H.; Goetz, R.; Mukherjee, S.; Biswas, A.; Zhu, L.; Kaubisch, A.; Wang, L.; Pullman, J.; et al. Pregnane X receptor activation induces FGF19-dependent tumor aggressiveness in humans and mice. J. Clin. Investig. 2011, 121, 3220–3232. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.L.; Lim, Y.P.; Hu, M.L. Fucoxanthin attenuates rifampin-induced cytochrome P450 3A4 (CYP3A4) and multiple drug resistance 1 (MDR1) gene expression through pregnane X receptor (PXR)-mediated pathways in human hepatoma HepG2 and colon adenocarcinoma LS174T cells. Mar. Drugs 2012, 10, 242–257. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, P.; Hamada, M.; Takahashi, S.; Xing, G.; Liu, J.; Sugiura, N. Potential chemoprevention effect of dietary fucoxanthin on urinary bladder cancer EJ-1 cell line. Oncol. Rep. 2008, 20, 1099–1103. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zeng, Y.; Liu, Y.; Hu, X.; Li, S.; Wang, Y.; Li, L.; Lei, Z.; Zhang, Z. Fucoxanthin induces growth arrest and apoptosis in human bladder cancer T24 cells by up-regulation of p21 and down-regulation of mortalin. Acta Biochim. Biophys. Sin. 2014, 46, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Kachuri, L.; De, P.; Ellison, L.F.; Semenciw, R. Advisory Committee on Canadian Cancer Statistics Cancer incidence, mortality and survival trends in Canada, 1970–2007. Chronic Dis. Inj. Can. 2013, 33, 69–80. [Google Scholar] [PubMed]
- Kim, K.-N.; Ahn, G.; Heo, S.J.; Kang, S.M.; Kang, M.C.; Yang, H.M.; Kim, D.; Roh, S.W.; Kim, S.K.; Jeon, B.T.; et al. Inhibition of tumor growth in vitro and in vivo by fucoxanthin against melanoma B16F10 cells. Environ. Toxicol. Pharmacol. 2013, 35, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, H.; Tanaka, J.; Shan, S.J.; Maoka, T. Anti-pigmentary activity of fucoxanthin and its influence on skin mRNA expression of melanogenic molecules. J. Pharm. Pharmacol. 2010, 62, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Gao, C.; Chen, L.; Hu, G.; Xie, S. Essential role of autophagy in fucoxanthin-induced cytotoxicity to human epithelial cervical cancer HeLa cells. Acta Pharmacol. Sin. 2013, 34, 1403–1410. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.; Lu, Q.; Zhao, W.; Du, D.; Jin, L.; Liu, Y. Fucoxanthin induces apoptosis in human cervical cancer cell line HeLa via PI3K/Akt pathway. Tumour Biol. 2014, 35, 11261–11267. [Google Scholar] [CrossRef] [PubMed]
- Lordan, S.; Ross, R.P.; Stanton, C. Marine bioactives as functional food ingredients: Potential to reduce the incidence of chronic diseases. Mar. Drugs 2011, 9, 1056–1100. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Yuan, J.P.; Wu, C.F.; Wang, J.H. Fucoxanthin, a marine carotenoid present in brown seaweeds and diatoms: Metabolism and bioactivities relevant to human health. Mar. Drugs 2011, 9, 1806–1828. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Shnimizu, M.; Moriwaki, H. Cancer chemoprevention by carotenoids. Molecules 2012, 17, 3202–3242. [Google Scholar] [CrossRef] [PubMed]
- Ayyad, S.E.N.; Ezmirly, S.T.; Basaif, S.A.; Alarif, W.M.; Badria, A.F.; Badria, F.A. Antioxidant, cytotoxic, antitumor, and protective DNA damage metabolites from the red sea brown alga Sargassum sp. Pharmacogn. Res. 2011, 3, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Rwigemera, A.; Mamelona, J.; Martin, L.J. Inhibitory effects of fucoxanthinol on the viability of human breast cancer cell lines MCF-7 and MDA-MB-231 are correlated with modulation of the NF-κB pathway. Cell Biol. Toxicol. 2014, 30, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Rwigemera, A.; Mamelona, J.; Martin, L.J. Comparative Effects Between Fucoxanthinol and its Precursor Fucoxanthin on Viability and Apoptosis of Breast Cancer Cell Lines MCF-7 and MDA-MB-231. Anticancer Res. 2015, 35, 207–219. [Google Scholar] [PubMed]
- Frasor, J.; Weaver, A.; Pradhan, M.; Dai, Y.; Miller, L.D.; Lin, C.Y.; Stanculescu, A. Positive cross-talk between estrogen receptor and NF-κB in breast cancer. Cancer Res. 2009, 69, 8918–8925. [Google Scholar] [CrossRef] [PubMed]
- Kalaitzidis, D.; Gilmore, T.D. Transcription factor cross-talk: The estrogen receptor and NF-κB. Trends Endocrinol. Metab. 2005, 16, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Zubair, A.; Frieri, M. Role of nuclear factor-κB in breast and colorectal cancer. Curr. Allergy Asthma Rep. 2013, 13, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Cogswell, P.C.; Guttridge, D.C.; Funkhouser, W.K.; Baldwin, A.S., Jr. Selective activation of NF-κB subunits in human breast cancer: Potential roles for NF-κB2/p52 and for Bcl-3. Oncogene 2000, 19, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- Dejardin, E.; Bonizzi, G.; Bellahcène, A.; Castronovo, V.; Merville, M.P.; Bours, V. Highly-expressed p100/p52 (NFKB2) sequesters other NF-κB-related proteins in the cytoplasm of human breast cancer cells. Oncogene 1995, 11, 1835–1841. [Google Scholar] [PubMed]
- Nakshatri, H.; Bhat-Nakshatri, P.; Martin, D.A.; Goulet, R.J., Jr.; Sledge, G.W., Jr. Constitutive activation of NF-κB during progression of breast cancer to hormone-independent growth. Mol. Cell. Biol. 1997, 17, 3629–3639. [Google Scholar] [PubMed]
- Fan, Y.; Dutta, J.; Gupta, N.; Fan, G.; Gélinas, C. Regulation of programmed cell death by NF-κB and its role in tumorigenesis and therapy. Adv. Exp. Med. Biol. 2008, 615, 223–250. [Google Scholar] [PubMed]
- Wang, C.Y.; Cusack, J.C.; Liu, R.; Baldwin, A.S. Control of inducible chemoresistance: Enhanced anti-tumor therapy through increased apoptosis by inhibition of NF-κB. Nat. Med. 1999, 5, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Mayo, M.W.; Baldwin, A.S. TNF- and cancer therapy-induced apoptosis: Potentiation by inhibition of NF-κB. Science 1996, 274, 784–787. [Google Scholar] [CrossRef] [PubMed]
- Saegusa, M.; Hashimura, M.; Suzuki, E.; Yoshida, T.; Kuwata, T. Transcriptional up-regulation of Sox9 by NF-κB in endometrial carcinoma cells, modulating cell proliferation through alteration in the p14(ARF)/p53/p21(WAF1) pathway. Am. J. Pathol. 2012, 181, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Mathews, L.A.; Cabarcas, S.M.; Zhang, X.; Yang, A.; Zhang, Y.; Young, M.R.; Klarmann, K.D.; Keller, J.R.; Farrar, W.L. Epigenetic Regulation of SOX9 by the NF-κB Signaling Pathway in Pancreatic Cancer Stem Cells. Stem Cells 2013, 31, 1454–1466. [Google Scholar] [CrossRef] [PubMed]
- Matheu, A.; Collado, M.; Wise, C.; Manterola, L.; Cekaite, L.; Tye, A.J.; Canamero, M.; Bujanda, L.; Schedl, A.; Cheah, K.S.E.; et al. Oncogenicity of the developmental transcription factor Sox9. Cancer Res. 2012, 72, 1301–1315. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, G.; Moroz, K.; Makridakis, N.M.; Lloyd, S.A.; Galvez, S.E.; Canavello, P.R.; Lacey, M.R.; Agrawal, K.; Mondal, D. Prognostic significance of cytoplasmic SOX9 in invasive ductal carcinoma and metastatic breast cancer. Exp. Biol. Med. 2011, 236, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Bellizzi, A.; Mangia, A.; Chiriatti, A.; Petroni, S.; Quaranta, M.; Schittulli, F.; Malfettone, A.; Cardone, R.A.; Paradiso, A.; Reshkin, S.J. RhoA protein expression in primary breast cancers and matched lymphocytes is associated with progression of the disease. Intl. J. Mol. Med. 2008, 22, 25–31. [Google Scholar] [CrossRef]
- Katoh, M. Cancer genomics and genetics of FGFR2 (Review). Intl. J. Oncol. 2008, 33, 233–237. [Google Scholar] [CrossRef]
- Kubo, M.; Nakamura, M.; Tasaki, A.; Yamanaka, N.; Nakashima, H.; Nomura, M.; Kuroki, S.; Katano, M. Hedgehog signaling pathway is a new therapeutic target for patients with breast cancer. Cancer Res. 2004, 64, 6071–6074. [Google Scholar] [CrossRef] [PubMed]
- Moore, L.D.; Isayeva, T.; Siegal, G.P.; Ponnazhagan, S. Silencing of transforming growth factor-β1 in situ by RNA interference for breast cancer: Implications for proliferation and migration in vitro and metastasis in vivo. Clin. Cancer Res. 2008, 14, 4961–4970. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wang, W.; Deng, C.X.; Man, Y.G. Aberrant p63 and WT-1 expression in myoepithelial cells of pregnancy-associated breast cancer: Implications for tumor aggressiveness and invasiveness. Intl. J. Biol. Sci. 2009, 5, 82–96. [Google Scholar] [CrossRef]
- Zardawi, S.J.; O’Toole, S.A.; Sutherland, R.L.; Musgrove, E.A. Dysregulation of Hedgehog, Wnt and Notch signalling pathways in breast cancer. Histol. Histopathol. 2009, 24, 385–398. [Google Scholar] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
