Entry Inhibition of Influenza Viruses with High Mannose Binding Lectin ESA-2 from the Red Alga Eucheuma serra through the Recognition of Viral Hemagglutinin
Abstract
:1. Introduction
2. Results and Discussion
2.1. Anti-Influenza Activity of Various Lectins with Diverse Carbohydrate Specificity
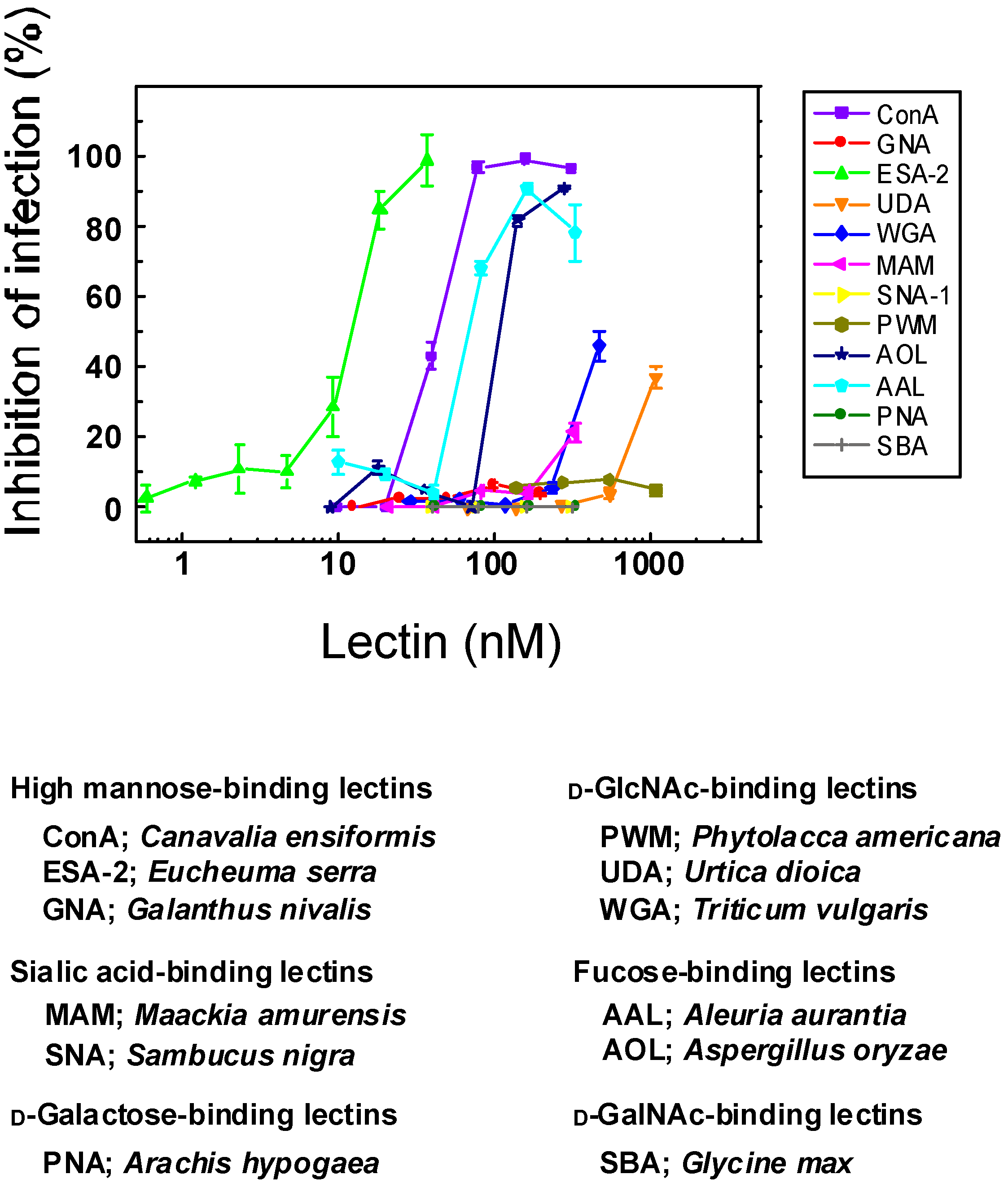
2.2. Anti-Influenza Activity of Red Algal Lectin ESA-2
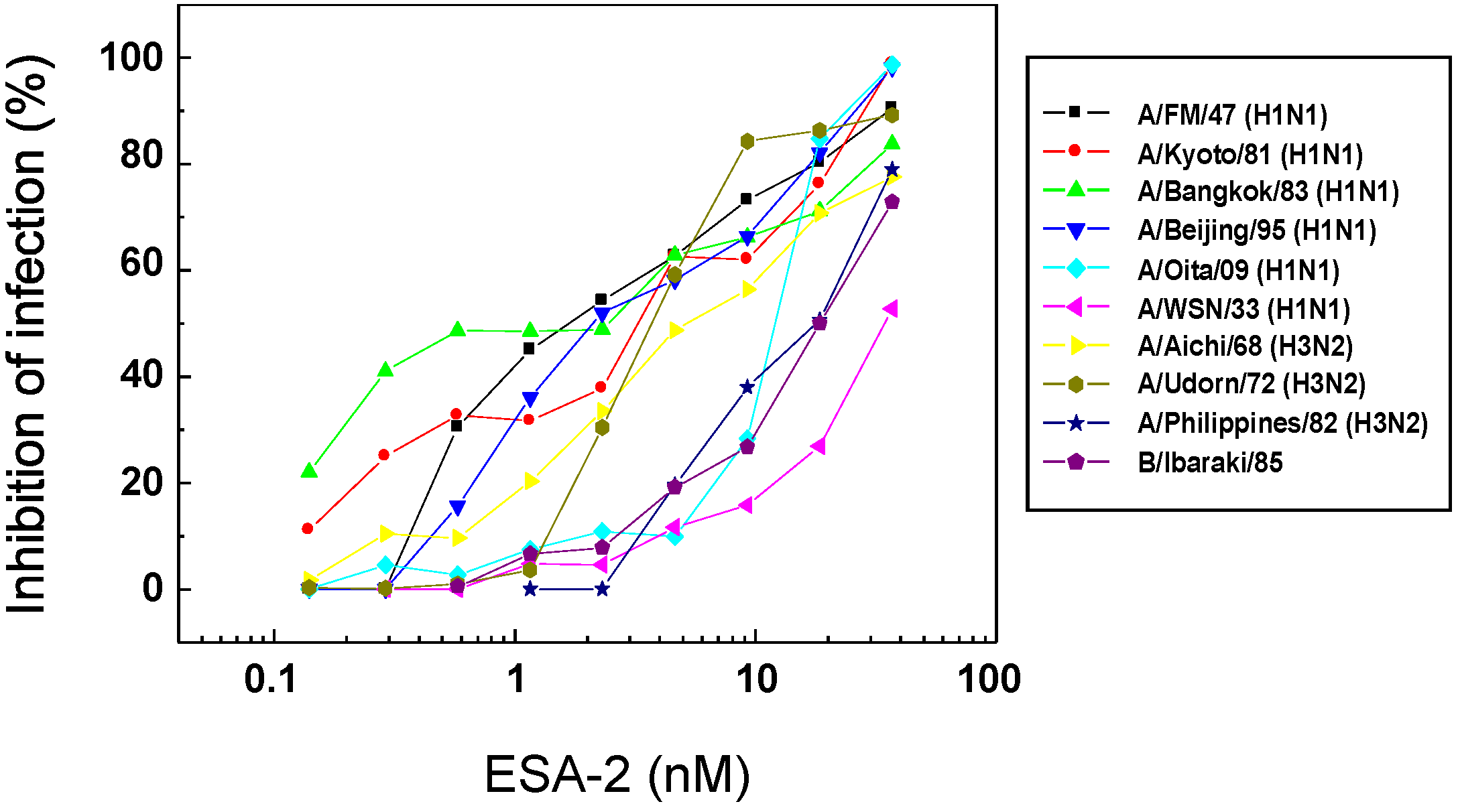
| Virus | Strain | ESA-2 | AOL |
|---|---|---|---|
| EC50 (nM) | EC50 (nM) | ||
| Influenza A | PR8/34 (H1N1) | −a | − |
| FM/1/47 (H1N1) | 0.8 ± 0.2 | 202.9 ± 26.8 | |
| Kyoto/1/81 (H1N1) | 2.7 ± 0.9 | 196.8 ± 26.6 | |
| Bangkok/10/83 (H1N1) | 2.8 ± 1.5 | 78.4 ± 23.6 | |
| Beijing/262/95 (H1N1) | 1.7 ± 0.4 | 19.2 ± 10.5 | |
| Oita/OU1 P3-3/09 (H1N1) | 12.4 ± 0.4 | 123.1 ± 25.4 | |
| WSN/33 (H1N1) | 34.6 ± 2.7 | 1.5 ± 3.2 | |
| Aichi/2/68 (H3N2) | 5.2 ± 1.5 | 200.8 ± 25.7 | |
| Udorn/72 (H3N2) | 3.7 ± 0.4 | − | |
| Philippines/2/82 (H3N2) | 17.2 ± 3.9 | 1.1 ± 2.5 | |
| Influenza B | Ibaraki/2/85 | 20.4 ± 3.3 | − |
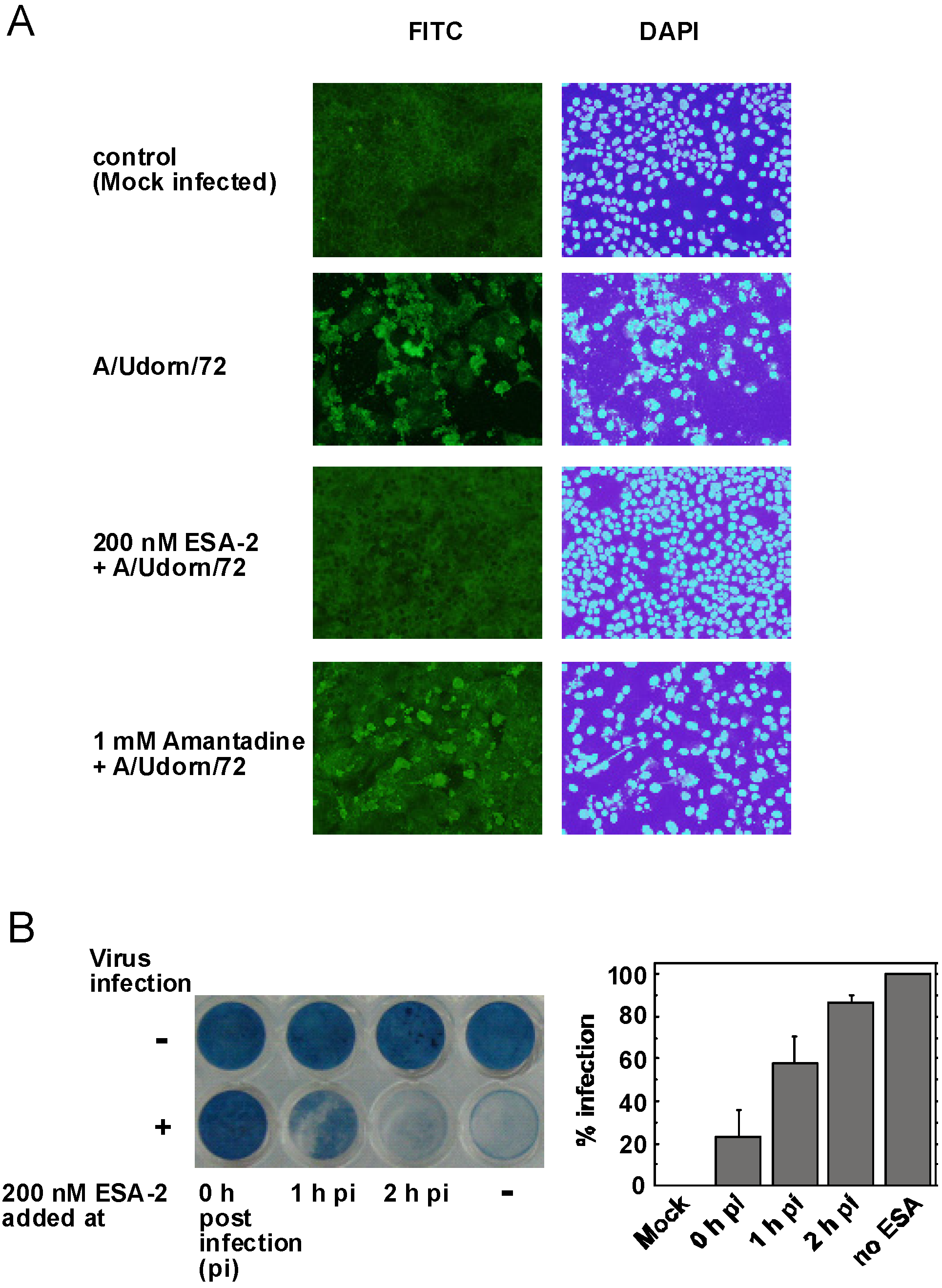
2.3. Evaluation of ESA-2 Potency as an Entry Inhibitor for Influenza Virus
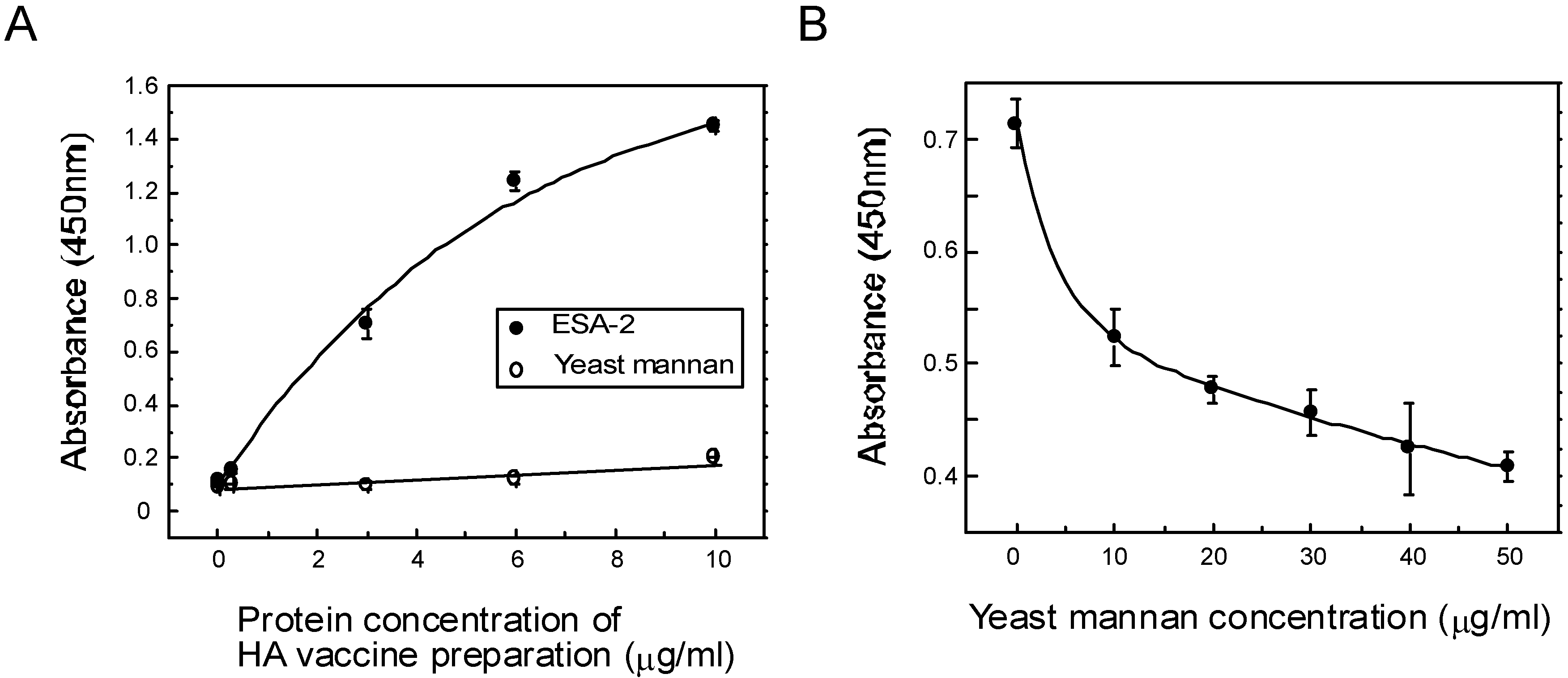
2.4. ESA-2 Binding Studies with Viral Hemagglutinin (HA)
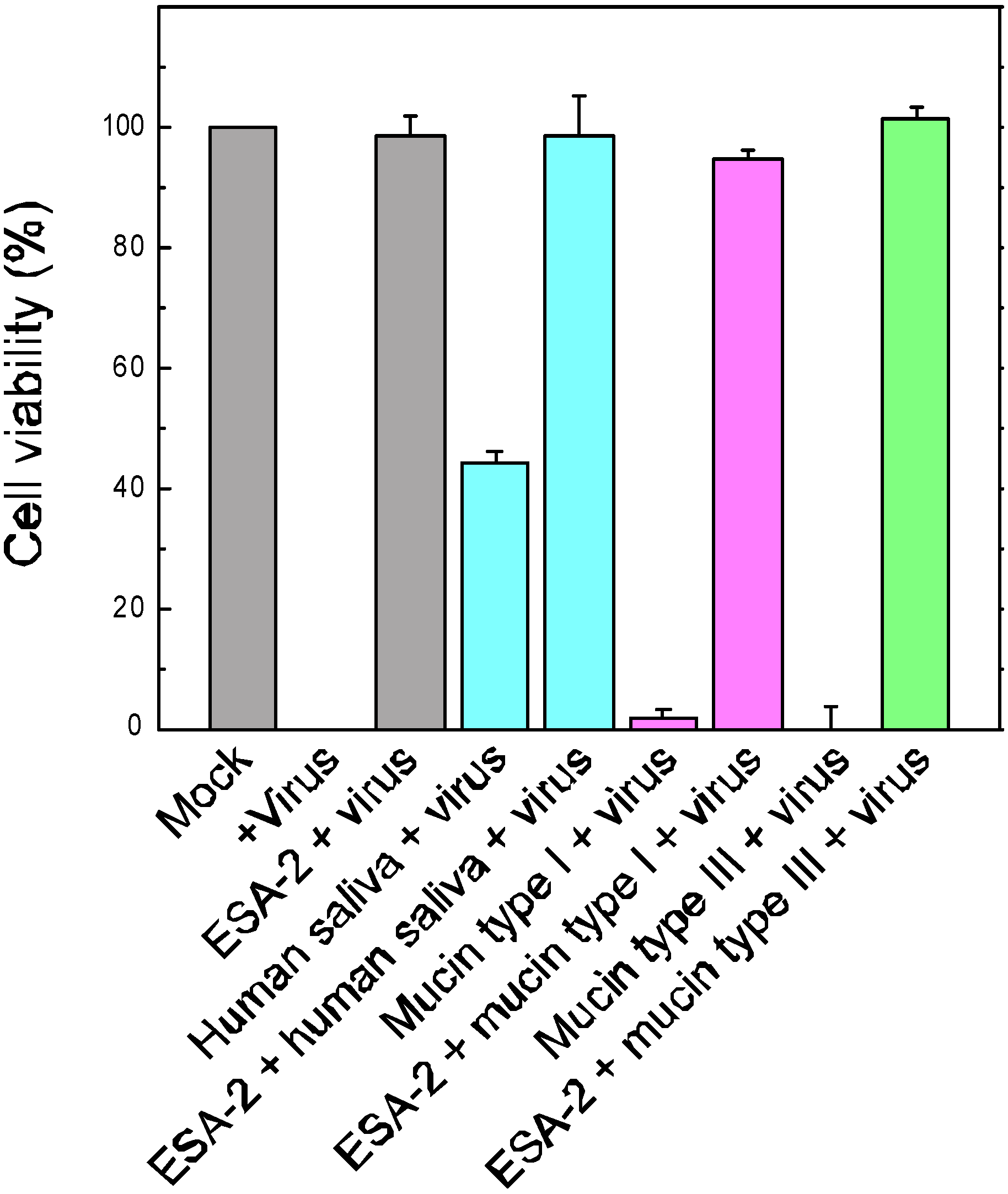
3. Experimental Section
3.1. Materials
3.2. Cells and Viruses
3.3. Determination of Anti-Influenza Activity of Various Lectins
3.4. Evaluation of ESA-2 Potency as an Entry Inhibitor for Influenza Virus
3.5. ESA-2 Binding Studies with Viral Hemagglutinin (HA)
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Neumann, G.; Kawaoka, Y. The first influenza pandemic of the new millennium. Influ. Other Respir. Viruses 2011, 5, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cleveland, B.; Klots, I.; Travis, B.; Richardson, B.A.; Anderson, D.; Montefiori, D.; Polacino, P.; Hu, S.L. Removal of a single N-linked glycan in human immunodeficiency virus type 1 gp120 results in an enhanced ability to induce neutralizing antibody responses. J. Virol. 2008, 82, 638–651. [Google Scholar] [CrossRef] [PubMed]
- Duvet, S.; Cocquerel, L.; Pillez, A.; Cacan, R.; Verbert, A.; Moradpour, D.; Wychowski, C.; Dubuisson, J. Hepatitis C virus glycoprotein complex localization in the endoplasmic reticulum involves a determinant for retention and not retrieval. J. Biol. Chem. 1998, 273, 32088–32095. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, G.; Harvey, D.J.; Feldmann, F.; Stroeher, U.; Feldmann, H.; Royle, L.; Dwek, R.A.; Rudd, P.M. Identification of N-linked carbohydrates from severe acute respiratory syndrome (SARS) spike glycoprotein. Virology 2010, 399, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Mir-Shekari, S.Y.; Ashford, D.A.; Harvey, D.J.; Dwek, R.A.; Schulze, I.T. The glycosylation of the influenza A virus hemagglutinin by mammalian cells. A site-specific study. J. Biol. Chem. 1997, 272, 4027–4036. [Google Scholar] [CrossRef] [PubMed]
- Balzarini, J. Targeting the glycans of glycoproteins: A novel paradigm for antiviral therapy. Nat. Rev. Microbiol. 2007, 5, 583–597. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Okuyama, S.; Hori, K. Primary structure and carbohydrate binding specificity of a potent anti-HIV lectin isolated from the filamentous cyanobacterium Oscillatoria agardhii. J. Biol. Chem. 2007, 282, 11021–11029. [Google Scholar] [CrossRef] [PubMed]
- Koharudin, L.M.; Furey, W.; Gronenborn, A.M. Novel fold and carbohydrate specificity of the potent anti-HIV cyanobacterial lectin from Oscillatoria agardhii. J. Biol. Chem. 2011, 286, 1588–1597. [Google Scholar] [CrossRef] [PubMed]
- Koharudin, L.M.; Kollipara, S.; Aiken, C.; Gronenborn, A.M. Structural insights into the anti-HIV activity of the Oscillatoria agardhii agglutinin homolog lectin family. J. Biol. Chem. 2012, 287, 33796–33811. [Google Scholar] [CrossRef] [PubMed]
- Ferir, G.; Huskens, D.; Noppen, S.; Koharudin, L.M.; Gronenborn, A.M.; Schols, D. Broad anti-HIV activity of the Oscillatoria. agardhii agglutinin homologue lectin family. J. Antimicrob. Chemother. 2014, 69, 2746–2758. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Morimoto, K.; Kubo, T.; Yanagihara, K.; Seyama, T. High mannose-binding antiviral lectin PFL from Pseudomonas fluorescens Pf0-1 promotes cell death of gastric cancer cell MKN28 via interaction with alpha2-integrin. PLoS ONE 2012, 7, e45922. [Google Scholar] [CrossRef] [PubMed]
- Whitley, M.J.; Furey, W.; Kollipara, S.; Gronenborn, A.M. Burkholderia oklahomensis agglutinin is a canonical two-domain OAA-family lectin: Structures, carbohydrate binding and anti-HIV activity. FEBS J. 2013, 280, 2056–2067. [Google Scholar] [CrossRef] [PubMed]
- Hori, K.; Sato, Y.; Ito, K.; Fujiwara, Y.; Iwamoto, Y.; Makino, H.; Kawakubo, A. Strict specificity for high-mannose type N-glycans and primary structure of a red alga Eucheuma serra lectin. Glycobiology 2007, 17, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Morimoto, K.; Hirayama, M.; Hori, K. High mannose-specific lectin (KAA-2) from the red alga Kappaphycus alvarezii potently inhibits influenza virus infection in a strain-independent manner. Biochem. Biophys. Res. Commun. 2011, 405, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Bewley, C.A.; Gustafson, K.R.; Boyd, M.R.; Covell, D.G.; Bax, A.; Clore, G.M.; Gronenborn, A.M. Solution structure of cyanovirin-N, a potent HIV-inactivating protein. Nat. Struct. Biol. 1998, 5, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, L.G.; O’Keefe, B.R.; Bray, M.; Sanchez, A.; Gronenborn, A.M.; Boyd, M.R. Cyanovirin-N binds to the viral surface glycoprotein, GP1,2 and inhibits infectivity of Ebola virus. Antivir. Res. 2003, 58, 47–56. [Google Scholar] [CrossRef]
- Helle, F.; Wychowski, C.; Vu-Dac, N.; Gustafson, K.R.; Voisset, C.; Dubuisson, J. Cyanovirin-N inhibits hepatitis C virus entry by binding to envelope protein glycans. J. Biol. Chem. 2006, 281, 25177–25183. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, B.R.; Smee, D.F.; Turpin, J.A.; Saucedo, C.J.; Gustafson, K.R.; Mori, T.; Blakeslee, D.; Buckheit, R.; Boyd, M.R. Potent anti-influenza activity of cyanovirin-N and interactions with viral hemagglutinin. Antimicrob. Agents Chemother. 2003, 47, 2518–2525. [Google Scholar] [CrossRef] [PubMed]
- Kouokam, J.C.; Huskens, D.; Schols, D.; Johannemann, A.; Riedell, S.K.; Walter, W.; Walker, J.M.; Matoba, N.; O’Keefe, B.R.; Palmer, K.E. Investigation of griffithsin’s interactions with human cells confirms its outstanding safety and efficacy profile as a microbicide candidate. PLoS ONE 2011, 6, e22635. [Google Scholar] [CrossRef] [PubMed]
- Kelley, B.S.; Chang, L.C.; Bewley, C.A. Engineering an obligate domain-swapped dimer of cyanovirin-N with enhanced anti-HIV activity. J. Am. Chem. Soc. 2002, 124, 3210–3211. [Google Scholar] [CrossRef] [PubMed]
- Moulaei, T.; Shenoy, S.R.; Giomarelli, B.; Thomas, C.; McMahon, J.B.; Dauter, Z.; O’Keefe, B.R.; Wlodawer, A. Monomerization of viral entry inhibitor griffithsin elucidates the relationship between multivalent binding to carbohydrates and anti-HIV activity. Structure 2010, 18, 1104–1115. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Hoorelbeke, B.; Kagiampakis, I.; Demeler, B.; Balzarini, J.; Liwang, P.J. The griffithsin dimer is required for high-potency inhibition of HIV-1: Evidence for manipulation of the structure of gp120 as part of the griffithsin dimer mechanism. Antimicrob. Agents Chemother. 2013, 57, 3976–3989. [Google Scholar] [CrossRef] [PubMed]
- Shinya, K.; Ebina, M.; Yamada, S.; Ono, M.; Kasai, N.; Kawaoka, Y. Avian flu: Influenza virus receptors in the human airway. Nature 2006, 440, 435–436. [Google Scholar] [CrossRef] [PubMed]
- Fukuyama, S.; Kawaoka, Y. The pathogenesis of influenza virus infections: The contributions of virus and host factors. Curr. Opin. Immunol. 2011, 23, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Imai, M.; Kawaoka, Y. The role of receptor binding specificity in interspecies transmission of influenza viruses. Curr. Opin. Virol. 2012, 2, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Hiono, T.; Okamatsu, M.; Nishihara, S.; Takase-Yoden, S.; Sakoda, Y.; Kida, H. A chicken influenza virus recognizes fucosylated alpha2,3 sialoglycan receptors on the epithelial cells lining upper respiratory tracts of chickens. Virology 2014, 456–457, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Kachko, A.; Loesgen, S.; Shahzad-Ul-Hussan, S.; Tan, W.; Zubkova, I.; Takeda, K.; Wells, F.; Rubin, S.; Bewley, C.A.; Major, M.E. Inhibition of hepatitis C virus by the cyanobacterial protein Microcystis. viridis lectin: Mechanistic differences between the high-mannose specific lectins MVL, CV-N, and GNA. Mol. Pharmacol. 2013, 10, 4590–4602. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sato, Y.; Morimoto, K.; Kubo, T.; Sakaguchi, T.; Nishizono, A.; Hirayama, M.; Hori, K. Entry Inhibition of Influenza Viruses with High Mannose Binding Lectin ESA-2 from the Red Alga Eucheuma serra through the Recognition of Viral Hemagglutinin. Mar. Drugs 2015, 13, 3454-3465. https://doi.org/10.3390/md13063454
Sato Y, Morimoto K, Kubo T, Sakaguchi T, Nishizono A, Hirayama M, Hori K. Entry Inhibition of Influenza Viruses with High Mannose Binding Lectin ESA-2 from the Red Alga Eucheuma serra through the Recognition of Viral Hemagglutinin. Marine Drugs. 2015; 13(6):3454-3465. https://doi.org/10.3390/md13063454
Chicago/Turabian StyleSato, Yuichiro, Kinjiro Morimoto, Takanori Kubo, Takemasa Sakaguchi, Akira Nishizono, Makoto Hirayama, and Kanji Hori. 2015. "Entry Inhibition of Influenza Viruses with High Mannose Binding Lectin ESA-2 from the Red Alga Eucheuma serra through the Recognition of Viral Hemagglutinin" Marine Drugs 13, no. 6: 3454-3465. https://doi.org/10.3390/md13063454
APA StyleSato, Y., Morimoto, K., Kubo, T., Sakaguchi, T., Nishizono, A., Hirayama, M., & Hori, K. (2015). Entry Inhibition of Influenza Viruses with High Mannose Binding Lectin ESA-2 from the Red Alga Eucheuma serra through the Recognition of Viral Hemagglutinin. Marine Drugs, 13(6), 3454-3465. https://doi.org/10.3390/md13063454






