Abstract
Natural anionic polysaccharides fucosylated chondroitin sulfates (FCS) from sea cucumbers attract great attention nowadays due to their ability to influence various biological processes, such as blood coagulation, thrombosis, angiogenesis, inflammation, bacterial and viral adhesion. To determine pharmacophore fragments in FCS we have started systematic synthesis of oligosaccharides with well-defined structure related to various fragments of these polysaccharides. In this communication, the synthesis of non-sulfated and selectively O-sulfated di- and trisaccharides structurally related to branching sites of FCS is described. The target compounds are built up of propyl β-d-glucuronic acid residue bearing at O-3 α-l-fucosyl or α-l-fucosyl-(1→3)-α-l-fucosyl substituents. O-Sulfation pattern in the fucose units of the synthetic targets was selected according to the known to date holothurian FCS structures. Stereospecific α-glycoside bond formation was achieved using 2-O-benzyl-3,4-di-O-chloroacetyl-α-l-fucosyl trichloroacetimidate as a donor. Stereochemical outcome of the glycosylation was explained by the remote participation of the chloroacetyl groups with the formation of the stabilized glycosyl cations, which could be attacked by the glycosyl acceptor only from the α-side. The experimental results were in good agreement with the SCF/MP2 calculated energies of such participation. The synthesized oligosaccharides are regarded as model compounds for the determination of a structure-activity relationship in FCS.
1. Introduction
Different types of natural anionic polysaccharides attract increasing attention nowadays due to their biological activity of different types that makes possible their use as pharmacological regulators of several diseases related to biological processes, such as blood coagulation, thrombosis, angiogenesis, inflammation, bacterial and viral adhesion and some others. The most famous biopolymer of the discussed type is glycosaminoglycan heparin, which was found to be a leader on anticoagulant and antithrombotic market for decades [1,2]. Besides, this biopolymer was shown to attenuate metastasis and inflammation in a way of inhibition of P- and l-selectins binding to their cellular ligands [3,4].
Due to several side effects of heparin treatment, new biologically active compounds are intensively searched for and are under development as potential alternative drugs. Among them are fucosylated chondroitin sulfates (FCS) isolated from different sea cucumber species. These polysaccharides demonstrated a wide spectrum of biological activities including anticoagulant, antithrombotic, antitumor, immunostimulatory, anti-hyperglycemia, antiangiogenic, antibacterial, antiviral and some others [5,6,7,8,9,10].
A number of studied to date FCSs are glycosaminoglycans built up of alternating →4)-linked β-d-glucuronic acid and →3)-linked N-acetyl β-d-galactosamine residues in a backbone. Unlike mammalian chondroitin sulfates, these polysaccharides bear side chains containing O-sulfated fucosyl residues attached to O-3 of glucuronic acid units (Figure 1) [5,11]. It should be noted that the presence of side chains is essential for biological properties of FCS [12]. The structures of fucosyl-branches vary accordingly to the type of sea cucumber species and determine in many respects the level and the character of its biological activity [5,11]. Moreover, the degree of O-sulfation and fucose content in FCS also depend on the geographic range and season of harvesting [13,14]. The known structures of non-sulfated and selectively O-sulfated α-l-fucosyl and α-l-fucosyl-(1→3)-α-l-fucosyl fragments which were discovered as the side chains in FCS from sea cucumbers [5,11,15] are shown in Figure 1.
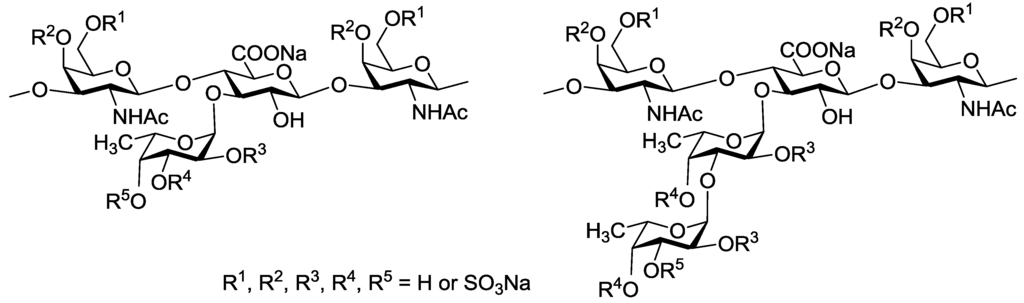
Figure 1.
Branched fragments of fucosylated chondroitin sulfates from sea cucumbers.
Figure 1.
Branched fragments of fucosylated chondroitin sulfates from sea cucumbers.
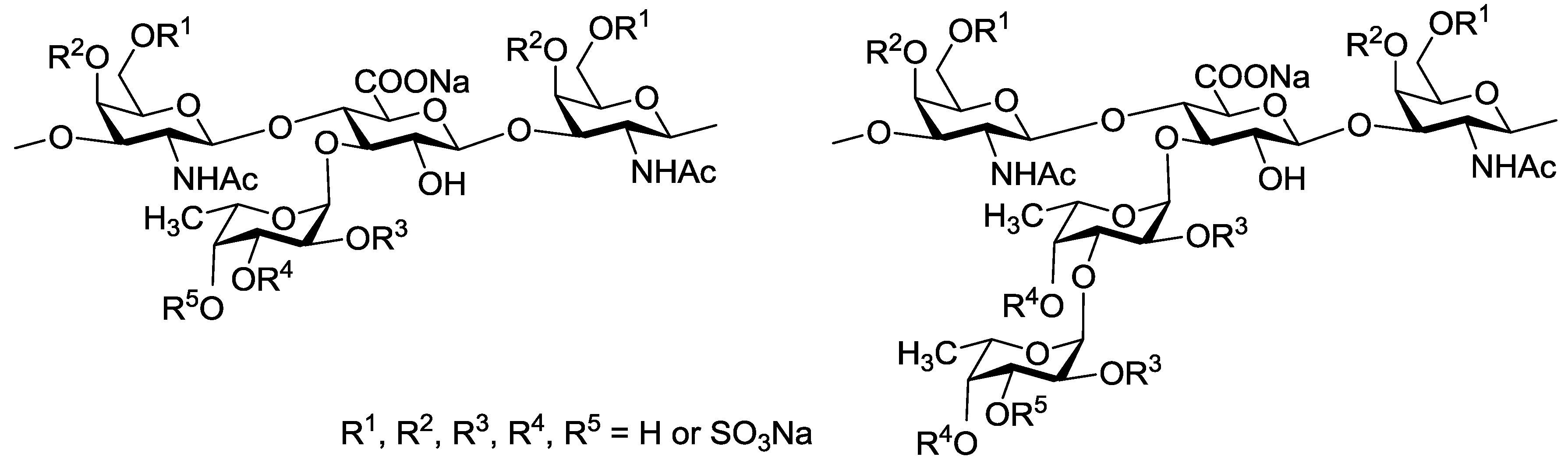
In 2013 Tamura et al. reported synthesis of the trisaccharide β-d-GalNAc(4,6-diS)(1→4) [α-l-Fuc(2,4-diS)(1→3)]-β-d-GlcA related to branching site of FCS [16]. But to determine pharmacophore fragments of FCS, a series of compounds with well-defined structure are required. We have started the systematic synthesis of oligosaccharides related to various fragments of these types of natural polysaccharides. In this communication, the first synthesis of non-sulfated and selectively O-sulfated di- and trisaccharides 1–8 related to the known structures of branching sites of FCS is described (Figure 2). The target compounds are built up of the propyl β-d-glucuronic acid residue bearing at O-3 the α-l-fucosyl or α-l-fucosyl-(1→3)-α-l-fucosyl substituents. The sulfation pattern of the fucosyl units was selected according to the data of holothurian FCS structures described in literature (see above for citations).
Figure 2.
The target compounds 1–8 related to branching sites of fucosylated chondroitin sulfates (FCS).
Figure 2.
The target compounds 1–8 related to branching sites of fucosylated chondroitin sulfates (FCS).
2. Results and Discussion
Two monosaccharides 9 and 10 (Figure 3) bearing free hydroxyl group at C-3 were studied as glycosyl acceptors for assembling of target compounds 1–8. These compounds were prepared from 2,4-di-O-acylated derivatives of 3,6-lactone of allyl glucuronide as described previously [17].
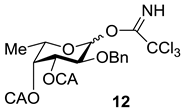
Since the target compounds contain α-l-fucosyl residues, an efficient method for α-l-fucosylation should be applied in their synthesis. Earlier we have shown that the presence of acyl groups at O-3 and O-4 of the fucosyl donor was essential for α-glycoside formation [18,19,20]. Thus, the use of 2-O-benzyl-3-O-acetyl-4-O-benzoyl-l-fucosyl trichloroacetimidate 13 gave the only α-isomeric glycosylation product [20]. The stereochemical result of the reaction was explained by the remote participation of acyl groups with the formation of the stabilized glycosyl cation (similar to cations II and III in Figure 4 below), which could be attacked by an acceptor only from the α-side. This approach was quite different then what was used by Tamura et al., where fucosyl fluoride with non-participating allyl and benzyl groups was applied [16].
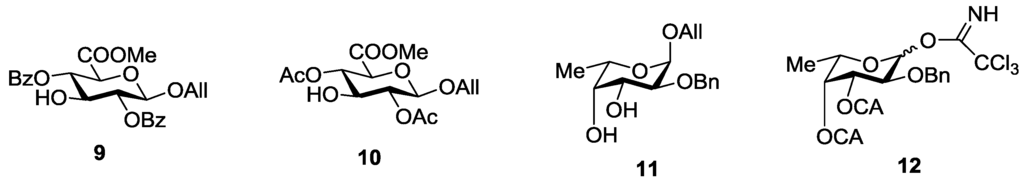
Figure 3.
Monosaccharide building blocks applied for the synthesis of FCS related oligosaccharides.
Figure 3.
Monosaccharide building blocks applied for the synthesis of FCS related oligosaccharides.
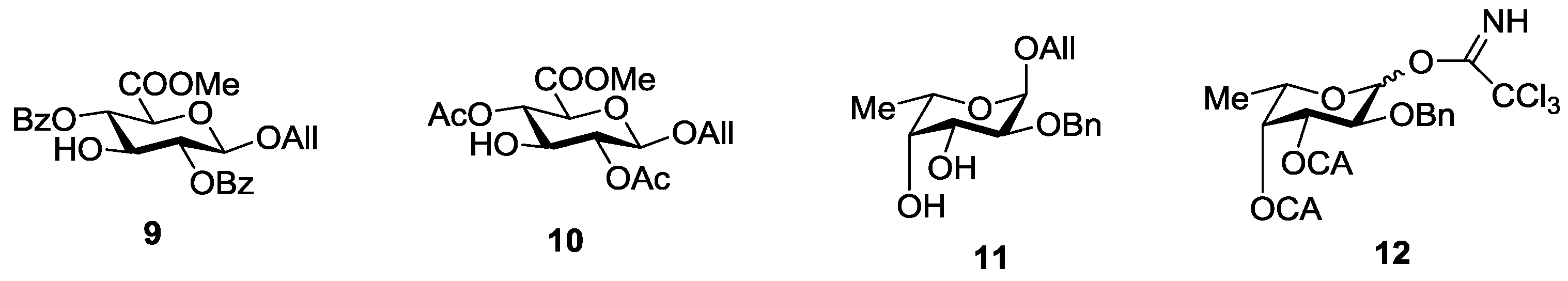
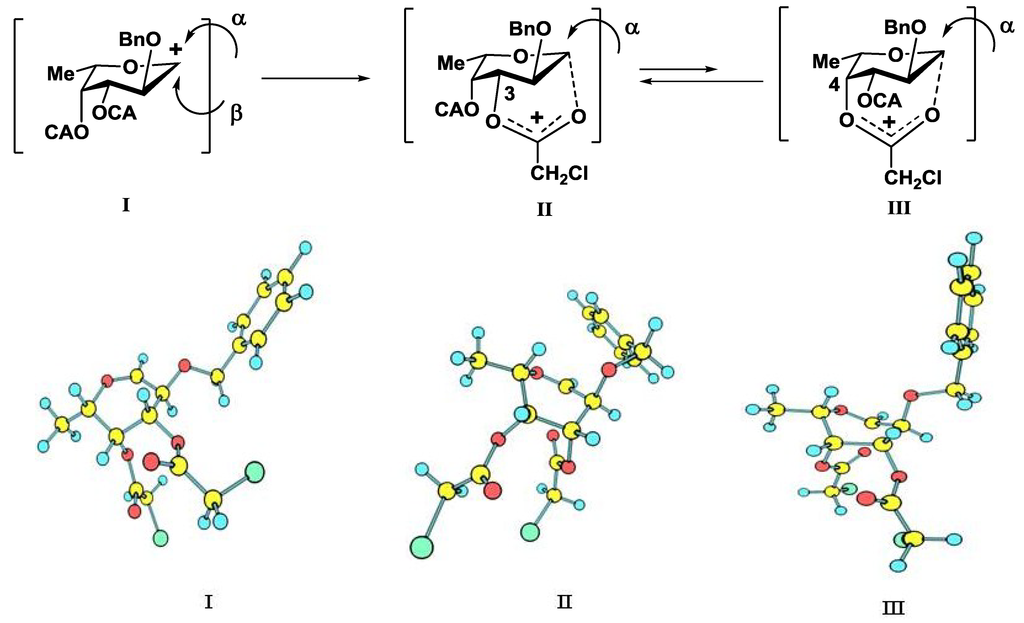
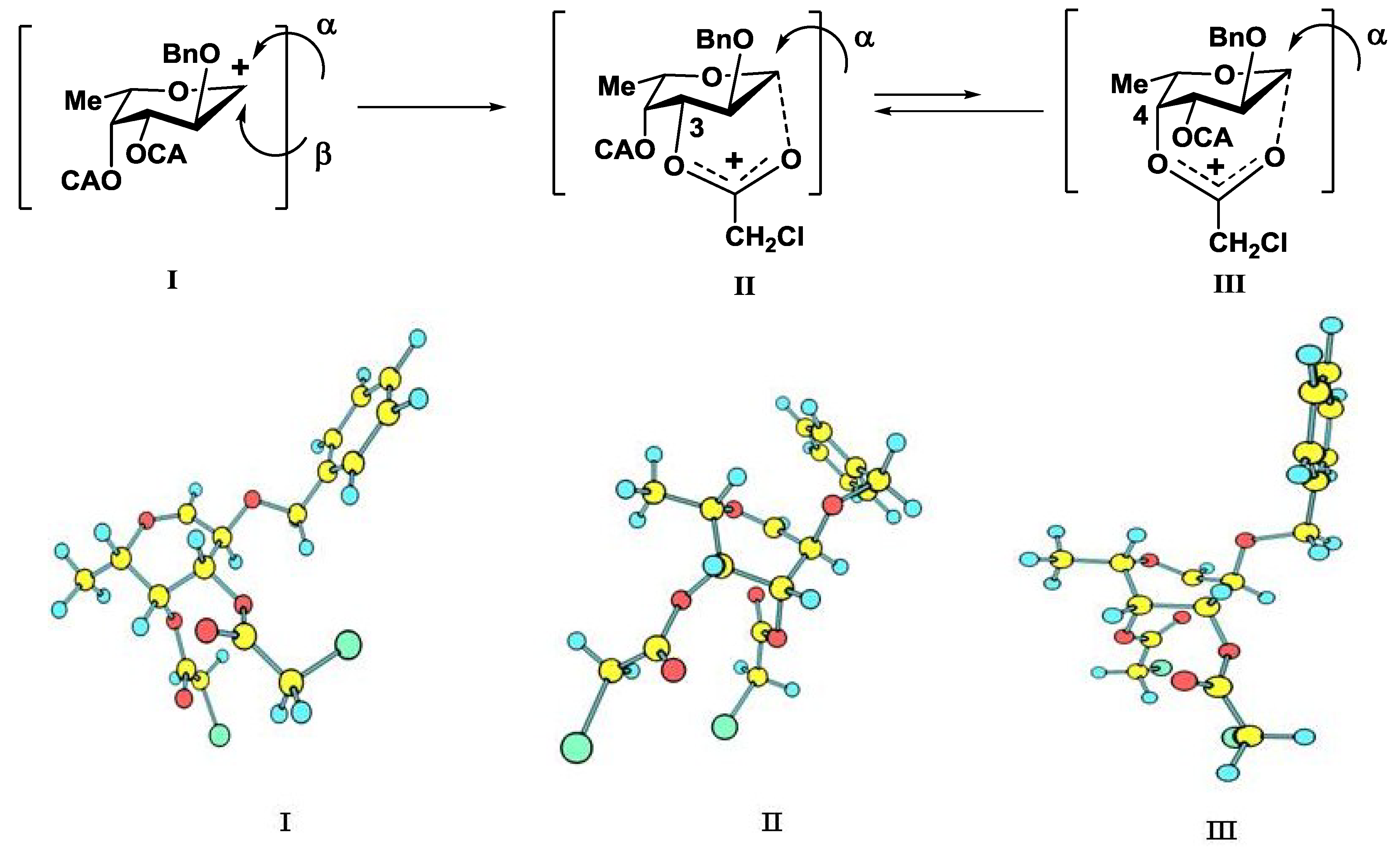
Figure 4.
Expected non-stabilized (I) and stabilized (II), (III) fucosyl cations in glycosylation reactions of fucosyl donor 12. 3D structures of cations were obtained by SCF/MP2 method.
Figure 4.
Expected non-stabilized (I) and stabilized (II), (III) fucosyl cations in glycosylation reactions of fucosyl donor 12. 3D structures of cations were obtained by SCF/MP2 method.
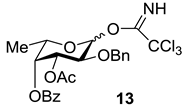
An additional requirement to the structure of the fucosyl donor is the presence of selectively removable protective groups at O-3 and O-4, which are necessary for the preparation of the partially O-sulfated oligosaccharides 3–5 and 8. It is known that chloroacetyl group (CA) could be selectively removed in the presence of benzoyl and acetyl groups. Thus, we investigated first whether the CA group could stabilize glycosyl cation in the same way as had been observed for other acyls and could thus direct the glycosylation towards the formation of the α-isomer. Following our synthetic strategy, 2-O-benzyl-3,4-di-O-chloroacetyl trichloroacetimidate 12 was regarded as a donor of choice (Figure 3).
We conducted theoretical calculations to investigate a possibility of the discussed stereocontrolling effect of remote CA groups. At the primary level, as very coarse estimate, we performed molecular mechanics calculations following the technique described in [21] using the evaluation version of HyperChem 1.0 for Linux (HyperChem is the trademark of Hypercube, Inc. (Gainesville, FL, USA) [22]). The MM+ force field (HyperChem version of MM2 [23]) was employed with the electrostatic term using charge-charge interactions. Atomic charges were obtained from single point calculations using semiempirical PM3 approximation [24,25]. Geometry optimizations of a non-stabilized and stabilized forms of the oxocarbenium ion (Figure 4) were conducted and the corresponding “stabilization energies” were calculated as energy differences in pairs E(I)—E(II) and E(I)—E(III). At this very low level of theory, it was found that the CA group (especially that at O-3) might have the ability to stabilize efficiently the oxocarbenium ion. Stabilization energies were computed as 11.1 and 6.2 kcal/mol for the CA groups at O-3 and O-4, respectively. Calculated values were only slightly lower than those found for the stabilizing groups in donor 13 (Table 1).
Then the study was carried out at the ab initio level with the account for electron correlation. Geometries of all the cations were optimized using the SCF/MP2 approximation with the 6-31+G* basis set as provided along with the NWChem 6.3 software package [26]. This calculation resulted in an increase in the difference in stabilization energies between 12 and 13 (Table 1), but the stabilization energies for the CA groups at O-3 and O-4 of 12 still remained rather high, suggesting their ability to interact with the cationic center.

Table 1.
Stabilization energy of the fucosyl cations.
| Donor | The Stabilizing Group | Stabilization Energy Calculated at MM+ Level, Kcal/Mole | Stabilization Energy Calculated at SCF/MP2 Level, Kcal/Mole |
|---|---|---|---|
 | 3-O-Ac | 11.6 | 15.5 |
| 4-O-Bz | 9.7 | 15.5 | |
 | 3-O-C(O)CH2Cl | 11.1 | 10.4 |
| 4-O-C(O)CH2Cl | 6.2 | 8.6 |
Compound 12 was prepared from diol 11 [27] by per-O-chloroacetylation followed by deallylation and subsequent trichloroacetimidation in a yield of 78% over three steps. The efficiency of this donor was then studied in direct experiments.
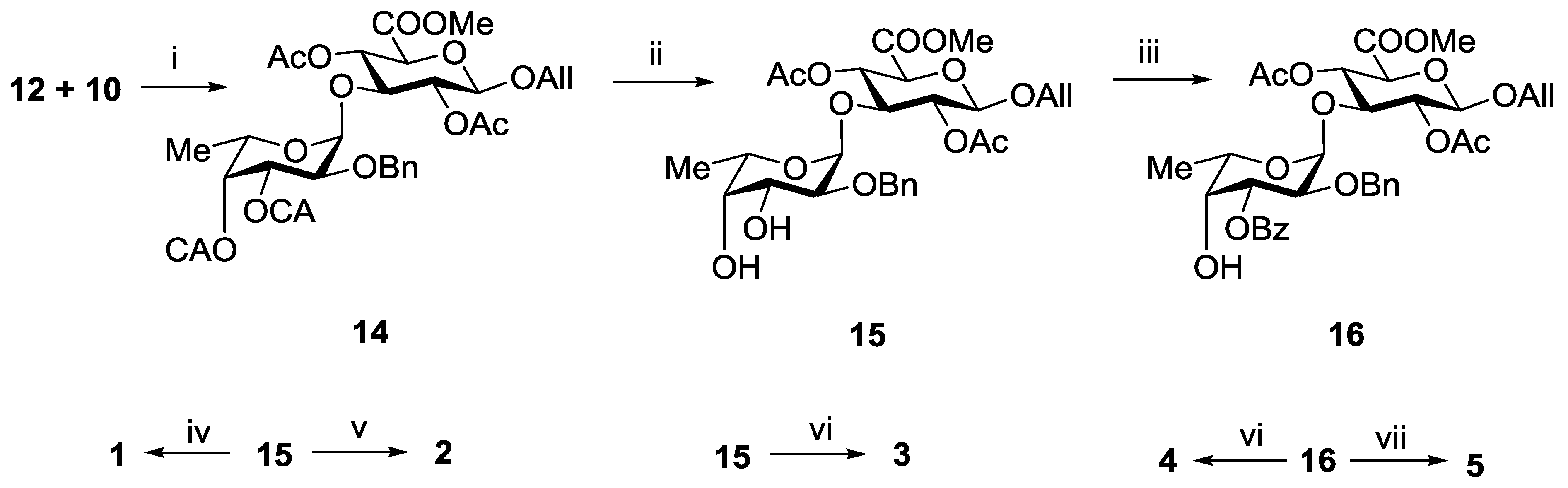
An attempt to involve the 2,4-di-O-benzoylated glucuronyl acceptor 9 into glycosylation with donor 12 in the presence of TMSOTf failed. The main product of the reaction was the respective N-glycosylated trichloroacetamide, while compound 9 was recovered unchanged. On the contrary, the 2,4-di-O-acetylated glucuronyl acceptor 10 under the same conditions reacted rapidly with the formation of the desired α-linked disaccharide 14 exclusively in a yield of 92% (Scheme 1). The difference in the reactivity of the acceptors 9 and 10 could be explained by the different steric availability of the hydroxyl group at C-3. Bulk benzoyl groups at O-2 and O-4 in 9 hindered the access of glycosyl donor to hydroxyl group. It should be noted that the stereochemical result of the glycosylation was still in good agreement with the theoretical prediction.
Selective removal of CA-groups in 14 using thiourea in the presence of collidine gave diol 15 in a yield of 94% (Scheme 1). This compound was used as the precursor of all target disaccharides 1–5. Thus, hydrogenolysis of 15 followed by saponification gave the non-sulfated propyl glycoside 1. Per-O-sulfation of 15 with subsequent hydrogenolysis and saponification led to the 3′,4′-di-O-sulfated disaccharide 3. Acetylation of 15 followed by hydrogenolysis, O-sulfation and saponification gave the 2′-O-sulfated disaccharide 2.
To prepare compounds 4 and 5, selective 3′-O-benzoylation of diol 15 via a stannylidene intermediate was performed with the formation of disaccharide 16 in a yield of 80%. Further O-sulfation of 16 and deprotection of the sulfated derivative gave target disaccharide 4. Hydrogenolysis of 15 followed by O-sulfation and saponification gave the 2′,4′-di-O-sulfated disaccharide 5.
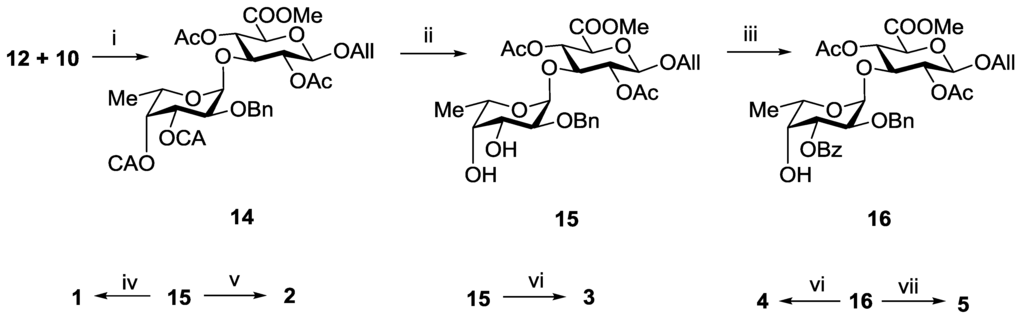
Scheme 1.
Synthesis of the disaccharides 1–5. Reagents and conditions: (i) TMSOTf, CH2Cl2, −30 °C, 92%; (ii) (NH2)2CS, collidine, MeOH, 94%; (iii) Bu2SnO, BzCl (1.1 eq), 80%; (iv) (a) H2, Pd(OH)2/C; (b) LiOH, H2O, THF; (c) NaOH, H2O, 79%; (v) (a) AcCl, Py; (b) H2, Pd(OH)2/C; (c) SO3·Py, DMF; (d) Amberlite IR-120 (Na+), NaHCO3; (e) LiOH, H2O, THF; (f) NaOH, H2O, 64%; (vi) (a) SO3·Py, DMF; (b) Amberlite IR-120 (Na+), NaHCO3; (c) H2, Pd(OH)2/C; (d) LiOH, H2O, THF; (e) NaOH, H2O, 62%; (vii) (a) H2, Pd(OH)2/C; (b) SO3·Py, DMF; (c) Amberlite IR-120 (Na+); NaHCO3; (d) LiOH, H2O, THF; (e) NaOH, H2O, 52%.
Scheme 1.
Synthesis of the disaccharides 1–5. Reagents and conditions: (i) TMSOTf, CH2Cl2, −30 °C, 92%; (ii) (NH2)2CS, collidine, MeOH, 94%; (iii) Bu2SnO, BzCl (1.1 eq), 80%; (iv) (a) H2, Pd(OH)2/C; (b) LiOH, H2O, THF; (c) NaOH, H2O, 79%; (v) (a) AcCl, Py; (b) H2, Pd(OH)2/C; (c) SO3·Py, DMF; (d) Amberlite IR-120 (Na+), NaHCO3; (e) LiOH, H2O, THF; (f) NaOH, H2O, 64%; (vi) (a) SO3·Py, DMF; (b) Amberlite IR-120 (Na+), NaHCO3; (c) H2, Pd(OH)2/C; (d) LiOH, H2O, THF; (e) NaOH, H2O, 62%; (vii) (a) H2, Pd(OH)2/C; (b) SO3·Py, DMF; (c) Amberlite IR-120 (Na+); NaHCO3; (d) LiOH, H2O, THF; (e) NaOH, H2O, 52%.
The synthesis of trisaccharides 6–8 was performed using [2 + 1] strategy for the assembling of the carbohydrate chain. Allyl glucuronide 10 was used as an acceptor, and difucoside 18 was chosen as a donor. Compound 18 was prepared by stereo- and regioselective glycosylation of diol 11 with monosaccharide 12 followed by 4-O-chloroacetylation, deallylation and trichloacetimidation steps (Scheme 2). Finally, the anomeric mixture of trichloroacetimidates 18 was obtained in a yield of 66%.
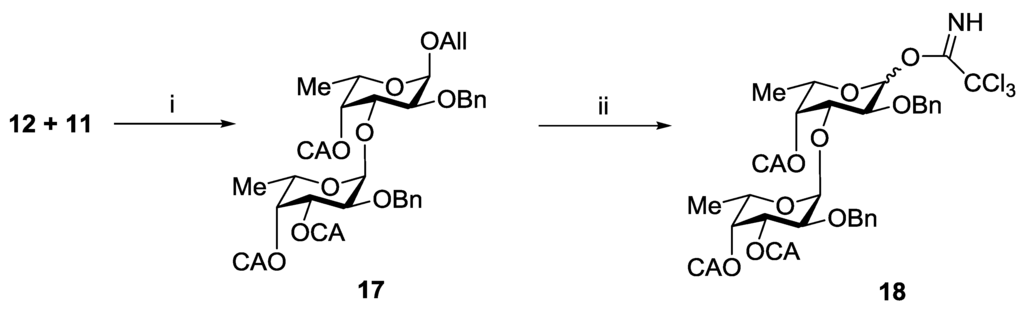
Scheme 2.
Preparation of the difucoside donor 18. Reagents and conditions: (i) (a) TMSOTf, CH2Cl2, −30 °C; (b) (ClСH2CO)2O, Py, 78%; (ii) (a) PdCl2, MeOH; (b) CCl3CN, Cs2CO3, 84%.
Scheme 2.
Preparation of the difucoside donor 18. Reagents and conditions: (i) (a) TMSOTf, CH2Cl2, −30 °C; (b) (ClСH2CO)2O, Py, 78%; (ii) (a) PdCl2, MeOH; (b) CCl3CN, Cs2CO3, 84%.
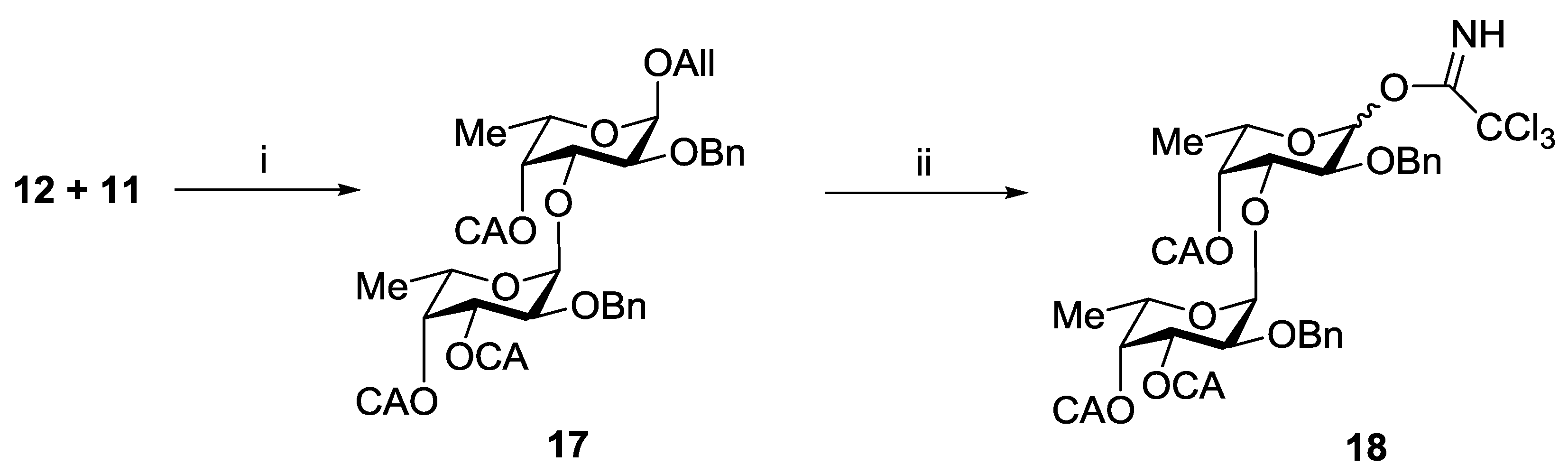
Coupling of thus obtained compounds 18 and 10 in the presence of TMSOTf gave exclusively trisaccharide 19 in a yield of 90% (Scheme 3). Removal of chloroacetyl groups in 19 gave corresponding triol 20, which was further transformed into target trisaccharides 6–8 using the procedure sequences applied for the synthesis of the disaccharides 1–3, respectively.
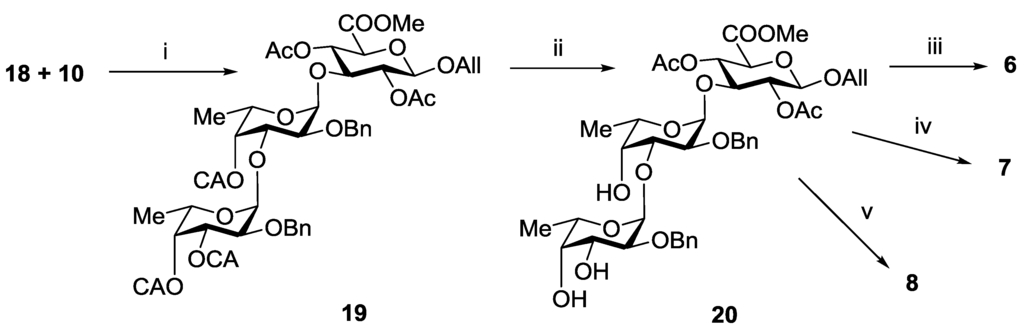
Scheme 3.
Synthesis of the trisaccharides 6–8. Reagents and conditions: (i) TMSOTf, CH2Cl2, −30 °C, 90%; (ii) (NH2)2CS, collidine, MeOH, 92%; (iii) (a) H2, Pd(OH)2/C; (b) LiOH, H2O, THF; (c) NaOH, H2O, 82%; (iv) (a) AcCl, Py, (b) H2, Pd(OH)2/C; (c) SO3·Py, DMF; (d) Amberlite IR-120 (Na+), NaHCO3; (e) LiOH, H2O, THF; (f) NaOH, H2O, 54%; (v) (a) SO3·Py, DMF; (b) Amberlite IR-120 (Na+), NaHCO3; (c) H2, Pd(OH)2/C; (d) LiOH, H2O, THF; (e) NaOH, H2O, 62%.
Scheme 3.
Synthesis of the trisaccharides 6–8. Reagents and conditions: (i) TMSOTf, CH2Cl2, −30 °C, 90%; (ii) (NH2)2CS, collidine, MeOH, 92%; (iii) (a) H2, Pd(OH)2/C; (b) LiOH, H2O, THF; (c) NaOH, H2O, 82%; (iv) (a) AcCl, Py, (b) H2, Pd(OH)2/C; (c) SO3·Py, DMF; (d) Amberlite IR-120 (Na+), NaHCO3; (e) LiOH, H2O, THF; (f) NaOH, H2O, 54%; (v) (a) SO3·Py, DMF; (b) Amberlite IR-120 (Na+), NaHCO3; (c) H2, Pd(OH)2/C; (d) LiOH, H2O, THF; (e) NaOH, H2O, 62%.
All the target compounds were characterized with 1H and 13C NMR spectroscopy (Table 2 and Table 3). The presence of the O-sulfate group was confirmed by the downfield shift of the signal of the neighbor proton and carbon atoms in the 1H and 13C NMR spectra. Introduction of the sulfate group at O-2 also influenced the shift of the signals of anomeric proton and carbon atoms.

Table 2.
13C data for the target compounds 1–8.
| Compound | Residue | C1 | C2 | C3 | C4 | C5 | C6 |
|---|---|---|---|---|---|---|---|
| 1 | →3)-β-d-GlcA | 103.2 | 74.8 | 83.5 | 71.7 | 78.0 | 176.9 |
| α-l-Fuc-(1→3 | 100.7 | 69.6 | 70.8 | 73.2 | 68.1 | 16.5 | |
| 2 | →3)-β-d-GlcA | 103.0 | 74.7 | 83.2 | 71.6 | 77.9 | 176.8 |
| α-l-Fuc-(1→3 | 98.4 | 76.8 | 68.7 | 73.3 | 68.0 | 16.5 | |
| 3 | →3)-β-d-GlcA | 103.5 | 74.6 | 83.5 | 71.6 | 78.1 | 176.8 |
| α-l-Fuc-(1→3 | 100.5 | 67.8 | 76.6 | 80.4 | 67.8 | 17.0 | |
| 4 | →3)-β-d-GlcA | 102.1 | 73.4 | 82.2 | 70.5 | 76.8 | 176.8 |
| α-l-Fuc-(1→3 | 99.1 | 68.6 | 68.6 | 81.0 | 66.4 | 17.0 | |
| 5 | →3)-β-d-GlcA | 103.0 | 74.7 | 83.2 | 71.5 | 77.9 | 176.8 |
| α-l-Fuc-(1→3 | 98.3 | 76.6 | 67.8 | 82.1 | 67.4 | 16.9 | |
| 6 | →3)-β-d-GlcA | 103.1 | 74.6 | 83.6 | 71.7 | 78.0 | 176.8 |
| →3)-α-l-Fuc-(1→3 | 100.7 | 70.7 | 73.2 | 69.5 | 68.1 | 16.5 | |
| α-l-Fuc-(1→3 | 96.5 | 69.3 | 68.2 | 75.9 | 67.9 | 16.5 | |
| 7 | →3)-β-d-GlcA | 103.1 | 75.2 | 83.2 | 71.6 | 78.0 | 176.9 |
| →3)-α-l-Fuc-(1→3 | 98.5 | 74.7 | 74.3 | 70.0 | 68.2 | 16.5 | |
| α-l-Fuc-(1→3 | 95.5 | 76.5 | 68.6 | 73.4 | 67.6 | 16.5 | |
| 8 | →3)-β-d-GlcA | 102.1 | 73.4 | 82.2 | 70.5 | 76.8 | 176.8 |
| →3)-α-l-Fuc-(1→3 | 99.1 | 68.6 | 74.0 | 81.2 | 66.4 | 16.9 | |
| α-l-Fuc-(1→3 | 100.5 | 67.8 | 76.5 | 80.4 | 67.8 | 17.0 |

Table 3.
1H NMR data for the target compounds 1–8.
| Compound | Residue | H1 | H2 | H3 | H4 | H5 | H6 |
|---|---|---|---|---|---|---|---|
| 1 | →3)-β-d-GlcA | 4.48 | 3.51 | 3.60 | 3.63 | 3.74 | - |
| α-l-Fuc-(1→3 | 5.27 | 3.79 | 3.92 | 3.82 | 4.37 | 1.20 | |
| 2 | →3)-β-d-GlcA | 4.49 | 3.55 | 3.65 | 3.65 | 3.74 | - |
| α-l-Fuc-(1→3 | 5.57 | 4.48 | 4.05 | 3.90 | 4.44 | 1.21 | |
| 3 | →3)-β-d-GlcA | 4.48 | 3.55 | 3.67 | 3.63 | 3.74 | - |
| α-l-Fuc-(1→3 | 5.36 | 4.00 | 4.65 | 4.90 | 4.52 | 1.27 | |
| 4 | →3)-β-d-GlcA | 4.47 | 3.50 | 3.62 | 3.62 | 3.73 | - |
| α-l-Fuc-(1→3 | 5.30 | 3.83 | 4.02 | 4.62 | 4.50 | 1.23 | |
| 5 | →3)-β-d-GlcA | 4.47 | 3.55 | 3.63 | 3.66 | 3.73 | - |
| α-l-Fuc-(1→3 | 5.60 | 4.47 | 4.17 | 4.69 | 4.55 | 1.27 | |
| 6 | →3)-β-d-GlcA | 4.49 | 3.55 | 3.63 | 3.65 | 3.74 | - |
| →3)-α-l-Fuc-(1→3 | 5.31 | 3.98 | 3.84 | 4.04 | 4.31 | 1.21 | |
| α-l-Fuc-(1→3 | 5.09 | 3.82 | 3.95 | 3.95 | 4.37 | 1.21 | |
| 7 | →3)-β-d-GlcA | 4.49 | 3.63 | 3.65 | 3.67 | 3.74 | - |
| →3)-α-l-Fuc-(1→3 | 5.60 | 4.57 | 4.07 | 4.10 | 4.42 | 1.24 | |
| α-l-Fuc-(1→3 | 5.34 | 4.45 | 4.12 | 3.91 | 4.49 | 1.26 | |
| 8 | →3)-β-d-GlcA | 4.46 | 3.55 | 3.63 | 3.65 | 3.73 | - |
| →3)-α-l-Fuc-(1→3 | 5.32 | 4.00 | 4.08 | 4.76 | 4.46 | 1.24 | |
| α-l-Fuc-(1→3 | 5.20 | 3.92 | 4.62 | 4.90 | 4.46 | 1.27 |
The synthesized oligosaccharides are regarded as model compounds for the investigation of a structure-activity relationship in FCS. The results of conformational analysis and biological studies of the compounds 1–8 will be published elsewhere. Synthesis and interdisciplinary studies of larger oligosaccharides related to structural fragments of FCS are in progress at this laboratory.
3. Experimental Section
3.1. Materials
Dimethylformamide (DMF, ≥99.5%) and pyridine (≥99.5%) were purchased from Sigma-Aldrich (Saint Louis, MO, USA). Dichloromethane (CH2Cl2) was successively distilled from diethanolamine, P2O5, and CaH2 under argon. Analytical thin-layer chromatography (TLC) was performed on Silica Gel 60 F254 aluminum sheets (Merck, Darmstadt, Germany), and visualization was accomplished using UV light or by charring at ~150 C with 10% (v/v) H3PO4 in ethanol. Liquid column chromatography was performed on Silica Gel 60, 40–63 µm (Merck, Darmstadt, Germany). Gel chromatography was performed on the Sephadex G-15 column (260 cm) by elution with water at a flow rate of 1 mL/min.
3.2. Characterization of Compounds
1H and 13C NMR spectra were recorded on Bruker DRX500 and AV600 spectrometers at 303 K in CDCl3 and D2O. Chemical shifts were reported in ppm referenced to the residual CHCl3 peak (δ 7.27) for substituted compounds and to acetone peak (0.05% as internal standard, 1H δ 2.22 and 13C δ 30.9) for the oligosaccharides 1–8. Signal assignment in 1H and 13C NMR spectra were made using COSY, TOCSY, ROESY and 1H-13C HSQC techniques. High-resolution mass spectra were acquired by electrospray ionization on a Bruker Daltonics micrOTOF II instrument [28]. Optical rotation values were measured using a JASCO DIP-360 polarimeter at the ambient temperature in solvents specified.
3.3. Chemical Synthesis
All glycosylation reactions were carried out under dry argon. Molecular sieves for glycosylation reactions were activated prior to application at 180 °C in vacuum of an oil pump during 2 h.
3.3.1. 2-O-Benzyl-3,4-di-O-chloroacetyl-l-fucopyranosyl Trichloroacetimidate (12)
To a solution of the monosaccharide 11 (800 mg, 2.72 mmol) in CH2Cl2 (20 mL), Py (2 mL) and (ClCH2C(O))2O (1 g, 5.9 mmol) were added. The reaction mixture was kept at room temperature (rt) for 1 h, then diluted with EtOAc (50 mL) and washed with HCl (0.1 M) (20 mL) and distilled water (2 × 30 mL). Organic layer was separated and concentrated in vacuo. Chromatography of the residue on a silica gel column gave the totally protected monosaccharide as an amorphous solid. The product was dissolved in MeOH (30 mL), and PdCl2 (127 mg, 0.8 mmol) was added. The mixture was stirred for 3 h at rt, then it was filtered through a celite pad and the filtrate was concentrated in vacuo. Flash column chromatography of the residue on silica gel gave the respective semiacetales as an amorphous solid, which was then dissolved in CH2Cl2 (15 mL), and CCl3CN (300 µL, 3.0 mmol) together with Cs2CO3 (50 mg, 0.15 mmol) were added. The reaction mixture was stirred for 1 h at rt, then it was filtered through a celite pad and the filtrate was concentrated in vacuo. Column chromatography of the residue on a silica gel inactivated by Et3N (0.1%) gave the compound 12 as a mixture of α and β isomers in a ratio of 1:1 (1.16 g, 2.12 mmol, 78%). HRMS (ESI) C19H20Cl5NO7 [M + Na]+ calc.: 571.9580, found: 571.9575.
α-Isomer: Rf 0.7 (Toluene–EtOAc 3:1); 1H NMR (600 MHz, CDCl3): δ 1.20 (d, J5,6 6.5 Hz, 3H, H-6), 3.99 (d, Jgem 2.0 Hz, 2H, ClCH2CO), 4.05 (dd, J1,2 3.5 Hz, J2,3 10.4 Hz, 1H, H-2), 4.15 (s, 2H, ClCH2CO), 4.42 (q, 1H, H-5), 4.65 (d, Jgem 12.0 Hz, 1H, PhCHH), 4.74 (d, Jgem 12.0 Hz, 1H, PhCHH), 5.45 (d, J3,4 3.1 Hz, 1H, H-4), 5.50 (dd, 1H, H-3), 6.56 (d, J1,2 3.5 Hz, 1H, H-1), 7.20–7.40 (m, 5H, Ar), 8.68 (s, 1H, NH).
β-Isomer: Rf 0.6 (Toluene–EtOAc 3:1); 1H NMR (600 MHz, CDCl3): δ 1.29 (d, J5,6 6.5 Hz, 3H, H-6), 3.83 (d, Jgem 11.7 Hz, 1H, ClCHHCO), 3.90 (d, Jgem 11.7 Hz, 1H, ClCHHCO), 3.95 (dd, J1,2 8.1 Hz, J2,3 10.0 Hz, 1H, H-2), 4.03 (q, 1H, H-5), 4.19 (s, 2H, ClCH2CO), 4.68 (d, Jgem 11.4 Hz, 1H, PhCHH), 4.92 (d, Jgem 11.4 Hz, 1H, PhCHH), 5.18 (dd, 1H, J3,4 3.4 Hz, H-3), 5.34 (d, 1H, H-4), 5.86 (d, J1,2 8.1 Hz, 1H, H-1), 7.20–7.40 (m, 5H, Ar), 8.78 (s, 1H, NH).
3.3.2. Allyl 2-O-benzyl-3,4-di-O-chloroacetyl-α-l-fucopyranosyl-(1→3)-(methyl 2,4-di-O-acetyl-β-d-glucopyranosyl Uronate) (14)
A solution of the monosaccharide 12 (274 mg, 0.5 mmol) and the monosaccharide 10 (166 mg, 0.5 mmol) in CH2Cl2 (5 mL) was stirred at rt under argon atmosphere with molecular sieves 4 Å (500 mg) for 1 h. The mixture was cooled to −30 °С and TMSOTf of (3 µL) was added. The mixture was stirred for 15 min at −30 °С, then Et3N (0.05 mL) was added. The mixture was filtered through a celite pad, and the filtrate was concentrated in vacuo. Column chromatography of the residue on silica gel gave the disaccharide 14 as an amorphous solid (330 mg, 0.46 mmol, 92%): Rf 0.4 (Toluene–EtOAc 3:1), [α]D = −63° (c 1, EtOAc); 1H NMR (600 MHz, CDCl3): δ 1.08 (d, J5,6 6.6 Hz, 3H, H-6), 1.85 (s, 3H, COCH3), 2.08 (s, 3H, COCH3), 3.78 (m, 5H, C(O)OCH3, H-2Fuc, H-3GlcA), 3.93 (m, 3H, H-5GlcA, ClCH2CO), 4.10 (s, 2H, ClCH2CO), 4.12 (m, 1H, CHHCH=CH2), 4.23 (q, 1H, H-5Fuc), 4.37 (m, 1H, CHHCH=CH2), 4.58 (d, J1,2 7.7 Hz, 1H, H-1GlcA), 4.62 (s, 2H, PhCH2), 4.93 (d, J1,2 3.3 Hz, 1H, H-1Fuc), 5.10 (t, J2,3 7.5 Hz, 1H, H-2GlcA), 5.20 (m, 3H, H-4GlcA, CH2CH=CH2), 5.30 (m, 2H, H-3Fuc, H-4Fuc), 5.85 (m, 1H, CH2CH=CH2), 7.20–7.40 (m, 5H, Ar). HRMS (ESI) C31H38Cl2O15 [M + Na]+ calc.: 743.1479, found: 743.1480.
3.3.3. Allyl 2-O-benzyl-α-l-fucopyranosyl-(1→3)-(methyl 2,4-di-O-acetyl-β-d-glucopyranosyl Uronate) (15)
To a solution of the disaccharide 14 (300 mg, 0.42 mmol) in MeOH (5 mL) thiourea (96 mg, 1.26 mmol) and collidine (50 µL) were added. The mixture was kept at 40 °С for 2 h, then it was diluted with EtOAc (20 mL) and washed with HCl (0.1 M) (10 mL) and distilled water (2 × 15 mL). Organic layer was separated and concentrated in vacuo. Chromatography of the residue on a silica gel column gave the diol 15 as an amorphous solid (222 mg, 0.39 mmol, 94%): Rf 0.2 (Toluene–EtOAc 1:1), [α]D = −87° (c 1, EtOAc); 1H NMR (600 MHz, CDCl3): δ 1.09 (d, J5,6 6.6 Hz, 3H, H-6), 1.82 (s, 3H, COCH3), 1.98 (s, 3H, COCH3), 3.23 (s, 2H, 2OH), 3.54 (dd, J1,2 3.5 Hz, J2,3 10.0 Hz, 1H, H-2Fuc), 3.60 (d, J3,4 3.3 Hz, 1H, H-4Fuc), 3.66 (m, 4H, C(O)OCH3, H-3GlcA), 3.74 (dd, 1H, H-3Fuc), 3.83 (d, J4,5 9.8 Hz, 1H, H-5GlcA), 3.91 (q, 1H, H-5Fuc), 4.04 (m, 1H, CHHCH=CH2), 4.28 (m, 1H, CHHCH=CH2), 4.49 (d, J1,2 7.9 Hz, 1H, H-1GlcA), 4.55 (d, Jgem 11.9 Hz, 1H, PhCHH), 4.60 (d, Jgem 11.9 Hz, 1H, PhCHH), 4.77 (d, J1,2 3.5 Hz, 1H, H-1Fuc), 4.98 (dd, J2,3 9.1 Hz, J3,4 7.9 Hz, 1H, H-2GlcA), 5.03 (t, J3,4 = J4,5 9.3 Hz, 1H, H-4GlcA), 5.14 (m, 1H, CH2CH=CHH), 5.21 (m, 1H, CH2CH=CHH), 5.76 (m, 1H, CH2CH=CH2), 7.20-7.40 (m, 5H, Ar). HRMS (ESI) C27H36O13 [M + Na]+ calc.: 591.2054, found: 591.2050.
3.3.4. Allyl 2-O-benzyl-3-O-benzoyl-α-l-fucopyranosyl-(1→3)-(methyl 2,4-di-O-acetyl-β-d-glucopyranosyl Uronate) (16)
A mixture of the compound 15 (210 mg, 0.37 mmol) and Bu2SnO (102 mg, 0.41 mmol) in toluene (5 mL) was refluxed with aseotropic removal of water to a volume of 3 mL, treated with benzoyl chloride (50 µL, 0.41 mmol), and the mixture was kept for 1 h. Then MeOH (5 mL) was added, and the mixture was concentrated. Column chromatography of the residue on silica gel afforded the compound 16 (200 mg, 0.30 mmol, 80%): Rf 0.5 (Toluene–EtOAc 1:1), [α]D = −72° (c 1, EtOAc); 1H NMR (600 MHz, CDCl3): δ 1.20 (d, J5,6 6.4 Hz, 3H, H-6), 1.86 (s, 3H, COCH3), 2.08 (s, 3H, COCH3), 3.78 (s, 3H, C(O)OCH3), 3.82 (t, J2,3 8.6 Hz, J3,4 9.0 Hz, 1H, H-3GlcA), 3.94 (d, J4,5 9.6 Hz, 1H, H-5GlcA), 4.02 (m, 2H, H-2Fuc, H-4Fuc), 4.13 (m, 1H, CHHCH=CH2), 4.22 (q, 1H, H-5Fuc), 4.39 (m, 1H, CHHCH=CH2), 4.60 (d, J1,2 7.6 Hz, 1H, H-1GlcA), 4.68 (dd, 2H, PhCH2), 5.00 (s, 1H, H-1Fuc), 5.16 (t, 1H, H-2GlcA), 5.22 (m, 2H, H-3Fuc, H-4GlcA), 5.30 (d, 1H, CH2CH=CHH), 5.40 (m, 1H, CH2CH=CHH), 5.88 (m, 1H, CH2CH=CH2), 7.20–8.00 (m, 10H, Ar). HRMS (ESI) C34H40O14 [M + Na]+ calc.: 695.2316, found: 695.2321.
3.3.5. Allyl 2-O-benzyl-3,4-di-O-chloroacetyl-α-l-fucopyranosyl-(1→3)-2-O-benzyl-4-O-cloroacetyl-α-l-fucopyranoside (17)
A solution of the monosaccharide 12 (300 mg, 0.55 mmol) and the monosaccharide 11 (162 mg, 0.55 mmol) in CH2Cl2 (5 mL) was stirred at rt under argon atmosphere with molecular sieves 4 Å (500 mg) for 1 h. The mixture was cooled to −30 °С and TMSOTf of (5 µL) was added. The mixture was stirred for 15 min −30 °С, then Et3N (0.05 mL) was added. The mixture was filtered through a celite pad, and the filtrate was concentrated in vacuo. Column chromatography of the residue on a silica gel gave the disaccharide, which was dissolved in CH2Cl2 (5 mL), and Py (0.5 mL) together with (ClCH2C(O))2O (150 mg, 0.9 mmol) were added. The reaction mixture was kept at rt for 1 h, then diluted with EtOAc (20 mL) and washed with HCl (0.1 M) (10 mL) and distilled water (2 × 20 mL). Organic layer was separated and concentrated in vacuo. Chromatography of the residue on a silica gel column gave the disaccharide 17 as an amorphous solid (326 mg, 0.43 mmol, 78%): Rf 0.6 (Toluene–EtOAc 4:1), [α]D = −170° (c 1, EtOAc); 1H NMR (600 MHz, CDCl3): δ 0.70 (d, J5,6 6.4 Hz, 3H, H-6), 1.15 (d, J5,6 6.4 Hz, 3H, H-6), 3.80 (m, 2H, H-2Fuc1, H-2Fuc2), 3.92–4.30 (m, 10H, H-3Fuc1, H-5Fuc1, 3xClCH2CO, CH2CH=CH2), 4.50 (q, 1H, H-5Fuc2), 4.52–4.80 (m, 4H, 2x PhCH2), 5.00 (d, 1H, H-1Fuc1), 5.10 (d, 1H, H-1Fuc2), 5.35 (m, 5H, H-3Fuc2, H-4Fuc1, H-4Fuc2, CH2CH=CH2), 5.92 (m, 1H, CH2CH=CH2), 7.20–7.42 (m, 10H, Ar). HRMS (ESI) C35H41Cl3O12 [M + Na]+ calc.: 781.1561, found: 781.1557.
3.3.6. 2-O-Benzyl-3,4-di-O-chloroacetyl-α-l-fucopyranosyl-(1→3)-2-O-benzyl-4-O-cloroacetyl-l-fucopyranosyl Trichloroacetimidate (18)
To a solution of the disaccharide 17 (230 mg, 0.30 mmol) in MeOH (5 mL) PdCl2 (22 mg, 0.12 mmol) was added. The mixture was stirred for 3 h at rt, then it was filtered through a celite pad and the filtrate was concentrated in vacuo. Flash column chromatography of the residue on a silica gel gave the respective semiacetales as an amorphous solid, which was then dissolved in CH2Cl2 (3 mL), and CCl3CN (50 µL, 0.49 mmol) together with Cs2CO3 (10 mg, 0.03 mmol) were added. The reaction mixture was stirred for 1 h at rt, then it was filtered through a celite pad and the filtrate was concentrated in vacuo. Column chromatography of the residue on a silica gel inactivated by Et3N (0.1%) gave the compound 18 as a mixture of α and β isomers in a ratio of 1:1 (215 mg, 0.25 mmol, 84%). HRMS (ESI) C34H37Cl6NO12 [M + Na]+ calc.: 884.0345, found: 884.0350.
α-Isomer: Rf 0.7 (Toluene–EtOAc 4:1); 1H NMR (600 MHz, CDCl3): δ 0.62 (d, J5,6 6.5 Hz, 3H, H-6Fuc2), 1.2 (d, J5,6 6.5 Hz, 3H, H-6Fuc1), 3.80 (dd, J1,2 3.4 Hz, J2,3 10.5 Hz, 1H, H-2Fuc2), 3.84 (d, Jgem 15.0 Hz, 1H, ClCHHCO), 3.90 (d, Jgem 15.0 Hz, 1H, ClCHHCO), 3.94 (d, Jgem 15.0 Hz, 1H, ClCHHCO), 4.04 (m, 4H, H-2Fuc1, ClCH2CO, ClCHHCO), 4.33 (m, H-3, H-3Fuc1, H-5Fuc1, H-5Fuc2), 4.50 (d, Jgem 12.2 Hz, 1H, PhCHH), 4.58 (d, Jgem 10.1 Hz, 1H, PhCHH), 4.72 (d, Jgem 12.2 Hz, 1H, PhCHH), 4.80 (d, Jgem 10.1 Hz, 1H, PhCHH), 4.90 (d, J3,4 2.7 Hz, 1H, H-4Fuc2), 5.10 (d, J1,2 3.4 Hz, 1H, H-1Fuc2), 5.39 (dd, 1H, H-3Fuc2), 5.49 (d, 1H, H-4Fuc1), 6.68 (d, J1,2 3.4 Hz, 1H, H-1Fuc1), 7.20–7.40 (m, 10H, Ar), 8.62 (s, 1H, NH).
β-Isomer: Rf 0.3 (Toluene–EtOAc 4:1); 1H NMR (600 MHz, CDCl3): δ 0.60 (d, J5,6 6.5 Hz, 3H, H-6Fuc2), 1.29 (d, J5,6 6.5 Hz, 3H, H-6Fuc1), 3.78 (dd, J1,2 3.5 Hz, J2,3 9.5 Hz, 1H, H-2Fuc2), 3.81 (d, Jgem 15.0 Hz, 1H, ClCHHCO), 3.92 (m, 4H, H-2Fuc1, H-3Fuc1, H-5Fuc1, ClCHHCO), 4.01 (m, 3H, ClCH2CO, ClCHHCO), 4.10 (d, Jgem 15.2 Hz, 1H, ClCHHCO), 4.15 (q, 1H, H-5Fuc2), 4.50 (d, Jgem 12.2 Hz, 1H, PhCHH), 4.58 (d, Jgem 10.1 Hz, 1H, PhCHH), 4.72 (d, Jgem 12.2 Hz, 1H, PhCHH), 4.82 (d, J3,4 2.9 Hz, 1H, H-4Fuc2), 5.05 (d, J1,2 3.5 Hz, 1H, H-1Fuc2), 5.08 (d, Jgem 10.1 Hz, 1H, PhCHH), 5.37 (dd, 1H, H-3Fuc2), 5.40 (d, 1H, H-4Fuc1), 5.82 (d, J1,2 7.8 Hz, 1H, H-1Fuc1), 7.20–7.40 (m, 10H, Ar), 8.75 (s, 1H, NH).
3.3.7. Allyl 2-O-benzyl-3,4-di-O-chloroacetyl-α-l-fucopyranosyl-(1→3)-2-O-benzyl-4-O-chloroacetyl-α-l-fucopyranosyl-(1→3)-(methyl 2,4-di-O-acetyl-β-d-glucopyranosyl Uronate) (19)
A solution of the disaccharide 18 (200 mg, 0.23 mmol) and the monosaccharide 10 (76 mg, 0.23 mmol) in CH2Cl2 (2 mL) was stirred at rt under argon atmosphere with molecular sieves 4 Å (200 mg) for 1 h. The mixture was cooled to −30 °С and TMSOTf of (2 µL) was added. The mixture was stirred for 15 min at −30 °С, then Et3N (0.05 mL) was added. The mixture was filtered through a celite pad, and the filtrate was concentrated in vacuo. Column chromatography of the residue on a silica gel gave the trisaccharide 19 as an amorphous solid (214 mg, 0.20 mmol, 90%): Rf 0.4 (Toluene–EtOAc 4:1), [α]D = −152° (c 1, EtOAc); 1H NMR (600 MHz, CDCl3): δ 1.06 (d, J5,6 6.5 Hz, 6H, H-6Fuc1, H-6Fuc2), 1.76 (s, 3H, COCH3), 2.06 (s, 3H, COCH3), 3.76 (m, 4H, C(O)OCH3, H-2Fuc1), 3.83 (m, 2H, H-3GlcA, H-2Fuc2), 3.94 (m, 3H, H-5GlcA, ClCH2CO), 4.12 (m, 5H, H-3Fuc1, H-5Fuc1, CHHCH=CH2, ClCH2CO), 4.16 (s, 2H, ClCH2CO), 4.30 (q, 1H, H-5Fuc2), 4.38 (m, 1H, CHHCH=CH2), 4.55 (d, Jgem 12.1 Hz, 1H, PhCHH), 4.58 (d, J1,2 7.7 Hz, 1H, H-1GlcA), 4.64 (d, Jgem 11.2 Hz, 1H, PhCHH), 4.69 (d, Jgem 11.2 Hz, 1H, PhCHH), 4.73 (d, Jgem 12.1 Hz, 1H, PhCHH), 4.94 (d, J1,2 3.5 Hz, 1H, H-1Fuc1), 5.12 (m, 3H, H-2GlcA, H-4GlcA, H-1Fuc2), 5.21 (m, 2H, H-4Fuc2, CH2CH=CHH), 5.30 (m, 1H, CH2CH=CHH), 5.37 (d, J3,4 3.3 Hz, 1H, H-4Fuc1), 5.53 (dd, J2,3 10.5 Hz, J3,4 3.2 Hz, 1H, H-3Fuc2), 5.85 (m, 1H, CH2CH=CH2), 7.29–7.44 (m, 10H, Ar). HRMS (ESI) C46H55Cl3O20 [M + Na]+ calc.: 1055.2250, found: 1055.2246.
3.3.8. Allyl 2-O-benzyl-α-l-fucopyranosyl-(1→3)-2-O-benzyl-α-l-fucopyranosyl-(1→3)-(methyl 2,4-di-O-acetyl-β-d-glucopyranosyl Uronate) (20)
To a solution of the trisaccharide 19 (200 mg, 0.19 mmol) in MeOH (3 mL) thiourea (60 mg, 0.78 mmol) and collidine (100 µL) were added. The mixture was kept at 40 °С for 2 h, then it was diluted with EtOAc (20 mL) and washed with HCl (0.1 M) (10 mL) and distilled water (2 × 20 mL). Organic layer was separated and concentrated in vacuo. Chromatography of the residue on a silica gel column gave the triol 20 as an amorphous solid (140 mg, 0.17 mmol, 92%): Rf 0.1 (TolueneEtOAc, 1:1), [α]D = −168 (c 1, EtOAc); 1H NMR (600 MHz, CDCl3): δ 1.18 (d, J5,6 6.5 Hz, 3H, H-6Fuc2), 1.21 (d, J5,6 6.5 Hz, 3H, H-6Fuc1), 1.89 (s, 3H, COCH3), 2.05 (s, 3H, COCH3), 3.11 (s, 3H, 3OH), 3.64 (d, J3,4 2.2 Hz, 1H, H-4Fuc2), 3.73 (m, 4H, H-3GlcA, H-2Fuc1, H-2Fuc2, H-4Fuc1), 3.75 (s, 3H, C(O)OCH3), 3.89 (m, 2H, H-5GlcA, H-3Fuc2), 3.98 (m, 2H, H-3Fuc1, H-5Fuc1), 4.13 (m, 2H, H-5Fuc2, CHHCH=CH2), 4.38 (m, 1H, CHHCH=CH2), 4.58 (m, 3H, H-1GlcA, PhCH2), 4.72 (s, 2H, PhCH2), 4.86 (d, J1,2 3.4 Hz, 1H, H-1Fuc2), 4.89 (d, J1,2 3.6 Hz, 1H, H-1Fuc1), 5.10 (t, J2,3 = J3,4 8.5 Hz, 1H, H-2GlcA), 5.14 (t, J3,4 = J4,5 8.5 Hz, 1H, H-4GlcA), 5.22 (m, 1H, CH2CH=CHH), 5.30 (m, 1H, CH2CH=CHH), 5.87 (m, 1H, CH2CH=CH2), 7.20–7.40 (m, 10H, Ar). HRMS (ESI) C40H52O17 [M + Na]+ calc.: 827.3102, found: 827.3105.
3.3.9. Propyl α-l-fucopyranosyl-(1→3)-β-d-glucopyranosyl Uronic Acid Sodium Salt (1)
To a solution of the disaccharide 15 (57 mg, 0.10 mmol) in MeOH (1.5 mL) and EtOAc (1.5 mL) Pd(OH)2/C (15 mg) was added. The mixture was stirred for 1 h at rt under hydrogen atmosphere, then it was filtered through a celite pad, and the filtrate was concentrated in vacuo. Column chromatography of the residue on a silica gel (Toluene–EtOAc) gave an amorphous solid, which was dissolved in THF (1.0 mL), and 0.1N(aq) LiOH (0.5 mL) was added. The mixture was kept for 1 h at rt and then 0.1N(aq) NaOH (0.5 mL) was added, and the solution was kept at 40 °C for 1 h. After the mixture was filtered through the Whatman paper filter, the filtrate was concentrated in vacuo to a volume of 1 mL. Column chromatography of the residue on the Sephadex G-15 gel in water gave the disaccharide 1 (32 mg, 0.08 mmol, 79%). [α]D = −44° (c 0.5, H2O). HRMS (ESI) C15H25NaO11 [M − Na]− calc.: 381.1397, found: 381.1402.
3.3.10. Propyl 2-O-sulfonato-α-l-fucopyranosyl-(1→3)-β-d-glucopyranosyl Uronic Acid Disodium Salt (2)
To a solution of the disaccharide 15 (57 mg, 0.10 mmol) in CH2Cl2 (1 mL) Py (0.1 mL) and AcCl (0.1 mL) were added. The mixture was kept for 2 h at rt, then it was diluted with EtOAc (20 mL) and washed with HCl (0.1 M) (10 mL) and distilled water (2 × 15 mL). Organic layer was separated and concentrated in vacuo. Chromatography of the residue on a silica gel column (Toluene–EtOAc) gave the totally protected disaccharide, which was dissolved in a 1:1 mixture MeOH–EtOAc (3 mL) and Pd(OH)2/C (15 mg) was added. The mixture was stirred for 1 h at rt under hydrogen atmosphere, then it was filtered through a celite pad, and the filtrate was concentrated in vacuo. Flash chromatography of the residue on a silica gel column (Toluene–EtOAc) gave an amorphous solid, which was dissolved in DMF (1 mL) and PySO3 (80 mg, 0.5 mmol) was added. The reaction mixture was kept for 1 h at rt and then quenched with NaHCO3 (200 mg). The resin Amberlite IR-120 (Na+) and MeOH (1 mL) were added, and the mixture was stirred for 1 h. Then the resin was filtered off, and the filtrate was concentrated in vacuo to a volume of 1 mL. Chromatography of the residue on a silica gel column (CH2Cl2–MeOH) gave the sulfated disaccharide, which was dissolved in THF (1.0 mL), and 0.1N(aq) LiOH (0.5 mL) was added. The mixture was kept for 1 h at rt and then 0.1N(aq) NaOH (0.5 mL) was added, and the solution was kept at 40 °C for 1 h. After the mixture was filtered through the Whatman paper filter, the filtrate was concentrated in vacuo to a volume of 1 mL. Column chromatography of the residue on the Sephadex G-15 gel in water gave the disaccharide 2 (33 mg, 0.065 mmol, 64%). [α]D = −38° (c 0.6, H2O). HRMS (ESI) C15H24Na2O14S [M − Na]− calc.: 483.0784, found: 483.0790.
3.3.11. Propyl 3,4-di-O-sulfonato-α-l-fucopyranosyl-(1→3)-β-d-glucopyranosyl Uronic Acid Trisodium Salt (3)
To a solution of the disaccharide 15 (57 mg, 0.10 mmol) in DMF (1 mL) PySO3 (80 mg, 0.5 mmol) was added. The reaction mixture was kept for 1 h at rt and then quenched with NaHCO3 (200 mg). The resin Amberlite IR-120 (Na+) and MeOH (1 mL) were added, and the mixture was stirred for 1 h. Then the resin was filtered off, and the filtrate was concentrated in vacuo to a volume of 1 mL. Chromatography of the residue on a silica gel column (CH2Cl2–MeOH) gave the sulfated disaccharide, which was dissolved in THF (1.0 mL) and H2O (50 µL), and Pd(OH)2/C (15 mg) was added. The mixture was stirred for 30 min at rt under hydrogen atmosphere, then it was filtered through a celite pad. To the filtrate 0.1N(aq) LiOH (0.5 mL) was added. The mixture was kept for 1 h at rt and then 0.1N(aq) NaOH (0.5 mL) was added, and the solution was kept at 40 C for 1 h. Then the mixture was filtered through the Whatman paper filter, and the filtrate was concentrated in vacuo to a volume of 1 mL. Column chromatography of the residue on the Sephadex G-15 gel in water gave the disaccharide 3 (37 mg, 0.062 mmol, 62%). [α]D = −30° (c 0.7, H2O). HRMS (ESI) C15H23Na3O17S2 [M − Na]− calc.: 585.0172, found: 585.0178.
3.3.12. Propyl 4-O-sulfonato-α-l-fucopyranosyl-(1→3)-β-d-glucopyranosyl Uronic Acid Disodium Salt (4)
To a solution of the disaccharide 16 (55 mg, 0.08 mmol) in DMF (1 mL) PySO3 (65 mg, 0.4 mmol) was added. The reaction mixture was kept for 1 h at rt and then quenched with NaHCO3 (200 mg). The resin Amberlite IR-120 (Na+) and MeOH (1 mL) were added, and the mixture was stirred for 1 h. Then the resin was filtered off, and the filtrate was concentrated in vacuo to a volume of 1 mL. Chromatography of the residue on a silica gel column (CH2Cl2–MeOH) gave the sulfated disaccharide, which was dissolved in THF (1.0 mL) and H2O (50 µL), and Pd(OH)2/C (15 mg) was added. The mixture was stirred for 30 min at rt under hydrogen atmosphere, then it was filtered through a celite pad. To the filtrate 0.1N(aq) LiOH (0.5 mL) was added. The mixture was kept for 1 h at rt and then 0.1N(aq) NaOH (0.5 mL) was added, and the solution was kept at 40 C for 1 h. Then the mixture was filtered through the Whatman paper filter, and the filtrate was concentrated in vacuo to a volume of 1 mL. Column chromatography of the residue on the Sephadex G-15 gel in water gave the disaccharide 4 (24 mg, 0.048 mmol, 62%). [α]D = −32° (c 0.6, H2O). HRMS (ESI) C15H24Na2O14S [M − Na]− calc.: 483.0784, found: 483.0790.
3.3.13. Propyl 2,4-di-O-sulfonato-α-l-fucopyranosyl-(1→3)-β-d-glucopyranosyl Uronic Acid Trisodium Salt (5)
To a solution of the disaccharide 16 (47 mg, 0.07 mmol) in a 1:1 mixture MeOH–EtOAc (3 mL) Pd(OH)2/C (15 mg) was added. The mixture was stirred for 1 h at rt under hydrogen atmosphere, then it was filtered through a celite pad, and the filtrate was concentrated in vacuo. Flash chromatography of the residue on a silica gel column (Toluene–EtOAc) gave an amorphous solid, which was dissolved in DMF (1 mL) and PySO3 (110 mg, 0.7 mmol) was added. The reaction mixture was kept for 1 h at rt and then quenched with NaHCO3 (200 mg). The resin Amberlite IR-120 (Na+) and MeOH (1 mL) were added, and the mixture was stirred for 1 h. Then the resin was filtered off, and the filtrate was concentrated in vacuo to a volume of 1 mL. Chromatography of the residue on a silica gel column (CH2Cl2–MeOH) gave the sulfated disaccharide, which was dissolved in THF (1.0 mL), and 0.1N(aq) LiOH (0.5 mL) was added. The mixture was kept for 1 h at rt and then 0.1N(aq) NaOH (0.5 mL) was added, and the solution was kept at 40 C for 1 h. Then the mixture was filtered through the Whatman paper filter, and the filtrate was concentrated in vacuo to a volume of 1 mL. Column chromatography of the residue on the Sephadex G-15 gel in water gave the disaccharide 5 (25 mg, 0.04 mmol, 58%). [α]D = −28° (c 0.5, H2O). HRMS (ESI) C15H23Na3O17S2 [M − Na]− calc.: 585.0172, found: 585.0176.
3.3.14. Propyl α-l-fucopyranosyl-(1→3)-α-l-fucopyranosyl-(1→3)-β-d-glucopyranosyl Uronic Acid Sodium Salt (6)
Treatment of the trisaccharide 20 (40 mg, 0.05 mmol) as described for the preparation of the compound 1 gave the trisaccharide 6 (19 mg, 0.035 mmol, 69%). [α]D = −68° (c 0.5, H2O). HRMS (ESI) C21H35NaO15 [M − Na]− calc.: 527.1976, found: 527.1981.
3.3.15. Propyl 2-O-sulfonato-α-l-fucopyranosyl-(1→3)-2-O-sulfonato-α-l-fucopyranosyl-(1→3)-β-d-glucopyranosyl Uronic Acid Trisodium Salt (7)
Treatment of the trisaccharide 20 (40 mg, 0.05 mmol) as described for the preparation of the compound 2 gave the trisaccharide 7 (20 mg, 0.027 mmol, 54%). [α]D = −48° (c 0.6, H2O). HRMS (ESI) C21H33Na3O21S2 [M − Na]− calc.: 731.0731, found: 731.0736.
3.3.16. Propyl 3,4-di-O-sulfonato-α-l-fucopyranosyl-(1→3)-4-O-sulfonato-α-l-fucopyranosyl-(1→3)-β-d-glucopyranosyl Uronic Acid Tetrasodium Salt (8)
Treatment of the trisaccharide 20 (45 mg, 0.056 mmol) as described for the preparation of the compound 3 gave the trisaccharide 8 (30 mg, 0.035 mmol, 62%). [α]D = −42° (c 0.6, H2O). HRMS (ESI) C21H32Na4O24S3 [M − Na]− calc.: 833.0139, found: 833.0143.
3.4. Calculations
Starting structures for geometry optimizations were built as follows: for the forms without stabilization, torsional angles H3-C3-O3-CO were set to −40°, C3-O3-CO-O to 0°; and H4-C4-O4-CO to +40°, C4-O4-CO-O to 0°. For the forms with supposed participation of the carbonyl oxygen at O3, torsion H3-C3-O3-CO were set to 180°, and for the supposed participation from O4, torsion H4-C4-O4-CO were set to 180°, putting the corresponding carbonyl oxygen into the proximity of the cationic center.
MM+ optimizations were carried out as described in the text until the RMS gradient attained 0.01 kcal/mol·Å.
Ab initio calculations used the SCF/MP2 approach with frozen core orbitals: 1s were frozen for all carbons and oxygens, 1s, 2s and 2p were frozen for chlorines. Optimizations were carried out until the RMS gradient attained the value of 0.0001. After that, vibrational analysis was performed to check that no negative frequencies were present.
4. Conclusions
The efficient synthesis of a series of non-sulfated and selectively O-sulfated di- and trisaccharides related to branching sites of fucosylated chondroitin sulfates from holothurias has been performed. The synthesized compounds are built up of the propyl β-d-glucuronic acid residue bearing at O-3 α-l-fucosyl or α-l-fucosyl-(1→3)-α-l-fucosyl substituents. The sulfation pattern in the fucosyl units was selected according to the known to date holothurian FCS structures. Stereospecific formation of the α-glycoside bond was achieved using 2-O-benzyl-3,4-di-O-chloroacetyl-α-l-fucosyl trichloroacetimidate as a donor. Stereochemical outcome of the glycosylation was explained by the possible remote participation of the chloroacetyl groups at O-3 and O-4 with the intermediate formation of the stabilized glycosyl cations, which are hindered from the β-side, and thus could only be attacked by the glycosyl acceptor from the α-side. The experimental results in glycosylation reactions were in good agreement with the SCF/MP2 calculated energies of such participation. Obtained NMR characteristics of synthesized oligosaccharides can be used as model in further analyses of natural FCSs.
Acknowledgments
The work was supported by the Russian Scientific Foundation (Grant 14-13-01325). The authors are grateful to A.O. Chizhov for recording of high-resolution mass spectra.
Author Contributions
NEN and NEU conceived the study. NEU synthesized and characterized compounds 9–12, 16–19 and 6–8. PAF synthesized and characterized compounds 14–15 and 1–5. AGG performed calculations of the values of the energy of stabilization of fucosyl cations. ASD recorded NMR spectra of all compounds and made signal assignment. All authors participated in interpreting the results and preparing the final version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Galanaud, J.P.; Laroche, J.P.; Righini, M. The history and historical treatments of deep vein thrombosis. J. Thomb. Haemost. 2013, 3, 402–411. [Google Scholar]
- Petitou, M.; van Boeckel, C.A. A synthetic antithrombin III binding pentasaccharide is now a drug! What comes next? Angew. Chem. Int. Ed. Engl. 2004, 43, 3118–3133. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, J.L.; Varki, A.; Borsig, L. Heparin attenuates metastasis mainly due to inhibition of P- and L-selectin, but non-anticoagulant heparins can have additional effects. Thromb. Res. 2007, 120, S107–S111. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Brown, J.R.; Varki, A.; Esko, J.D. Heparin’s anti-inflammatory effects require glucosamine 6-O-sulfation and are mediated by blockade of L- and P-selectins. J. Clin. Invest. 2002, 110, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Pomin, V.H. Holothurian Fucosylated Chondroitin Sulfate. Mar. Drugs 2014, 12, 232–254. [Google Scholar] [CrossRef] [PubMed]
- Borsig, L.; Wang, L.; Cavalcante, M.C.; Cardilo-Reis, L.; Ferreira, P.L.; Mourão, P.A.; Esko, J.D.; Pavão, M.S. Selectin blocking activity of a fucosylated chondroitin sulfate glycosaminoglycan from sea cucumber. Effect on tumor metastasis and neutrophil recruitment. J. Biol. Chem. 2007, 282, 14984–14991. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, R.J.; Oliveira, S.N.; Pomin, V.H.; Mecawi, A.S.; Araujo, I.G.; Mourão, P.A. Effects of oversulfated and fucosylated chondroitin sulfates on coagulation. Challenges for the study of anticoagulant polysaccharides. Thromb. Haemost. 2010, 103, 994–1004. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, D.; Wang, S.; Tao, L.; Wang, A.; Chen, W.; Zhu, Z.; Zheng, S.; Gao, X.; Lu, Y. Holothurian glycosaminoglycan inhibits metastasis and thrombosis via targeting of nuclear factor-κB/tissue factor/Factor Xa pathway in melanoma B16F10 cells. PLoS One 2013, 8, e56557. [Google Scholar] [CrossRef] [PubMed]
- Panagos, C.G.; Thomson, D.S.; Moss, C.; Hughes, A.D.; Kelly, M.S.; Liu, Y.; Chai, W.; Venkatasamy, R.; Spina, D.; Page, C.P.; et al. Fucosylated chondroitin sulfates from the body wall of the sea cucumber Holothuria. forskali: Conformation, selectin binding, and biological activity. J. Biol. Chem. 2014, 289, 28284–28298. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Wu, M.; Xu, L.; Lian, W.; Xiang, J.; Lu, F.; Gao, N.; Xiao, C.; Wang, S.; Zhao, J. Comparison of physicochemical characteristics and anticoagulant activities of polysaccharides from three sea cucumbers. Mar. Drugs 2013, 11, 399–417. [Google Scholar] [CrossRef] [PubMed]
- Myron, P.; Siddiquee, S.; Al Azad, S. Fucosylated chondroitin sulfate diversity in sea cucumbers: A review. Carbohydr. Polym. 2014, 112, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Mourão, P.A.; Giumarães, B.; Mulloy, B.; Thomas, S.; Gray, E. Antithrombotic activity of a fucosylated chondroitin sulphate from echinoderm: Sulphated fucose branches on the polysaccharide account for its antithrombotic action. Br. J. Haematol. 1998, 101, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Kang, H.; Wu, M.; Zeng, W.; Li, Z.; Gao, Y.; Cui, J.; Wang, J.; Feng, H.; Yu, L. Depolymerized Glycosaminoglycan from Thelenota. ananas and Preparation Method Thereof. US Patent 20120270834 A1, 25 October 2012. [Google Scholar]
- Chen, S.; Xue, C.; Yin, L.; Tang, Q.; Yu, G.; Chai, W. Comparison of structures and anticoagulant activities of fucosylated chondroitin sulfates from different sea cucumbers. Carbohydr. Polym. 2011, 83, 688–695. [Google Scholar] [CrossRef]
- Kariya, Y.; Watabe, S.; Kyogashima, M.; Ishihara, M.; Ishii, T. Structure of fucose branches in the glycosaminoglycan from the body wall of the sea cucumber Stichopus. japonicus. Carbohydr. Res. 1997, 297, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Tamura, J.; Tanaka, H.; Nakamura, A.; Takeda, N. Synthesis of β-d-GalNAc(4,6-diS)(1→4) [α-l-Fuc(2,4-diS)(1→3)]-β-d-GlcA, a novel trisaccharide unit of chondroitin sulfate with a fucose branch. Tetrahedr. Lett. 2013, 54, 3940–3943. [Google Scholar] [CrossRef]
- Kornilov, A.V.; Sukhova, E.V.; Nifantiev, N.E. Preparative route to glucuronyl donors bearing temporary protecting group at O-3 via 6,3-lactonisation by Bz2O or Piv2O. Carbohydr. Res. 2001, 336, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Gerbst, A.G.; Ustuzhanina, N.E.; Grachev, A.A.; Tsvetkov, D.E.; Khatuntseva, E.A.; Whitefield, D.M.; Berces, A.; Nifantiev, N.E. Synthesis, NMR and conformational studies of fucoidan fragments. Part 3. Effect of benzoyl group at O-3 on stereoselectivity of glycosylation by 3-O- and 3,4-di-O-benzoylated 2-O-benzylfucosyl bromides. J. Carbohydr. Chem. 2001, 20, 821–831. [Google Scholar] [CrossRef]
- Komarova, B.S.; Ustyuzhanina, N.E.; Tsvetkov, Y.E.; Nifantiev, N.E. Stereocontrol of 1,2-cis-Glycosylation by remote O-Acyl protecting groups. In Modern Synthetic Methods in Carbohydrate Chemistry—From Monosaccharides to Complex Glycoconjugates; Vidal, S., Wertz, D., Eds.; Wiley-VCH: Weinheim, Germany, 2013; pp. 125–159. [Google Scholar]
- Ustyuzhanina, N.; Krylov, V.; Grachev, A.; Gerbst, A.; Nifantiev, N. Synthesis, NMR and conformational studies of fucoidan fragments. part 8: Convergent block-wise synthesis of long chain linear and 2,3-branched oligosaccharides. Synthesis 2006, 23, 4017–4031. [Google Scholar]
- Gerbst, A.G.; Ustuzhanina, N.E.; Grachev, A.A.; Tsvetkov, D.E.; Khatuntseva, E.A.; Nifant’ev, N.E. Effect of the nature of protecting group at O-4 on stereoselectivity of glycosylation by 4-O-substituted 2,3-di-O-benzylfucosyl bromides. Mend. Commun. 1999, 9, 114–116. [Google Scholar] [CrossRef]
- Hypercube, Inc. Available online: http://www.hyper.com (accessed on 27 January 2015).
- Allinger, N.L. Conformational analysis. 130. MM2. A hydrocarbon force field utilizing V1 and V2 torsional terms. J. Am. Chem. Soc. 1977, 99, 8127–8134. [Google Scholar] [CrossRef]
- Stewart, J.J.P. Optimization of parameters for semiempirical methods I. Method. J. Comp. Chem. 1989, 10, 209–220. [Google Scholar] [CrossRef]
- Stewart, J.J.P. Optimization of parameters for semiempirical methods II. Applications. J. Comp. Chem. 1989, 10, 221–264. [Google Scholar] [CrossRef]
- Valiev, M.; Bylaska, E.J.; Govind, N.; Kowalski, K.; Straatsma, T.P.; van Dam, H.J.J.; Wang, D.; Nieplocha, J.; Apra, E.; Windus, T.L.; et al. NWChem: A comprehensive and scalable open-source solution for large scale molecular simulations. Comput. Phys. Commun. 2010, 181, 1477–1489. [Google Scholar] [CrossRef]
- Khatuntseva, E.A.; Ustuzhanina, N.E.; Zatonskii, G.V.; Shashkov, A.S.; Usov, A.I.; Nifant’ev, N.E. Synthesis, NMR and conformational studies of fucoidan fragments. Part 1: Desulfated 2,3- and 3,4-branched trisaccharide fragments and constituting disaccharides. J. Carbohydr. Chem. 2000, 19, 1151–1173. [Google Scholar] [CrossRef]
- Belyakov, P.A.; Kadentsev, V.I.; Chizhov, A.O.; Kolotyrkina, N.G.; Shashkov, A.S.; Ananikov, V.P. Mechanistic insight into organic and catalytic reactions by joint studies using mass spectrometry and NMR spectroscopy. Mendel. Commun. 2010, 20, 125–131. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
