Dolabelladienols A–C, New Diterpenes Isolated from Brazilian Brown Alga Dictyota pfaffii
Abstract
:1. Introduction
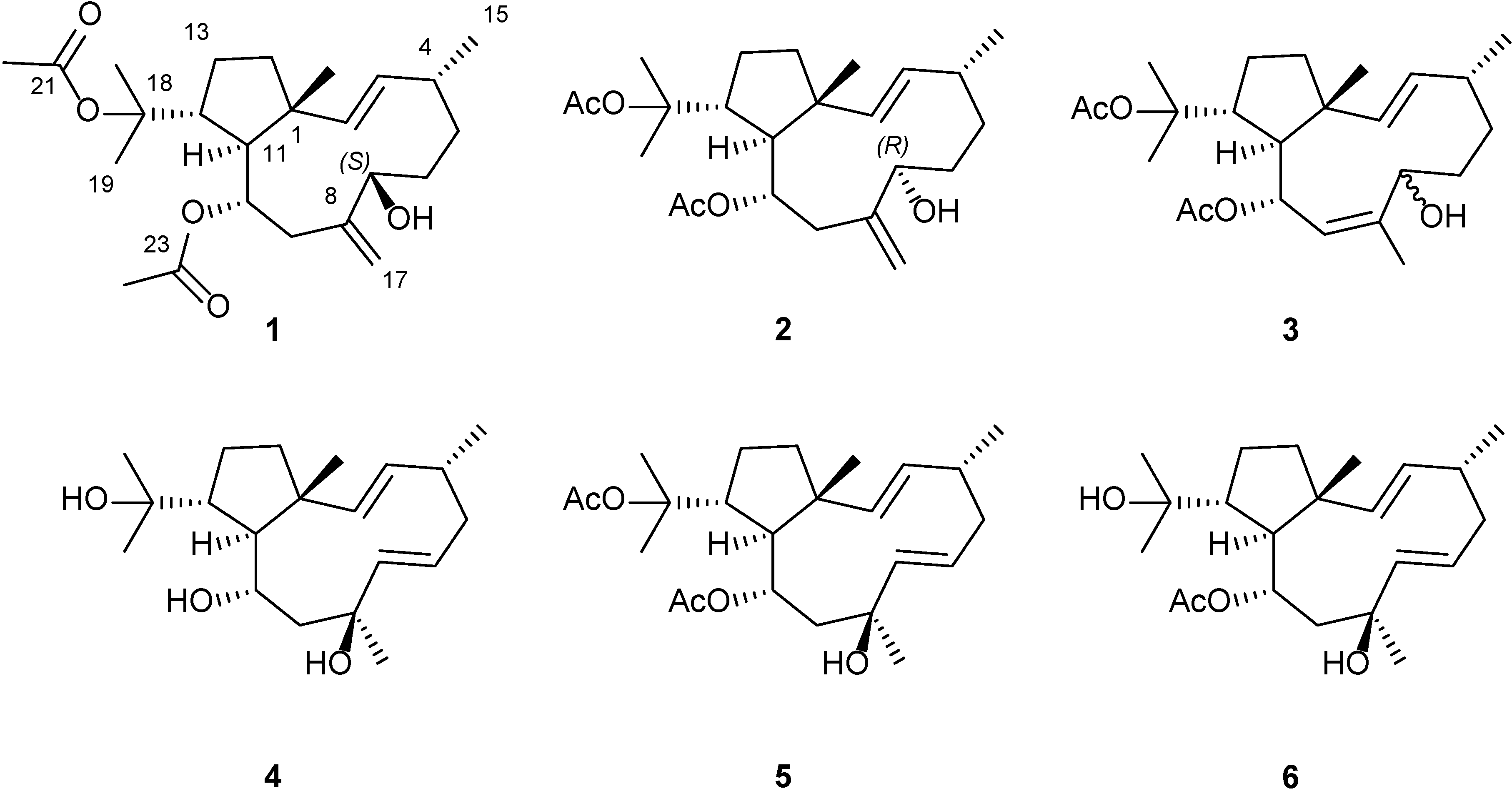
2. Results and Discussion
2.1. Structural Elucidation
| Position | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| δC | δH | δC | δH | δC | δH | |
| 1 | 48.4 | - | 48.1 | - | 48.9 | - |
| 2 | 137.5 | 5.17 (d, 16.4) | 138.9 | 5.24 (d, 16.4) | 136.9 | 5.23 (dd, 16.4, 0.7) |
| 3 | 133.6 | 5.66 (dd, 16.4, 7.2) | 134.5 | 5.53 (dd, 16.4, 8.5) | 134.1 | 5.70 (dd, 16.4, 6.9) |
| 4 | 34.8 | 2.20 (m) | 34.7 | 2.04 (m) | 34.9 | 2.39 (ddd, 13.9, 7.7, 3.3) |
| 5 | 32.0 | 1.58 (m) 1.63 (m) | 33.2 | 1.76 (dd, 11.2, 2.3) 1.08 (dd, 11.2, 4.9) | 33.5 | 2.34 (m) 1.71 (m) |
| 6 | 32.6 | 1.62 (m) 1.90 (m) | 33.3 | 1.85 (dd, 9.0, 4.9) 1.64 (m) | 30.7 | 1.45 (m) 1.66 (m) |
| 7 | 70.2 | 4.37 (dd, 8.4, 3.5) | 76.7 | 3.95 (dd, 11.4, 4.9) | 69.0 | 5.08 (t, 6.6) |
| 8 | 146.2 | - | 144.8 | - | 140.1 | - |
| 9 | 41.0 | 2.43 (dd, 13.2, 4.7) 2.46 (dd, 13.2, 8.9) | 36.5 | 2.65 (dd, 13.8, 10.1) 2.50 (br d, 13.8) | 125.8 | 5.31 (d, 10.4) |
| 10 | 68.6 | 4.91 (ddd, 8.9, 4.7, 2.4) | 70.4 | 4.88 (ddd, 10.1, 3.2, 2.1) | 67.9 | 5.58 (dd, 10.4, 2.3) |
| 11 | 51.1 | 1.84 (dd, 10.8, 2.4) | 50.6 | 1.94 (dd, 10.5, 3.2) | 53.3 | 1.76 (dd, 10.1, 2.1) |
| 12 | 44.2 | 2.95 (td, 10.8, 4.7) | 44.2 | 3.06 (td, 10.5, 4.9) | 44.3 | 2.98 (m) |
| 13 | 26.4 | 1.95 (m) 1.45 (m) | 26.5 | 1.96 (m) 1.48 (m) | 26.2 | 1.95 (m), 1.46 (m) |
| 14 | 38.9 | 1.46 (m) 1.39 (m) | 38.7 | 1.51 (m) 1.47 (m) | 39.2 | 1.49 (m) 1.39 (m) |
| 15 | 16.8 | 0.83 (s) | 18.4 | 0.83 (s) | 16.9 | 0.93 (s) |
| 16 | 22.2 | 0.97 (d 6.9 ) | 21.1 | 1.00 (d, 6.7) | 19.8 | 0.96 (d, 6.9) |
| 17 | 113.5 | 5.12 (brs) 4.99 (brs) | 117.0 | 5.04 (brs) 4.89 (brs) | 17.0 | 1.66 (d, 1.4) |
| 18 | 84.8 | - | 84.8 | - | 84.9 | - |
| 19 | 23.4 | 1.42 (s) | 23.2 | 1.45 (s) | 26.0 | 1.59 (s) |
| 20 | 26.4 | 1.60 (s) | 26.6 | 1.61 (s) | 23.6 | 1.43 (s) |
| 21 | 170.5 | - | 171.5 | - | 170.2 | - |
| 22 | 21.0 | 2.04 (s) | 21.0 | 2.05 (s) | 23.0 | 1.97 (s) |
| 23 | 170.1 | - | 170.0 | - | 170.8 | - |
| 24 | 23.0 | 1.93 (s) | 22.9 | 1.92 (s) | 21.1 | 2.02 (s) |
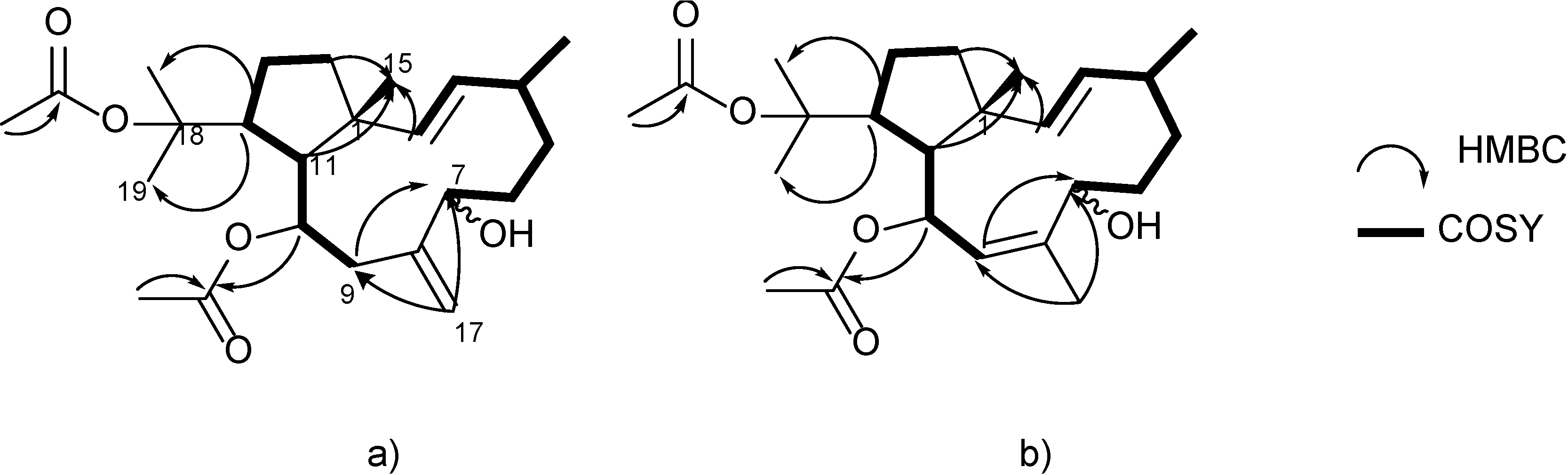
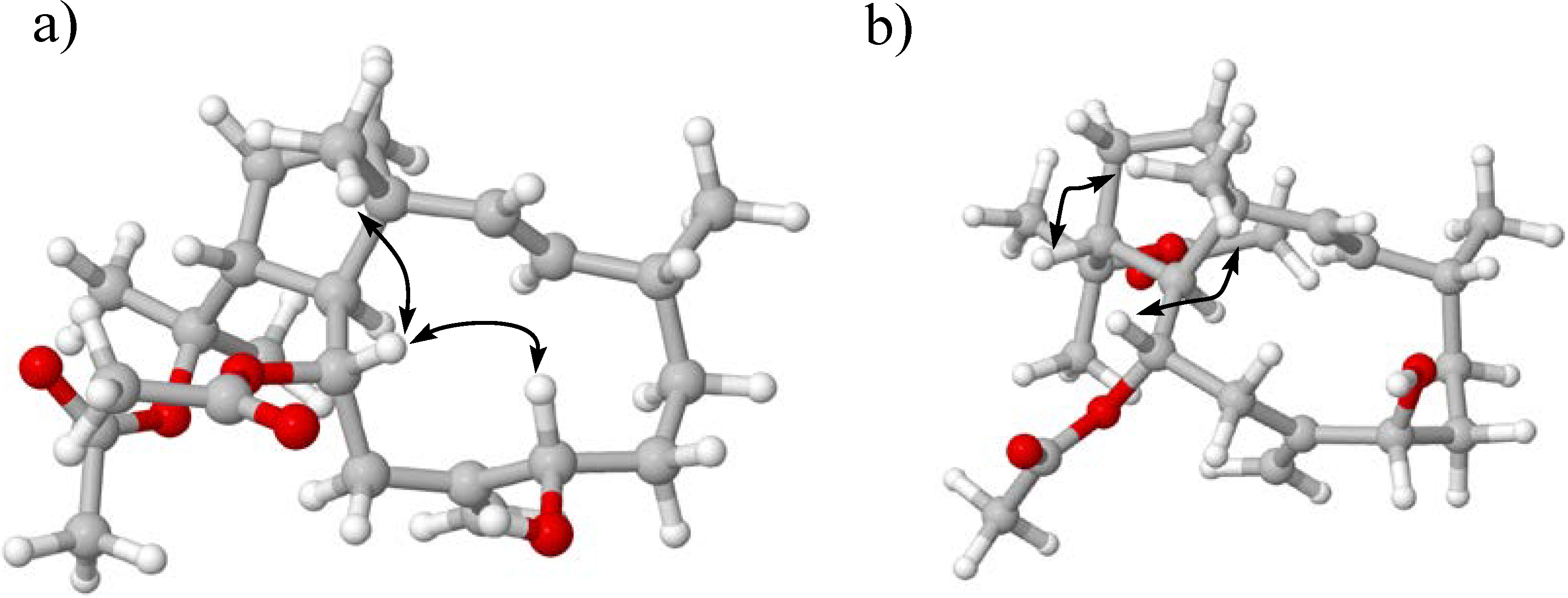
3. Experimental Section
3.1. General Experimental Procedures
3.2. Sample Collection
3.3. Extraction, Isolation and Structural Elucidation of Compounds
3.4. Conformational Searches
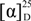 +11° (c 0.002, CHCl3), RI 2593. 1H and 13C NMR data, see Table 1. EIMS m/z (relative intensity) 406 (4), 346 (3), 304 (4), 286 (100), 271 (46), 243 (61), 215 (25), 173 (34), 145 (32), 133 (30), 107 (75), 93 (80), 55 (65). HRESIMS: [M + Na]+ 429.2631 (C24H38O5Na 429.2611, ∆1.95 mmu).
+11° (c 0.002, CHCl3), RI 2593. 1H and 13C NMR data, see Table 1. EIMS m/z (relative intensity) 406 (4), 346 (3), 304 (4), 286 (100), 271 (46), 243 (61), 215 (25), 173 (34), 145 (32), 133 (30), 107 (75), 93 (80), 55 (65). HRESIMS: [M + Na]+ 429.2631 (C24H38O5Na 429.2611, ∆1.95 mmu).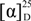 +4° (c 0.002, CHCl3), RI 2567. 1H and 13C NMR data, see Table 1. HRESIMS 835.5353 [2M + Na]+; 429.2627 [M + Na]+; EIMS m/z (relative intensity) 406 [M]+ (3), 328 [M – AcOH − H2O]+ (5), 304 (7), 286 [M − AcOH − AcOH]+ (45), 243 (43), 215 (20), 187 (28), 161 (47), 135 (65), 107 (77), 93 (100), 55 (60).
+4° (c 0.002, CHCl3), RI 2567. 1H and 13C NMR data, see Table 1. HRESIMS 835.5353 [2M + Na]+; 429.2627 [M + Na]+; EIMS m/z (relative intensity) 406 [M]+ (3), 328 [M – AcOH − H2O]+ (5), 304 (7), 286 [M − AcOH − AcOH]+ (45), 243 (43), 215 (20), 187 (28), 161 (47), 135 (65), 107 (77), 93 (100), 55 (60).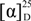 +14° (c 0.002, CHCl3), RI 2475. 1H and 13C NMR data, see Table 1 EIMS m/z (relative intensity) 406 (4) 304 (5) 286 (38) 271 (31) 243 (32) 229 (12) 203 (23) 175 (51) 133 (52) 121 (71) 107 (75) 95 (100) 79 (58) 69 (53) 55 (63).
+14° (c 0.002, CHCl3), RI 2475. 1H and 13C NMR data, see Table 1 EIMS m/z (relative intensity) 406 (4) 304 (5) 286 (38) 271 (31) 243 (32) 229 (12) 203 (23) 175 (51) 133 (52) 121 (71) 107 (75) 95 (100) 79 (58) 69 (53) 55 (63).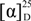 −27° (c, 0.5, CHCl3), RI 2257, NMR, and MS data are consistent with literature values [15].
−27° (c, 0.5, CHCl3), RI 2257, NMR, and MS data are consistent with literature values [15].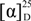 −123° (c 0.002, CHCl3), RI 2475, NMR, and MS data are consistent with literature values [15].
−123° (c 0.002, CHCl3), RI 2475, NMR, and MS data are consistent with literature values [15]. −70° (c, 0.5, CHCl3), RI 2314, NMR, and MS data are consistent with literature values [15].
−70° (c, 0.5, CHCl3), RI 2314, NMR, and MS data are consistent with literature values [15].3.5. X-Ray Diffraction Analysis of Compound 4
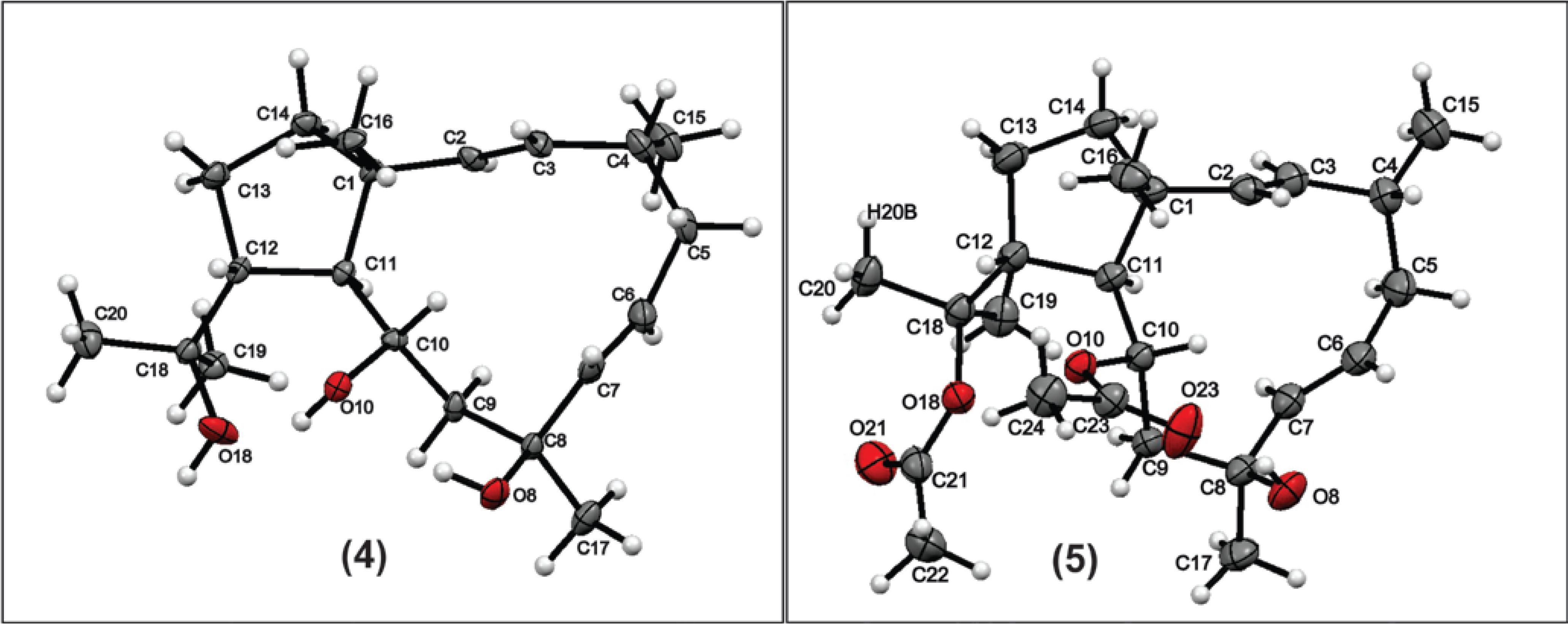
3.6. Cells and Viruses
3.7. Cytotoxicity Assays
4. Conclusions
Supplementary Files
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhou, X.; Liu, J.; Yang, B.; Lin, X.; Yang, X.W.; Liu, Y. Marine natural products with anti-HIV activities in the last decade. Curr. Med. Chem. 2013, 20, 953–973. [Google Scholar]
- Cirne-Santos, C.C.; Souza, T.M.; Teixeira, V.L.; Fontes, C.F.; Rebello, M.A.; Castello-Branco, L.R.; Abreu, C.M.; Tanuri, A.; Frugulhetti, I.C.; Bou-Habib, D.C.; et al. The dolabellane diterpene Dolabelladienetriol is a typical noncompetitive inhibitor of HIV-1 reverse transcriptase enzyme. Antivir. Res. 2008, 77, 64–71. [Google Scholar] [CrossRef]
- Huang, L.; Chen, C.H. Molecular targets of anti-HIV-1 triterpenes. Curr. Drug Targets Infect. Disord. 2002, 2, 33–36. [Google Scholar]
- Pereira, H.S.; Leão-Ferreira, L.R.; Moussatché, N.; Teixeira, V.L.; Cavalcanti, D.N.; Costa, L.J.; Diaz, R.; Frugulhetti, I.C.P.P. Antiviral activity of diterpenes isolated from the Brazilian marine alga Dictyota menstrualis against human immunodeficiency virus type 1 (HIV-1). Antivir. Res. 2004, 64, 69–76. [Google Scholar]
- Aiken, C.; Chen, C.H. Betulinic acid derivatives as HIV-1 antivirals. Trends Mol. Med. 2005, 11, 31–36. [Google Scholar] [CrossRef]
- Dang, Z.; Qian, K.; Ho, P.; Zhu, L.; Lee, K.H.; Huang, L.; Chen, C.H. Synthesis of betulinic acid derivatives as entry inhibitors against HIV-1 and bevirimat-resistant HIV-1 variants. Bioorg. Med. Chem. Lett. 2012, 22, 5190–5194. [Google Scholar]
- Xu, H.X.; Zeng, F.Q.; Wan, M.; Sim, K.Y. Anti-HIV triterpene acids from Geum japonicum. J. Nat. Prod. 1996, 59, 643–645. [Google Scholar] [CrossRef]
- Gochfeld, D.J.; el Sayed, K.A.; Yousaf, M.; Hu, J.F.; Bartyzel, P.; Dunbar, D.C.; Wilkins, S.P.; Zjawiony, J.K.; Schinazi, R.F.; Schlueter Wirtz, S.; et al. Marine natural products as lead anti-HIV agents. Mini Rev. Med. Chem. 2003, 3, 401–424. [Google Scholar] [CrossRef]
- Ioannou, E.; Quesada, A.; Rahman, M.M.; Gibbons, S.; Vagias, C.; Roussis, V. Dolabellanes with antibacterial activity from the brown alga Dilophus spiralis. J. Nat. Prod. 2011, 74, 213–222. [Google Scholar]
- Wei, X.; Rodríguez, A.D.; Baran, P.; Raptis, R.G. Dolabellane-Type Diterpenoids with Antiprotozoan Activity from a Southwestern Caribbean Gorgonian Octocoral of the Genus Eunicea. J. Nat. Prod. 2010, 73, 925–934. [Google Scholar] [CrossRef]
- Konig, G.M.; Wright, A.D.; Sticher, O.; Angerhofer, C.K.; Pezzuto, J.M. Biological activities of selected marine natural products. Planta Med. 1994, 60, 532–537. [Google Scholar] [CrossRef]
- Yodsaoue, O.; Sonprasit, J.; Karalai, C.; Ponglimanont, C.; Tewtrakul, S.; Chantrapromma, S. Diterpenoids and triterpenoids with potential anti-inflammatory activity from the leaves of Aglaia odorata. Phytochemistry 2012, 76, 83–91. [Google Scholar] [CrossRef]
- Konig, G.M.; Wright, A.D.; Franzblau, S.G. Assessment of antimycobacterial activity of a series of mainly marine derived natural products. Planta Med. 2000, 66, 337–342. [Google Scholar] [CrossRef]
- Cirne-Santos, C.C.; Teixeira, V.L.; Castello-Branco, L.R.; Frugulhetti, I.C.; Bou-Habib, D.C. Inhibition of HIV-1 replication in human primary cells by a dolabellane diterpene isolated from the marine algae Dictyota pfaffii. Planta Med. 2006, 72, 295–299. [Google Scholar] [CrossRef]
- Barbosa, J.P.; Pereira, R.C.; Abrantes, J.L.; Cirne dos Santos, C.C.; Rebello, M.A.; Frugulhetti, I.C.; Texeira, V.L. In vitro antiviral diterpenes from the Brazilian brown alga Dictyota pfaffii. Planta Med. 2004, 70, 856–860. [Google Scholar] [CrossRef]
- Vallim, M.A.; de Paula, J.C.; Pereira, R.C.; Teixeira, V.L. The diterpenes from Dictyotacean marine brown algae in the Tropical Atlantic American region. Biochem. Syst. Ecol. 2005, 33, 1–16. [Google Scholar]
- Ireland, C.; Faulkner, D.J. Diterpenes from Dolabella californica. J. Org. Chem. 1977, 42, 3157–3162. [Google Scholar] [CrossRef]
- Lu, Q.; Faulkner, D.J. Three dolabellanes and a macrolide from the sponge Dysidea sp. from Palau. J. Nat. Prod. 1998, 61, 1096–1100. [Google Scholar] [CrossRef]
- Abrantes, J.L.; Barbosa, J.; Cavalcanti, D.; Pereira, R.C.; Frederico Fontes, C.L.; Teixeira, V.L.; Moreno Souza, T.L.; Paixao, I.C. The effects of the diterpenes isolated from the Brazilian brown algae Dictyota pfaffii and Dictyota menstrualis against the herpes simplex type-1 replicative cycle. Planta Med. 2010, 76, 339–344. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef]
- Parsons, S.; Flack, H. Precise absolute-structure determination in light-atom crystals. Acta Crystallogr. A 2004, A60, s61. [Google Scholar]
- Scudiero, D.A.; Shoemaker, R.H.; Paull, K.D.; Monks, A.; Tierney, S.; Nofziger, T.H.; Currens, M.J.; Seniff, D.; Boyd, M.R. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 1988, 48, 4827–4833. [Google Scholar]
- Richman, D.D.; Johnson, V.A.; Mayers, D.M.; Shirasaka, T.; O’Brien, M.C.; Mitsuya, H. In Vitro Evaluation of Experimental Agents for Anti-HIV Activity. In Current Protocols in Immunology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2001. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Pardo-Vargas, A.; De Barcelos Oliveira, I.; Stephens, P.R.S.; Cirne-Santos, C.C.; De Palmer Paixão, I.C.N.; Ramos, F.A.; Jiménez, C.; Rodríguez, J.; Resende, J.A.L.C.; Teixeira, V.L.; et al. Dolabelladienols A–C, New Diterpenes Isolated from Brazilian Brown Alga Dictyota pfaffii. Mar. Drugs 2014, 12, 4247-4259. https://doi.org/10.3390/md12074247
Pardo-Vargas A, De Barcelos Oliveira I, Stephens PRS, Cirne-Santos CC, De Palmer Paixão ICN, Ramos FA, Jiménez C, Rodríguez J, Resende JALC, Teixeira VL, et al. Dolabelladienols A–C, New Diterpenes Isolated from Brazilian Brown Alga Dictyota pfaffii. Marine Drugs. 2014; 12(7):4247-4259. https://doi.org/10.3390/md12074247
Chicago/Turabian StylePardo-Vargas, Alonso, Ingrid De Barcelos Oliveira, Paulo Roberto Soares Stephens, Claudio Cesar Cirne-Santos, Izabel Christina Nunes De Palmer Paixão, Freddy Alejandro Ramos, Carlos Jiménez, Jaime Rodríguez, Jackson Antonio Lamounier Camargos Resende, Valeria Laneuville Teixeira, and et al. 2014. "Dolabelladienols A–C, New Diterpenes Isolated from Brazilian Brown Alga Dictyota pfaffii" Marine Drugs 12, no. 7: 4247-4259. https://doi.org/10.3390/md12074247
APA StylePardo-Vargas, A., De Barcelos Oliveira, I., Stephens, P. R. S., Cirne-Santos, C. C., De Palmer Paixão, I. C. N., Ramos, F. A., Jiménez, C., Rodríguez, J., Resende, J. A. L. C., Teixeira, V. L., & Castellanos, L. (2014). Dolabelladienols A–C, New Diterpenes Isolated from Brazilian Brown Alga Dictyota pfaffii. Marine Drugs, 12(7), 4247-4259. https://doi.org/10.3390/md12074247








