A New Lyngbyatoxin from the Hawaiian Cyanobacterium Moorea producens
Abstract
:1. Introduction

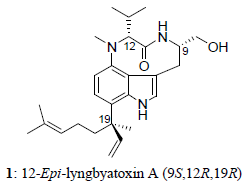
2. Results and Discussion

| No. | δC a | δH (J in Hz) b | HMBC |
|---|---|---|---|
| 1 | 8.45, s | 3, 3a, 7a | |
| 2 | 121.8 | 6.81, d (1.9) | 3, 3a, 7a, 8 |
| 3 | 113.8 | ||
| 3a | 120.9 | ||
| 4 | 146.7 | ||
| 5 | 109.0 | 6.73, d (8.1) | 3, 3a, 4, 7 |
| 6 | 119.8 | 6.95, d (8.1) | 4, 5, 7a, 19 |
| 7 | 122.7 | ||
| 7a | 136.6 | ||
| 8 | 32.3 | 3.27, dd (15.6, 2.5) | 2, 3, 4, 9, 14 |
| 2.90, dd (15.6, 2.5) | |||
| 9 | 57.6 | 3.84, br, m | |
| 10 | 7.45, br, s | 9, 12, 14 | |
| 11 | 175.2 | ||
| 12 | 69.0 | 3.91, d (10.6) | 4, 11, 15, 17 |
| 14 | 65.4 | 3.88, dd (10.4, 3.6) | 8, 9 |
| 3.82, dd (10.4, 6.5) | |||
| 15 | 28.1 | 2.61, m | 12, 16, 17 |
| 16 | 20.0 | 0.67, d (6.6) | 12, 15, 17 |
| 17 | 20.4 | 0.74, d (6.5) | 12, 15, 16 |
| 18 | 31.6 | 3.09, s | 4, 12 |
| 19 | 43.4 | ||
| 20 | 24.7 | 1.44, s | 7, 19, 21, 23 |
| 21 | 148.8 | 6.21, dd (17.7, 10.6) | 7, 19, 23 |
| 22 | 112.2 | 5.29, dd (17.9, 1.4) | 19, 21 |
| 5.26, dd (10.7, 1.4) | |||
| 23 | 38.2 | 1.98, m | 7, 19, 21, 24, 25 |
| 1.81, m | |||
| 24 | 22.7 | 1.91, br, m | 23, 25, 26, 28 |
| 1.71, br, m | |||
| 25 | 124.6 | 5.08, t (7.1) | 23, 24, 27, 28 |
| 26 | 131.5 | ||
| 27 | 17.4 | 1.40, s | 25, 26, 28 |
| 28 | 25.7 | 1.64, s | 25, 26, 27 |
| OH on 14 | Not observed |


3. Experimental Section
3.1. General Experimental Procedures
3.2. Marine Cyanobacterium Moorea producens
3.3. Extraction and Isolation
3.4. 12-Epi-Lyngbyatoxin A (1) and Lyngbyatoxin A (2)
3.5. Cytotoxicity Assay
3.6. Crustacean Lethality Test
3.7. Binding Assay of PKC Ligands Using PKC-C1B Peptide
4. Conclusions
Supplementary Files
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Engene, N.; Rottacker, E.C.; Kaštovský, J.; Byrum, T.; Choi, H.; Ellisman, M.H.; Komárek, J.; Gerwick, W.H. Moorea producens gen. nov., sp. nov. and Moorea bouillonii comb. nov., tropical marine cyanobacteria rich in bioactive secondary metabolites. Int. J. Syst. Evol. Microbiol. 2012, 62, 1171–1178. [Google Scholar] [CrossRef]
- Burja, A.M.; Banaigs, B.; Abou-Mansour, E.; Grant Burgess, J.; Wright, P.C. Marine cyanobacteria—Aprolific source of natural products. Tetrahedron 2001, 57, 9347–9377. [Google Scholar] [CrossRef]
- Cardellina, J.H.; Marner, F.J.; Moore, R.E. Seaweed dermatitis: Structure of lyngbyatoxin A. Science 1979, 204, 193–195. [Google Scholar]
- Moore, R.E.; Blackman, A.J.; Cheuk, C.E.; Mynderse, J.S.; Matsumoto, G.K.; Clardy, J.; Woodard, R.W.; Craig, J.C. Absolute stereochemistries of the aplysiatoxins and oscillatoxin A. J. Org. Chem. 1984, 49, 2484–2489. [Google Scholar] [CrossRef]
- Mynderse, J.S.; Moore, R.E.; Kashiwagi, M.; Norton, T.R. Antileukemia activity in the Osillatoriaceae: Isolation of debromoaplysiatoxin from Lyngbya. Science 1977, 196, 538–540. [Google Scholar]
- Fujiki, H.; Mori, M.; Nakayasu, M.; Terada, M.; Sugimura, T.; Moore, R.E. Indole alkaloids: Dihydroteleocidin B, teleocidin, and lyngbyatoxin A as members of a new class of tumor promoters. Proc. Natl. Acad. Sci. USA 1981, 78, 3872–3876. [Google Scholar] [CrossRef]
- Fujiki, H.; Tanaka, Y.; Miyake, R.; Kikkawa, U.; Nishizuka, Y.; Sugimura, T. Activation of calcium-activated, phospholipid-dependent protein kinase (protein kinase C) by new classes of tumor promoters: Teleocidin and debromoaplysiatoxin. Biochem. Biophys. Res. Commun. 1984, 120, 339–343. [Google Scholar] [CrossRef]
- Yasumoto, T. Fish poisoning due to toxins of microalgal origins in the Pacific. Toxicon 1998, 36, 1515–1518. [Google Scholar] [CrossRef]
- Ito, E.; Satake, M.; Yasumoto, T. Pathological effects of lyngbyatoxin A upon mice. Toxicon 2002, 40, 551–556. [Google Scholar] [CrossRef]
- Nagai, H.; Yasumoto, T.; Hokama, Y. Aplysiatoxin and debromoaplysiatoxin as the causative agents of a red alga Gracilaria. coronopifolia poisoning in Hawaii. Toxicon 1996, 34, 753–761. [Google Scholar] [CrossRef]
- Nagai, H.; Yasumoto, T.; Hokama, Y. Manauealides, some of the causative agents of a red alga Gracilaria. coronopifolia poisoning in Hawaii. J. Nat. Prod. 1997, 60, 925–928. [Google Scholar] [CrossRef]
- Nagai, H.; Kan, Y.; Fujita, T.; Sakamoto, B.; Hokama, Y. Manauealide C and anhydrodebromoaplysiatoxin, toxic constituents of the Hawaiian red alga, Gracilaria Coronopifolia. Biosci. Biotechnol. Biochem. 1998, 62, 1011–1013. [Google Scholar] [CrossRef]
- Arthur, K.; Limpus, C.; Balazs, G.; Capper, A.; Udy, J.; Shaw, G.; Keuper-Bennett, U.; Bennett, P. The exposure of green turtles (Chelonia mydas) to tumour promoting compounds produced by the cyanobacterium Lyngbya. majuscula and their potential role in the aetiology of fibropapillomatosis. Harmful Algae 2008, 7, 114–125. [Google Scholar] [CrossRef]
- Harr, K.E.; Szabo, N.J.; Cichra, M.; Phlips, J.E. Debromoaplysiatoxin in Lyngbya-dominated mats on manatees (Trichechus manatus latirostris) in the Florida King’s Bay ecosystem. Toxicon 2008, 52, 385–388. [Google Scholar] [CrossRef]
- Namikoshi, M.; Rinehart, K.L. Bioactive compounds produced by cyanobacteria. J. Ind. Microbiol. 1996, 17, 373–384. [Google Scholar] [CrossRef]
- Gademann, K.; Portmann, C. Secondary metabolites from cyanobacteria: Complex structures and powerful bioactivities. Curr. Org. Chem. 2008, 12, 326–341. [Google Scholar] [CrossRef]
- Molinski, T.F.; Dalisay, D.S.; Lievens, S.L.; Saludes, J.P. Drug development from marine natural products. Nat. Rev. Drug. Discov. 2008, 8, 69–85. [Google Scholar]
- Sakai, S.I.; Hitotsuyanagi, Y.; Aimi, N.; Fujiki, H.; Suganuma, M.; Sugimura, T.; Endo, Y.; Shudo, K. Absolute configuration of lyngbyatoxin A (teleocidin A-1) and teleocidin A-2. Tetrahedron Lett. 1986, 27, 5219–5220. [Google Scholar] [CrossRef]
- Aimi, N.; Odaka, H.; Sakai, S.I.; Fujiki, H.; Suganuma, M.; Moore, R.E.; Patterson, G.M.L. Lyngbyatoxins B and C, two new irritants from Lyngbya majuscula. J. Nat. Prod. 1990, 53, 1593–1596. [Google Scholar] [CrossRef]
- Muratake, H.; Okabe, K.; Natsume, M. Synthesis of teleocidins A, B and their congeners. Part 2. Synthesis of lyngbyatoxin A (teleocidin A-1), teleocidin A-2, pendolmycin, and (R,E)- and (S,E)-7-(3,7,11-trimethyl-1,6,10-dodecatrien-3-yl)-(−)-indolactams V. Tetrahedron 1991, 47, 8545–8558. [Google Scholar] [CrossRef]
- Endo, Y.; Shudo, K.; Okamoto, T. Molecular requirements for epigenetic modulators. Synthesis of active fragments of teleocidins and lyngbyatoxin. Chem. Pharm. Bull. 1982, 30, 3457–3460. [Google Scholar] [CrossRef]
- Irie, K.; Hirota, M.; Hagiwara, N.; Koshimizu, K.; Hayashi, H.; Murao, S.; Tokuda, H.; Ito, Y. The Epstein-Barr virus early antigen inducing indole alkaloids, (−)-indolactam-V and its related compounds, produced by Actinomycetes. Agric. Biol. Chem. 1984, 48, 1269–1274. [Google Scholar]
- Fujiki, H.; Suganuma, M.; Nakayasu, M.; Tahira, T.; Endo, Y.; Shudo, K.; Sugimura, T. Structure-activity studies on synthetic analogues (indolactams) of the tumor promoter teleocidin. Jpn. J. Cancer Res. 1984, 75, 866–870. [Google Scholar]
- Kawai, T.; Ichinose, T.; Takeda, M.; Tomioka, N.; Endo, Y.; Yamaguchi, K.; Shudo, K.; Itai, A. Prediction of ring conformations of indolactams. Crystal and solution structures. J. Org. Chem. 1992, 57, 6150–6155. [Google Scholar] [CrossRef]
- Sakai, S.I.; Aimi, N.; Yamaguchi, K.; Hitotsuyanagi, Y.; Watanabe, C.; Yokose, K.; Koyama, Y.; Shudo, K.; Itai, A. Elucidation of the structure of olivoretin A and D (teleocidin B). Chem. Pharm. Bull. 1984, 32, 354–357. [Google Scholar] [CrossRef]
- Endo, Y.; Shudo, K.; Furuhata, K.; Ogura, H.; Sakai, S.I.; Aimi, N.; Hitotsuyanagai, Y.; Koyama, Y. Synthesis of optically active teleocidin derivatives. Absolute configuration of teleocidin B and olivoretin A. Chem. Pharm. Bull. 1984, 32, 358–361. [Google Scholar] [CrossRef]
- Hitotsuyanagi, Y.; Yamaguchi, K.; Ogata, K.; Aimi, N.; Sakai, S.I.; Koyama, Y.; Endo, Y.; Shudo, K.; Itai, A.; Iitaka, Y. Elucidation of the structures of olivoretin B and C. Chem. Pharm. Bull. 1984, 32, 3774–3778. [Google Scholar] [CrossRef]
- Endo, Y.; Shudo, K.; Itai, A.; Hasegawa, M.; Sakai, S.I. Synthesis and stereochemistry of indolactam-V, an active fragment of teleocidins. Structural requirements for tumor-promoting activity. Tetrahedron 1986, 42, 5905–5924. [Google Scholar] [CrossRef]
- Fujiki, H.; Sugimura, T. New classes of tumor promoters: Teleocidin, aplysiatoxin, and palytoxin. Adv. Cancer Res. 1987, 49, 223–264. [Google Scholar] [CrossRef]
- Irie, K.; Kajiyama, S.-I.; Funaki, A.; Koshimizu, K.; Hayashi, H.; Arai, M. Biosynthesis of indole alkaloid tumor promoter teleocidins (I): Possible biosynthetic pathway of the monoterpenoid moieties of teleocidins. Tetrahedron 1990, 46, 2773–2788. [Google Scholar] [CrossRef]
- Irie, K.; Isaka, T.; Iwata, Y.; Yanai, Y.; Nakamura, Y.; Koizumi, F.; Ohigashi, H.; Wender, P.A.; Satomi, Y.; Nishino, H. Synthesis and biological activities of new conformationally restricted analogues of (−)-indolactam-V: Elucidation of the biologically active conformation of the tumor-promoting teleocidins. J. Am. Chem. Soc. 1996, 118, 10733–10743. [Google Scholar] [CrossRef]
- Izumikawa, M.; Khan, S.T.; Komaki, H.; Takagi, M.; Shin-ya, K. JBIR-31, a new teleocidin analog, produced by salt-requiring Streptomyces sp. NBRC 105896 isolated from a marine sponge. J. Antibiot. 2009, 63, 33–36. [Google Scholar]
- Pu, J.; Deng, K.; Butera, J.; Chlenov, M.; Gilbert, A.; Kagan, M.; Mattes, J.; Resnick, L. De novo synthesis of teleocidin B analogs. Tetrahedron 2010, 66, 1963–1972. [Google Scholar] [CrossRef]
- Irie, K.; Hagiwara, N.; Koshimizu, K. New probes for receptor analysis of tumor promoters synthesis of fluorescent derivatives of (−)-indolactam V, the basic ring-structure of teleocidins. Tetrahedron 1987, 43, 5251–5260. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Yanagita, R.C.; Hamada, N.; Murakami, A.; Takahashi, H.; Saito, N.; Nagai, H.; Irie, K. A simple analogue of tumor-promoting aplysiatoxin is an antineoplastic agent rather than a tumor promoter: Development of a synthetically accessible protein kinase C activator with bryostatin-like activity. J. Am. Chem. Soc. 2009, 131, 7573–7579. [Google Scholar] [CrossRef]
- Yanagita, R.C.; Kamachi, H.; Tanaka, K.; Murakami, A.; Nakagawa, Y.; Tokuda, H.; Nagai, H.; Irie, K. Role of the phenolic hydroxyl group in the biological activities of simplified analogue of aplysiatoxin with antiproliferative activity. Bioorg. Med. Chem. Lett. 2010, 20, 6064–6066. [Google Scholar] [CrossRef]
- Kikumori, M.; Yanagita, R.C.; Tokuda, H.; Suzuki, N.; Nagai, H.; Suenaga, K.; Irie, K. Structure-activity studies on the spiroketalmoiety of a simplified analogue of debromoaplysiatoxin with antiproliferative activity. J. Med. Chem. 2012, 55, 5614–5626. [Google Scholar] [CrossRef]
- Hanaki, Y.; Kikumori, M.; Ueno, S.; Tokuda, H.; Suzuki, N.; Irie, K. Structure-activity studies at position 27 of aplog-1, a simplified analog of debromoaplysiatoxin with anti-proliferative activity. Tetrahedron 2013, 69, 7636–7645. [Google Scholar] [CrossRef]
- Kawabata, T.; Lindsay, D.J.; Kitamura, M.; Konishi, S.; Nishikawa, J.; Nishida, S.; Kamio, M.; Nagai, H. Evaluation of the bioactivities of water-soluble extracts from twelve deep-sea jellyfish species. Fish. Sci. 2013, 79, 487–494. [Google Scholar] [CrossRef]
- Sharkey, N.A.; Blumberg, P.M. Highly lipophilic phorbolesters as inhibitors of specific [3H]phorbol 12,13-dibutyrate binding. Cancer Res. 1985, 45, 19–24. [Google Scholar]
- Irie, K.; Oie, K.; Nakahara, A.; Yanai, Y.; Ohigashi, H.; Wender, P.A.; Fukuda, H.; Konishi, H.; Kikkawa, U. Molecular basis for protein kinase C isozyme-selective binding: The synthesis, folding, and phorbol ester binding of the cysteine-rich domains of all protein kinase C isozymes. J. Am. Chem. Soc. 1998, 120, 9159–9167. [Google Scholar] [CrossRef]
- Shindo, M.; Irie, K.; Nakahara, A.; Ohigashi, H.; Konishi, H.; Kikkawa, U.; Fukuda, H.; Wender, P.A. Toward the identification of selective modulators of protein kinase C (PKC) isozymes: Establishment of a binding assay for PKC isozymes using synthetic C1 peptide receptors and identification of the critical residues involved in the phorbolester binding. Bioorg. Med. Chem. 2001, 9, 2073–2081. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jiang, W.; Zhou, W.; Uchida, H.; Kikumori, M.; Irie, K.; Watanabe, R.; Suzuki, T.; Sakamoto, B.; Kamio, M.; Nagai, H. A New Lyngbyatoxin from the Hawaiian Cyanobacterium Moorea producens. Mar. Drugs 2014, 12, 2748-2759. https://doi.org/10.3390/md12052748
Jiang W, Zhou W, Uchida H, Kikumori M, Irie K, Watanabe R, Suzuki T, Sakamoto B, Kamio M, Nagai H. A New Lyngbyatoxin from the Hawaiian Cyanobacterium Moorea producens. Marine Drugs. 2014; 12(5):2748-2759. https://doi.org/10.3390/md12052748
Chicago/Turabian StyleJiang, Weina, Wei Zhou, Hajime Uchida, Masayuki Kikumori, Kazuhiro Irie, Ryuichi Watanabe, Toshiyuki Suzuki, Bryan Sakamoto, Michiya Kamio, and Hiroshi Nagai. 2014. "A New Lyngbyatoxin from the Hawaiian Cyanobacterium Moorea producens" Marine Drugs 12, no. 5: 2748-2759. https://doi.org/10.3390/md12052748
APA StyleJiang, W., Zhou, W., Uchida, H., Kikumori, M., Irie, K., Watanabe, R., Suzuki, T., Sakamoto, B., Kamio, M., & Nagai, H. (2014). A New Lyngbyatoxin from the Hawaiian Cyanobacterium Moorea producens. Marine Drugs, 12(5), 2748-2759. https://doi.org/10.3390/md12052748