Abstract
In order to determine the absolute configuration of naturally occurring alloxanthin, a HPLC analytical method for three stereoisomers 1a–c was established by using a chiral column. Two authentic samples, (3S,3′S)- and meso-stereoisomers 1b and 1c, were chemically synthesized according to the method previously developed for (3R,3′R)-alloxanthin (1a). Application of this method to various alloxanthin specimens of aquatic animals demonstrated that those isolated from shellfishes, tunicates, and crucian carp are identical with (3R,3′R)-stereoisomer 1a, and unexpectedly those from lake shrimp, catfish, biwa goby, and biwa trout are mixtures of three stereoisomers of 1a–c.
1. Introduction
Alloxanthin (1) (Figure 1) was first isolated from Cryptomonas algae [1] and its structure was determined to be 7,8,7′,8′-tetreradehydro-β,β-carotene-3,3′-diol by MS, IR and 1H-NMR spectroscopies [2]. Additionally, cynthiaxanthin [3] from the tunicate Cynthia rorezi (Halocynthia rorezi) and pectenoxanthin [4] from giant scallop Pecten maximus were isolated by Japanese scientists. In 1967, Campbel et al. demonstrated that these two carotenoids were identical with alloxanthin [5]. Therefore, cynthiaxanthin and pectenoxanthin were synonyms of alloxanthin. The absolute configuration of alloxanthin isolated form algae was deduced to be 3R,3′R by X-ray analysis of degradation product of fucoxanthin and in view of biogenetic grounds [6]. Bartlett et al. reported that the ORD spectra of alloxanthin specimens from Cryptomonas algae and tunicate showed an identical shape each other and that both specimens are assumed to have an identical absolute configuration [7].
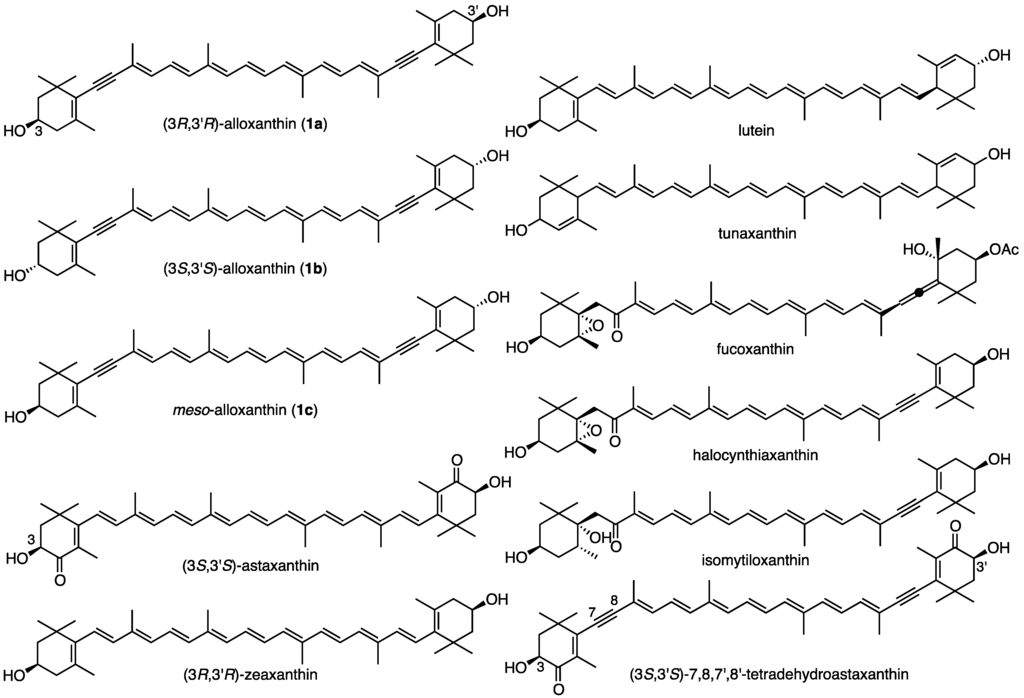
Figure 1.
Structures of stereoisomers of alloxanthin (1a–c) and other related carotenoids.
Since then, alloxanthin was isolated from several aquatic animals, such as shellfishes [8,9], starfishes [10], tunicates [11,12] and freshwater fishes [13,14], etc. These alloxanthin specimens showed similar non-conservative CD with weak negative Cotton effects.
Carotenoids such as astaxanthin, zeaxanthin, lutein, and tunaxanthin in animals are known to exist as a mixture of stereoisomers. Namely, astaxanthin in crustaceans and marine fishes exists as a mixture of three stereoisomers at C3 and C3′-positions [15,16]. Zeaxanthin [17], lutein [18], and tunaxanthin [19] in marine fishes also consist of these stereoisomers. Their absolute configurations were determined by CD spectra and chiral HPLC analyses. Due to its non-conservative CD, absolute configurations of alloxanthin in several origins could not be determined exactly by CD spectra.
In order to determine the absolute configuration of naturally occurring alloxanthin, we synthesized stereoisomers of alloxanthin (1a–c) and established a HPLC analytical method using a chiral column. Applying this method, the absolute configurations of alloxanthin specimens isolated from shellfishes, tunicates and fishes were investigated. Here, we describe these results.
2. Results and Discussion
2.1. Synthesis of (3S,3′S)-Alloxanthin (1b) and meso-Alloxanthin (1c)
We previously reported [20] stereoselective total synthesis of (3R,3′R)-alloxanthin (1a) by use of C15-acetylenic tri-n-butylphosphonium salt 5a (Scheme 1) as a versatile synthon for syntheses of acetylenic carotenoids. This time, (3S,3′S)-alloxanthin (1b) and its meso-stereoisomer 1c were newly synthesized using (3S)-phosphonium salt 5b, which was prepared from 3-epi-actinol 6 [21] in the same procedure [20] as preparation of (3R)-one 5a.
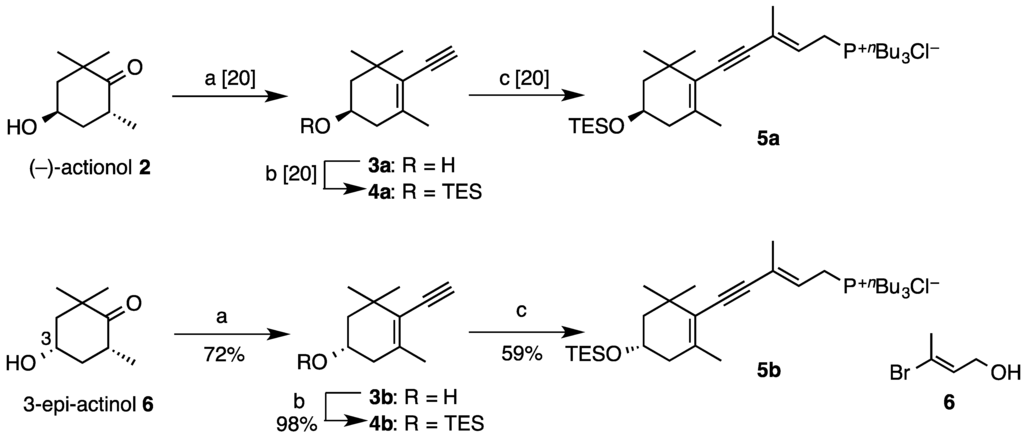
Scheme 1.
Synthesis of C15-acetylenic tri-n-butylphosphonium salts 5a and 5b.
Compound 6 was converted into terminal alkyne 3b via the addition of lithium acetylide in 72% yield over six steps. The high enantiomeric purity of 3b (99% ee) was confirmed by HPLC analysis [CHIRALPAK AY-H; Daicel, 2-PrOH–n-hexane (5:95)]. Compound 3b was then transformed into the phoshonium salt 5b via Sonogashira cross-coupling of the triethylsilyl (TES)-protected terminal alkyne 4b with vinylbromide 6 in 59% over four steps.
Wittig condensation of C10-dialdehyde 7 with excess amount of (3S)-phosphonium salt 5b in the presence of sodium methoxide in dichloromethane at room temperature and subsequent desilylation stereoselectively provided (3S,3′S)-alloxanthin (1b) (Scheme 2). On the other hand, meso-alloxanthin (1c) was synthesized via condensation between (3S)-phosphonium salt 5b and (3R)-C25-acetylenic apocarotenal 8, which was prepared by Wittig reaction of C10-dialdehyde 7 with (3R)-phosphonium salt 5a in the presence of sodium methoxide in dichloromethane at 0 °C.
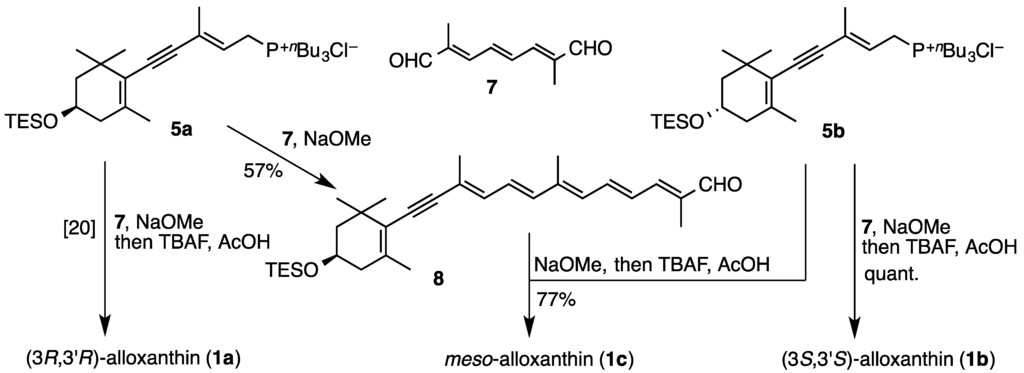
Scheme 2.
Synthesis of three stereoisomers of alloxanthin (1a–c).
CD spectrum of (3S,3′S)-alloxanthin (1b) showed an antisymetrical curve having week Cotton effects to that of previously synthesized [20] (3R,3′R)-alloxanthin (1a) as shown in Figure 2.
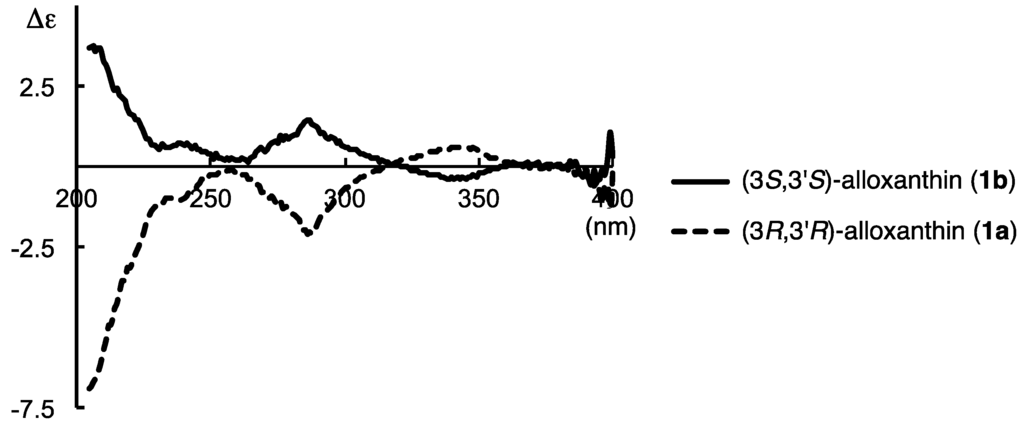
Figure 2.
CD spectra in Et2O–isopentane–EtOH (5:5:2) of synthesized (3R,3′R)-alloxanthin (1a) and (3S,3′S)-alloxanthin (1b).
2.2. Determination of Absolute Configuration of Alloxanthin Isolated from Aquatic Animals by HPLC
In order to determine the absolute configuration of naturally occurring alloxanthin, a HPLC analytical method for three stereoisomers 1a–c was investigated. As a result, three synthetic stereoisomers of alloxanthin can be separated using a chiral column (CHIRALPAK AD-H; Daicel) as shown in Figure 3.
Next, alloxanthin specimens isolated from scallop Mizuhopecten yessoensis, oyster Crassostrea gigas, pacific pearl oyster Pinctada margaritifera, freshwater bivalve Unio douglasiae, tunicate Halocynthia roretzi, and crucian carp Carassius auratus grandoculis were subjected to the HPLC method to find that these consist of only (3R,3′R)-stereoisomer 1a. On the other hand, alloxanthin specimens isolated from lake shrimp Palaemon paucidens, catfish Silurus asotus, biwa goby Gymnogobius isaza, and biwa trout Oncorhynchus masou rhodurus consisted of three stereoisomers 1a–c (Table 1).
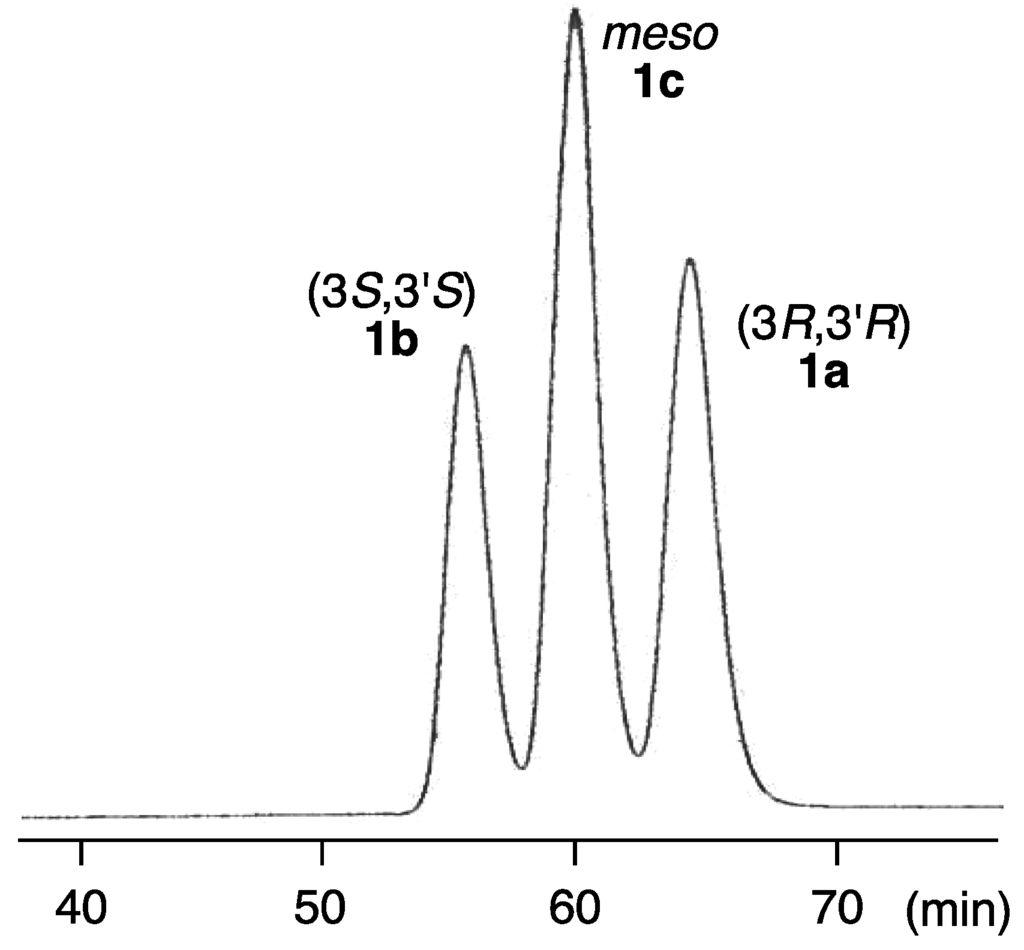
Figure 3.
HPLC elution profile of a mixture of three stereoisomers of alloxanthin (1a–c).

Table 1.
Occurrence and percentage composition of alloxanthin stereoisomers in aquatic animals.
| Species | 3R,3′R | 3S,3′S | meso | |
|---|---|---|---|---|
| 1a | 1b | 1c | ||
| Shellfish | ||||
| Scallop | Mizuhopecten yessoensis | 100 | n.d. | n.d. |
| Oyster | Crassostrea gigas | 100 | n.d. | n.d. |
| Pacific pearl oyster | Pinctada margaritifera | 100 | n.d. | n.d. |
| Freshwater bivalves | Unio douglasiae | 100 | n.d. | n.d. |
| Tunicate | ||||
| Sea squirt | Halocynthia roretzi | 100 | n.d. | n.d. |
| Crustacean | ||||
| Lake shrimp | Palaemon paucidens | 53.7 | 9.6 | 36.7 |
| Fish | ||||
| Crucian carp | Carassius auratus grandoculis | 100 | n.d. | n.d. |
| Biwa goby | Gymnogobius isaza | 91.4 | 0.9 | 7.7 |
| Biwa trout | Oncorhynchus masou rhodurus | >99.9 | trace | trace |
| Catfish | Silurus asotus | 82.9 | 1.5 | 15.6 |
n.d.: not detected.
Previously, one of the authors reported that zeaxanthin in plants, shellfishes, and tunicates consisted of only (3R,3′R)-stereoisomer, whereas zeaxanthin in fishes consisted of three stereoisomers [17]. Similar results were obtained in the case of alloxanthin in aquatic animals. Alloxanthin is de novo synthesized in Chryptophyceae and Euglenophyceae micro algae [22]. However, origin of alloxanthin in aquatic animals was remained uncertain. Patrali et al. (1989) [22] and Liaaen-Jensen (1998) [23] reported that alloxanthin in Mytilus edulis might be a terminal metabolite of fucoxanthin through intermediates, halocynthiaxanthin and isomytiloxanthin, based on observation in feeding experiment. However, conversion of isomytiloxanthin into alloxanthin is too complex and there were no direct evidences for the conversion, especially in aquatic animals. In our experience, isomytiloxanthin has not been isolated from these animals [24].
Shellfishes (bivalves) and tunicates are filter-feeders, which accumulate carotenoids from micro algae. Therefore, alloxanthin in these animals is assumed to originate from Chryptophyceae and Euglenophyceae micro algae, etc. Thus, these alloxanthin specimes consist of only (3R,3′R)-stereoisomer. Crucian carp is omnivorous and feeds not only animal planktons belonging to Cladocera but also micro algae. Therefore, alloxanthin in crucian carp is also assumed to originate from micro algae. On the other hand, alloxanthin in lake shrimp, catfish, biwa goby, and biwa trout exist as a mixture of three stereoisomres. These crustacean and fishes are carnivorous. Especially, lake shrimp contains a large amount of (3S,3′S)- and meso-alloxanthin (Table 1). Lake shrimp is a one of the major food of catfish and biwa trout. Therefore, (3S,3′S)- and meso-alloxanthin in these fishes might be originated from lake shrimp. However, origin of (3S,3′S)- and meso-alloxanthin in lake shrimp is uncertain.
Catfish is a top predator in Japanese freshwater ecosystems. Catfish ingests astaxanthin from crustaceans whose astaxanthin exists as a mixture of three stereoisomers. Catfish can convert astaxanthin into zeaxanthin [24]. Therefore, zeaxanthin in catfish exists as a mixture of three stereoisomers. Although the origin of stereoisomers of alloxanthin in catfish is uncertain, it might be naturally formed by epimerization of 7,8,7′,8′-tetradehydroastaxanthin originated from crustacean at C3 and C3′-positions and subsequent reduction at C4 and C4′-positions. Further studies are need to reveal the origin of (3S,3′S)- and meso-alloxanthin in crustaceans and fishes.
This is the first report of the occurrence of (3S,3′S) and meso-alloxanthin in nature.
3. Experimental Section
3.1. General
IR spectrum was measured on a Perkin-Elmer FT-IR spectrometer (Perkin-Elmer, Yokohama, Japan), spectrum 100. 1H and 13C NMR spectra were determined on a Varian Gemini-300 superconducting FT-NMR spectrometer (Agilent Technologies, Santa Clara, CA, USA) and the chemical shifts were referenced to tetramethylsilane. Mass spectrum was taken on a Thermo Fisher Scientific Exactive spectrometer (Thermo Fisher Scientific, Bremen, Germany). CD spectra were measured on a Shimadzu-AVIN 62A DS circular dichroism spectrometer (Shimadzu, Kyoto, Japan).
HPLC analyses were performed on Simadzu-LC-20AT instrument (Shimadzu, Kyoto, Japan) with a photodiode array detector (Waters 996, Tokyo, Japan) and column oven (GL Sciences Model 552, Tokyo, Japan).
NMR assignments are given using the carotenoid nmbering system.
3.2. Synthesis of (3S,3′S)-Alloxanthin (1b) and meso-Alloxanthin (1c)
In the same procedure [20] as preparation of (3R)-phosphonium salt 5a and (3R,3′R)-alloxanthin (1a), (3S)-5b and (3S,3′S)-alloxanthin (1b) were prepared. Spectral data except for optical data of compounds 1b, 3b, 4b and 5b were identical with the corresponding previous reported [20] enantiomers 1a, 3a, 4a and 5a.
(3S,3′S)-Alloxanthin (1b): HRMS (ESI) m/z calcd for C40H53O2 [M + H]+ 565.4040, found 565.4038.
Compound 3b: [α]D26 102.9 (c 1.03, MeOH); HRMS (ESI) m/z calcd for C11H17O [M + H]+ 165.1274, found 165.1277.
Compound 4b: [α]D23 68.1 (c 1.00, MeOH); HRMS (ESI) m/z calcd for C17H31OSi [M + H]+ 279.2139, found 279.2139.
Compound 5b: HRMS (ESI) m/z calcd for C33H62OPSi [M − Cl]+ 533.4302, found 533.4293.
meso-Alloxanthin (1c) was synthesized via condensation between 5b and (3R)-C25-acetylenic apocarotenal 8, which was prepared by Wittig reaction of C10-dialdehyde 7 with 5a as follows.
(2E,4E,6E,8E,10E)-2,7,11-trimethyl-13-[(R)-2,6,6-trimethyl-4-triethylsilyloxycyclohex-1-en-1-yl]trideca-2,4,6,8,10-pentaen-12-ynal (8). NaOMe (1 M in MeOH; 1.2 mL, 1.2 mmol) was added to a solution of the (3R)-phosphonium salt 5a (409 mg, 0.73 mmol) and C10-dialdehyde 7 (100 mg, 0.61 mmol) in CH2Cl2 (10 mL) at 0 °C. After being stirred at 0 °C for 15 min, the mixture was poured into saturated aq. NH4Cl and extracted with AcOEt. The extracts were washed with brine, dried over Na2SO4 and evaporated to afford a residue, which was purified by flash column chromatography (AcOEt–n-hexane, 1:4) to give the (3R)-C25-acetylenic apocarotenal 8 (165 mg, 57%) as an orange viscous oil: UV-VIS λmax (EtOH)/nm 420; IR νmax (CHCl3)/cm−1 2170 (C≡C), 1663 (conj. CHO), 1610 and 1599 (split) (C=C), 1552 (C=C); 1H-NMR (CDCl3, 300 MHz) δ 0.61 (6H, q, J = 8 Hz, SiCH2 × 3), 0.97 (9H, t, J = 8 Hz, CH2CH3 × 3), 1.14 and 1.18 (each 3H, s, 1-gem-Me), 1.49 (1H, t, J = 12 Hz, 2-Hβ), 1.74 (1H, ddd, J = 12, 3.5, 2 Hz, 2-Hα), 1.89 (3H), 1.91 (3H) and 2.03 (6H) (each s, 5-Me, 9-Me, 13-Me and 13′-Me), 2.11 (1H, br dd, J = 17.5, 9.5 Hz, 4-Hβ), 2.30 (1H, br dd, J = 17.5, 5.5 Hz, 4-Hα), 3.94 (1H, m, 3-H), 6.32 (1H, br d, J = 12 Hz, 14-H), 6.37 (1H, d, J = 15 Hz, 12-H), 6.46 (1H, br d, J = 11.5 Hz, 10-H), 6.66 (1H, dd, J = 15, 11.5 Hz, 11-H), 6.70 (1H, dd, J = 14.5, 11.5 Hz, 15′-H), 6.96 (1H, br d, J = 11.5 Hz, 14′-H), 7.03 (1H, dd, J = 14.5, 12 Hz, 15-H), 9.46 (1H, s, CHO); 13C-NMR (CDCl3, 75 MHz) δ 4.82 (C × 3), 6.83 (C × 3), 9.59, 12.96, 18.17, 22.53, 28.61, 30.45, 36.53, 42.11, 47.04, 65.01, 90.10, 98.16, 121.15, 123.84, 126.60, 127.73, 131.75, 134.51, 137.02, 137.07, 137.47, 138.70, 141.26, 148.75, 194.45; HRMS (ESI) m/z calcd for C31H47O2Si (MH)+ 479.3340, found 479.3347.
Preparation of meso-alloxanthin (1c). NaOMe (1 M in MeOH; 0.24 mL, 0.24 mmol) was added to a solution of the (3S)-phosphonium salt 5b (113 mg, 0.20 mmol) and (3R)-C25-acetylenic apocarotenal 8 (59 mg, 0.12 mmol) in CH2Cl2 (10 mL) at room temperature. After being stirred for further 15 min, the mixture was poured into saturated aq. NH4Cl and extracted with AcOEt. The extracts were washed with brine, dried over Na2SO4 and evaporated to afford a residue, which was purified by flash column chromatography (AcOEt–n-hexane, 1:4) to give the TES-protected condensed product. Subsequently, to a solution of this condensed product in dry THF (5 mL) were added AcOH (1 M in THF; 0.20 mL, 0.20 mmol) and then tetrabutylammonium fluoride (TBAF) (1 M in THF, 0.40 mL, 0.40 mmol). After being stirred at room temperature for 2 h, the mixture was concentrated to give a residue, which was purified by flash column chromatography (AcOEt–n-hexane–MeOH, 50:45:5) to provide meso-alloxanthin (1c) (70 mg, quant.) as red solids. Its spectral data were identical with those of (3R,3′R)-alloxanthin (1a) [20]. HRMS (ESI) m/z calcd for C40H53O2 [M + H]+ 565.4040, found 565.4033.
3.3. Configurational Analysis of Natural Alloxanthin
3.3.1. Animal Materials
Scallop Mizuhopecten yessoensis was provided from Hokkaido Research Organization, Abashiri Fisheries Research Institute, Hokkaido, Japan. Oyster Crassostrea gigas, and sea squirt Halocynthia roretzi were purchased from fisheries market at Kyoto city. Pacific pearl oyster Pinctada margaritifera was provided from a pearl aquaculture industry, Ishigaki city, Okinawa Prefecture. Freshwater bivalve Unio douglasiae, crucian carp Carassius auratus grandoculis, and catfish Silurus asotus were purchased from Katata fisheries cooperative, Shiga Prefecture. Biwa trout Oncorhynchus masou rhodurus was purchased from Nango Fisheries Center, Shiga Prefecture. Biwa goby Gymnogobius isaza and lake shrimp Palaemon paucidens were purchased from fisheries market at Maibara city.
3.3.2. Isolation of Alloxanthin from Aquatic Animals
According to our routine methods, carotenoid was extracted with acetone from animal tissue. The extract was partitioned between Et2O–n-hexane (1:1) and water in separating funnel. The organic phase was evaporated and saponified with 5% KOH/MeOH at room temperature for 2 h. Then, unsaponifiable compounds were extracted with Et2O–n-hexane (1:1, v/v) from the reaction mixture by addition of water. The organic layer was dried over Na2SO4 and evaporated. The residue was subjected to silica gel column chromatography increasing percentage of Et2O in n-hexane. The fraction eluted with Et2O was subjected to HPLC on silica gel with acetone–n-hexane (3:7) to afford alloxanthin. Purity of alloxanthin was checked by UV-Vis, 1H-NMR, and MS spectral data. Then alloxanthin obtained from aquatic animals was subject to configurational analysis using a chiral column described above.
4. Conclusions
In conclusion, we synthesized stereoisomers of alloxanthin (1a–c) and established a HPLC analytical method using a chiral column to identify them for naturally occurring alloxanthin. Application of this method to various alloxanthin specimens of aquatic animals demonstrated that those isolated from shellfishes, tunicates, and crucian carp are identical with (3R,3′R)-stereoisomer 1a, and unexpectedly those from lake shrimp, catfish, biwa goby, and biwa trout are mixtures of three stereoisomers of 1a–c. This is the first report of the occurrence of (3S,3′S) and meso-alloxanthin in nature. The analytical method can be a powerful tool to identify stereoisomers of alloxanthin in nature in a straightforward manner.
Acknowledgments
We thank M. Kurimoto and M Shoji for technical assistance.
Author Contributions
Basic idea of the research was proposed by three authors collaboratively. The synthetic and analytical experiments were designed and performed by Y. Yamano. The isolation of natural products was designed and carried out by T. Maoka.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Haxo, F.T.; Fork, D.C. Photosynthetically active accessory pigments of cryptomonads. Nature 1959, 184, 1051–1052. [Google Scholar] [CrossRef]
- Mallams, A.K.; Waight, E.S.; Weedon, B.C.L.; Chapman, D.J.; Haxo, F.T.; Goodwin, T.W.; Thomas, B.M. A new class of carotenoids. Chem. Commun. 1967, 301–302. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Suzuki, Y. Biochemical studies of the ascidian, Cynthia rorezi variety drasche. IV Carotenoids in test. Tohoku J. Agric Res. 1959, 10, 397–407. [Google Scholar]
- Nishibori, K. Pigments of marine animlas—VIII. Carotenoids of some shellfish. Publ. Seto Mar. Biol. Lab. 1960, 8, 317–326. [Google Scholar]
- Campbell, S.A.; Mallams, A.K.; Waight, E.S.; Weedon, B.C.L. Pectenoxanthin, cynthiaxanthin, and a new acetylenic carotenoid, pectenolone. Chem. Commun. 1967, 941–942. [CrossRef]
- DeVille, T.E.; Hursthouse, M.B.; Russell, S.W.; Weedon, B.C.L. Absolute configuration of carotenoids. Chem. Commun. 1969, 1311–1312. [Google Scholar] [CrossRef]
- Bartlett, L.; Klyne, W.; Mose, W.P.; Scopes, P.M.; Galasko, G.; Mallams, A.K.; Weedon, B.C.L.; Szabolcs, J.; Toth, G. Optical rotatory dispersion of carotenoids. J. Chem. Soc. Perkin Trans. 1 1969, 2527–2544. [Google Scholar] [CrossRef]
- Hertzberg, S.; Partali, V.; Liaaen-Jensen, S. Animal carotenoids. 32. Carotenoids of Mytilus edulis (edible mussel). Acta Chem. Scand. 1988, B42, 495–503. [Google Scholar]
- Maoka, T.; Matsuno, T. Carotenoids of shellfishes—IX. Isolation and structural elucidation of three new acetylenic carotenoids from the Japanese sea mussel Mytilus coruscus. Nippon Suisan Gakkaishi 1988, 54, 1443–1447. [Google Scholar] [CrossRef]
- Maoka, T.; Tsushima, M.; Matsuno, T. New acetylenic carotenoids from the starfishes Asterina pectinifera and Asterias amurensis. Comp. Biochem. Physiol. 1989, 93B, 829–834. [Google Scholar]
- Matsuno, T.; Ookubo, M.; Nishizawa, T.; Shimizu, I. Carotenoids of sea squirts. I. New marine carotenoids, halocynthiaxanthin and mytiloxanthinone from Halocynthia roretzi. Chem. Pharm. Bull. 1984, 32, 4309–4315. [Google Scholar] [CrossRef]
- Ookubo, M.; Matsuno, T. Carotenoids of sea squirts—II. Comparative biochemical studies of carotenoids in sea squirts. Comp. Biochem. Physiol. 1985, 81B, 137–141. [Google Scholar]
- Matsuno, T.; Maoka, T.; Ikuno, Y. Comparative biochemical studies of carotenoids in fish—XXVII. Carotenoids in the eggs of three species of Cyprinidae. Comp. Biochem. Physiol. 1986, 83B, 335–337. [Google Scholar]
- Maoka, T.; Akiomoto, N. Structures of minor carotenoids from the Japanese common catfish, Silurus asotus. Chem. Phram. Bull. 2011, 59, 140–145. [Google Scholar] [CrossRef]
- Ronneberg, H.; Renstrom, B.; Aareskjold, K.; Liaaen-Jensen, S.; Vecchi, M.; Leuenberger, F.J.; Mȕller, R.K.; Mayer, H. Naturally occurrence of enantiomeric and meso-astaxanthin 1. Ex lobster eggs (Homarus gammarus). Helv. Chim. Acta 1980, 63, 711–715. [Google Scholar] [CrossRef]
- Matsuno, T.; Maoka, T.; Katsuyama, M.; Ookubo, M.; Katagiri, K.; Jimura, H. The occurrence of enantiomeric and meso-astaxanthin in aquatic animals. Nippon Suisan Gakkaishi 1984, 50, 1589–1592. [Google Scholar] [CrossRef]
- Maoka, T.; Arai, A.; Shimizu, M.; Matsuno, T. The first isolation of enantiomeric and meso-zeaxanthin in nature. Comp. Biochem. Physiol. 1986, 83B, 121–124. [Google Scholar]
- Matsuno, T.; Maoka, T.; Katsuyama, M.; Hirono, T.; Ikuno, Y.; Shimizu, M.; Komori, T. Comparative biochemical studies of carotenoids in fishes—XXIX. Isolation of new luteins, lutein F and lutein G from marine fishes. Comp. Biochem. Physiol. 1986, 85B, 77–80. [Google Scholar]
- Ikuno, Y.; Shimizu, M.; Koshino, Y.; Maoka, T.; Matsuno, T. Comparative biochemical studies of carotenoids in fishes—XXVII. Stereochemical investigation of carotenoids from yellow-tail rockfish Sebastes flavidus. Nippon Suisan Gakkaishi 1985, 51, 2033–2035. [Google Scholar] [CrossRef]
- Yamano, Y.; Chary, V.M.; Wada, A. Stereoselective total synthesis of the acetylenic carotenoids alloxanthin and triophaxanthin. Org. Biomol. Chem. 2012, 10, 4103–4108. [Google Scholar] [CrossRef]
- Leuenberger, H.G.W.; Boguth, W.; Widmer, E.; Zell, R. Synthesis of optically active natural carotenoids and structurally related compounds. I. Synthesis of the chiral key compound (4R,6R)-4-hydroxy-2,2,6-trimethylcyclohexanone. Helv. Chim. Acta 1976, 59, 1832–1849. [Google Scholar] [CrossRef]
- Partali, V.; Tangen, K.; Liaaen-Jensen, S. Carotenoids in food chain studies. III. Resorption and metabolic transformation of carotenoids in Mytilus edulis (edible mussel). Comp. Biochem. Physiol. 1989, 92B, 239–246. [Google Scholar]
- Liaaen-Jensen, S. Carotenoids in food chain. In Carotenoids Volume 3: Biosynthesis and Metabolism; Britton, G., Liaaen-Jensen, S., Pfander, H., Eds.; Birkhäuser: Basel, Switzerland, 1998; pp. 359–371. [Google Scholar]
- Maoka, T. Carotenoids in marine animals. Mar. Drugs 2011, 9, 278–293. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).