The Anticancer Effect of Fucoidan in PC-3 Prostate Cancer Cells
Abstract
:1. Introduction
2. Results
2.1. Inhibitory Effect of Fucoidan on the Growth of PC-3 Cells
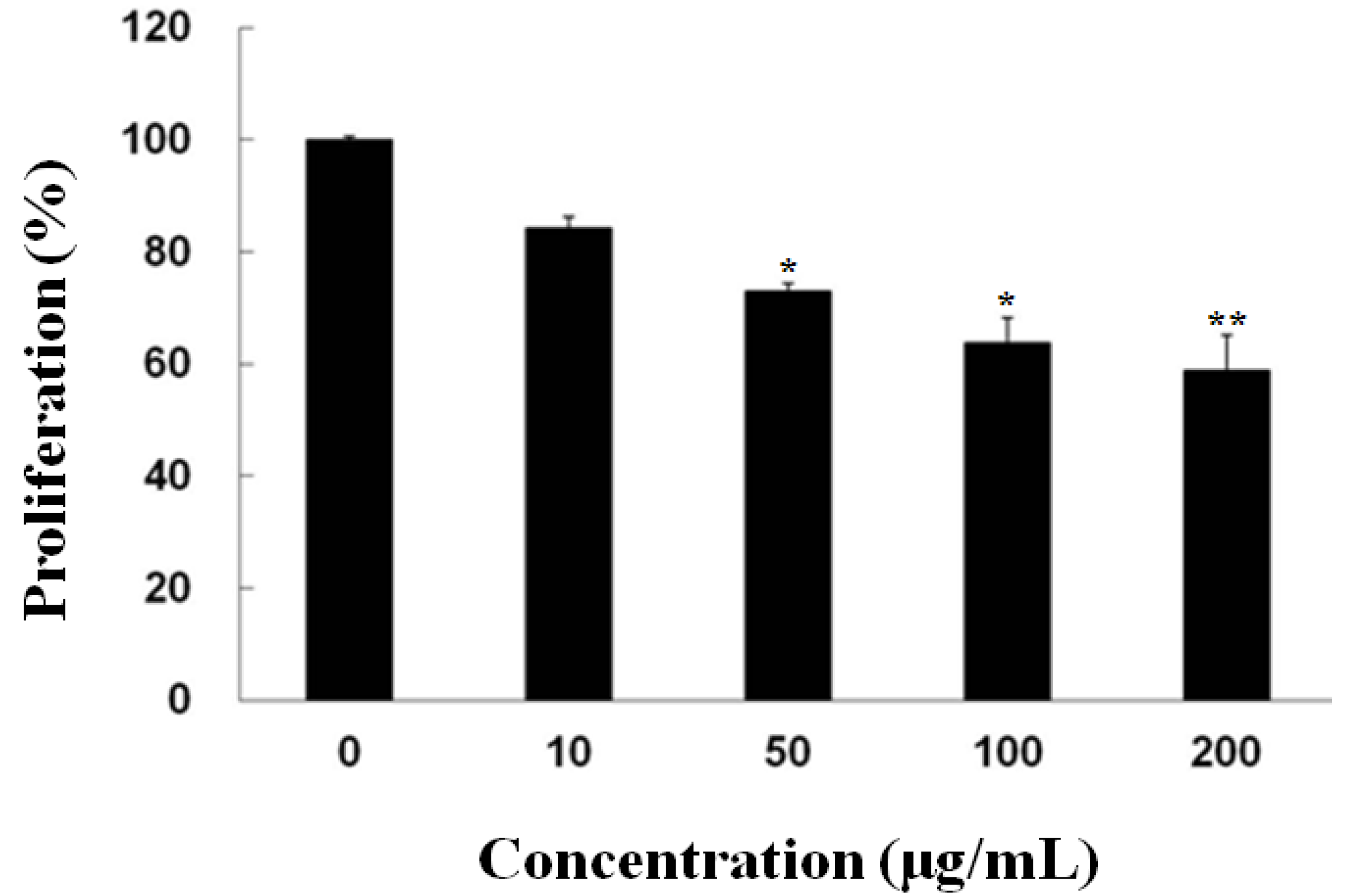
2.2. Fucoidan Induced Apoptotic Characteristics in PC-3 Cells
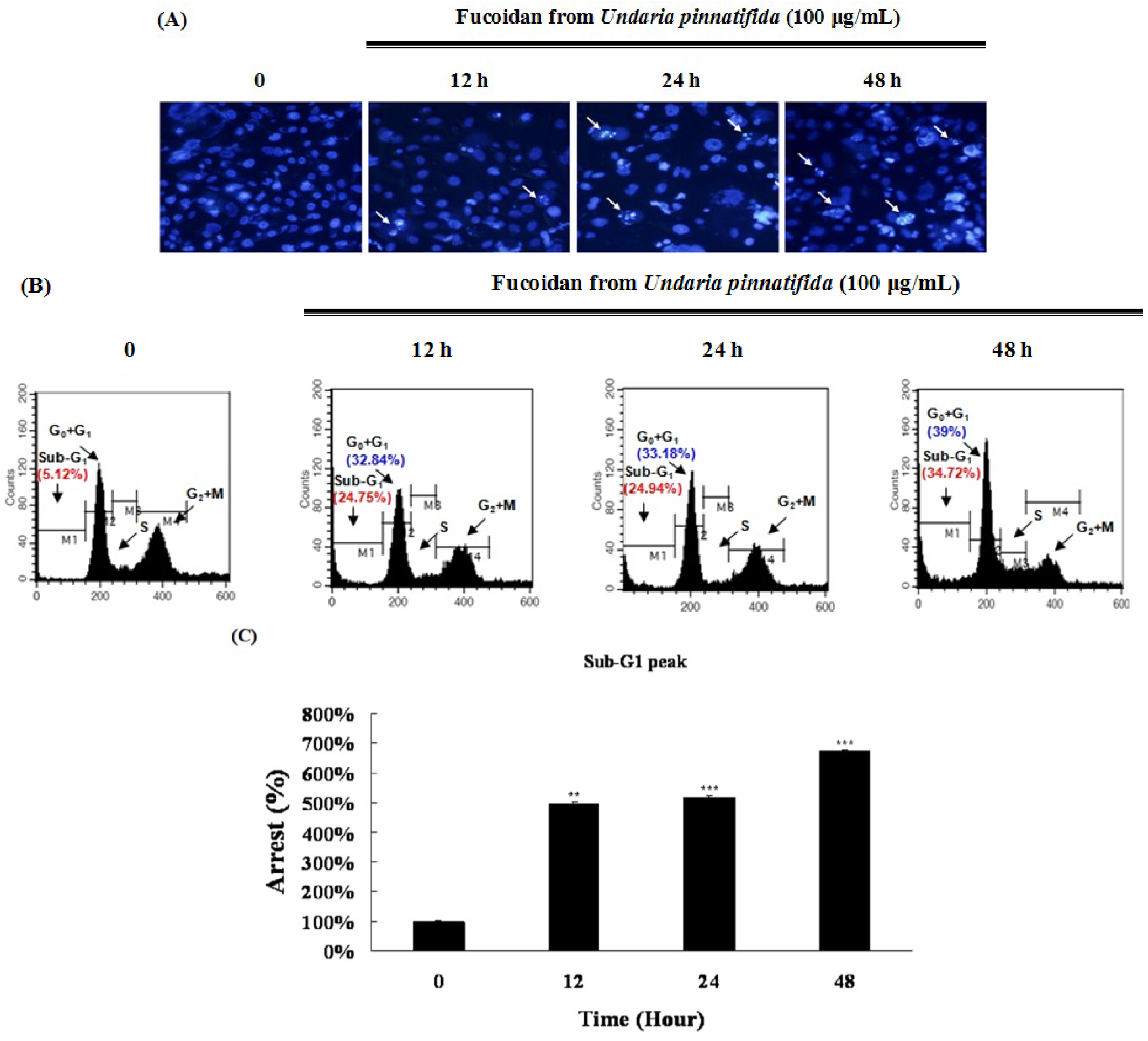
2.3. Fucoidan Induced Apoptosis through Extrinsic and Intrinsic Apoptosis Pathways in PC-3 Cells
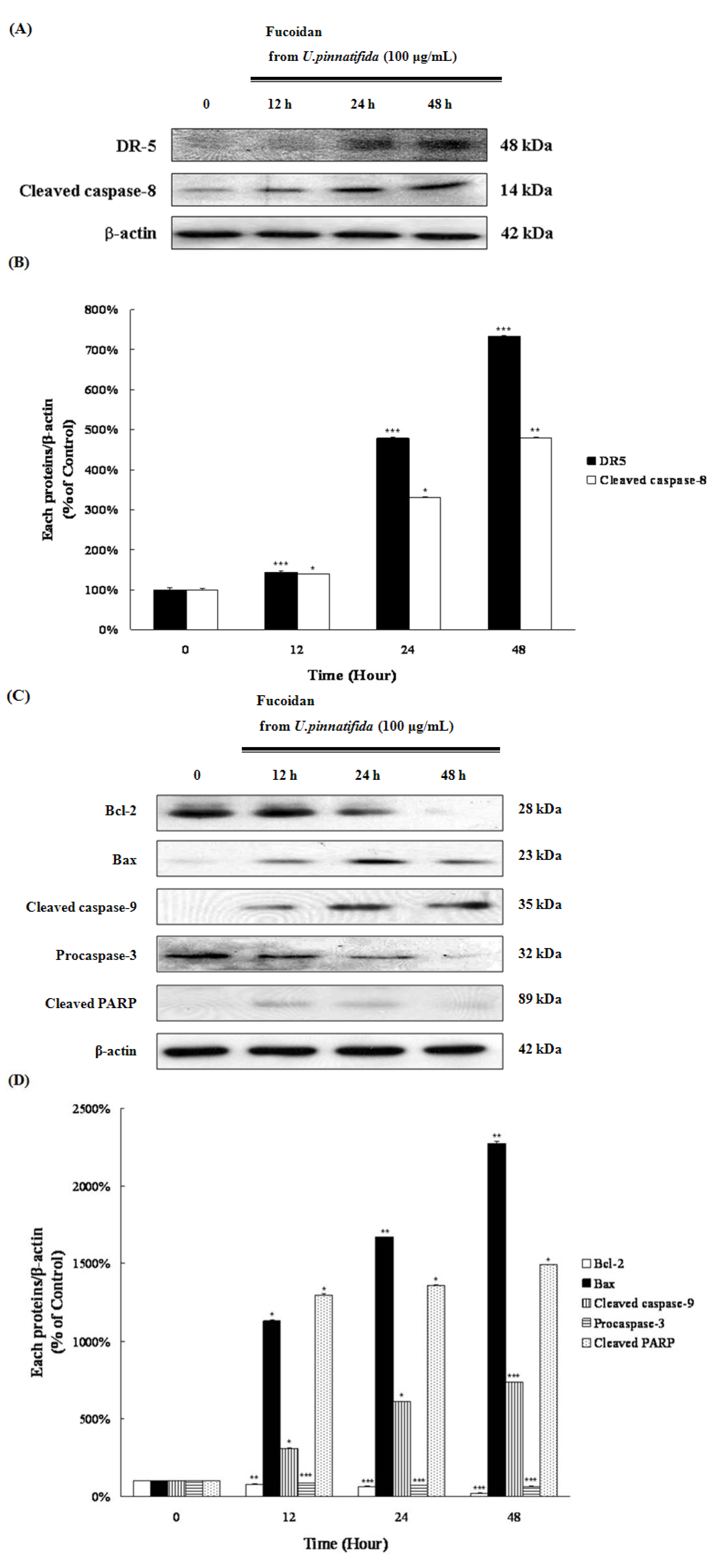
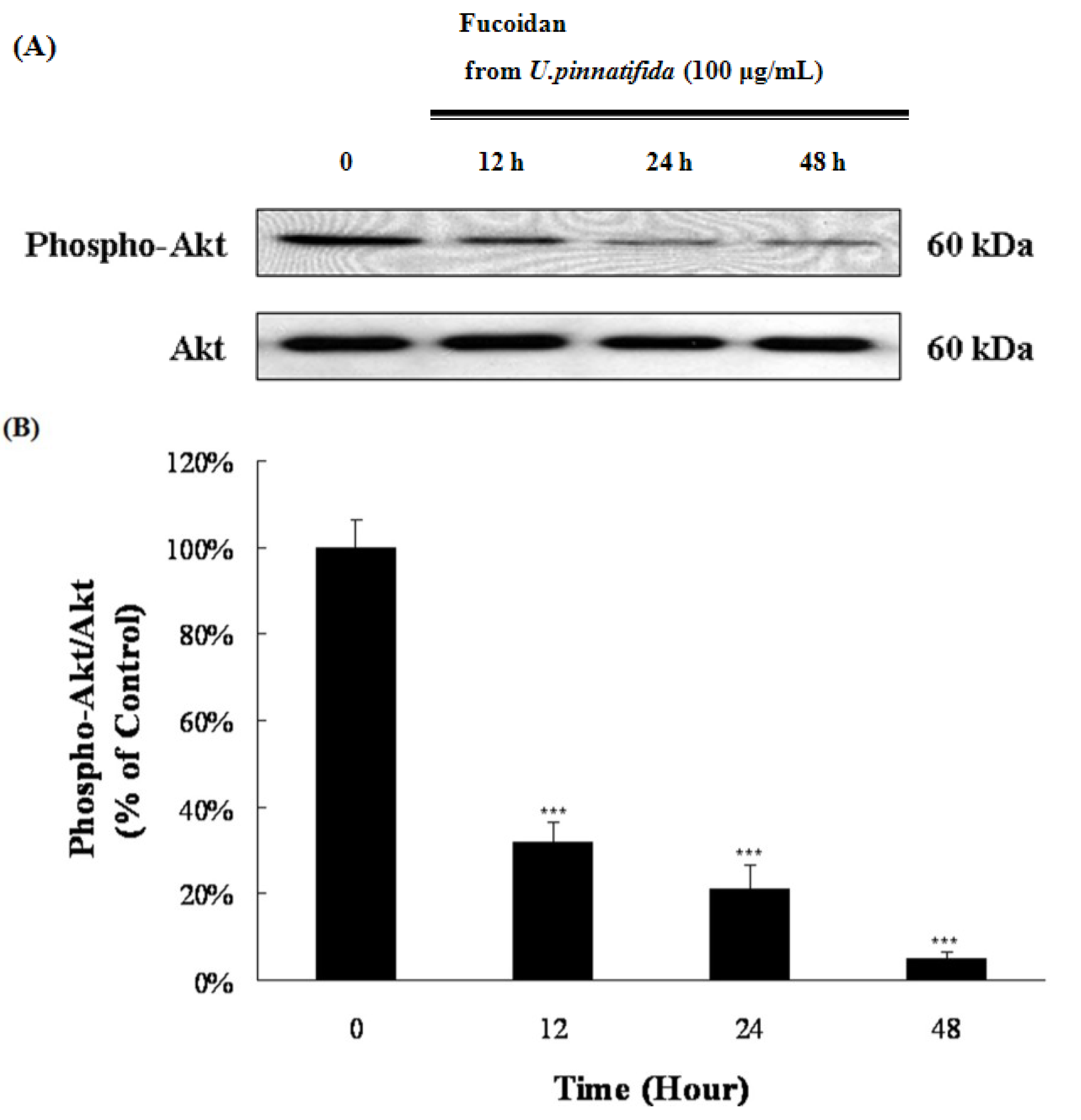
2.4. Effect of Fucoidan on MAP Kinase and PI3K/Akt Signaling in PC-3 Cells
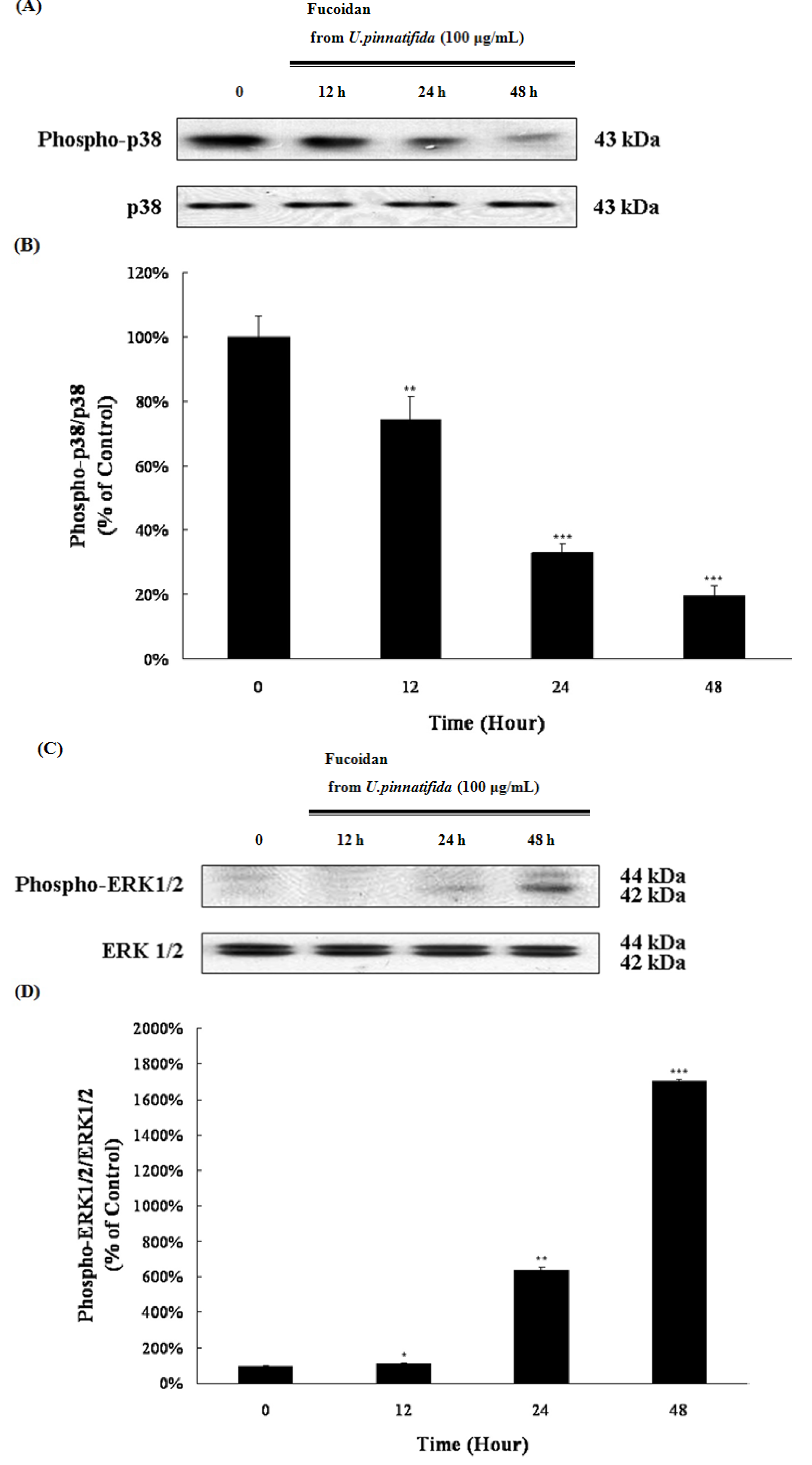
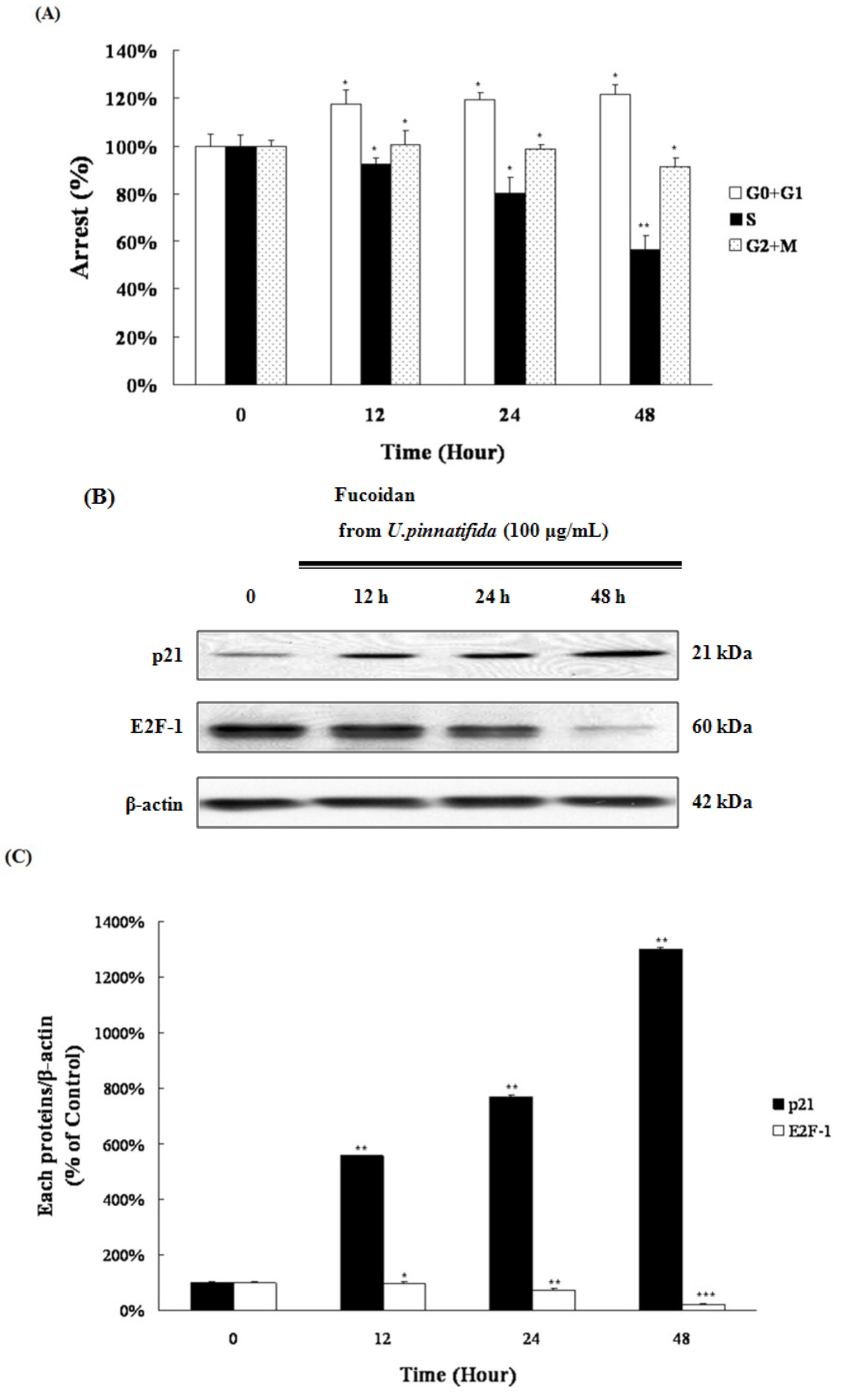
2.5. Fucoidan Induced G0/G1 Phase Arrest of PC-3 Cells
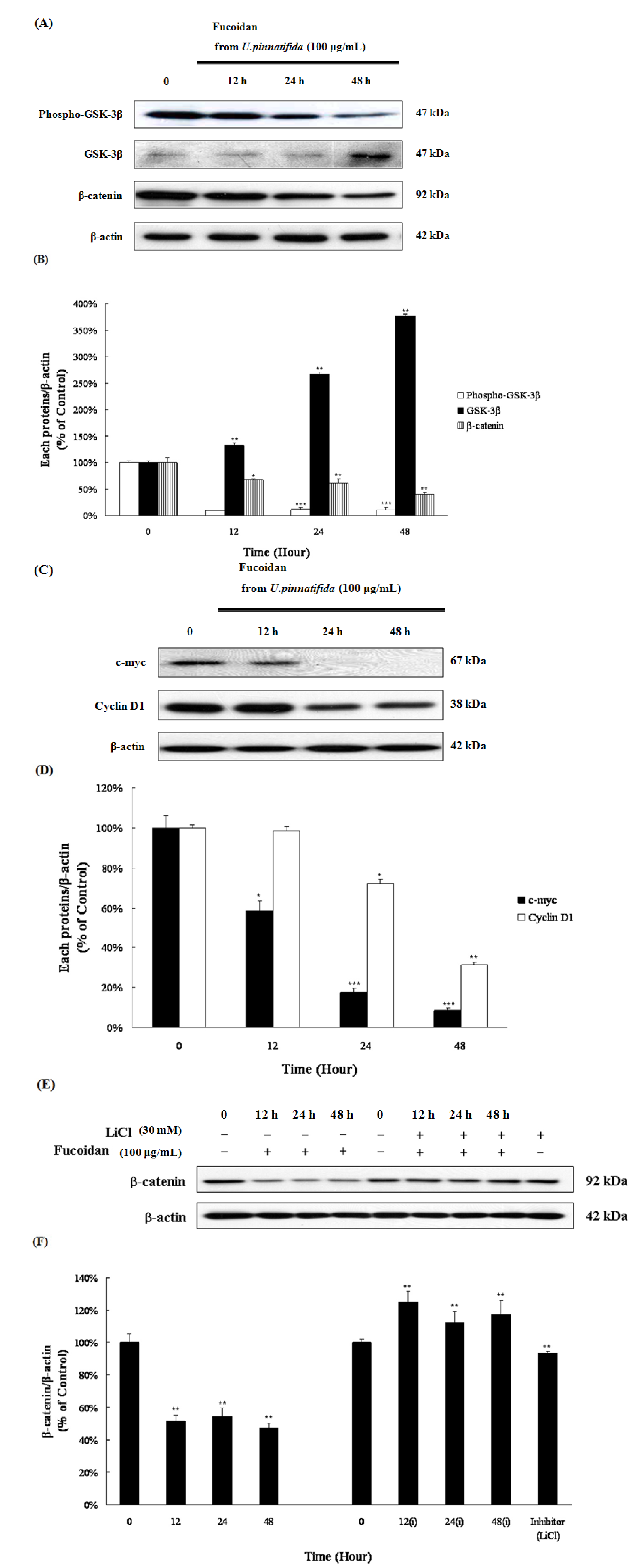
2.6. Fucoidan Induced Apoptosis via Down-Regulation of Wnt/β-Catenin Pathway in PC-3 Cells
3. Discussion
4. Experimental Section
4.1. Materials
4.2. Cell Culture
4.3. Cell Viability Assay
4.4. Flow Cytometric Analysis of Apoptosis
4.5. Morphological Analysis of Apoptosis by Hoechst 33342 Staining
4.6. Western Blot Analyses
4.7. Statistical Analyses
5. Conclusions
Acknowledgments
Conflict of Interest
References
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef]
- Yamasaki-Miyamoto, Y.; Yamasaki, M.; Tachibana, H.; Yamada, K. Fucoidan induces apoptosis through activation of caspase-8 on human breast cancer MCF-7 cells. J. Agric. Food Chem. 2009, 57, 8677–8682. [Google Scholar] [CrossRef]
- Bilan, M.I.; Grachev, A.A.; Ustuzhanina, N.E.; Shashkov, A.S.; Nifantiev, N.E.; Usov, A.I. Structure of a fucoidan from the brown seaweed Fucus evanescens C.Ag. Carbohydr. Res. 2002, 337, 719–730. [Google Scholar] [CrossRef]
- Gideon, T.P.; Rengasamy, R. Toxicological evaluation of fucoidan from Cladosiphon okamuranus. J. Med. Food 2008, 11, 638–642. [Google Scholar] [CrossRef]
- Lee, J.B.; Hayashi, K.; Hashimoto, M.; Nakano, T.; Hayashi, T. Novel antiviral fucoidan from sporophyll of Undaria pinnatifida (Mekabu). Chem. Pharm. Bull. 2004, 52, 1091–1094. [Google Scholar] [CrossRef]
- Asia, Y.; Miyakawa, Y.; Nakazato, T.; Shibata, H.; Saito, K.; Ikeda, Y.; Kizaki, M. Fucoidan induces apoptosis of human HS-sultan cells accompanied by activation of caspase-3 and down-regulation of ERK pathways. Am. J. Hematol. 2005, 78, 7–14. [Google Scholar] [CrossRef]
- Koyanagi, S.; Tanigawa, N.; Nakagawa, H.; Soeda, S.; Shimeno, H. Oversulfation of fucoidan enhances its anti-angiogenic and antitumor activities. Biochem. Pharmacol. 2003, 65, 173–179. [Google Scholar] [CrossRef]
- Durig, J.; Bruhn, T.; Zurborn, K.H.; Gutensohn, K.; Bruhn, H.D.; Beress, L. Anticoagulant fucoidan fractions from Fucus vesiculosus induce platelet activation in vitro. Thromb. Res. 1997, 85, 479–491. [Google Scholar] [CrossRef]
- McClure, M.O.; Moore, J.P.; Blance, D.F.; Scotting, P.; Cook, G.M.; Keynes, R.J.; Weber, J.N.; Davies, D.; Weiss, R.A. Investigations into the mechanism by which sulfated polysaccharides inhibit HIV infection in vitro. AIDS Res. Hum. Retrovir. 1992, 8, 19–26. [Google Scholar] [CrossRef]
- Dehm, S.M.; Tindall, D.J. Molecular regulation of androgen action in prostate cancer. J. Cell Biochem. 2006, 99, 333–344. [Google Scholar] [CrossRef]
- Nieto, M.; Finn, S.; Loda, M.; Hahn, W.C. Prostate cancer: Re-focusing on androgen receptor signaling. Int. J. Biochem. Cell Biol. 2007, 39, 1562–1568. [Google Scholar] [CrossRef]
- Feldman, B.J.; Feldman, D. The development of androgen-independent prostate cancer. Nat. Rev. Cancer 2001, 1, 34–45. [Google Scholar] [CrossRef]
- Tilley, W.D.; Wilson, C.M.; Marcelli, M.; McPhaul, M.J. Androgen receptor gene expression in human prostate carcinoma cell lines. Cancer Res. 1990, 50, 5382–5386. [Google Scholar]
- Zi, X.; Guo, Y.; Simoneau, A.R.; Hope, C.; Xie, J.; Holcombe, R.F.; Hoang, B.H. Expression of Frzb/secreted frizzled-related protein 3, a secreted Wnt antagonist, in human androgen-independent prostate cancer PC-3 cells suppresses tumor growth and cellular invasivenes. Cancer Res. 2005, 65, 9762–9770. [Google Scholar]
- Verras, M.; Brown, J.; Li, X.; Nusse, R.; Sun, Z. Wnt3a growth factor induces androgen receptor-mediated transcription and enhances cell growth in human prostate cancer cells. Cancer Res. 2004, 64, 8860–8866. [Google Scholar] [CrossRef]
- Polakis, P. Wnt signaling and cancer. Genes Dev. 2000, 14, 1837–1851. [Google Scholar]
- Morin, P.J. Beta-catenin signaling and cancer. Bioessays 1999, 21, 1021–1230. [Google Scholar]
- Debatin, K.M. Apoptosis pathways in cancer and cancer therapy. Cancer Immunol. Immunother. 2004, 53, 153–159. [Google Scholar] [CrossRef]
- Wada, T.; Penninger, J.M. Mitogen-activated protein kinases in apoptosis regulation. Oncogene 2004, 23, 2838–2849. [Google Scholar] [CrossRef]
- Jin, S.; Pang, R.P.; Shen, J.N.; Huang, G.; Wang, J.; Zhou, J.G. Grifolin induces apoptosis via inhibition of PI3K/AKT signaling pathway in human osteosarcoma cells. Apoptosis 2007, 12, 1317–1326. [Google Scholar] [CrossRef]
- Toker, A.; Yoeli-Lerner, M. Akt signaling and cancer: Surviving but not moving on. Cancer Res. 2006, 66, 3963–3966. [Google Scholar] [CrossRef]
- Kaseb, A.O.; Chinnakannu, K.; Chen, D.; Sivanandam, A.; Tejwani, S.; Menon, M.; Dou, Q.P.; Reddy, G.P. Androgen receptor and E2F-1 targeted thymoquinone therapy for hormone-refractory prostate cancer. Cancer Res. 2007, 67, 7782–7788. [Google Scholar] [CrossRef]
- Cayrol, C.; Knibiehler, M.; Ducommun, B. p21 binding to PCNA causes G1 and G2 cell cycle arrest in p53-deficient cells. Oncogene 1998, 16, 311–320. [Google Scholar]
- Lu, W.; Tinsley, H.N.; Keeton, A.; Qu, Z.; Piazza, G.A.; Li, Y. Suppression of Wnt/beta-catenin signaling inhibits prostate cancer cell proliferation. Eur. J. Pharmacol. 2009, 602, 8–14. [Google Scholar] [CrossRef]
- Teruya, T.; Konishi, T.; Uechi, S.; Tamaki, H.; Tako, M. Anti-proliferative activity of oversulfated fucoidan from commercially cultured Cladoshiphon okamuranus TOKIDA in U937 cells. Int. J. Biol. Macromol. 2007, 41, 221–226. [Google Scholar] [CrossRef]
- Hyun, J.H.; Kim, S.C.; Kang, J.I.; Kim, M.K.; Boo, H.J.; Kwon, J.M.; Koh, Y.S.; Hyun, J.W.; Park, D.B.; Yoo, E.S.; et al. Apoptosis inducing activity of fucoidan in HCT-15 colon carcinoma cells. Biol. Pharm. Bull. 2009, 32, 1760–1764. [Google Scholar] [CrossRef]
- Boo, H.J.; Hyun, J.H.; Kim, S.C.; Kang, J.I.; Kim, M.K.; Kim, S.Y.; Cho, H.; Yoo, E.S.; Kang, H.K. Fucoidan from Undaria pinnatifida induces apoptosis in A549 human lung carcinoma cells. Phytother. Res. 2011, 25, 1082–1086. [Google Scholar] [CrossRef]
- Yang, L.; Wang, P.; Wang, H.; Li, Q.; Teng, H.; Liu, Z.; Yang, W.; Hou, L.; Zou, X. Fucoidan derived from Undaria pinnatifida induces apoptosis in human hepatocellular carcinoma SMMC-7721 cells via the ROS-mediated mitochondrial pathway. Mar. Drugs 2013, 11, 1961–1976. [Google Scholar] [CrossRef]
- Chen, J.H.; Lim, J.D.; Sohn, E.H.; Choi, Y.S.; Han, E.T. Growth-inhibitory effect of a fucoidan from brown seaweed Undaria pinnatifida on Plasmodium parasites. Parasitol. Res. 2009, 104, 245–250. [Google Scholar] [CrossRef]
- Ashkenazi, A. Targeting the extrinsic apoptosis pathway in cancer. Cytokine Growth Factor Rev. 2008, 19, 325–331. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, X.; Chen, Y.; Wang, X.; Chen, R. p38 and JNK MAPK, but not ERK1/2 MAPK, play important role in colchicines-induced cortical neurons apoptosis. Eur. J. Pharmacol. 2007, 576, 26–33. [Google Scholar] [CrossRef]
- Hsieh, T.C.; Wu, J.M. Differential effects on growth, cell cycle arrest, and induction of apoptosis by resveratrol in human prostate cancer cell lines. Exp. Cell Res. 1999, 249, 109–115. [Google Scholar] [CrossRef]
- Keyse, S.M. Dual-specificity MAP kinase phosphatases (MKPs) and cancer. Cancer Metastasis Rev. 2008, 27, 253–261. [Google Scholar] [CrossRef]
- Seger, R.; Krebs, E.G. The MAPK signaling cascade. FASEB J. 1995, 9, 726–735. [Google Scholar]
- Cheung, E.C.; Slack, R.S. Emerging role for ERK as a key regulator of neuronal apoptosis. Sci. STKE 2004, 2004. [Google Scholar] [CrossRef]
- Wang, X.; Martindale, J.L.; Holbrook, N.J. Requirement for ERK activation in cisplatin-induced apoptosis. J. Biol. Chem. 2009, 275, 39435–39443. [Google Scholar]
- Bassi, R.; Heads, R.; Marber, M.S.; Clark, J.E. Targeting p38-MAPK in the ischaemic heart: Kill or cure? Curr. Opin. Pharmacol. 2008, 8, 141–146. [Google Scholar] [CrossRef]
- Serini, S.; Trombino, S.; Oliva, F.; Piccioni, E.; Monego, G.; Resci, F.; Boninsegna, A.; Picci, N.; Ranelletti, F.O.; Calviello, G. Docosahexaenoic acid induces apoptosis in lung cancer cells by increasing MKP-1 and down-regulating p-ERK1/2 and p-p38 expression. Apoptosis 2008, 13, 1172–1183. [Google Scholar] [CrossRef]
- Xue, M.; Ge, Y.; Zhang, J.; Liu, Y.; Wang, Q.; Hou, L.; Zheng, Z. Fucoidan inhibited 4T1 mouse breast cancer cell growth in vivo and in vitro via downregulation of Wnt/β-catenin signaling. Nutr. Cancer 2013, 65, 460–468. [Google Scholar] [CrossRef]
- Xue, M.; Ge, Y.; Zhang, J.; Wang, Q.; Hou, L.; Liu, Y.; Sun, L.; Li, Q. Anticancer properties and mechanisms of fucoidan on mouse breast cancer in vitro and in vivo. PLoS One 2012, 7, e43483. [Google Scholar]
- Hsu, H.Y.; Hajjar, D.P.; Khan, K.M.; Falcone, D.J. Ligand binding to macrophage scavenger receptor-A induces urokinase-type plasminogen activator expression by a protein kinase-dependent signaling pathway. J. Biol. Chem. 1998, 273, 1240–1246. [Google Scholar] [CrossRef]
- Nakamura, T.; Suzuki, H.; Wada, Y.; Kodama, T.; Doi, T. Fucoidan induces nitric oxide production via p38 mitogen-activated protein kinase and NF-kappaB-dependent signaling pathways through macrophage scavenger receptors. Biochem. Biophys. Res. Commun. 2006, 343, 286–294. [Google Scholar] [CrossRef]
- Do, H.; Pyo, S.; Sohn, E.H. Suppression of iNOS expression by fucoidan is mediated by regulation of p38 MAPK, JAK/STAT, AP-1 and IRF-1, and depends on up-regulation of scavenger receptor B1 expression in TNF-alpha- and IFN-gamma-stimulated C6 glioma cells. J. Nutr. Biochem. 2010, 21, 671–679. [Google Scholar] [CrossRef]
- Dillard, P.R.; Lin, M.F.; Khan, S.A. Androgen-independent prostate cancer cells acquire the complete steroidogenic potential of synthesizing testosterone from cholesterol. Mol. Cell. Endocrinol. 2008, 295, 115–120. [Google Scholar] [CrossRef]
- Leon, C.G.; Locke, J.A.; Adomat, H.H.; Etinger, S.L.; Twiddy, A.L.; Neumann, R.D.; Nelson, C.C.; Guns, E.S.; Wasan, K.M. Alterations in cholesterol regulation contribute to the production of intratumoral androgens during progression to castration-resistant prostate cancer in a mouse xenograft model. Prostate 2010, 70, 390–400. [Google Scholar]
- Scudiero, D.A.; Shoemaker, R.H.; Paull, K.D.; Monks, A.; Tierney, S.; Nofziger, T.H.; Currens, M.J.; Seniff, D.; Boyd, M.R. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 1988, 48, 4827–4833. [Google Scholar]
- Fried, J.; Perez, A.G.; Clarkson, B.D. Flow cytofluorometric analysis of cell cycle distributions using propidium iodide. Properties of the method and mathematical analysis of the data. J. Cell Biol. 1976, 71, 172–181. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Boo, H.-J.; Hong, J.-Y.; Kim, S.-C.; Kang, J.-I.; Kim, M.-K.; Kim, E.-J.; Hyun, J.-W.; Koh, Y.-S.; Yoo, E.-S.; Kwon, J.-M.; et al. The Anticancer Effect of Fucoidan in PC-3 Prostate Cancer Cells. Mar. Drugs 2013, 11, 2982-2999. https://doi.org/10.3390/md11082982
Boo H-J, Hong J-Y, Kim S-C, Kang J-I, Kim M-K, Kim E-J, Hyun J-W, Koh Y-S, Yoo E-S, Kwon J-M, et al. The Anticancer Effect of Fucoidan in PC-3 Prostate Cancer Cells. Marine Drugs. 2013; 11(8):2982-2999. https://doi.org/10.3390/md11082982
Chicago/Turabian StyleBoo, Hye-Jin, Ji-Young Hong, Sang-Cheol Kim, Jung-Il Kang, Min-Kyoung Kim, Eun-Ji Kim, Jin-Won Hyun, Young-Sang Koh, Eun-Sook Yoo, Jung-Mi Kwon, and et al. 2013. "The Anticancer Effect of Fucoidan in PC-3 Prostate Cancer Cells" Marine Drugs 11, no. 8: 2982-2999. https://doi.org/10.3390/md11082982
APA StyleBoo, H.-J., Hong, J.-Y., Kim, S.-C., Kang, J.-I., Kim, M.-K., Kim, E.-J., Hyun, J.-W., Koh, Y.-S., Yoo, E.-S., Kwon, J.-M., & Kang, H.-K. (2013). The Anticancer Effect of Fucoidan in PC-3 Prostate Cancer Cells. Marine Drugs, 11(8), 2982-2999. https://doi.org/10.3390/md11082982



