Influence of Fucoidans on Hemostatic System
Abstract
:1. Introduction
2. Results and Discussion
2.1. Sulfated Carbohydrate Samples
| Sample | Source | Fuc | Xyl | Man | Gal | Uronic acids | SO3Na | Degree of Sulfation ** |
|---|---|---|---|---|---|---|---|---|
| SL | S. latissima | 36.7 | 1.8 | 0.7 | 8.4 | 1.9 | 39.8 | 1.3 |
| FV | F. vesiculosus | 28.5 | 5.6 | 2.8 | 2.9 | 7.9 | 26.0 | 0.9 |
| CO | C. okamuranus | 42.7 | 2.0 | 1.1 | 1.9 | 15.1 | 16.9 | 0.4 |
| SL-S | Sulfation of SL | 26.9 | - | 1.3 | 7.1 | 1.0 | 46.7 | 2.0 |
| CO-S | Sulfation of CO | 19.7 | 0.8 | 0.9 | 0.7 | 8.1 | 45.7 | 2.4 |
| OS | Synthetic compound | 39.4 | - | - | - | - | 59.1 | 2.1 |
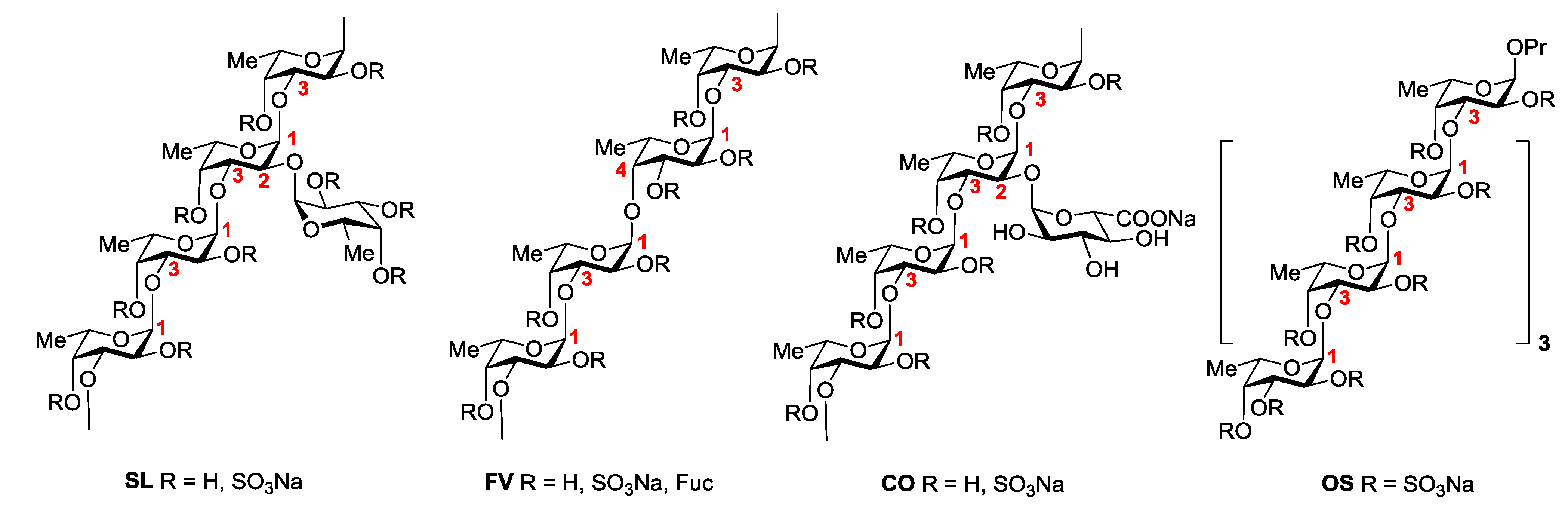
2.2. Clotting Assays
| Sample | 2APTT (μg/mL) | 2TT (μg/mL) |
|---|---|---|
| Clexane® | 3.32 ± 0.12 | 2.25 ± 0.03 |
| SL | 1.07 ± 0.05 | 2.43 ± 0.05 |
| SL-S | 2.01 ± 0.07 | 2.20 ± 0.07 |
| FV | 6.81 ± 0.03 | 9.15 ± 0.11 |
| CO | >100 | >100 |
| CO-S | 2.51 ± 0.09 | 4.28 ± 0.07 |
| OS | 5.10 ± 0.12 | 16.08 ± 0.23 |

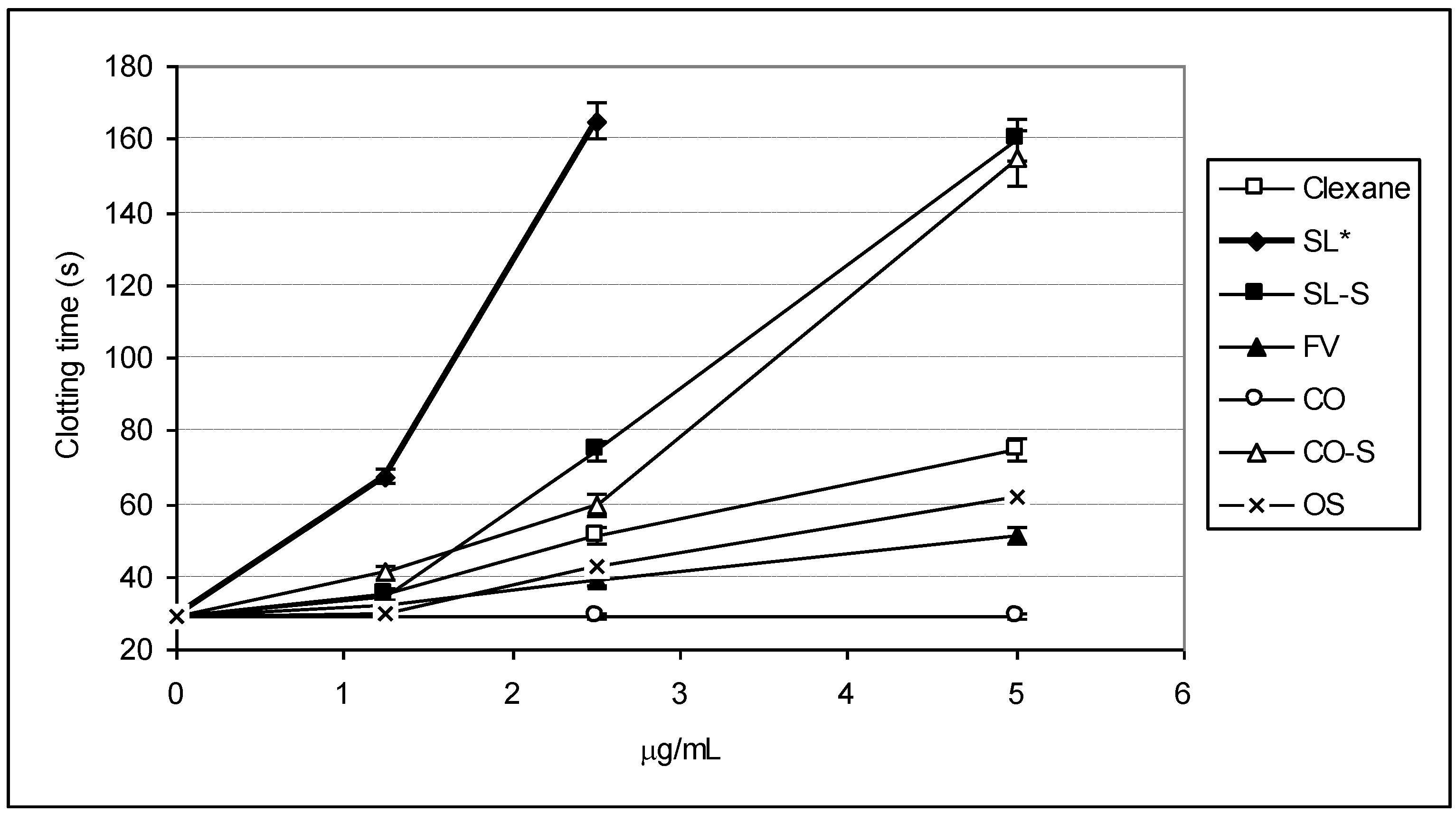
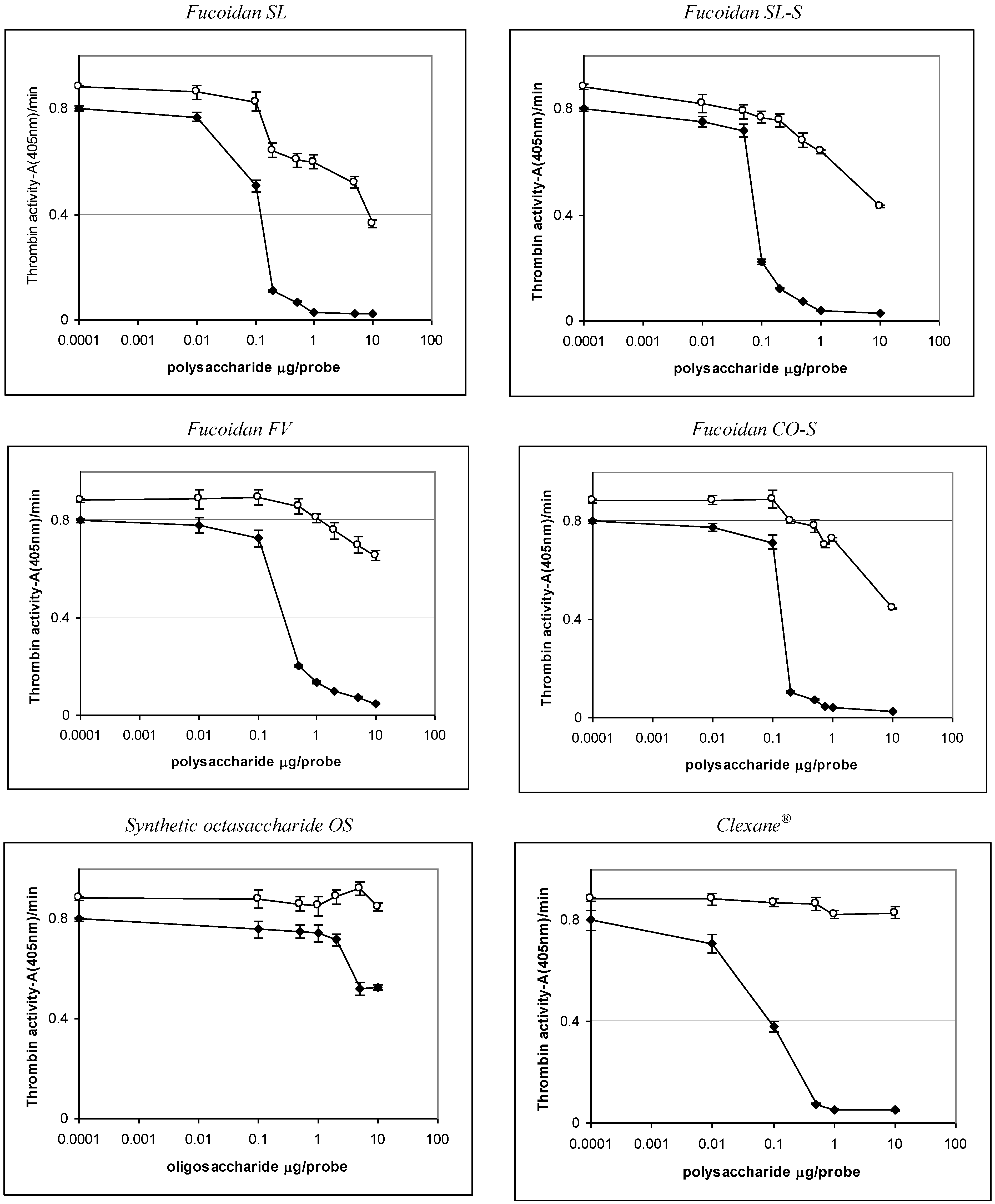
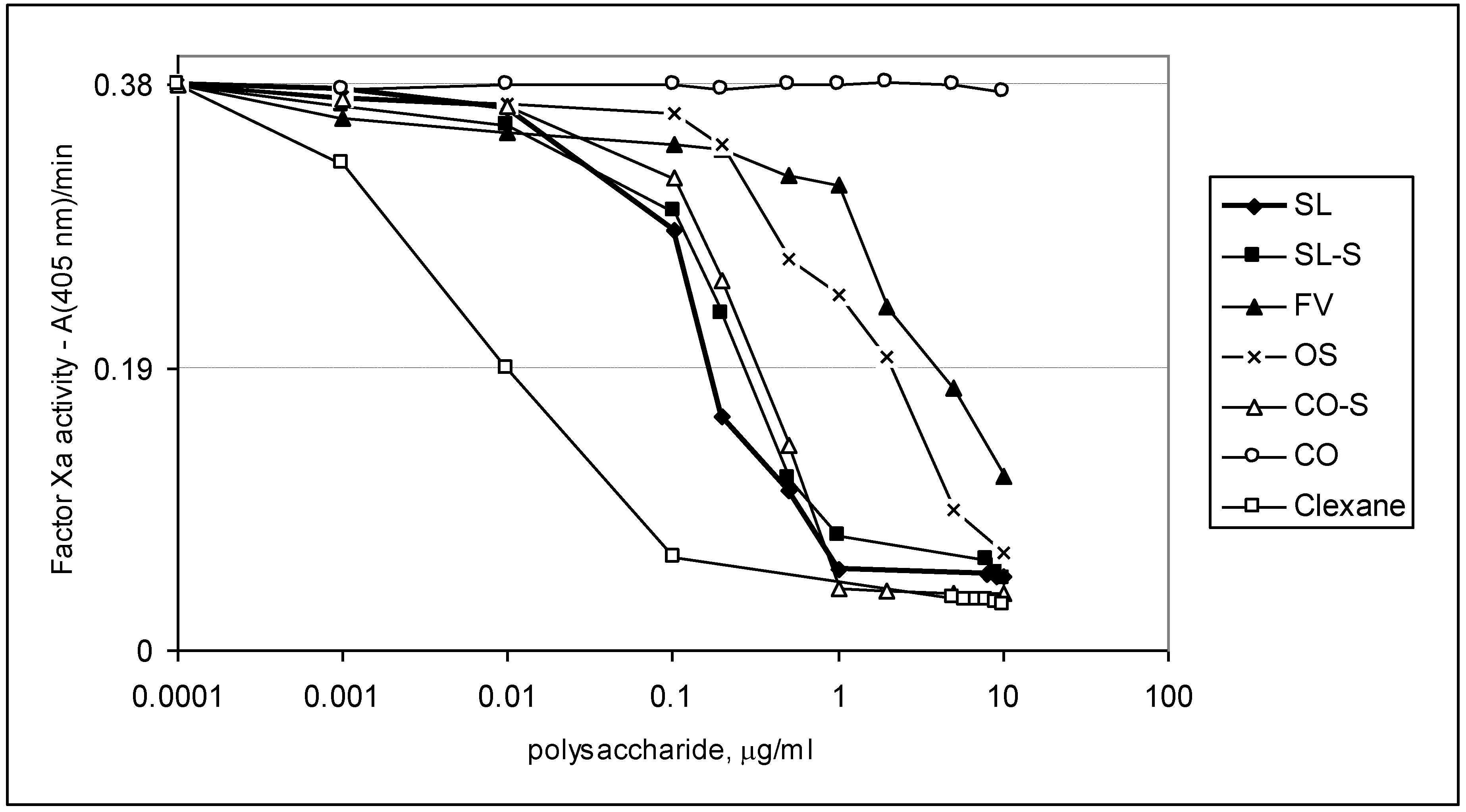
2.3. Effect of the Compounds on the Inactivation of Thrombin and Factor Xa in the Presence and in the Absence of Antithrombin III
| Sample | IC50 (μg/mL) | ||
|---|---|---|---|
| АТIII + thrombin | +thrombin | АТIII + Xa | |
| SL | 0.76 ± 0.04 | 45.86 ± 0.58 | 1.06 ± 0.04 |
| SL-S | 0.47 ± 0.02 | 55.86 ± 1.07 | 1.94 ± 0.08 |
| FV | 2.1 ± 0.08 | No * | 28.22 ± 0.95 |
| CO | no | no | No |
| CO-S | 0.88± 0.03 | 58.81 ± 1.12 | 2.06 ± 0.09 |
| OS | No ** | no | 12.94 ± 0.98 |
| Clexane® | 0.59 ± 0.02 | no | 0.059 ± 0.002 |
| Arixtra® | no | no | 0.0065 ± 0.0003 |
2.4. Influence on Platelets Aggregation
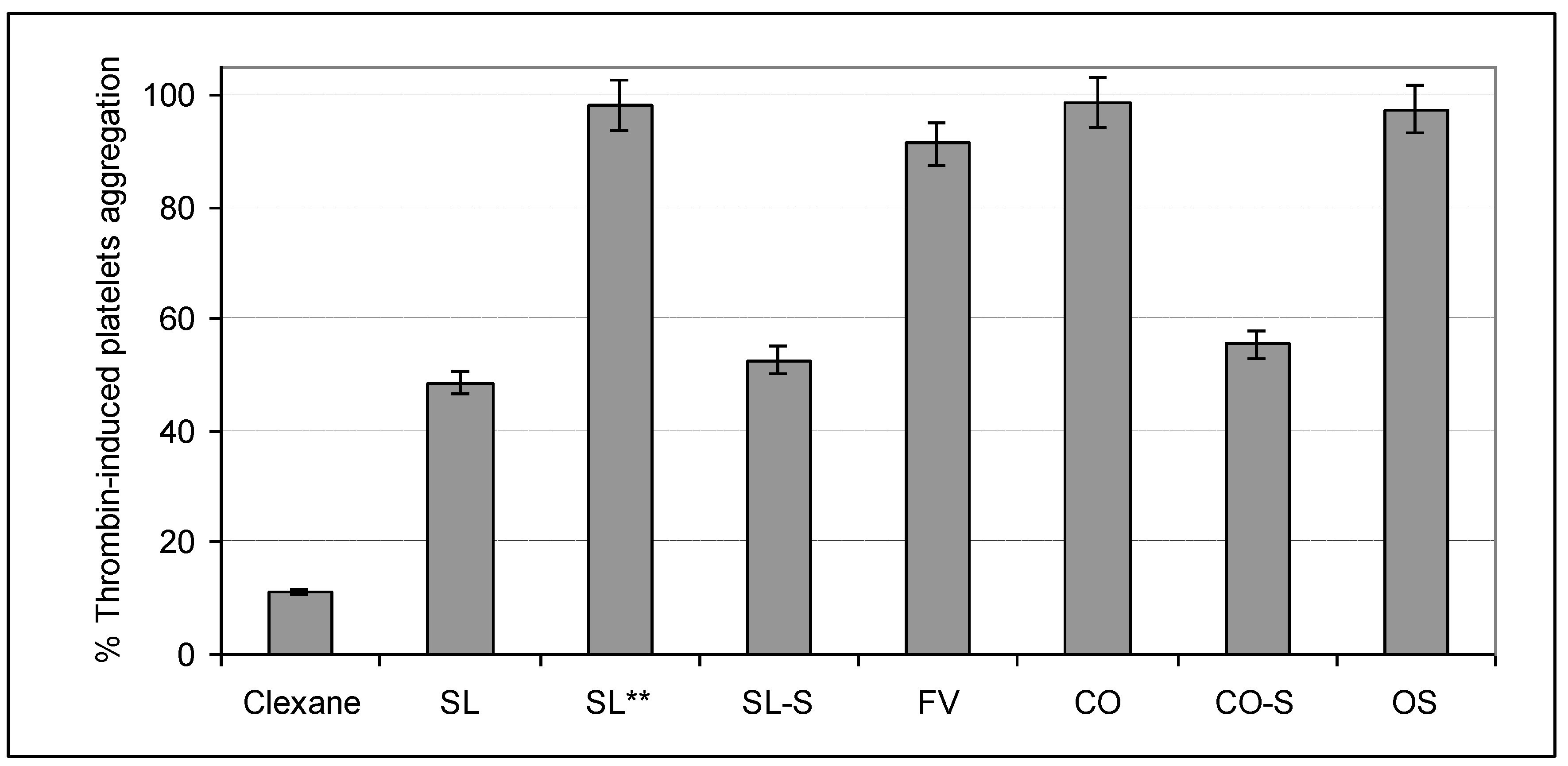
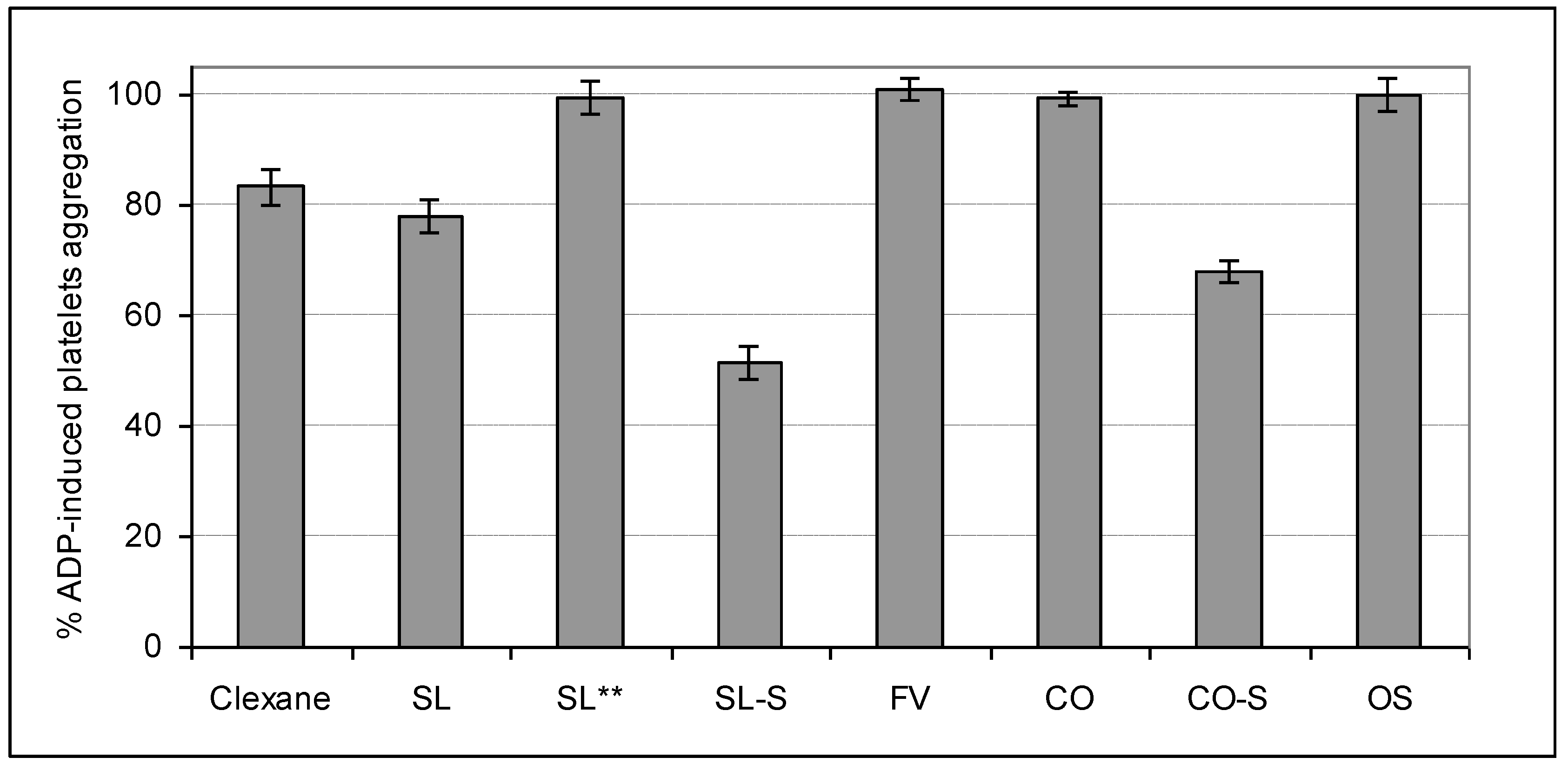
3. Experimental Section
3.1. Preparation of the Samples
3.2. General Coagulation Assays
3.3. Amidolytic Assays
3.4. Inhibition of Platelets Aggregation
3.5. Statistical Analysis
4. Conclusions
Acknowledgments
References
- Galanaud, J.P.; Laroche, J.P.; Righini, M. The history and historical treatments of deep vein thrombosis. J. Thomb. Haemost. 2013, 3, 402–411. [Google Scholar] [CrossRef]
- Falanga, A.; Vignoli, A.; Diani, E.; Marchetti, M. Comparative assessment of low-molecular-weight heparins in cancer from the perspective of patient outcomes and survival. Patient Related Outcome Meas. 2011, 2, 175–188. [Google Scholar]
- Pomin, V.H. Fucanomics and galactanomics: Current status in drug discovery, mechanisms of action and role of the well defined structures. Biochim. Biopys. Acta 2012, 1820, 1971–1979. [Google Scholar] [CrossRef]
- Pereira, M.S.; Melo, F.R.; Mourão, P.A. Is there a correlation between structure and anticoagulant action of sulfated galactans and sulfated fucans? Glycobiology 2002, 12, 573–580. [Google Scholar] [CrossRef]
- Melo, F.R.; Pereira, M.S.; Foguel, D.; Mourão, P.A. Antithrombin-mediated anticoagulant activity of sulfated polysaccharides: Different mechanisms for heparin and sulfated galactans. J. Biol. Chem. 2004, 279, 20824–20835. [Google Scholar]
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, S. Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar. Drugs 2011, 9, 196–223. [Google Scholar] [CrossRef]
- Ciancia, M.; Quintana, I.; Cerezo, A.S. Overview of anticoagulant activity of sulfated polysaccharides from seaweeds in relation to their structures, focusing on those of green seaweeds. Curr. Med. Chem. 2010, 17, 2503–2529. [Google Scholar] [CrossRef]
- Fernandez, P.V.; Quintana, I.; Cerezo, A.S.; Caramelo, J.J.; Pol-Fachin, L.; Verli, H.; Estevez, J.M.; Ciancia, M. Anticoagulant activity of a unique sulfated pyranosic (1→3)-β-l-arabinan through direct interaction with thrombin. J. Biol. Chem. 2013, 288, 223–233. [Google Scholar] [CrossRef]
- Fonseca, R.J.; Santos, G.R.; Mourão, P.A. Effects of polysaccharides enriched in 2,4-disulfated fucose units on coagulation, thrombosis and bleeding. Practical and conceptual implications. Thromb. Haemost. 2009, 102, 829–836. [Google Scholar]
- Fitton, J.H. Therapies from fucoidan; Multifunctional marine polymers. Mar. Drugs 2011, 9, 1731–1760. [Google Scholar] [CrossRef]
- Ale, M.T.; Mikkelsen, J.D.; Meyer, A.S. Important determinants for fucoidan bioactivity: A critical review of structure-function relations and extraction methods for fucose-containing sulfated polysaccharides from brown seaweeds. Mar. Drugs 2011, 9, 2106–2130. [Google Scholar] [CrossRef]
- Cumashi, A.; Ushakova, N.A.; Preobrazhenskaya, M.E.; D’Incecco, A.; Piccoli, A.; Totani, L.; Tinari, N.; Morozevich, G.E.; Berman, A.E.; Bilan, M.A.; et al. Comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology 2007, 17, 541–552. [Google Scholar]
- Usov, A.I.; Bilan, M.I. Fucoidans—Sulfated polysaccharides of brown algae. Russ. Chem. Rev. 2009, 78, 785–799. [Google Scholar] [CrossRef]
- Pereira, M.S.; Mulloy, B.; Mourão, P.A. Structure and anticoagulant activity of sulfated fucans. J. Biol. Chem. 1999, 274, 7656–7667. [Google Scholar] [CrossRef]
- Ushakova, N.A.; Morozevich, G.E.; Ustyuzhanina, N.E.; Bilan, M.I.; Usov, A.I.; Nifantiev, N.E.; Preobrazhenskaya, M.E. Anticoagulant activity of fucoidans from brown seaweeds (in Russian). Biomed. Chem. 2008, 54, 597–606. [Google Scholar]
- Croci, D.O.; Cumashi, A.; Ushakova, N.A.; Preobrazhenskaya, M.E.; Piccoli, A.; Totani, L.; Ustyuzhanina, N.E.; Bilan, M.I.; Usov, A.I.; Grachev, A.A.; et al. Fucans, but not fucomannoglucuronans, determine the biological activities of sulfated polysaccharides from Laminaria saccharina brown seaweed. PLoS One 2011, 6. [Google Scholar] [CrossRef]
- Nishino, T.; Nagumo, T. The sulfate-content dependence of the anticoagulant activity of a fucan sulfate from the brown seaweed Ecklonia kurome. Carbohydr. Res. 1991, 214, 193–197. [Google Scholar] [CrossRef]
- Haroun-Bouhedja, F.; Ellouali, M.; Sinquin, C.; Boisson-Vidal, C. Relationship between sulfate groups and biological activities of fucans. Thromb. Res. 2000, 100, 453–459. [Google Scholar] [CrossRef]
- Pomin, V.H.; Pereira, M.S.; Valente, A.P.; Tollefsen, D.M.; Pavao, M.S.G.; Mourão, P.A.S. Selective cleavage and anticoagulant activity of a sulfated fucan: Stereospecific removal of a 2-sulfate ester from the polysaccharide by mild acid hydrolysis, preparation of oligosaccharides, and heparin cofactor II-dependent anticoagulant activity. Glycobiology 2005, 15, 369–381. [Google Scholar]
- Bilan, M.I.; Grachev, A.A.; Shashkov, A.S.; Kelly, M.; Sanderson, C.J.; Nifantiev, N.E.; Usov, A.I. Further studies on the composition and structure of a fucoidan preparation from the brown alga Saccharina latissima. Carbohydr. Res. 2010, 345, 2038–2047. [Google Scholar] [CrossRef]
- Chevolot, L.; Mulloy, B.; Ratiskol, J.; Foucault, A.; Colliec-Jouault, S. A disaccharide repeat unit is the major structure in fucoidans from two species of brown algae. Carbohydr. Res. 2001, 330, 529–535. [Google Scholar] [CrossRef]
- Nagaoka, M.; Shibata, H.; Kimura-Takagi, I.; Hashimoto, S.; Kimura, K.; Makino, T.; Aiyama, R.; Ueyama, S.; Yokokura, T. Structural study of fucoidan from Cladosiphon okamuranus TOKIDA. Glycoconj. J. 1999, 16, 19–26. [Google Scholar] [CrossRef]
- Krylov, V.B.; Ustyuzhanina, N.E.; Grachev, A.A.; Nifantiev, N.E. Efficient acid-promoted per-O-sulfation of organic polyols. Tetrahedron Lett. 2008, 49, 5877–5879. [Google Scholar] [CrossRef]
- Krylov, V.B.; Kaskova, Z.M.; Vinnitskiy, D.Z.; Ustyuzhanina, N.E.; Grachev, A.A.; Chizhov, A.O.; Nifantiev, N.E. Acid-promoted total sulfation of fucoidan fragments. Carbohydr. Res. 2011, 346, 540–550. [Google Scholar] [CrossRef]
- McGarry, L.J.; Thompson, D. Retrospective database analysis of the prevention of venous thromboembolism with low-molecular-weight heparin in acutely III medical inpatients in community practice. Clin. Ther. 2004, 26, 419–430. [Google Scholar] [CrossRef]
- Sobieraj, D.M.; Coleman, C.I.; Tongbram, V.; Chen, W.; Colby, J.; Lee, S.; Kluger, J.; Makanji, S.; Ashaye, A.; White, C.M. Comparative effectiveness of low-molecular-weight heparins versus other anticoagulants in major orthopedic surgery: A systematic review and meta-analysis. Pharmacotherapy 2012, 32, 799–808. [Google Scholar] [CrossRef]
- Gray, E.; Hogwood, J.; Mulloy, B. The anticoagulant and antithrombotic mechanisms of heparin. Handb. Exp. Pharmacol. 2012, 207, 43–61. [Google Scholar] [CrossRef]
- Imberty, A.; Lortat-Jacob, H.; Perez, S. Structural view on glycosaminoglycan-protein interactions. Carbohydr. Res. 2007, 342, 430–439. [Google Scholar] [CrossRef]
- Petitou, M.; Casu, B.; Lindahl, U. 1976–1983, a critical period in the history of heparin: The discovery of the antithrombin binding site. Biochimie 2003, 85, 83–89. [Google Scholar] [CrossRef]
- Usov, A.I.; Smirnova, G.P.; Klochkova, N.G. Polysaccharides of algae. 55. Polysaccharide composition of some brown algae from Kamchatka. Russ. J. Bioorg. Chem. 2001, 27, 395–399. [Google Scholar] [CrossRef]
- Usov, A.I.; Smirnova, G.P.; Bilan, M.I.; Shashkov, A.S. Polysaccharides of algae. 53. Brown alga Laminaria saccharina (L.) Lam. as a source of fucoidan. Russ. J. Bioorg. Chem. 1998, 24, 437–445. [Google Scholar]
- Bilan, M.I.; Grachev, A.A.; Ustuzhanina, N.E.; Shashkov, A.S.; Nifantiev, N.E.; Usov, A.I. Structure of a fucoidan from the brown seaweed Fucus evanescens C. Ag. Carbohydr. Res. 2002, 337, 719–730. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ustyuzhanina, N.E.; Ushakova, N.A.; Zyuzina, K.A.; Bilan, M.I.; Elizarova, A.L.; Somonova, O.V.; Madzhuga, A.V.; Krylov, V.B.; Preobrazhenskaya, M.E.; Usov, A.I.; et al. Influence of Fucoidans on Hemostatic System. Mar. Drugs 2013, 11, 2444-2458. https://doi.org/10.3390/md11072444
Ustyuzhanina NE, Ushakova NA, Zyuzina KA, Bilan MI, Elizarova AL, Somonova OV, Madzhuga AV, Krylov VB, Preobrazhenskaya ME, Usov AI, et al. Influence of Fucoidans on Hemostatic System. Marine Drugs. 2013; 11(7):2444-2458. https://doi.org/10.3390/md11072444
Chicago/Turabian StyleUstyuzhanina, Nadezhda E., Natalia A. Ushakova, Ksenia A. Zyuzina, Maria I. Bilan, Anna L. Elizarova, Oksana V. Somonova, Albina V. Madzhuga, Vadim B. Krylov, Marina E. Preobrazhenskaya, Anatolii I. Usov, and et al. 2013. "Influence of Fucoidans on Hemostatic System" Marine Drugs 11, no. 7: 2444-2458. https://doi.org/10.3390/md11072444
APA StyleUstyuzhanina, N. E., Ushakova, N. A., Zyuzina, K. A., Bilan, M. I., Elizarova, A. L., Somonova, O. V., Madzhuga, A. V., Krylov, V. B., Preobrazhenskaya, M. E., Usov, A. I., Kiselevskiy, M. V., & Nifantiev, N. E. (2013). Influence of Fucoidans on Hemostatic System. Marine Drugs, 11(7), 2444-2458. https://doi.org/10.3390/md11072444





