Abstract
Five non-toxin producing cyanobacterial isolates from the genera Synechococcus, Trichormus, Microcystis, Leptolyngbya and Chlorogloea were examined in terms of quantity and quality as lipid feedstock for biofuel production. Under the conditions used in this study, the biomass productivity ranged from 3.7 to 52.7 mg·L−1·day−1 in relation to dry biomass, while the lipid productivity varied between 0.8 and 14.2 mg·L−1·day−1. All cyanobacterial strains evaluated yielded lipids with similar fatty acid composition to those present in the seed oils successfully used for biodiesel synthesis. However, by combining biomass and lipid productivity parameters, the greatest potential was found for Synechococcus sp. PCC7942, M. aeruginosa NPCD-1 and Trichormus sp. CENA77. The chosen lipid samples were further characterized using Fourier Transform Infrared spectroscopy (FTIR), viscosity and thermogravimetry and used as lipid feedstock for biodiesel synthesis by heterogeneous catalysis.
1. Introduction
Cyanobacteria are a large group of oxygenic photoautotrophic bacteria and, like plants and algae, can capture CO2 via the Calvin-Benson cycle and convert it to a suite of organic compounds. They are important primary producers of organic material and play significant roles in biochemical cycles of carbon, nitrogen, and oxygen [1]. Cyanobacteria are well suited for synthetic biology and metabolic engineering approaches for the phototrophic production of various desirable biomolecules, including substances with cytotoxic, antifungal, antibacterial and antiviral activities [2,3]. Lipids, carotenoids, pigments, vitamins and aromatic compounds are also found in cyanobacteria. Lipids (accumulated in the thylakoid membranes) are associated with high levels of photosynthesis and rapid growth rate and are of particular interest, since they can be used as lipid feedstock for biodiesel production [4,5,6]. Microalgae accumulate large amounts of lipids as reserve material, but only in conditions of stress and slow growth [7]. Thus, cyanobacteria have a natural advantage to produce lipids in high-speed growth.
Currently, biodiesel derived from microbial biomass is attracting much attention and investment and can meet the demand necessary for biodiesel supply without commitment to the food chain. The biosynthesis of fatty acid-based biofuels in cyanobacteria includes two steps, production and transesterification of fatty acids (FAs) to form alkyl FA esters [8]. Considering that fuel properties are largely dependent on the FA composition of the feedstock from which biodiesel is prepared, FA profile was employed as a screening tool for selection of cyanobacterial lipids with high amounts of monounsaturated FAs. The presence of double bonds in the FAs from cyanobacterial lipids is related to their morphological complexity [9]. It has been reported that unicellular forms are characterized by the presence of mono and polyenoic acids [10].
In order to compare representatives of four different cyanobacterial orders [11], five strains isolated from extreme biomes were chosen: Synechococcus sp. PCC7942 (Synechococcales); Microcystis aeruginosa NPCD-1 and Chlorogloea sp. CENA170 (Chroococcales), isolated from a sewage treatment plant and the mangrove, respectively; Leptolyngbya sp. CENA104 (Pseudanabaenales), which is classified as a filamentous cyanobacteria without heterocytes, also isolated from a sewage treatment plant; Trichormus sp. CENA77 (Nostocales), a heterocystous filamentous strain, isolated from a contaminated environment containing the herbicide propanil.
In addition, three of them are unicellular strains (Synechococcus sp. PCC7942, M. aeruginosa NPCD-1 and Chlorogloea sp. CENA170), which allow easy genetic manipulation in order to increase the photosynthetic efficiency, growth rate and lipid content in the biomass [12]. On the other hand, the two filamentous strains (Leptolyngbya sp. CENA104 and Trichormus sp. CENA77) have the advantage of easy procedures for biomass harvesting [13].
Although cyanobacteria have several advantages for biofuel production, including easy genetic manipulation to increase photosynthetic efficiency, fast growth rate and high lipid content in the biomass, there are few specific studies on their use as a source of lipids. In this context, this study aims to evaluate the potential of five cyanobacterial strains as producers of lipid feedstock for the synthesis of biodiesel. Biomass and lipid productivities and FA profiles were evaluated. Other selection criteria included were iodine value, degree of unsaturation and long chain saturated factor.
2. Results and Discussion
2.1. Biomass and Lipid Productivity
The biomass productivity ranged from 3.7 to 52.7 mg·L−1·day−1 in relation to dry biomass while the lipid productivity varied between 0.8 and 14.2 mg·L−1·day−1 (Table 1). The highest lipid productivity was obtained by Synechococcus sp. PCC7942 and the lowest by Leptolyngbya sp. CENA104. High biomass and lipid productivities were also found for M. aeruginosa NPCD-1 (46.9 and 13.1 mg·L−1·day−1, respectively) and Trichormus sp. CENA77 (30.8 and 7.3 mg·L−1·day−1, respectively). The biomass from Chlorogloea sp. CENA170 and Leptolyngbya sp. CENA104 attained low lipid productivity (lower than 1.0 mg·L−1·day−1) and were considered unsuitable as a source of lipid feedstock.

Table 1.
Cyanobacterial strains used in this study and their respective biomass and lipid productivity.
| Cyanobacterium | Micrograph | Strain source | Biomass productivity | Lipid productivity |
|---|---|---|---|---|
| Scale bar: 20 µm | (mg·L−1·day−1) | (mg·L−1·day−1) | ||
| Microcystis aeruginosa NPCD-1 |  | Sewage treatment
plant of Cidade de Deus, RJ, Brazil | 46.9 | 13.1 |
| Synechococcus sp. PCC7942 |  | Pasteur Culture
Collection, France | 52.7 | 14.2 |
| Chlorogloea sp. CENA170 |  | Mangrove soil from
Cardoso Island, SP, Brazil | 6.8 | 0.9 |
| Trichormus sp. CENA77 |  | Flooded rice field,
SC, Brazil | 30.8 | 7.3 |
| Leptolyngbya sp. CENA104 |  | Sewage treatment
plant of Cajati, SP, Brazil | 3.7 | 0.8 |
For the three selected isolates, lipid content (28.0% for M. aeruginosa NPCD-1, 26.9% for Synechococcus sp. PCC7942 and 23.7% for Trichormus sp. CENA77) were similar to those reported in a study in which 13 different species of cyanobacteria isolated from aquatic habitats showed lipid content between 10.5 and 28.1 wt% [14]. The highest lipid content (28.1%) was obtained by M. aeruginosa followed by Phormidium purpurascens (26.4%), while P. ambiguum had the lowest value.
Another study showed that biomass productivity attained by Aphanotece microscopic Nägeli was 753.6 mg·L−1·day−1, but lipid content was low as 8.0 wt% [15]. The microalgae Scenedesmus sp. yielded 220 mg·L−1·day−1 of biomass productivity and 9.5 wt% of lipid content [16], whereas the cyanobacterium Spirulina maxima LB 2342 presented biomass productivity of 210 mg·L−1·day−1 and 4.1% of lipid content [17]. Scenedesmus obliquus sp. resulted in a biomass productivity of 60 mg·L−1·day−1 and 12.7% of lipids [18].
The lipid content of cyanobacteria can range from 5 to 13 wt% [19]. However, other authors reveal that the lipid content accumulated by cyanobacteria can vary from 38 to 45 wt%, depending on the species and environmental conditions, including nutrients and light intensity [4,20]. Thus, the results of lipid content (from 13% to 28%) obtained in the present study for the assessed species are favorable compared to those reported for other species and genera of cyanobacteria.
2.2. Fatty Acids Profile
Besides the favorable lipid productivity, the selected strains should have a FA profile that allows obtaining biodiesel with the physico-chemical properties required to be used as a fuel. The FA profile of cyanobacterial lipid characterized by GC yielded 21 FAs with carbon chains ranging from C6 to C24 and different degrees of unsaturation (Table 2). The percentage of unidentified FAs was approximately 1%, with the exception of lipid biomass from M. aeruginosa NPCD-1, which showed 3.4% of unidentified FAs.

Table 2.
Fatty acid (FA) profile (% wt) present in the cyanobacterial lipid.
| FA | Microcystis aeruginosa NPCD-1 | Synechococcus sp. PCC7942 | Chlorogloea sp. CENA170 | Trichormus sp. CENA77 | Leptolyngbya sp. CENA104 | |
|---|---|---|---|---|---|---|
| Saturated FA (%wt) | ||||||
| C6:0 | Caproic | 0.1 | 0.06 | 0.05 | 0.08 | 0.09 |
| C8:0 | Caprilic | 0.6 | 0.06 | 0.4 | 0.6 | 0.2 |
| C10:0 | Capric | 0.9 | 0.06 | 0.6 | 0.6 | 0.2 |
| C12:0 | Lauric | 13.2 | 0.7 | 8.7 | 9.7 | 3.9 |
| C14:0 | Myristic | 5.2 | 0.7 | 3.4 | 3.9 | 1.7 |
| C15:0 | Pentadecanoic | 0.1 | 0.06 | 0.06 | 0.06 | 0.08 |
| C16:0 | Palmitic | 24.3 | 23.5 | 15.4 | 24.9 | 14.6 |
| C17:0 | Margaric | 0.2 | 0.1 | 0.1 | 0.2 | 0.15 |
| C18:0 | Stearic | 4.9 | 3.6 | 3.8 | 3.4 | 2.8 |
| C20:0 | Arachidic | 0.3 | 0.3 | 0.2 | 0.37 | 0.4 |
| C22:0 | Behenic | 0.2 | 0.3 | 0.2 | 0.15 | 0.3 |
| C24:0 | Lignoceric | 0.1 | 0.2 | 0.1 | 0.10 | 0.15 |
| Total | 50.1 | 29.6 | 33.0 | 44.1 | 24.6 | |
| Monounsaturated FA | ||||||
| C16:1 | Palmitoleic | 1.1 | 3.3 | 5.4 | 1.5 | 3.9 |
| C17:1 | cis-10-Heptadecenoic | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 |
| C18:1 | Oleic | 26.8 | 31.5 | 28.8 | 36.9 | 38.8 |
| C20:1 | Gadoleic | 0.3 | 0.2 | 0.1 | 0.3 | 0.42 |
| C22:1 | Erucic | 2.5 | nd | nd | nd | nd |
| C24:1 | Nervonic | 0.8 | nd | nd | nd | nd |
| Total | 31.6 | 35.1 | 34.4 | 38.8 | 43.3 | |
| Polyunsaturated FA | ||||||
| C18:2 | Linoleic | 12.5 | 30.9 | 29.4 | 10.7 | 26.4 |
| C18:3 | .-Linolenic | 0.8 | 0.5 | 0.27 | nd | 0.4 |
| C18:3 | Linolenic | 1.6 | 2.9 | 1.63 | 5.1 | 4.3 |
| Total | 14.9 | 34.3 | 31.3 | 15.8 | 31.1 | |
| N.I. | Unidentified | 3.4 | 1.0 | 1.3 | 1.3 | 1.0 |
n.d. = not detected.
Most FAs present in the five cyanobacterial isolates studied were unsaturated (46%–74%) although saturated FAs were also found (24%–50%). The majority FA were palmitic acid (C16:0), ranging from 14% to 25%, and oleic (C18:1) and linoleic (C18:2) acids, ranging from 26% to 38% and from 10% to 30%, respectively.
M. aeruginosa NPCD-1 biomass had approximately 50% of saturated FAs, with a higher proportion of palmitic (24.3%) and lauric (13.2%) acids. Unsaturated FAs obtained were 31% of monounsaturated and 14.9% polyunsaturated, with a higher proportion of oleic (C18:1) and linoleic (C18:2) acids, which represented 26.8% and 12.5%, respectively. A similar profile was obtained for Trichormus sp. CENA77 biomass, which contained 44.1% of saturated and 54.6% of unsaturated FAs. Oleic acid was present in a higher proportion (36.9%), followed by palmitic (24.9%), linoleic (10.7%) and lauric (9.7%) acids.
Synechococcus sp. PCC7942 showed 29.6% of saturated FAs and 69.4% of unsaturated FAs, with a greater proportion of oleic (31.5%), linoleic (30.9%) and palmitic (23.5%) acids. Chlorogloea sp. CENA170 biomass had 33% of saturated FAs (palmitic = 15.4% and lauric = 8.7%) and 65.7% of unsaturated FAs, including linoleic (29.4%) and oleic (28.8%) acids in the higher proportion. The lipid from Leptolyngbya sp. CENA104 presented 24.6% of saturated and 74.4% of unsaturated FAs, with oleic (38.8%) and linoleic (26.4%) acids in greater proportion, followed by palmitic acid (14.6%).
These results confirm previously reported data regarding the ability of cyanobacteria to synthesize simpler FAs than eukaryotic microalgae [20]. It can be noticed that all tested strains produced high amounts of FAs, such as palmitic (C16-saturated), oleic (C18:1-monounsaturated) and linoleic (C18:2-polyunsaturated) acids. All cyanobacteria strains evaluated in this work showed a FA profile similar to the seed oils successfully used as lipid feedstocks for biodiesel synthesis [21,22].
Biodiesel is produced by reacting a short chain alcohol with biologically derived oils that consist of FAs ester-linked to a glycerol molecule. Considering that the FAs themselves are not modified by the reaction, the fatty esters composition of the fuel is directly equivalent to the FAs composition of the biomass feedstock. The fatty esters composition determines the fuel properties [23] and the most important attributes that affect the fuel properties are the length of the carbon chain and the number of double bonds. These also affected the viscosity of biodiesel, which is about an order of magnitude lower than that of the parent oil and depends on the composition of alkyl esters [24]. High viscosity is the major fuel property why neat vegetable oils have been largely abandoned as alternative diesel fuel. Viscosity increases with chain length (number of carbon atoms) and with increasing degree of saturation. This holds also for the alcohol moiety as the viscosity of ethyl esters is slightly higher than that of methyl esters [23].
2.3. Cyanobacterial Fatty Acid Composition: Implications for Biodiesel Quality
Using data on the content of individual FAs, the quality of biodiesel could be predicted by calculating IV (iodine value), SV (Saponification value), DU (Degree of unsaturation) and LCSF (long chain saturated factor) using empirical equations described by Ramos et al. [24] as displayed in Table 3.

Table 3.
Properties of lipid feedstock from cyanobacteria.
| Property | M. aeruginosa NPCD-1 | Synechococcus sp. PCC7942 | Chlorogloea sp. CENA170 | Trichormus sp. CENA77 | Leptolyngbya sp. CENA104 |
|---|---|---|---|---|---|
| Iodine value (g I2/100 g) | 57 | 97 | 90 | 68 | 100 |
| Saponification value | 210 | 203 | 210 | 213 | 205 |
| Degree of unsaturation (%) | 60.7 | 103.7 | 97 | 70.4 | 105.5 |
| Long chain saturated factor | 5.7 | 5.2 | 4.1 | 5.0 | 4.0 |
Values for IV ranged from 57 to 100 g I2/100 g, which are in accordance with European Standard UNE-EN 14214 [25] Lipids from M. aeruginosa NPCD-1, which yielded approximately 50% of saturated FAs and 46% of unsaturated FAs, resulted in an IV of 57 g I2/100 g and a DU equal to 60.7%. Similar results were obtained for lipids from Trichormus sp. CENA77, which resulted in an IV of 68 g I2/100 g and a DU of 70.4%. All of these parameters comply with the limits established by US Standard (ASTM D6751-02) [26], European Standard (EN 14214) [25] and Brazilian National Petroleum Agency Standard (ANP Resolution N° 14/2012) [27] for biodiesel quality.
LCSF of lipid feedstock is a critical parameter for oxidation stability, cetane number, IV and cold filter plugging point (CFPP) of the biodiesel obtained. Some authors reported that the longer the biodiesel carbon chains, the worse their low-temperature properties. In addition, when a liquid biodiesel is cooled, the alkyl esters of stearic and palmitic acids are the first to precipitate and, therefore, typically constitute a major share of material recovered from clogged biodiesel fuel filters [15].
Lipids from M. aeruginosa NPCD-1, Synechococcus sp. PCC7942 and Trichormus sp. CENA77 have the highest LCSF, since they contain a higher concentration of palmitic and stearic FAs (Table 2).
Low-temperature properties depend mostly on the saturated ester content and the effect of unsaturated ester composition can be considered negligible. In this sense, the CFPP of the biodiesel is correlated with the LCSF [24].
As shown by the FA profile, IV, SV, DU and LCSF, all tested strains are promising sources of lipid feedstock for biodiesel production, as well as satisfying biodiesel quality standards. However, Synechococcus sp. PCC7942, M. aeruginosa NPCD-1 and Trichormus sp. CENA77 had better properties as feedstock for biodiesel production. The highest values for biomass and lipid productivity were attained by Synechococcus sp. PCC7942 (52.7 and 14.2 mg·L−1·day−1, respectively) and M. aeruginosa NPCD-1 (46.9 and 13.1 mg·L−1·day−1, respectively) followed by Trichormus sp. CENA77 (30.8 and 7.3 mg·L−1·day−1, respectively).
2.4. Characterization of Lipid Materials
Rheological studies concerning the behavior and properties of lipid materials can be very useful in the design and evaluation of equipment used for the development of lipid-based diesel fuel substitutes derived from microbial oils. In this context the lipid from the selected cyanobacterial isolates were evaluated to verify the rheological behavior of the liquid oils. Figure 1 (a,b,c) shows the change in viscosity as a function of shear rate for the behavior of the absolute viscosity of the lipid material at 50 °C. The amount of viscosity (cP) decreases as the shear rate increase, characterizing the behavior of the non-Newtonian liquid oil. The analysis of the curve allowed an adjustment of the shear stress as a function of shear rate according to the power law.
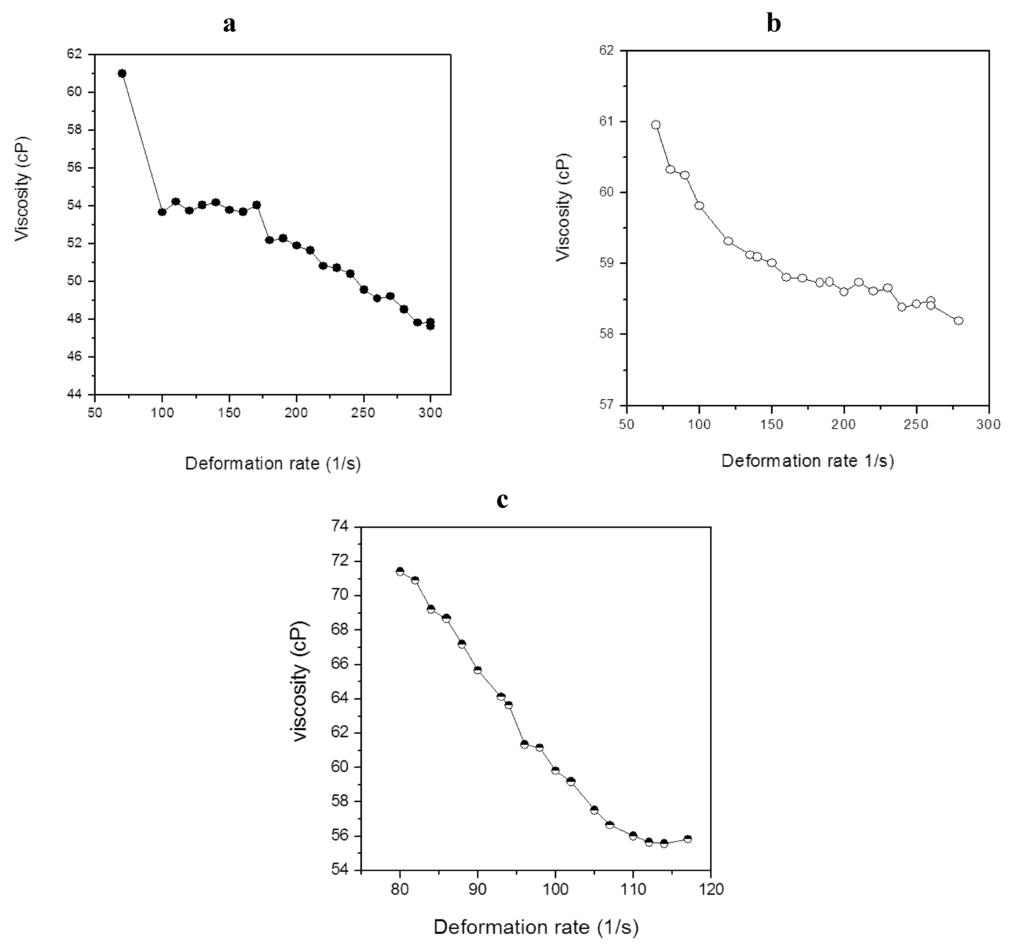
Figure 1.
Absolute viscosity as function of shear rate for cyanobacterial lipid from M. aeruginosa NPCD-1 (a), Trichormus sp. CENA77 (b) and Synechococcus sp. PCC7942 (c).
Table 4 shows the adjustment coefficients for the log t data as a function of log γ [28]. For all lipids analyzed, the value of the angular coefficient was lower than 1, characterizing them as pseudoplastic fluids, where the viscosity decreases with an increasing shear rate [28].

Table 4.
Values of viscosity coefficient and adjustment of power law [28] data.
| Strain | Viscosity (cP) | η * |
|---|---|---|
| M. aeruginosa NPCD-1 | 52.7 | 0.84 |
| Trichormus sp. CENA77 | 59.1 | 0.97 |
| Synechococcus sp. PCC7942 | 62.3 | 0.37 |
* Calculated according to equation 5 at 50 °C described at the experimental section (item 3.4).
Within the applied range of deformation (70 to 300 s−1), the lipid materials from M. aeruginosa NPCD-1 and Trichormus sp. CENA77 showed absolute viscosity average of 52.7 cP and 59.1 cP at 50 °C, respectively, while lipid from Synechococcus sp. PCC7942 biomass showed 62.3 cP (deformation range between 70 and 120 s−1). When comparing these values with the viscosity of traditional feedstocks, such as palm oil (31.1 cP) and beef tallow (43.8 cP), they present a different rheological behavior, since vegetable oils and residual fat generally behave as a Newtonian fluid [21,22].
Biodiesel synthesis from high viscosity lipids such as beef tallow, for example, results in viscosity values that meet the fuel standards [29]. Therefore, it can be predicted that the high viscosity of the lipid biomass is not a limiting parameter for dimensioning reactors to produce biodiesel. However, it is a limitation that should be considered and that requires the use of solvents in the reaction, driven mainly by the non-Newtonian fluid behavior. Previous work carried out by our group [12] has demonstrated that the use of iso-octane as solvent allowed almost full conversion of M. aeruginosa NPCD-1 lipid (52.7 cP) into ethyl esters by enzymatic route.
Thermal analysis (TGA) generates a curve of thermal decomposition that gives the degradation stages of the samples as a function of temperature [30]. By analyzing these data, it is possible to establish parameters for the thermal stability of oils and fats and compare it to the stability of biodiesel. Furthermore, the TGA has been used to study the behavior of biomass combustion [31].
Figure 2 displays the curves of TG of the lipid materials, which demonstrate the percentage of mass loss versus temperature. The mass losses from M. aeruginosa NPCD-1, Trichormus sp. CENA77 and Synechococcus sp. PCC7942 show only one stage of thermal degradation that began at about 280, 200 and 250 °C, respectively, and extended to approximately 500, 400 and 430 °C, respectively. These steps relate to the decomposition and volatilization of lipid present in the samples (triglycerides and free FAs). During this process, the temperature of maximum mass loss observed by the TG curve occurred at 497 °C for M. aeruginosa NPCD-1, 380 °C for Trichormus sp. CENA77 and 405 °C for Synechococcus sp. PCC7942.
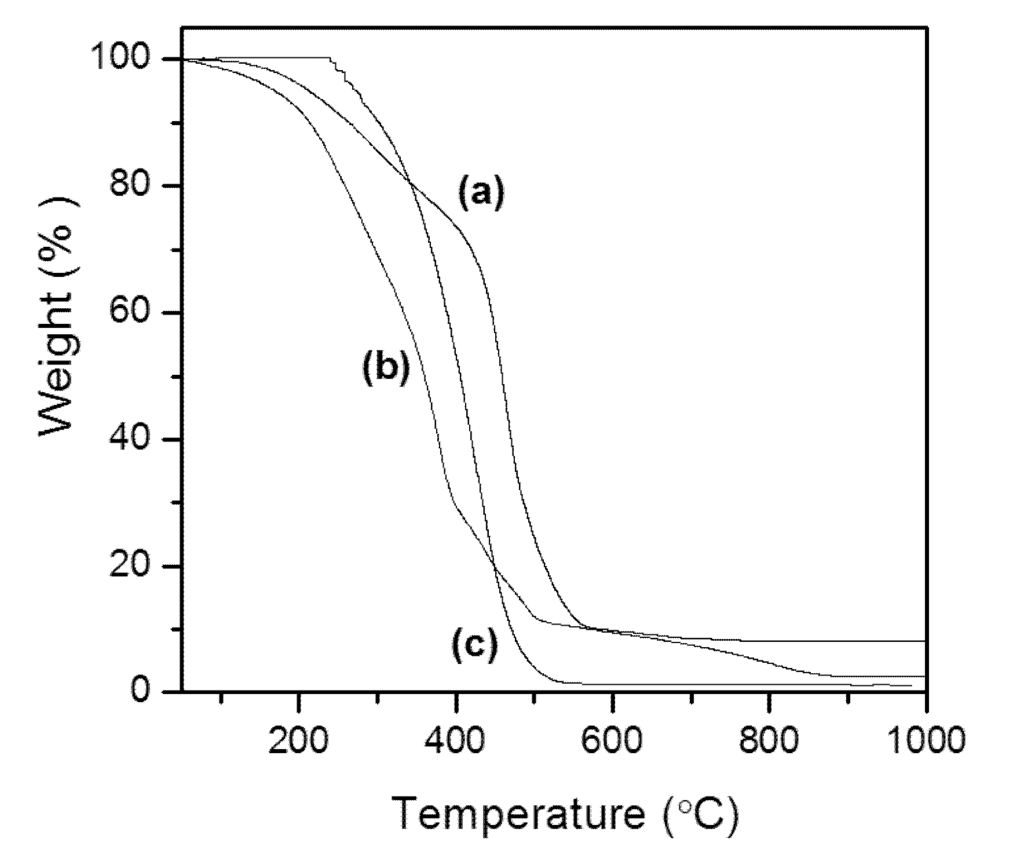
Figure 2.
Thermal study (TGA) for cyanobacterial lipid from M. aeruginosa NPCD-1 (a), Trichormus sp. CENA77 (b) and Synechococcus sp. PCC7942 (c).
These results corroborate to those reported by Carvalho [32], in which various traditional feedstocks, such as andiroba, babassu, jatropha oils and beef tallow were evaluated by TGA. The results also indicated a similarity between the intervals at which mass loss occurs for babassu and andiroba oils and beef tallow: 200 to 321 °C, 321 to 442 °C and 210 to 560 °C, respectively. However, the initial temperature of mass loss for andiroba oil was much lower, with a range between 97 and 376 °C [32].
The use of FT-IR became a powerful tool in lipids analysis, since other analytical methods can be time-consuming. The FAs identification can be assessed through this methodology by the correlation with other analytical data, as used in this study which correlated the IR spectroscopy results with GC. This procedure is a way of cross-checking the results. The measurement is performed by infrared absorption according to a range of molecular vibrational modes [33]. The contents and functional groups of the lipid extracts from cyanobacterial biomass were observed between 400 and 4000 cm−1. The spectra of the cyanobacterial lipid from M. aeruginosa NPCD-1, Synechococcus sp. PCC7942 and Trichormus sp. CENA77 are displayed in Figure 3. The assignments for the infrared bands were based on the correlations with FAs, esters and triglycerides of FAs and similar results were found for andiroba oil as displayed in Table 5.
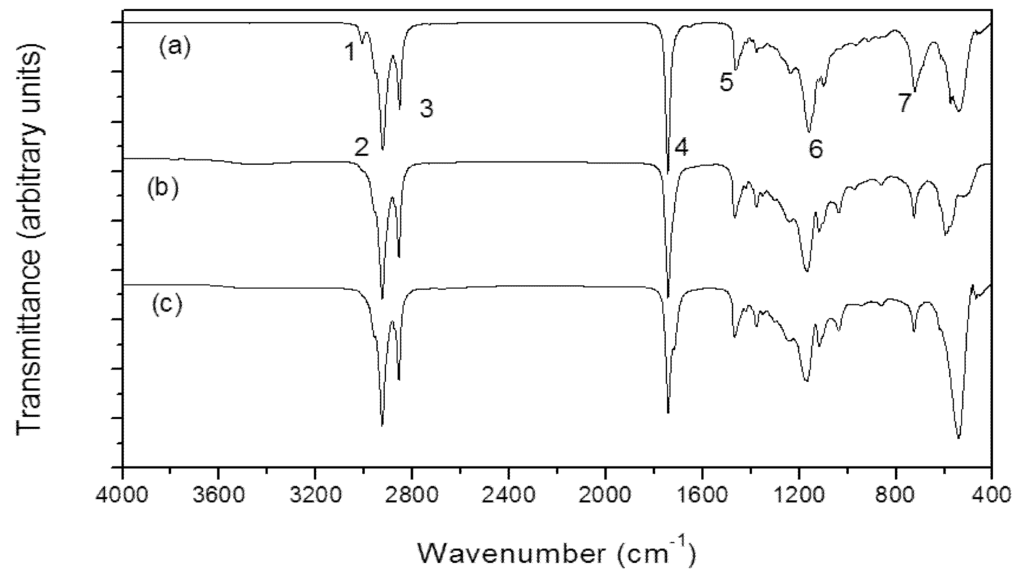
Figure 3.
Infrared spectra for cyanobacterial lipid from M. aeruginosa NPCD-1 (a), Trichormus sp. CENA77 (b) and Synechococcus sp. PCC7942 (c).

Table 5.
Assignment of principal infrared bands identified by FT-IR spectroscopy to evaluate cyanobacterial lipids.
| Band | Assignment | Lipid from cyanobacteria (this study) | Andiroba oil (cm−1) * |
|---|---|---|---|
| 1 | (=C–H) stretching | 3006 | 3005 |
| 2 | CH2 asymmetry stretching | 2928 | 2923 |
| 3 | CH2 symmetry stretching | 2852 | 2854 |
| 4 | C=O stretching | 1744 | 1741 |
| 5 | CH2 scissors | 1464 | 1463 |
| 6 | C=C–C–O | 1150 | 1040–1290 |
| 7 | CH2 rocking | 724 | 722 |
* Reference [33].
All samples showed a spectrum with vibration band at 3006 cm−1 related to the CH stretching of =C–H bonding (band 1). The two intense bands at 2928 cm−1 (band 2) and 2852 cm−1 (band 3) are due to CH2 asymmetric and symmetric stretching vibrations, respectively. These vibrations were also observed at 2926 and 2855 cm−1 for the spectra of several vegetal oils such as soybean, palm and palm kernel oils [34]. The band at 1744 cm−1 (band 4) is assigned to the C=O stretching vibration of the carboxylic groups, and occurs in the range of 1750–1735 cm−1 [34,35,36]. It appears around the same frequency for methyl esters and triglycerides; for long-chain FAs it appears at around 1700 cm−1.
Hence, the observation of this band in the spectrum of the cyanobacterial lipids from M. aeruginosa NPCD-1, Synechococcus sp. PCC7942 and Trichormus sp. CENA77 indicates the presence of the ester function. At 1464 cm–1 a band 5 was observed, which is assigned to CH2 scissors deformation vibration [34]. This vibration was observed as a single band at 1475 cm−1. The band at 1150 cm−1 (band 6) is due to C–O (esters), stretch in two or more bands, one stronger and broader than the other, occurred in the range 1300–1000 cm−1 [33]. The band at 726 cm−1 (band 7) observed in the spectrum is assigned to the CH2 rocking mode [34]. The bending motion associated with four or more CH2 groups in an open chain and occurs at about 724 cm−1 (called a long-chain band) [36].
2.5. Ethanolysis of Lipid Feedstocks
The feasibility of the selected cyanobacteria lipid to yield biodiesel using ethanol as acyl acceptor was performed using niobium impregnated with NaOH as catalyst. This procedure was based on the efficiency of this catalyst to carry out transesterification reactions of several vegetal oils [32]. Experiments were conducted under a preliminary set of reaction conditions (oil-to-ethanol proportion of 1:4; 78.5 °C and 10% of catalyst) that may not have been the optimum set for all the feedstocks. Biodiesel samples were quantified by 1H NMR, which allowed evaluating the conversion of raw materials into biodiesel, based on the monitoring of changes in the sign of the hydrogens of glycerin, thus enabling confirm the occurrence of the transesterification reaction of triglycerides and its conversion into ethyl esters. Figure 4 shows the 1H NMR spectra related to the purified biodiesel samples from M. aeruginosa NPCD-1 (a), Synechococcus sp. PCC7942 (b) and Trichormus sp. CENA77 (c).
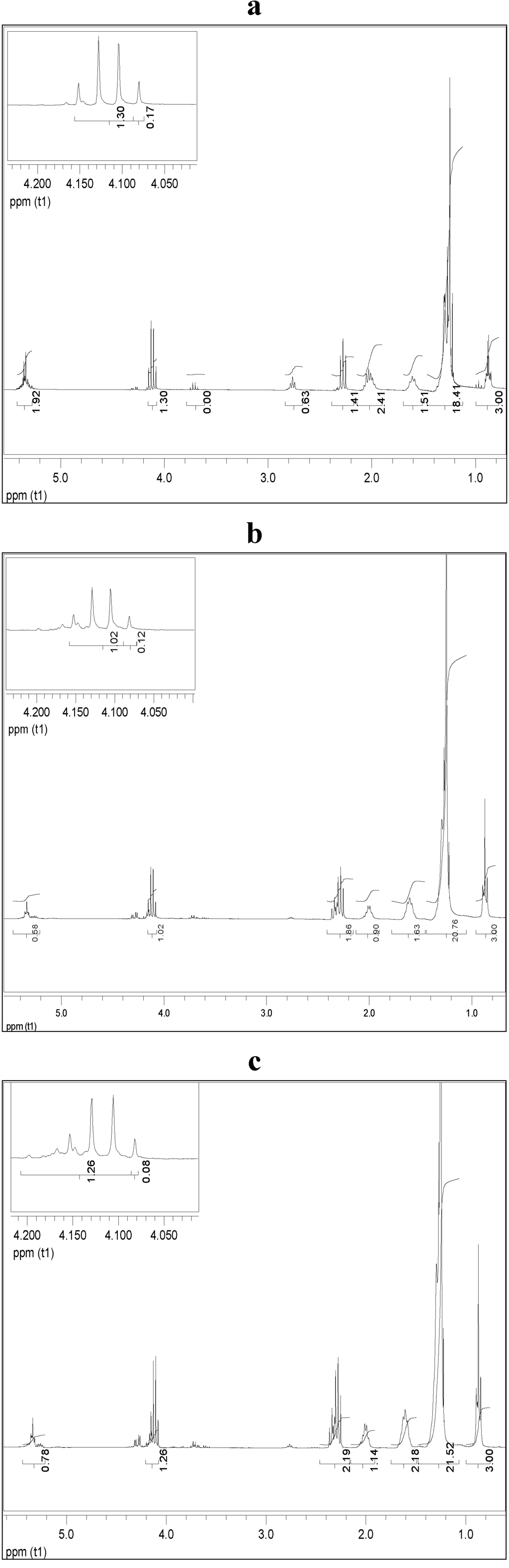
Figure 4.
1H NMR spectra for purified biodiesel from M. aeruginosa NPCD-1 (a), Synechococcus sp. PCC7942 (b) and Trichormus sp. CENA77 (c).
In the spectra corresponding to the ethanolysis of lipid material of M. aeruginosa NPCD-1 (Figure 4a), multiples at 4.10–4.32 ppm, attributed to the protons on the 1 and 3 carbon atoms of the glycerol group are totally absent due to the conversion of triglycerides into ethyl esters. This also shows that the formation of ethyl esters occurred at the region from 4 to 4.2 ppm, being represented by a quartet. This spectrum provided evidence that a complete transesterification reaction had occurred (100%). The ethanolysis from lipid material of Synechococcus sp. PCC7942 provided a similar conversion (94.2%) as shown in spectrum (Figure 4b).
On the other hand, much lower conversion (≈50%) was obtained for ethanolysis from lipid material of Trichormus sp. CENA77 (Figure 4c) which was associated to the acid value determined as 15.7 mg KOH/g of the sample which corresponded to a level of 9 wt % FFA. Lipid with FFA content higher than 5 wt% is not suitable for base catalyzed transesterification as the FFA will tend to consume the catalyst and form soap which will cause serious separation problem to the product [37]. For this particular case, acid catalyzed transesterification is recommended.
Similar results were reported by Carvalho [32] in the ethanolysis from macaw palm using the same heterogeneous catalyst (Nb/Na). The high acidity present in macaw palm feedstock (16.1 mg KOH/g) resulted in poor transesterification yield (20.7%).
3. Experimental Section
3.1. Cyanobacterial Strains and Cultivation
Five cyanobacterial strains from the culture collection of the Laboratory of Molecular Ecology of Cyanobacteria (CENA/USP, São Paulo, Brazil) as follows: Synechococcus sp. PCC7942, Microcystis aeruginosa NPCD-1, Chlorogloea sp. CENA170, Leptolyngbya sp. CENA104 and Trichormus sp. CENA77 were tested (Table 1). Cyanobacteria were grown in flasks containing 8 L of appropriated culture media for each strain: BG-11 [38], ASM-1 [39] and SWBG-11 [40] and kept under constant aeration, light intensity equivalent to 100 µmol photons m−2·s−1 and controlled temperature at 24 ± 1 °C. The period of cultivation was from 15 to 30 days depending on the strain studied. Cells were harvested by centrifugation and lyophilized.
3.2. Total Lipids Extraction and FA Analysis
Total lipids were extracted using the methodology described by Bligh and Dyer [41]. The total lipids were measured gravimetrically and yields were calculated. The lipid extracted was dried in a rotary evaporator to remove remaining residues of solvent and subsequently dried at 60 °C to constant weight. The total lipids were measured gravimetrically, and then lipids contents and yields were calculated. Analysis of fatty acid composition was performed in a capillary gas chromatography (CGC Agilent 6850 Series GC System) according to AOCS procedure [42].
3.3. Empirical Parameters
Iodine value (IV), saponification value (SV), degree of unsaturation (DU) and long chain saturated factor (LCSF) were empirically determined according to Equations 1–4 as described by Ramos et al. [24].
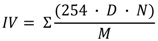
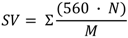
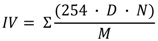
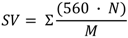
DU = (wt.% monounsaturated) + 2 × ( wt.% polyunsaturated)
LCSF = (0.1 × C16) + (0.5 × C18) + (1 × C20) + (1.5 × C22) + (2 × C24)
3.4. Viscosity Determination
The absolute viscosity of lipid was determined with LVDV-II cone and plate spindle Brookfield viscosimeter (Brookfield Viscometers Ltd., Harlow, England) using a CP 52 cone. The shear stress measurements were taken as a function of shear rate and the dynamic viscosity was determined as a constant slope [28]. All measurements were performed in triplicate. The data obtained (viscosity, rate of deformation and shear stress) were adjusted according to the power law (Equation 5) to verify possible deviations from Newtonian behavior [28].
where: τ is the shear stress, γ is the applied deformation rate, . is the value of angular coefficient and K is the consistency index.
τ = K × γn
3.5. Thermal Study (TG)
TG curves were recorded using thermal balance (TGA-50 Shimadzu thermogravimetric analyzer, Shimadzu, Kyoto, Japan. A dynamic method was used at a heating rate of 10 °C·min−1. The initial sample mass was 10.00 ± 0.5 mg, in a nitrogen atmosphere with a flow rate of 50 mL·min−1, in a temperature range of 25–1000 °C.
3.6. Infrared Spectroscopy (IR)
IR spectra were obtained from a FT-IR Spectrum GX (Perkin Elmer, Perdizes, São Paulo, Brazi) with a spectral resolution of 4 cm−1. The spectrum was obtained through the horizontal attenuated total reflectance (HATR) technique using a ZnSe crystal. Spectra in the region 400–4000 cm−1 were run and plotted as a percent transmittance curve versus wavenumber.
3.7. Biodiesel Synthesis
Biodiesel synthesis from the three selected cyanobacterial biomass were performed in jacket cylindrical glass reactor (6 mm high × 4 mm internal diameter and 150 mL capacity, coupled with a reflux condenser) containing 20 g of substrate (4 g of lipid feedstock and 15 g of ethanol). Niobium oxide impregnated with sodium (Nb2O5/Na) previously activated in an oven (200 °C for 24 h) was used as catalyst at proportion of 10% in relation to the mass of lipid feedstock. The experiments were carried out at 78.5 °C under mechanical agitation 600 rpm for 10 h [32].
3.8. Downstream Procedure
When the reaction was completed the catalyst was separated from the medium and the organic phase was washed twice with one volume of water to remove both the remaining ethanol and the free glycerol as a by-product. The residual ethanol was removed by rotary evaporator to attain the final fatty acid ethyl ester product.
3.9. Proton Nuclear Magnetic Resonance Spectrometry (1H NMR)
The conversion into ethyl esters was evaluated by 1H NMR in a Mercury 300 MHz (Varian spectrometer, Varian, Palo Alto, CA, USA) with 5 mm glass tubes, using CDCl3 as the solvent and 0.3% TMS as the internal standard. The calculations involving the conversion of esters were performed using the equation according to methodology described by Paiva and collaborators [43]. This methodology allowed for the identification of molecules by 1H NMR, with peaks present in the region from 4.05 to 4.35 ppm during the transesterification reaction.
4. Conclusions
Synechococcus sp. PCC7942, M. aeruginosa NPCD-1 and Trichormus sp. CENA77 had the best set of properties to be used as a feedstock source in the synthesis of biodiesel. They showed appropriate values of biomass and lipid productivity, as well as FA profiles similar to the oil seeds already used successfully in the synthesis of biodiesel. Analytical techniques, including infrared spectroscopy, viscosimetry and thermogravimetry showed to be a practical tool to confirm the feasibility of these cyanobacterial lipids for biofuel synthesis. Although further practical investigation regarding the growth of cyanobacterial biomass is required, ethyl esters of cyanobacterial lipids were successfully attained by heterogeneous catalysis.
Acknowledgments
The authors would like to thank the Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP), the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for financial support and scholarships. We thank Diego B. Genuário for cyanobacterial micrographs.
Conflict of Interest
The authors declare that they have no conflict of interests.
References
- Jansson, C.; Northen, T. Calcifying cyanobacteria—The potential of biomineralization for carbon capture and storage. Curr. Opin. Biotechnol. 2010, 21, 365–371. [Google Scholar]
- Burja, A.M.; Banaigs, B.; Abou-Mansour, E.; Burgess, J.G.; Wright, P.C. Marine cyanobacteria: A prolific source of natural products. Tetrahedron Lett. 2001, 57, 9347–9377. [Google Scholar] [CrossRef]
- Silva-Stenico, M.E.; Silva, C.S.P.; Lorenzi, A.S.; Shishido, T.K.; Etchegaray, A.; Lira, S.P.; Moraes, L.A.B.; Fiore, M.F. Non-ribosomal peptides produced by Brazilian cyanobacterial isolates with antimicrobial activity. Microbiol. Res. 2011, 166, 161–175. [Google Scholar] [CrossRef]
- Karatay, S.E.; Donmez, G. Microbial oil production from thermophile cyanobacteria for biodiesel production. Appl. Energ. 2011, 88, 3632–3635. [Google Scholar] [CrossRef]
- Costa, J.A.V.; Morais, M.G. The role of biochemical engineering in the production of biofuels from microalgae. Bioresour. Technol. 2011, 102, 2–9. [Google Scholar] [CrossRef]
- Taher, H.; Al-Zuhair, S.; Al-Marzouqi, A.H.; Haik, Y.; Farid, M.M. A review of enzymatic transesterification of microalgal oil-based biodiesel using supercritical technology. Enzyme Res. 2011, 2011, 468292. [Google Scholar] [CrossRef]
- Rittmann, B.E. Opportunities for renewable bioenergy using microorganisms. Biotechnol. Bioeng. 2008, 2, 203–212. [Google Scholar]
- Balasubramanian, L.; Subramanian, G.; Nazeer, T.T.; Simpson, H.S.; Rahuman, S.T.; Raju, P. Cyanobacteria cultivation in industrial wastewaters and biodiesel production from their biomass: A review. Biotechnol. Appl. Biochem. 2012, 59, 220–225. [Google Scholar]
- Vargas, M.A.; Rodriguez, H.; Moreno, J.; Olivares, H.; Delcampo, J.A.; Rivas, J.; Guerrero, M.G. Biochemical composition and fatty acid content of filamentous nitrogen-fixing cyanobacteria. J. Phycol. 1998, 34, 812–817. [Google Scholar] [CrossRef]
- Murata, N.; Wada, H.; Gombos, Z. Modes of fatty acid desaturation in cyanobacteria. Plant Cell Physiol. 1993, 33, 933–941. [Google Scholar]
- Hoffmann, L.; Komárek, J.; Kastovsky, J. System of cyanoprokaryotes (cyanobacteria)—State in 2004. Algol. Stud. 2005, 117, 95–115. [Google Scholar] [CrossRef]
- Da Rós, P.C.M.; Silva, C.S.P.; Silva-Stenico, M.E.; Fiore, M.F.; de Castro, H.F. Microcystis aeruginosa lipids as feedstock for biodiesel synthesis by enzymatic route. J. Mol. Catal. B Enzym. 2012, 84, 177–182. [Google Scholar] [CrossRef]
- Bruno, L.; di Pippo, F.; Antonaroli, S.; Gismondi, A.; Valentini, C.; Albertano, P. Characterization of biofilm-forming cyanobacteria for biomass and lipid production. J. Appl. Microb. 2012, 113, 1052–1064. [Google Scholar] [CrossRef]
- Sharathchandra, K.; Rajashekhar, M. Total lipid and fatty acid composition in some freshwater cyanobacteria. J. Algal Biomass Util. 2011, 2, 83–97. [Google Scholar]
- Francisco, E.C.; Neves, D.B.; Jacob-Lopes, E.; Franco, T.T. Microalgae as feedstock for biodiesel production: Carbon dioxide sequestration, lipid production and biofuel quality. J. Chem. Technol. Biotechnol. 2010, 85, 395–403. [Google Scholar] [CrossRef]
- Rodolfi, L.; Zittelli, G.C.; Bassi, N.; Padovani, G.; Biondi, N.; Bonini, G.; Tredici, M.R. Microalgae for oil: Strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol. Bioeng. 2009, 102, 100–112. [Google Scholar]
- Gouveia, L.; Oliveira, A.C. Microalgae as a raw material for biofuels production. J. Ind. Microbiol. Biotechnol. 2009, 36, 269–274. [Google Scholar] [CrossRef]
- Mandal, S.; Mallick, N. Microalga Scenedesmus obliquus as a potential source for biodiesel production. Appl. Microbiol. Biotechnol. 2009, 84, 281–291. [Google Scholar] [CrossRef]
- Griffiths, M.J.; Harrison, S.T.L. Lipid productivity as a key characteristic for choosing algal species for biodiesel production. J. Appl. Phycol. 2009, 21, 493–507. [Google Scholar] [CrossRef]
- Gunstone, F.D.; Harwood, J.L.; Dijkstra, A.J. The Lipid Handbook; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Moreira, A.B.R.; Perez, V.H.; Zanin, G.M.; de Castro, H.F. Biodiesel synthesis by enzymatic transesterification of palm oil with ethanol using lipases from several sources immobilized on silica-PVA composite. Energy Fuels 2007, 21, 3689–3694. [Google Scholar] [CrossRef]
- Da Rós, P.C.M.; Silva, G.A.M.; Mendes, A.A.; Santos, J.C.; de Castro, H.F. Evaluation of the catalytic properties of Burkholderia cepacia lipase immobilized on non-commercial matrices to be used in biodiesel synthesis from different feedstocks. Bioresour. Technol. 2010, 101, 5508–5516. [Google Scholar] [CrossRef]
- Knothe, G. Dependence of biodiesel fuel properties on the structures of fatty acid alkyl esters. Fuel Process. Technol. 2005, 86, 1059–1070. [Google Scholar] [CrossRef]
- Ramos, M.J.; Fernández, C.M.; Casas, A.; Rodríguez, L.; Pérez, Á. Influence of fatty acid composition of raw materials on biodiesel properties. Bioresour. Technol. 2009, 100, 261–268. [Google Scholar] [CrossRef]
- UNE-EN 14214, European Standard. In Automotive Fuels. Fatty Acid Methyl Esters (FAME) for Diesel Engines. Requirements and Test Methods; CEN-European Committee for Standardization: Brussels, Belgium, 2003.
- ASTM D6751-02, Standard Specification for Biodiesel Fuel (B100) Blend Stock for Distillate Fuels; ASTM International: West Conshohocken, PA, USA, 2002.
- RESOLUÇÃO ANP N° 14, DE 11.5.2012-DOU 18.5.2012. Available online: http://nxt.anp.gov.br/nxt/gateway.dll/leg/resolucoes_anp/2012/maio/ranp%2014%20-%202012.xml (accessed on 20 June 2013).
- De Nevers, N. Fluid Mechanics for Chemical Engineers, 3rd ed; McGraw-Hill: Boston, MA, USA, 2005. [Google Scholar]
- Silva, G.A.M.; da Rós, P.C.M.; Souza, L.T.A.; Costa, A.P.O.; de Castro, H.F. Physico-chemical, spectroscopical and thermal characterization of biodiesel obtained by enzymatic route as a tool to select the most efficient immobilized lipase. Braz. J. Chem. Eng. 2012, 29, 39–47. [Google Scholar] [CrossRef]
- Miranda, T.; Esteban, A.; Rojas, S.; Montero, I.; Ruiz, A. Combustion analysis of different olive residues. Int. J. Mol. Sci. 2008, 9, 512–525. [Google Scholar] [CrossRef]
- Marcilla, A.; Gómez-Siurana, A.; Gomis, C.; Chápuli, E.; Catalá, M.C.; Valdés, F.J. Characterization of microalgal species through TGA/FTIR analysis: Application to Nannochloropsis sp. Thermochim. Acta 2009, 484, 41–47. [Google Scholar] [CrossRef]
- Carvalho, A.K.F. Synthesis of Biodiesel by Transesterification by Ethylic Route: A Comparison of Heterogeneous Catalysts. M.Sc Dissertation, Engineering School of Lorena-USP, Lorena, Brazil, 2011. [Google Scholar]
- Dean, A.P.; Sigee, D.C.; Estrada, B.; Pittman, J.K. Using FTIR spectroscopy for rapid determination of lipid accumulation response to nitrogen limitation in freshwater microalgae. Bioresour. Technol. 2010, 101, 4499–4507. [Google Scholar] [CrossRef]
- Albuquerque, M.L.S.; Guedes, I.; Alcantara, J., Jr.; Moreira, S.G.C. Infrared absorption spectra of Buriti (Mauritia flexuosa L.) oil. Vibrat. Spectrosc. 2003, 33, 127–131. [Google Scholar]
- Kaplan, M.; Davidson, G.; Poliakoff, M. Capillary supercritical fluid chromatography-Fourier transform infrared spectroscopy study of triglycerides and the qualitative analysis of normal and “unsaturated” cheeses. J. Chromatogr. 1994, 673, 231–237. [Google Scholar]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S. Introduction to Spectroscopy, 2nd ed; Saunders College Publishing: Orlando, FL, USA, 1996. [Google Scholar]
- Porphy, S.J.; Farid, M.M. Feasibility study for production of biofuel and chemicals from marine microalgae Nannochloropsis sp. based on basic mass and energy analysis. ISRN Renew. Energy 2012, 2012, 156824. [Google Scholar] [CrossRef]
- Allen, M.B. Simple conditions for growth of unicellular blue-green algae on plates. J. Phycol. 1968, 4, 1–4. [Google Scholar]
- Gorham, P.R.; Mclahlan, J.R.; Hammer, V.T.; Kim, W.K. Isolation and culture of toxic strains of Anabaena flos-aquae (Lyngb.) de Bréb. Verh. Int. Ver. Theor. Angew Limnol 1964, 15, 796–804. [Google Scholar]
- Castenholz, R.W. Culturing methods for cyanobacteria. Methods Enzymol. 1988, 167, 68–95. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method for total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar]
- American Oil Chemists’ Society. In Official Methods and Recommended Practices of the AOCS, 5th ed; AOCS Press: Boulder, CO, USA, 2004.
- Paiva, E.J.M.; Silva, M.L.C.P.; Barboza, J.C.S.; Oliveira, P.C.; Castro, H.F.; Giordani, D.S. Non-edible babassu oil as a new source for energy production—A feasibility transesterification survey assisted by ultrasound. Ultrason. Sonochem. 2013, 20, 833–838. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).