The Extract of Rhodobacter sphaeroides Inhibits Melanogenesis through the MEK/ERK Signaling Pathway
Abstract
:1. Introduction
2. Results
2.1. Evaluation of Anti-Melanogenic Activity of Lycogen™ in vitro
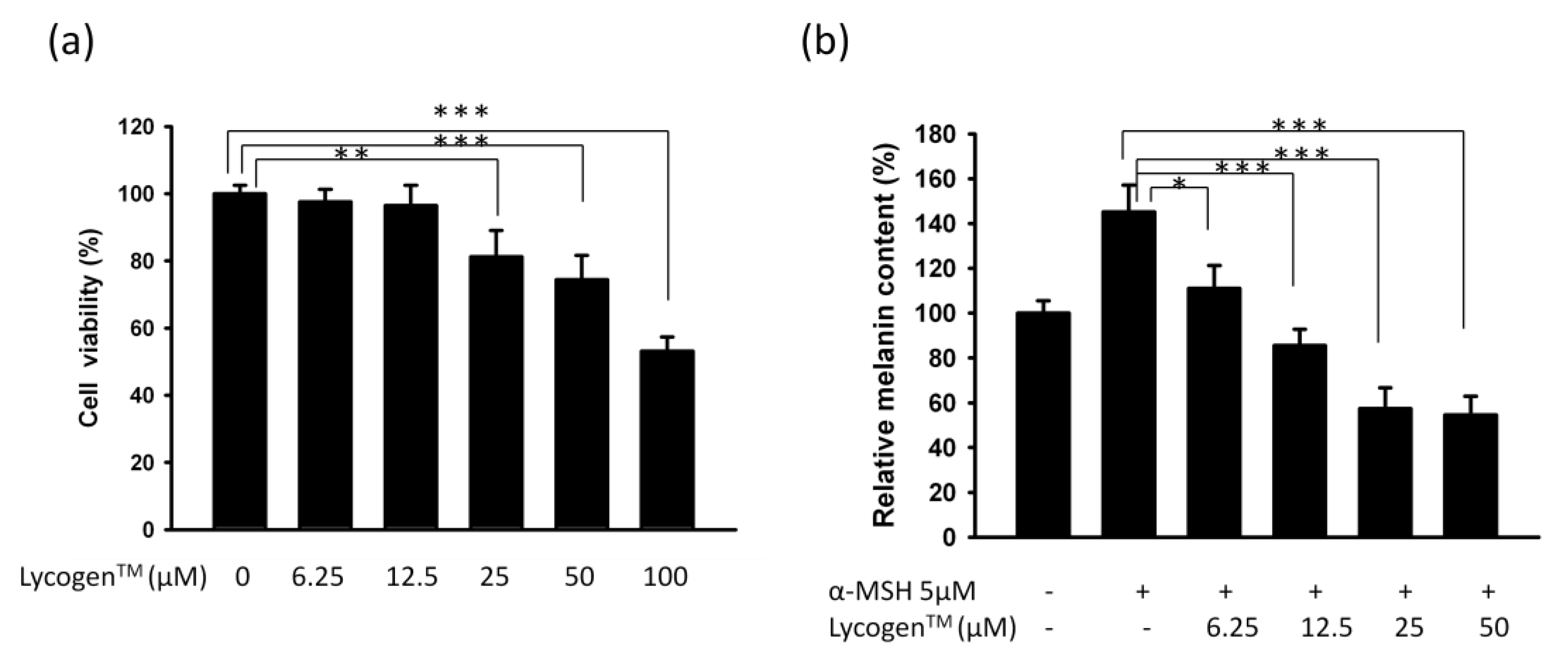
2.2. Lycogen™ Dose-Dependently Downregulated the Expression Levels of Tyrosinase and MITF

2.3. Effects of Lycogen™ on the Mitogen-Activated Protein Kinase (MAPKs) Signaling Pathway
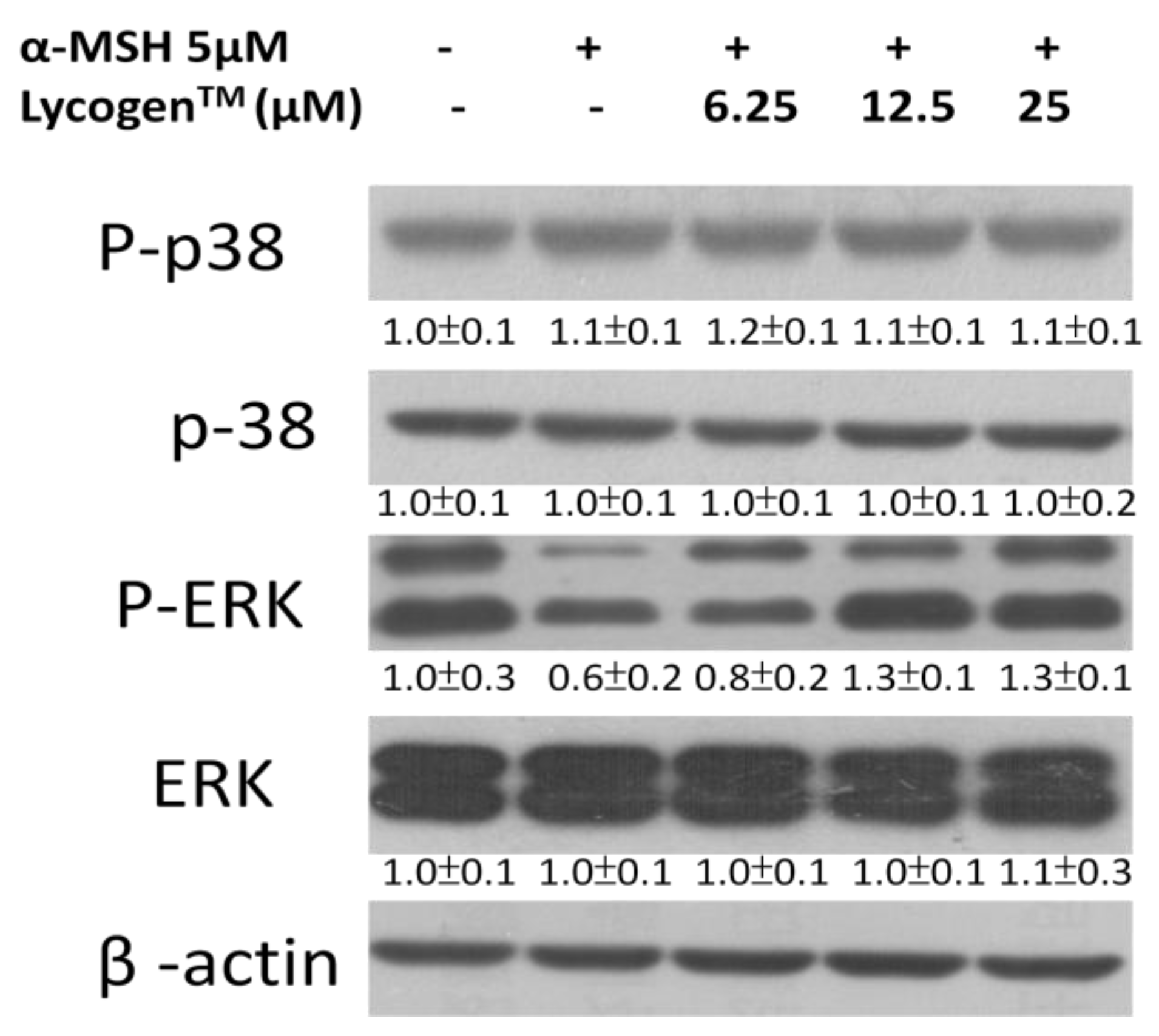
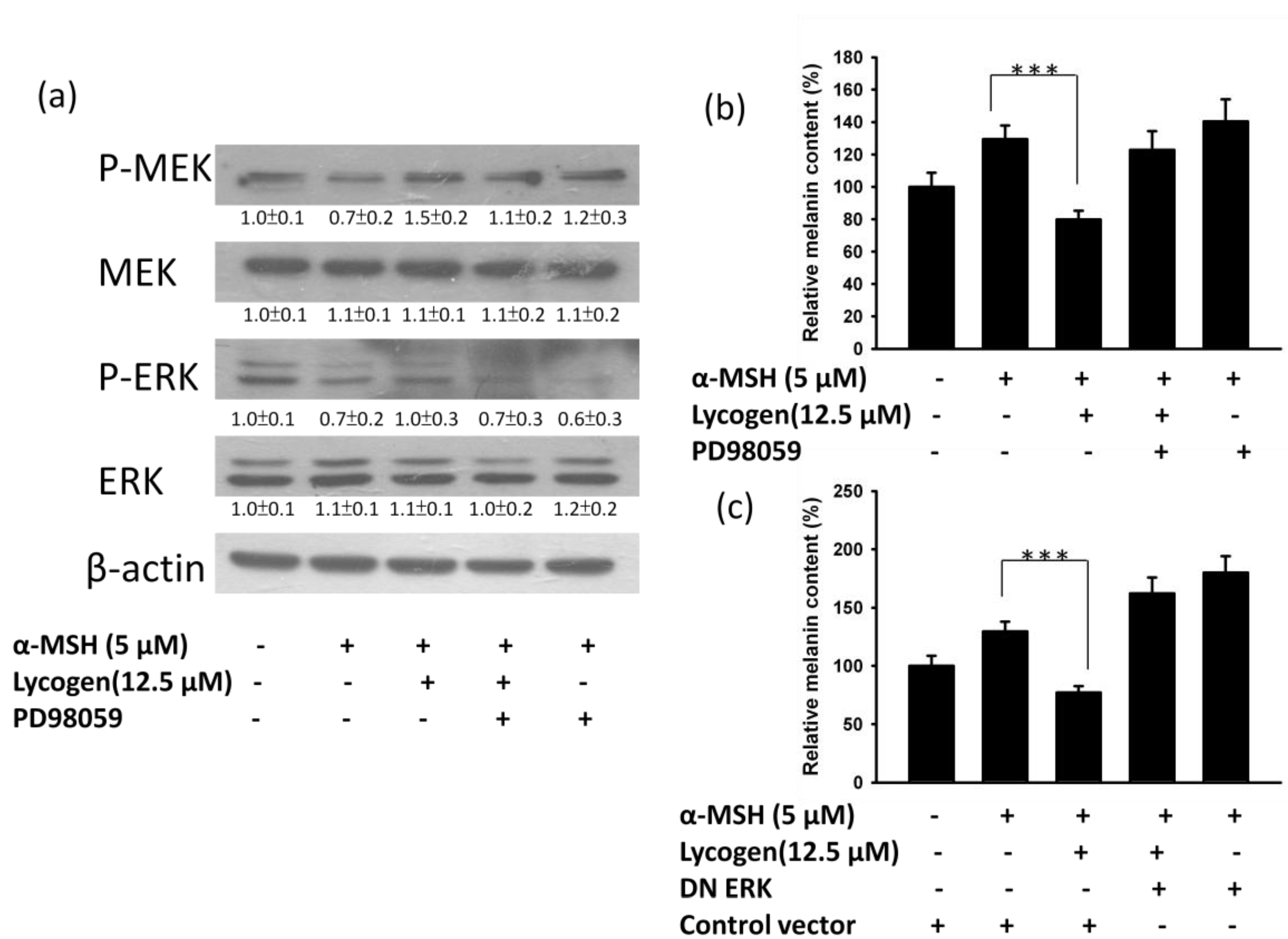
2.4. Evaluation of Anti-Melanogenic Activity of Lycogen™ in vitro
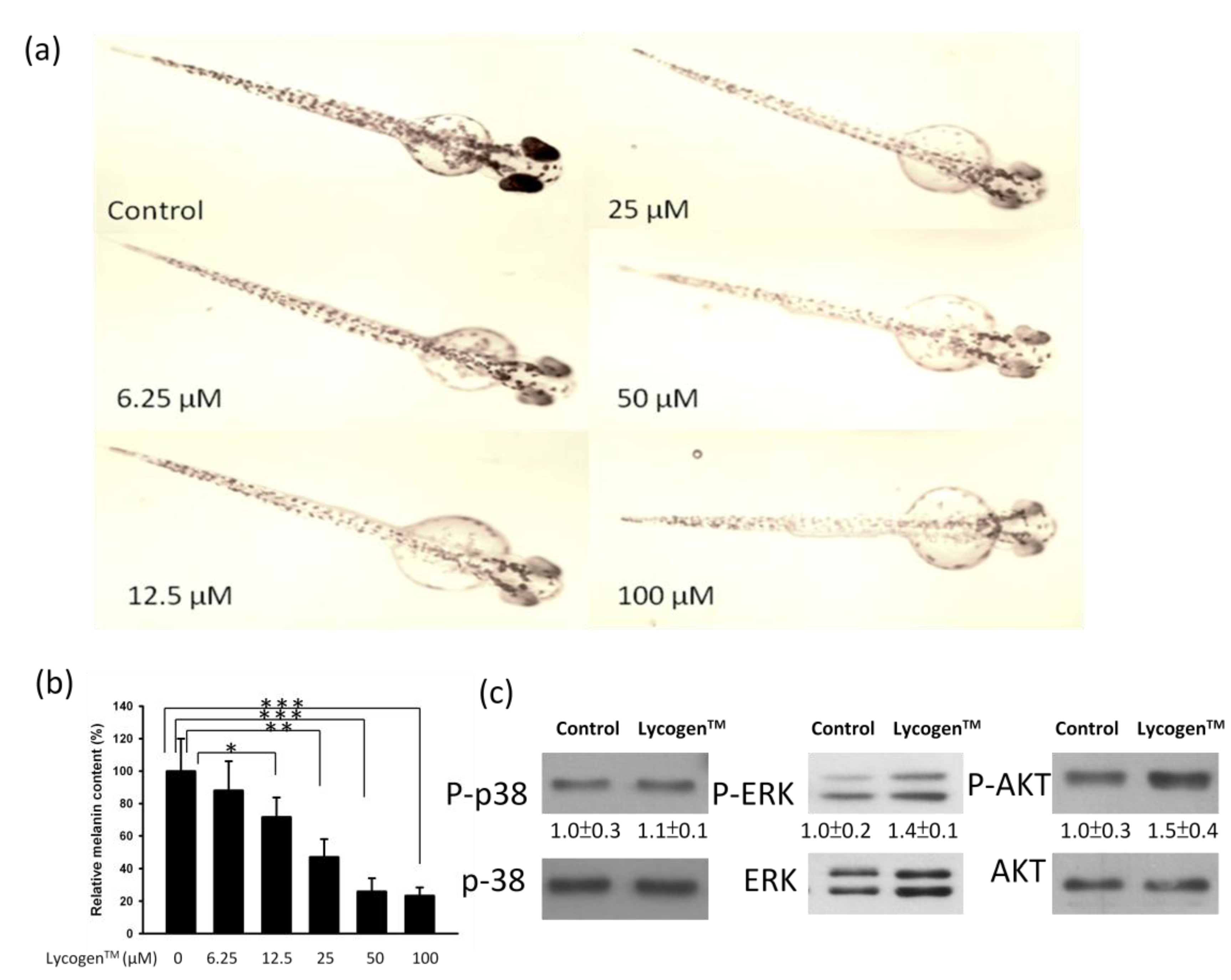
3. Discussion
4. Experimental Section
4.1. Lycogen™, Cells, Mice and Zebrafish
4.2. Determinations of Anti-Melanogenic Activity in Zebrafish
4.3. Assay of Cell Proliferation
4.4. Determination of Melanin Content
4.5. Western Blot Analysis
4.6. Statistical Analysis
5. Conclusions
Acknowledgments
Conflict of Interest
References
- Jang, J.Y.; Lee, J.H.; Kang, B.W.; Chung, K.T.; Choi, Y.H.; Choi, B.T. Dichloromethane fraction of Cimicifuga heracleifolia decreases the level of melanin synthesis by activating the ERK or AKT signaling pathway in B16F10 cells. Exp. Dermatol. 2009, 18, 232–237. [Google Scholar] [CrossRef]
- Lee, H.D.; Lee, W.H.; Roh, E.; Seo, C.S.; Son, J.K.; Lee, S.H.; Hwang, B.Y.; Jung, S.H.; Han, S.B.; Kim, Y. Manassantin A inhibits cAMP-induced melanin production by down-regulating the gene expressions of MITF and tyrosinase in melanocytes. Exp. Dermatol. 2011, 20, 761–763. [Google Scholar] [CrossRef]
- Wu, W.T.; Liu, W.S. Anti-inflammstory property of biomaterial carotenoids production by Rhodobacter sphaeroidese WL-APD911. Adv. Mater. Res. 2011, 287–290, 2028–2031. [Google Scholar] [CrossRef]
- Liu, W.S.; Chen, M.C.; Chiu, K.H.; Wen, Z.H.; Lee, C.H. Amelioration of dextran sodium sulfate-induced colitis in mice by Rhodobacter sphaeroides extract. Molecules 2012, 17, 13622–13630. [Google Scholar] [CrossRef]
- Jang, J.Y.; Kim, H.N.; Kim, Y.R.; Choi, W.Y.; Choi, Y.H.; Shin, H.K.; Choi, B.T. Partially purified components of Nardostachys chinensis suppress melanin synthesis through ERK and Akt signaling pathway with cAMP down-regulation in B16F10 cells. J. Ethnopharmacol. 2011, 137, 1207–1214. [Google Scholar] [CrossRef]
- Ye, Y.; Wang, H.; Chu, J.H.; Chou, G.X.; Yu, Z.L. Activation of p38 MAPK pathway contributes to the melanogenic property of apigenin in B16 cells. Exp. Dermatol. 2011, 20, 755–757. [Google Scholar] [CrossRef]
- Wu, L.C.; Lin, Y.Y.; Yang, S.Y.; Weng, Y.T.; Tsai, Y.T. Antimelanogenic effect of c-phycocyanin through modulation of tyrosinase expression by upregulation of ERK and downregulation of p38 MAPK signal pathways. J. Biomed. Sci. 2011, 18, 74. [Google Scholar] [CrossRef]
- Albrecht, M.; Klein, A.; Hugueney, P.; Sandmann, G.; Kuntz, M. Molecular cloning and functional expression in E. coli of a novel plant enzyme mediating zeta-carotene desaturation. FEBS Lett. 1995, 372, 199–202. [Google Scholar] [CrossRef]
- Smit, N.; Vicanova, J.; Cramers, P.; Vrolijk, H.; Pavel, S. The combined effects of extracts containing carotenoids and vitamins E and C on growth and pigmentation of cultured human melanocytes. Skin Pharmacol. Physiol. 2004, 17, 238–245. [Google Scholar] [CrossRef]
- Sandmann, G.; Kuhn, S.; Böger, P. Evaluation of structurally different carotenoids in Escherichia coli transformants as protectants against UV-B radiation. Appl. Environ. Microbiol. 1998, 64, 1972–1974. [Google Scholar]
- Huang, H.C.; Wang, H.F.; Yih, K.H.; Chang, L.Z.; Chang, T.M. Dual bioactivities of essential oil extracted from the leaves of Artemisia argyi as an antimelanogenic versus antioxidant agent and chemical composition analysis by GC/MS. Int. J. Mol. Sci. 2012, 13, 14679–14697. [Google Scholar] [CrossRef]
- Hseu, Y.C.; Tsou, H.T.; Kumar, K.J.; Lin, K.Y.; Chang, H.W.; Yang, H.L. The antitumor activity of Antrodia camphorata in melanoma cells: Modulation of Wnt/β-Catenin signaling pathways. Evid. Based Complement. Altern. Med. 2012, 2012, 197309. [Google Scholar] [CrossRef]
- ImageJ Software, version 1.46; National Institutes of Health (NIH): Bethesda, MA, USA, 2012.
- Lee, C.H.; Lin, Y.H.; Hsieh, J.L.; Chen, M.C.; Kuo, W.L. A polymer coating applied to Salmonella prevents the binding of Salmonella-specific antibodies. Int. J. Cancer 2013, 132, 717–725. [Google Scholar] [CrossRef]
- Ito, S.; Nakanishi, Y.; Valenzuela, R.K.; Brilliant, M.H.; Kolbe, L.; Wakamatsu, K. Usefulness of alkaline hydrogen peroxide oxidation to analyze eumelanin and phenomelanin in various tissue sample: Application to chemical analysis of human hair melanins. Pigment Cell Melanoma Res. 2011, 24, 605–613. [Google Scholar] [CrossRef]
- Chen, M.C.; Chang, W.W.; Kuan, Y.D.; Lin, S.T.; Hsu, H.C.; Lee, C.H. Resveratrol inhibits LPS-induced epithelial-mesenchymal transition in mouse melanoma model. Innate Immun. 2012, 18, 685–693. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Liu, W.-S.; Kuan, Y.-D.; Chiu, K.-H.; Wang, W.-K.; Chang, F.-H.; Liu, C.-H.; Lee, C.-H. The Extract of Rhodobacter sphaeroides Inhibits Melanogenesis through the MEK/ERK Signaling Pathway. Mar. Drugs 2013, 11, 1899-1908. https://doi.org/10.3390/md11061899
Liu W-S, Kuan Y-D, Chiu K-H, Wang W-K, Chang F-H, Liu C-H, Lee C-H. The Extract of Rhodobacter sphaeroides Inhibits Melanogenesis through the MEK/ERK Signaling Pathway. Marine Drugs. 2013; 11(6):1899-1908. https://doi.org/10.3390/md11061899
Chicago/Turabian StyleLiu, Wen-Sheng, Yu-Diao Kuan, Kuo-Hsun Chiu, Wei-Kuang Wang, Fu-Hsin Chang, Chen-Hsun Liu, and Che-Hsin Lee. 2013. "The Extract of Rhodobacter sphaeroides Inhibits Melanogenesis through the MEK/ERK Signaling Pathway" Marine Drugs 11, no. 6: 1899-1908. https://doi.org/10.3390/md11061899
APA StyleLiu, W.-S., Kuan, Y.-D., Chiu, K.-H., Wang, W.-K., Chang, F.-H., Liu, C.-H., & Lee, C.-H. (2013). The Extract of Rhodobacter sphaeroides Inhibits Melanogenesis through the MEK/ERK Signaling Pathway. Marine Drugs, 11(6), 1899-1908. https://doi.org/10.3390/md11061899



