Abstract
Five new eunicellin-based diterpenoids, krempfielins E–I (1–5) and seven known compounds (6–12) were isolated from the organic extract of a Taiwanese soft coral Cladiella krempfi. The structures of the new metabolites were elucidated on the basis of extensive spectroscopic analysis. Metabolites 5, 6, 10 and 12 were shown to exhibit cytotoxicity against a limited panel of cancer cell lines. Furthermore, compounds 6 and 10 could potently inhibit the accumulation of the pro-inflammatory iNOS protein, and 6 and 12 could significantly reduce the expression of COX-2 protein in LPS-stimulated RAW264.7 macrophage cells.
1. Introduction
Previous studies showed that many eunicellin-based diterpenes discovered from soft corals exhibited cytotoxic and anti-inflammatory activities [1,2,3,4,5,6,7,8,9,10,11,12,13,14]. The soft coral Cladiella krempfi has been found to generate several types of metabolites including eunicellin-type diterpenoids [15] and pregnane-type steroids [16,17,18]. Our previous chemical investigation of C. krempfi had led to the isolation of four new eunicellin-based diterpenoids, krempfielins A–D [19]. In this paper, we further report the isolation of five new eunicellin-based diterpenoids, krempfielins E–I (1–5) (Chart 1) and known compounds 6-methyl ether of litophynol B (6) [13], sclerophytin A (7) [20], sclerophytin B (8) [20], litophynin I monoacetate (9) [21], 6-acetoxy litophynin E (10) [22], (1R*, 2R*, 3R*, 6S*, 9R*, 10R*, 14R*)-3-acetoxycladiell-7(16),11(17)-dien-6-ol (11) [23] and litophynin F (12) [24]. The structures of metabolites 1–12 were characterized by extensive spectroscopic analysis. Cytotoxicity of all compounds against five human tumor cell lines, lung adenocarcinoma (A549 and H1299), breast carcinoma (BT483), liver carcinoma (HepG2), oral cancer (SAS) and one human lung bronchial cell (BEAS2B) lines was studied. The ability of them to inhibit the up-regulation of pro-inflammatory iNOS (inducible nitric oxide synthase) and COX-2 (cyclooxygenase-2) proteins in LPS (lipopolysaccharide)-stimulated RAW264.7 macrophage cells was also evaluated. The results showed that compounds 5, 6, 10 and 12, in particular 10, are cytotoxic towards the above cancer cell’s compounds. Except 2, 4 and 11, these compounds were found to significantly reduce the levels of iNOS protein; among them, 6 and 10 are most active. Furthermore, 6 and 12 also could significantly reduce the expression of COX-2 protein.
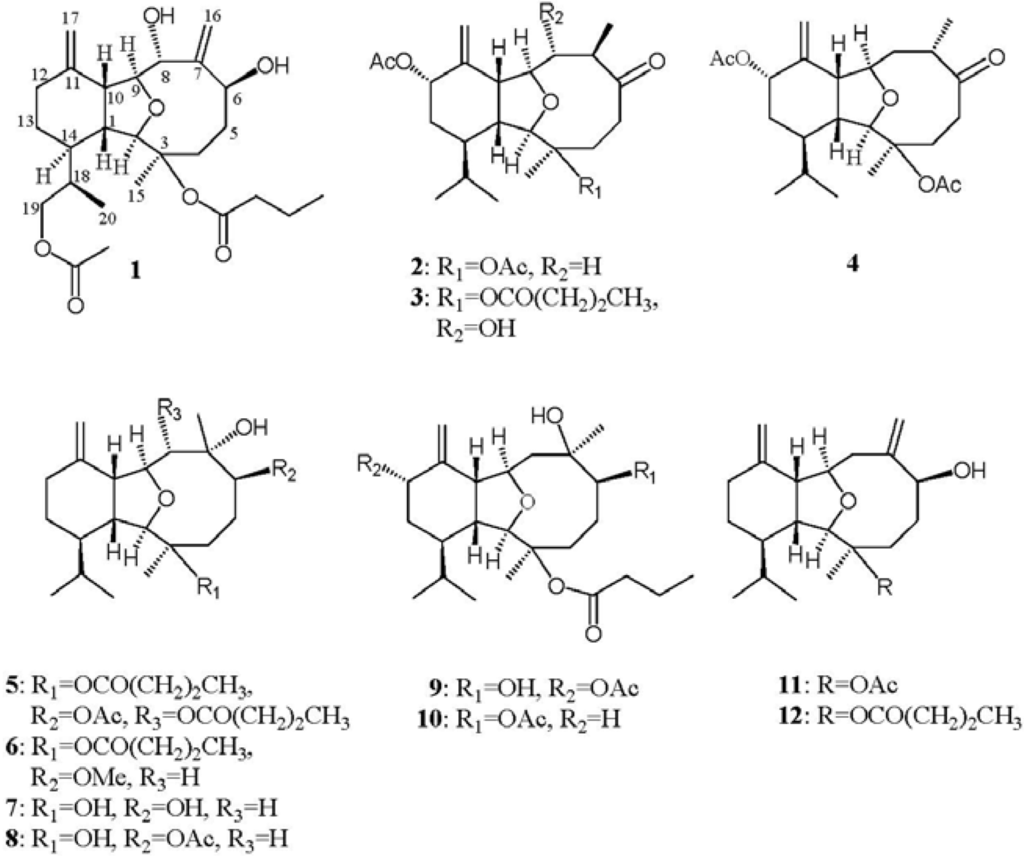
Chart 1.
Structures of metabolites 1–12.
2. Results and Discussion
The new metabolite krempfielin E (1) showed the molecular ion peak [M + Na]+ at m/z 487.2669 in the HRESIMS and established a molecular formula of C26H40O7, implying seven degrees of unsaturation. The IR absorptions bands at nmax 3421 and 1732 cm−1 revealed the presence of hydroxy and ester carbonyl functionalities. The 13C NMR spectrum measured in CDCl3 showed signals of twenty-six carbons (Table 1) which were assigned by the assistance of the DEPT spectrum to four methyls (including one acetate methyl δC 21.0), seven sp3 methylenes, two sp2 methylenes, eight sp3 methines (including four oxymethines), one sp3 and four sp2 quaternary carbons (including two ester carbonyls).The NMR spectroscopic data of 1 (Table 1, Table 2) showed the presence of two 1,1-disubstituted double bonds (δC 118.1 CH2, 112.0 CH2, 152.2 C, and 145.2 C; δH 5.51 s, 5.22 s, 4.81 s, and 4.65 s). Two ester carbonyls (δC 172.5 and 171.2) were assigned from the 13C NMR spectrum and their signals were correlated with the methylene protons (δH 2.10, 2H, m) of an n-butyrate and protons of an acetate methyl (δH 2.07 s, 3H), respectively. Therefore, the remaining three degrees of unsaturation identified 1 as a tricyclic molecule. The 1H–1H COSY and HMBC correlations (Figure 1) were further used for establishing the molecular skeleton of 1. The COSY experiment assigned three isolated consecutive proton spin systems. Above evidences and the analysis of HMBC spectrum (Figure 1) suggested that 1 is an eunicellin-based diterpenoid. Furthermore, the acetoxy group attaching at C-19 was confirmed by the HMBC correlations from oxymethylene [δH 3.94 (H2-19)] and acetate methyl protons (δH 2.07) to the ester carbonyl carbon appearing at δ 171.2 (C). Thus, the remaining one n-butyryloxy group had to be positioned at C-3, an oxygen-bearing quaternary carbon resonating at δ 84.4 ppm. On the basis of above analysis, the planar structure of 1 was established.

Table 1.
13C NMR Data for Compounds 1–5.
| C | 1 a | 2 a | 3 b | 4 b | 5 a |
|---|---|---|---|---|---|
| 1 | 43.3, CH c | 45.8, CH | 44.7, CH | 44.9, CH | 46.6, CH |
| 2 | 91.4, CH | 90.6, CH | 90.9, CH | 90.8, CH | 92.6, CH |
| 3 | 84.4, C | 84.7, C | 84.2, C | 84.6, C | 85.6, C |
| 4 | 28.4, CH2 | 33.2, CH2 | 33.2, CH2 | 32.6, CH2 | 35.8, CH2 |
| 5 | 35.3, CH2 | 30.2, CH2 | 29.7, CH2 | 38.3, CH2 | 28.5, CH2 |
| 6 | 66.9, CH | 214.8, C | 217.0, C | 213.4, C | 82.3, CH |
| 7 | 152.2, C | 48.3, CH | 40.7, CH | 40.6, CH | 78.0, C |
| 8 | 77.3, CH | 42.4, CH2 | 78.5, CH | 37.7, CH2 | 78.5, CH |
| 9 | 83.7, CH | 81.9, CH | 85.5, CH | 78.9, CH | 78.3, CH |
| 10 | 47.9, CH | 52.1, CH | 47.5, CH | 49.4, CH | 52.0, CH |
| 11 | 145.2, C | 142.1, C | 141.0, C | 141.6, C | 148.5, C |
| 12 | 31.3, CH2 | 73.1, CH | 72.8, CH | 73.0, CH | 31.6, CH2 |
| 13 | 25.7, CH2 | 28.5, CH2 | 28.9, CH2 | 28.7, CH2 | 25.5, CH2 |
| 14 | 39.1, CH | 35.3, CH | 35.6, CH | 35.7, CH | 44.0, CH |
| 15 | 22.2, CH3 | 22.3, CH3 | 22.6, CH3 | 22.6, CH3 | 22.8, CH3 |
| 16 | 118.1, CH2 | 17.8, CH3 | 14.1, CH3 | 15.4, CH3 | 18.5, CH3 |
| 17 | 112.0, CH2 | 118.1, CH2 | 119.5, CH2 | 118.7, CH2 | 109.2, CH2 |
| 18 | 32.6, CH | 28.0, CH | 27.2, CH | 27.5, CH | 29.0, CH |
| 19 | 67.5, CH2 | 21.5, CH3 | 21.6, CH3 | 21.5, CH3 | 21.9, CH3 |
| 20 | 10.6, CH3 | 16.6, CH3 | 14.9, CH3 | 14.9, CH3 | 15.4, CH3 |
| 3-n-butyrate | 172.5, C | 172.5, C | 173.1, C | ||
| 37.4, CH2 | 37.3, CH2 | 36.5, CH2 | |||
| 18.5, CH2 | 18.4, CH2 | 18.4, CH2 | |||
| 13.6, CH3 | 13.7, CH3 | 13.8, CH3 | |||
| 3-OAc | 169.6, C | 169.7, C | |||
| 22.4, CH3 | 22.3, CH3 | ||||
| 6-OAc | 171.6, C | ||||
| 21.4, CH3 | |||||
| 8-n-butyrate | 173.3, C | ||||
| 36.7, CH2 | |||||
| 18.2, CH2 | |||||
| 13.5, CH3 | |||||
| 12-OAc | 170.2, C | 170.1, C | 170.2, C | ||
| 21.6, CH3 | 21.5, CH3 | 21.4, CH3 | |||
| 19-OAc | 171.2, C | ||||
| 21.0, CH3 |
a Spectra recorded at 100 MHz in CDCl3. b Spectra recorded at 125 MHz in CDCl3. c Deduced from DEPT.

Table 2.
1H NMR Data for Compounds 1–5.
| H | 1 a | 2 a | 3 b | 4 b | 5 a |
|---|---|---|---|---|---|
| 1 | 2.25 m | 2.25 dd | 2.28 m | 2.29 m | 2.17 m |
| (12.4, 7.2) | |||||
| 2 | 3.71 br s | 3.67 br s | 3.81 br s | 3.73 br s | 3.63 br s |
| 4 | 1.64 m 2.25 m | 2.03 m 2.75 dd (14.4, 8.8) | 2.23 m 2.46 m | 2.19 m 2.52 dd (5.5, 2.0) | 1.96 m 2.65 m |
| 5 | 1.74 m 2.19 m | 1.91 m 2.48 dd (13.2, 11.2) | 1.25 m | 2.25 m 2.43 t (10.5) | 1.56 m |
| 6 | 4.73 dd | 5.71 d (5.6) | |||
| (11.2, 4.0) c | |||||
| 7 | 2.58 m | 2.32 m | 2.68 m | ||
| 8 | 4.18 d (3.2) | 1.88 m | 3.77 d (8.5) | 1.87 t (5.0) | 5.27 d (9.2) |
| 2.00 m | 2.24 m | ||||
| 9 | 4.19 d (6.8) | 4.12 ddd | 4.43 d (11.0) | 4.23 dt | 4.11 t (8.8) |
| (11.6, 8.8, 4.4) | (10.0, 5.0) | ||||
| 10 | 2.87 dd | 3.12 t (7.6) | 2.91 t (7.5) | 3.07 td | 3.39 t (7.2) |
| (10.8, 8.0) | (10.0, 1.5) | ||||
| 12 | 2.10 m | 5.49 t (2.8) | 5.52 br s | 5.49 t (2.5) | 2.03 m |
| 2.27 m | 2.23 m | ||||
| 13 | 1.11 m 1.70 m | 1.26 m 1.92 m | 1.32 m 1.97 dt (14.5, 3.0) | 1.29 m 1.94 dt (14.5, 3.0) | 1.08 m 1.76 m |
| 14 | 1.55 m | 1.71 m | 1.76 m | 1.71 m | 1.23 m |
| 15 | 1.63 s | 1.40 s | 1.49 s | 1.48 s | 1.38 s |
| 16 | 5.22 s | 1.06 d (7.2) | 1.26 d (6.5) | 1.07 d (7.0) | 1.08 s |
| 5.51 s | |||||
| 17 | 4.65 s | 4.99 d (1.6) | 4.96 s | 4.97 s | 4.51 s |
| 4.81 s | 5.91 d (1.6) | 5.27 s | 5.28 s | 4.64 s | |
| 18 | 2.08 m | 1.76 m | 1.84 m | 1.81 m | 1.69 m |
| 19 | 3.94 t (6.4) | 0.91 d (6.8) | 0.96 d (7.0) | 0.93 d (6.5) | 0.96 d (6.8) |
| 20 | 0.80 s | 0.75 d (6.8) | 0.78 d (7.0) | 0.77 d (6.5) | 0.77 d (6.8) |
| 3-n-butyrate | 2.10 m | 2.28 m | 2.25 m | ||
| 1.57 m | 1.68 m | 1.68 m | |||
| 0.92 t (7.2) | 0.99 t (7.5) | 0.98 t (7.6) | |||
| 3-OAc | 2.18 s | 2.14 s | |||
| 6-OAc | 2.09 s | ||||
| 8-n-butyrate | 2.55 m | ||||
| 1.61 m | |||||
| 0.96 t (7.2) | |||||
| 12-OAc | 2.00 s | 2.03 s | 1.98 s | ||
| 19-OAc | 2.07 s |
a Spectra recorded at 400 MHz in CDCl3. b Spectra recorded at 500 MHz in CDCl3. c J values (Hz) in parentheses.
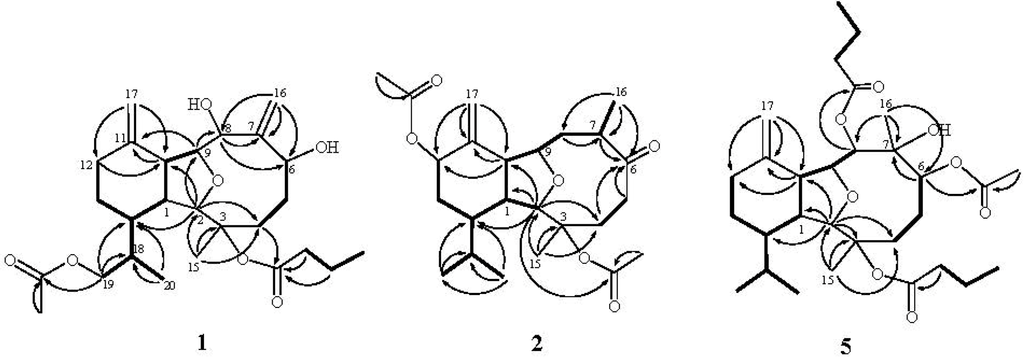
Figure 1.
Selected 1H–1H COSY (▬) and HMBC (→) correlations of 1, 2 and 5.
The relative structure of 1 was elucidated by the analysis of NOE correlations, as shown in Figure 2. The observation of the NOE correlations of H-1 with H-10 and H3-20 suggested that these protons had the same orientation and were assumed to be β-oriented. The NOE interactions found between the oxymethine proton H-8 and H-10 assigned the α-orientation of the hydroxy group. Furthermore, the NOE correlations of H-2 with both H-14 and H3-15; H-14 with both H-9 and H2-19; and H3-15 with H-6, suggested that H-2, H-6, H-9, H-14, and H3-15 are α-oriented. Furthermore, the configuration of C-18 was to be R* on the basis of NOE correlations of H-1/H3-20 and H-14/H2-19. The relative configuration of 1 was thus established. Comparison of the 1H and 13C NMR spectroscopic data of 1 with those of its 19-deacetoxyl derivative, litophynol A [21], further confirmed the structure of 1.
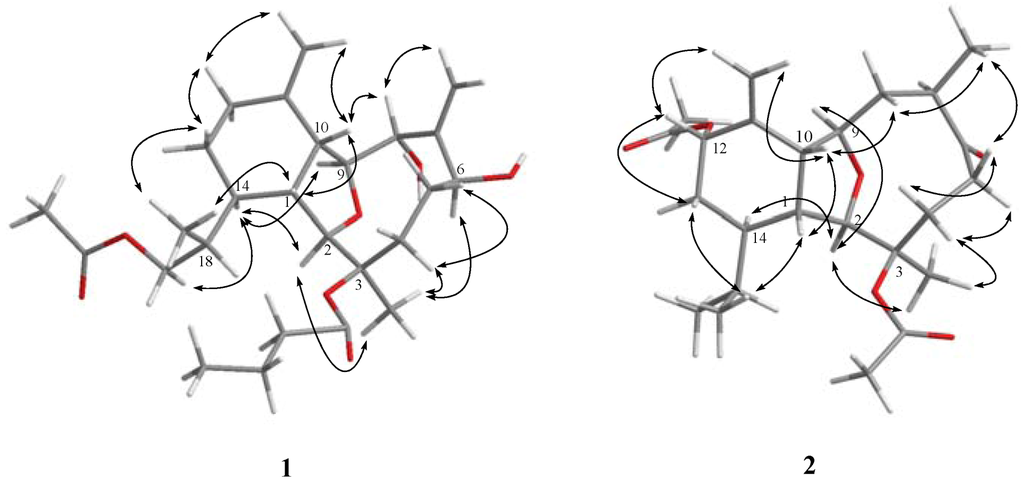
Figure 2.
Key NOESY correlations for 1 and 2.
Krempfielin F (2) was found to have the molecular formula C24H36O6 and seven degrees of unsaturation, as indicated from the HRESIMS (m/z 443.2408 [M + Na]+). The 13C NMR spectrum of 2 showed signals of twenty-four carbons (Table 1), which were characterized by the DEPT spectrum as six methyls, five methylenes (including one sp2 methylene), eight methines (including three oxygenated carbons), and five quaternary carbons (including one ketone carbonyl, two ester carbonyls, and one sp2 quaternary carbon of an olefinic group). The presence of two acetoxy groups was indicated by the 1H NMR signals (Table 2) the 1H NMR signals at δH 2.18 (s, 3H) and 2.00 (s, 3H), and the 13C NMR signals at δC 22.4 (CH3), 21.6 (CH3), 169.6 (C), and 170.2 (C). The remaining three degrees of unsaturation again identified 2 as a tricyclic diterpenoid. The molecular framework was established by 1H–1H COSY and HMBC experiments (Figure 1). The stereochemistry of compound 2 was also determined by the NOESY spectrum (Figure 3), which exhibited NOE correlations of H-1 and with H-10 and H3-20, H-13β (δH 1.26) and with H-12 and H3-20, H-8β (δH 1.88) with H-5β (δH 2.48), H-10 and H3-16, H-5α with H-4α and H3-15, and H3-16 with both H-8β (δH 1.88) and H-5β (δH 2.48), establishing the β-orientation of H-12 and H3-16. On the basis of these results and other observed NOE correlations (Figure 2), the structure of metabolite 2 was determined.
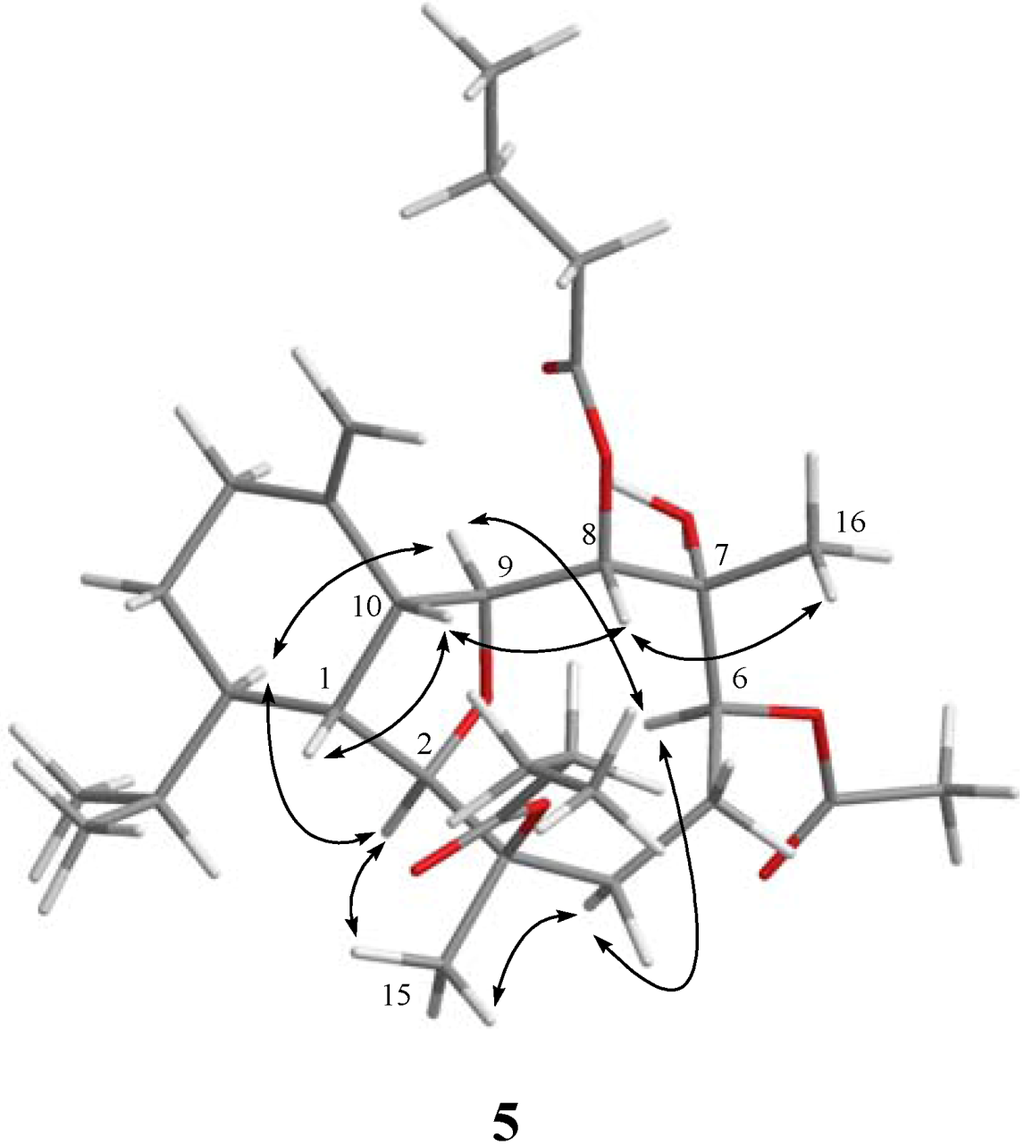
Figure 3.
Key NOESY correlations for 5.
The HRESIMS (m/z 487.2675 [M + Na]+) of 3 established the molecular formula of C26H40O7. Comparison of the NMR data of 3 with those of 2 revealed the replacement of one acetoxy group (δH 2.18, 3H, s; δC 169.6, C and 22.4, CH3) in 2 by an n-butyryloxy group in 3 (δH 0.99, 3H, t, J = 7.5 Hz; 1.68, 2H, m; 2.28, 2H, m; and δC 172.5, C; 37.3, CH2; 18.4, CH2 and 13.7, CH3), and an additional hydroxy group substituted at C-8 of 3 that downfielded H-8 to δH 3.77 and C-8 to δC 78.5 ppm. The placement of the n-butyryloxy group at C-3 was confirmed by the HMBC experiment which showed a correlation between H-2 and the carbonyl carbon (δC 172.5 C) of this n-butyryloxy group. The NOE correlations of 3 also showed that the stereochemistry of this metabolite is identical with that of 2 excepted for the presence of the α-oriented hydroxy group at C-8.
The HRESIMS of krempfielin H (4) exhibited a [M + Na]+ ion peak at m/z 443.2408, appropriate for a molecular formula of C24H36O6. By analysis of 2D NMR spectra, including 1H–1H COSY, HMQC, and HMBC, compound 4 was shown to possess the same molecular framework as that of 2. Furthermore, it was found that the NMR data of 4 were very similar to those of 2 (Table 1, Table 2), suggesting that 4 might be an isomer of 2. From NOESY spectrum, it was found that the β-oriented H-10 showed NOE interactions with both H-7 and H-8β (δH 1.87), while H-8β showed NOE interactions with H-7, indicating the β-orientation of H-7. This inferred the α-orientation of methyl substituent at C-7. Further analysis of other NOE interactions revealed that 4 possessed the same relative configuration sat C-1, C-2, C-3, C-9, C-10, C-12 and C-14 as those of 2. Therefore, 4 was found to be the C-7 epimer of 2.
The related metabolite, krempfielin I (5), had a molecular formula of C30H48O8 as indicated by the HRESIMS (m/z 559.3243, [M + Na]+) and NMR data (Table 1, Table 2). The 13C NMR spectrum of 5 revealed the appearance of three ester carbonyls (δC 173.3, 173.1 and 171.6), which were correlated with protons of two methylenes (δH 2.55, 2.25, m, each 2H; and δC 36.7 and 36.5) of two n-butyrates and the methyl protons (δH 2.09 s, 3H and δC 21.4) of one acetate group, respectively. The planar structure of 5 was established by 1H–1H COSY and HMBC correlations (Figure 2). The HMBC connectivities from H-2 (δH 3.63 br s, 1H) and H-8 (δH 5.27 d, 1H, J = 9.2 Hz) to two carbonyl carbons δC 173.3 (C) and 173.1 (C) determined the positions of the two n-butyrates at C-8 and C-3. Also, the location of an acetate group at C-6 was supported by the HMBC connectivities from both of the acetate methyl protons (δH 2.09 s, 3H) and oxygenated methine proton (δH 5.71 d, 1H, J = 5.6 Hz) to the carbon resonating at δC 171.6 (C). The relative configuration of 5 was confirmed by analyzing the key NOE correlations (Figure 3).
The cytotoxicity of the diterpenoids 1–12 against the growth of five human carcinoma cells A549, BT483, H1299, HepG2, SAS and one human normal cell line BEAS2B was studied (Table 3). The results showed that 1–4, 7–9 and 11 are not cytotoxic toward the above cancer and normal cells. Compounds 5, 6, 10 and 12 exhibited cytotoxicity toward the above five cancer cell lines and the human normal cell line; 10, being the most cytotoxic. The in vitro anti-inflammatory effects of compounds 1–12 were also tested by examining the inhibitory activity of these compounds toward the LPS-induced up-regulation of pro-inflammatory proteins iNOS and COX-2, in RAW264.7 macrophage cells (Figure 4). At a concentration of 10 μM, compounds except 2, 4, and 11 were found to significantly reduce the expression of iNOS protein, relative to the control cells stimulated with LPS only. Among them, 6 and 10 could potently reduce the levels of iNOS protein to 6.4 ± 0.8% and 12.8 ± 2.9%, respectively. Compounds 6 and 12 also effectively reduced COX-2 expression (52.5 ± 8.0% and 48.1 ± 10.8%, respectively) in the same LPS-stimulated cells. These results revealed that n-butyryloxy group at C-3 could significantly enhance the cytotoxic and anti-inflammatory activities in eunicellin-type compounds. Overall, compounds 6, 10 and 12 exhibited interesting cytotoxic and anti-inflammatory activity and could become lead compounds in the future drug development.

Table 3.
Cytotoxicity (ED50 μg/mL) of compounds 5, 6, 10 and 12.
| Cell Lines | Normal Cell Line | |||||
|---|---|---|---|---|---|---|
| Compounds | A549 | BT483 | H1299 | HepG2 | SAS | BEAS2B |
| 5 | 15.0 ± 3.5 | 11.5 ± 1.8 | 19.2 ± 4.0 | 12.9 ± 3.1 | 10.2 ± 3.5 | – a |
| 6 | 16.1 ± 1.2 | 10.0 ± 1.8 | 11.8 ± 1.0 | – a | 17.2 ± 0.4 | 10.4 ± 0.3 |
| 10 | 6.8 ± 1.0 | 11.6 ± 2.8 | 6.7 ± 0.7 | 8.5 ± 1.3 | 9.5 ± 3.7 | 4.8 ± 0.7 |
| 12 | 12.2 ± 1.1 | 6.8 ± 0.6 | 12.8 ± 1.2 | 11.1 ± 0.4 | 10.3 ± 0.5 | 13.6 ± 0.5 |
| Taxol | 1.5 ± 0.9 | 3.9 ± 0.8 | 1.2 ± 0.1 | 1.4 ± 0.7 | 2.3 ± 1.5 | 2.3 ± 1.5 |
a IC50 > 20 μg/mL.
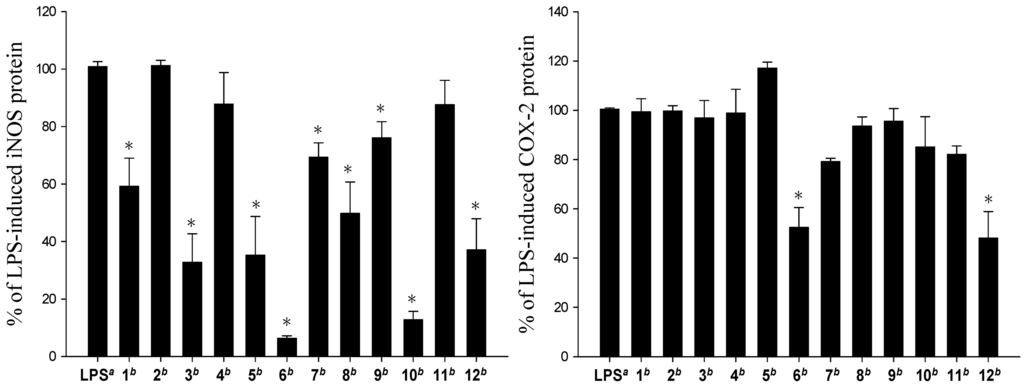
Figure 4.
Effect of compounds 1–12 on LPS-induced iNOS and COX-2 proteins expression in RAW264.7 macrophage cells by immunoblot analysis. The values are mean ± SEM. (n = 6). Relative intensity of the LPS alone stimulated group was taken as 100%.* Significantly different from LPS alone stimulated group (* P < 0.05). a stimulated with LPS, b stimulated with LPS in the presence of 1–12 (10 μM).
3. Experimental Section
3.1. General Experimental Procedures
Optical rotations were measured on a JASCO P-1020 polarimeter. IR spectra were recorded on a JASCO FT/IR-4100 infrared spectrophotometer. ESIMS were obtained with a Bruker APEX II mass spectrometer. NMR spectra were recorded either on a Varian UNITY INOVA-500 FT-NMR, a Varian 400MR FT-NMR. Silica gel (Merck, 230–400 mesh) was used for column chromatography. Precoated silica gel plates (Merck, Kieselgel 60 F-254, 0.2 mm) were used for analytical TLC. High performance liquid chromatography was performed on a Hitachi L-7100 HPLC apparatus with a ODS column (25.0 × 21.2 mm, 5 μm).
3.2. Animal Material
C. krempfi was collected by hand using scuba off the coast of Penghu islands of Taiwan in June 2008, at a depth of 5–10 m, and stored in a freezer until extraction. A voucher sample (specimen no. 200806CK) was deposited at the Department of Marine Biotechnology and Resources, National Sun Yat-sen University.
3.3. Extraction and Separation
The octocoral (1.1 kg fresh wt) was collected and freeze-dried. The freeze-dried material was minced and extracted exhaustively with EtOH (3 × 10 L). The EtOH extract of the frozen organism was partitioned between CH2Cl2 and H2O. The CH2Cl2-soluble portion (14.4 g) was subjected to column chromatography on silica gel and eluted with EtOAc in n-hexane (0%–100% of EtOAc, stepwise) and then further with MeOH in EtOAc with increasing polarity to yield 41 fractions. Fraction 28, eluted with n-hexane–EtOAc (1:2), was rechromatoraphed over a reversed-phase column (RP-18) using acetone–H2O (10:1) as the mobile phase to afford six subfractions (A1–A6). Subfraction A1 was repeatedly separated by reverse phase HPLC (CH3CN–H2O, 1:1 to 2:1) to afford compounds 1 (3.5 mg), 2 (4.9 mg), 3 (7.6 mg), 4 (4.8 mg), and 9 (3.6 mg). Subfraction A2 separated by reverse phase HPLC (CH3CN–H2O, 3.8: 1) to afford compounds 5 (7.1 mg), 6 (16.4 mg), 10 (30.2 mg), 11 (5.2 mg) and 12 (5.4 mg). Subfraction A3 by reverse phase HPLC (CH3CN–H2O, 1:1) to afford compound 7 (13.2 mg). Subfraction A4 by reverse phase HPLC (MeOH–H2O, 2.4:1) to afford compound 8 (5.5 mg).
3.3.1. Krempfielin E (1)
3.3.2. Krempfielin F (2)
3.3.3. Krempfielin G (3)
3.3.4. Krempfielin H (4)
3.3.5. Krempfielin I (5)
3.4. Cytotoxicity Testing
Cell lines were purchased from the American Type Culture Collection (ATCC). Cytotoxicity assays of compounds 1–12 were performed using the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetra-zolium bromide] colorimetric method [25,26].
3.5. In Vitro Anti-Inflammatory Assay
Macrophage (RAW264.7) cell line was purchased from ATCC. In vitro anti-inflammatory activities of compounds 1–12 were measured by examining the inhibition of lipopolysaccharide (LPS) induced upregulation of iNOS (inducible nitric oxide synthetase) and COX-2 (cyclooxygenase-2) proteins in macrophages cells using western blotting analysis [27,28].
4. Conclusions
New eunicellin-based diterpenoids were isolated together with known ones from the soft coral Cladiella krempfi. Compounds 5, 6, 10 and 12 showed cytotoxicity toward the above five cancer cell lines, and one human normal cell line. Also, 6, 10 and 12 could significantly reduce the accumulation of pro-inflammatory proteins iNOS and COX-2. Thus, these compounds, in particular 6, 10 and 12 could be promising bioactive agents and may warrant further biomedical investigation.
Acknowledgements
This research was supported by grants from the National Science Council (100-2320-B-110-001-MY2), Taiwan, awarded to J.-H. Sheu.
References
- Ahmed, A.F.; Wu, M.-H.; Wang, G.-H.; Wu, Y.-C.; Sheu, J.-H. Eunicellin-based diterpenoids, australins A–D, isolated from the soft coral Cladiella australis. J. Nat. Prod. 2005, 68, 1051–1055. [Google Scholar] [CrossRef]
- Wu, S.-L.; Su, J.-H.; Wen, Z.-H.; Hsu, C.-H.; Chen, B.-W.; Dai, C.-F.; Kuo, Y.-H.; Sheu, J.-H. Simplexins A–I, eunicellin-based diterpenoids from the soft coral Klyxum simplex. J. Nat. Prod. 2009, 72, 994–1000. [Google Scholar] [CrossRef]
- Chen, B.-W.; Wu, Y.-C.; Chiang, M.Y.; Su, J.-H.; Wang, W.-H.; Fan, T.-Y.; Sheu, J.-H. Eunicellin-based diterpenoids from the cultured soft coral Klyxum simplex. Tetrahedron 2009, 65, 7016–7022. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Tai, C.-Y.; Hwang, T.-L.; Weng, C.-F.; Li, J.-J.; Fang, L.-S.; Wang, W.-H.; Wu, Y.-C.; Sung, P.-J. Cladielloides A and B: New eunicellin-type diterpenoids from an Indonesian octocoral Cladiella sp. Mar. Drugs 2010, 8, 2936–2945. [Google Scholar] [CrossRef]
- Chen, B.-W.; Chang, S.-M.; Huang, C.-Y.; Chao, C.-H.; Su, J.-H.; Wen, Z.-H.; Hsu, C.-H.; Dai, C.-F.; Wu, Y.-C.; Sheu, J.-H. Hirsutalins A–H, eunicellin-based diterpenoids from the soft coral Cladiella hirsuta. J. Nat. Prod. 2010, 73, 1785–1791. [Google Scholar] [CrossRef]
- Hassan, H.M.; Khanfar, M.A.; Elnagar, A.Y.; Mohammed, R.; Shaala, L.A.; Youssef, D.T.A.; Hifnawy, M.S.; El Sayed, K.A. Pachycladins A–E, prostate cancer invasion and migration inhibitory eunicellin-based diterpenoids from the Red Sea soft coral Cladiella pachyclados. J. Nat. Prod. 2010, 73, 848–853. [Google Scholar] [CrossRef]
- Chen, B.-W.; Chao, C.-H.; Su, J.-H.; Wen, Z.-H.; Sung, P.-J.; Sheu, J.-H. Anti-Inflammatory eunicellin-based diterpenoids from the cultured soft coral Klyxum simplex. Org. Biomol. Chem. 2010, 8, 2363–2366. [Google Scholar] [CrossRef]
- Wu, S.-L.; Su, J.-H.; Lu, Y.; Chen, B.-W.; Huang, C.-Y.; Wen, Z.-H.; Kuo, Y.-H.; Sheu, J.-H. Simplexins J–O, eunicellin-based diterpenoids from a Dongsha Atoll soft coral Klyxum simplex. Bull. Chem. Soc. Jpn. 2011, 84, 626–632. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Tai, C.-Y.; Su, Y.-D.; Chang, Y.-C.; Lu, M.-C.; Weng, C.-F.; Su, J.-H.; Hwang, T.-L.; Wu, Y.-C.; Sung, P.-J. Discovery of new eunicellins from an Indonesian octocoral Cladiella sp. Mar. Drugs 2011, 9, 934–943. [Google Scholar] [CrossRef]
- Hsu, F.-J.; Chen, B.-W.; Wen, Z.-H.; Huang, C.-Y.; Dai, C.-F.; Su, J.-H.; Wu, Y.-C.; Sheu, J.-H. Klymollins A–H, bioactive eunicellin-based diterpenoids from the Formosan soft coral Klyxum molle. J. Nat. Prod. 2011, 74, 2467–2471. [Google Scholar] [CrossRef]
- Chen, B.-W.; Huang, C.-Y.; Wen, Z.-H.; Su, J.-H.; Wang, W.-H.; Sung, P.-J.; Wu, Y.-C.; Sheu, J.-H. Klysimplexins U–X, eunicellin-based diterpenoids from the cultured soft coral Klyxum simplex. Bull. Chem. Soc. Jpn. 2011, 84, 1237–1242. [Google Scholar] [CrossRef]
- Chen, B.-W.; Chao, C.-H.; Su, J.-H.; Tsai, C.-W.; Wang, W.-H.; Wen, Z.-H.; Huang, C.-Y.; Sung, P.-J.; Wu, Y.-C.; Sheu, J.-H. Klysimplexins I–T, eunicellin-based diterpenoids from the cultured soft coral Klyxum simplex. Org. Biomol. Chem. 2011, 9, 834–844. [Google Scholar] [CrossRef]
- Iwagawa, T.; Kusatsu, T.; Tsuha, K.; Hamada, T.; Okamura, H.; Furukawa, T.; Akiyama, S.; Doe, M.; Morimoto, Y.; Iwase, F.; et al. Cytotoxic eunicellin-type diterpenes from the soft coral Litophyton Viscudium. Heterocycles 2011, 83, 2149–2155. [Google Scholar] [CrossRef]
- Wu, S.-L.; Su, J.-H.; Huang, C.-Y.; Tai, C.-J.; Sung, P.-J.; Liaw, C.-C.; Sheu, J.-H. Simplexins P–S, eunicellin-based diterpenes from the soft coral Klyxum simplex. Mar. Drugs 2012, 10, 1203–1211. [Google Scholar] [CrossRef]
- Sarma, N.S.; Chavakula, R.; Rao, I.N.; Kadirvelraj, R.; Row, T.N.G.; Saito, I. Crystal and molecular structure of sclerophytin F methyl ether from the soft coral Cladiella krempfi. J. Nat. Prod. 1993, 56, 1977–1980. [Google Scholar] [CrossRef]
- Lan, W.-J.; Lin, C.-W.; Su, J.-Y.; Zeng, L.-M. Two steroidal glycosides from the soft coral Cladiella krempfi. Chem. J. Chin. Univ. 2003, 24, 2019–2021. [Google Scholar]
- Huang, X.-P.; Deng, Z.-W.; Ofwegen, L.V.; Li, J.; Fu, H.-Z.; Zhu, X.-B.; Lin, W.-H. Two new pregnane glycosides from soft coral Cladiella krempfi. J. Asian Nat. Prod. Res. 2006, 8, 287–291. [Google Scholar] [CrossRef]
- Huang, X.-P.; Deng, Z.-W.; Zhu, X.-B.; Ofwegen, L.V.; Proksch, P.; Lin, W.-H. Krempenes A–D: A series of unprecedented pregnane-type steroids from the marine soft coral Cladiella krempfi. Helv. Chim. Acta 2006, 89, 2020–2026. [Google Scholar] [CrossRef]
- Tai, C.-J.; Su, J.-H.; Huang, M.-S.; Wen, Z.-H.; Dai, C.-F.; Sheu, J.-H. Bioactive eunicellin-based diterpenoids from the soft coral Cladiella krempfi. Mar. Drugs 2011, 9, 2036–2045. [Google Scholar] [CrossRef]
- Friedrich, D.; Doskotch, R.W.; Paquette, L.A. Revised constitution of sclerophytins A and B. Org. Lett. 2000, 2, 1879–1882. [Google Scholar] [CrossRef]
- Miyamoto, T.; Yamada, K.; Ikeda, N.; Komori, T.; Higuchi, R. Bioactive terpenoids from octocorallia, I. Bioactive diterpenoids: Litophynols A and B from the mucus of the soft coral Litophyton sp. J. Nat. Prod. 1994, 57, 1212–1219. [Google Scholar]
- Ochi, M.; Yamada, K.; Futatsugi, K.; Kotsuki, H.; Shibata, K. Litophynin D and E, two new diterpenoids from a soft coral Litophyton sp. Chem. Lett. 1990, 19, 2183–2186. [Google Scholar]
- Rao, C.B.; Rao, D.S.; Satyanarayana, C.; Rao, D.V.; Kassühlke, K.E.; Faulkner, D.J. New cladiellane diterpenes from the soft coral Cladiella australis of the Andaman and Nicobar Islands. J. Nat. Prod. 1994, 57, 574–580. [Google Scholar] [CrossRef]
- Ochi, M.; Yamada, K.; Futatsugi, K.; Kotsuki, H.; Shibata, K. Litophynins F, G, and H, three new diterpenoids from a soft coral Litophyton sp. Heterocycles 1991, 32, 29–32. [Google Scholar] [CrossRef]
- Alley, M.C.; Scudiero, D.A.; Monks, A.; Hursey, M.L.; Czerwinski, M.J.; Fine, D.L.; Abbott, B.J.; Mayo, J.G.; Shoemaker, R.H.; Boyd, M.R. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988, 48, 589–601. [Google Scholar]
- Scudiero, D.A.; Shoemaker, R.H.; Paull, K.D.; Monks, A.; Tierney, S.; Nofziger, T.H.; Currens, M.J.; Seniff, D.; Boyd, M.R. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 1988, 48, 4827–4833. [Google Scholar]
- Lu, Y.; Huang, C.-Y.; Lin, Y.-F.; Wen, Z.-H.; Su, J.-H.; Kuo, Y.-H.; Chiang, M.Y.; Sheu, J.-H. Anti-inflammatory cembranoids from the soft corals Sinularia querciformis and Sinularia granosa. J. Nat. Prod. 2008, 71, 1754–1759. [Google Scholar] [CrossRef]
- Jean, Y.-H.; Chen, W.-F.; Duh, C.-Y.; Huang, S.-Y.; Hsu, C.-H.; Lin, C.-S.; Sung, C.-S.; Chen, I.-M.; Wen, Z.-H. Inducible nitric oxide synthase and cyclooxygenase-2 participate in anti-inflammatory and analgesic effects of the natural marine compound lemnalol from Formosan soft coral Lemnalia cervicorni. Eur. J. Pharmacol. 2008, 578, 323–331. [Google Scholar] [CrossRef]
- Samples Availability: Not available.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).