Abstract
Two novel chlorinated pyrones, halomadurones A and B, and two novel brominated analogues, halomadurones C and D, were isolated from a marine Actinomadura sp. cultivated from the ascidian Ecteinascidia turbinata. Additionally, a non-halogenated analogue, 2-methyl-6-((E)-3-methyl-1,3-hexadiene)-γ-pyrone, was synthesized to understand the role of the halogens for activity. Halomadurones C and D demonstrated potent nuclear factor E2-related factor antioxidant response element (Nrf2-ARE) activation, which is an important therapeutic approach for treatment of neurodegenerative diseases.
1. Introduction
The prevalence of neurodegenerative diseases, such as Alzheimer’s disease (AD), Parkinson’s disease, Huntington’s disease, and amyotrophic lateral sclerosis (ALS) is on the rise worldwide [1,2,3]; cases of AD, the most common neurodegenerative disease, are projected to reach 115 million by the year 2050 [3]. Meanwhile, only a handful of therapeutics are available that target these diseases, most of which only treat the symptoms not the underlying cause. Many neurodegenerative diseases are caused by mitochondrial DNA mutation or oxidative stress damage [4]. Therefore, determining methods of minimizing oxidative stress is a potential strategy towards preventing many neurodegenerative diseases [5,6].
The transcription factor Nrf2 (nuclear factor E2-related factor) activates the antioxidant response element (ARE), which is located in the promoter region of genes that encode cytoprotective and antioxidant enzymes including many phase II detoxification enzymes [7,8,9]. Therefore, activation of Nrf2 represents a promising therapeutic strategy for combatting neurodegenerative diseases. In order to identify activators of this pathway in conjunction with having an in vivo model for evaluating therapeutics for neurodegenerative diseases, a transgenic mouse model was created that contained a human placental alkaline phosphatase reporter under the control of the ARE. For in vitro testing, primary neuronal cells from ARE-hPAP transgenic reporter were used to test for Nrf2/ARE activation [10,11]. The use of primary neuronal cells has provided an in vitro model that most closely resembles the in vivo pathology, albeit without the blood brain barrier. In parallel, the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium inner salt (MTS) assay can be used to test toxicity of the compounds. There have been only limited reports on natural product pharmacophores that activate the Nrf2/ARE pathway. The vast majority have been electrophiles, such as Michael acceptors [12,13,14,15,16]. More recently, activation of the Nrf2-ARE pathway with small molecules was also investigated by Luesch and co-workers leading to the discovery of Nrf2-ARE activators from seaweed extracts [17] and an electrophile ARE activator with in vivo activity [16].
In our pursuit of Nrf2/ARE activators, we isolated four novel halogenated electrophilic polyketides that were named halomadurones A–D (1–4) (Figure 1), from a marine Actinomadura sp. (Strain WMMB499) cultivated from the ascidian Ecteinascidia turbinata (Herdman, 1880). Halomadurones C (3) and D (4) demonstrated potent nuclear factor E2-related factor antioxidant response element (Nrf2-ARE) activation in the ARE-hPAP assay. The cytotoxicity studies and activity in the ARE-hPAP assay was suggestive of a narrow therapeutic window. Synthesis of a non-halogenated pyrone demonstrated that bromination was a key feature of the pharmacophore.
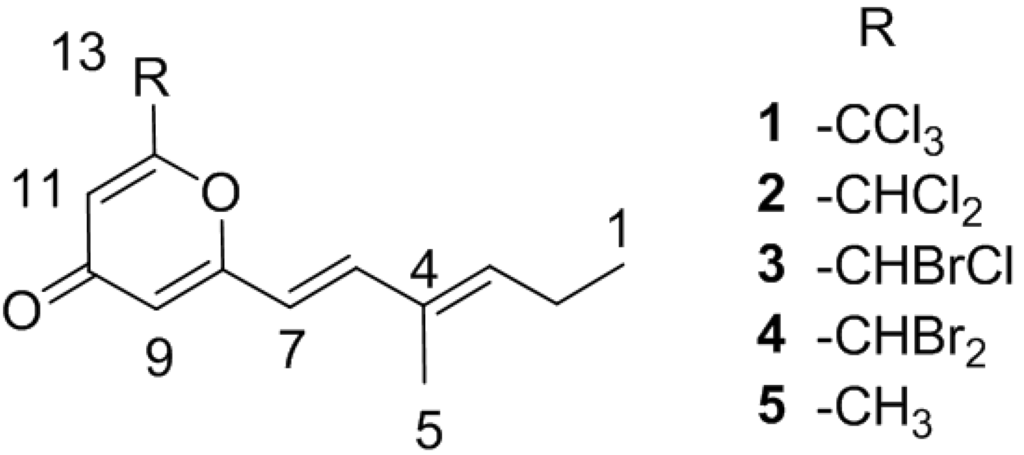
Figure 1.
Structures of 1–5.
In addition to the promising in vitro activity, halomadurones A–D (1–4) represent a new carbon skeleton due to the position of the methyl group. Additionally, halomadurone A (1), contains a trichloromethyl group, which is a rare moiety in natural products. Most natural products that contain a trichloromethyl group, such as barbamide A [18] and dysidenamide [19], were isolated from cyanobacteria and also contain a thiazole ring. However, cytotoxic trichlorinated polyenones neocarzilin A and B [20] were produced by the soil-derived bacterium Streptomyces carzinostaticus and do not contain a thiazole ring. Consequently, neocarzilins A and B, which have been the target for various synthetic [21] and biosynthetic studies [22], are now joined by halomadurone A (1) as the sole members of actinomycete-derived trichlorinated natural products. The role of the trichloromethyl group in bioactivity was investigated with halomadurones A–D (1–4) and a non-halogenated analogue, 2-methyl-6-((E)-3-methyl-1,3-hexadiene)-γ-pyrone (5), which we synthesized.
2. Results and Discussion
2.1. Bacterial Strain Selection and Structure Elucidation
Our attention was drawn to strain WMMB499 after analysis of thirty-four marine-derived bacterial extracts using LCMS-based metabolomics relying on principal component analysis (PCA) [23]. When compared to the other 33 LCMS chromatograms using PCA, WMMB499 was identified as having unique chemical signatures and putative novel natural products; after saline fermentation and isolation, WMMB499 was found to produce halomadurones A (1) and B (2). Halomadurones C (3) and D (4) were produced by WMMB499 by increasing the ratio KBr/NaCl. Previous studies have demonstrated that many halogenases are promiscuous with respect to using Br and Cl [24,25].
HRMS supported the molecular formula of C13H13Cl3O2 and C13H14Cl2O2 for halomadurone A (1) and B (2), respectively. Analysis of 1H and 13C NMR data (Table 1, Figure S1–S12) allowed for determination of the structures of halomadurones A (1) and B (2). The 1H and 13C NMR data for halomadurone A (1) supported the conclusion that C-13 was connected to only one carbon, and in combination with the 13C chemical shift at C-13 (90.3 ppm) and molecular formula, allowed for the assignment of the trichloromethyl group. A large vicinal coupling constant (3JH 15.6) for H-6 and H-7 supported the assignment of the E conformation [26]. ROESY NMR data allowed for the assignment of the E olefin at C-3 and C-4 (Figure S13). A comparison of 1H and 13C NMR shifts between halomadurones A (1) and B (2) showed that the only difference between the two structures was at C-13. C-13 in halomadurone B (2) contained two chlorine atoms and one hydrogen as evidenced by the upfield shift of C-13 to 65.8 ppm and a methine proton at 6.35 ppm. The relative configuration of the two olefins was assigned the same as halomadurone A (1) on the basis of vicinal coupling constants and ROESY NMR data.
After the structural elucidation of halomadurones A (1) and B (2), the amount of KBr was increased from 0.1 g/L to 10 g/L and NaCl was reduced from 20 g/L to 0 g/L in fermentation medium ASW-A, resulting in the production of brominated analogues. Many halogenases have low specificity for the halide substrate, and the incorporation of a specific halogen often depends on the relative concentration of each halogen anion in the fermentation medium [27,28]. HRMS of the two brominated analogues, halomadurones C (3) and D (4), supported the molecular formulas of C13H14BrClO2 and C13H14Br2O2, respectively. A comparison of the 1H and 13C NMR shifts (Table 1, Figure S14–S25) of halomadurones C (3) and D (4) with halomadurone B (2) confirmed that the only difference in the structures was the halogenated atoms at C-13. The relative configuration of the two olefins for halomadurones C (3) and D (4) was assigned the same as halomadurone A (1) on the basis of vicinal coupling constants and ROESY NMR data. The lack of optical rotation by halomadurone C (3) suggested that a racemic mixture of both enantiomers existed.

Table 1.
1H and 13C NMR data for 1–4 (600 MHz for 1H, 150 MHz for 13C, CDCl3).
| No. | 1 | 2 | 3 | 4 | ||||
|---|---|---|---|---|---|---|---|---|
| δC, mult. | δH (J in Hz) | δC, mult. | δH (J in Hz) | δC, mult. | δH (J in Hz) | δC, mult. | δH (J in Hz) | |
| 1 | 13.8, CH3 | 1.04, t (7.3) | 13.8, CH3 | 1.04, t (7.3) | 13.8, CH3 | 1.04, t (7.5) | 13.8, CH3 | 1.05, t (7.5) |
| 2 | 22.4, CH2 | 2.24, qn (7.3) | 22.4, CH2 | 2.24, qn (7.3) | 22.4, CH2 | 2.24, qn (7.5) | 22.5, CH2 | 2.24, qn (7.5) |
| 3 | 144.3, CH | 5.93, t (7.3) | 144.0, CH | 5.92, t (7.3) | 144.0, CH | 5.93, t (7.2) | 144.0, CH | 5.94, t (7.5) |
| 4 | 132.6, C | 132.6, C | 132.7, C | 132.7, C | ||||
| 5 | 12.2, CH3 | 1.82, s | 12.2, CH3 | 1.82, s | 12.2, CH3 | 1.82, s | 12.2, CH3 | 1.82, s |
| 6 | 143.3, CH | 7.14, d (15.6) | 142.9, CH | 7.14, d (15.6) | 142.9, CH | 7.14, d (15.8) | 142.9, CH | 7.17, d (15.8) |
| 7 | 115.8, CH | 6.07, d (15.6) | 116.1, CH | 6.07, d (15.6) | 116.2, CH | 6.04, d (15.8) | 116.3, CH | 6.05, d (15.8) |
| 8 | 163.2, C | 162.9, C | 163.4, C | 163.4, C | ||||
| 9 | 112.3, CH | 6.17, d (1.8) | 112.7, CH | 6.17, d (2.0) | 112.2, CH | 6.14, d (2.3) | 113.1, CH | 6.13, d (2.3) |
| 10 | 179.5, C | 179.5, C | 179.6, C | 179.5, C | ||||
| 11 | 111.8, CH | 6.85, d (1.8) | 112.7, CH | 6.42, d (2.0) | 112.1, CH | 6.39, d (2.3) | 111.4, CH | 6.32, d (2.3) |
| 12 | 160.5, C | 160.2, C | 160.7, C | 160.8, C | ||||
| 13 | 90.3, C | 65.8, CH | 6.35, s | 50.1, CH | 6.35, s | 32.5, CH | 6.24, s | |
2.2. Synthetic Analogue
After isolation and structure elucidation of halomadurones A–D (1–4), a non-halogenated analogue, 2-methyl-6-((E)-3-methyl-1,3-hexadiene)-γ-pyrone (5) was synthesized to compare the bioactivity of the novel structures (Scheme 1). In particular, the effect of substitution on the pyrone methyl was investigated. 2-6-Dimethyl-γ-pyrone in sodium ethoxide was added to 2-methyl-2-pentenal and stirred at room temperature for 5 hours [29]. Purification of the product (see Experimental Section) resulted in 4.0 mg 2-methyl-6-((E)-3-methyl-1,3-hexadiene)-γ-pyrone (5). The structure was confirmed by analysis of 1H and 13C NMR and MS data (Table S1, Figure S26–S31). ROESY NMR data and vicinal coupling constants confirmed the assignment of the E olefin at C-3 and C-4. Although sufficient compound yield was achieved for initial in vitro studies, the overall yield was low (~2%) as expected.
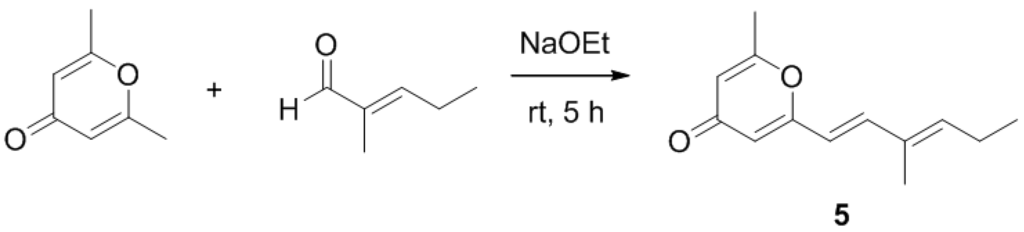
Scheme 1.
Synthesis of 5.
2.3. Biological Activity
On the basis of structural motifs that were present in other natural product activators, we hypothesized that the halomadurones could be activators and would contribute further knowledge surrounding SAR. Therefore, halomadurones A–D (1–4) and 2-methyl-6-((E)-3-methyl-1,3-hexadiene)-γ-pyrone (5) were evaluated for Nrf2-ARE activation (Figure 2). Tert-butylhydroquinone (tBHQ) was used as a positive control. Halomadurones C (3) and D (4) demonstrated potent Nrf2-ARE activation in the hPAP assay, but toxicity was apparent at higher concentrations (Figure 2). Nrf2 activation did not increase in a dose-dependent manner with increasing concentrations of halomadurones C (3) and D (4) due to cytotoxicity. For example, treatment with concentrations of 7.5 and 15 µM resulted in greater Nrf2 activation than treatment with 30 µM. This decrease in activation at higher concentrations of halomadurones C (3) and D (4) correlated with an increase in cytotoxicity indicative of a narrow therapeutic window. More specifically, halomadurone D (4) had a dramatic decrease in hPAP activity at 30 µM in comparison to halomadurone C (3); yet, halomadurone D (4) had greater hPAP activity than halomadurone C (3) at 15 and 7.5 µM. Halomadurones A (1) and B (2) and 2-methyl-6-((E)-3-methyl-1,3-hexadiene)-γ-pyrone (5) demonstrated less than a ten-fold increase in Nrf2-ARE activation, considerably less than tBHQ. The four halogenated pyrones (1–4) demonstrated greater Nrf2 activation than the non-halogenated pyrone (5), suggesting the importance of the halogen atoms in their activity. Among the halogenated pyrones, brominated halomadurones C (3) and D (4) demonstrated a considerable increase in activation compared to chlorinated halomadurones A (1) and B (2), potentially due to the increased electrophilicity from the bromine atoms. However, at higher concentrations, electrophiles sometimes react with thiol groups, which can lead to toxicity [30]. Neuroprotection assays were conducted for halomadurone C (3) and D (4) at 3 µM, but neither compound demonstrated neuroprotection.
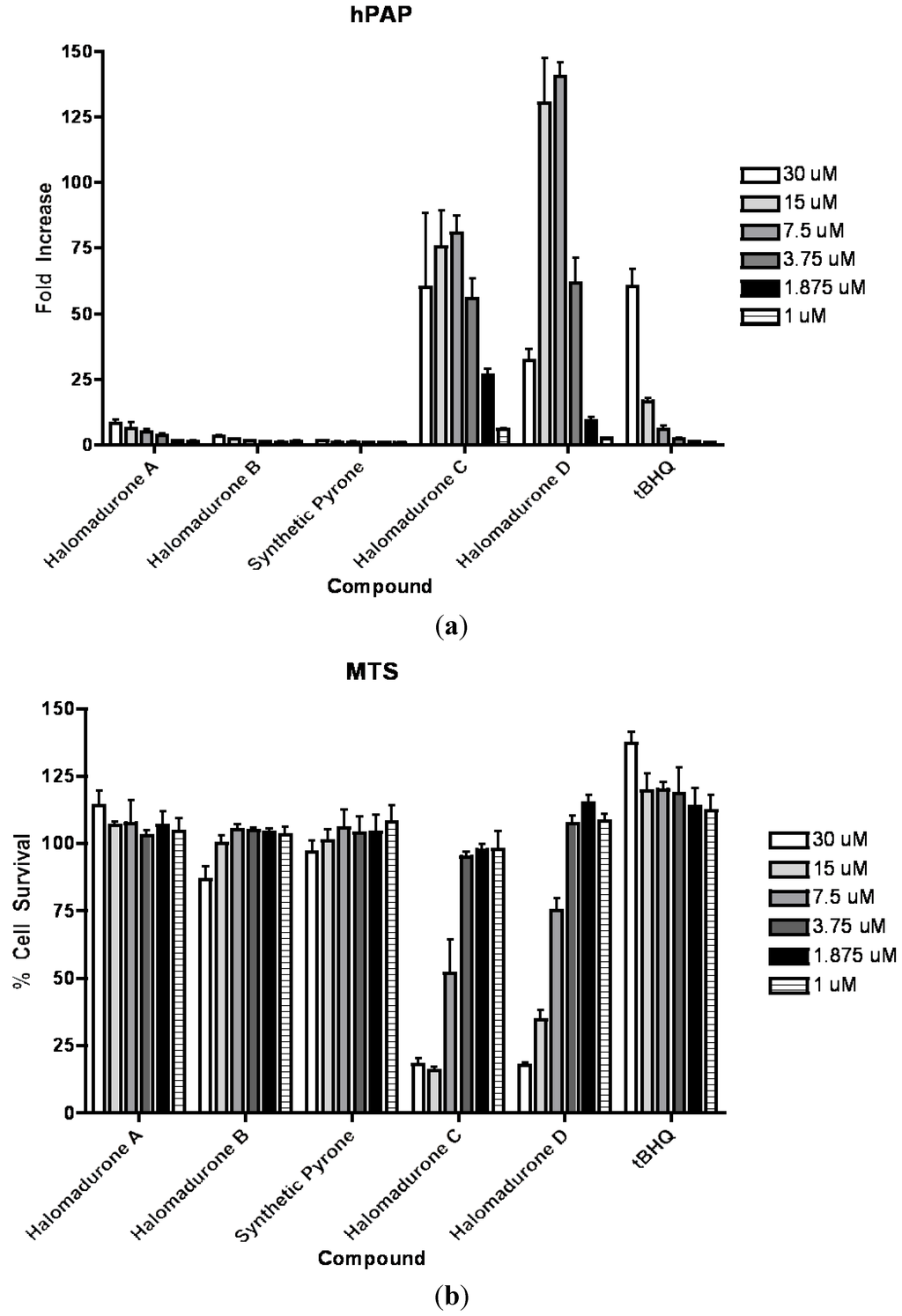
Figure 2.
Nrf2 activation and cytotoxicity of 1–5. Primary cortical cultures were prepared and treated with vehicle or compounds at increasing concentrations for 48 h. (a) hPAP activity was measured. All values are standardized to the vehicle treated value and presented as fold change; Mean ± SEM. (b) Cell viability was assessed using MTS assay. All values are standardized to the vehicle treated value and presented as percent of Mean ± SEM.
3. Experimental Section
3.1. General Experimental Procedures
Optical rotations were measured on a Perkin–Elmer 241 Polarimeter. UV spectra were recorded on an Aminco/OLIS UV-Vis spectrophotometer. IR spectra were measured with a Bruker Equinox 55/S FT–IR spectrophotometer. NMR spectra were obtained in CDCl3 with a Bruker Avance 600 MHz spectrometer equipped with a 1.7 mm 1H{13C/15N} cryoprobe, a Bruker Avance 500 MHz spectrometer equipped with a 13C/15N{1H} cryoprobe, and a Varian Unity-Inova 500 MHz spectrometer. HRMS data were acquired with a Bruker MaXis 4G QTOF mass spectrometer. RP HPLC was performed using a Shimadzu Prominence HPLC system and a Phenomenex Onyx Monolithic C18 column (100 × 4.6 mm).
3.2. Biological Material
Ascidian specimens were collected on 10 August 2011, in the Florida Keys (24°39.591′, 81°25.217′). A voucher specimen (FLK10-5-6) for Ecteinascidia turbinata (Herdman, 1880) is housed at the University of Wisconsin-Madison. For cultivation, a sample of ascidian (1 cm3) was rinsed with sterile seawater, macerated using a sterile pestle in a micro-centrifuge tube, and dilutions were made in sterile seawater, with vortexing between steps to separate bacteria from heavier tissues. Dilutions were separately plated on three media: ISP2, R2A, and M4. Each medium was supplemented with 50 µg/mL cycloheximide and 25 µg/mL nalidixic acid. Plates were incubated at 28 °C for at least 28 days, and strain WMMB499 was purified from an ISP2 isolation plate.
3.3. Sequencing
16S rDNA sequencing was conducted as previously described [31]. WMMB499 was identified as an Actinomadura sp. and demonstrated 99% sequence similarity to Actinomadura sp. 13679C (accession number EU741239). The 16S sequence for WMMB499 was deposited in GenBank (accession number JX101467).
3.4. Fermentation, Extraction, and Isolation
Two 10 mL seed cultures (25 × 150 mm tubes) in medium ASW-A (20 g soluble starch, 10 g glucose, 5 g peptone, 5 g yeast extract, 5 g CaCO3 per liter of artificial seawater) were inoculated with strain WMMB499 and shaken (200 RPM, 28 °C) for seven days. Two hundred fifty mL baffled flasks (12 × 50 mL) containing ASW-A were inoculated with 1 mL seed culture and were incubated (200 RPM, 28 °C) for seven days. Two-liter flasks (6 × 500 mL) containing medium ASW-A with Diaion HP20 (4% by weight) were inoculated with 25 mL from the 50 mL culture and shaken (200 RPM, 28 °C) for seven days. Filtered HP20 and cells were washed with H2O and extracted with acetone. The acetone extract (3.5 g) was subjected to liquid-liquid partitioning using 30% aqueous MeOH and CHCl3 (1:1). The CHCl3-soluble partition (2.5 g) was fractionated by Sephadex LH20 column chromatography (column size CHCl3:MeOH, 1:1). Fractions containing 1 and 2 were subjected to RP HPLC (55%/45% to 75%/25% MeOH/H2O, 15 min, 3 mL/min) using a Phenomenex Onyx Monolithic C18 column (100 × 4.6 mm), yielding 2 (3.0 mg, tR 7.1 min) and 1 (3.2 mg, tR 11.0 min). For production of 3 and 4, the amount of KBr in medium ASW-A was increased from 0.1 g/L to 10 g/L, and no NaCl was included. After fermentation of 500 mL ASW-A (with KBr), the same isolation procedure was used. One additional purification step by RP HPLC (70%/30% to 76%/24% ACN/H2O, 11 min, 4 mL/min) using a Phenomenex Luna C18 column (250 × 10 mm, 5 µm) yielded 3 (1.3 mg, tR 8.8 min) and 4 (1.2 mg, tR 9.1 min).
Halomadurone A (1). White solid; UV (MeOH) λmax (log ε) 204 (3.96), 223 (3.99), 255 (3.90), 315 (3.92) nm; IR (ATR) νmax 1644, 1635, 1394, 1215 cm−1; 1H and 13C NMR (See Table 1); HRMS [M + H]+ m/z 307.0044 (calcd. for C13H14Cl3O2, 307.0054).
Halomadurone B (2). White solid; UV (MeOH) λmax (log ε) 204 (4.04), 222 (3.94), 253 (3.79), 315 (3.83) nm; IR (ATR) νmax 1641, 1632, 1602, 1369, 932 cm−1; 1H and 13C NMR (See Table 1); HRMS [M + H]+ m/z 273.0453 (calcd. for C13H15Cl2O2, 273.0444).
Halomadurone C (3). White solid;  ±0 UV (MeOH) λmax (log ε) 206 (4.02), 253 (3.40), 324 (3.50) nm; IR (ATR) νmax 1650, 1601, 1396, 1215, 934 cm−1; 1H and 13C NMR (See Table 1); HRMS [M + H]+ m/z 316.9937 (calcd. for C13H15BrClO2, 316.9938).
±0 UV (MeOH) λmax (log ε) 206 (4.02), 253 (3.40), 324 (3.50) nm; IR (ATR) νmax 1650, 1601, 1396, 1215, 934 cm−1; 1H and 13C NMR (See Table 1); HRMS [M + H]+ m/z 316.9937 (calcd. for C13H15BrClO2, 316.9938).
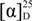 ±0 UV (MeOH) λmax (log ε) 206 (4.02), 253 (3.40), 324 (3.50) nm; IR (ATR) νmax 1650, 1601, 1396, 1215, 934 cm−1; 1H and 13C NMR (See Table 1); HRMS [M + H]+ m/z 316.9937 (calcd. for C13H15BrClO2, 316.9938).
±0 UV (MeOH) λmax (log ε) 206 (4.02), 253 (3.40), 324 (3.50) nm; IR (ATR) νmax 1650, 1601, 1396, 1215, 934 cm−1; 1H and 13C NMR (See Table 1); HRMS [M + H]+ m/z 316.9937 (calcd. for C13H15BrClO2, 316.9938).Halomadurone D (4). White solid; (c 0.12, MeOH); UV (MeOH) λmax (log ε) 206 (4.30), 255 (3.42), 324 (3.42) nm; IR (ATR) νmax 1651, 1601, 1396, 1215, 934 cm−1; 1H and 13C NMR (See Table 1); HRMS [M + H]+ m/z 360.9433 (calcd. for C13H15Br2O2, 360.9433).
2-Methyl-6-((E)-3-methyl-1,3-hexadiene)-γ-pyrone (5). To a solution of 2-6-dimethyl-γ-pyrone (1.24 g) in 15 mL ethanol was added 2-methyl-2-pentenal (2.28 mL) and sodium ethoxide (680 mg) in 7.5 mL ethanol. The reaction mixture was stirred for 5 h at rt. After 5 h, dilute HCl was added to the mixture and dried under vacuum. The reaction mixture was subjected to RP HPLC (40%/60% to 100%/0% MeOH/H2O containing 0.1% acetic acid, 20 min) using a Phenomenex Gemini C18 column (100 × 30 mm, 5 µm). Additional purification of the fraction containing 5 was conducted by Normal Phase HPLC (60%/40% to 100%/0% ethyl acetate/hexanes, 30 min) using a Phenomenex Luna Silica column (250 × 10 mm, 5 µm), yielding 5 (4.0 mg, 2.0% yield, tR 25.5 min) as a white solid; UV (MeOH) λmax (log ε) 208 (3.99), 227 (4.00), 314 (4.17) nm; IR (ATR) νmax 1655, 1597, 1398, 1217, 925 cm−1; 1H and 13C NMR (See Supporting Information); HRMS [M + H]+ m/z 205.1230 (calcd. for C13H17O2, 205.1223).
3.5. Bioasssay hPAP and MTS
Primary cortical neuronal cultures were derived from ARE-hPAP reporter mice as previously described [10,11]. Compounds were dissolved in 100% DMSO and administered to cells for 48 h. Tert-butylhydroquinone (tBHQ) was used as a control. After 48 h, Nrf2 activation was determined by measuring for hPAP activity as previously described [32]. Using one-second integration luminescence was measured on a Berthold Orion microplate luminometer (Berthold Technologies GmbH & Co., Bad Wildbad, Germany). Baseline signals from hPAP negative control culture samples were subtracted from all values. Cell viability was assayed using the MTS assay following the manufacturer’s suggested protocol (Promega, Madison, Wisconsin, USA). All hPAP and MTS data are represented as mean ± SEM (n = 4). Results of hPAP assays are expressed as the fold increase in hPAP activity over basal levels. Statistical analysis was performed using one-way ANOVA followed by Newman–Keuls multiple comparison (GraphPad Prism, version 4). A p < 0.05 was considered statistically significant.
4. Conclusions
We reported the isolation and structure elucidation of halomadurones A–D (1–4), novel halogenated electrophilic pyrones from an Actinomadura sp. and the synthesis of a non-halogenated analogue, 2-methyl-6-((E)-3-methyl-1,3-hexadiene)-γ-pyrone (5). Halomadurones C (3) and D (4) demonstrated potent Nrf2-ARE activation. Therefore, halomaudrones A–D (1–4) could play an important role in the discovery of new therapeutics, especially considering the ever-present need for therapeutics for neurodegenerative diseases.
Acknowledgments
This work was supported by funding from the University of Wisconsin-Madison School of Pharmacy, the Graduate School at the University of Wisconsin, and the UW College of Agriculture and Life Sciences. This work was also funded by the NIH, NIGMS Grant R01 GM092009, and in part by R01 GM104192. We would like to thank the Analytical Instrumentation Center at the University of Wisconsin-Madison for the facilities to acquire spectroscopic data. This study made use of the National Magnetic Resonance Facility at Madison, which is supported by NIH grants P41RR02301 (BRTP/NCRR) and P41GM66326 (NIGMS). Additional equipment was purchased with funds from the University of Wisconsin, the NIH (RR02781, RR08438), the NSF (DMB-8415048, OIA-9977486, BIR-9214394), and the USDA. We would like to thank Don Demaria for assistance with collection, and Weiping Tang for helpful synthesis discussions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Menken, M.; Munsat, T.L.; Toole, J.F. The global burden of disease study: Implications for neurology. Arch. Neurol. 2000, 57, 418–420. [Google Scholar] [CrossRef]
- Hebert, L.E.; Weuve, J.; Scherr, P.A.; Evans, D.A. Alzheimer disease in the United States (2010–2050) estimated during the 2010 census. Neurology 2013, 80, 1778–1783. [Google Scholar] [CrossRef]
- Alzheimer’s Disease Internationa. World Alzheimer Report 2009; Prince, M., Jackson, J., Eds.; Alzheimer’s Disease International: London, UK, 2009. [Google Scholar]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef]
- De Vries, H.E.; Witte, M.; Hondius, D.; Rozemuller, A.J.M.; Drukarch, B.; Hoozemans, J.; van Horssen, J. Nrf2-induced antioxidant protection: A promising target to conteract ROS-mediated damage neurodegenerative disease? Free Radic. Biol. Med. 2008, 45, 1375–1383. [Google Scholar] [CrossRef]
- Calkins, M.J.; Johnson, D.A.; Townsend, J.A.; Vargas, M.R.; Dowell, J.A.; Williamson, T.P.; Kraft, A.D.; Lee, J.-M.; Li, J.; Johnson, J.A. The Nrf2/ARE pathway as a potential therapeutic target in neurodegenerative disease. Antioxid. Redox Signal. 2009, 11, 497–508. [Google Scholar] [CrossRef]
- Vanugopal, R.; Jaiswal, A.K. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc. Natl. Acad. Sci. USA 1996, 93, 14960–14965. [Google Scholar] [CrossRef]
- Itoh, K.; Ishii, T.; Wakabayashi, N.; Yamamoto, M. Regulatory mechanism of cellular response to oxidative stress. Free Radical Res. 1999, 31, 319–324. [Google Scholar] [CrossRef]
- Wild, A.C.; Moinova, H.R.; Mulcahy, R.T. Regulation of γ-glutamylcysteine synthetase subunit gene expression by the transription factor Nrf2. J. Biol. Chem. 1999, 274, 33627–33636. [Google Scholar] [CrossRef]
- Kraft, A.D.; Johnson, D.A.; Johnson, J.A. Nuclear factor E2-related factor 2-dependent antioxidant response element activation by tert-butylhydroquinone and sulforaphane occurring preferentially in astrocytes conditions neurons against oxidative insult. J. Neurosci. 2004, 24, 1101–1112. [Google Scholar] [CrossRef]
- Johnson, D.A.; Andrews, G.K.; Xu, W.; Johnson, J.A. Activation of the antioxidant response element in primary cortical neuronal cultures derived from transgenic reporter mice. J. Neurochem. 2002, 81, 1233–1241. [Google Scholar] [CrossRef]
- Balogun, E.; Hoque, M.; Gong, P.; Killeen, E.; Green, C.J.; Foresti, R.; Alam, J.; Motterlini, R. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem. J. 2003, 371, 887–895. [Google Scholar] [CrossRef]
- Haridas, V.; Hanausek, M.; Nishimura, G.; Soehnge, H.; Gaikwad, A.; Narog, M.; Spears, E.; Zoltaszek, R.; Walaszek, Z.; Gutterman, J.U. Triterpenoid electrophiles (avicins) activate the innate stress response by redox regulation of a gene battery. J. Clin. Invest. 2004, 113, 65–73. [Google Scholar]
- Shibuya, A.; Onda, K.; Kawahara, H.; Uchiyama, Y.; Nakayama, H.; Omi, T.; Nagaoka, M.; Matsui, H.; Hirano, T. Sofalcone, a gastric mucosa protective agent, increases vascular endothelial growth factor via the Nrf2-heme-oxygenase-1 dependent pathway in gastric epithelial cells. Biochem. Biophys. Res. Commun. 2010, 398, 581–584. [Google Scholar] [CrossRef]
- Lee, Y.M.; Jeong, G.S.; Lim, H.D.; An, R.B.; Kim, Y.C.; Kim, E.C. Isoliquiritigenin 2′-methyl ether induces growth inhibition and apoptosis in oral cancer cells via heme oxygenase-1. Toxicol. Vitro 2010, 24, 776–782. [Google Scholar] [CrossRef]
- Wang, R.; Mason, D.E.; Choe, K.P.; Lewin, A.S.; Peters, E.C.; Luesch, H. In vitro and in vivo characterization of a tunable dual-reactivity probe of the Nrf2-ARE Pathway. ACS Chem. Biol. 2013, 8, 1764–1774. [Google Scholar] [CrossRef]
- Wang, R.; Paul, V.; Luesch, H. Seaweed extracts and unsaturated fatty acid constituents from the green alga Ulva lactuca as activators of the cytoprotective Nrf2-ARE pathway. Free Radic. Biol. Med. 2013, 57, 141–153. [Google Scholar] [CrossRef]
- Orjala, J.; Gerwick, W.H. Barbamide, a chlorinated metabolite with molluscicidal activity from the Caribbean cyanobacterium Lyngbya majuscula. J. Nat. Prod. 1996, 59, 427–430. [Google Scholar] [CrossRef]
- Jiménez, J.I.; Scheuer, P.J. New lipopeptides from the Caribbean cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2001, 64, 200–203. [Google Scholar] [CrossRef]
- Nozoe, S.; Ishii, N.; Kusano, G.; Kikuchi, K.; Ohta, T. Neocarzilins A and B, novel polyenones from Streptomyces carzinostaticus. Tetrahedron Lett. 1992, 33, 7547–7550. [Google Scholar] [CrossRef]
- Nozoe, S.; Kikuchi, K.; Ishii, N.; Ohta, T. Synthesis of neocarzilin A: An absolute sterochemistry. Tetrahedron Lett. 1992, 33, 7551–7552. [Google Scholar] [CrossRef]
- Otsuka, M.; Ichinose, K.; Fujii, I.; Ebizuka, Y. Cloning, sequencing, and functional analysis of an iterative type I polyketide synthase gene cluster for biosynthesis of the antitumor chlorinated polyenone neocarzilin in “Streptomyces carzinostaticus”. Antimicrob. Agents Chemother. 2004, 48, 3468–3476. [Google Scholar] [CrossRef]
- Hou, Y.; Braun, D.R.; Michel, C.R.; Klassen, J.L.; Adnani, N.; Wyche, T.P.; Bugni, T.S. Microbial strain prioritization using metabolomics tools for the discovery of the natural products. Anal. Chem. 2012, 84, 4277–4283. [Google Scholar] [CrossRef]
- Zeng, J.; Zhan, J. A novel fungal flavin-dependent halogenase for natural product biosynthesis. ChemBioChem 2010, 11, 2119–2123. [Google Scholar] [CrossRef]
- Vaillancourt, F.H.; Vosburg, D.A.; Walsh, C.T. Dichlorination and bromination of a Threonyl-S-carrier protein by the non-heme FeII halogenase SYRB2. Chembiochem 2006, 7, 748–752. [Google Scholar] [CrossRef]
- Williams, D.H.; Fleming, I. Spectroscopic Methods in Organic Chemistry, 4th ed.; McGraw-Hill Book Company Limited: London, UK, 1989. [Google Scholar]
- Eustaquio, A.S.; Pojer, F.; Noel, J.P.; Moore, B.S. Discovery and characterization of a marine bacterial SAM-dependent chlorinase. Nat. Chem. Biol. 2008, 4, 69–74. [Google Scholar] [CrossRef]
- Wagner, C.; El Omari, M.; König, G.M. Biohalogenation: Nature’s way to synthesize halogenated metabolites. J. Nat. Prod. 2009, 72, 540–553. [Google Scholar]
- Ghandi, M.; Bayat, Y.; Teimuri-mofrad, R. A novel method for the synthesis of formyl and hydroxymethyl derivatives of 4H-pyran-4-one. Org. Prep. Proced. Int. 2002, 34, 525–530. [Google Scholar] [CrossRef]
- Satoh, T.; McKercher, S.R.; Lipton, S.A. Nrf2/ARE-mediated antioxidant actions of pro-electrophilic drugs. Free Radical Biol. Med. 2013, 65, 645–657. [Google Scholar] [CrossRef]
- Wyche, T.P.; Hou, Y.; Braun, D.; Cohen, H.C.; Xiong, M.P.; Bugni, T.S. First natural analogs of the cytotoxic thiodepsipeptide thiocoraline A from a marine Verrucosispora sp. J. Org. Chem. 2011, 76, 6542–6547. [Google Scholar] [CrossRef]
- Fischedick, J.T.; Standiford, M.; Johnson, D.A.; Johnson, J.A. Structure activity relationship of phenolic diterpenes from Salvia officinalis as activators of the nuclear factor E2-related factor 2 pathway. Bioorg. Med. Chem. 2013, 21, 2618–2622. [Google Scholar] [CrossRef]
Supplementary Files
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).