Chitosan Nanoparticles Attenuate Hydrogen Peroxide-Induced Stress Injury in Mouse Macrophage RAW264.7 Cells
Abstract
:1. Introduction
2. Results
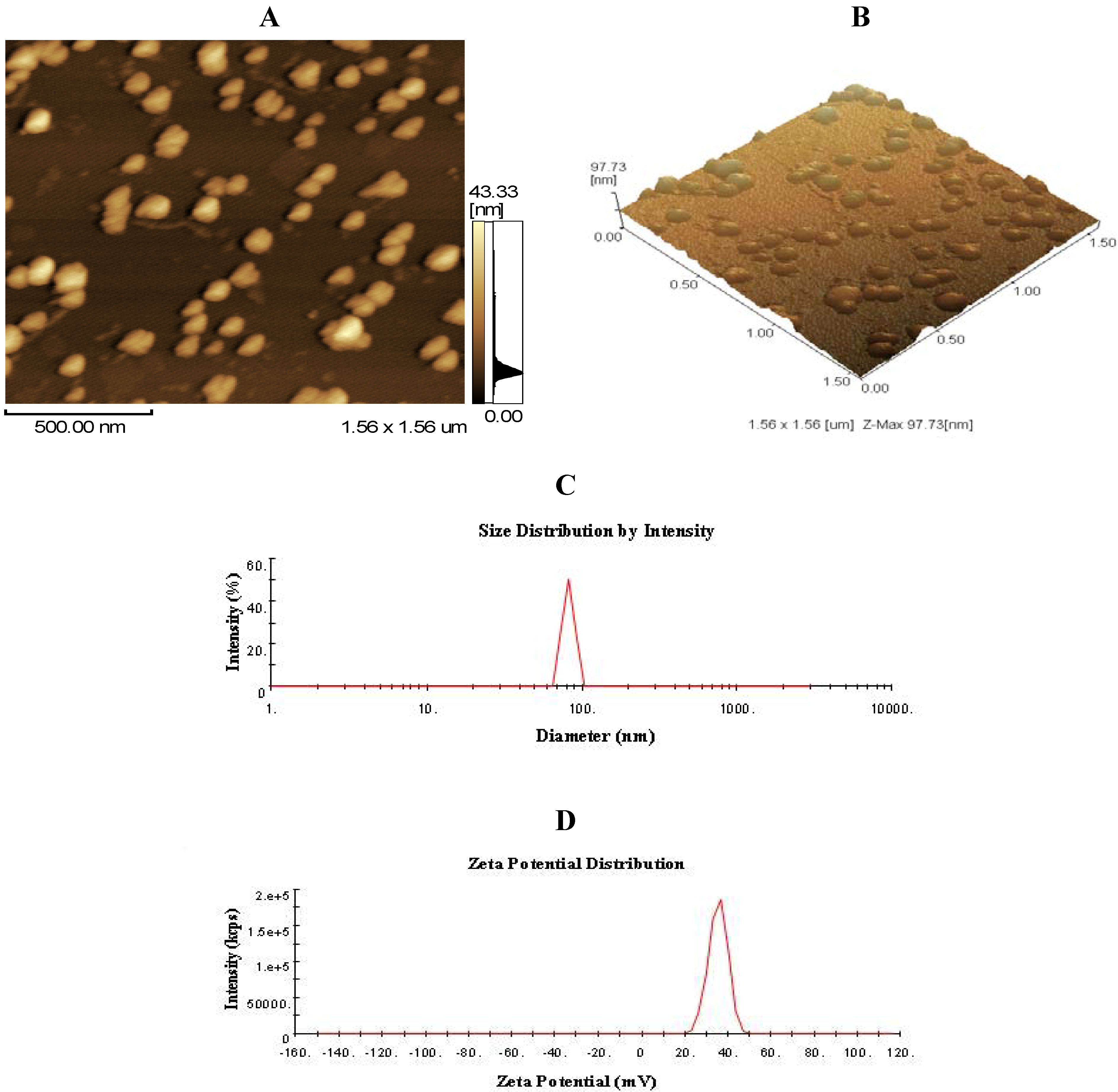
2.1. Morphology, Size, and Zeta Potential of CNP
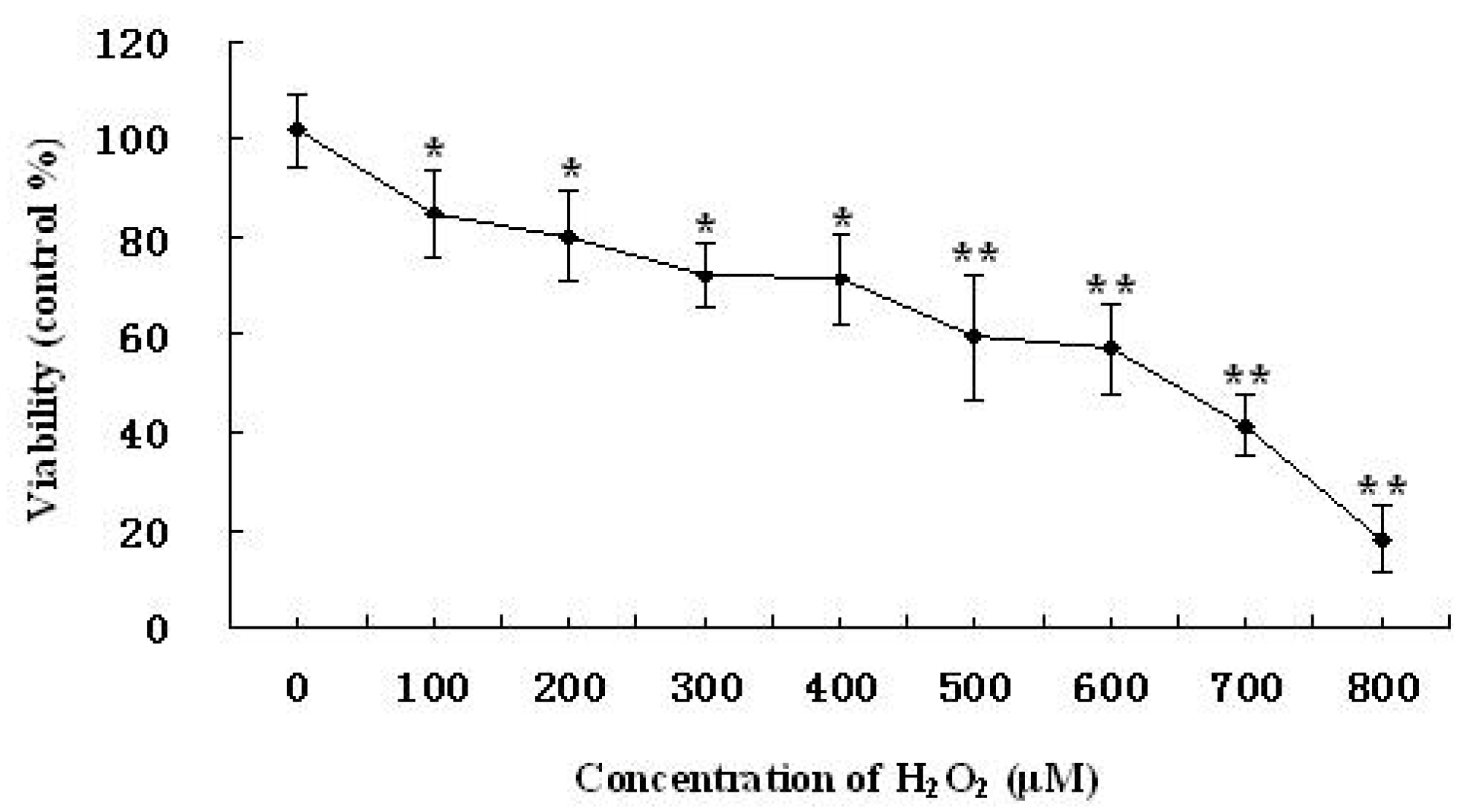
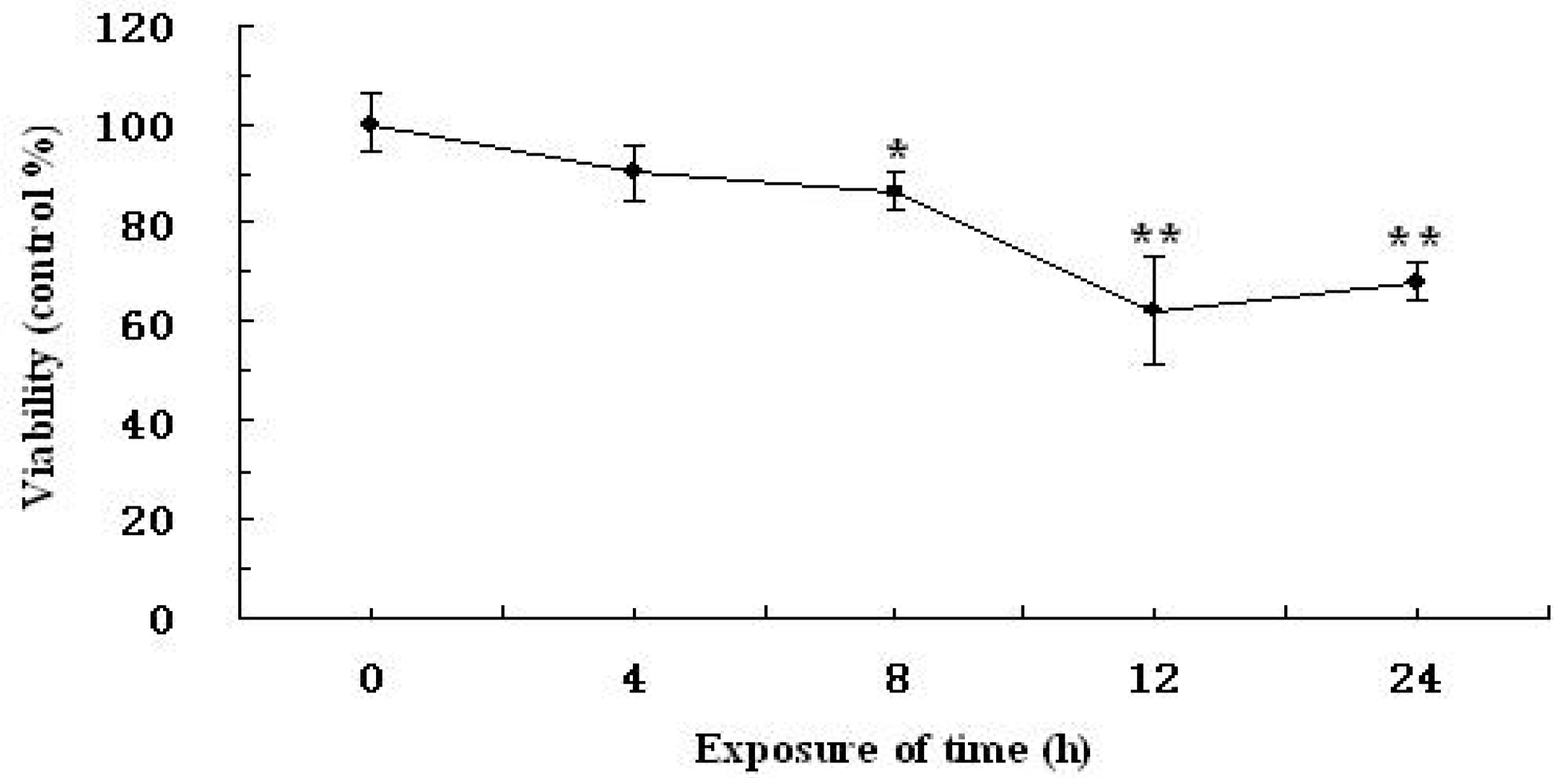
2.2. Time-Dependent and Concentration-Dependent Viability Losses in RAW264.7 Cells Induced by H2O2
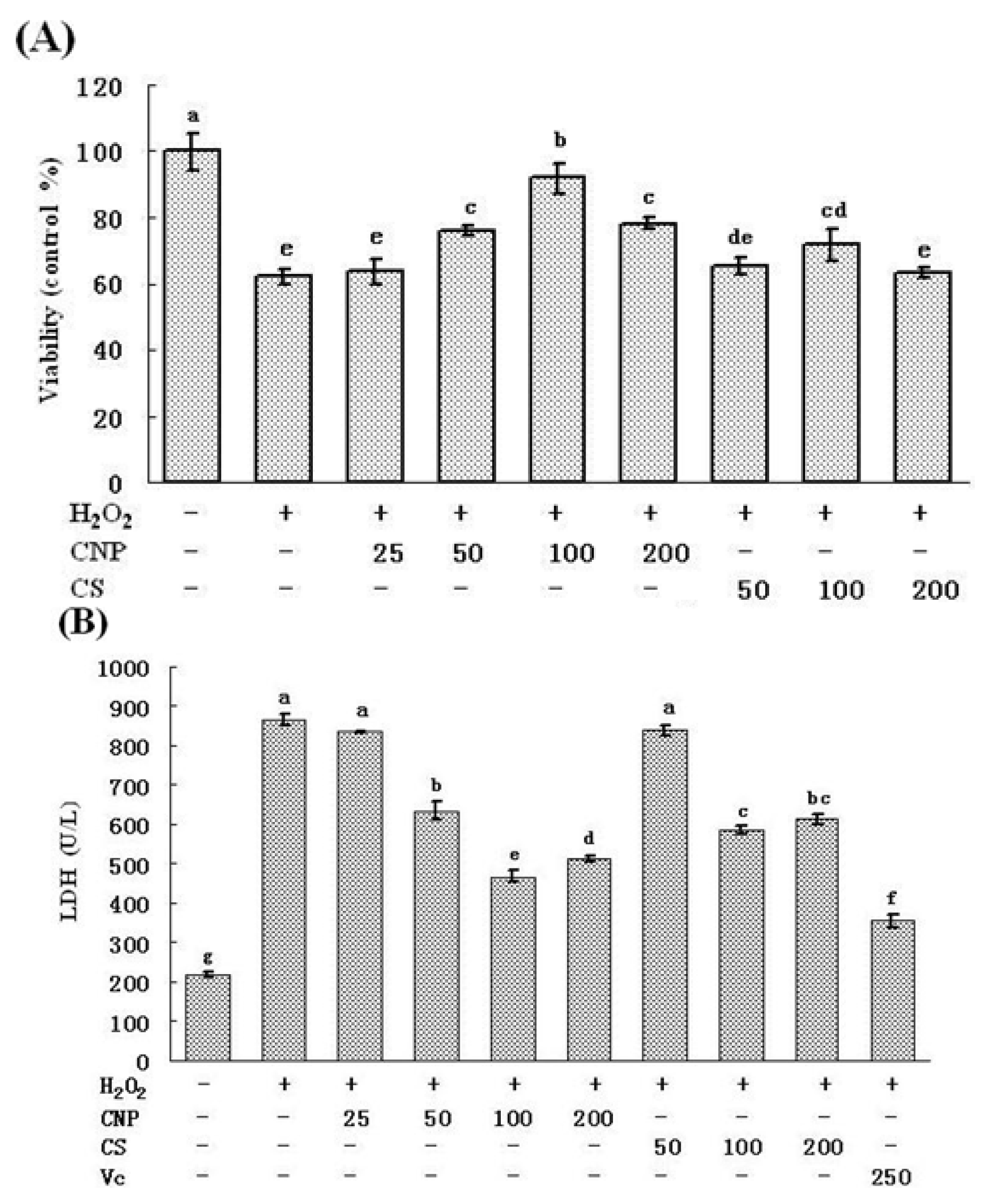
2.3. Effect of CNP on the Viability of H2O2-Induced RAW264.7 Cells
2.4. Effects of CNP on Morphology of RAW264.7 Cells

2.5. Measurement of SOD, GSH-Px, GSH, and T-AOC Activities as Well as MnSOD and GSH-Px mRNA Expression Levels
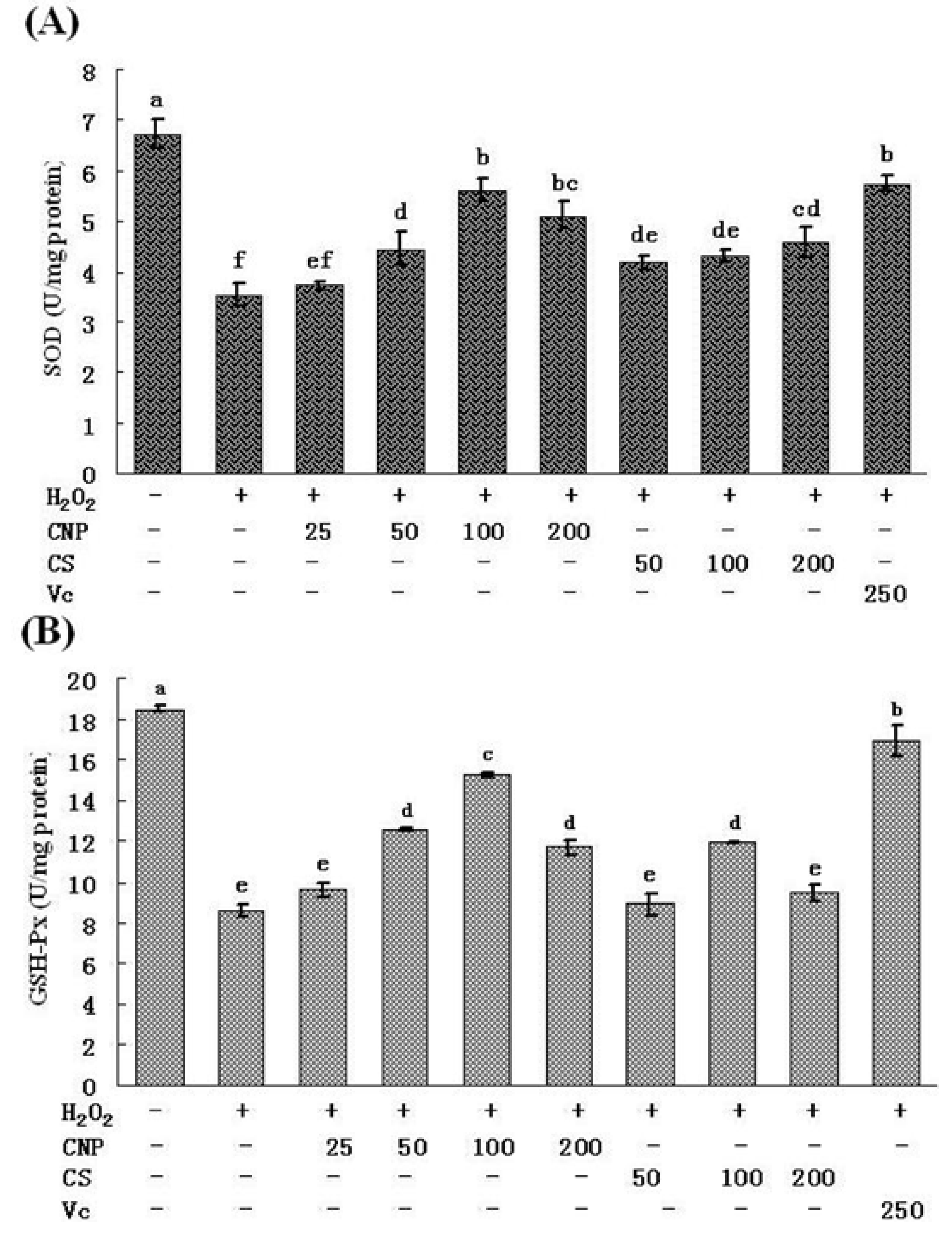
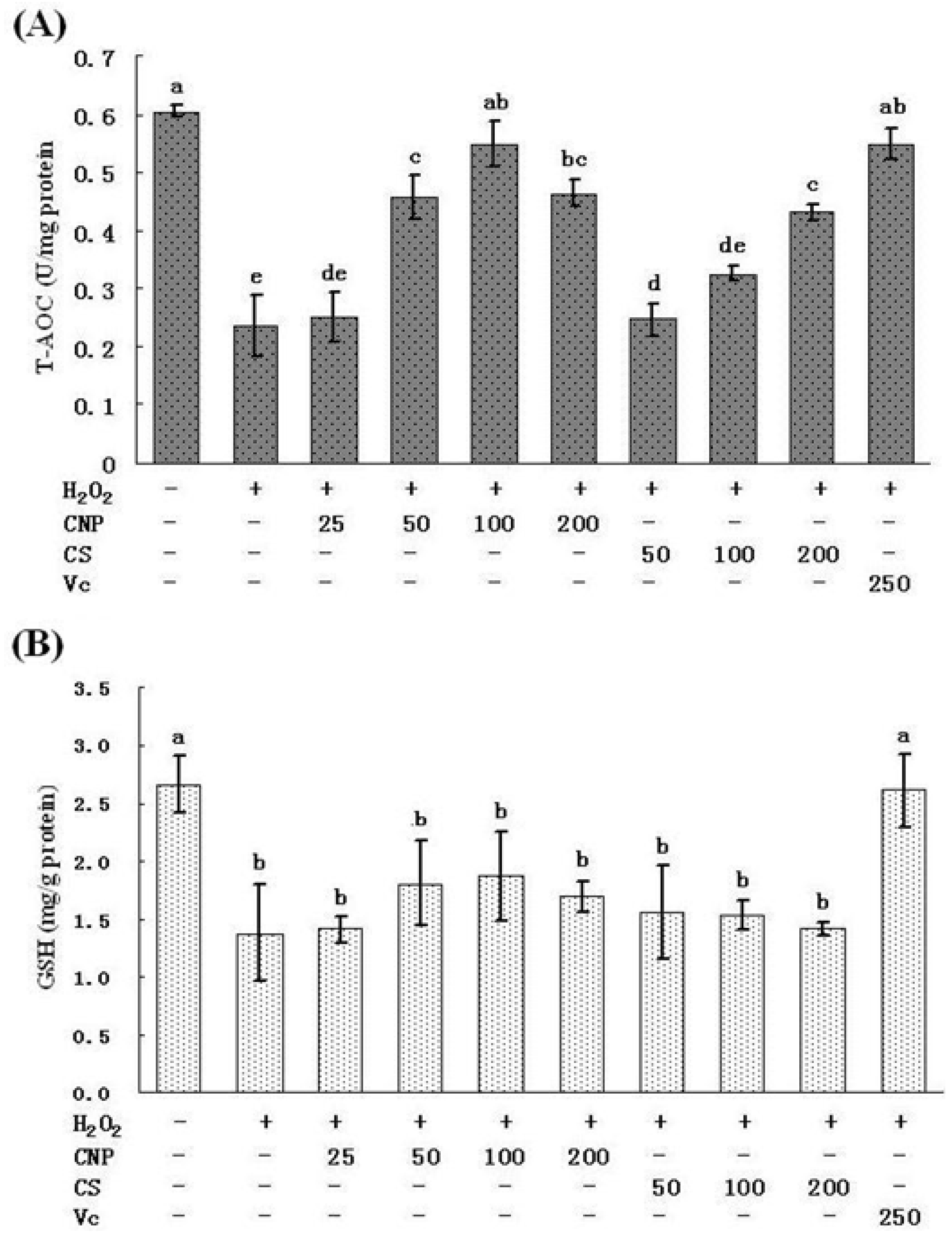
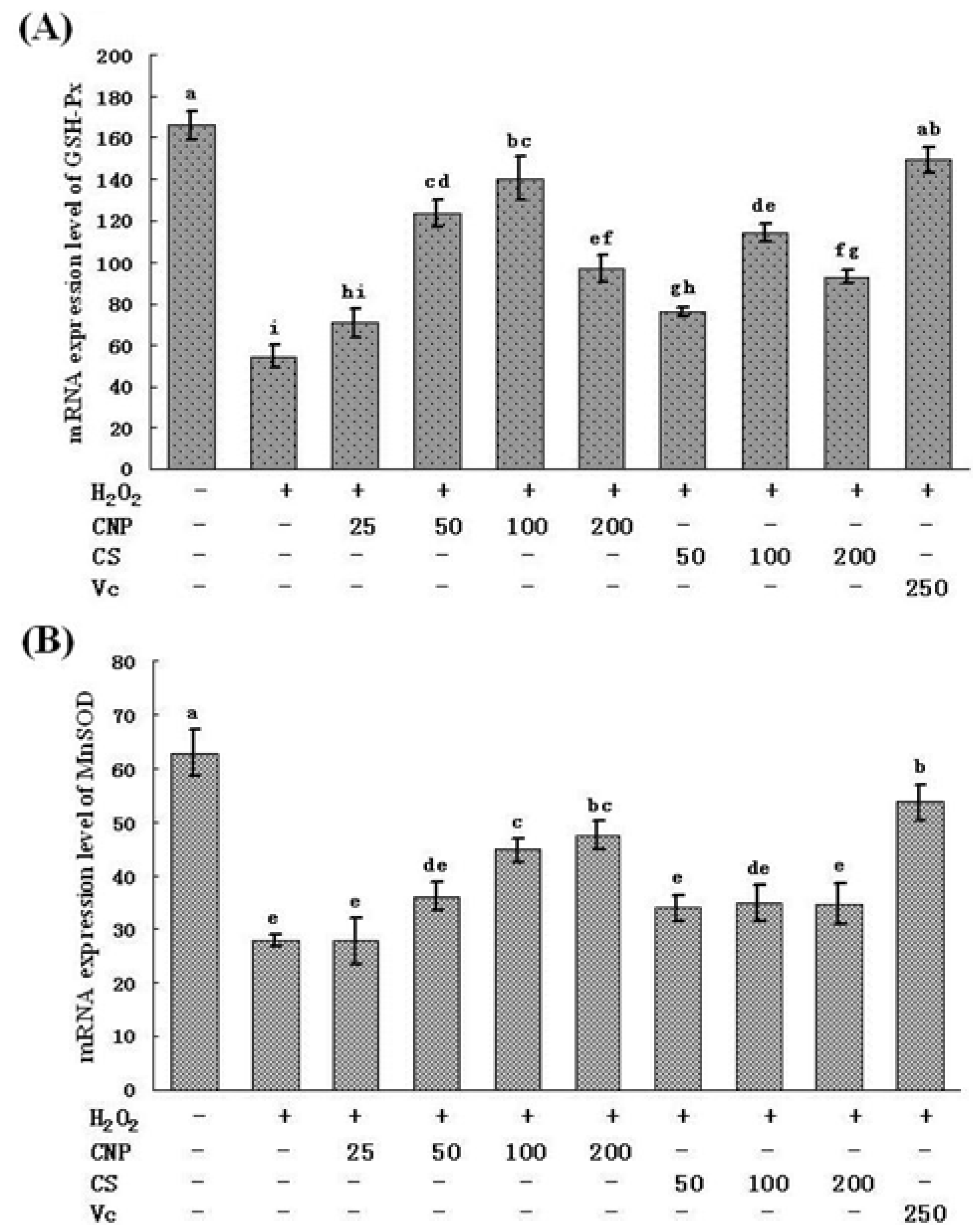
2.6. Detection of Nitric Oxide (NO) Release in Cell Culture Medium as Well as MDA Content
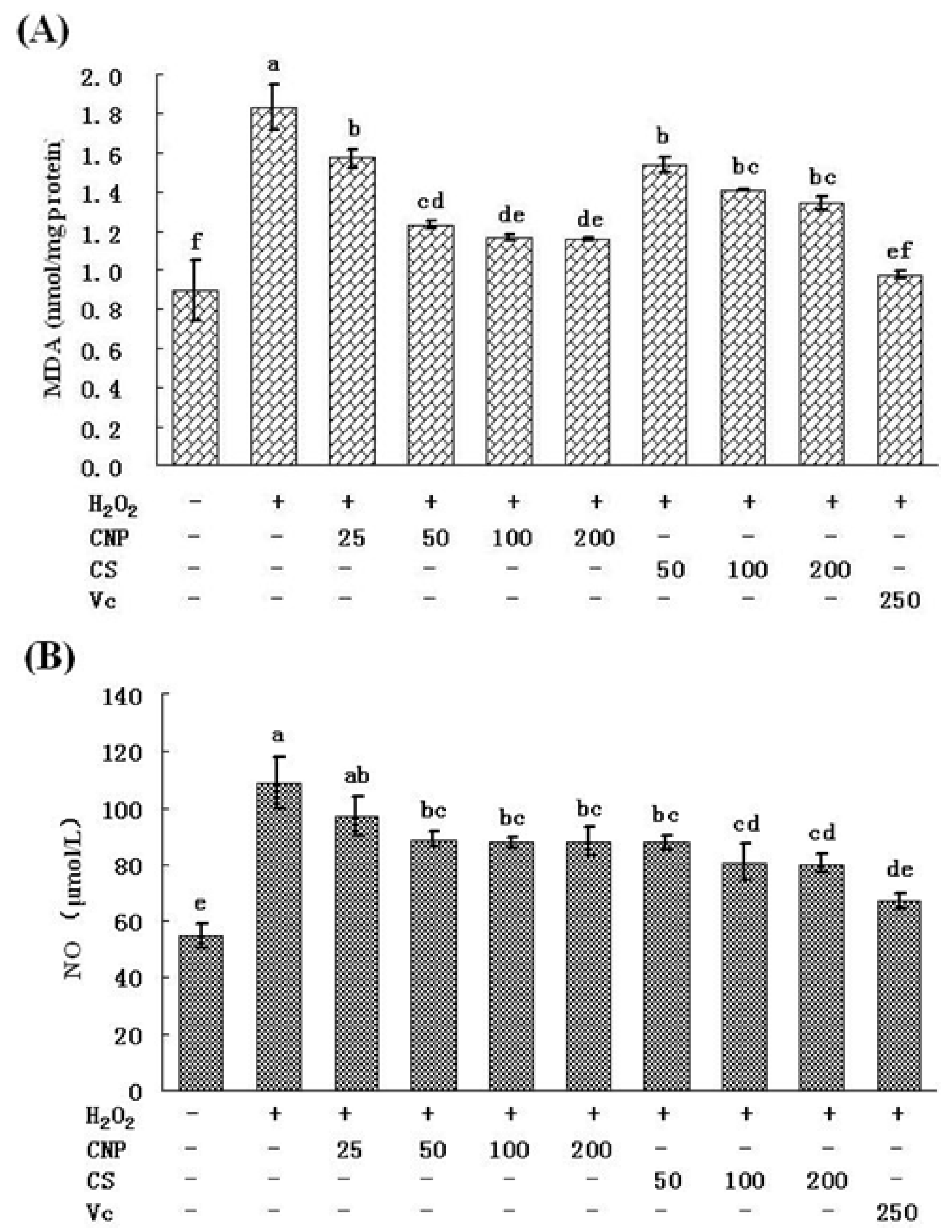
3. Discussion
4. Experimental Section
4.1. Chemicals and Reagents
4.2. Cell Culture and Treatment
4.3. Preparation and Characterization of CNP
4.4. Cell Viability Measurement
4.5. Morphology of RAW264.7 Cells
4.6. Preparation of Cell Lysates
4.7. Measurement of LDH and Nitric Oxide (NO) Release
4.8. Assay for Intracellular Contents of SOD, GSH-Px, and MDA
4.9. Measurement of the mRNA Expression Levels of MnSOD and GSH-Px by Real-Time PCR
| Gene | Genbank Accession | Primer sequence | Product size (bp) | Annealing (°C) |
|---|---|---|---|---|
| GSH-Px | NM_008160 | 5′ ACAGTCCACCGTGTATGCCTTC 3′ | 238 | 60 |
| 5′ CTCTTCATTCTTGCCATTCTCCTG 3′ | ||||
| MnSOD | X04972 | 5′ CTGTGGGAGTCCAAGGTTCA 3′ | 79 | 60 |
| 5′ GAGCAGGCAGCAATCTGTAAG 3′ | ||||
| 18S | NR_003278 | 5′ CGGACACGGACAGGATTGACA 3′ | 94 | 62 |
| 5′ CCAGACAAATCGCTCCACCAACTA 3′ |
4.10. Effect of CNP on the Antioxidant Capacity of H2O2-Induced RAW264.7 Cells
4.11. Statistical Analysis
5. Conclusions
Acknowledgments
Conflict of Interest
References
- Butterfield, D.A.; Abdul, H.M.; Opii, W.; Newman, S.F.; Joshi, G.; Ansari, M.A.; Sultana, R. Pin1 in Alzheimer’s disease. J. Neurochem. 2006, 98, 1697–1706. [Google Scholar] [CrossRef]
- Dhalla, N.S.; Temsah, R.M.; Netticadan, T. Role of oxidative stress in cardiovascular diseases. J. Hypertens. 2000, 18, 655–673. [Google Scholar] [CrossRef]
- Seven, A.; Guzel, S.; Aslan, M.; Hamuryudan, V. Lipid, protein, DNA oxidation and antioxidant status in rheumatoid arthritis. Clin. Biochem. 2008, 41, 538–543. [Google Scholar] [CrossRef]
- Suzuki, K.; Mikami, T.; Okawa, Y.; Tokoro, T.; Suzuki, S.; Suzuki, M. Antitumor effect of hexa-N-acetylchitohexaose and chitohexaose. Carbohyd. Polym. 1986, 151, 403–408. [Google Scholar]
- Jeon, Y.J.; Kim, S.K. Potential immuno-stimulating effect of antitumoral fraction of chitosan oligosaccharides. J. Chtin Chitosan 2001, 6, 163–167. [Google Scholar]
- Schipper, N.G.; Varum, M.K.M.; Stenberg, P.; Cklind, G.O.; Lennernas, H.; Artursson, P. Chitosans as absorption enhancers of poorly absorbable drugs: 3. Influence of mucus on absorption enhancement. Eur. J. Pharm. Sci. 1999, 8, 335–343. [Google Scholar] [CrossRef]
- Park, P.J.; Je, J.Y.; Byun, H.G.; Moon, S.H.; Kim, S.K. Antimicrobial activity of hetero-chitosans and their oligosaccharides with different molecular weights. J. Mol. Microb. Biotechnol. 2004, 14, 317–323. [Google Scholar]
- Porporatto, C.; Bianco, I.D.; Riera, C.M.; Correa, S.G. Chitosan induces different l-arginine metabolic pathways in resting and inflammatory macrophages. Biochem. Biophys. Res. Commun. 2003, 304, 266–272. [Google Scholar] [CrossRef]
- Anraku, M.; Kabashima, M.; Namura, H.; Maruyama, T.; Otagiri, M.; Gebicki, J.M.; Furutani, N.; Tomida, H. Antioxidant protection of human serum albumin by chitosan. Int. J. Biol. Macromol. 2008, 43, 159–164. [Google Scholar] [CrossRef]
- Xie, W.; Xu, P.; Liu, Q. Antioxidant activity of watersoluble chitosan derivatives. Bioorg. Med. Chem. Lett. 2001, 11, 1699–1701. [Google Scholar] [CrossRef]
- Santhosh, S.; Sini, T.K.; Anandan, R.; Mathew, P.T. Effect of chitosan supplementation on antitubercular drugs-induced hepatotoxicity in rats. Toxicology 2006, 219, 53–59. [Google Scholar] [CrossRef]
- Yoon, H.J.; Moon, M.E.; Park, H.S.; Kim, H.W.; Im, S.Y.; Lee, J.H.; Kim, Y.H. Effects of chitosan oligosaccharide (COS) on the glycerol-induced acute renal failure in vitro and in vivo. Food Chem. Toxicol. 2008, 46, 710–716. [Google Scholar] [CrossRef]
- Liu, J.N. Study on the hypolipidemic mechanism of chitosan. Ph.D. Thesis, Jiangnan University, Wuxi, China, 2008. [Google Scholar]
- Wen, Z.S.; Xu, Y.L.; Zou, X.T.; Xu, Z.R. Chitosan nanoparticles act as an adjuvant to promote both Th1 and Th2 immune responses induced by ovalbumin in mice. Mar. Drugs 2011, 9, 1038–1055. [Google Scholar] [CrossRef]
- Qi, L.; Xu, Z.; Chen, M. In vitro and in vivo suppression of hepatocellular carcinoma growth by chitosan nanoparticles. Eur. J. Cancer 2007, 43, 184–193. [Google Scholar] [CrossRef]
- Qi, L.; Xu, Z.; Jiang, X.; Hu, C.; Zou, X. Preparation and antibacterial activity of chitosan nanoparticles. Carbohyd. Res. 2004, 339, 2693–2700. [Google Scholar]
- Droge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar]
- Griendling, K.K.; Sorescu, D.; Lassegue, B.; Ushino-Fukai, M. Modulation of protein kinase activity and gene expression by reactive oxygen species and their role in vascular physiology and pathophysiology. Arterioscl. Throm. Vas. 2000, 20, 2175–2183. [Google Scholar] [CrossRef]
- Kang, K.B.; Lee, K.H.; Chae, S.W.; Zhang, R.; Jung, M.S.; Lee, Y.K.; Kim, S.Y.; Kim, H.S.; Joo, H.G.; Park, J.W.; et al. Eckol isolated from Ecklonia cava attenuates oxidative stress induced cell damage in lung fibroblast cells. FEBS Lett. 2005, 579, 6295–6304. [Google Scholar] [CrossRef]
- Calabrese, V.; Lodi, R.; Tonon, C.; D’Agata, V.; Sapienza, M.; Scapagnini, G.; Mangiameli, A.; Pennisi, G.; Giuffrida Stella, A.M.; Butterfield, D.A. Oxidative stress, mitochondrial dysfunction and cellular stress response in Friedreich’s ataxia. J. Neurol. Sci. 2005, 233, 145–162. [Google Scholar] [CrossRef]
- Je, J.Y.; Park, P.J.; Kim, S.K. Free radical scavenging properties of hetero-chitooligosaccharides using an ESR spectroscopy. Food Chem. Toxicol. 2004, 42, 381–387. [Google Scholar] [CrossRef]
- Vajragupta, O.; Boonchoong, P.; Wongkrajang, Y. Comparative quantitative structure activity study of radical scavengers. Bioorgan. Med. Chem. 2000, 8, 2617–2628. [Google Scholar] [CrossRef]
- Huang, M.; Khor, E.; Lim, L.Y. Uptake and cytotoxicity of chitosan molecules and nanoparticles: effects of molecular weight and degree of deacetylation. Pharm. Res. 2004, 21, 344–353. [Google Scholar] [CrossRef]
- Anraku, M.; Fujii, T.; Furutani, N.; Kadowaki, D.; Maruyama, T.; Otagiri, M.; Gebicki, J.M.; Tomida, H. Antioxidant effects of a dietary supplement: Reduction of indices of oxidative stress in normal subjects by water-soluble chitosan. Food Chem. Toxicol. 2009, 47, 104–109. [Google Scholar] [CrossRef]
- Khodagholi, F.; Eftekharzadeh, B.; Maghsoudi, N.; Rezaei, P.F. Chitosan prevents oxidative stress-induced amyloid β formation and cytotoxicity in NT2 neurons: involvement of transcription factors Nrf2 and NF-κB. Mol. Cell. Biochem. 2010, 337, 39–51. [Google Scholar] [CrossRef]
- Anraku, M.; Fujii, T.; Kondo, Y.; Kojima, E.; Hata, T.; Tabuchi, N.; Tsuchiya, D.; Goromaru, T.; Tsutsumi, H.; Kadowaki, D.; et al. Antioxidant properties of high molecular weight dietary chitosan in vitro and in vivo. Carbohyd. Polym. 2011, 83, 501–505. [Google Scholar] [CrossRef]
- Maeng, O.; Kim, Y.C.; Shin, H.I.; Lee, J.O.; Huh, T.L.; Kang, K.I.; Kim, Y.S.; Paik, S.G.; Lee, H. Cytosolic NADP(+)-dependent isocitrate dehydrogenase protects macrophages from LPS-induced nitric oxide and reactive oxygen species. Biochem. Biophys. Res. Commun. 2004, 317, 558–564. [Google Scholar] [CrossRef]
- Thoeni, G.; Werner, E.R.; Werner-Felmayer, G. Tetrahydropteridines suppress gene expression and induce apoptosis of activated RAW264.7 cells via formation of hydrogen peroxide. Free Radical Biol. Med. 2004, 37, 375–385. [Google Scholar] [CrossRef]
- Gupta, A.; Singh, S.; Jamal, F.; Nath, S.; Mehrotra, S.; Sharma, B. Synergistic effects of glutathione and Vitamin E on ROS mediated ethanol toxicity in isolated rat hepatocytes. Asian J. Biochem. 2011, 6, 347–356. [Google Scholar] [CrossRef]
- Shi, G.F.; An, L.J.; Jiang, B.; Guan, S.; Bao, Y.M. Alpinia protocatechuic acid protects against oxidative damage in vitro and reduces oxidative stress in vivo. Neurosci. Lett. 2006, 403, 206–210. [Google Scholar] [CrossRef]
- Aguilar, A.; Alvarez-Vijande, R.; Capdevila, S.; Alcoberro, J.; Alcaraz, A. Antioxidant patterns (superoxide dismutase, glutathione reductase and glutathione peroxidase) in kidneys from non-heart-beating-donors: experimental study. Transplant Proc. 2007, 39, 249–252. [Google Scholar] [CrossRef]
- Cini, M.; Fariello, R.G.; Bianchetti, A.; Moretti, A. Studies on lipid peroxidation in the rat brain. Neurochem. Res. 1994, 19, 283–288. [Google Scholar] [CrossRef]
- Surapaneni, K.M.; Venkataramana, G. Status of lipid peroxidation, glutathione, ascorbic acid, vitamin E and antioxidant enzymes in patients with osteoarthritis. Indian J. Med. Sci. 2007, 61, 9–14. [Google Scholar] [CrossRef]
- Luo, T.; Xia, Z. A small dose of hydrogen peroxide enhances tumor necrosis factor- alpha toxicity in inducing human vascular endothelial cell apoptosis: reversal with propofol. Anesth. Analg. 2006, 103, 110–116. [Google Scholar] [CrossRef]
- Valdivia, A.; Pérez-Álvarez, S.; Aroca-Aguilar, J.D.; Ikuta, I.; Jordán, J. Superoxide dismutases: a physiopharmacological update. J. Physiol. Biochem. 2009, 65, 195–208. [Google Scholar] [CrossRef]
- Brown, G.C. Nitric oxide and neuronal death. Nitric Oxide 2010, 23, 153–165. [Google Scholar] [CrossRef]
- Brown, G.C. Reversible binding and inhibition of catalase by nitric oxide. Eur. J. Biochem. 1995, 232, 188–191. [Google Scholar] [CrossRef]
- Savvides, S.N.; Scheiwein, M.; Bohme, C.C.; Arteel, G.E.; Karplus, P.A.; Becker, K.; Schirmer, R.H. Crystal structure of the antioxidant enzyme glutathione reductase inactivated by peroxynitrite. J. Biol. Chem. 2002, 277, 2779–2784. [Google Scholar]
- McBride, A.G.; Borutaite, V.; Brown, G.C. Superoxide dismutase and hydrogen peroxide cause rapid nitric oxide breakdown, peroxynitrite production and subsequent cell death. Biochim. Biophys. Acta 1999, 1454, 275–288. [Google Scholar]
- Thomas, D.D.; Ridnour, L.A.; Espey, M.G.; Donzelli, S.; Ambs, S.; Hussain, S.P.; Harris, C.C.; DeGraff, W.; Roberts, D.D.; Mitchell, J.B.; et al. Superoxide fluxes limit nitric oxide-induced signaling. J. Biol. Chem. 2006, 281, 25984–25993. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCt Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wen, Z.-S.; Liu, L.-J.; Qu, Y.-L.; OuYang, X.-K.; Yang, L.-Y.; Xu, Z.-R. Chitosan Nanoparticles Attenuate Hydrogen Peroxide-Induced Stress Injury in Mouse Macrophage RAW264.7 Cells. Mar. Drugs 2013, 11, 3582-3600. https://doi.org/10.3390/md11103582
Wen Z-S, Liu L-J, Qu Y-L, OuYang X-K, Yang L-Y, Xu Z-R. Chitosan Nanoparticles Attenuate Hydrogen Peroxide-Induced Stress Injury in Mouse Macrophage RAW264.7 Cells. Marine Drugs. 2013; 11(10):3582-3600. https://doi.org/10.3390/md11103582
Chicago/Turabian StyleWen, Zheng-Shun, Li-Jia Liu, You-Le Qu, Xiao-Kun OuYang, Li-Ye Yang, and Zi-Rong Xu. 2013. "Chitosan Nanoparticles Attenuate Hydrogen Peroxide-Induced Stress Injury in Mouse Macrophage RAW264.7 Cells" Marine Drugs 11, no. 10: 3582-3600. https://doi.org/10.3390/md11103582
APA StyleWen, Z.-S., Liu, L.-J., Qu, Y.-L., OuYang, X.-K., Yang, L.-Y., & Xu, Z.-R. (2013). Chitosan Nanoparticles Attenuate Hydrogen Peroxide-Induced Stress Injury in Mouse Macrophage RAW264.7 Cells. Marine Drugs, 11(10), 3582-3600. https://doi.org/10.3390/md11103582





