Abstract
Five new cembrane diterpenoids, named sinuflexibilins A–E (1–5), along with nine other known diterpenoids (6–14), have been isolated from the organic extract of a Hainan soft coral Sinularia sp. Their structures were determined on the basis of extensive spectroscopic analyses and by comparison of their spectral data with those of related metabolites. Compound 13, flexibilide, exhibited significant inhibitory activity of NF-κB activation using the cell-based HEK293 NF-κB luciferase reporter gene assay.
1. Introduction
Cembrane diterpenoids and their cyclized derivatives are the most abundant secondary metabolites of soft corals and gorgonians [1,2,3]. There is a wide range of structural complexity within this series. These cembranes represent the main chemical defense tools of animals against their natural predators such as other corals, and fishes, as well as the settlement of microorganisms [4,5]. In addition, cembranes also exhibit a wide range of biological activities including anti-inflammatory [6,7,8], and antitumor properties [9,10].
Genus Sinularia is one of the most widely distributed soft corals. It constitutes a dominant portion of the biomass in the tropical reef environment. Sinularia elaborates a rich harvest of secondary metabolites, including sesquiterpenes, diterpenoids, polyhydroxylated steroids, and polyamine compounds [11,12,13,14]. These metabolites were recently shown to possess a range of biological activities [15]. Cembranes are the most frequent secondary metabolites isolated from various Sinularia species [16,17,18].
Nuclear factor-kappa B (NF-κB) is a protein complex that controls the transcription of DNA. NF-κB plays a key role in regulating the immune response to infection. Incorrect regulation of NF-κB has been linked to cancer, inflammatory and autoimmune diseases, septic shock, viral infection, and improper immune development [19]. Our recent investigation of bioactive natural products from a Hainan soft coral, Sinularia sp., has led to the isolation of five new cembranes (1–5), along with nine other known diterpenoids (6–14) (Figure 1). In this paper, we report the isolation and structural elucidation of these diterpenoids and their activities as inhibitors of NF-κB.
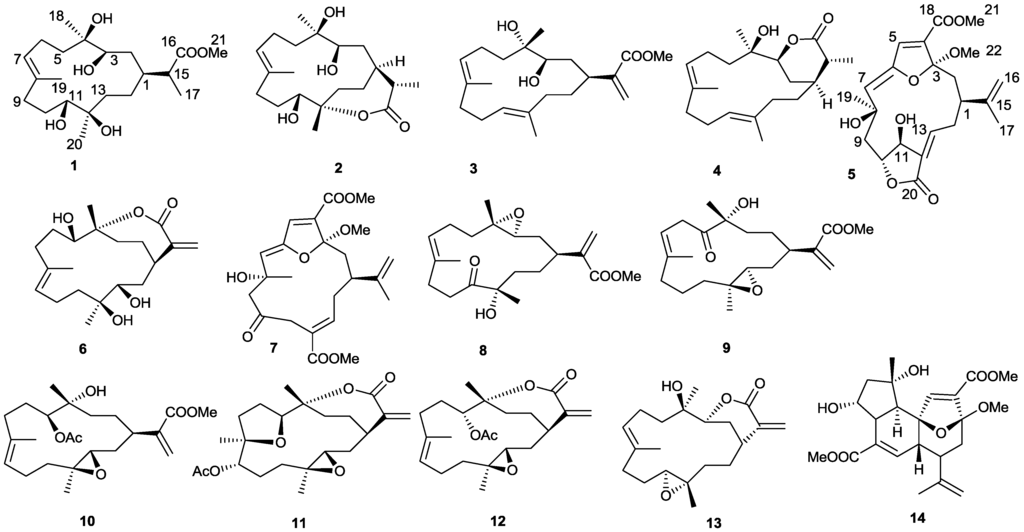
Figure 1.
Structures of compounds 1–14 from Sinularia sp.
2. Results and Discussion
Compound 1 was isolated as a colorless oil. The HRESIMS of 1 established its molecular formula as C21H38O6, indicating three unsaturations. Resonances due to olefinic carbons (δC 133.9, 128.7), and one carboxyl (δC 178.9) in the 13C NMR spectrum accounted for two double-bond equivalents, indicating that 1 was a monocyclic compound (Table 1). Signals for a vinyl methyl at δ 1.68 (3H, s), one methoxy group at δ 3.68 (3H, s), a methyl doublet at δ 1.17 (3H, d, J = 7.0 Hz), and two tertiary oxygenated methyl groups at δ 1.15 (3H, s), and 1.08 (3H, s) were observed in the 1H NMR spectrum (Table 2). Carbon signals at δ 70.6, 71.0, 75.3, and 75.7, and two proton signals at δ 3.52, and 3.65 indicated the presence of two secondary and two tertiary hydroxyl groups. A signal at δ 5.43 attributed to an olefinic proton, together with a methyl carbon signal at δ 17.0, indicated the E configuration for this double bond. These data suggested that 1 was a rearranged cembrane. Key HMBC correlations from H3-20 to C-13, C-12, and C-11; H-11 to C-12, C-10, and C-20; H3-19 to C-7, C-8, and C-9; H-7 to C-9, C-6, and C-19; H3-18 to C-5, C-4, and C-3; and H-13 to C-14, and C-1 established the 14-membered ring structure of 1 (Figure 2). The isopropyl acid group was found based on the HMBC correlations observed from H3-21 to C-1, C-15, and C-16; H-15 to C-1, C-2, C-21, and C-16. Furthermore, the methoxyl substituent was shown to be connected to position C-16 on the basis of the HMBC correlation between the oxymethyl protons (δH 3.68) and the carbonyl carbon (δC 178.9). The NMR spectra of compound 1 were almost identical with those of sinuflexibilin [20] with the exception that the exo-methylene proton resonances of the latter were replaced by a methyl doublet. The stereochemistry of 1 was determined on the basis of the chemical shift and NOESY spectrum (Figure 3). NOE correlations from H-3 to H-1, H-11, H3-18 and from H-11 to H-20 indicated that all four hydroxy groups in 1 were β-oriented and H-1, H-3, H-11, H3-18, and CH3-20 were α-oriented with respect to this ring.

Table 1.
13C NMR (125 MHz) data for compounds 1–5 in CDCl3.
| Position | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 1 | 38.4 CH | 34.8 CH | 39.4 CH | 36.4 CH | 40.9 CH |
| 2 | 33.3 CH2 | 37.2 CH2 | 35.1 CH2 | 36.6 CH2 | 38.3 CH2 |
| 3 | 71.0 CH | 74.2 CH | 71.7 CH | 84.3 CH | 116.6 C |
| 4 | 75.7 C | 76.3 C | 75.2 C | 74.3 C | 131.1 C |
| 5 | 39.4 CH2 | 39.9 CH2 | 35.2 CH2 | 37.8 CH2 | 139.5 CH |
| 6 | 24.1 CH2 | 23.7 CH2 | 25.3 CH2 | 23.9 CH2 | 150.0 C |
| 7 | 128.7 CH | 128.9 CH | 124.1 CH | 124.6 CH | 117.2 CH |
| 8 | 133.9 C | 135.4 C | 135.9 C | 134.5 C | 71.5 C |
| 9 | 35.6 CH2 | 37.5 CH2 | 39.5 CH2 | 39.4 CH2 | 40.9 CH2 |
| 10 | 27.9 CH2 | 28.8 CH2 | 26.4 CH2 | 22.4 CH2 | 81.9 CH |
| 11 | 70.6 CH | 68.6 CH | 126.1 CH | 126.5 CH | 75.4 CH |
| 12 | 75.3 C | 88.7 C | 134.1 C | 132.3 C | 131.5 C |
| 13 | 34.9 CH2 | 33.2 CH2 | 34.7 CH2 | 26.8 CH2 | 145.4 CH |
| 14 | 22.2 CH2 | 26.5 CH2 | 28.2 CH2 | 30.4 CH2 | 32.5 CH2 |
| 15 | 44.5 CH | 42.2 CH2 | 144.3 C | 42.0 CH | 147.3 C |
| 16 | 178.9 C | 181.5 C | 168.8 C | 175.1 C | 112.9 CH2 |
| 17 | 15.2 CH3 | 11.0 CH3 | 124.3 CH2 | 16.3 CH3 | 18.4 CH3 |
| 18 | 23.6 CH3 | 24.0 CH3 | 23.4 CH3 | 24.8 CH3 | 162.2 C |
| 19 | 17.0 CH3 | 15.8 CH3 | 16.4 CH3 | 14.1 CH3 | 30.3 CH3 |
| 20 | 24.1 CH3 | 23.1 CH3 | 15.6 CH3 | 15.2 CH3 | 168.1 C |
| 21 | 51.9 CH3 | 52.0 CH3 | 51.9 CH3 | ||
| 22 | 50.2 CH3 |

Table 2.
1H NMR (500 MHz) data for compounds 1–5 in CDCl3, δ in ppm and J in Hz.
| Position | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 1 | 1.98 m | 1.96 m | 2.90 m | 1.30 m | 2.27 m |
| 2 | 1.64 m | 1.87 m | 2.05 m | 2.12 m | 2.48 dd (8.0, 6.0) |
| 1.19 m | 1.23 m | 1.42 m | 1.87 d (14.0) | ||
| 3 | 3.65 d (10.5) | 3.36 m | 3.67 m | 4.03 d (10.5) | |
| 5 | 2.02 m | 1.82 m | 2.26 m | 1.75 m | 7.02 s |
| 1.57 m | 1.42 m | 1.48 m | 1.65 m | ||
| 6 | 2.32 m | 2.10 m | 2.32 m | 2.26 m | |
| 2.40 m | 1.84 m | 2.12 m | 2.14 m | ||
| 7 | 5.43 t (6.5) | 5.12 d (8.0) | 5.11 m | 5.06 t (8.0) | 5.03 s |
| 9 | 2.18 m | 2.20 m | 2.18 m | 2.19 m | 3.02 m |
| 2.09 m | 1.98 m | 1.94 dd (5.5, 9.0) | |||
| 10 | 1.81 m | 1.98 m | 1.68 m | 1.89 m | 4.75 dd (5.5, 6.0) |
| 1.45 m | 1.42 m | 1.30 m | |||
| 11 | 3.52 d (10.0) | 4.17 d (7.5) | 5.11 m | 5.12 t (7.0) | 4.38 s |
| 13 | 1.15 m | 2.14 m | 1.95 m | 2.09 m | 6.72 t (7.5) |
| 1.65 m | 1.69 m | 1.28 m | |||
| 14 | 1.47 m | 1.90 m | 1.25 m | 1.77 m | 2.77 m |
| 1.35 m | 1.14 m | 2.16 m | |||
| 15 | 2.43 m | 2.92 m | 2.09 m | ||
| 16 | 6.25 s | 4.70 s | |||
| 5.56 s | 4.67 s | ||||
| 17 | 1.17 d (7.0) | 1.28 d (7.5) | 1.32 d (7.0) | 1.64 s | |
| 18 | 1.08 s | 1.25 s | 1.04 s | 1.39 s | |
| 19 | 1.68 s | 1.51 s | 1.58 s | 1.56 s | 1.39 s |
| 20 | 1.15 s | 1.23 s | 1.60 s | 1.56 s | |
| 21 | 3.68 s | 3.76 s | 3.69 s | ||
| 22 | 3.06 s |
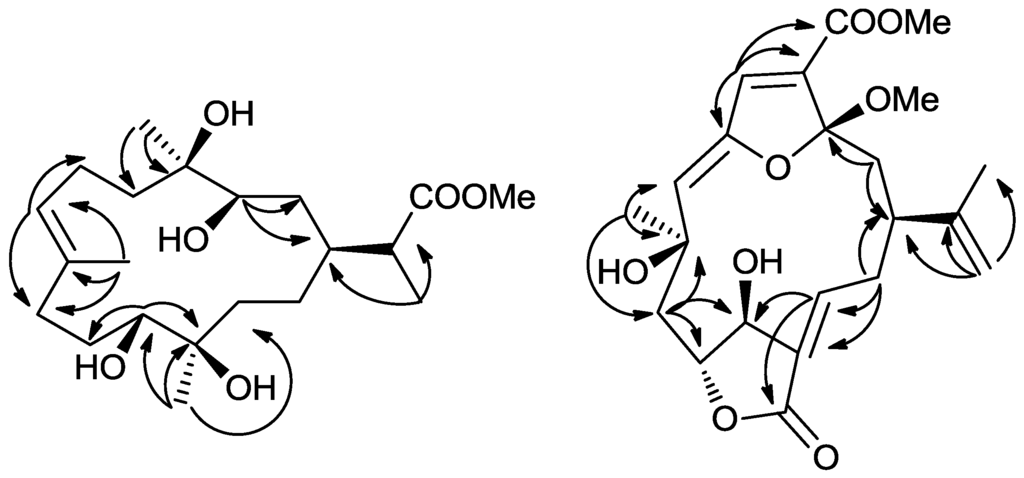
Figure 2.
Key HMBC correlations 1 and 5.
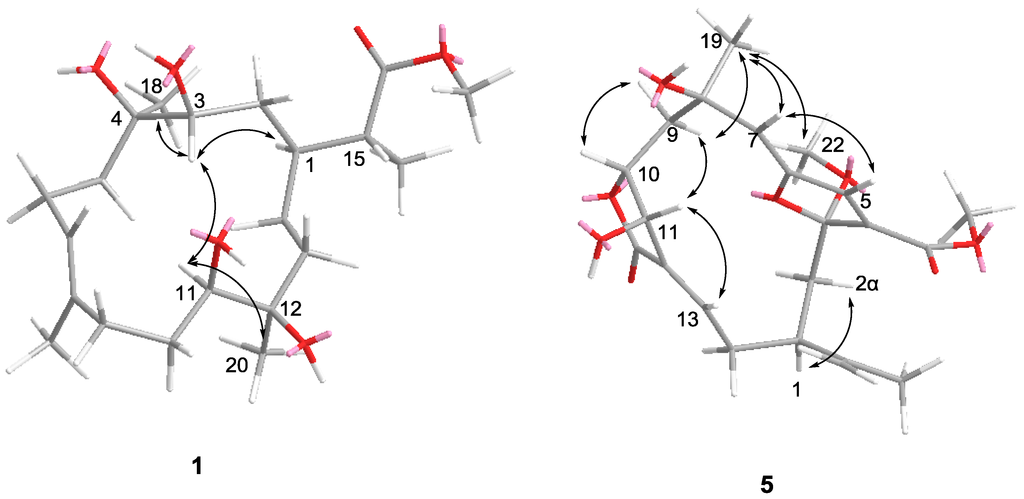
Figure 3.
Key NOE correlations 1 and 5.
Compound 2 was isolated as a colorless oil. The HRESIMS of 2 established its molecular formula as C20H34O5, indicating four unsaturations. The 1H and 13C NMR spectra of 2 were similar to those of capillolide [21], with the exception that the exo-methylene proton resonances of the latter were replaced by a methyl doublet at δ 1.28 (3H, d, J = 7.5 Hz) coupled to a signal at δ 2.92. The relative stereochemistry of 2 was deduced mainly by NOESY and by comparison with that found for capillolide. Both H-3 and H-11 showed NOEs with H-1, which further correlated with H-15. Moreover, H3-18 shared mutual NOE enhancement with H-3 and H-15. These observations indicated that all H-1, H-3, H-11, H3-18, and H-15 were α-oriented with respect to this ring. Furthermore, it was found that the H3-20 did not exhibit NOE response with H-11, indicating the β-configuration.
Compound 3 was isolated as a colorless oil. The HRESIMS of 3 established its molecular formula as C21H34O4, indicating five unsaturations. The 1H and 13C NMR spectra of 3 were similar to those of pseudoplexauric acid methyl ester except for the downfield shifts of C-3 (62.8→71.7), C-4 (60.7→75.2), and C-18 (16.9→23.4) (Table 1) [22], which indicated that two hydroxylated carbons replaced the 3,4-epoxy carbons of the known analogue. This was also supported by the molecular weight of 3, which was 18 amu higher than that of pseudoplexauric acid methyl ester, as indicated by the HRESIMS data. In the NOESY spectrum of 3, H-3 showed NOE with H-1, but not with H-18, justifying the assigned relative stereochemistry at C-1, C-3, and C-4.
Compound 4 was isolated as a colorless oil. The HRESIMS of 4 established its molecular formula as C20H32O3, indicating five unsaturations. The 1H and 13C NMR spectra of 4 were similar to those of 14-deoxycrassin [22,23], with the exception that the exo-methylene proton resonances of the latter were replaced by a methyl doublet at δ 1.32 (3H, d, J = 7.0 Hz) coupled to a signal at δ 2.09, which was confirmed by the HMBC experiment. Analyses of NOESY and NMR data revealed that the relative stereochemistry of 4 was the same as 14-deoxycrassin. The relative configuration of the secondary methyl group at C-15 was assigned to be on the same side as the H-1 proton of the δ-lactone ring by comparison of 1H NMR spectral data with those of dihydrosinularin [δ 1.35 (3H, d, J = 7 Hz, H3-17), 2.2 (1H, m, H-15)] and its 15-epimer [δ 1.21 (3H, H3-17), 2.80 (1H, m, H-15)] [20,24].
Compound 5 was isolated as a colorless oil. The HRESIMS of 5 established its molecular formula as C22H28O8, indicating nine unsaturations. The 1H and 13C NMR spectra of 5 showed great similarity to those of 3α-ethoxyfuranocembrane [25] except that the ethoxyl was replaced by a methoxyl at C-3, and the acetoxy group was replaced by a hydroxy at C-11. The determination of the structure of 5 was further supported by detailed analysis of its 2D NMR data (Figure 2). The relative configuration of 5 was deduced by a NOESY experiment (Figure 3) and by comparison with those of 3α-ethoxyfuranocembranoid [25]. One proton attaching at C-2 and resonating at δH 2.48 was found to show NOE interactions with H-1 and was assigned arbitrarily as H-2α. The isopropenyl group located at C-1 should be β-oriented. NOE correlations between H-5 and H-7, H-7 and H-19 revealed the α-orientation of Me-19, and the Z configuration of the double bond Δ6(7). The NOE interaction between H3-19 and H3-22 indicated the α-orientation of C-3. The trans-arrangement of H-10, and H-11 was implied by the coupling constant J (10, 11) ≈ 0 Hz. The NOE interaction between H-11 and H-13 indicated the Z configuration of the double bond at Δ12(13) [25]. The 3α-ethoxyfuranocembranoid is an artifact created during isolation by reaction of the solvent ethanol with the natural product danielid [25]. However the danielid and its analogues have not been isolated in our investigation from Sinularia sp. In this context, whether compound 5 is created from danielid or its analogues remains to be established.
The identities of compounds 6–14 were established by comparison of their spectral data with those of the known compounds reported. They are capilloloid (6) [21], sethukarailin (7) [26], sinuladiterpenes I (8) [27], sinulaflexiolides H (9) [28], flexibilisin A (10) [29], (1R,13S,12S,9S,8R,5S,4R)-9-acetoxy-5,8:12,13-diepoxycembr-15(17)-en-16,4-olide (11) [21], 11-epi-sinulariolide acetate (12) [30], flexibilide (13) [21], mandapamate (14) [31].
Compounds 1–14 were evaluated for inhibition of NF-κB activation using the cell-based HEK293 NF-κB luciferase reporter gene assay. The results showed that only 13 exhibited a potent effect with IC50 value of 5.30 μg/mL, while other compounds showed only marginal effects.
3. Experimental Section
3.1.General Experimental Procedures
The NMR spectra were recorded on a Bruker AC 500 NMR spectrometer with TMS as an internal standard. HR-ESI-MS data were measured on an AQUITY UPLC/Q-TOF micro spectrometer. IR spectra were recorded on a Nicolet 6700 FT-IR spectrometer. Optical rotations were measured on a PerKin Elmer 341 polarimeter using a 1 dm path length cell. ESI-MS data were measured on Bruker’s amaZon SL ion trap LC/MS. Materials for column chromatography were silica gel (Qingdao Marine Chemical Factory, Qingdao, China), Sephadex LH20 (Amersham Pharmacia Biotech AB, Uppsala, Sweden), and YMC Gel ODS-A (YMC, MA, USA). The silica gel GF254 used for TLC was supplied by the Qingdao Marine Chemical Factory, Qingdao, China. HPLC was carried out on SHIMEDZU LC-10ATvp with YMC ODS SERIES.
3.2. Animal Material
The soft coral Sinularia sp. was collected from Dongluo Island, Hainan province of China in March 2010 (7–10 m depth) and identified by Professor Hui Huang, South China Sea Institute of Oceanology, Chinese Academy of Sciences. A voucher specimen (No. M100301) was deposited in the Key Laboratory of Marine Bio-resources Sustainable Utilization, South China Sea Institute of Oceanology, Chinese Academy of Sciences, Guangzhou, China.
3.3. Extraction and Isolation
The soft coral Sinularia sp. (7 kg) was extracted three times with 95% EtOH. The extract was concentrated under reduced pressure, and partitioned between H2O (4 L) and CHCl3 (4 L); the CHCl3 layer (101 g) was further partitioned between 85% EtOH (4 L) and petroleum ether (PE; 4 L) to yield 85% EtOH (30 g) and PE (55.3 g) fractions.
The PE extract was subjected to silica gel column chromatography, using a gradient of EtOAc in PE, to give 13 fractions (X1–X13). X8 (2.7 g) was subjected to silica gel column chromatography, using a gradient of EtOAc in PE, to give 7 fractions (X8-1–X8-7). X8-2 was purified by RP HPLC (70% MeOH in H2O) to afford 7 (7.2 mg), and 11 (5.2 mg). X8-3 was purified by RP HPLC (70% MeOH in H2O) to afford 9 (15.5 mg), and 12 (17.7 mg). X8-4 (430 mg) was further purified on a Sephadex LH20 column to give three subfractions (X8-4-1–X8-4-6). X8-4-2 was purified by RP HPLC (66.5% MeOH in H2O) to afford 3 (5.1 mg). X8-4-6 was purified by RP HPLC (70% MeOH in H2O) to afford 4 (15.0 mg). X8-6 was purified by RP HPLC (70% MeOH in H2O) to afford 6 (11.7 mg).
The 85% EtOH extract was subjected to silica gel column chromatography, using a gradient of MeOH in CDCl3, to give 12 fractions (Y1–Y12). Y3 (1.1 g) was subjected to silica gel column chromatography, using a gradient of EtOAc in PE, to give nine fractions (Y3-1–Y3-9). Y3-3 was further purified on a Sephadex LH20 column to give three subfractions (Y3-3-1–Y3-3-4). Y3-3-4 was purified by RP HPLC (66.5% MeOH in H2O) to afford 5 (5.1 mg), and 10 (11.1 mg). Y3-7 was purified by RP HPLC (66.5% MeOH in H2O) to afford 13 (5.0 mg), and 14 (4.8 mg). Y4 (1.2 g) was subjected to silica gel column chromatography, using a gradient of EtOAc in PE, to give six fractions (Y4-1–Y4-6). Y4-1 was purified by RP HPLC (55% MeOH in H2O) to afford 2 (17.2 mg). Y4-3 was purified by RP HPLC (55% MeOH in H2O) to afford 1 (4.3 mg), and 8 (3.9 mg).
Sinuflexibilin A (1): colorless oil; [α]D25 = +16.7 (c = 0.03, CHCl3); IR (KBr) νmax 3364, 2967, 1709, 1650, 1453 cm−1; 1H and 13C NMR in Table 1 and Table 2; ESIMS m/z 409 [M + Na]+, 795 [2M + Na]+, HRESIMS m/z 409.2574 (calcd for C21H38O6Na, 409.2566).
Sinuflexibilin B (2): colorless oil; [α]D25 = +23.0 (c = 0.10, CHCl3); IR (KBr) νmax 3440, 2937, 1687, 1465 cm−1; 1H and 13C NMR in Table 1 and Table 2; HRESIMS m/z 377.2015 (calcd for C20H34O5Na, 377.2018).
Sinuflexibilin C (3): colorless oil; [α]D25 = +5.0 (c = 0.01, CHCl3); IR (KBr) νmax 3428, 2928, 1720, 1439 cm−1; 1H and 13C NMR in Table 1 and Table 2; HRESIMS m/z 373.2231 (calcd for C21H34O4Na, 373.2202).
3.4. The Cell-Based HEK293 NF-κB Luciferase Reporter Gene Assay
All compounds were evaluated for inhibition of NF-κB activation using the cell-based HEK 293 NF-κB luciferase reporter gene assay according to the previously reported procedures [19].
4. Conclusions
The investigation of bioactive natural products from a Hainan soft coral, Sinularia sp., has led to the isolation of five new cembranes, sinuflexibilins A–E (1–5), along with nine other known diterpenoids (6–14). Compound 13 exhibited significant inhibition activity of NF-κB activation using the cell-based HEK293 NF-κB luciferase reporter gene assay with an IC50 of 5.30 μg/mL.
Supplementary Files
Acknowledgments
This study was supported by grants from the National Key Basic Research Program of China (973)’s Project (2010CB833800, 2011CB915503), the National High Technology Research and Development Program (863 Program, 2012AA092104), National Natural Science Foundation of China (30973679, 20902094, 21172230, 41176148, 21002110), Knowledge Innovation Program of Chinese Academy of Science (SQ201117, SQ200904), Knowledge Innovation Program of Chinese Academy of Science (KSCX2-YW-G-073 and KSCX2-EW-G-12B), Guangdong Province-CAS Joint Research Program (2011B090300023). Our thanks are due to Jia Li, the National Center for Drug Screening, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, for the biological activity screening.
References
- Li, G.Q.; Zhang, Y.L.; Deng, Z.W.; van Ofwegen, L.; Proksch, P.; Lin, W.H. Cytotoxic cembranoid diterpenes from a soft coral Sinularia gibberosa. J. Nat. Prod. 2005, 68, 649–652. [Google Scholar] [CrossRef]
- Yang, B.; Zhou, X.F.; Lin, X.P.; Liu, J.; Peng, Y.; Yang, X.W.; Liu, Y.H. Cembrane diterpenes chemistry and biological properties. Curr. Org. Chem. 2012, 16, 1512–1539. [Google Scholar] [CrossRef]
- Chao, C.H.; Chou, K.J.; Huang, C.Y.; Wen, Z.H.; Hsu, C.H.; Wu, Y.C.; Dai, C.F.; Sheu, J.H. Bioactive Cembranoids from the Soft Coral Sinularia crassa. Mar. Drugs 2011, 9, 1955–1968. [Google Scholar] [CrossRef]
- Li, Y.; Gao, A.H.; Huang, H.; Li, J.; Mollo, E.; Gavagnin, M.; Cimino, G.; Gu, Y.C.; Guo, Y.W. Diterpenoids from the Hainan soft coral Sinularia parva. Helv. Chim. Acta 2009, 92, 1341–1348. [Google Scholar] [CrossRef]
- Coll, J.C.; Price, I.R.; Konig, G.M.; Bowden, B.F. Algal overgrowth of alcyonacean soft corals. Mar. Biol. 1987, 96, 129–135. [Google Scholar] [CrossRef]
- Lu, Y.; Huang, C.Y.; Lin, Y.F.; Wen, Z.H.; Su, J.H.; Kuo, Y.H.; Chiang, M.Y.; Sheu, J.H. Anti-inflammatory cembranoids from the soft corals Sinularia querciformis and Sinularia granosa. J. Nat. Prod. 2008, 71, 1754–1759. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Chuang, C.T.; Wen, Z.H.; Wang, S.K.; Chiou, S.F.; Hsu, C.H.; Dai, C.F.; Duh, C.Y. Bioactive norditerpenoids from the soft coral Sinularia gyrosa. Bioorg. Med. Chem. 2010, 18, 3379–3386. [Google Scholar]
- Lu, Y.; Su, J.H.; Huang, C.Y.; Liu, Y.C.; Kuo, Y.H.; Wen, Z.H.; Hsu, C.H.; Sheu, J.H. Cembranoids from the soft corals Sinularia granosa and Sinularia querciformis. Chem. Pharm.Bull. 2010, 58, 464–466. [Google Scholar] [CrossRef]
- Liu, C.I.; Chen, C.C.; Chen, J.C.; Su, J.H.; Huang, H.H.; Chen, J.Y.F.; Wu, Y.J. Proteomic analysis of anti-tumor effects of 11-dehydrosinulariolide on CAL-27 cells. Mar. Drugs 2011, 9, 1254–1272. [Google Scholar] [CrossRef]
- Lin, Y.S.; Chen, C.H.; Liaw, C.C.; Chen, Y.C.; Kuo, Y.H.; Shen, Y.C. Cembrane diterpenoids from the Taiwanese soft coral Sinularia flexibilis. Tetrahedron 2009, 65, 9157–9164. [Google Scholar] [CrossRef]
- Chao, C.H.; Chou, K.J.; Huang, C.Y.; Wen, Z.H.; Hsu, C.H.; Wu, Y.C.; Dai, C.F.; Sheu, J.H. Steroids from the soft coral Sinularia crassa. Mar. Drugs 2012, 10, 439–450. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Huang, K.J.; Wang, S.K.; Duh, C.Y. Capilloquinol: A novel farnesyl quinol from the Dongsha atoll soft coral Sinularia capillosa. Mar. Drugs 2011, 9, 1469–1476. [Google Scholar] [CrossRef]
- Shi, H.Y.; Yu, S.J.; Liu, D.; van Ofwegen, L.; Proksch, P.; Lin, W.H. Sinularones A-I, new cyclopentenone and butenolide derivatives from a marine soft coral Sinularia sp. and their antifouling activity. Mar. Drugs 2012, 10, 1331–1344. [Google Scholar] [CrossRef]
- Iwagawa, T.; Shibata, Y.; Okamura, H.; Nakatani, M.; Shiro, M. Novel cembranoids with a 13-membered carbocyclic skeleton from a soft coral, Sarcophyton species. Tetrahedron Lett. 1994, 35, 8415–8416. [Google Scholar] [CrossRef]
- Lakshmi, V.; Kumar, R. Metabolites from Sinularia species. Nat. Prod. Res. 2009, 23, 801–850. [Google Scholar] [CrossRef]
- Ahmed, A.F.; Wen, Z.H.; Su, J.H.; Hsieh, Y.T.; Wu, Y.C.; Hu, W.P.; Sheu, J.H. Oxygenated cembranoids from a Formosan soft coral Sinulatia gibberosa. J. Nat. Prod. 2008, 71, 179–185. [Google Scholar] [CrossRef]
- Su, J.H.; Wen, Z.H. Bioactive cembrane-based diterpenoids from the soft coral Sinularia triangular. Mar. Drugs 2011, 9, 944–951. [Google Scholar] [CrossRef]
- Lin, Y.S.; Lee, N.L.; Lu, M.C.; Su, J.H. Two new cembrane-based diterpenoids from the marine soft coral Sinularia crassa. Molecules 2012, 17, 5422–5429. [Google Scholar] [CrossRef]
- Peddibhotla, S.; Shi, R.X.; Khan, P.; Smith, L.H.; Mangravita-Novo, A.; Vicchiarelli, M.; Su, Y.; Okolotowicz, K.J.; Cashman, J.R.; Reed, J.C.; Roth, G.P. Inhibition of protein kinase C-driven nuclear factor-kappa B activation: Synthesis, structure-activity relationship, and pharmacological profiling of pathway specific benzimidazole probe molecules. J. Med. Chem. 2010, 53, 4793–4797. [Google Scholar]
- Duh, C.Y.; Wang, S.K.; Tseng, H.K.; Sheu, J.H.; Chiang, M.Y. Novel cytotoxic cembranoids from the soft coral Sinularia flexibilis. J. Nat. Prod. 1998, 61, 844–847. [Google Scholar] [CrossRef]
- Su, J.Y.; Yang, R.L.; Kuang, Y.Y.; Zeng, L.M. A new cembranolide from the soft coral Sinularia capillosa. J. Nat. Prod. 2000, 63, 1543–1545. [Google Scholar] [CrossRef]
- Rodriguez, A.D.; Li, Y.X.; Dhasmana, H.; Barnes, C.L. New marine cembrane diterpenoids isolated from the Caribbean gorgonian Eunicea mammosa. J. Nat. Prod. 1993, 56, 1101–1113. [Google Scholar] [CrossRef]
- Rodriguez, A.D.; Martinez, N. Marine antitumor agents-14: Deoxycrassin and pseudoplexaurol, new cembranoid diterpenes from the Caribbean gorgonian Pseudoplexaura porosa. Cell. Mol. Life Sci. 1993, 49, 179–181. [Google Scholar] [CrossRef]
- Su, J.H.; Ahmed, A.F.; Sung, P.J.; Chao, C.H.; Kuo, Y.H.; Sheu, J.H. Manaarenolides A-I, diterpenoids from the soft coral Sinularia manaarensis. J. Nat. Prod. 2006, 69, 1134–1139. [Google Scholar] [CrossRef]
- Grote, D.; Dahse, H.M.; Seifert, K. Furanocembranoids from the soft corals Sinularia asterolobata and Litophyton arboreum. Chem. Biodivers. 2008, 5, 2449–2456. [Google Scholar] [CrossRef]
- Venkateswarlu, Y.; Sridevi, K.V.; Rao, M.R. New furanocembranoid diterpenes from the soft coral Sinularia maxima. J. Nat. Prod. 1999, 62, 756–758. [Google Scholar] [CrossRef]
- Lo, K.L.; Khalil, A.T.; Chen, M.H.; Shen, Y.C. New cembrane diterpenes from Taiwanese soft coral Sinularia flexibilis. Helv. Chim. Acta 2010, 93, 1329–1335. [Google Scholar] [CrossRef]
- Wen, T.; Ding, Y.; Deng, Z.W.; van Ofwegen, L.P.; Proksch, P.; Lin, W.H. Sinulaflexiolides A-K, cembrane-type diterpenoids from the Chinese soft coral Sinularia flexibilis. J. Nat. Prod. 2008, 71, 1133–1140. [Google Scholar] [CrossRef]
- Su, J.H.; Lin, Y.F.; Lu, Y.; Yeh, H.C.; Wang, W.H.; Fan, T.Y.; Sheu, J.H. Oxygenated cembranoids from the cultured and wild-type soft corals Sinularia flexibilis. Chem. Pharm.Bull. 2009, 57, 1189–1192. [Google Scholar] [CrossRef]
- Hsieh, P.W.; Chang, F.R.; McPhail, A.T.; Lee, K.; Wu, Y.C. New cembranolide analogues from the formosan soft coral Sinularia flexibilis and their cytotoxicity. Nat. Prod. Res. 2003, 17, 409–418. [Google Scholar] [CrossRef]
- Venkateswarlu, Y.; Biabani, M.A.F.; Reddy, M.V.R.; Rao, T.P.; Kunwar, A.C.; Faulkner, D.J. Mandapmate, a diterpenolid from the soft coral sinularia dissecta. Tetrahedron Lett. 1994, 35, 2249–2252. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).