Terpenoids from the Octocorals Menella sp. (Plexauridae) and Lobophytum crassum (Alcyonacea)
Abstract
:1. Introduction
2. Results and Discussion
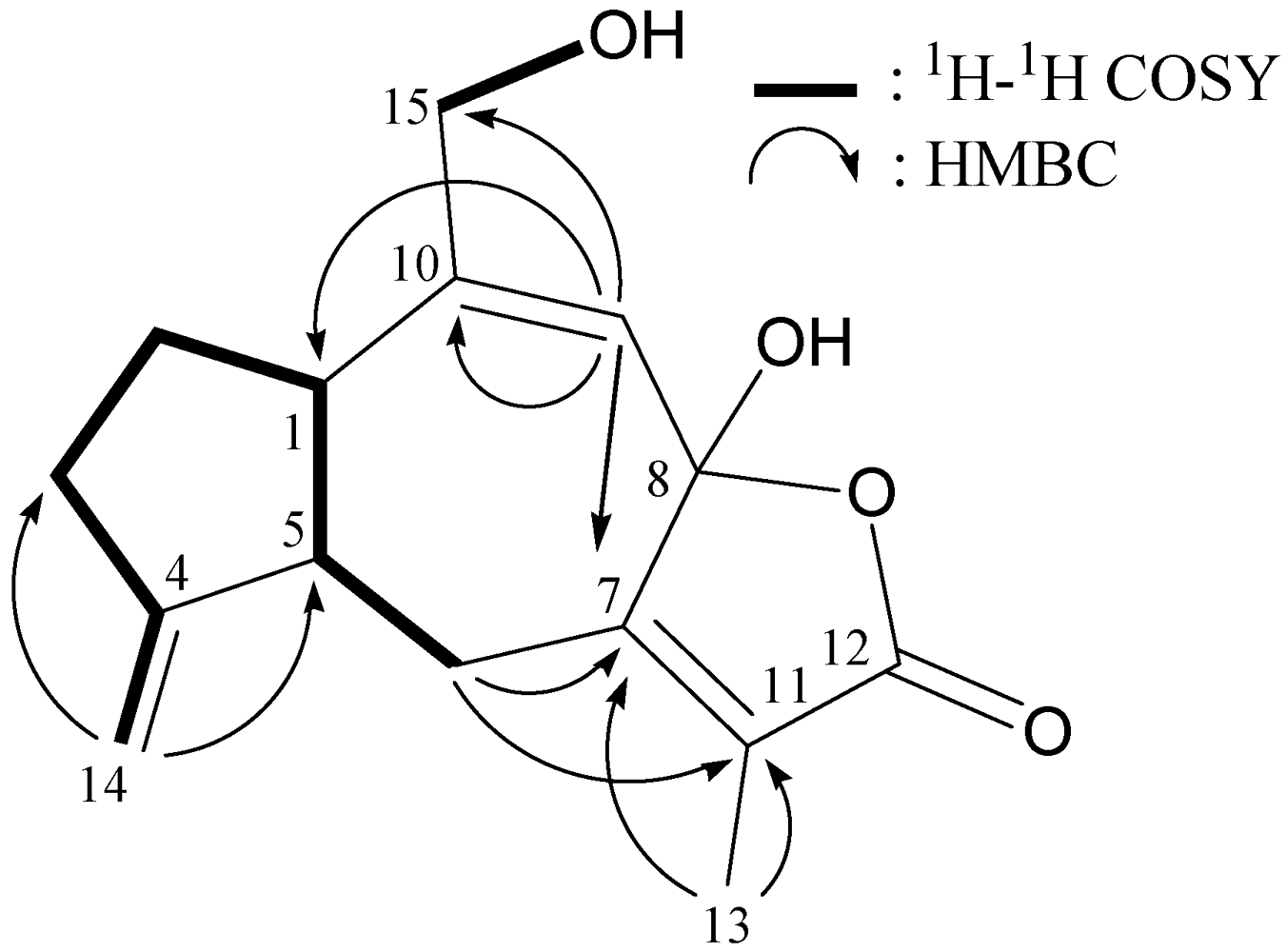
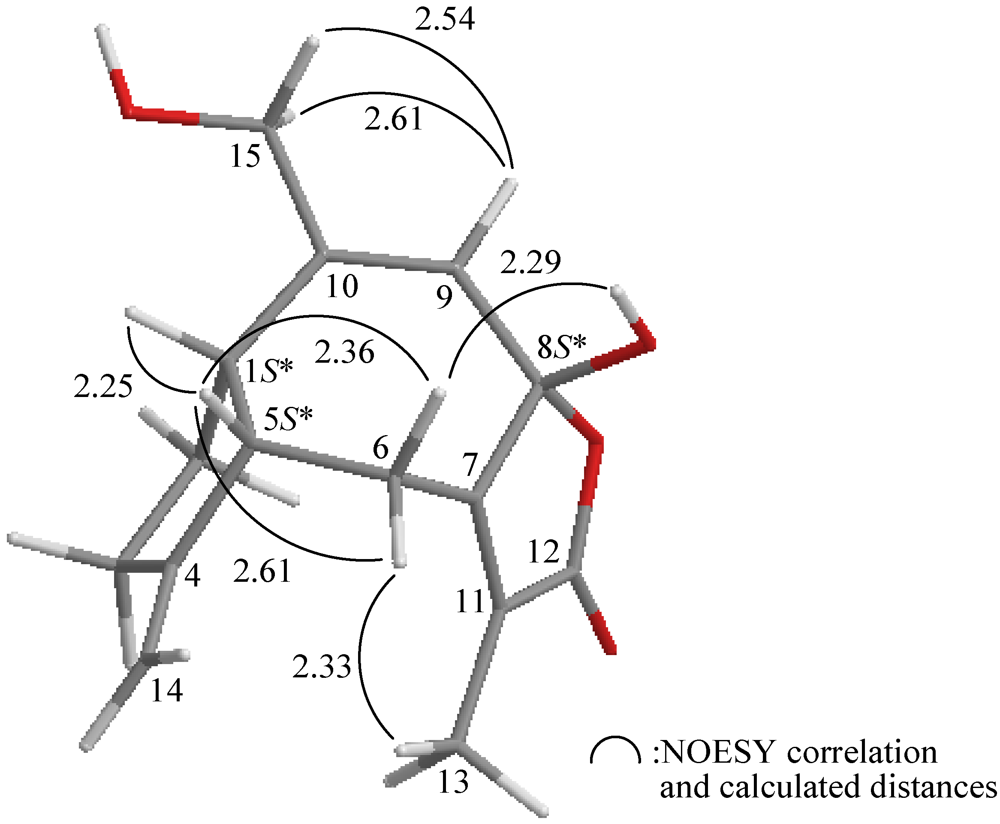
2.1. Isolation and Structure Determination of Menelloide E (1) from Menella sp.

| Position | δH (J in Hz) | δC, Mult. |
|---|---|---|
| 1 | 2.85 td (11.5, 8.0) | 48.3, CH |
| 2α/β | 1.19 m; 1.74 m | 28.7, CH2 |
| 3 | 2.25 m | 33.8, CH2 |
| 4 | 157.6, qC | |
| 5 | 3.21 m | 40.7, CH |
| 6α/β | 3.00 dd (15.0, 3.5); 3.51 dd (15.0, 3.5) | 26.2, CH2 |
| 7 | 149.8, qC | |
| 8 | 102.7, qC | |
| 9 | 5.68 s | 111.4, CH |
| 10 | 154.5, qC | |
| 11 | 127.4, qC | |
| 12 | 175.1, qC | |
| 13 | 1.93 s | 8.7, CH3 |
| 14a/b | 4.89 s; 4.66 s | 104.5, CH2 |
| 15a/b | 3.67 dd (10.5, 5.5); 3.61 dd (10.5, 5.5) | 70.2, CH2 |
| OH-8 | 2.49 s | |
| OH-15 | 2.04 t (5.5) |
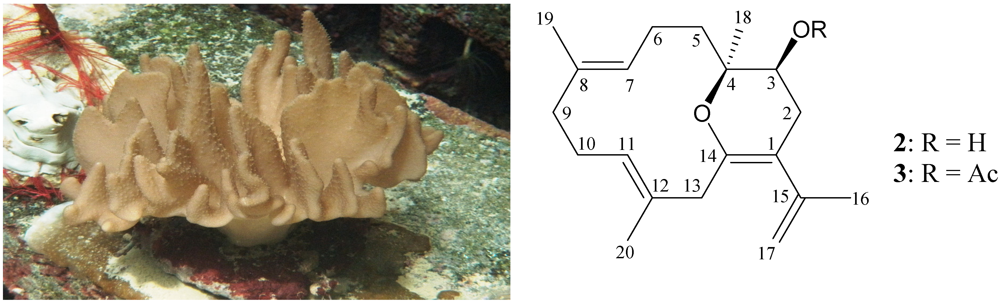
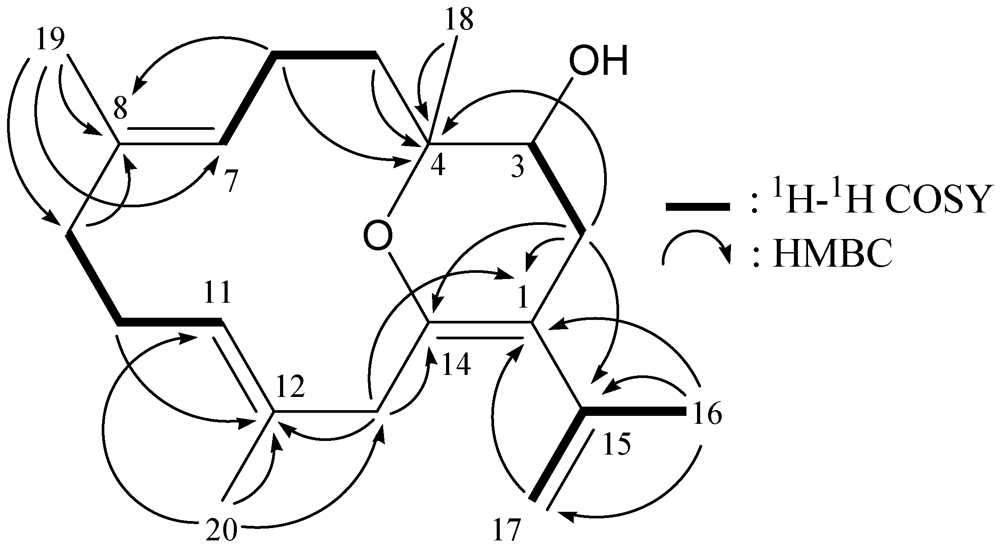
2.2. Isolation and Structure Determination of Lobocrassin F (2) from Lobophytum crassum
| Position | δH (J in Hz) | δC, Mult. |
|---|---|---|
| 1 | 108.2, qC | |
| 2α/β | 2.01 dd (16.5, 6.5); 2.39 dd (16.5, 4.0) | 31.5, CH2 |
| 3 | 3.54 br s | 67.7, CH |
| 4 | 77.6, qC | |
| 5 | 1.60 m | 35.0, CH2 |
| 6 | 2.21 m | 23.4, CH2 |
| 7 | 5.11 t (7.5) | 127.8, CH |
| 8 | 130.0, qC | |
| 9 | 2.05 m | 39.5, CH2 |
| 10 | 2.15 m | 25.6, CH2 |
| 11 | 5.02 t (7.5) | 127.7, CH |
| 12 | 131.5, qC | |
| 13α/β | 2.98 d (14.0); 2.72 d (14.0) | 41.1, CH2 |
| 14 | 144.2, qC | |
| 15 | 144.3, qC | |
| 16 | 1.81 s | 22.7, CH3 |
| 17a/b | 4.72 d (1.5); 4.93 d (1.5) | 113.7, CH2 |
| 18 | 1.17 s | 16.5, CH3 |
| 19 | 1.59 s | 15.6, CH3 |
| 20 | 1.43 s | 15.7, CH3 |

| Superoxide anion | Elastase release | ||||
|---|---|---|---|---|---|
| Compounds | IC50 (µg/mL) | Inh % a | IC50 (µg/mL) | Inh % a | |
| 1 | >10 | 19.85 ± 6.65 | >10 | 26.99 ± 4.99 | |
| 2 | >10 | 7.80 ± 5.23 | 6.27 ± 1.91 | 58.29 ± 5.47 | |
| DPI b | 0.82 ± 0.31 | ||||
| Elastatinal b | 31.82 ± 5.92 | ||||
3. Experimental Section
3.1. General Experimental Procedures
3.2. Animal Material
3.2.1. Menella sp.
3.2.2. Lobophytum crassum
3.3. Extraction and Isolation
3.3.1. Menella sp.
3.3.2. Lobophytum crassum
3.4. Molecular Mechanics Calculations
3.5. Superoxide Anion Generation and Elastase Release by Human Neutrophils
4. Conclusions
Acknowledgments
References
- Fraga, B.M. Natural sesquiterpenoids. Nat. Prod. Rep. 2011, 28, 1580–1610. [Google Scholar]
- Blunt, J.W.; Copp, B.R.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2011, 28, 196–268. [Google Scholar]
- Harper, M.K.; Bugni, T.S.; Copp, B.R.; James, R.D.; Lindsay, B.S.; Richardson, A.D.; Schnabel, P.C.; Tasdemir, D.; VanWagoner, R.M.; Verbitski, S.M.; Ireland, C.M. Introduction to the Chemical Ecology of Marine Natural Products. In Marine Chemical Ecology; McClintock, J.B., Baker, B.J., Eds.; CRC Press: Washington, DC, USA, 2001; pp. 3–69. [Google Scholar]
- Bayer, F.M. Key to the genera of Octocorallia exclusive of Pennatulacea (Coelenterata: Anthozoa), with diagnoses of new taxa. Proc. Biol. Soc. Wash. 1981, 94, 902–947. [Google Scholar]
- Fabricius, K.; Alderslade, P. Soft Corals and Sea Fans—A Comprehensive Guide to the Tropical Shallow-Water Genera of the Central-West Pacific, the Indian Ocean and the Red Sea, 1st ed; Australian Institute of Marine Science: Queensland, Australia, 2001; pp. 49-50, 59-60, 94-97, 206-207. [Google Scholar]
- Deng, S.; Peng, S.; Li, F.; Tan, X.; Chen, J. A study on chemical constituents of South China Sea gorgonian Menella spinifera Kukenthal (I). Guangzhou Chem. 1993, 44–47. [Google Scholar]
- Li, F.; Deng, S.; Rao, Z.; Wu, H.; Xu, J. Studies on chemical constituents of South China Sea gorgonian Menella spinifera Kukenthal (II). Guangzhou Chem. 1996, 49–51. [Google Scholar]
- Deng, S.; Li, F.; Peng, S.; Rao, Z.; Wu, H.; Xu, J. Chemical constituents of the South China Sea gorgonian Menella spinifera Kukenthal. Chin. J. Appl. Chem. 1997, 14, 80–82. [Google Scholar]
- Zhang, W.; Guo, Y.-W.; Mollo, E.; Cimino, G. Menverins A–D, new highly oxygenated guaiane lactones from Hainan gorgonian Menella verrucosa (Brundin). Helv. Chim. Acta 2004, 87, 2919–2925. [Google Scholar]
- Zhang, W.; Huang, H.; Ding, Y.; Gavagnin, M.; Mollo, E.; Cimino, G.; Guo, Y.-W. Three new polyoxygenated steroids from two species of the South China Sea gorgonian Muricella flexuosa and Menella verrucosa Brundin. Helv. Chim. Acta 2006, 89, 813–820. [Google Scholar]
- Li, L.; Wang, C.-Y.; Huang, H.; Mollo, E.; Cimino, G.; Guo, Y.-W. Further highly oxygenated guaiane lactones from the South China Sea gorgonian Menella sp. Helv. Chim. Acta 2008, 91, 111–117. [Google Scholar]
- Chai, X.-Y.; Sun, J.-F.; Tang, L.-Y.; Yang, X.-W.; Li, Y.-Q.; Huang, H.; Zhou, X.-F.; Yang, B.; Liu, Y. A novel cyclopentene derivative and a polyhydroxylated steroid from a South China Sea gorgonian Menella sp. Chem. Pharm. Bull. 2010, 58, 1391–1394. [Google Scholar]
- Kao, S.-Y.; Chang, Y.-C.; Su, J.-H.; Lu, M.-C.; Chen, Y.-H.; Sheu, J.-H.; Wen, Z.-H.; Wang, W.-H.; Kuo, Y.-H.; Hwang, T.-L.; Sung, P.-J. (−)-Hydroxylindestrenolide, a new sesquiterpenoid from a gorgonian coral Menella sp. (Plexauridae). Chem. Pharm. Bull. 2011, 59, 1048–1050. [Google Scholar] [CrossRef]
- Kao, S.-Y.; Su, J.-H.; Hwang, T.-L.; Sheu, J.-H.; Su, Y.-D.; Lin, C.-S.; Chang, Y.-C.; Wang, W.-H.; Fang, L.-S.; Sung, P.-J. Discovery of novel sesquiterpenoids from a gorgonian Menella sp. Tetrahedron 2011, 67, 7311–7315. [Google Scholar]
- Kao, S.-Y.; Su, J.-H.; Hwang, T.-L.; Sheu, J.-H.; Wen, Z.-H.; Wu, Y.-C.; Sung, P.-J. Menelloides C and D, new sesquiterpenoids from the gorgonian coral Menella sp. Mar. Drugs 2011, 9, 1534–1542. [Google Scholar]
- Sung, P.-J.; Kao, S.-Y.; Kao, C.-Y.; Chang, Y.-C.; Chen, Y.-H.; Su, Y.-D. Seco-germacrane anhydride: Occurrence of a sesquiterpene lactone in the gorgonian coral Menella sp. (Plexauridae). Biochem. Syst. Ecol. 2012, 40, 53–55. [Google Scholar] [CrossRef]
- Imbs, A.B.; Demidkova, D.A.; Dautova, T.N.; Latyshev, N.A. Fatty acid biomarkers of symbionts and unusual inhibition of tetracosapolyenoic acid biosynthesis in corals (octocorallia). Lipids 2009, 44, 325–335. [Google Scholar]
- Allinger, N.L. Conformational analysis. 130. MM2. A hydrocarbon force field utilizing V1 and V2 torsional terms. J. Am. Chem. Soc. 1977, 99, 8127–8134. [Google Scholar] [CrossRef]
- Duh, C.-Y.; Wang, S.-K.; Huang, B.-T.; Dai, C.-F. Cytotoxic cembrenolide diterpenes from the Formosan soft coral Lobophytum crassum. J. Nat. Prod. 2000, 63, 884–885. [Google Scholar]
- Chao, C.-H.; Wen, Z.-H.; Wu, Y.-C.; Yeh, H.-C.; Sheu, J.-H. Cytotoxic and anti-inflammatory cembranoids from the soft coral Lobophytum crassum. J. Nat. Prod. 2008, 71, 1819–1824. [Google Scholar]
- Lin, S.-T.; Wang, S.-K.; Cheng, S.-Y.; Duh, C.-Y. Lobocrasol, a new diterpenoid from the soft coral Lobophytum crassum. Org. Lett. 2009, 11, 3012–3014. [Google Scholar]
- Liao, Z.-J.; Su, H.-J.; Shyue, Y.-C.; Wen, Z.-H.; Sheu, J.-H.; Su, J.-H. Two new cembranoids from the soft coral Lobophytum crassum. Bull. Chem. Soc. Jpn. 2011, 84, 653–655. [Google Scholar]
- Tseng, Y.-J.; Wen, Z.-H.; Hsu, C.-H.; Dai, C.-F.; Sheu, J.-H. Bioactive cembranoids from the Dongsha atoll soft coral Lobophytum crassum. Bull. Chem. Soc. Jpn. 2011, 84, 1102–1106. [Google Scholar]
- Kao, C.-Y.; Su, J.-H.; Lu, M.-C.; Hwang, T.-L.; Wang, W.-H.; Chen, J.-J.; Sheu, J.-H.; Kuo, Y.-H.; Weng, C.-F.; Fang, L.-S.; Wen, Z.-H.; Sung, P.-J. Lobocrassins A–E: New cembrane-type diterpenoids from the soft coral Lobophytum crassum. Mar. Drugs 2011, 9, 1319–1331. [Google Scholar]
- Lee, N.-L.; Su, J.-H. Tetrahydrofuran cembranoids from the cultured soft coral Lobophytum crassum. Mar. Drugs 2011, 9, 2526–2536. [Google Scholar]
- Lin, S.-T.; Wang, S.-K.; Duh, C.-Y. Cembranoids from the Dongsha atoll soft coral Lobophytum crassum. Mar. Drugs 2011, 9, 2705–2716. [Google Scholar]
- Chao, C.-H.; Huang, H.-C.; Wu, Y.-C.; Lu, C.-K.; Dai, C.-F.; Sheu, J.-H. Glycolipids from the Formosan soft coral Lobophytum crassum. Chem. Pharm. Bull. 2007, 55, 1720–1723. [Google Scholar]
- Cheng, S.-Y.; Lin, S.-T.; Wang, S.-K.; Duh, C.-Y. α-Tocopherols from the Formosan soft coral Lobophytum crassum. Bull. Chem. Soc. Jpn. 2011, 84, 783–787. [Google Scholar]
- Lu, Y.; Huang, C.-Y.; Lin, Y.-F.; Wen, Z.-H.; Su, J.-H.; Kuo, Y.-H.; Chiang, M.Y.; Sheu, J.-H. Anti-inflammatory cembranoids from the soft coral Sinularia querciformis and Sinularia granosa. J. Nat. Prod. 2008, 71, 1754–1759. [Google Scholar]
- Marrero, J.; Benítez, J.; Rodríguez, A.D.; Zhao, H.; Raptis, R.G. Bipinnatins K–Q, minor cembrane-type diterpenes from the West Indian gorgonian Pseudopterogorgia kallos: Isolation, structure assignment, and evaluation of biological activities. J. Nat. Prod. 2008, 71, 381–389. [Google Scholar]
- Chen, S.-Y.; Wen, Z.-H.; Wang, S.-K.; Chiou, S.-F.; Hsu, C.-H.; Dai, C.-F.; Chiang, M.Y.; Duh, C.-Y. Unprecedented hemiketal cembranolides with anti-inflammatory activity from the soft coral Lobophytum durum. J. Nat. Prod. 2009, 72, 152–155. [Google Scholar]
- Chao, C.-H.; Chou, K.-J.; Huang, C.-Y.; Wen, Z.-H.; Hsu, C.-H.; Wu, Y.-C.; Dai, C.-F.; Sheu, J.-H. Bioactive cembranoids from the soft coral Sinularia crassa. Mar. Drugs 2011, 9, 1955–1968. [Google Scholar]
- Yu, H.-P.; Hsieh, P.-W.; Chang, Y.-J.; Chung, P.-J.; Kuo, L.-M.; Hwang, T.-L. 2-(2-Fluorobenzamido)benzoate ethyl ester (EFB-1) inhibits superoxide production by human neutrophils and attenuates hemorrhagic shock-induced organ dysfunction in rats. Free Radic. Biol. Med. 2011, 50, 1737–1748. [Google Scholar]
- Hwang, T.-L.; Wang, C.-C.; Kuo, Y.-H.; Huang, H.-C.; Wu, Y.-C.; Kuo, L.-M.; Wu, Y.-H. The hederagenin saponin SMG-1 is a natural FMLP receptor inhibitor that suppresses human neutrophil activation. Biochem. Pharmacol. 2010, 80, 1190–1200. [Google Scholar]
- Samples Availability: Not available.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lee, C.-H.; Kao, C.-Y.; Kao, S.-Y.; Chang, C.-H.; Su, J.-H.; Hwang, T.-L.; Kuo, Y.-H.; Wen, Z.-H.; Sung, P.-J. Terpenoids from the Octocorals Menella sp. (Plexauridae) and Lobophytum crassum (Alcyonacea). Mar. Drugs 2012, 10, 427-438. https://doi.org/10.3390/md10020427
Lee C-H, Kao C-Y, Kao S-Y, Chang C-H, Su J-H, Hwang T-L, Kuo Y-H, Wen Z-H, Sung P-J. Terpenoids from the Octocorals Menella sp. (Plexauridae) and Lobophytum crassum (Alcyonacea). Marine Drugs. 2012; 10(2):427-438. https://doi.org/10.3390/md10020427
Chicago/Turabian StyleLee, Cheng-Hung, Chia-Ying Kao, Shih-Yao Kao, Chih-Han Chang, Jui-Hsin Su, Tsong-Long Hwang, Yueh-Hsiung Kuo, Zhi-Hong Wen, and Ping-Jyun Sung. 2012. "Terpenoids from the Octocorals Menella sp. (Plexauridae) and Lobophytum crassum (Alcyonacea)" Marine Drugs 10, no. 2: 427-438. https://doi.org/10.3390/md10020427
APA StyleLee, C.-H., Kao, C.-Y., Kao, S.-Y., Chang, C.-H., Su, J.-H., Hwang, T.-L., Kuo, Y.-H., Wen, Z.-H., & Sung, P.-J. (2012). Terpenoids from the Octocorals Menella sp. (Plexauridae) and Lobophytum crassum (Alcyonacea). Marine Drugs, 10(2), 427-438. https://doi.org/10.3390/md10020427






