Potentiation of the Cytotoxic Activity of Copper by Polyphosphate on Biofilm-Producing Bacteria: A Bioinspired Approach
Abstract
:1. Introduction
2. Results
2.1. Toxic Effect of Copper on S. mutans
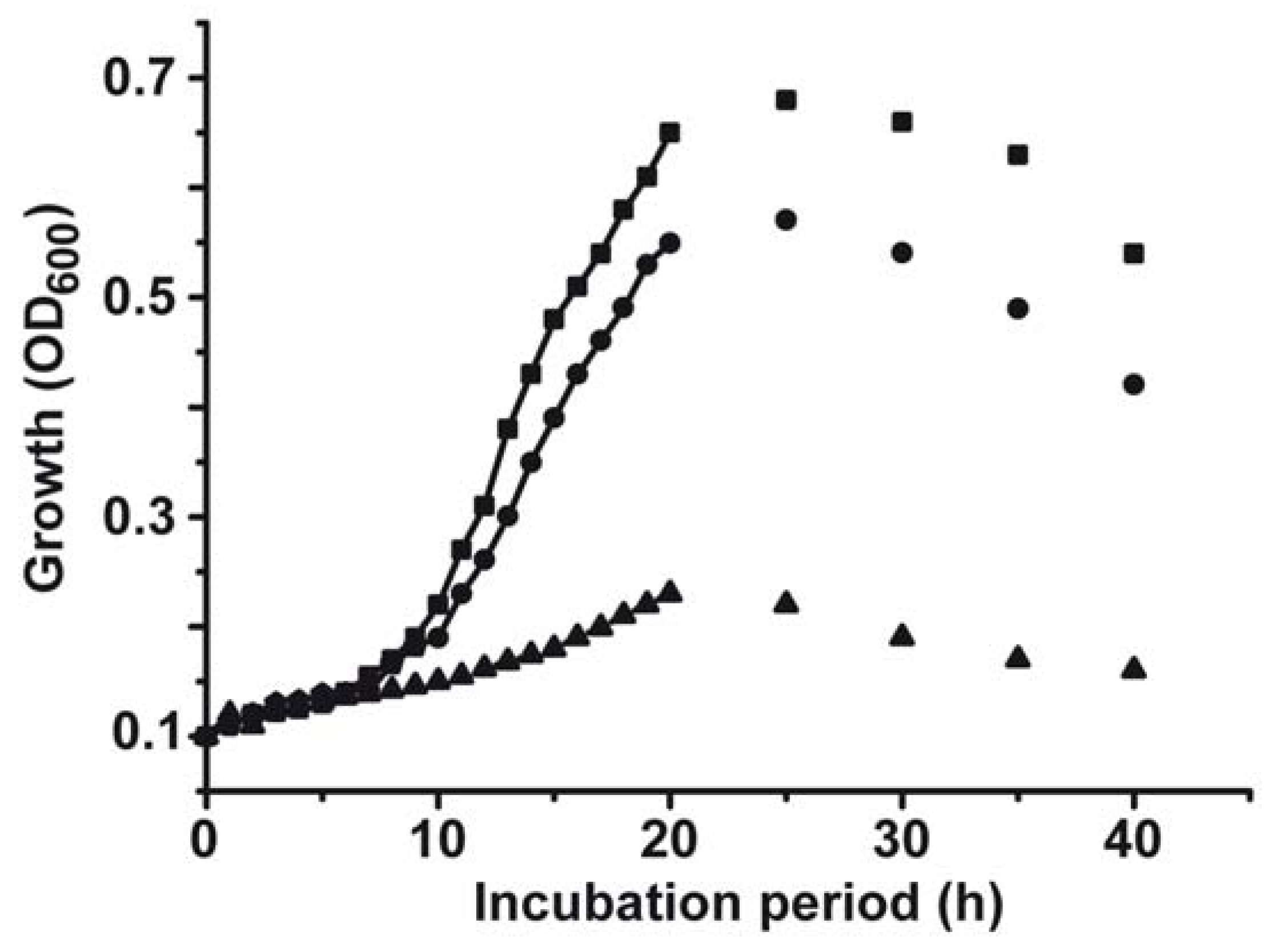
2.2. Effect of PolyP on the Gram-Positive S. mutans
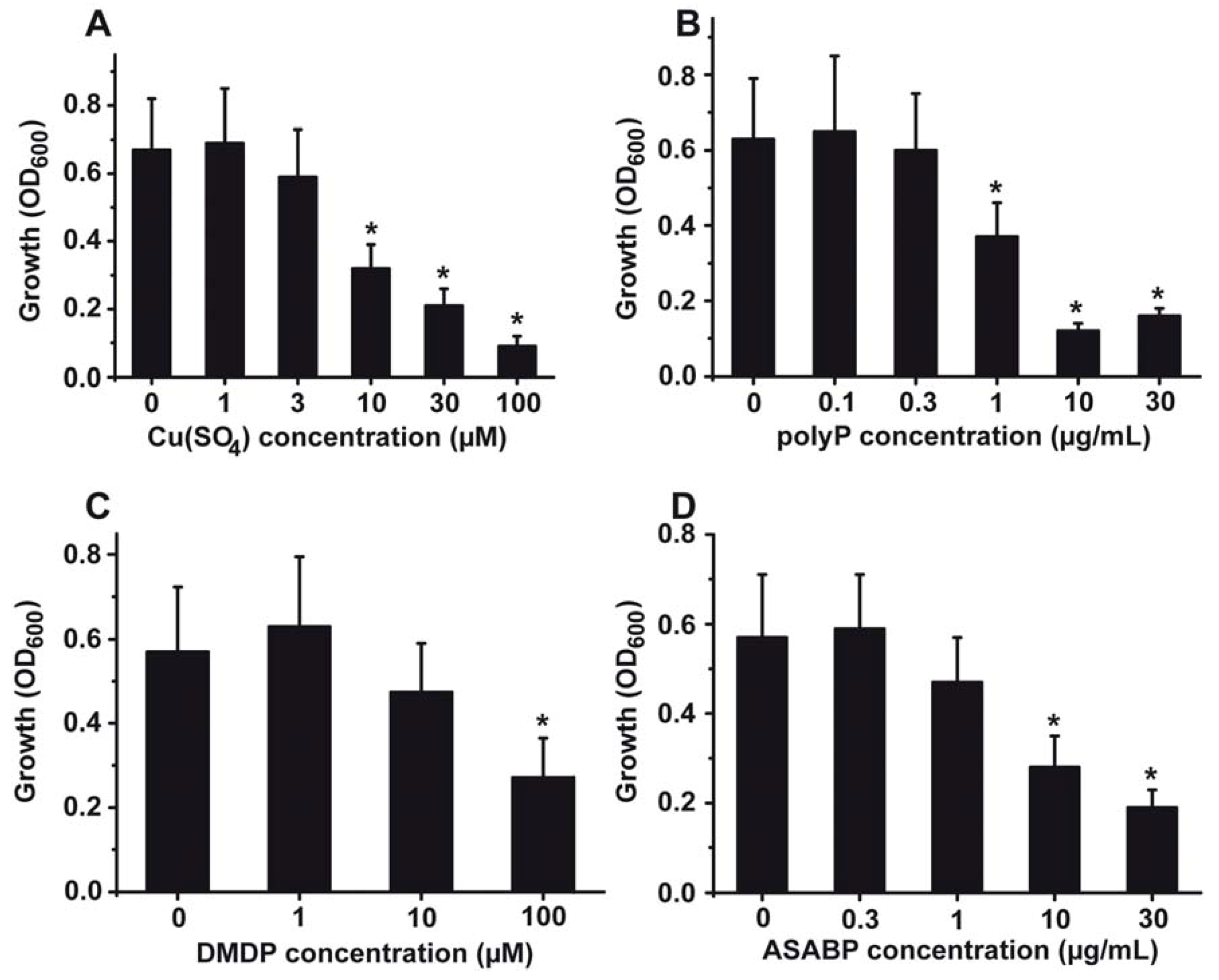
2.3. Effect of Bisphosphonates
2.4. The Inhibitory Activity of the Antimicrobial Peptide from S. domuncula
2.5. Evaluation of the Combinatorial Potential of Copper Together with the Other Antimicrobial Agents Against S. mutans Growth

2.6. Effect of Copper on PolyP Content in S. mutans

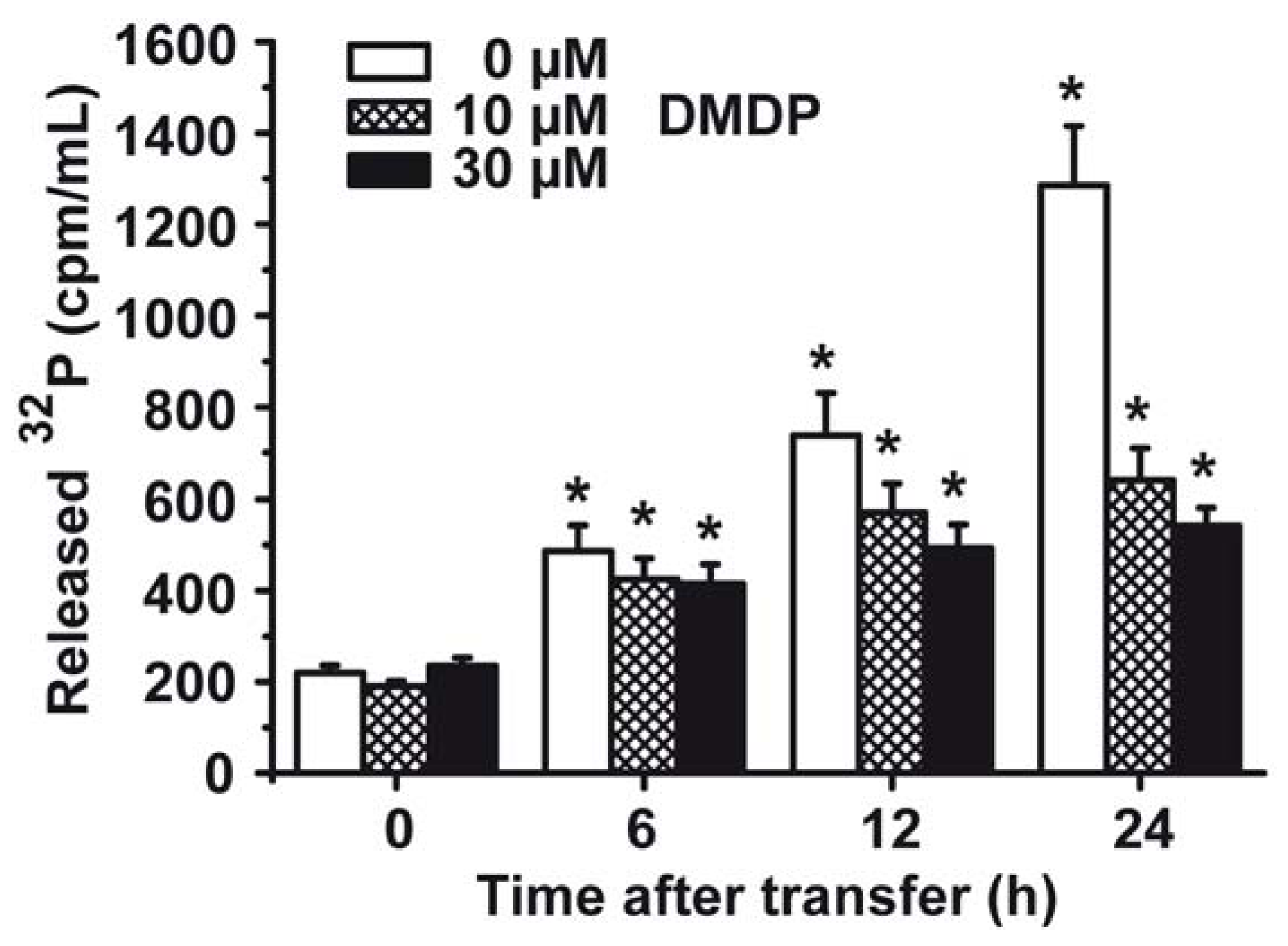
2.7. Phosphate (Pi) Efflux from S. mutans
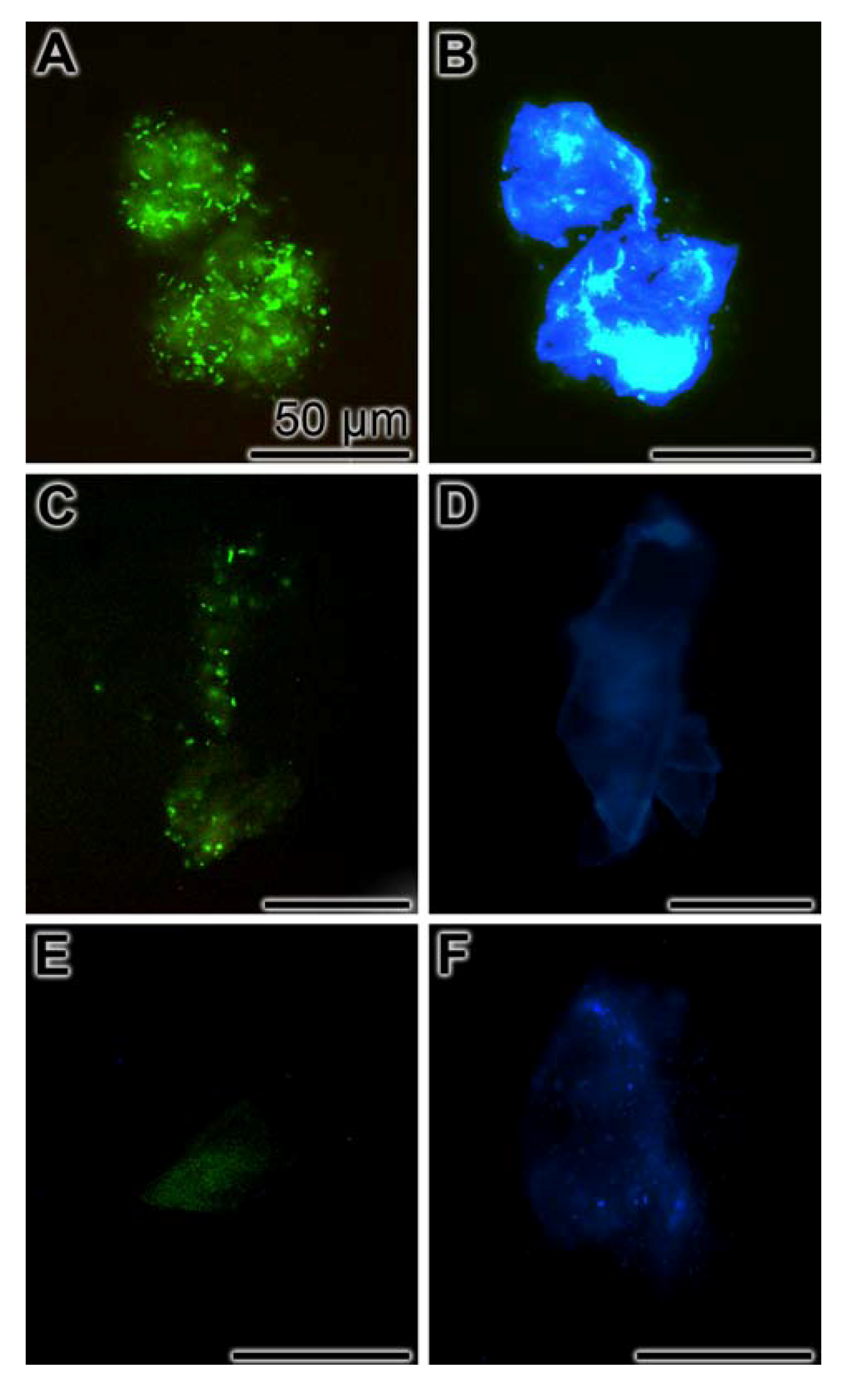
2.8. Inhibition of Biofilm Production
3. Discussion
4. Experimental Section
4.1. Materials
4.2. Antimicrobial Peptide from S. domuncula
4.3. S. mutans and Culture Condition
4.4. Inhibition Studies
4.5. Evaluation of the Combinatorial Potential of Copper Together with the Other Antimicrobial Agents Against S. mutans Growth
4.6. Cultivation of S. mutans on Microscope Slides for Biofilm Formation
4.7. Quantification of PolyP
4.8. Phosphate Efflux Determinations
4.9. Further Methods
5. Conclusions

Acknowledgments
References
- Batel, R.; Bihari, N.; Rinkevich, B.; Dapper, J.; Schäcke, H.; Schröder, H.C.; Müller, W.E.G. Modulation of organotin-induced apoptosis by the water pollutant methyl mercury in a human lymphoblastoid tumor cell line and a marine sponge. Mar. Ecol. Prog. Ser. 1993, 93, 245–251. [Google Scholar] [CrossRef]
- Zobell, C.E. The effect of solid surfaces upon bacterial activity. J. Bacteriol. 1943, 46, 39–56. [Google Scholar]
- Characklis, W.G.; Cooksey, K.E. Biofilms and microbial fouling. In Applied Microbiology; Laskin, A.I., Ed.; Academic Press: New York, NY, USA, 1983; Volume 29, pp. 93–138. [Google Scholar]
- Marshall, K.C. History of bacterium-particle interaction studies. Am. Soc. Microbiol. News 1993, 59, 377–378. [Google Scholar] [CrossRef]
- Cooksey, K.E.; Wigglesworth-Cooksey, B. Adhesion of bacteria and diatoms to surfaces in the sea: A review. Aquat. Microb. Ecol. 1995, 9, 87–96. [Google Scholar]
- Cooksey, K.E. Requirement of calcium in adhesion of a fouling diatom to glass. Appl. Environ. Microbiol. 1981, 41, 1378–1382. [Google Scholar]
- Callow, M.E.; Callow, J.A. Marine biofouling: A sticky problem. Biologist 2002, 49, 1–5. [Google Scholar]
- Kirschner, C.M.; Brennan, A.B. Bio-Inspired antifouling strategies. Annu. Rev. Mater. Res. 2012, 42, 1–19. [Google Scholar] [CrossRef]
- Fusetani, N. Biofouling and antifouling. Nat. Prod. Rep. 2004, 21, 94–104. [Google Scholar] [CrossRef]
- Schultz, M.P. Effects of coating roughness and biofouling on ship resistance and powering. Biofouling 2007, 23, 331–341. [Google Scholar] [CrossRef]
- Schultz, M.; Bendick, J.; Holm, E.; Hertel, W. Economic impact of biofouling on a naval surface ship. Biofouling 2011, 27, 87–98. [Google Scholar] [CrossRef]
- Jung, C.W. Surface properties of superparamagnetic iron oxide MR contrast agents: Ferumoxides, ferumoxtran, ferumoxsil. Magn. Reson. Imaging 1995, 13, 675–691. [Google Scholar] [CrossRef]
- Jung, C.W.; Jacobs, P. Physical and chemical properties of superparamagnetic iron oxide MR contrast agents: Ferumoxides, ferumoxtran, ferumoxsil. Magn. Reson. Imaging 1995, 13, 661–674. [Google Scholar] [CrossRef]
- Karmali, P.P.; Chao, Y.; Park, J.H.; Sailor, M.J.; Ruoslahti, E.; Esener, S.C.; Simberg, D. Different effect of hydrogelation on antifouling and circulation properties of dextran—iron oxide nanoparticles. Mol. Pharm. 2012, 9, 539–545. [Google Scholar] [CrossRef]
- Swain, G. Biofouling control: A critical component of drag reduction. In Proceedings of the International Symposium on Sea Water Drag Reduction, Newport, RI, USA, 22–23 July 1998; The Naval Undersea Warfare Center: Newport, RI, USA, 1998; pp. 155–161. [Google Scholar]
- Evans, S.M.; Leksono, T.; McKinnel, P.D. Tributyltin pollution: A diminishing problem following legislation limiting the use of TBT-based anti-fouling paints. Mar. Pollut. Bull. 1995, 30, 14–21. [Google Scholar] [CrossRef]
- Mičić, M.; Bihari, N.; Labura, Ž.; Müller, W.E.G.; Batel, R. Induction of apoptosis in the blue mussel Mytilus galloprovincialis by tri-n-butyltin chloride. Aquat. Toxicol. 2001, 55, 61–73. [Google Scholar] [CrossRef]
- Champ, M.A. A review of organotin regulatory strategies pending actions related costs and benefits. Sci. Total Environ. 2000, 258, 21–71. [Google Scholar]
- Yebra, D.M.; Kiil, S.; Dam-Johansen, K. Antifouling technology. Past, present and future steps towards efficient and environmentally friendly antifouling coatings. Prog. Org. Coat. 2004, 50, 75–104. [Google Scholar]
- Champ, M.A. The status of the treaty to ban TBT in marine antifouling paints and alternatives. In Proceedings of the 24th UJNR (US/Japan) Marine Facilities Panel Meeting, Hawaii, HI, USA, 7–8 November 2001; 2001; pp. 1–24. [Google Scholar]
- Almeida, E.; Diamantino, T.C.; de Sousa, O. Marine paints: The particular case of antifouling paints. Prog. Org. Coat. 2007, 59, 2–20. [Google Scholar]
- Singh, A.K.; Singh, P.; Mishra, S.; Shahi, V.K. Anti-biofouling organic-inorganic hybrid membrane for water treatment. J. Mater. Chem. 2012, 22, 1834–1844. [Google Scholar]
- Kiaune, L.; Singhasemanon, N. Pesticidal copper (I) oxide: Environmental fate and aquatic toxicity. Rev. Environ. Contam. Toxicol. 2011, 213, 1–26. [Google Scholar] [CrossRef]
- Ebada, S.S.; Proksch, P. The chemistry of marine sponges. In Handbook of Marine Natural Products; Fattorusso, E., Gerwick, W.H., Taglialatela-Scafati, T., Eds.; Springer Science: Dordrecht, The Netherlands, 2012; pp. 191–293. [Google Scholar]
- Bringmann, G.; Gulder, T.; Hentschel, U.; Meyer, F.; Moll, H.; Morschhäuser, J.; Vanegas, P.S.; Ziebuhr, W.; Stich, A.; Brunn, R.; et al. Biofilm-Inhibition Effect and Anti-Infective Activity of N,C-Linked Aryl Isoquinolines and the Use Thereof. U.S. Patent 8,173,673, 8 May 2012. [Google Scholar]
- Althoff, K.; Schütt, C.; Steffen, R.; Batel, R.; Müller, W.E.G. Evidence for a symbiosis between bacteria of the genus Rhodobacter and the marine sponge Halichondria panicea: Harbor also for putatively-toxic bacteria? Mar. Biol. 1998, 130, 529–536. [Google Scholar] [CrossRef]
- Lorenz, B.; Batel, R.; Bachinski, N.; Müller, W.E.G.; Schröder, H.C. Purification of two exopolyphosphatases from the marine sponge Tethya lyncurium. Biochim. Biophys. Acta 1995, 1245, 17–28. [Google Scholar] [CrossRef]
- Lorenz, B.; Müller, W.E.G.; Kulaev, I.S.; Schröder, H.C. Purification and characterization of an exopolyphosphatase activity from Saccharomyces cerevisiae. J. Biol. Chem. 1994, 269, 22198–22204. [Google Scholar]
- Kornberg, A.; Rao, N.N.; Ault-Riché, D. Inorganic polyphosphate: A molecule of many functions. Annu. Rev. Biochem. 1999, 68, 89–125. [Google Scholar]
- Schröder, H.C.; Müller, W.E.G. Inorganic Polyphosphates: Biochemistry, Biology, Biotechnology; Springer Press: Berlin, Germany, 1999; pp. 1–317. [Google Scholar]
- Kulaev, I.S.; Vagabov, V.; Kulakovskaya, T. The Biochemistry of Inorganic Polyphosphates; John Wiley & Sons Inc.: New York, NY, USA, 2004; pp. 1–294. [Google Scholar]
- Kulakovskaya, T.V.; Vagabov, V.M.; Kulaev, I.S. Inorganic polyphosphate in industry, agriculture and medicine: Modern state and outlook. Proc. Biochem. 2012, 47, 1–10. [Google Scholar] [CrossRef]
- Wang, X.H.; Schröder, H.C.; Wiens, M.; Ushijima, H.; Müller, W.E.G. Bio-Silica and bio-polyphosphate: Applications in biomedicine (bone formation). Curr. Opin. Biotechnol. 2012, 23, 570–578. [Google Scholar] [CrossRef]
- Wang, G.; Yang, J. Influences of binder on fire protection and anticorrosion properties of intumescent fire resistive coating for steel structure. Surf. Coat. Technol. 2010, 204, 1186–1192. [Google Scholar] [CrossRef]
- Alvarez, S.; Jerez, C.A. Copper ions stimulate polyphosphate degradation and phosphate efflux in Acidithiobacillus ferrooxidans. Appl. Environ. Microbiol. 2004, 70, 5177–5182. [Google Scholar] [CrossRef]
- Harwood, V.J.; Gordon, A.S. Regulation of extracellular copper-binding proteins in copper-resistant and copper-sensitive mutants of Vibrio alginolyticus. Appl. Environ. Microbiol. 1994, 60, 1749–1753. [Google Scholar]
- Yoshida, A.; Kuramitsu, H.K. Multiple Streptococcus mutans genes are involved in biofilm formation. Appl. Environ. Microbiol. 2002, 68, 6283–6291. [Google Scholar] [CrossRef]
- Reszka, A.A.; Rodan, G.A. Mechanism of action of bisphosphonates. Curr. Osteoporos. Rep. 2003, 1, 45–52. [Google Scholar]
- Rogers, M.J. New insights into the molecular mechanisms of action of bisphosphonates. Curr. Pharm. Des. 2003, 9, 2643–2658. [Google Scholar]
- Moon, J.H.; Park, J.H.; Lee, J.Y. Antibacterial action of polyphosphate on Porphyromonas gingivalis. Antimicrob. Agents Chemother. 2011, 55, 806–812. [Google Scholar] [CrossRef]
- Wiens, M.; Schröder, H.C.; Korzhev, M.; Wang, X.H.; Batel, R.; Müller, W.E.G. Inducible ASABF-type antimicrobial peptide from the sponge Suberites domuncula: Microbicidal and hemolytic activity in vitro and toxic effect on molluscs in vivo. Mar. Drugs 2011, 9, 1969–1994. [Google Scholar] [CrossRef]
- Elion, G.B.; Singer, S.; Hitchings, G.H. Antagonists of nucleic acid derivatives. VIII. Synergism in combinations of biochemically related antimetabolites. J. Biol. Chem. 1954, 208, 477–488. [Google Scholar]
- Rao, N.N.; Gómez-Garcıa, M.R.; Kornberg, A. Inorganic polyphosphate: Essential for growth and survival. Annu. Rev. Biochem. 2009, 78, 605–647. [Google Scholar]
- Lee, W.; De La Barca, A.M.; Drake, D.; Doyle, R.J. Lectin-Oral streptococci interactions. J. Med. Microbiol. 1998, 47, 29–37. [Google Scholar] [CrossRef]
- Johnsen, A.R.; Hausner, M.; Schnell, A.; Wuertz, S. Evaluation of fluorescently labeled lectins for noninvasive localization of extracellular polymeric substances in Sphingomonas biofilms. Appl. Environ. Microbiol. 2000, 66, 3487–3491. [Google Scholar] [CrossRef]
- Miller, M.B.; Bassler, B.L. Quorum sensing in bacteria. Annu. Rev. Microbiol. 2001, 55, 165–199. [Google Scholar]
- Ling, V. Multidrug resistance: Molecular mechanisms and clinical relevance. Cancer Chemother. Pharmacol. 1997, 40, S3–S8. [Google Scholar] [CrossRef]
- Kurelec, B.; Krca, S.; Pivcevic, B.; Ugarkovic, D.; Bachmann, M.; Imsiecke, G.; Müller, W.E.G. Expression of P-glycoprotein gene in marine sponges. Identification and characterization of the 125-kDa drug-binding glycoprotein. Carcinogenesis 1992, 13, 69–76. [Google Scholar] [CrossRef]
- Waldmann, P.; Pivcevic, B.; Müller, W.E.G.; Zahn, R.K.; Kurelec, B. Increased genotoxicity of aminoanthracene by modulators of multixenobiotic resistance mechanism: Studies with the fresh water clam Corbicula fluminea. Mutat. Res. 1995, 342, 113–123. [Google Scholar] [CrossRef]
- Kurelec, B.; Pivcevic, B.; Müller, W.E.G. Determination of pollutants with multixenobiotic-resistance inhibiting properties. Mar. Environ. Res. 1995, 39, 261–265. [Google Scholar] [CrossRef]
- Müller, W.E.G.; Steffen, R.; Kurelec, B.; Smodlaka, N.; Puskaric, S.; Jagic, B.; Müller-Niklas, G.; Queric, N.V. Chemosensitizers of the multixenobiotic resistance in the amorphous aggregates (marine snow): Etiology of mass killing on the benthos in the Northern Adriatic? Environ. Toxicol. Pharmacol. 1998, 6, 229–238. [Google Scholar] [CrossRef]
- Zhu, J.; Winans, S.C. The quorum-sensing transcriptional regulator TraR requires its cognate signaling ligand for protein folding, protease resistance, and dimerization. Proc. Natl. Acad. Sci. USA 2001, 98, 1507–1512. [Google Scholar]
- Rashid, M.H.; Rao, N.N.; Kornberg, A. Inorganic polyphosphate is required for motility of bacterial pathogens. J. Bacteriol. 2000, 182, 225–227. [Google Scholar] [CrossRef]
- Domenico, P.; Reich, J.; Madonia, W.; Cunha, B.A. Resistance to bismuth among gram-negative bacteria is dependent upon iron and its uptake. J. Antimicrob. Chemother. 1996, 38, 1031–1040. [Google Scholar] [CrossRef]
- Santo, C.E.; Morais, P.V.; Grass, G. Isolation and characterization of bacteria resistant to metallic copper surfaces. Appl. Environ. Microbiol. 2010, 76, 1341–1348. [Google Scholar] [CrossRef]
- Georgopoulos, P.G.; Roy, A.; Yonone-Lioy, M.J.; Opiekun, R.E.; Lioy, P.J. Copper: Environmental dynamics and human exposure issues. J. Toxicol. Environ. Health Part B 2001, 4, 341–394. [Google Scholar] [CrossRef]
- Aston, N.S.; Watt, N.; Morton, I.E.; Tanner, M.S.; Evans, G.S. Copper toxicity affects proliferation and viability of human hepatoma cells (HepG2 line). Hum. Exp. Toxicol. 2000, 19, 367–376. [Google Scholar] [CrossRef]
- Lim, C.Y.; Yoo, Y.H.; Sidharthan, M.; Ma, C.W.; Bang, I.C.; Kim, J.M.; Lee, K.S.P.; Nam, S.; Shin, H.W. Effects of copper(I) oxide on growth and biochemical compositions of two marine microalgae. J. Environ. Biol. 2006, 27, 461–466. [Google Scholar]
- Tepel, M.; Heidenreich, S.; Schlüter, H.; Beinlich, A.; Nofer, J.R.; Walter, M.; Assmann, G.; Zidek, W. Diadenosine polyphosphates induce transplasma membrane calcium influx in cultured glomerular mesangial cells. Eur. J. Clin. Invest. 1996, 26, 1077–1084. [Google Scholar]
- Reusch, R.N. Biological complexes of poly-beta-hydroxybutyrate. FEMS Microbiol. Rev. 1992, 9, 119–129. [Google Scholar]
- Santi, I.; Grifantini, R.; Jiang, S.M.; Brettoni, C.; Grandi, G.; Wessels, M.R.; Soriani, M. CsrRS regulates group B Streptococcus virulence gene expression in response to environmental pH: A new perspective on vaccine development. J. Bacteriol. 2009, 191, 5387–5397. [Google Scholar] [CrossRef]
- Burne, R.A.; Wen, Z.T.; Chen, Y.Y.; Penders, J.E. Regulation of expression of the fructan hydrolase gene of Streptococcus mutans GS-5 by induction and carbon catabolite repression. J. Bacteriol. 1999, 181, 2863–2871. [Google Scholar]
- Müller, W.E.G.; Wang, X.H.; Diehl-Seifert, B.; Kropf, K.; Schloßmacher, U.; Lieberwirth, I.; Glasser, G.; Wiens, M.; Schröder, H.C. Inorganic polymeric phosphate/polyphosphate as an inducer of alkaline phosphatase and a modulator of intracellular Ca2+ level in osteoblasts (SaOS-2 cells) in vitro. Acta Biomater. 2011, 7, 2661–2671. [Google Scholar] [CrossRef]
- Sachs, L. Angewandte Statistik; Springer: Berlin, Germany, 1984; p. 242. [Google Scholar]
- Müller, W.E.G.; Zahn, R.K. Metabolism of 1-β-D-arabinofuranosyluracil in mouse L5178y cells. Cancer Res. 1979, 39, 1102–1107. [Google Scholar]
- King, T.C.; Schlessinger, D.; Krogstad, D.J. The assessment of antimicrobial combinations. Rev. Infect. Dis. 1981, 3, 627–633. [Google Scholar]
- Ault-Riché, D.; Fraley, C.D.; Tzeng, C.M.; Kornberg, A. A novel assay reveals multiple pathways regulating stress-induced accumulations of inorganic polyphosphate in Escherichia coli. J. Bacteriol. 1998, 180, 1841–1847. [Google Scholar]
- Müller, W.E.G.; Kasueske, M.; Wang, X.H.; Schröder, H.C.; Wang, Y.; Pisignano, D.; Wiens, M. Luciferase a light source for the silica-based optical waveguides (spicules) in the demosponge Suberites domuncula. Cell. Mol. Life Sci. 2009, 66, 537–552. [Google Scholar] [CrossRef]
- Compton, S.; Jones, C.G. Mechanism of dye response and interference in the Bradford protein assay. Anal. Biochem. 1985, 151, 369–374. [Google Scholar]
- Samples Availability: Available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Müller, W.E.G.; Wang, X.; Guo, Y.-W.; Schröder, H.C. Potentiation of the Cytotoxic Activity of Copper by Polyphosphate on Biofilm-Producing Bacteria: A Bioinspired Approach. Mar. Drugs 2012, 10, 2369-2387. https://doi.org/10.3390/md10112369
Müller WEG, Wang X, Guo Y-W, Schröder HC. Potentiation of the Cytotoxic Activity of Copper by Polyphosphate on Biofilm-Producing Bacteria: A Bioinspired Approach. Marine Drugs. 2012; 10(11):2369-2387. https://doi.org/10.3390/md10112369
Chicago/Turabian StyleMüller, Werner E. G., Xiaohong Wang, Yue-Wei Guo, and Heinz C. Schröder. 2012. "Potentiation of the Cytotoxic Activity of Copper by Polyphosphate on Biofilm-Producing Bacteria: A Bioinspired Approach" Marine Drugs 10, no. 11: 2369-2387. https://doi.org/10.3390/md10112369
APA StyleMüller, W. E. G., Wang, X., Guo, Y.-W., & Schröder, H. C. (2012). Potentiation of the Cytotoxic Activity of Copper by Polyphosphate on Biofilm-Producing Bacteria: A Bioinspired Approach. Marine Drugs, 10(11), 2369-2387. https://doi.org/10.3390/md10112369






