Glycine Betaine and β-Aminobutyric Acid Mitigate the Detrimental Effects of Heat Stress on Chinese Cabbage (Brassica rapa L. ssp. pekinensis) Seedlings with Improved Photosynthetic Performance and Antioxidant System
Abstract
:1. Introduction
2. Results
2.1. Effects of GB and BABA on the Growth of Chinese Cabbage Seedlings under Heat Stress
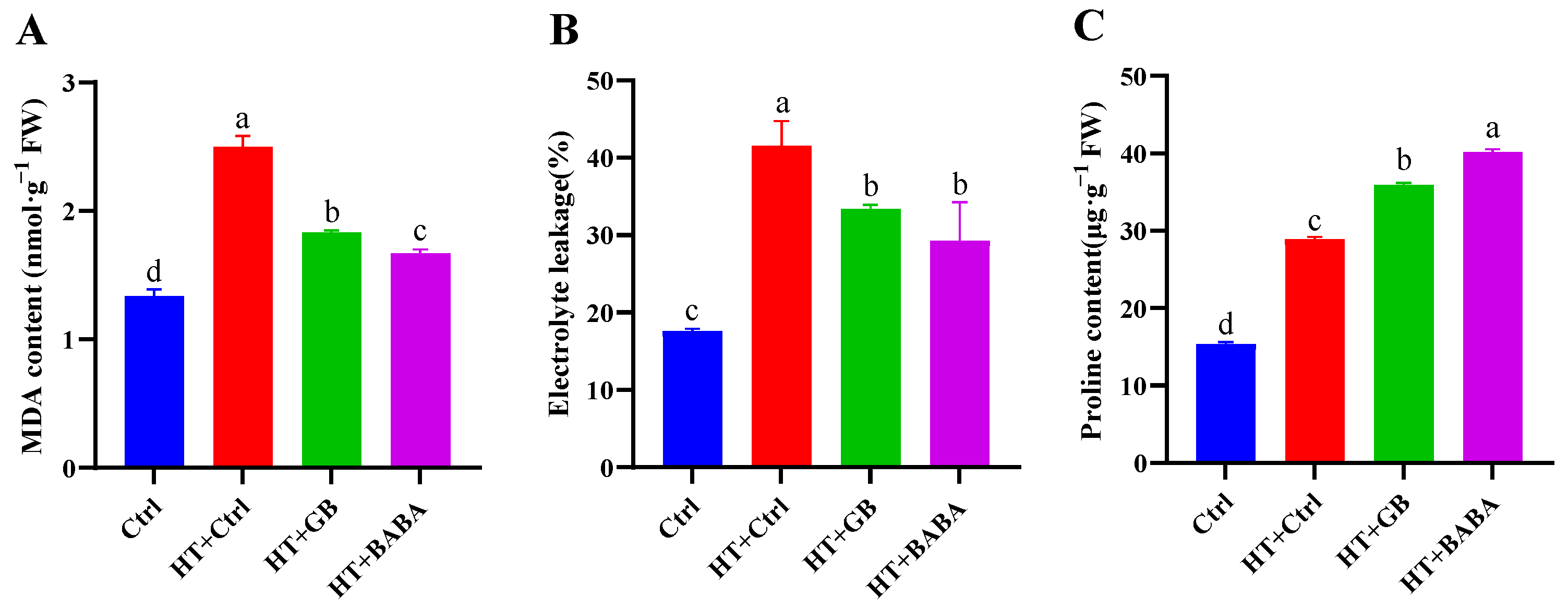
2.2. Effects of GB and BABA on MDA, Electrolyte Leakage, and Proline Content in Chinese Cabbage under Heat Stress
2.3. Effects of GB and BABA on the Photochemical Efficiency of Chinese Cabbage under Heat Stress
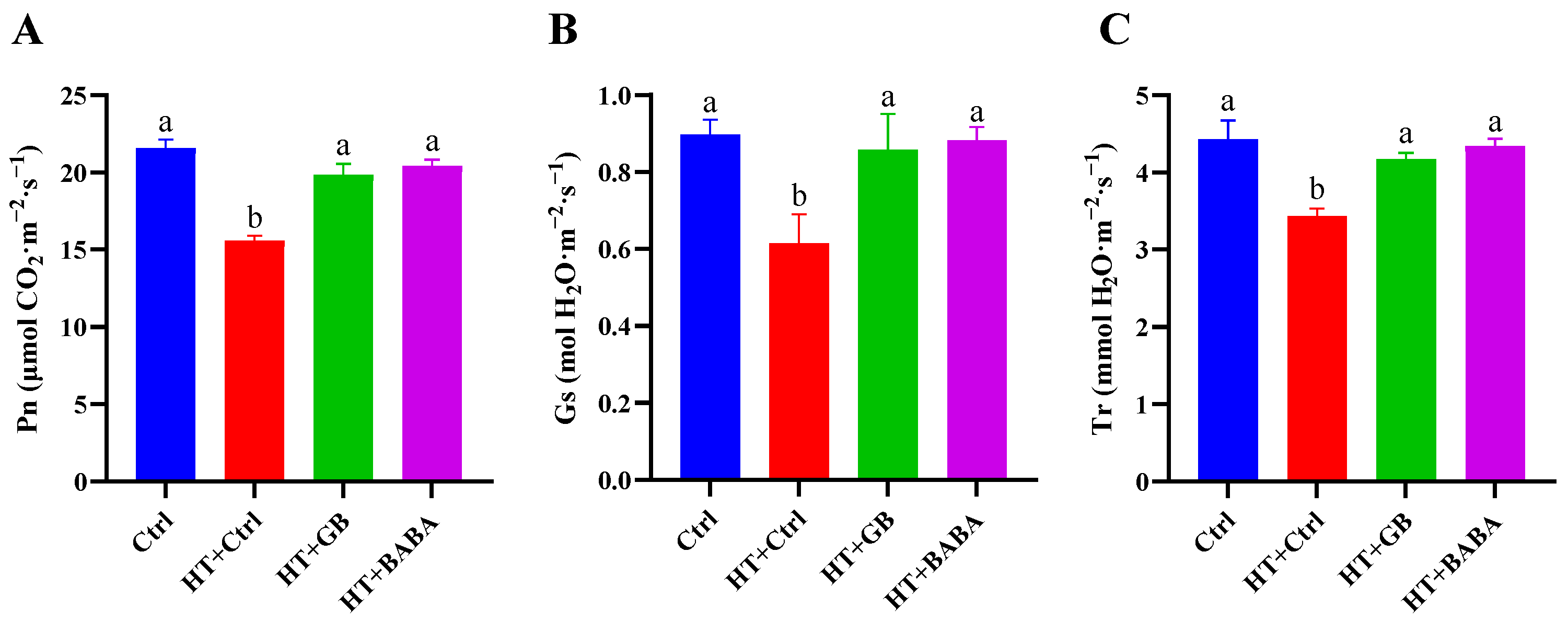
2.4. Effects of GB and BABA on the Gas Exchange Parameters of Chinese Cabbage under Heat Stress
2.5. Effects of GB and BABA on Antioxidant Enzyme Activity in Chinese Cabbage under Heat Stress
3. Discussion
3.1. GB and BABA Promote Plant Growth by Enhancing Thermotolerance in Chinese Cabbage Seedlings
3.2. GB and BABA Maintain Higher Photosynthesis in Chinese Cabbage Seedlings under Heat Stress
3.3. GB and BABA Increase the Activity Levels of Antioxidant Enzymes and Proline Content in Chinese Cabbage Seedlings under Heat Stress
4. Materials and Methods
4.1. Plant Materials and Treatments
4.2. Measurement of Growth Parameters
4.3. Measurement of Gas Exchange Parameters
4.4. Measurement of Chlorophyll Fluorescence Parameters
4.5. Chlorophyll Content
4.6. Electrolyte Leakage, and MDA (Malondialdehyde) and Proline Contents
4.7. Antioxidant Enzyme Activity Assays
4.8. Statistical Analyses
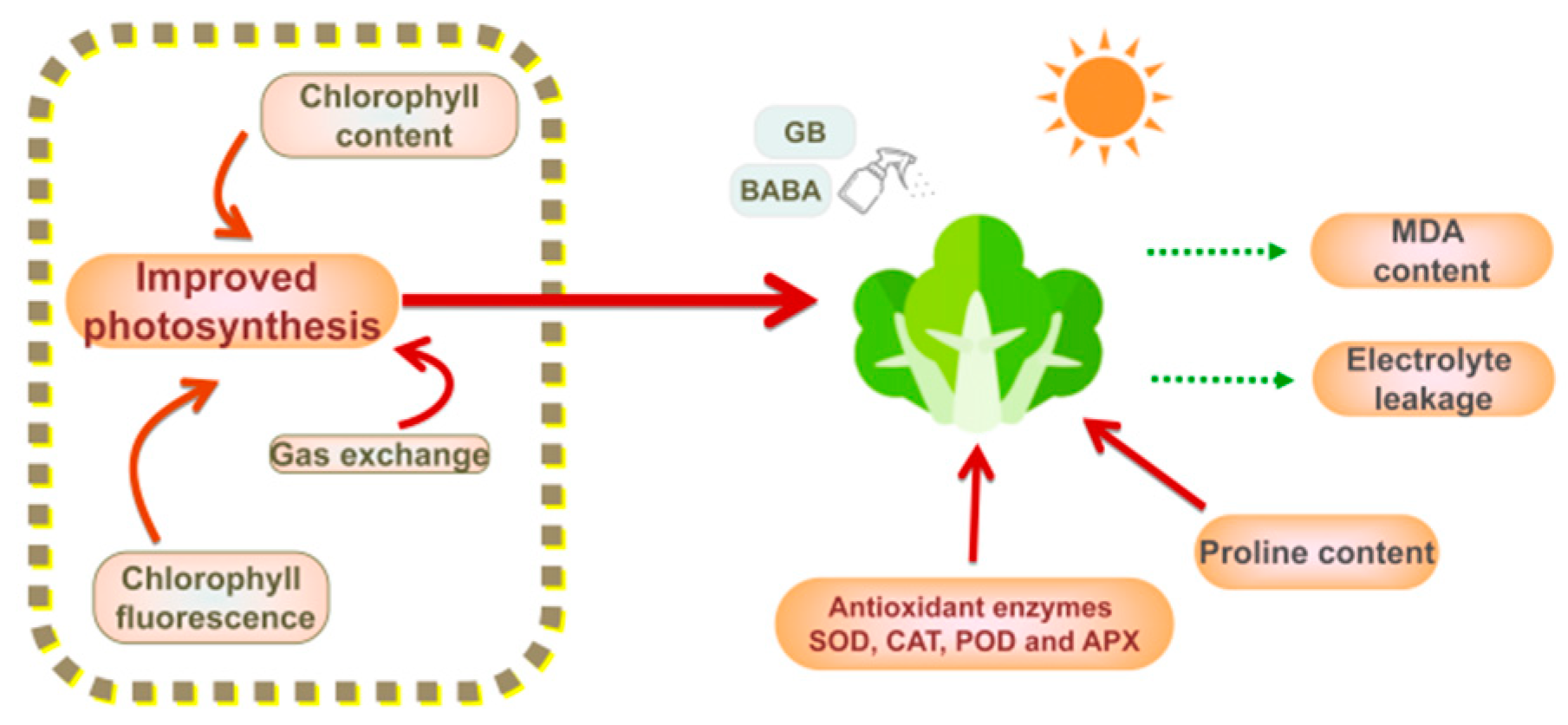
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhou, B.; Chao, Q.; Huang, L. The core conclusions and interpretation of working group I contribution to the fifth assessment report of the intergovernmental panel on climate change. Chin. J. Urban Env. Stu. 2015, 3, 1550003. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change (IPCC). Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, C.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; p. 1535. [Google Scholar]
- Rajametov, S.N.; Yang, E.Y.; Jeong, H.B.; Cho, M.C.; Chae, S.Y.; Paudel, N. Heat treatment in two tomato cultivars: A study of the effect on physiological and growth recovery. Horticulturae 2021, 7, 119. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Alam, M.M.; Rahman, A.; Hasanuzzaman, M.; Nahar, K.; Fujita, M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef] [PubMed]
- Jahan, M.S.; Guo, S.; Sun, J.; Shu, S.; Wang, Y.; El-Yazied, A.A.; Alabdallah, N.M.; Hikal, M.; Mohamed, M.H.M.; Ibrahim, M.F.M.; et al. Melatonin-mediated photosynthetic performance of tomato seedlings under high-temperature stress. Plant Physiol. Bioch. 2021, 167, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Battisti, D.S.; Naylor, R.L. Historical warnings of future food insecurity with unprecedented seasonal heat. Science 2009, 323, 240–244. [Google Scholar] [CrossRef] [Green Version]
- Mathur, S.; Agrawal, D.; Jajoo, A. Photosynthesis: Response to high temperature stress. J. Photoch. Photobio. B 2014, 137, 116–126. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef] [Green Version]
- Sharma, D.K.; Andersen, S.B.; Ottosen, C.O.; Rosenqvist, E. Phenotyping of wheat cultivars for heat tolerance using chlorophyll a fluorescence. Funct. Plant Biol. 2012, 39, 936–947. [Google Scholar] [CrossRef]
- Wang, Q.L.; Chen, J.H.; He, N.Y.; Guo, F.Q. Metabolic reprogramming in chloroplasts under heat stress in plants. Int. J. Mol. Sci. 2018, 19, 849. [Google Scholar] [CrossRef] [Green Version]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic stress and reactive oxygen species: Generation, signaling, and defense mechanisms. Antioxidants 2021, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Roychoudhury, A. reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef] [Green Version]
- Buttar, Z.A.; Wu, S.N.; Arnao, M.B.; Wang, C.; Ullah, I.; Wang, C. Melatonin suppressed the heat stress-induced damage in wheat seedlings by modulating the antioxidant machinery. Plants 2020, 9, 809. [Google Scholar] [CrossRef] [PubMed]
- Stephenie, S.; Chang, Y.P.; Gnanasekaran, A.; Esa, N.M.; Gnanaraj, C. An insight on superoxide dismutase (SOD) from plants for mammalian health enhancement. J. Funct. Foods 2020, 68, 103917. [Google Scholar] [CrossRef]
- Jouili, H.; Bouazizi, H.; Ferjani, E.E. Plant peroxidases: Biomarkers of metallic stress. Acta. Physiol. Plant 2011, 33, 2075. [Google Scholar] [CrossRef]
- Almagro, L.; Gómez Ros, L.V.; Belchi-Navarro, S.; Bru, R.; Ros Barceló, A.; Pedreño, M.A. Class III peroxidases in plant defence reactions. J. Exp Bot. 2009, 2, 377–390. [Google Scholar] [CrossRef] [Green Version]
- Sofo, A.; Scopa, A.; Nuzzaci, M.; Vitti, A. Ascorbate peroxidase and catalase activities and their genetic regulation in plants subjected to drought and salinity stresses. Int. J. Mol. Sci. 2015, 16, 13561–13578. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Euring, D.; Cha, J.Y.; Lin, Z.; Lu, M.; Huang, L.-J.; Kim, W.Y. Plant hormone-mediated regulation of heat tolerance in response to global climate change. Front. Plant Sci. 2021, 11, 2318. [Google Scholar] [CrossRef]
- Halpern, M.; Bar-Tal, A.; Ofek, M.; Minz, D.; Muller, T.; Yermiyahu, U. The use of biostimulants for enhancing nutrient uptake. Adv. Agron. 2015, 130, 141–174. [Google Scholar] [CrossRef]
- Kumari, R.; Kaur, I.; Bhatnagar, A.K. Effect of aqueous extract of Sargassum johnstonii Setchell & Gardner on growth, yield and quality of Lycopersicon esculentum Mill. J. Appl. Phycol. 2011, 23, 623–633. [Google Scholar] [CrossRef]
- Kałużewicz, A.; Krzesiński, W.; Spiżewski, T.; Zaworska, A. Effect of biostimulants on several physiological characteristics and chlorophyll content in broccoli under drought stress and re-watering. Not. Bot. Horti Agrobo. 2017, 45, 197–202. [Google Scholar] [CrossRef] [Green Version]
- Sadeghi, H.; Taban, A. Crushed maize seeds enhance soil biological activity and salt tolerance in caper (Capparis spinosa L.). Ind. Crop Prod. 2021, 160, 113103. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Toward a sustainable agriculture through plant biostimulants: From experimental data to practical applications. Agronomy 2020, 10, 1461. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef] [Green Version]
- Colla, G.; Hoagland, L.; Ruzzi, M.; Cardarelli, M.; Bonini, P.; Canaguier, R.; Rouphael, Y. Biostimulant action of protein hydrolysates: Unraveling their effects on plant physiology and microbiome. Front. Plant Sci. 2017, 8, 2202. [Google Scholar] [CrossRef] [Green Version]
- Nardi, S.; Pizzeghello, D.; Schiavon, M.; Ertani, A. Plant biostimulants: Physiological responses induced by protein hydrolyzed-based products and humic substances in plant metabolism. Sci. Agric. 2016, 73, 18–23. [Google Scholar] [CrossRef] [Green Version]
- Islam, S.; Parrey, Z.A.; Shah, S.H.; Mohammad, F. Glycine betaine mediated changes in growth, photosynthetic efficiency, antioxidant system, yield and quality of mustard. Sci. Hortic. 2021, 285, 110170. [Google Scholar] [CrossRef]
- Annunziata, M.G.; Ciarmiello, L.F.; Woodrow, P.; Aversana, E.D.; Carillo, P. Spatial and temporal profile of glycine betaine accumulation in plants under abiotic stresses. Front. Plant Sci. 2019, 10, 230. [Google Scholar] [CrossRef] [Green Version]
- Min, K.; Cho, Y.; Kim, E.; Lee, M.; Lee, S.R. Exogenous glycine betaine application improves freezing tolerance of cabbage (Brassica oleracea L.) leaves. Plants 2021, 10, 2821. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Ashraf, M.; Siddique, K.H.M. Role of glycine betaine in the thermotolerance of plants. Agronomy 2022, 12, 276. [Google Scholar] [CrossRef]
- Li, D.; Wang, M.; Zhang, T.; Chen, X.; Li, C.; Liu, Y.; Brestic, M.; Yang, X. Glycinebetaine mitigated the photoinhibition of photosystem II at high temperature in transgenic tomato plants. Photosynth Res. 2021, 147, 301–315. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hou, Y.; Wang, Y.; Hu, S.; Zheng, Y.; Jin, P. Genome-wide identification of heat shock transcription factors and potential role in regulation of antioxidant response under hot water and glycine betaine treatments in cold-stored peaches. J. Sci. Food Agric. 2022, 102, 628–643. [Google Scholar] [CrossRef] [PubMed]
- Allakhverdiev, S.I.; Los, D.A.; Mohanty, P.; Nishiyama, Y.; Murata, N. Glycinebetaine alleviates the inhibitory effect of moderate heat stress on the repair of photosystem II during photoinhibition. Biochim. Biophys. Acta (BBA) Bioenerg. 2007, 1767, 1363–1371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakamoto, A.; Murata, N. The role of glycine betaine in the protection of plants from stress: Clues from transgenic plants. Plant Cell Environ. 2002, 25, 163–171. [Google Scholar] [CrossRef]
- Zhang, T.; Li, Z.; Li, D.; Li, C.; Wei, D.; Li, S.; Liu, Y.; Chen, T.H.H.; Yang, X. Comparative effects of glycinebetaine on the thermotolerance in codA- and BADH-transgenic tomato plants under high temperature stress. Plant Cell Rep. 2020, 39, 1525–1538. [Google Scholar] [CrossRef]
- Wang, G.P.; Li, F.; Zhang, J.; Zhao, M.R.; Hui, Z.; Wang, W. Overaccumulation of glycine betaine enhances tolerance of the photosynthetic apparatus to drought and heat stress in wheat. Photosynthetica 2010, 48, 30–41. [Google Scholar] [CrossRef]
- Wang, G.; Wang, J.; Xue, X.; Lu, C.; Chen, R.; Wang, L.; Han, X. Foliar spraying of glycine betaine lowers photosynthesis inhibition of Malus hupehensis leaves under drought and heat stress. Int. J. Agric. Biol. 2020, 23, 1121–1128. [Google Scholar] [CrossRef]
- Sorwong, A.; Sakhoneasee, S. Foliar application of glycine betaine mitigates the effect of heat stress in three Marigold (Tagetes erecta) cultivars. Hortic. J. 2015, 84, 161–171. [Google Scholar] [CrossRef] [Green Version]
- Wahid, A.; Shabbir, A. Induction of heat stress tolerance in barley seedlings by pre-sowing seed treatment with glycinebetaine. Plant Growth Regul. 2005, 46, 133–141. [Google Scholar] [CrossRef]
- Thevenet, D.; Pastor, V.; Baccelli, I.; Balmer, A.; Vallat, A.; Neier, R.; Glauser, G.; Mauch-Mani, B. The priming molecule β-aminobutyric acid is naturally present in plants and is induced by stress. New Phytol. 2017, 213, 552–559. [Google Scholar] [CrossRef]
- Ma, X.H.; Xu, J.Y.; Han, D.; Huang, W.X.; Dang, B.J.; Jia, W.; Xu, Z.C. Combination of β-aminobutyric acid and Ca2+ alleviates chilling stress in tobacco (Nicotiana tabacum L.). Front. Plant Sci. 2020, 11, 556. [Google Scholar] [CrossRef] [PubMed]
- Zimmerli, L.; Hou, B.H.; Tsai, C.H.; Jakab, G.; Mauch-Mani, B.; Somerville, S. The xenobiotic beta-aminobutyric acid enhances Arabidopsis thermotolerance. Plant J. 2008, 53, 144–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mostek, A.; Börner, A.; Weidner, S. Comparative proteomic analysis of β-aminobutyric acid-mediated alleviation of salt stress in barley. Plant Physiol. Biochem. 2016, 99, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Cao, S.; Wang, K.; Lei, C.; Ji, N.; Xu, F.; Jiang, Y.; Qiu, L.; Zheng, Y. Heat shock protein HSP24 Is Involved in the BABA-induced resistance to fungal pathogen in postharvest grapes underlying an NPR1-dependent manner. Front. Plant Sci. 2021, 12, 812672. [Google Scholar] [CrossRef]
- Cohen, Y.R. β-Aminobutyric acid-induced resistance against plant pathogens. Plant Dis. 2002, 86, 448–457. [Google Scholar] [CrossRef] [Green Version]
- Hamiduzzaman, M.; Jakab, G.; Barnavon, L.; Neuhaus, J.M.; Brigitte, M.M. β-Aminobutyric acid-induced resistance against downy mildew in grapevine acts through the potentiation of callose formation and jasmonic acid signaling. Mol. Plant Microbe Interact. 2005, 18, 819–829. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Cao, S.; Wang, L.; Wang, X.; Jin, P.; Zheng, Y. Effect of β-aminobutyric acid on disease resistance against Rhizopus rot in harvested peaches. Front. Microbiol. 2018, 9, 1505. [Google Scholar] [CrossRef]
- Park, E.J.; Jeknic, Z.; Chen, T.H.H. Exogenous application of glycinebetaine increases chilling tolerance in tomato plants. Plant Cell Physiol. 2006, 47, 706–714. [Google Scholar] [CrossRef] [Green Version]
- Fu, I.M.; Shennan, C.; Welbaum, G.E. Evaluating Chinese cabbage cultivars for high temperature tolerance. In New Crops, Proceedings of the Second National Symposium New Crops: Exploration, Research, and Commercialization Indianapolis, IN, USA, 6–9 October 1993; Wiley: Hoboken, NJ, USA, 1993; pp. 570–573. [Google Scholar]
- Guo, J.; Jin, J.; Tang, Y.; Wu, X. Design of temperature insurance index and risk zonation for single-season rice in response to high-temperature and low-temperature damage: A case study of Jiangsu province, China. Int. J. Environ. Res. Public Health 2019, 16, 1187. [Google Scholar] [CrossRef] [Green Version]
- Rehmani, M.I.A.; Ding, C.; Li, G.; Ata-Ul-Karim, S.T.; Hadifa, A.; Bashir, M.A.; Hashem, M.; Alamri, S.; Al-Zubair, F.; Ding, Y. Vulnerability of rice production to temperature extremes during rice reproductive stage in Yangtze River Valley, China. J. King Saud. Univ. Sci. 2021, 33, 101599. [Google Scholar] [CrossRef]
- Kuo, C.G.; Tsay, J.S. Physiological responses of Chinese cabbage under high temperature. In Chinese Cabbage. Proceedings of the First International Symposium, Budapest, Hungary, 30 September–2 October 1981; Avrdc: Shanhua, Taiwan, 1981; pp. 217–224. [Google Scholar]
- Rimmer, R.S.; Shattuck, V.I.; Buchwaldt, L. Compendium of Brassica Diseases; APS Press: New York, NY, USA, 2007; ISBN -13978-0-89054-344-3. [Google Scholar]
- Daly, P.; Tomkins, B. Production and postharvest handling of Chinese cabbage (Brassica rapa var. pekinensis); Rural Industries Research and Development Corporation: Kingston, Australia, 1997; No. 97/1. [Google Scholar]
- El-Afifi, S.T.; Zaghloul, M.M.; El-Sawy, M.B.I.; Hashim, A.M.A. Effect of starter solutions in soil and foliar spray with some stimulants on growth and productivity of Chinese cabbage. Int. J. Plant Prod. 2014, 5, 21–40. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oukarroum, A.; El Madidi, S.; Strasser, R.J. Exogenous glycine betaine and proline play a protective role in heat-stressed barley leaves (Hordeum vulgare L.): A chlorophyll a fluorescence study. Plant Biosyst. 2012, 146, 1037–1043. [Google Scholar] [CrossRef]
- Sós-Hegedűs, A.; Juhász, Z.; Poór, P.; Kondrák, M.; Antal, F.; Tari, I.; Mauch-Mani, B.; Bánfalvi, Z. Soil drench treatment with ß-aminobutyric acid increases drought tolerance of potato. PLoS ONE 2014, 9, e114297. [Google Scholar] [CrossRef] [PubMed]
- Shafiq, S.; Akram, N.A.; Ashraf, M.; García-Caparrós, P.; Ali, O.M.; Latef, A.A.H.A. Influence of glycine betaine (natural and synthetic) on growth, metabolism and yield production of drought-stressed maize (Zea mays L.) plants. Plants 2021, 10, 2540. [Google Scholar] [CrossRef]
- Shaw, A.K.; Bhardwaj, P.K.; Ghosh, S.; Roy, S.; Saha, S.; Sherpa, A.R.; Saha, S.K.; Hossain, Z. β-aminobutyric acid mediated drought stress alleviation in maize (Zea mays L.). Environ. Sci. Pollut. R. 2016, 23, 2437–2453. [Google Scholar] [CrossRef]
- Bokari, U.G. Chlorophyll, dry matter, and photosynthetic conversion-efficiency relationships in warm-season grasses. J. Range Manage. 1983, 36, 431–434. [Google Scholar] [CrossRef]
- Cristofano, F.; El-Nakhel, C.; Rouphael, Y. Biostimulant substances for sustainable agriculture: Origin, operating mechanisms and effects on cucurbits, leafy greens, and nightshade vegetables species. Biomolecules 2021, 11, 1103. [Google Scholar] [CrossRef]
- Li, D.; Zhang, T.; Wang, M.; Liu, Y.; Brestic, M.; Chen, T.H.H.; Yang, X. Genetic engineering of the biosynthesis of glycine betaine modulates phosphate homeostasis by regulating phosphate acquisition in tomato. Front. Plant Sci. 2019, 9, 1995. [Google Scholar] [CrossRef] [Green Version]
- Krouk, G.; Kiba, T. Nitrogen and phosphorus interactions in plants: From agronomic to physiological and molecular insights. Curr. Opin. Plant Biol. 2020, 57, 104–109. [Google Scholar] [CrossRef]
- Collado-González, J.; Piñero, M.C.; Otálora, G.; López-Marín, J.; Del Amor, F.M. Effects of different nitrogen forms and exogenous application of putrescine on heat stress of cauliflower: Photosynthetic gas exchange, mineral concentration and lipid peroxidation. Plants 2021, 10, 152. [Google Scholar] [CrossRef] [PubMed]
- Kader, M.H.A.; CJea, S.X. Reduction in photosynthesis of cotton seedling under water and salinity stresses is induced by both stomatal and non-stomatal limitations. J. Irrig. Drain. Eng. 2020, 39, 13–18. [Google Scholar] [CrossRef]
- Hamani, A.K.M.; Li, S.; Chen, J.; Amin, A.S.; Wang, G.; Xiaojun, S.; Zain, M.; Gao, Y. Linking exogenous foliar application of glycine betaine and stomatal characteristics with salinity stress tolerance in cotton (Gossypium hirsutum L.) seedlings. BMC Plant Biol. 2021, 21, 146. [Google Scholar] [CrossRef] [PubMed]
- Rajasekaran, L.R.; Kriedemann, P.E.; Aspinall, D.; Paleg, L.G. Physiological significance of proline and glycinebetaine: Maintaining photosynthesis during NaCl stress in wheat. Photosynthetica 1998, 34, 357–366. [Google Scholar] [CrossRef]
- Shemi, R.; Wang, R.; Gheith, E.M.S.; Hussain, H.A.; Hussain, S.; Irfan, M.; Cholidah, L.; Zhang, K.; Zhang, S.; Wang, L. Effects of salicylic acid, zinc and glycine betaine on morpho-physiological growth and yield of maize under drought stress. Sci. Rep. 2021, 11, 3195. [Google Scholar] [CrossRef]
- Li, C.; Ma, M.; Zhang, T.; Feng, P.; Chen, X.; Liu, Y.; Brestic, M.; Galal, T.M.; Al-Asi, H.M.; Yang, X. Comparison of photosynthetic activity and heat tolerance between near isogenic lines of wheat with different photosynthetic rates. PLoS ONE 2021, 16, e0255896. [Google Scholar] [CrossRef]
- Brestic, M.; Zivcak, M.; Hauptvogel, P.; Misheva, S.; Kocheva, K.; Yang, X.; Li, X.; Allakhverdiev, S.I. Wheat plant selection for high yields entailed improvement of leaf anatomical and biochemical traits including tolerance to non-optimal temperature conditions. Photsynth. Res. 2018, 136, 245–255. [Google Scholar] [CrossRef]
- Haworth, M.; Marino, G.; Centritto, M. An introductory guide to gas exchange analysis of photosynthesis and its application to plant phenotyping and precision irrigation to enhance water use efficiency. J. Water Clim. Chang. 2018, 4, 786–808. [Google Scholar] [CrossRef] [Green Version]
- Müller, M.; Munné-Bosch, S. Hormonal impact on photosynthesis and photoprotection in plants. Plant Physiol. 2021, 185, 1500–1522. [Google Scholar] [CrossRef]
- Woodward, F. Stomatal numbers are sensitive to increase in CO2 from pre-industrial levels. Nature 1987, 327, 617–618. [Google Scholar] [CrossRef]
- Bita, C.E.; Gerats, T. Plant tolerance to high temperature in a changing environment: Scientific fundamentals and production of heat stress-tolerant crops. Front. Plant Sci. 2013, 4, 273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faseela, P.; Sinisha, A.K.; Brestic, M.; Puthur, J.T. Special issue in honour of Prof. Reto J. Strasser—Chlorophyll a fluorescence parameters as indicators of a particular abiotic stress in rice. Photosynthetica 2020, 58, 293–300. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Zhang, W.; Li, D.; Zhou, F.; Chen, X.; Li, C.; Yang, X. Glycinebetaine: A versatile protectant to improve rice performance against aluminium stress by regulating aluminium uptake and translocation. Plant Cell Rep. 2021, 40, 2397–2407. [Google Scholar] [CrossRef] [PubMed]
- Yudina, L.; Sukhova, E.; Gromova, E.; Nerush, V.; Vodeneev, V.; Sukov, V. A light-induced decrease in the photochemical reflectance index (PRI) can be used to estimate the energy-dependent component of non-photochemical quenching under heat stress and soil drought in pea, wheat, and pumpkin. Photosynth. Res. 2020, 146, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Ibaraki, Y. Time course of the photochemical reflectance index during photosynthetic induction: Its relationship with the photochemical yield of photosystem II. Physiol Plant. 2019, 165, 524–536. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.K.; Desai, T.S.; Mohanty, P. Temperature dependent alterations in the pattern of photochemical and non-photochemical quenching and associated changes in the photosystem II conditions of the leaves. Plant Cell Physiol. 1995, 36, 1221–1227. [Google Scholar] [CrossRef]
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef] [Green Version]
- Raza, M.A.S.; Saleem, M.F.; Shah, G.M.; Khan, I.H.; Raza, A. Exogenous application of glycinebetaine and potassium for improving water relations and grain yield of wheat under drought. J. Soil Sci. Plant Nut. 2014, 14, 348–364. [Google Scholar] [CrossRef] [Green Version]
- Shehzadi, A.; Akram, N.A.; Ali, A.; Ashraf, M. Exogenously applied glycinebetaine induced alteration in some key physio-biochemical attributes and plant anatomical features in water stressed oat (Avena sativa L.) plant. J. Arid Land 2019, 11, 292–305. [Google Scholar] [CrossRef] [Green Version]
- Ali, S.; Abbas, Z.; Seleiman, M.F.; Rizwan, M.; Yavaş, İ.; Alhammad, B.A.; Kalderis, D. Glycine betaine accumulation, significance and interests for heavy metal tolerance in plants. Plants 2020, 9, 896. [Google Scholar] [CrossRef]
- Larkindale, J.; Hall, J.D.; Knight, M.R.; Vierling, E. Heat stress phenotypes of Arabidopsis mutants implicate multiple signalling pathways in the acquisition of thermotolerance. Plant Physiol. 2005, 138, 882–897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Z.; Wei, X.; Wan, D.; He, W.; Wang, X.; Xiong, Y. Effect of molybdenum on plant physiology and cadmium uptake and translocation in rape (Brassica napus L.) under different levels of cadmium stress. Int. J. Environ. Res. Public Health 2020, 17, 2355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rolfe, S.A.; Scholes, J.D. Chlorophyll fluorescence imaging of plant-pathogen interactions. Protoplasma 2010, 247, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Zafari, M.; Ebadi, A.; Jahanbakhsh, S.; Sedghi, M. Safflower (Carthamus tinctorius) biochemical properties, yield, and oil content affected by 24-epibrassinosteroid and genotype under drought stress. J. Agric. Food Chem. 2020, 68, 6040–6047. [Google Scholar] [CrossRef] [PubMed]

| Treatment | Fresh Weight (g) | Dry Weight (g) | Leaf Length (cm) | Leaf Width (cm) | Plant Height (cm) | Hypocotyl Diameter (mm) | SPAD |
|---|---|---|---|---|---|---|---|
| Ctrl | 5.78 ± 0.24 a | 0.59 ± 0.02 a | 17.47 ± 0.28 a | 7.40 ± 0.14 a | 17.97 ± 0.29 a | 3.02 ± 0.02 a | 44.57 ± 0.38 a |
| HT + Ctrl | 3.51 ± 0.17 c | 0.33 ± 0.02 c | 11.63 ± 0.35 c | 4.67 ± 0.31 c | 12.60 ± 0.36 c | 2.25 ± 0.06 c | 29.13 ± 0.49 c |
| HT + GB | 4.42 ± 0.16 b | 0.45 ± 0.01 b | 14.59 ± 0.36 b | 5.06 ± 0.24 c | 14.79 ± 0.32 b | 2.41 ± 0.06 b | 31.97 ± 0.33 b |
| HT + BABA | 4.72 ± 0.10 b | 0.48 ± 0.01 b | 15.83 ± 0.32 b | 5.93 ± 0.29 b | 15.07 ± 0.33 b | 2.52 ± 0.05 b | 33.63 ± 0.25 b |
| Treatment | Fv/Fm | ETR | ΦPSII | qN | qP |
|---|---|---|---|---|---|
| Ctrl | 0.82 ± 0.04 a | 40.18 ± 1.03 a | 0.55 ± 0.79 a | 0.39 ± 0.01 ab | 0.76 ± 0.05 a |
| HT + Ctrl | 0.77 ± 0.04 c | 31.70 ± 0.13 c | 0.37 ± 0.01 c | 0.54 ± 0.03 b | 0.56 ± 0.02 c |
| HT + GB | 0.79 ± 0.02 b | 36.43 ± 0.91 b | 0.45 ± 0.03 b | 0.48 ± 0.05 a | 0.67 ± 0.03 b |
| HT + BABA | 0.80 ± 0.04 b | 38.73 ± 0.86 b | 0.47 ± 0.06 b | 0.48 ± 0.04 a | 0.68 ± 0.01 b |
| Treatments | Antioxidant Enzyme Activity (U·g−1 FW) | |||
|---|---|---|---|---|
| SOD | APX | CAT | POD | |
| Ctrl | 205.26 ± 0.52 c | 0.60 ± 0.31 b | 248.55 ± 0.21 c | 27.23 ± 1.39 c |
| HT + Ctrl | 255.31 ± 0.32 b | 0.56 ± 0.13 c | 333.44 ± 0.12 b | 32.29 ± 1.45 b |
| HT + GB | 261.54 ± 0.42 a | 0.63 ± 0.25 a | 367.89 ± 0.14 a | 37.58 ± 1.61 a |
| HT + BABA | 272.33 ± 0.49 a | 0.65 ± 0.26 a | 381.61 ± 0.11 a | 39.36 ± 1.63 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quan, J.; Zheng, W.; Wu, M.; Shen, Z.; Tan, J.; Li, Z.; Zhu, B.; Hong, S.-B.; Zhao, Y.; Zhu, Z.; et al. Glycine Betaine and β-Aminobutyric Acid Mitigate the Detrimental Effects of Heat Stress on Chinese Cabbage (Brassica rapa L. ssp. pekinensis) Seedlings with Improved Photosynthetic Performance and Antioxidant System. Plants 2022, 11, 1213. https://doi.org/10.3390/plants11091213
Quan J, Zheng W, Wu M, Shen Z, Tan J, Li Z, Zhu B, Hong S-B, Zhao Y, Zhu Z, et al. Glycine Betaine and β-Aminobutyric Acid Mitigate the Detrimental Effects of Heat Stress on Chinese Cabbage (Brassica rapa L. ssp. pekinensis) Seedlings with Improved Photosynthetic Performance and Antioxidant System. Plants. 2022; 11(9):1213. https://doi.org/10.3390/plants11091213
Chicago/Turabian StyleQuan, Jin, Weiwei Zheng, Meifang Wu, Zhuojun Shen, Jingru Tan, Zewei Li, Biao Zhu, Seung-Beom Hong, Yanting Zhao, Zhujun Zhu, and et al. 2022. "Glycine Betaine and β-Aminobutyric Acid Mitigate the Detrimental Effects of Heat Stress on Chinese Cabbage (Brassica rapa L. ssp. pekinensis) Seedlings with Improved Photosynthetic Performance and Antioxidant System" Plants 11, no. 9: 1213. https://doi.org/10.3390/plants11091213
APA StyleQuan, J., Zheng, W., Wu, M., Shen, Z., Tan, J., Li, Z., Zhu, B., Hong, S.-B., Zhao, Y., Zhu, Z., & Zang, Y. (2022). Glycine Betaine and β-Aminobutyric Acid Mitigate the Detrimental Effects of Heat Stress on Chinese Cabbage (Brassica rapa L. ssp. pekinensis) Seedlings with Improved Photosynthetic Performance and Antioxidant System. Plants, 11(9), 1213. https://doi.org/10.3390/plants11091213








