Abstract
Taraxerol is a pentacyclic triterpenoid that is actively produced by some higher plants as part of a defense mechanism. The biosynthesis of taraxerol in plants occurs through the mevalonate pathway in the cytosol, in which dimethylallyl diphosphate (DMAPP) and isopentyl pyrophosphate (IPP) are first produced, followed by squalene. Squalene is the primary precursor for the synthesis of triterpenoids, including taraxerol, β-amyrin, and lupeol, which are catalyzed by taraxerol synthase. Taraxerol has been extensively investigated for its medicinal and pharmacological properties, and various biotechnological approaches have been established to produce this compound using in vitro techniques. This review provides an in-depth summary of the hypothesized taraxerol biosynthetic pathway, the medicinal properties of taraxerol, and recent developments on tissue culture for the in vitro production of taraxerol.
1. Introduction
Despite recent advances in combinatorial chemistry, and other means of synthesis methods towards the production of essential drugs in healthcare, naturally derived compounds are still an invaluable source of medicine [1]. This is due to the minimal side effects and relatively higher biological activity of natural drugs compared to synthetic drugs [2]. Although the process of discovering effective drugs from natural raw materials is time consuming, costly, and less sustainable, advances in biotechnology could be helpful for such efforts [3]. In fact, natural products have played an important role in drug development whereby a considerable number of drugs are derived from naturally occurring compounds [4]. Today, there are more than 250 naturally derived drugs that are manufactured at large scales in the healthcare industries such as morphine, cephalosporin, and paclitaxel [5].
Taraxerol, an oleanane-type pentacyclic triterpene, is one of the natural compounds that have been investigated extensively for its potential utilization in drug development [6]. It has received major attention for its potential use as a therapeutic agent for the treatment of various diseases [7]. Plants containing taraxerol are Hypericum perforatum [8], Clitoria ternatea [9], Mangifera indica [10], and Strobilanthes crispus [11]. Taraxerol attracted wide interest among researchers due to its significant capabilities in modern pharmacology, such as its ability to act as an anti-tumor [12], anti-microbial [13], and anti-inflammatory agent [14], and in the treatment of Alzheimer’s disease [15].
Thus, this review aims to further explore the distribution of taraxerol in plants, their valuable properties and activities, as well as sustainable approaches in further producing this compound.
2. Taraxerol
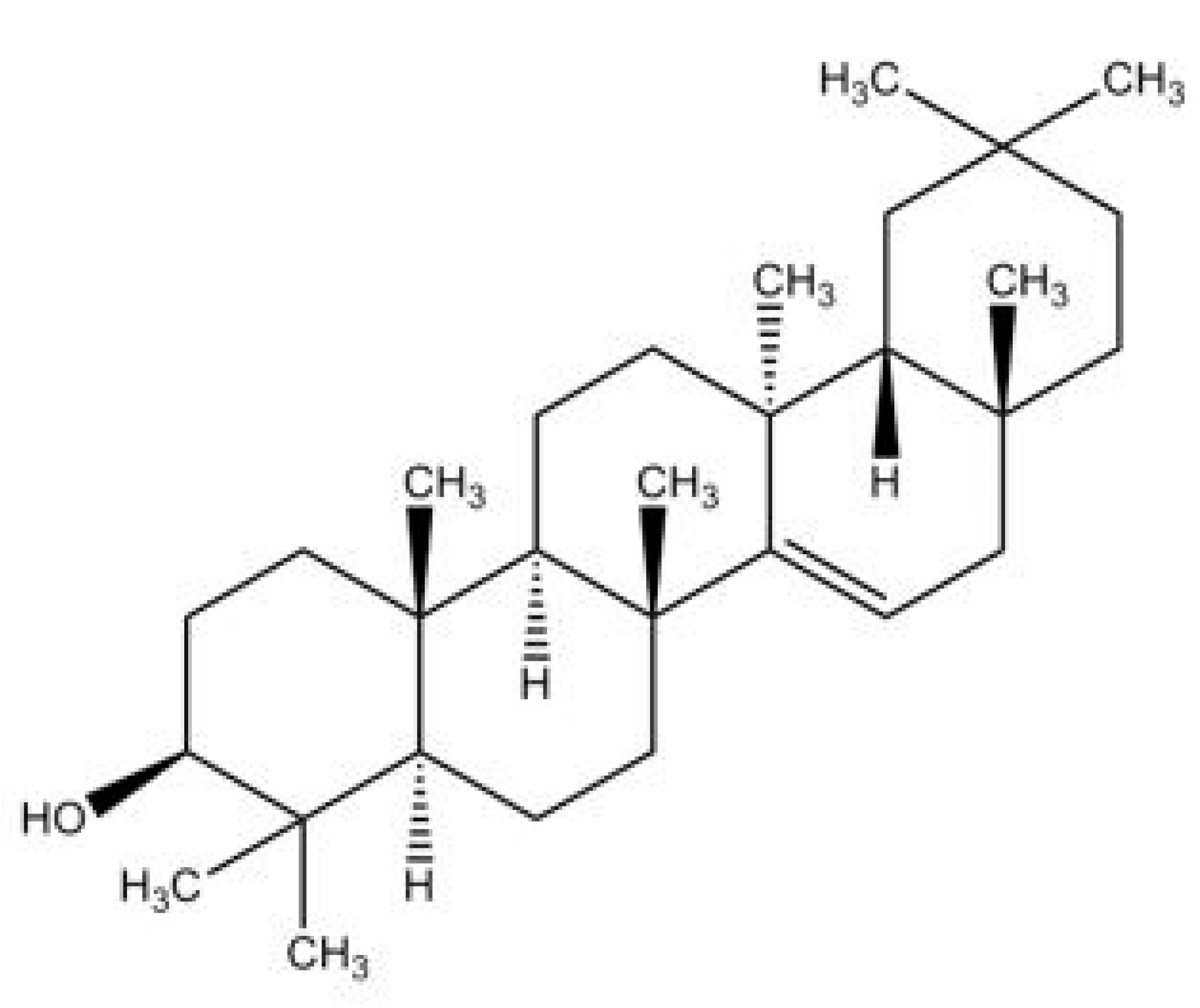
Taraxerol, (3β)-D-Friedoolean-14-en-3-ol, is a pentacylic triterpenoid [6,16]. Its chemical structure was first elucidated by Beaton et al. (1955) who identified that the oleanane-3-ol lacks the methyl group at position 14, with an α-methyl substituent at position 13 and a double bond between positions 14 and 15 [17] (Figure 1). This compound is also known by a few other synonymous names, which are isoolean-14-en-3b-ol, skimmiol, alnulin, and tiliadin. Taraxerol can be extracted from various plant families and species found in nature. However, the synthesis of taraxerol is challenging and depends on natural resources that have a negative impact on biological conservation. Hence, the ongoing research on taraxerol production and its distribution provides vital information for future investigations.
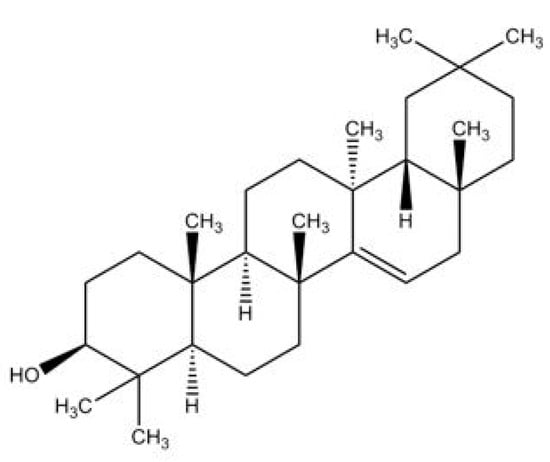
Figure 1.
The proposed structure of taraxerol. Adapted from Beaton et al. (1955) [17].
2.1. Distribution of Taraxerol in the Plant Kingdom
Members of the Asteraceae family comprise the greatest number of taraxerol-containing taxa, followed by the Euphorbiaceae and Malvaceae families (Table 1). It should be noted that within the Euphorbiaceae family, species in the Euphorbia genus have shown considerable accumulation of taraxerol. The most prominent source of taraxerol was found to be chiefly concentrated in the leaves for most taxa [11,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37], followed by the roots [9,12,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52] and finally the stems [36,38,53,54,55,56,57,58,59]. Some literature has also managed to isolate taraxerol from flowers (Table 1). However, the distribution of taraxerol is highly diverse in plants, and taraxerol content differ in different parts of plants and across different plant species.

Table 1.
The distribution of taraxerol isolated from different plant taxa.
2.2. Biosynthesis Pathway of Taraxerol
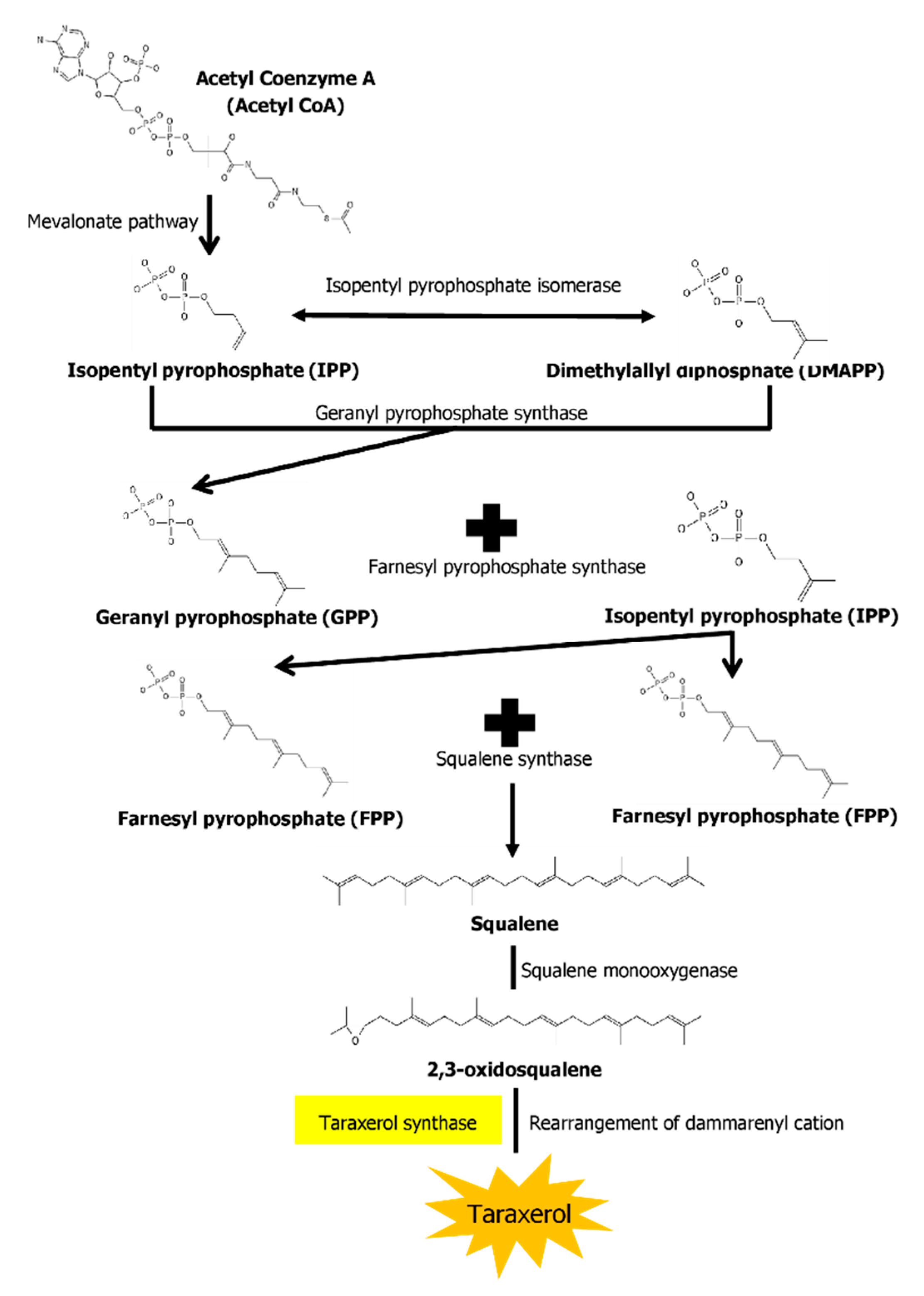
The biosynthesis pathways of taraxerol in plants have yet to be definitively elucidated. Swain et al. (2012) hypothesized that the biosynthesis of taraxerol in plants begins from the mevalonic acid pathway in the plant’s cell cytoplasm [94]. The mevalonate pathway begins with acetyl-CoA and ends with the production of IPP and DMAPP, which are the basic building blocks of various terpenoid compounds including taraxerol [95,96]. The DMAPP produced will then undergo condensation with IPP which is catalyzed by geranyl pyrophosphate synthase, producing geranyl pyrophosphate (GPP) that will be further subjected to condensation with IPP to produce farnesyl pyrophosphate (FPP) catalyzed by farnesyl diphosphate synthase (FPS) [29,97]. Squalene synthase catalyzes the condensation of the FPP molecules through reduction by NADPH to produce one molecule of squalene [98,99]. Squalene is then oxidised by NADPH and O2 to produce 2,3-oxidosqualene, which results in the reduction of NADPH into NADP+ and O2 to H2O [100]. 2,3-oxidosqualene is then utilised as a precursor for the biosynthesis of various triterpenoids, starting with a proton-initiated cyclization to produce dammarenyl cation, following which subsequent rearrangement leads to the pentacylic oleanyl cation via baccharenyl and lupenyl cation intermediates [101]. A series of 1,2-hyride shifts and/or methyl groups leads to compound rearrangements. Finally, the rearrangements of compounds via taraxerol synthase eventually lead to the formation of taraxerol in plants, more specifically in the cuticular waxes [72,100,102]. A summary of the biosynthesis pathway is illustrated in Figure 2.
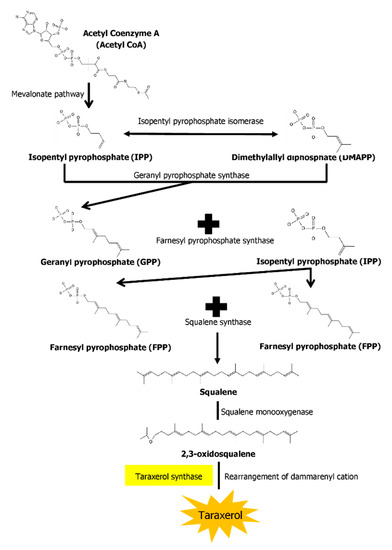
Figure 2.
A summary of the biosynthesis pathway of taraxerol. With the aid of taraxerol synthase, dammarenyl cation undergoes rearrangements to produce taraxerol.
3. Medicinal Properties of Taraxerol
3.1. Antioxidative Properties
‘Reactive oxygen species’ (ROS) is a term that encompasses various oxygen free radicals produced during cellular oxidative process. These compounds pose a significant risk factor for various diseases. Hence, antioxidants play an important role as a phytochemical that could inhibit the oxidative process. A study reported that taraxerol isolated from the bark of Styrax japonica exhibited weak radical-scavenging activity in the DPPH assay [103]. Increasing the concentration of taraxerol from 0.05–0.5 mg/mL yielded moderate radical scavenging activity in DPPH assay [85]. Jamila et al. (2015) supported the findings from Min et al. (2004), where taraxerol isolated from Garcinia hombroniana was found to be more potent than trolox and equipotent to gallic acid in DPPH radical scavenging activity, while in ABTS the scavenging activity of taraxerol was higher than trolox but less than gallic acid [71]. The reducing capacity of the extracts is related to the presence of biologically active compounds, particularly the hydrogendonating ability [17]. Owing to the potential chemical structure of taraxerol itself, this might explain the potent antioxidative capabilities of taraxerol. The current body of literature on taraxerol as an antioxidant provides valuable insight on this compound, but the work is not yet completed, and there are aspects that are under-explored.
3.2. Antimicrobial Properties
Singh et al. (2002) observed that 1 mg of taraxerol compound exhibited moderate antimicrobial activity against two Gram-positive (Staphylococcus aureus and Bacillus thuringiensis) and three Gram-negative bacteria (Escherichia coli, Enterobacter cloacae, and Klebsiella pneumonia) [13]. Koay et al. (2013) investigated the minimum inhibitory concentrations (MICs) of taraxerol on several bacteria and found that the compound is active against Gram-positive Bacillus subtilis and Staphylococcus aureus at a concentration of 15.6 µg/mL but is only moderately inhibitive to the Gram-negative Escherichia coli, Klebseilla pneumonia, and Salmonella typhimurium at a concentration of 62.5 µg/mL The taraxerol antimicrobial activity is comparable to that of positive control gentamicin [11]. Meanwhile, Hernandez-Chavez et al. (2012) reported on the anti-gardial activities of taraxerol towards Giardia lambia, a parasitic protozoan [104]. It was found that taraxerol possessed strong anti-gardial activity exhibiting a growth inhibition (IC50) of 50% at a concentration of 16.11 µg/mL and a growth inhibition of 90% at a concentration of 102.4 µg/mL, although the activity is lower compared to the positive control metronidazole. Another study on the cytotoxic activity of taraxerol against parasitic protozoans was conducted by Simelane et al. (2013), targeting malaria-causing Plasmodium falcifarum and Plasmodium berghei [105]. Anti-plasmodial activities were reported for taraxerol at a concentration of more than 100 µg/mL [105], but it was found to have no effect on mycobacteria (Mycobacterium Madagascar and M. indicuspranii), exhibiting a lower activity than the positive control chloroquine (IC50 = 14.1 ng/mL) [85]. Thus, future studies should focus mainly on the potential of taraxerol as an anti-protozoan drug. Warfield et al. (2014) conducted studies on the efficacy of taraxerol in combating the parasitic Trypanosoma cruzi [106]. The authors characterized the affinity of taraxerol with the sterol 14α-demethylase enzyme from Trypanosoma cruzi and found that the skeletal structure of taraxerol has higa affinity towards the enzyme, therefore providing potent inhibitory activity.
3.3. Anti-Fungal Properties
In an earlier study, taraxerol at a concentration of 1 mg/disc exhibited weak antifungal activities against four types of fungi namely Aspergillus niger, Aspergillus flavus, Rhizoctonia phaseoli, and Penicillium chrysogenum [13]. On the other hand, Aguilar-Guaddarama et al. (2009) shed some positive light on the anti-fungal potential of taraxerol [64]. The authors focused on another type of fungus known as dermatophytes, which are pathogens that cause skin diseases in animals and humans [107]. The compound exhibited strong anti-dermatophytic activities against various dermatophytes, at varying degrees of inhibition. Taraxerol was particularly effective against several species of Trichophyton, for instance T. rubrum and T. mentagrophytes, with an MIC of 12.5 µg/mL, as well as Candida albicans (MIC = 25 µg/mL) and Aspergillus niger at 100 µg/mL [64].
3.4. Cytotoxic Properties
Chaturvedula et al. (2004) found taraxerol at a concentration of 21.8 µg/mL was enough to inhibit 50% (IC50) of the growth of the A2780 ovarian carcinoma cell line, although it performed worse than the positive control doxorubicin (IC50 1–3 ng/mL) [88]. At concentrations lower than 20 µg/mL, it showed little to no effect on the A2780 cell line [30]. Taraxerol also showed cytotoxicity towards the A431 squamous carcinoma cell line at 2.65 µg/mL, even though it was found to be inactive against HeLa, MCF-7, and MRC-5 cancer cell lines. While taraxerol cytotoxicity exhibited low activity compared to positive control doxorubicin, the activity is comparable to that of cisplatin [42]. Taraxerol also showed little to no inhibitory potential against Hypoxia-Induced Factor-1 (HIF-1) protein to reduce hypoxic tumor growth compared to 17-DMAF [17-(dimethylaminoethylamino)-17-demethoxygeldanamycin] [53]. However, taraxerol exhibited strong cytotoxicity towards human AGS gastric epithelial cell line at a concentration of 100 µmol/L by elevating cells arresting from complete mitosis and promoting early cell apoptosis rate from 4.45% to 10.29% [108]. Moreover, the report by Kaennakam et al. (2013) [49] contradicted earlier results from Csupor-Lötfer et al. (2011) [42] whereby the former observed that taraxerol displayed potent cytotoxicity to HeLa cells at a concentration of 14.94 µg/mL and to KB cells at a concentration of 13.58 µg/mL. Based on these results, taraxerol shows potential as a chemotherapeutic agent in cancer therapy.
3.5. Anti-Diabetic Properties
The utility of taraxerol in the treatment of diabetes was reported by Kwon et al. (2008), in which the compound was tested against the protein tyrosine phosphatase 1B (PTP1B)—a negative regulator of the insulin-signalling pathway for the treatment of type 2 diabetes [92]. Taraxerol was shown to exhibit moderate inhibitory properties against PTP1B at concentrations higher than 50 µM. Yet, Sangeetha et al. (2010) discovered that instead of targeting the PTP1B protein, taraxerol holds the potential to treat type 2 diabetes by dual action: as a glucose transport activator and as a glycogen synthesis stimulant [109]. The authors also revealed that taraxerol could reverse the effects of dexamethasone-induced insulin resistance back to its normal homoeostasis state. These findings were supported by Gururaja et al. (2015) who claimed that taraxerol is one of the active compounds that shows inhibitory activities against cholesterol esterase enzyme [18]. The antidiabetic properties of taraxerol were mostly attributed to its high affinity towards proteins involved in glucose metabolism [71].
3.6. Anti-Inflammatory Properties
Perhaps the most potent pharmacological properties actively shown by taraxerol is as an anti-inflammatory agent. Singh et al. (2002) investigated the anti-inflammatory activity of taraxerol on carrageenan-induced paw edema on rats and found that applying the triterpenoid extract at a dosage of 20 mg/kg led to edema reduction by 49.66% after 7 h [13]. Naik et al. (2004) further uncovered the anti-inflammatory effects of taraxerol on TPA-induced local inflammation in Swiss Albino mice, in which development of ear edema in rat model was suppressed following its application. A dosage of 1 mg/ear showed the best suppressive effects with a 25.7 mm difference in ear thickness 4 h following the injection [31]. Apart from paw and ear inflammation, taraxerol was also found to be beneficial in inflammatory pulmonary diseases. By directly acting on airway epithelial cells, taraxerol regulates the expression of the Muc5a gene in the cells, thus regulating mucus production in the inflamed airway [45].
Other than in the treatment of edema, taraxerol’s neuroinflammation amelioration effect has also been studied. Tsao et al. (2008) examined the effect of taraxerol on the production of nitric oxide (NO) and reactive oxygen species (ROS) by activated microglial cells, which play a number of deleterious roles in central nervous system mediation [24]. The NO and ROS are produced by activated microglial cells through the induction of NADPH oxidase (NOX) and nitric oxide synthase (NOS), which the authors noted to have been inhibited by 11.6% at 50 µM concentration and 50% at 24.2 µM concentration, respectively. The mechanism through which taraxerol functions as an anti-inflammatory agent was further elucidated by Yao et al. (2013) who showed that taraxerol downregulates the expression of proinflammatory mediators in macrophages through the interference of TAK1 and Akt protein activation, thus preventing NF-κB activation from producing various proinflammatory mediators through a cascade effect [110]. Cellular redox reactions have a critical role in the regulation of immune response, which directly suggested that taraxerol could also mediate inflammatory responses [110].
3.7. Treatment for Neurodegenerative Diseases
Taraxerol has also been extensively studied for its potential in treating neurodegenerative diseases. Cholinesterase enzymes were targeted by various target compounds in drug development to find possible treatments for neurodegenerative diseases, particularly Alzheimer’s [111]. Lee et al. (2004) found the potential of taraxerol for this purpose by inhibiting acetylcholinesterase (AChE) activity in a dose-dependent manner, with an IC50 value of 33.6 µg/mL [75]. This finding was supported by Jamila et al. (2014) in which taraxerol could not exercise its inhibitory effects at concentrations higher than 33.6 µg/mL [71]. Nevertheless, at 50 µg/mL, taraxerol exhibited inhibitory effects on butyrylcholinesterase (BChE) with 98.4% inhibition [71]. The IC50 of taraxerol against BChE was found to be at 17.8 µM.
Furthermore, taraxerol displayed high binding affinity to the monomers and mature fibrils of amyloid peptides, which are critical proteins associated with neurodegenerative disorders [111]. Taraxerol can completely assimilate into the human body and cross the blood-brain barrier, which are the two prerequisites for the development of a potent neurodegenerative drug [15]. In silico analysis of taraxerol affinity towards acetylcholinesterase A and B revealed high affinity towards both enzymes through the formation of hydrogen bonds [71]. This might explain the ability of taraxerol to compete for the active site of acetylcholinesterase, thereby exhibiting potential as a treatment for neurodegenerative diseases.
3.8. Other Notable Pharmacological Properties of Taraxerol
Taraxerol also exhibited wound healing properties. Naik et al. (2004) tested taraxerol for its inhibition on glycogen synthase kinase-3β (GSK-3β) protein, a wound healing biomarker through molecular and dynamic approach [31]. In silico studies have indicated that taraxerol may be a potent inhibitor of GSK-3β due to its expressed minimum binding (−12.59 kJ/mol) and docking energy (−11.25 kJ/mol). On the other hand, in vivo studies have shown that taraxerol displayed an astounding capability in healing three types of wounds, namely excised wounds (18.28 days with 94.42% enclosure), incised wounds (epidermal tensile strength of 562.36 g after 10 days of wounding), and dead space wounds (increased weight of granuloma tissues up to 21.02 mg, tissue breaking strength at 657.12 g, and hydroxyproline content of 1455.93 µg/100 g). Thus, the therapeutic properties of taraxerol can be extended to wound healing and remain to be further explored.
Natural compounds and extracts have been an important source for alternative medicine. The specific chemical compounds that have been isolated from natural plants hold a great potential in medicine, as had been demonstrated by the high number of FDA-approved drugs or natural products as well as their derivatives [112]. The search for antivirals is gaining popularity due to the coronavirus disease 2019 (COVID-19) which have had a huge impact on human well-being. Several phytochemicals such as friedelin, stigmasterol, and taraxerol were reported to exhibit promising antiviral properties [113]. Molecular dynamics simulation demonstrated that taraxerol has a better binding energy with viral proteins such as spike protein, main protease enzyme Mpro, and the RNA-dependent RNA polymerase of COVID-19 [113]. This has shed light into further evaluation of taraxerol using in vitro and in vivo experiments for the development of a COVID-19 inhibitor.
4. In Vitro Production of Taraxerol
Cell culture techniques have emerged as an attractive alternative for the production of plants’ secondary metabolites, and various strategies have been developed for use in biomass accumulation as well as synthesis of a slew of secondary compounds [3]. However, taraxerol production through in vitro techniques has been limited so far. An example is the protocol developed by Swain et al. (2012) for producing taraxerol from Clitoria ternatea (Butterfly pea) through the establishment of transformed hairy root cultures [94]. Transformed hairy roots contained integrated TL-rolB gene and were able to increase taraxerol four-fold greater by dry weight basis compared to natural roots. Since transformation was involved in the process, the taraxerol isolated were ascertained by IR, 1H-NMR, and 1C-NMR spectroscopy as the modification of the Clitoria ternatea genetic make-up could change its phytochemical content.
Zafar & Sharma (2015) also used an in vitro approach to produce taraxerol through the establishment of callus cultures from the roots of Taraxacum officinale (Dandelion) [114]. Calluses were induced from the roots of Taraxacum officinale by using two types of MS media supplemented with 0.5 mg/L IAA + 1 mg/L BAP + 0.5 mg/L 2,4-D and 2 mg/L IAA + 1 mg/L BAP. The established root callus has successfully increased the taraxerol yield by 1.04 times. To further enhance taraxerol production, Zafar & Sharma (2015) established root callus suspension cultures using the same MS media and PGR combinations from Sharma and Zafar (2014) with the addition of methyl jasmonate (MJ) and β-cyclodextrin (CD) as elicitor agents [16,114]. According to the authors, both elicitors were able to elevate taraxerol production by 0.018% with MJ at 0.05 mM, 0.1 mM, and 0.2 mM, and by 0.023% with 25 mM β-CD compared to natural roots.
5. Conclusions
Taraxerol is a bioactive metabolite present in some higher plants which possesses multiple selective biological actions, especially in medicinal applications. Despite displaying little anti oxidative abilities and only moderate antimicrobial properties, various studies have reported the potential of taraxerol to act as an anti-plasmodial, antidiabetic, anticancer, anti-inflammatory, and anti-dermatophyte. These findings demonstrated the potential of taraxerol in the development of a novel and multipurpose drug. From a commercial point of view, taraxerol is hitherto a costly compound to chemically and biologically synthesize. With several pathways towards in vitro synthesis of taraxerol having already been established, it may not be a good use of resources to continue exploring more alternative synthesis pathways. Instead, research efforts should be directed towards optimizing known synthesis techniques through an experimental approach by the establishment of high-yielding cell lines, optimizing culture conditions, nutrient media, phytohormone contents and carbohydrate sources, elicitors, and precursors. With enhanced taraxerol production, further drug research and development works for various treatments using taraxerol can be performed.
Author Contributions
Conceptualization and supervision, H.M. and J.A.G.; investigation, resources and data curation, A.A.M. and L.P.W.G.; writing—original draft preparation, A.A.M.; writing—review and editing, A.A.M., L.P.W.G., H.M. and J.A.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Universiti Malaysia Sabah (SBK0247-SG-2015).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Salim, A.A.; Chin, Y.-W.; Kinghorn, A.D. Drug Discovery from Plants. In Bioactive Molecules and Medicinal Plants; Springer: Berlin/Heidelberg, Germany, 2008; pp. 1–24. [Google Scholar] [CrossRef]
- Lahlou, M. The Success of Natural Products in Drug Discovery. Pharmacol. Pharm. 2013, 4, 17–31. [Google Scholar] [CrossRef]
- Murthy, H.N.; Lee, E.-J.; Paek, K.-Y. Production of Secondary Metabolites from Cell and Organ Cultures: Strategies and Approaches for Biomass Improvement and Metabolite Accumulation. Plant Cell Tissue Organ Cult. 2014, 118, 1–16. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the 30 Years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.S. Natural Products to Drugs: Natural Product-Derived Compounds in Clinical Trials. Nat. Prod. Rep. 2008, 25, 475. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, A.; Hamid, K.; Kam, A.; Wong, K.H.; Abdelhak, Z.; Razmovski-Naumovski, V.; Chan, K.; Li, K.M.; Groundwater, P.W.; Li, G.Q. The Pentacyclic Triterpenoids in Herbal Medicines and Their Pharmacological Activities in Diabetes and Diabetic Complications. Curr. Med. Chem. 2013, 20, 908–931. [Google Scholar] [CrossRef] [PubMed]
- Sheng, H.; Sun, H. Synthesis, Biology and Clinical Significance of Pentacyclic Triterpenes: A Multi-Target Approach to Prevention and Treatment of Metabolic and Vascular Diseases. Nat. Prod. Rep. 2011, 28, 543. [Google Scholar] [CrossRef] [PubMed]
- Ganeva, Y.; Chanev, C.; Dentchev, T.; Vitanova, D. Triterpenoids and sterols from Hypericum perforatum. Comptes Rendus Acad. Bulg. 2003, 56, 37–40. [Google Scholar]
- Kumar, V.; Mukherjee, K.; Kumar, S.; Mal, M.; Mukherjee, P.K. Validation of HPTLC Method for the Analysis of Taraxerol in Clitoria ternatea. Phytochem. Anal. 2007, 19, 244–250. [Google Scholar] [CrossRef]
- Sangeetha, K.N.; Shilpa, K.; Jyothi Kumari, P.; Lakshmi, B.S. Reversal of Dexamethasone Induced Insulin Resistance in 3T3L1 Adipocytes by 3β-Taraxerol of Mangifera Indica. Phytomedicine 2013, 20, 213–220. [Google Scholar] [CrossRef]
- Koay, Y.C.; Wong, K.C.; Hasnah, O.; Ibrahim, E.M.S.; Mohammad, Z.A. Chemical constituents and biological activities of Strobilanthes crispus L. Rec. Nat. Prod. 2013, 7, 59–64. [Google Scholar]
- Takasaki, M.; Konoshima, T.; Tokuda, H.; Masuda, K.; Arai, Y.; Shiojima, K.; Ageta, H. Anti-Carcinogenic Activity of Taraxacum Plant. I. Biol. Pharm. Bull. 1999, 22, 602–605. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Sahu, P.M.; Sharma, M.K. Anti-Inflammatory and Antimicrobial Activities of Triterpenoids from Strobilanthes Callosus Nees. Phytomedicine 2002, 9, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Biswas, M.; Biswas, K.; Ghosh, A.K.; Haldar, P.K. A pentacyclic triterpenoid possessing anti-inflammatory activity from the fruits of Dregea volubilis. Pharmacogn. Mag. 2009, 5, 64–68. [Google Scholar] [CrossRef]
- Ngo, S.T.; Li, M.S. Top-Leads from Natural Products for Treatment of Alzheimer’s Disease: Docking and Molecular Dynamics Study. Mol. Simul. 2013, 39, 279–291. [Google Scholar] [CrossRef]
- Sharma, K.; Zafar, R. Optimization of Methyl Jasmonate and β-Cyclodextrin for Enhanced Production of Taraxerol and Taraxasterol in (Taraxacum officinale Weber) Cultures. Plant Physiol. Biochem. 2016, 103, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Beaton, J.M.; Spring, F.S.; Stevenson, R.; Stewart, J.L. Triterpenoids. Part XXXVII. The Constitution of Taraxerol. J. Chem. Soc. (Resumed) 1955, 2131–2137. [Google Scholar] [CrossRef]
- Gururaja, G.; Mundkinajeddu, D.; Dethe, S.; Sangli, G.; Abhilash, K.; Agarwal, A. Cholesterol Esterase Inhibitory Activity of Bioactives from Leaves of Mangifera indica L. Pharmacogn. Res. 2015, 7, 355. [Google Scholar] [CrossRef]
- Du, J.; Gao, L. Chemical constituents of the leaves of Acanthopanax trifoliatus (Linn) Merr. Zhongguo Zhong Yao Za Zhi 1992, 17, 356–357. [Google Scholar]
- Phan, M.G.; Chinh Truong, T.T.; Phan, T.S.; Matsunami, K.; Otsuka, H. Mangiferonic Acid, 22-Hydroxyhopan-3-One, and Physcion as Specific Chemical Markers for Alnus nepalensis. Biochem. Syst. Ecol. 2010, 38, 1065–1068. [Google Scholar] [CrossRef]
- Correia Da Silva, T.B.; Souza, V.K.T.; Da Silva, A.P.F.; Lyra Lemos, R.P.; Conserva, L.M. Determination of the Phenolic Content and Antioxidant Potential of Crude Extracts and Isolated Compounds from Leaves of Cordia multispicata and Tournefortia bicolor. Pharm. Biol. 2009, 48, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Pinto, N.d.C.C.; Machado, D.C.; da Silva, J.M.; Conegundes, J.L.M.; Gualberto, A.C.M.; Gameiro, J.; Moreira Chedier, L.; Castañon, M.C.M.N.; Scio, E. Pereskia aculeata Miller Leaves Present In Vivo Topical Anti-Inflammatory Activity in Models of Acute and Chronic Dermatitis. J. Ethnopharmacol. 2015, 173, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Mokoka, T.A.; McGaw, L.J.; Mdee, L.K.; Bagla, V.P.; Iwalewa, E.O.; Eloff, J.N. Antimicrobial Activity and Cytotoxicity of Triterpenes Isolated from Leaves of Maytenus undata (Celastraceae). BMC Complement. Altern. Med. 2013, 13, 111. [Google Scholar] [CrossRef]
- Tsao, C.-C.; Shen, Y.-C.; Su, C.-R.; Li, C.-Y.; Liou, M.-J.; Dung, N.-X.; Wu, T.-S. New Diterpenoids and the Bioactivity of Erythrophleum fordii. Bioorg. Med. Chem. 2008, 16, 9867–9870. [Google Scholar] [CrossRef]
- Macías-Rubalcava, M.L.; Hernández-Bautista, B.E.; Jiménez-Estrada, M.; Cruz-Ortega, R.; Anaya, A.L. Pentacyclic Triterpenes with Selective Bioactivity from Sebastiania adenophora Leaves, Euphorbiaceae. J. Chem. Ecol. 2006, 33, 147–156. [Google Scholar] [CrossRef]
- Falodun, A.; Qadir, M.I.; Chouldary, M.I. Isolation and characterization of xanthine oxidase inhibitory constituents of Pyrenacantha staudtii. Yao Xue Xue Bao 2009, 44, 390–394. [Google Scholar] [PubMed]
- Hu, H.; Liu, Q.; Yang, Y.; Yang, L.; Wang, Z. Chemical constituents of Clerodendrum trichotomum Leaves. Zhong Yao Cai 2014, 37, 1590–1593. [Google Scholar]
- Khanra, R.; Dewanjee, S.; Dua, T.K.; Sahu, R.; Gangopadhyay, M.; De Feo, V.; Zia-Ul-Haq, M. Abroma Augusta L. (Malvaceae) Leaf Extract Attenuates Diabetes Induced Nephropathy and Cardiomyopathy via Inhibition of Oxidative Stress and Inflammatory Response. J. Transl. Med. 2015, 13, 6. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Y.; Liu, D. Cloning and Characterization of Farnesyl Diphosphate Synthase Gene Involved in Triterpenoids Biosynthesis from Poria cocos. Int. J. Mol. Sci. 2014, 15, 22188–22202. [Google Scholar] [CrossRef]
- Cao, S.; Brodie, P.; Miller, J.S.; Birkinshaw, C.; Rakotondrafara, A.; Andriantsiferana, R.; Rasamison, V.E.; Kingston, D.G.I. Antiproliferative Compounds of Helmiopsis sphaerocarpa from the Madagascar Rainforest. Nat. Prod. Res. 2009, 23, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Naik, D.G.; Mujumdar, A.M.; Waghole, R.J.; Misar, A.V.; Bligh, S.W.; Bashall, A.; Crowder, J. Taraxer-14-En-3β-Ol, an Anti-Inflammatory Compound from Sterculia foetida L. Planta Med. 2004, 70, 68–69. [Google Scholar] [CrossRef]
- Manguro, L.O.A.; Onyango Okwiri, S.; Lemmen, P. Oleanane-Type Triterpenes of Embelia schimperi Leaves. Phytochemistry 2006, 67, 2641–2650. [Google Scholar] [CrossRef] [PubMed]
- Machado, K.E.; Cechinel Filho, V.; Cruz, R.C.B.; Meyre-Silva, C.; Cruz, A.B. Antifungal activity of Eugenia umbelliflora against dermatophytes. Nat. Prod. Commun. 2009, 4, 1181–1184. [Google Scholar] [CrossRef]
- Raja Naika, H.; Krishna, V.; Lingaraju, K.; Chandramohan, V.; Dammalli, M.; Navya, P.N.; Suresh, D. Molecular Docking and Dynamic Studies of Bioactive Compounds from Naravelia zeylanica (L.) DC against Glycogen Synthase Kinase-3β Protein. J. Taibah Univ. Sci. 2015, 9, 41–49. [Google Scholar] [CrossRef][Green Version]
- Yang, X.; Li, H.; Chen, H.; Li, P.; Ye, B. Chemical constituents in the leave of Rhizophora stylosa L and their biological activities. Yao Xue Xue Bao 2008, 43, 974–978. [Google Scholar]
- Williams, L.A.D. Rhizophora mangle (Rhizophoraceae) Triterpenoids with Insecticidal Activity. Naturwissenschaften 1999, 86, 450–452. [Google Scholar] [CrossRef] [PubMed]
- Pensec, F.; Szakiel, A.; Pączkowski, C.; Woźniak, A.; Grabarczyk, M.; Bertsch, C.; Fischer, M.J.C.; Chong, J. Characterization of Triterpenoid Profiles and Triterpene Synthase Expression in the Leaves of Eight Vitis vinifera Cultivars Grown in the Upper Rhine Valley. J. Plant Res. 2016, 129, 499–512. [Google Scholar] [CrossRef]
- Okoth, D.A.; Koorbanally, N.A. Cardanols, Long Chain Cyclohexenones and Cyclohexenols from Lannea schimperi (Anacardiaceae). Nat. Prod. Commun. 2015, 10, 1934578X1501000. [Google Scholar] [CrossRef]
- Padmaja, V.; Thankamany, V.; Hisham, A. Antibacterial, Antifungal and Anthelmintic Activities of Root Barks of Uvaria hookeri and Uvaria narum. J. Ethnopharmacol. 1993, 40, 181–186. [Google Scholar] [CrossRef]
- Chen, Y.; Tao, S.; Zeng, F.; Xie, L.; Shen, Z. Antinociceptive and Anti-Inflammatory Activities of Schefflera octophylla Extracts. J. Ethnopharmacol. 2015, 171, 42–50. [Google Scholar] [CrossRef]
- Rashid, M.-U.; Alamzeb, M.; Ali, S.; Ahmad Khan, A.; Igoli, J.O.; Ferro, V.A.; Gray, A.I.; Rafiullah Khan, M. A new ceramide along with eight known compounds from the roots of Artemisia incisa pamp. Rec. Nat. Prod. 2014, 9, 294–304. [Google Scholar]
- Csupor-Löffler, B.; Hajdú, Z.; Zupkó, I.; Molnár, J.; Forgo, P.; Vasas, A.; Kele, Z.; Hohmann, J. Antiproliferative Constituents of the Roots of Conyza canadensis. Planta Med. 2011, 77, 1183–1188. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-M.; Liu, X.-K.; Qing, C.; Wu, D.-G.; Zhu, D.-Y. Chemical constituents from the roots of Homonoia riparia. Yao Xue Xue Bao 2007, 42, 292–296. [Google Scholar] [PubMed]
- Duan, J.; Wang, L.; Qian, S.; Su, S.; Tang, Y. A New Cytotoxic Prenylated Dihydrobenzofuran Derivative and Other Chemical Constituents from the Rhizomes of Atractylodes lancea DC. Arch. Pharm. Res. 2008, 31, 965–969. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.P.; Lee, H.J.; Lee, D.-U.; Lee, S.K.; Hong, J.-H.; Lee, C.J. Effects of Lupenone, Lupeol, and Taraxerol Derived From Adenophora triphyllaon the Gene Expression and Production of Airway MUC5AC Mucin. Tuberc. Respir. Dis. 2015, 78, 210. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, X.-W. Studies on chemical constituents of root tuber of cultivated Pseudostellaria heterophylla (Zheshen No. 1). Zhongguo Zhong Yao Za Zhi 2008, 33, 2353–2355. [Google Scholar]
- Xiang, Y.; Zhang, C.; Zheng, Y. Studies on the chemical constituents of the roots of Rhododendron molle G. Don. J. Huazhong Univ. Sci. Technol. 2004, 24, 202–204. [Google Scholar]
- Liu, Y.-M.; Tian, D.; Bao, H.; Zhao, G.-L.; Wang, J.-X. Study on chemical constituents of root bark of Discocleidion rufescens. Zhong Yao Cai 2012, 35, 1795–1798. [Google Scholar]
- Kaennakam, S.; Sichaem, J.; Khumkratok, S.; Siripong, P.; Tip-pyang, S. A New Taraxerol Derivative from the Roots of Microcos tomentosa. Nat. Prod. Commun. 2013, 8, 1934578X1300801. [Google Scholar] [CrossRef]
- Li, S.; Shi, Y.; Shang, X.-Y.; Cui, B.-S.; Yuan, Y.; Chen, X.-G.; Yang, Y.-C.; Shi, J.-G. Triterpenoids from the Roots of Pterospermum heterophyllum Hance. J. Asian Nat. Prod. Res. 2009, 11, 652–657. [Google Scholar] [CrossRef]
- Ango, P.Y.; Kapche, D.W.F.G.; Fotso, G.W.; Fozing, C.D.; Yeboah, E.M.O.; Mapitse, R.; Demirtas, I.; Ngadjui, B.T.; Yeboah, S.O. Thonningiiflavanonol A and Thonningiiflavanonol B, Two Novel Flavonoids, and Other Constituents of Ficus thonningii Blume (Moraceae). Z. Naturforsch. 2016, 71, 65–71. [Google Scholar] [CrossRef]
- Paul, B.D.; Subba Rao, G.; Kapadia, G.J. Isolation of Myricadiol, Myricitrin, Taraxerol, and Taraxerone from Myrica cerifera L. Root Bark. J. Pharm. Sci. 1974, 63, 958–959. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Cai, X.F.; Na, M.; Lee, J.J.; Bae, K. Triterpenoids and Diarylheptanoids from Alnus hirsuta Inhibit HIF-1 in Ags Cells. Arch. Pharm. Res. 2007, 30, 412–418. [Google Scholar] [CrossRef]
- Jiang, J.; Li, Y.; Chen, Z.; Min, Z.; Lou, F. Two Novel C29-5β-Sterols from the Stems of Opuntia dillenii. Steroids 2006, 71, 1073–1077. [Google Scholar] [CrossRef] [PubMed]
- Al Muqarrabun, L.M.R.; Ahmat, N.; Aris, S.R.S.; Norizan, N.; Shamsulrijal, N.; Yusof, F.Z.M.; Suratman, M.N.; Yusof, M.I.M.; Salim, F. A New Triterpenoid from Sapium baccatum (Euphorbiaceae). Former. Nat. Prod. Res. 2014, 28, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Ragasa, C.Y.; Cornelio, K.B. Triterpenes from Euphorbia hirta and Their Cytotoxicity. Chin. J. Nat. Med. 2013, 11, 528–533. [Google Scholar] [CrossRef]
- Mawa, S.; Jantan, I.; Husain, K. Isolation of Terpenoids from the Stem of Ficus aurantiaca Griff and Their Effects on Reactive Oxygen Species Production and Chemotactic Activity of Neutrophils. Molecules 2016, 21, 9. [Google Scholar] [CrossRef]
- Somwong, P.; Suttisri, R.; Buakeaw, A. New Sesquiterpenes and Phenolic Compound from Ficus foveolata. Fitoterapia 2013, 85, 1–7. [Google Scholar] [CrossRef]
- Lin, L.-C.; Chou, C.-J.; Kuo, Y.-C. Cytotoxic Principles from Ventilago leiocarpa. J. Nat. Prod. 2001, 64, 674–676. [Google Scholar] [CrossRef]
- Si, X.; Wei, S.; Xu, X.; Fang, X.; Wu, W. Chemical constituents in the leaves of Mangifera persiciformis C.Y. Wu et Y.L. Ming. Zhongguo Zhong Yao Za Zhi 1995, 20, 295–296. [Google Scholar]
- Lu, R.-M.; Su, X.; Zhou, Y.-Y.; Wei, J.-H. Study on the chemical constituents of Uvaria microcarpa. Zhong Yao Cai 2009, 32, 1056–1059. [Google Scholar]
- Zhang, H.; Wang, S.; Chen, R.; Yu, D. Studies on chemical constituents of Uvaria macrophylia. Yao Xue Xue Bao 2002, 37, 124–127. [Google Scholar] [PubMed]
- Marzouk, A.M.; Osman, S.M.; Gohar, A.A. A New Pregnane Glycoside from Gomphocarpus fruticosus Growing in Egypt. Nat. Prod. Res. 2015, 30, 1060–1067. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Guadarrama, B.; Navarro, V.; León-Rivera, I.; Rios, M.Y. Active Compounds against Tinea Pedis Dermatophytes from Ageratina pichinchensis var. bustamenta. Nat. Prod. Res. 2009, 23, 1559–1565. [Google Scholar] [CrossRef] [PubMed]
- Gawrońska-Grzywacz, M.; Krzaczek, T. Identification and Determination of Triterpenoids in Hieracium pilosella L. J. Sep. Sci. 2007, 30, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Akihisa, T.; Yasukawa, K.; Oinuma, H.; Kasahara, Y.; Yamanouchi, S.; Takido, M.; Kumaki, K.; Tamura, T. Triterpene Alcohols from the Flowers of Compositae and Their Anti-Inflammatory Effects. Phytochemistry 1996, 43, 1255–1260. [Google Scholar] [CrossRef]
- Saeecd, M.T.; Agarwal, R.; Khan, M.W.Y.; Ahmad, F.; Osman, S.M.; Akihisa, T.; Suzuki, K.; Matsumoto, T. Unsaponifiable Lipid Constituents of Ten Indian Seed Oils. J. Am. Oil Chem. Soc. 1991, 68, 193–197. [Google Scholar] [CrossRef]
- Dharmaratne, H.R.W.; Sajeevani, J.R.D.M.; Marasinghe, G.P.K.; Ekanayake, E.M.H.G.S. Distribution of Pyranocoumarins in Calophyllum Cordato-Oblongum. Phytochemistry 1998, 49, 995–998. [Google Scholar] [CrossRef]
- Qi, H.; Wang, R.; Liu, Y.; Shi, Y. Studies on the chemical constituents of Codonopsis pilosula. Zhong Yao Cai 2011, 34, 546–548. [Google Scholar]
- Chen, Y.; Zhu, Y.; Wei, J.; Liang, N. Chemical components of Codonopsis pilosula (Franch.) Nannf. var. volubilis (Nannf.) L.T. Shen. Zhongguo Zhong Yao Za Zhi 1995, 20, 611–612. [Google Scholar]
- Jamila, N.; Khairuddean, M.; Yeong, K.K.; Osman, H.; Murugaiyah, V. Cholinesterase Inhibitory Triterpenoids from the Bark of Garcinia hombroniana. J. Enzym. Inhib. Med. Chem. 2014, 30, 133–139. [Google Scholar] [CrossRef]
- Wang, Z.; Yeats, T.; Han, H.; Jetter, R. Cloning and Characterization of Oxidosqualene Cyclases from Kalanchoe daigremontiana. J. Biol. Chem. 2010, 285, 29703–29712. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zhu, H.; Shen, D.; Yang, B.; Yang, X. Studies on the chemical constituents of Vaccinium iteophyllum. Zhong Yao Cai 2007, 30, 47–49. [Google Scholar] [PubMed]
- Feng, Z.; Wang, Y.; Zhang, P. The chemical constituents of Rhododendron ovatum Planch. Yao Xue Xue Bao 2005, 40, 150–152. [Google Scholar]
- Lee, J.H.; Lee, K.T.; Yang, J.H.; Baek, N.I.; Kim, D.K. Acetylcholinesterase Inhibitors from the Twigs of Vaccinium Oldhami Miquel. Arch. Pharm. Res. 2004, 27, 53–56. [Google Scholar] [CrossRef]
- Khiev, P.; Oh, S.-R.; Chae, H.-S.; Kwon, O.-K.; Ahn, K.-S.; Chin, Y.-W.; Lee, H.-K. Anti-Inflammatory Diterpene from Thyrsanthera suborbicularis. Chem. Pharm. Bull. 2011, 59, 382–384. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Tian, X.-J.; Li, Y.-F.; Yang, G.-Z. Terpenoids from Euphorbia antiquorum L. Yao Xue Xue Bao 2009, 44, 1118–1122. [Google Scholar] [PubMed]
- Jiang, C.; Mu, S.; Deng, B.; Ge, Y.; Zhang, J.; Hao, X. Studies on the chemical constituents from Euphorbia chrysocoma. Zhong Yao Cai 2009, 32, 1390–1392. [Google Scholar]
- Jang, D.S.; Cuendet, M.; Pawlus, A.D.; Kardono, L.B.S.; Kawanishi, K.; Farnsworth, N.R.; Fong, H.H.S.; Pezzuto, J.M.; Kinghorn, A.D. Potential Cancer Chemopreventive Constituents of the Leaves of Macaranga triloba. Phytochemistry 2004, 65, 345–350. [Google Scholar] [CrossRef]
- Setzer, W.N.; Shen, X.; Bates, R.B.; Burns, J.R.; MCClure, K.J.; Zhang, P.; Moriarity, D.M.; Lawton, R.O. A Phytochemical Investigation of Alchornea latifolia. Fitoterapia 2000, 71, 195–198. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Xie, J.-M.; Yao, H.; Lin, X.-Y.; Zhang, Y.-H. Studies on the triterpenoids of Vitex trifolia. Zhong Yao Cai 2010, 33, 908–910. [Google Scholar]
- Gao, L.; Wei, X.; He, Y. Studies on chemical constituents of Clerodendrum bungei. Zhongguo Zhong Yao Za Zhi 2003, 28, 1042–1044. [Google Scholar] [PubMed]
- Yang, Y.; Deng, Z.; Proksch, P.; Lin, W. Two new 18-en-oleane derivatives from marine mangrove plant, Barringtonia racemosa. Pharmazie 2006, 61, 365–366. [Google Scholar] [CrossRef] [PubMed]
- Lopes, L.G.; Tavares, G.L.; Thomaz, L.D.; Sabino, J.R.; Borges, K.B.; Vieira, P.C.; Veiga, T.A.M.; de Souza Borges, W. Taraxerol 4-Methoxybenzoate, Anin Vitro Inhibitor of Photosynthesis Isolated from Pavonia multiflora A. St-Hil. (Malvaceae). Chem. Biodivers. 2016, 13, 284–292. [Google Scholar] [CrossRef]
- Christopher, R.; Nyandoro, S.S.; Chacha, M.; de Koning, C.B. A New Cinnamoylglycoflavonoid, Antimycobacterial and Antioxidant Constituents from Heritiera littoralis leaf Extracts. Nat. Prod. Res. 2014, 28, 351–358. [Google Scholar] [CrossRef]
- Xu, L.-R.; Zhang, S.; Wu, J.; Qi, S.-H.; Huang, J.-S.; Xiao, Z.-H.; Zhang, D.-J.; Yang, J.; Tian, Y. A new triterpenoid: Taraxerol-3-beta-O-tridecyl ether from Derris triofoliata. Pharmazie 2004, 59, 655–656. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Zhang, B.; Xu, Q.; Li, L.; Hao, X. Study on the chemical constituents of Mitragyna rotundifolia. Zhong Yao Cai 2006, 29, 557–560. [Google Scholar]
- Chaturvedula, V.S.P.; Schilling, J.K.; Miller, J.S.; Andriantsiferana, R.; Rasamison, V.E.; Kingston, D.G.I. New Cytotoxic Terpenoids from the Wood of Vepris punctata from the Madagascar Rainforest. J. Nat. Prod. 2004, 67, 895–898. [Google Scholar] [CrossRef]
- Gachet, M.S.; Kunert, O.; Kaiser, M.; Brun, R.; Zehl, M.; Keller, W.; Muñoz, R.A.; Bauer, R.; Schuehly, W. Antiparasitic Compounds from Cupania cinerea with Activities against Plasmodium falciparum and Trypanosoma bruceirhodesiense. J. Nat. Prod. 2011, 74, 559–566. [Google Scholar] [CrossRef]
- Misra, G.; Mitra, C.R. Mimusops Hexandra—II. Phytochemistry 1966, 5, 535–538. [Google Scholar] [CrossRef]
- Haliński, Ł.P.; Paszkiewicz, M.; Gołębiowski, M.; Stepnowski, P. The Chemical Composition of Cuticular Waxes from Leaves of the Gboma Eggplant (Solanum macrocarpon L.). J. Food Compost. Anal. 2012, 25, 74–78. [Google Scholar] [CrossRef]
- Kwon, J.-H.; Chang, M.-J.; Seo, H.-W.; Lee, J.-H.; Min, B.-S.; Na, M.; Kim, J.C.; Woo, M.H.; Choi, J.S.; Lee, H.K.; et al. Triterpenoids and a Sterol from the Stem-Bark of Styrax japonicaand Their Protein Tyrosine Phosphatase 1B Inhibitory Activities. Phytother. Res. 2008, 22, 1303–1306. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Yang, J. A study on chemical components of Tetrastigma hemsleyanum Diels et Gilg. Native to China. Zhongguo Zhong Yao Za Zhi 1999, 24, 611–612. [Google Scholar] [PubMed]
- Swain, S.S.; Rout, K.K.; Chand, P.K. Production of Triterpenoid Anti-Cancer Compound Taraxerol in Agrobacterium-Transformed Root Cultures of Butterfly Pea (Clitoria ternatea L.). Appl. Biochem. Biotechnol. 2012, 168, 487–503. [Google Scholar] [CrossRef]
- Holstein, S.A.; Hohl, R.J. Isoprenoids: Remarkable Diversity of Form and Function. Lipids 2004, 39, 293–309. [Google Scholar] [CrossRef] [PubMed]
- Miziorko, H.M. Enzymes of the Mevalonate Pathway of Isoprenoid Biosynthesis. Arch. Biochem. Biophys. 2011, 505, 131–143. [Google Scholar] [CrossRef]
- Delourme, D.; Lacroute, F.; Karst, F. Cloning of an Arabidopsis thaliana CDNA Coding for Farnesyl Diphosphate Synthase by Functional Complementation in Yeast. Plant Mol. Biol. 1994, 26, 1867–1873. [Google Scholar] [CrossRef] [PubMed]
- Dewar, M.J.S.; Ruiz, J.M. Mechanism of the Biosynthesis of Squalene from Farnesyl Pyrophosphate. Tetrahedron 1987, 43, 2661–2674. [Google Scholar] [CrossRef]
- Tansey, T. Structure and Regulation of Mammalian Squalene Synthase. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2000, 1529, 49–62. [Google Scholar] [CrossRef]
- Abe, I. Enzymatic Synthesis of Cyclic Triterpenes. Nat. Prod. Rep. 2007, 24, 1311. [Google Scholar] [CrossRef]
- Abe, I.; Rohmer, M.; Prestwich, G.D. Enzymatic Cyclization of Squalene and Oxidosqualene to Sterols and Triterpenes. Chem. Rev. 1993, 93, 2189–2206. [Google Scholar] [CrossRef]
- Basyuni, M.; Oku, H.; Tsujimoto, E.; Kinjo, K.; Baba, S.; Takara, K. Triterpene Synthases from the Okinawan Mangrove Tribe, Rhizophoraceae. FEBS J. 2007, 274, 5028–5042. [Google Scholar] [CrossRef] [PubMed]
- Min, B.-S.; Na, M.-K.; Oh, S.-R.; Ahn, K.-S.; Jeong, G.-S.; Li, G.; Lee, S.-K.; Joung, H.; Lee, H.-K. New Furofuran and Butyrolactone Lignans with Antioxidant Activity from the Stem Bark of Styrax japonica. J. Nat. Prod. 2004, 67, 1980–1984. [Google Scholar] [CrossRef]
- Hernández-Chávez, I.; Torres-Tapia, L.W.; Simá-Polanco, P.; Cedillo-Rivera, R.; Moo-Puc, R.; Peraza-Sánchez, S.R. Antigiardial Activity of Cupania dentata Bark and Its Constituents. J. Mex. Chem. Soc. 2017, 56, 105–108. [Google Scholar] [CrossRef]
- Simelane, M.; Shonhai, A.; Shode, F.; Smith, P.; Singh, M.; Opoku, A. Anti-Plasmodial Activity of Some Zulu Medicinal Plants and of Some Triterpenes Isolated from Them. Molecules 2013, 18, 12313–12323. [Google Scholar] [CrossRef] [PubMed]
- Warfield, J.; Setzer, W.N.; Ogungbe, I.V. Interactions of Antiparasitic Sterols with Sterol 14α-Demethylase (CYP51) of Human Pathogens. SpringerPlus 2014, 3, 679. [Google Scholar] [CrossRef] [PubMed]
- Flores, F.C.; Beck, R.C.R.; da Silva, C.d.B. Essential Oils for Treatment for Onychomycosis: A Mini-Review. Mycopathologia 2015, 181, 9–15. [Google Scholar] [CrossRef]
- Tan, B.; Shi, H.-L.; Ji, G.; Xie, J.Q. Effects of Taraxerol and Taraxeryl Acetate on Cell Cycle and Apoptosis of Human Gastric Epithelial Cell Line AGS. Chin. J. Integr. Med. 2011, 9, 638–642. [Google Scholar] [CrossRef]
- Sangeetha, K.N.; Sujatha, S.; Muthusamy, V.S.; Anand, S.; Nithya, N.; Velmurugan, D.; Balakrishnan, A.; Lakshmi, B.S. 3β-Taraxerol of Mangifera Indica, a PI3K Dependent Dual Activator of Glucose Transport and Glycogen Synthesis in 3T3-L1 Adipocytes. Biochim. Biophys. Acta Gen. Subj. 2010, 1800, 359–366. [Google Scholar] [CrossRef]
- Yao, X.; Li, G.; Bai, Q.; Xu, H.; Lü, C. Taraxerol Inhibits LPS-Induced Inflammatory Responses through Suppression of TAK1 and Akt Activation. Int. Immunopharmacol. 2013, 15, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Godyń, J.; Jończyk, J.; Panek, D.; Malawska, B. Therapeutic Strategies for Alzheimer’s Disease in Clinical Trials. Pharmacol. Rep. 2016, 68, 127–138. [Google Scholar] [CrossRef]
- Patridge, E.; Gareiss, P.; Kinch, M.S.; Hoyer, D. An analysis of FDA-approved drugs: Natural products and their derivatives. Drug Discov. Today 2016, 21, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Kar, P.; Sharma, N.R.; Singh, B.; Sen, A.; Roy, A. Natural Compounds from Clerodendrum Spp. as Possible Therapeutic Candidates against SARS-CoV-2: An In Silico Investigation. J. Biomol. Struct. Dyn. 2020, 39, 4774–4785. [Google Scholar] [CrossRef] [PubMed]
- Zafar, R.; Sharma, K. Occurrence of Taraxerol and Taraxasterol in Medicinal Plants. Pharmacogn. Rev. 2015, 9, 19. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).