Suppressive Effects of Gelsemine on Anxiety-like Behaviors Induced by Chronic Unpredictable Mild Stress in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Mouse Model of CUMS
2.3. Behavioral Tests
2.3.1. Sucrose-Preference Test (SPT)
2.3.2. Open-Field Test (OFT)
2.3.3. Light/Dark-Transition (LDT) Test
2.3.4. Elevated Plus-Maze (EPM)
2.3.5. Forced-Swim Test (FST)
2.4. Brian-Tissue Collection
2.5. Measurement of Inflammatory Cytokine Levels
2.6. Histopathology
2.7. Western Blot Analysis
2.8. Data Analysis
3. Results
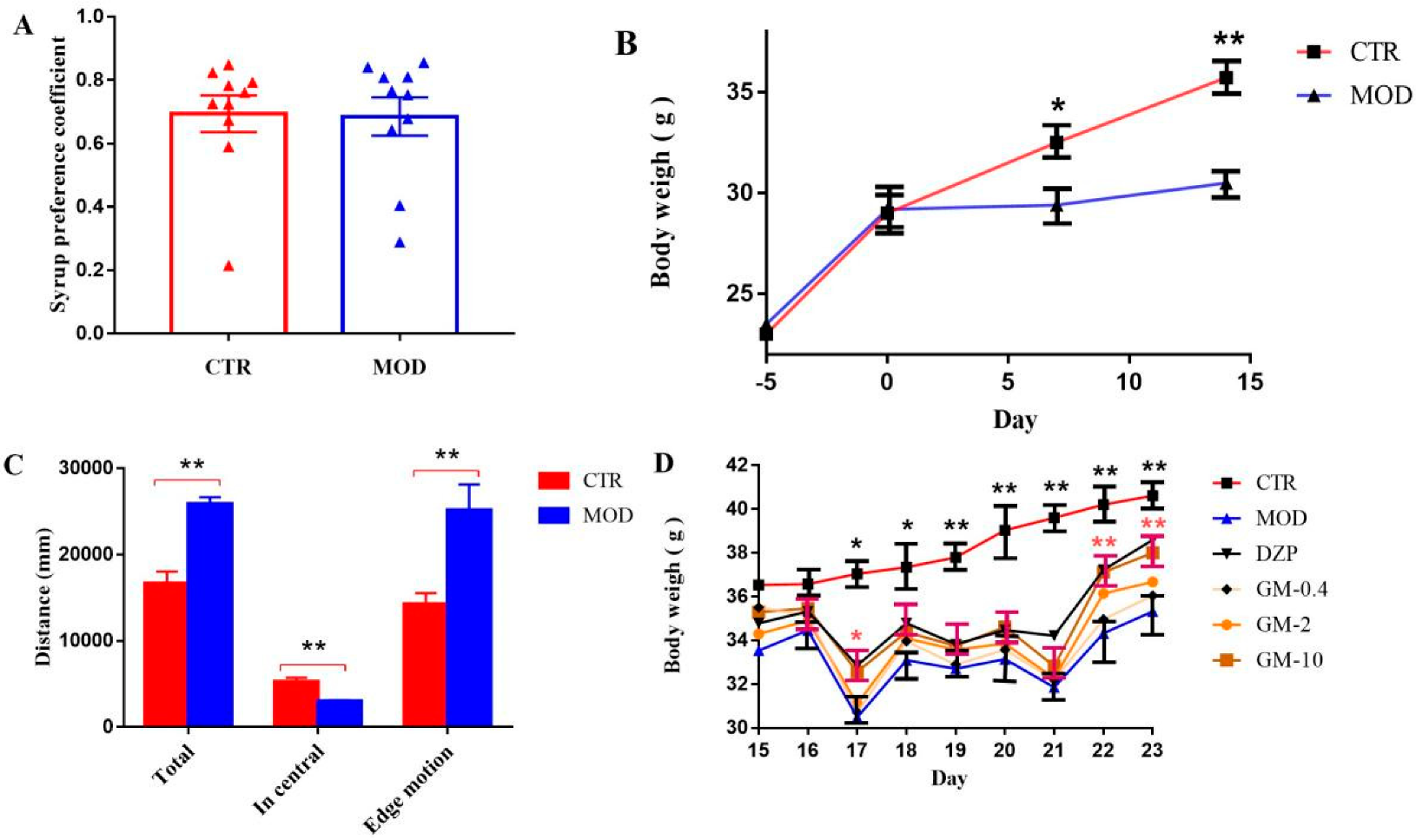
3.1. Establishment of the Mouse Model of CUMS-Induced Anxiety
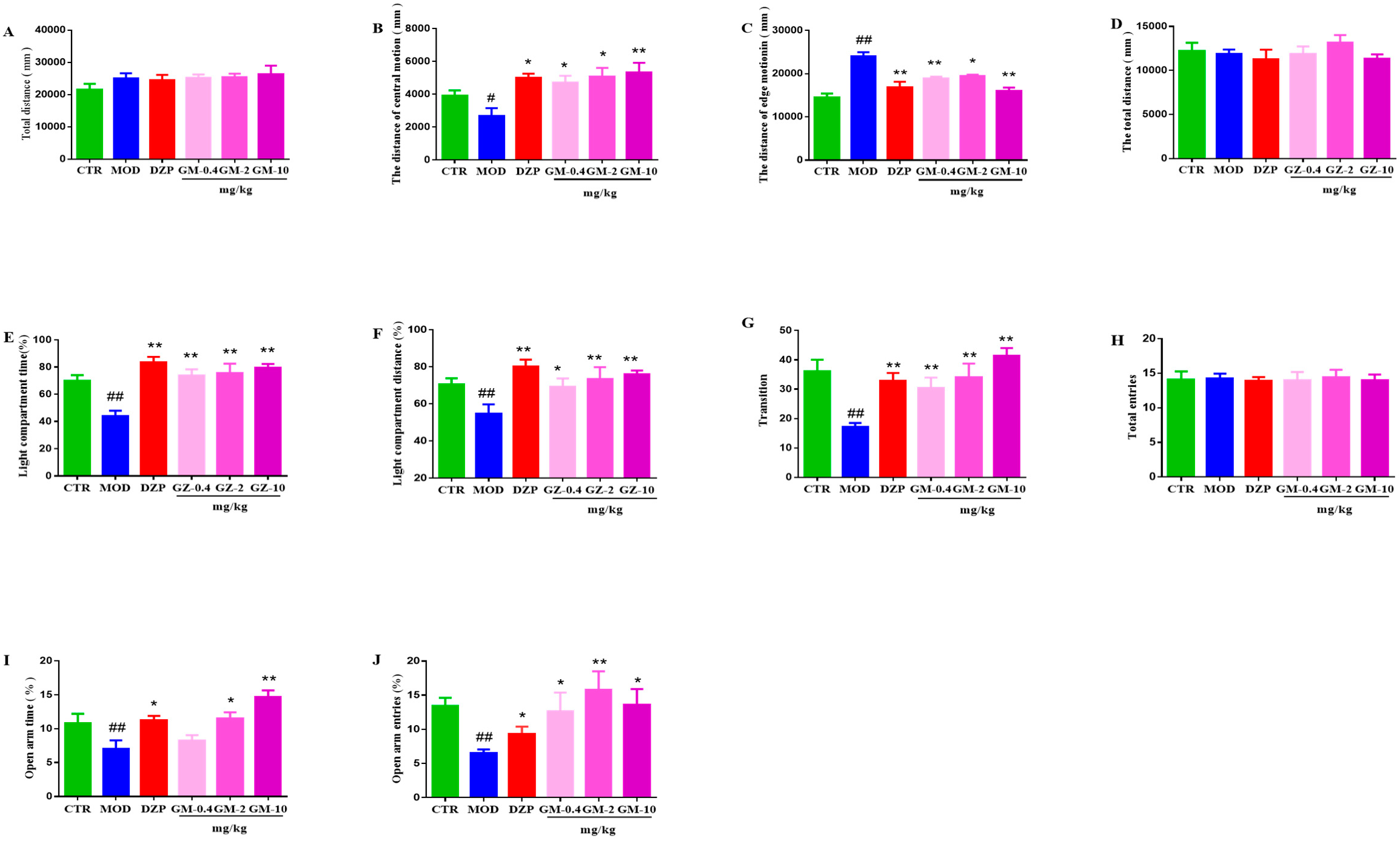
3.2. Effects of Gelsemine on Anxiety-like Behaviors in Mice
3.2.1. OFT
3.2.2. LDT
3.2.3. EPM
3.2.4. FST
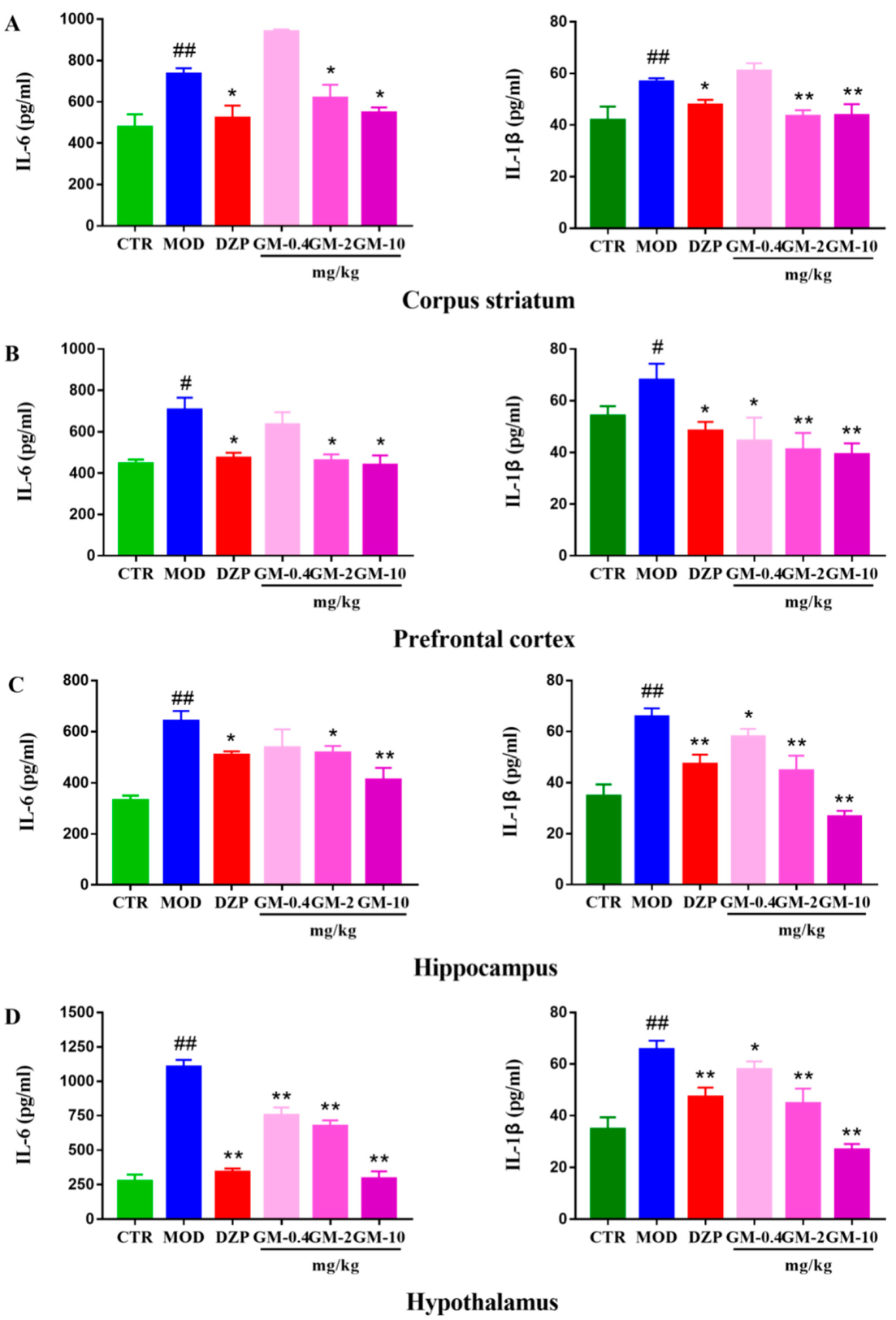
3.3. Gelsemine Decreased Inflammation in the Mouse Brain
3.4. Gelsemine Ameliorates Changes in the Brains of Anxious Mice, as Observed Using Electron Microscopy
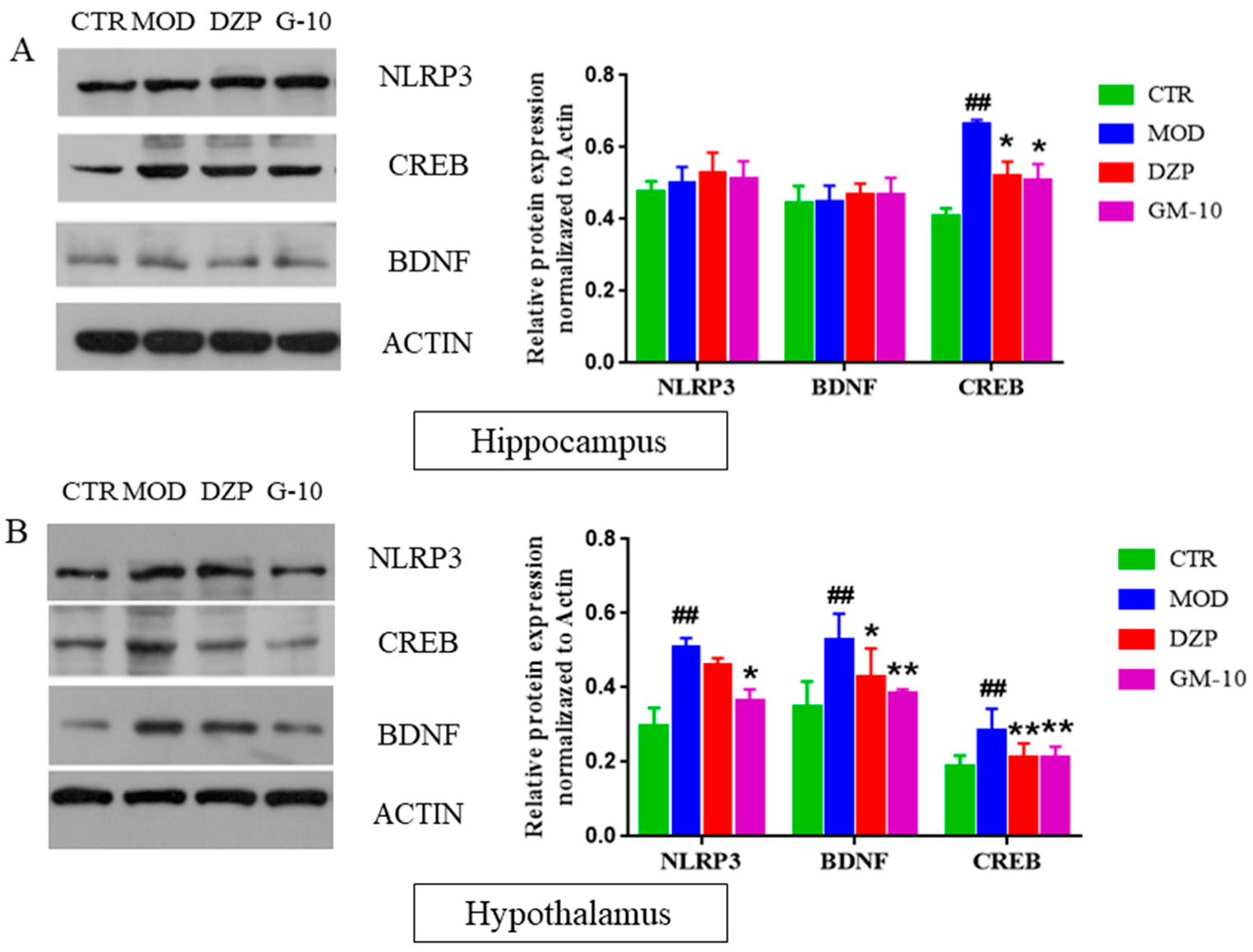
3.5. Gelsemine Inhibits the CUMS-Induced Increase in the Expression of CREB, BDNF and NLRP3 Inflammasomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
| CNS | Centra + B17 + A1:B17 + A1:B16 + A1:B17 |
| G. elegans | Gelsemium elegans |
| GM | Gelsemine |
| DZP | Diazepam |
| CUMS | Chronic unpredictable mild stress |
| MOD | Model group |
| CTR | Control group |
| CREB | cAMP-response element-binding protein |
| BDNF | Brain derived neurotrophic factor |
| SPT | Sucrose-preference test |
| OFT | open-field test |
| EPM | Elevated-plus-maze test |
| LDT | light/dark-choice test |
| FST | Forced-swim test |
| IL-6 | Interleukin 6 |
| IL-1β | Interleukin 1 Beta |
| NLRP-3 | NOD-like receptor protein 3 |
References
- Farzaei, M.H.; Bahramsoltani, R.; Rahimi, R.; Abbasabadi, F.; Abdollahi, M. A Systematic Review of Plant-Derived Natural Compounds for Anxiety Disorders. Curr. Top. Med. Chem. 2016, 16, 1924–1942. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; van Gerven, J.; Cohen, A.; Jacobs, G. Human pharmacology of positive GABA-A subtype-selective receptor modulators for the treatment of anxiety. Acta Pharmacol. Sin. 2019, 40, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Bandelow, B.; Michaelis, S.; Wedekind, D. Treatment of anxiety disorders. Dialogues Clin. Neurosci. 2017, 19, 93–107. [Google Scholar] [PubMed]
- Kumar, D.; Gupta, S.K.; Ganeshpurkar, A.; Singh, R.; Kumar, D.; Das, N.; Krishnamurthy, S.; Singh, S.K. Biological profiling of piperazinediones for the management of anxiety. Pharmacol. Biochem. Behav. 2019, 176, 63–71. [Google Scholar] [CrossRef]
- Garabadu, D.; Krishnamurthy, S. Asparagus racemosus attenuates anxiety-like behavior in experimental animal models. Cell. Mol. Neurobiol. 2014, 34, 511–521. [Google Scholar] [CrossRef]
- Eshaghi, E.; Sadigh-Eteghad, S.; Mohaddes, G.; Rasta, S.H. Transcranial photobiomodulation prevents anxiety and depression via changing serotonin and nitric oxide levels in brain of depression model mice: A study of three different doses of 810 nm laser. Lasers Surg. Med. 2019, 51, 634–642. [Google Scholar] [CrossRef]
- Vogelzangs, N.; Beekman, A.T.F.; de Jonge, P.; Penninx, B.W.J.H. Anxiety disorders and inflammation in a large adult cohort. Transl. Psychiat. 2013, 3, e249 . [Google Scholar] [CrossRef]
- Difrancesco, S.; Lamers, F.; Riese, H.; Merikangas, K.R.; Beekman, A.T.F.; van Hemert, A.M.; Schoevers, R.A.; Penninx, B. Sleep, circadian rhythm, and physical activity patterns in depressive and anxiety disorders: A 2-week ambulatory assessment study. Depress Anxiety 2019, 36, 975–986. [Google Scholar] [CrossRef]
- Slee, A.; Nazareth, I.; Bondaronek, P.; Liu, Y.; Cheng, Z.; Freemantle, N. Pharmacological treatments for generalised anxiety disorder: A systematic review and network meta-analysis. Lancet 2019, 393, 768–777. [Google Scholar] [CrossRef]
- Baldwin, D.; Woods, R.; Lawson, R.; Taylor, D. Efficacy of drug treatments for generalised anxiety disorder: Systematic review and meta-analysis. BMJ 2011, 342, d1199. [Google Scholar] [CrossRef]
- Liu, M.; Huang, H.H.; Yang, J.; Su, Y.P.; Lin, H.W.; Lin, L.Q.; Liao, W.J.; Yu, C.X. The active alkaloids of Gelsemium elegans Benth. are potent anxiolytics. Psychopharmacology 2013, 225, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.D.; Haroon, E.; Xu, X.; Woolwine, B.J.; Li, Z.; Felger, J.C. Inflammation negatively correlates with amygdala-ventromedial prefrontal functional connectivity in association with anxiety in patients with depression: Preliminary results. Brain Behav. Immun. 2018, 73, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Michopoulos, V.; Powers, A.; Gillespie, C.F.; Ressler, K.J.; Jovanovic, T. Inflammation in Fear- and Anxiety-Based Disorders: PTSD, GAD, and Beyond. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2017, 42, 254–270. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Tang, B.; Liao, X.; Su, Z.; Lee, S.M.; Cai, Y.; Li, C. Suppressive effects of the supercritical-carbon dioxide fluid extract of Chrysanthemum indicum on chronic unpredictable mild stress-induced depressive-like behavior in mice. Food Funct. 2019, 10, 1212–1224. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wu, Y.; Ye, L.; Wang, Y.; Zhang, K.; Wang, L.; Huang, Y.; Wang, L.; Xian, S.; Zhang, Y.; et al. Aspirin alleviates endothelial gap junction dysfunction through inhibition of NLRP3 inflammasome activation in LPS-induced vascular injury. Acta Pharm. Sin. B 2019, 9, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Ising, C.; Venegas, C.; Zhang, S.; Scheiblich, H.; Schmidt, S.V.; Vieira-Saecker, A.; Schwartz, S.; Albasset, S.; McManus, R.M.; Tejera, D.; et al. NLRP3 inflammasome activation drives tau pathology. Nature 2019, 575, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Giacobbo, B.L.; Doorduin, J.; Klein, H.C.; Dierckx, R.; Bromberg, E.; de Vries, E.F.J. Brain-Derived Neurotrophic Factor in Brain Disorders: Focus on Neuroinflammation. Mol. Neurobiol. 2019, 56, 3295–3312. [Google Scholar] [CrossRef] [PubMed]
- Autry, A.E.; Monteggia, L.M. Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol. Rev. 2012, 64, 238–258. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, A.; Rao, B.S.S. Transgenic brain-derived neurotrophic factor expression causes both anxiogenic and antidepressant effects. Proc. Natl. Acad. Sci. USA 2006, 103, 13208–13213. [Google Scholar] [CrossRef]
- Caracciolo, L.; Marosi, M.; Mazzitelli, J.; Latifi, S.; Sano, Y.; Galvan, L.; Kawaguchi, R.; Holley, S.; Levine, M.S.; Coppola, G.; et al. CREB controls cortical circuit plasticity and functional recovery after stroke. Nat. Commun. 2018, 9, 2250. [Google Scholar] [CrossRef]
- Zhang, J.; Little, C.J.; Tremmel, D.M.; Yin, J.C.; Wesley, C.S. Notch-inducible hyperphosphorylated CREB and its ultradian oscillation in long-term memory formation. J. Neurosci. 2013, 33, 12825–12834. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.L.; Han, M.H.; Graham, D.L.; Green, T.A.; Vialou, V.; Iniguez, S.D.; Cao, J.L.; Kirk, A.; Chakravarty, S.; Kumar, A.; et al. CREB regulation of nucleus accumbens excitability mediates social isolation-induced behavioral deficits. Nat. Neurosci. 2009, 12, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, H.; Wu, S.; Li, D.; Chen, S. Effect of Gelsemium elegans and Mussaenda pubescens, the Components of a Detoxification Herbal Formula, on Disturbance of the Intestinal Absorptions of Indole Alkaloids in Caco-2 Cells. Evid.-Based Complement. Altern. Med. 2017, 2017, 6947948. [Google Scholar]
- Wang, L.; Sun, Q.; Zhao, N.; Wen, Y.Q.; Song, Y.; Meng, F.H. Ultra-Liquid Chromatography Tandem Mass Spectrometry (UPLC-MS/MS)-Based Pharmacokinetics and Tissue Distribution Study of Koumine and the Detoxification Mechanism of Glycyrrhiza uralensis Fisch on Gelsemium elegans Benth. Molecules 2018, 23, 1693. [Google Scholar] [CrossRef]
- Bellavite, P.; Magnani, P.; Zanolin, E.; Conforti, A. Homeopathic Doses of Gelsemium sempervirens Improve the Behavior of Mice in Response to Novel Environments. Evid.-Based Complement. Altern. Med. 2011, 2011, 362517. [Google Scholar]
- Magnani, P.; Conforti, A.; Zanolin, E.; Marzotto, M.; Bellavite, P. Dose-effect study of Gelsemium sempervirens in high dilutions on anxiety-related responses in mice. Psychopharmacology 2010, 210, 533–545. [Google Scholar] [CrossRef][Green Version]
- Olioso, D.; Marzotto, M.; Bonafini, C.; Bellavite, P. Gelsemium sempervirens effects in vitro A bridge between homeopathy and molecular biology? HRI Res. Artic. 2014, 26, 535–539. [Google Scholar]
- Jin, G.L.; Su, Y.P.; Liu, M.; Xu, Y.; Yang, J.; Liao, K.J.; Yu, C.X. Medicinal plants of the genus Gelsemium (Gelsemiaceae, Gentianales)—A review of their phytochemistry, pharmacology, toxicology and traditional use. J. Ethnopharmacol. 2014, 152, 33–52. [Google Scholar] [CrossRef]
- Xiong, B.J.; Xu, Y.; Jin, G.L.; Liu, M.; Yang, J.; Yu, C.X. Analgesic effects and pharmacologic mechanisms of the Gelsemium alkaloid koumine on a rat model of postoperative pain. Sci. Rep. 2017, 7, 14269. [Google Scholar] [CrossRef]
- Chen, J.Z.; Li, Y.; Xiao, J.P.; Wu, S.S.; Song, H.W. Development of a sensitive and rapid UPLC-MS/MS method for the determination of koumine in rat plasma: Application to a pharmacokinetic study. Biomed. Chromatogr. 2013, 27, 736–740. [Google Scholar] [CrossRef]
- Bellavite, P.; Bonafini, C.; Marzotto, M. Experimental neuropharmacology of Gelsemium sempervirens: Recent advances and debated issues. J. Ayurveda Integr. Med. 2018, 9, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Meyer, L.; Boujedaini, N.; Patte-Mensah, C.; Mensah-Nyagan, A.G. Pharmacological effect of gelsemine on anxiety-like behavior in rat. Behav. Brain Res. 2013, 253, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Vandana, G.; Dhar, V.J.; Sharma, A.; Dutt, R. Antianxiety Activity of Methanol Extract of Gelsemium sempervirens (Linn.) Ait. J. Stress Physiol. Biochem. 2018, 8, 118–124. [Google Scholar]
- Zhao, T.T.; Shin, K.S.; Choi, H.S.; Lee, M.K. Ameliorating effects of gypenosides on chronic stress-induced anxiety disorders in mice. BMC Complement. Altern. Med. 2015, 15, 323. [Google Scholar] [CrossRef] [PubMed]
- Gawali, N.B.; Bulani, V.D.; Gursahani, M.S.; Deshpande, P.S.; Kothavade, P.S.; Juvekar, A.R. Agmatine attenuates chronic unpredictable mild stress-induced anxiety, depression-like behaviours and cognitive impairment by modulating nitrergic signalling pathway. Brain Res. 2017, 1663, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Nestler, E.J.; Gould, E.; Manji, H.; Buncan, M.; Duman, R.S.; Greshenfeld, H.K.; Hen, R.; Koester, S.; Lederhendler, I.; Meaney, M.; et al. Preclinical models: Status of basic research in depression. Biol. Psychiatry 2002, 52, 503–528. [Google Scholar] [CrossRef]
- Cunha, J.M.; Masur, J. Evaluation of psychotropic drugs with a modified open field test. Pharmacology 1978, 16, 259–267. [Google Scholar] [CrossRef]
- Walsh, R.N.; Cummins, R.A. The Open-Field Test: A critical review. Psychol. Bull. 1976, 83, 482–504. [Google Scholar] [CrossRef]
- Sanchez, C. Acute stress enhances anxiolytic-like drug responses of mice tested in a black and white test box. Eur. Neuropsychopharmacol. 1997, 7, 283–288. [Google Scholar] [CrossRef]
- Costall, B.; Jones, B.J.; Kelly, M.E.; Naylor, R.J.; Tomkins, D.M. Exploration of mice in a black and white test box: Validation as a model of anxiety. Pharmacol. Biochem. Behav. 1989, 32, 777–785. [Google Scholar] [CrossRef]
- Lister, R.G. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology 1987, 92, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Pellow, S.; Chopin, P.; File, S.E.; Briley, M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J. Neurosci. Methods 1985, 14, 149–167. [Google Scholar] [CrossRef]
- Leggio, G.M.; Micale, V.; Drago, F. Increased sensitivity to antidepressants of D3 dopamine receptor-deficient mice in the forced swim test (FST). Eur. Neuropsychopharmacol. 2008, 18, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Malyszczak, K.; Szechinski, M. Comorbidity of different forms of anxiety disorders and depression. Psychiatr. Pol. 2004, 38, 603–609. [Google Scholar] [PubMed]
- Jung, Y.H.; Hong, S.I.; Ma, S.X.; Hwang, J.Y.; Kim, J.S.; Lee, J.H.; Seo, J.Y.; Lee, S.Y.; Jang, C.G. Strain differences in the chronic mild stress animal model of depression and anxiety in mice. Biomol. Ther. 2014, 22, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Mineur, Y.S.; Belzung, C.; Crusio, W.E. Effects of unpredictable chronic mild stress on anxiety and depression-like behavior in mice. Behav. Brain Res. 2006, 175, 43–50. [Google Scholar] [CrossRef]
- Kraeuter, A.K.; Guest, P.C.; Sarnyai, Z. The Open Field Test for Measuring Locomotor Activity and Anxiety-Like Behavior. Methods Mol. Biol. 2019, 1916, 99–103. [Google Scholar]
- Kraeuter, A.K.; Guest, P.C.; Sarnyai, Z. The Elevated Plus Maze Test for Measuring Anxiety-Like Behavior in Rodents. Methods Mol. Biol. 2016, 1916, 69–74. [Google Scholar]
- Dutt, V.; Dhar, V.J.; Sharma, A. Antianxiety activity of Gelsemium sempervirens. Pharm. Biol. 2010, 48, 1091–1096. [Google Scholar] [CrossRef]
- Haroon, E.; Raison, C.L.; Miller, A.H. Psychoneuroimmunology meets neuropsychopharmacology: Translational implications of the impact of inflammation on behavior. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2012, 37, 137–162. [Google Scholar] [CrossRef]
- Liu, M.; Shen, J.; Liu, H.; Xu, Y.; Su, Y.P.; Yang, J.; Yu, C.X. Gelsenicine from Gelsemium elegans attenuates neuropathic and inflammatory pain in mice. Biol. Pharm. Bull. 2011, 34, 1877–1880. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Qiu, H.Q.; Liu, H.; Liu, M.; Huang, Z.Y.; Yang, J.; Su, Y.P.; Yu, C.X. Effects of koumine, an alkaloid of Gelsemium elegans Benth., on inflammatory and neuropathic pain models and possible mechanism with allopregnanolone. Pharmacol. Biochem. Behav. 2012, 101, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.K.; Tang, Q.; Dai, J.J.; Li, Y.Y.; Hei, M.Y. Effects of dopamine receptor regulation on anxiety-like behavior after hypoxic-ischemic brain injury in neonatal rats. Chin. J. Contemp. Pediatr. 2014, 16, 1045–1050. [Google Scholar]
- Li, Q.; Qu, F.L.; Gao, Y.; Jiang, Y.P.; Rahman, K.; Lee, K.H.; Han, T.; Qin, L.P. Piper sarmentosum Roxb. produces antidepressant-like effects in rodents, associated with activation of the CREB-BDNF-ERK signaling pathway and reversal of HPA axis hyperactivity. J. Ethnopharmacol. 2017, 199, 9–19. [Google Scholar] [CrossRef]
- Shen, Z.; Zhu, J.; Yuan, Y.; Ren, L.; Qian, M.; Lin, M.; Cai, M.; Zhang, Z.; Shen, X. The roles of brain-derived neurotrophic factor (BDNF) and glial cell line-derived neurotrophic factor (GDNF) in predicting treatment remission in a Chinese Han population with generalized anxiety disorder. Psychiatry Res. 2019, 271, 319–324. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, H.; Tang, M.-H.; Zeng, Z.-Y.; Huang, S.-J.; Zheng, X.-F.; Liu, Z.-Y. Suppressive Effects of Gelsemine on Anxiety-like Behaviors Induced by Chronic Unpredictable Mild Stress in Mice. Brain Sci. 2022, 12, 191. https://doi.org/10.3390/brainsci12020191
Yu H, Tang M-H, Zeng Z-Y, Huang S-J, Zheng X-F, Liu Z-Y. Suppressive Effects of Gelsemine on Anxiety-like Behaviors Induced by Chronic Unpredictable Mild Stress in Mice. Brain Sciences. 2022; 12(2):191. https://doi.org/10.3390/brainsci12020191
Chicago/Turabian StyleYu, Hui, Mo-Huan Tang, Zi-Yue Zeng, Si-Juan Huang, Xiao-Feng Zheng, and Zhao-Ying Liu. 2022. "Suppressive Effects of Gelsemine on Anxiety-like Behaviors Induced by Chronic Unpredictable Mild Stress in Mice" Brain Sciences 12, no. 2: 191. https://doi.org/10.3390/brainsci12020191
APA StyleYu, H., Tang, M.-H., Zeng, Z.-Y., Huang, S.-J., Zheng, X.-F., & Liu, Z.-Y. (2022). Suppressive Effects of Gelsemine on Anxiety-like Behaviors Induced by Chronic Unpredictable Mild Stress in Mice. Brain Sciences, 12(2), 191. https://doi.org/10.3390/brainsci12020191





