Molecular Physiology of the Neuronal Synapse
Abstract
1. Introduction
2. Presynaptic Molecular Mechanisms
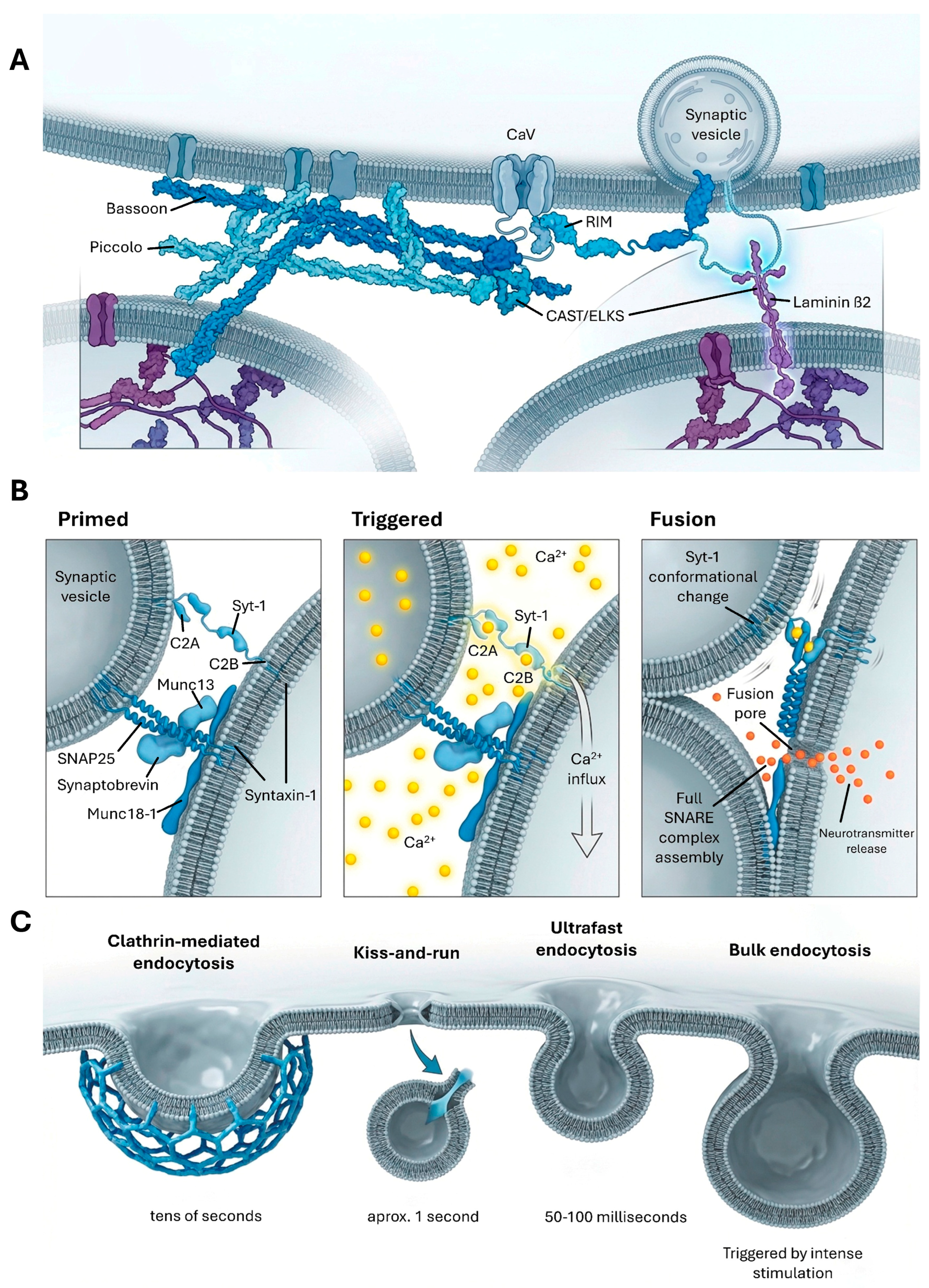
2.1. Active Zone Organization and Dynamics
2.2. Neurotransmitter Synthesis, Packaging, and Release
| Protein | Role | Interactions |
|---|---|---|
| SNARE Complex (Syntaxin-1, SNAP25, Synaptobrevin) | Core machinery for membrane fusion; pulls vesicle and plasma membranes together | Synaptotagmin-1, Munc18-1, Munc13-1 |
| Synaptotagmin-1 (Syt1) | Major Ca2+ sensor; triggers release via a “lever” mechanism | SNARE complex (primary interface regions I & II), membrane phospholipids |
| Munc13-1 | Facilitates SNARE complex assembly; vesicle priming | RIM1, CAST/ELKS, Munc18-1 |
| Rab3-Interacting Molecules (RIMs) | Active zone organization; synaptic vesicle docking and priming | Munc13-1, CAST/ELKS, Bassoon, Piccolo |
| Bassoon | Large scaffolding protein of the cytomatrix at the active zone (CAZ); AZ assembly, CaV localization, SV priming, ubiquitin-proteasome regulation | CAST/ELKS, RIMs, Piccolo |
| Piccolo | Large scaffolding protein of CAZ; AZ assembly, CaV localization, SV priming, actin cytoskeleton dynamics | CAST/ELKS, Bassoon |
| CAST/ELKS (Cytomatrix at Active Zone-Associated Structural Protein) | Key structural component of CAZ; links other AZ proteins | RIM1, Bassoon, Piccolo |
| Laminin β2 | Postsynaptic synapse organizer; anchors presynaptic complex | Extracellular domains of presynaptic calcium channels |
| Voltage-gated Calcium Channels (CaV) | Mediate Ca2+ influx for release; presynaptic scaffolding | Cytosolic AZ proteins, Laminin β2 |
2.3. Synaptic Vesicle Recycling Pathways
3. Postsynaptic Molecular Mechanisms
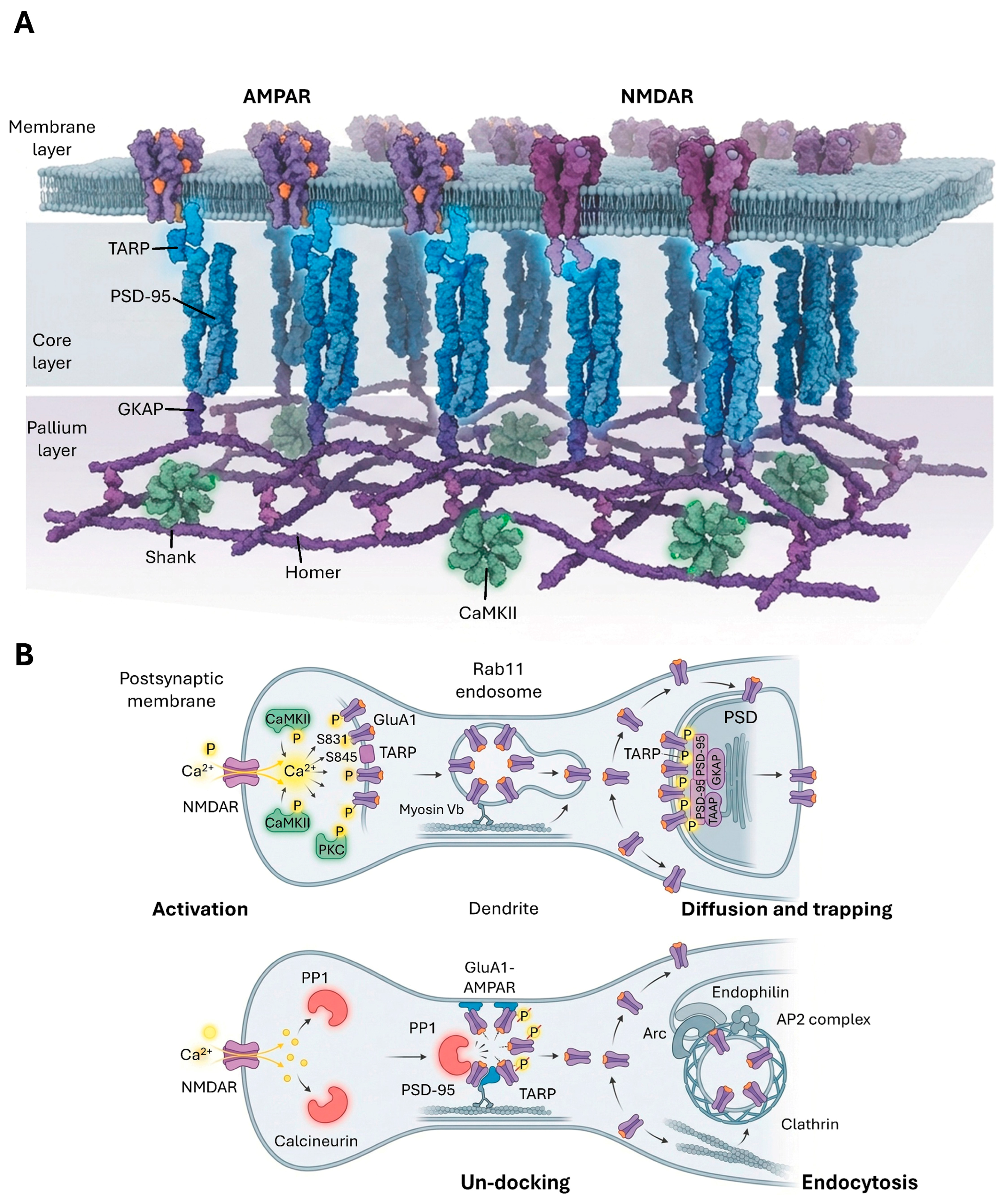
3.1. Postsynaptic Density (PSD) Structure and Protein Composition
3.2. Excitatory Receptor Trafficking and Regulation
3.2.1. AMPAR Trafficking and Regulation
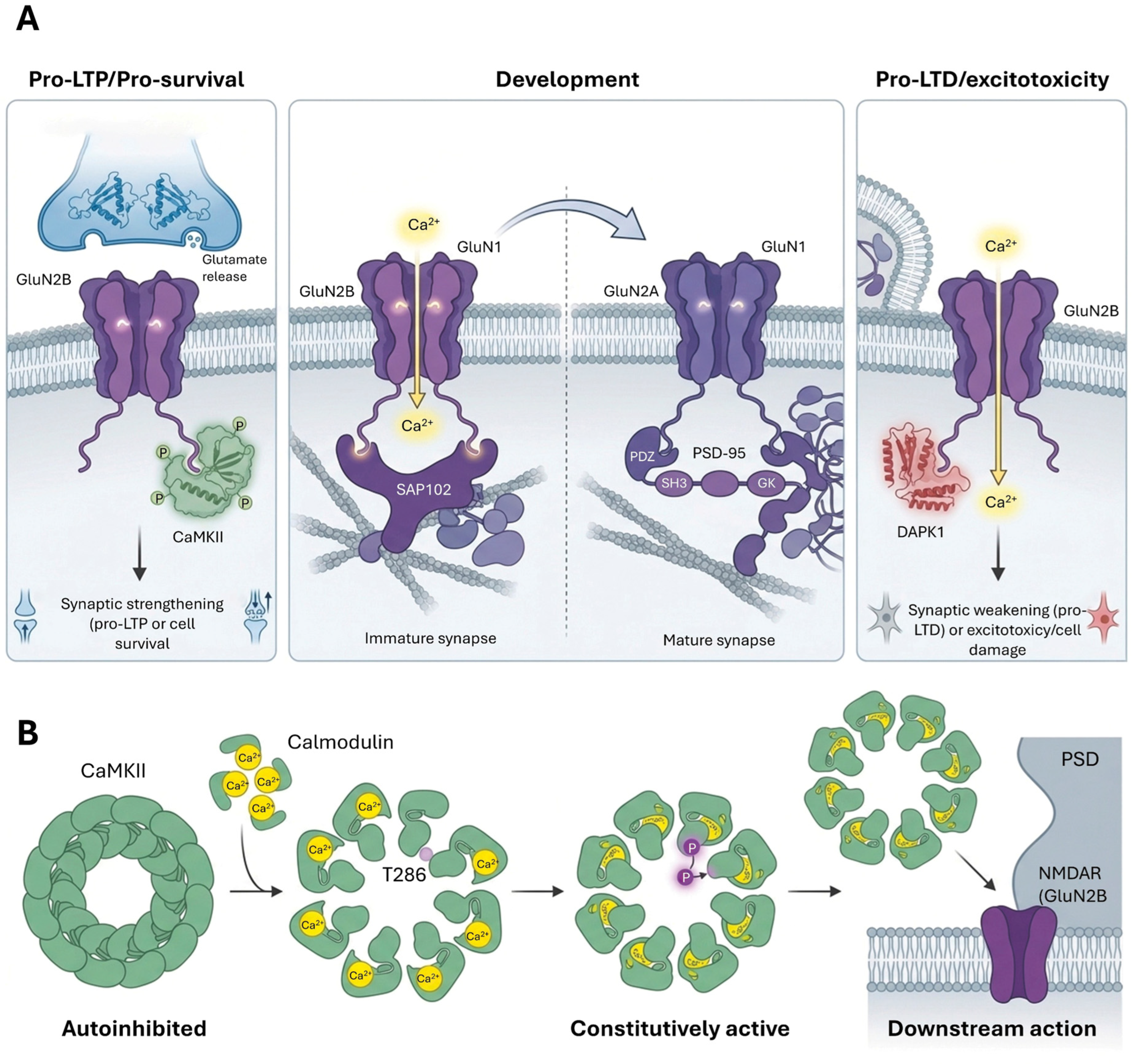
3.2.2. NMDAR Trafficking and Regulation
3.3. Inhibitory Receptor Trafficking and Regulation
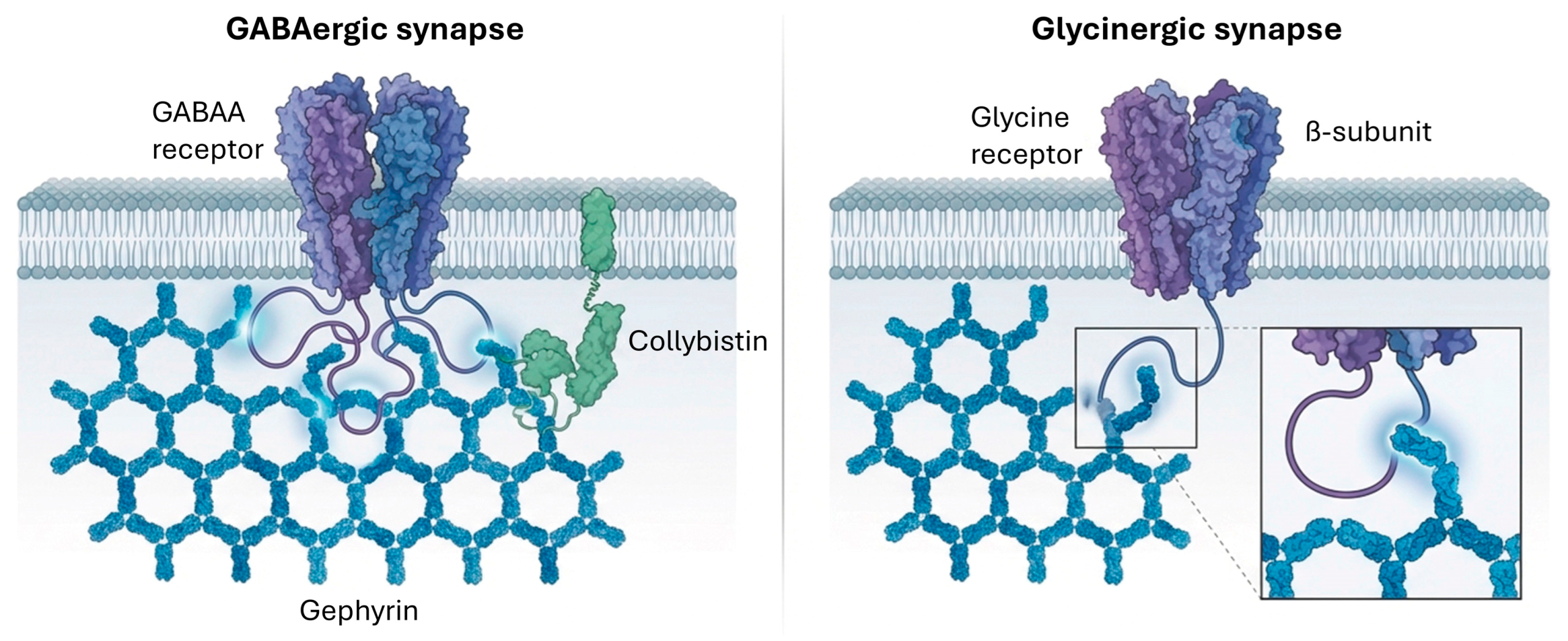
3.3.1. GABA-A Receptor Trafficking and Regulation
3.3.2. Glycine Receptor Trafficking and Regulation
4. Synaptic Plasticity: Molecular Mechanisms
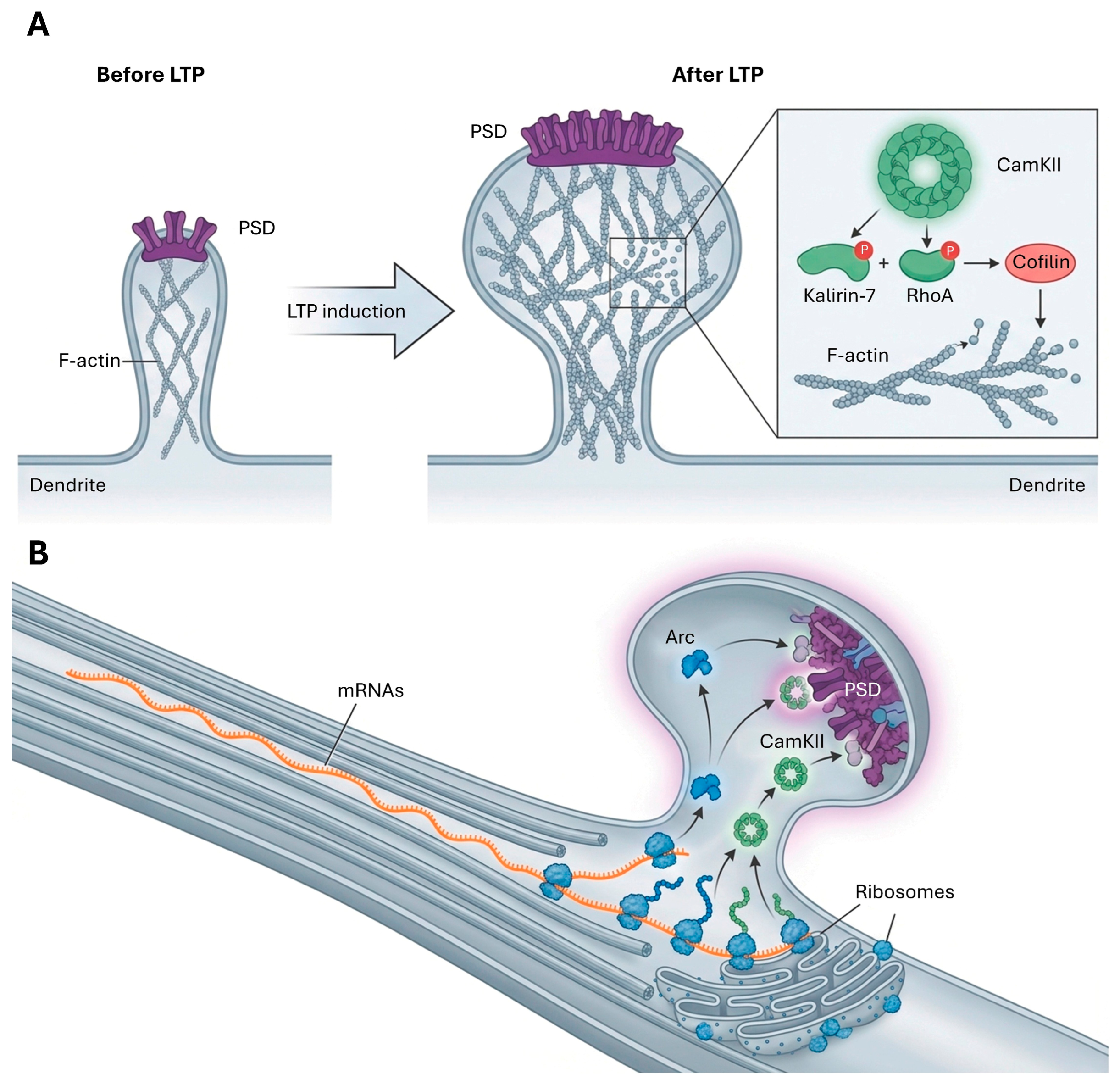
4.1. Long-Term Potentiation (LTP)
4.2. Long-Term Depression (LTD)
4.3. Structural Plasticity and Cytoskeletal Remodeling
4.4. Role of Local Protein Synthesis
5. Synapse Formation, Maturation, and Elimination
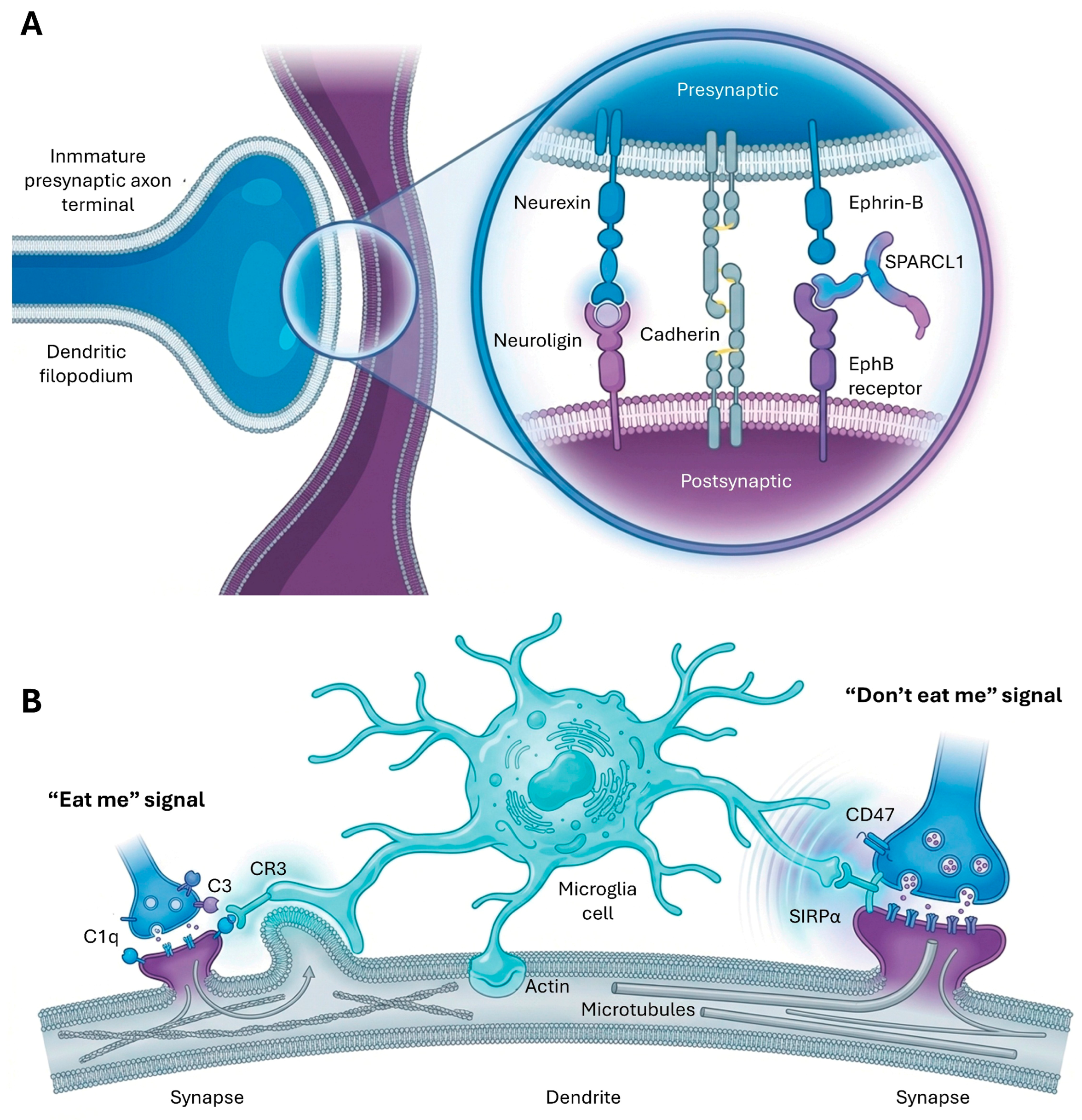
5.1. Molecular Cues and Cell Adhesion Molecules in Synaptogenesis
5.2. Role of Glial Cells in Synapse Development and Pruning
6. Synaptic Diversity and Specialization
6.1. Molecular Basis of Synaptic Heterogeneity
6.2. Role of Alternative Splicing and Post-Translational Modifications
7. Integration of Molecular Mechanisms
8. Gaps in Current Knowledge and Future Directions
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMPA | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid |
| AMPARs | AMPA receptor |
| AP2 | Adaptor protein-2 |
| AZ | Active zone |
| Arc | Activity-regulated cytoskeleton-associated protein |
| BAIs | Brain-specific angiogenesis inhibitors |
| BDNF | Brain-derived neurotrophic factor |
| C1q | Complement protein 1q |
| C3 | Complement protein 3 |
| CAMs | Cell adhesion molecules |
| CAST | Cytomatrix at the active zone-associated structural protein |
| CAZ | Cytomatrix at the active zone |
| CB | Collybistin |
| CD200 | Cluster of differentiation 200 |
| CD200R | CD200 receptor |
| CD47 | Cluster of differentiation 47 |
| CME | Clathrin-mediated endocytosis |
| CR3 | Complement receptor 3 |
| CTDs | Cytoplasmic carboxyl termini |
| CX3CL1 | Fractalkine |
| CX3CR1 | Fractalkine receptor |
| CaMKII | Calcium/calmodulin-dependent protein kinase II |
| DAPK1 | Death-associated protein kinase 1 |
| E-LTP | Early-phase LTP |
| ERK | Extracellular signal-regulated kinase |
| EphBs | Ephrin B receptors |
| F-actin | Filamentous actin |
| GABA | Gamma-aminobutyric acid |
| GABAARs | GABA-A receptors |
| GEF | Guanine nucleotide exchange factor |
| GKAP | Guanylate kinase-associated protein |
| GLAST | Glutamate aspartate transporter |
| GPCRs | G-protein coupled receptors |
| Geph | Gephyrin |
| GluA1-4 | AMPA receptor subunits |
| GluN1 | NMDA receptor subunit |
| GluN2 | NMDA receptor subunit |
| GluN3 | NMDA receptor subunit |
| GlyRs | Glycine receptors |
| HHL | Hidden hearing loss |
| JAK2 | Janus kinase 2 |
| L-LTP | Late-phase LTP |
| LTD | Long-term depression |
| LTP | Long-term potentiation |
| MAGUK | Membrane-associated guanylate kinase |
| NMDA | N-methyl-D-aspartate |
| NMDARs | NMDA receptors |
| NSF | N-ethylmaleimide sensitive factor |
| NZs | Nascent zones |
| OCT2 | Organic cation transporter 2 |
| PDZ | PSD-95/Dlg/ZO-1 |
| PET | Positron emission tomography |
| PICK1 | Protein interacting with C kinase 1 |
| PKA | Protein kinase A |
| PKC | Protein kinase C |
| PP1 | Protein phosphatase 1 |
| PS | Phosphatidylserine |
| PSD | Postsynaptic density |
| PSD-95 | Protein name (specific MAGUK) |
| PTMs | Post-translational modifications |
| RIMs | Rab3-interacting molecules |
| Rab11 | Ras-related protein Rab-11 |
| RhoA | Ras homolog family member A |
| SAP102 | Synapse-associated protein 102 |
| SAP97 | Synapse-associated protein 97 |
| SER | Smooth endoplasmic reticulum |
| SERT | Serotonin transporter |
| SIRP | Signal regulatory protein |
| SNAPs | Soluble NSF attachment proteins |
| SNARE | Soluble N-ethylmaleimide sensitive factor attachment protein receptor |
| SNX27 | Sorting Nexin 27 |
| SPARCL1 | SPARC-like protein 1 (Hevin) |
| STAT1 | Signal transducer and activator of transcription 1 |
| SV2A | Synaptic vesicle glycoprotein 2A |
| SVs | Synaptic vesicles |
| Syb2 | Synaptobrevin 2 |
| SynCAMs | Synaptic cell adhesion molecules |
| SynRIs | Synaptic reuptake inhibitors |
| Syt1 | Synaptotagmin-1 |
| TARPs | Transmembrane AMPA receptor regulatory proteins |
| TNF | Tumor necrosis factor |
| TREM2 | Triggering receptor expressed on myeloid cells 2 |
| VAChT | Vesicular acetylcholine transporter |
| VAMP2 | Vesicle-associated membrane protein 2 |
| VMAT2 | Vesicular monoamine transporter 2 |
| mGluRs | Metabotropic glutamate receptors |
| mRNAs | Messenger RNAs |
| t-SNARE | Target SNARE |
| v-SNARE | Vesicle SNARE |
References
- Sudhof, T.C. The cell biology of synapse formation. J. Cell Biol. 2021, 220, e202103052. [Google Scholar] [CrossRef]
- Sudhof, T.C.; Malenka, R.C. Understanding synapses: Past, present, and future. Neuron 2008, 60, 469–476. [Google Scholar] [CrossRef]
- Sudhof, T.C. Towards an Understanding of Synapse Formation. Neuron 2018, 100, 276–293. [Google Scholar] [CrossRef]
- van Oostrum, M.; Schuman, E.M. Understanding the molecular diversity of synapses. Nat. Rev. Neurosci. 2025, 26, 65–81. [Google Scholar] [CrossRef]
- Reig-Viader, R.; Del Castillo-Berges, D.; Burgas-Pau, A.; Arco-Alonso, D.; Zerpa-Rios, O.; Ramos-Vicente, D.; Picanol, J.; Castellanos, A.; Soto, D.; Roher, N.; et al. Synaptic proteome diversity is shaped by the levels of glutamate receptors and their regulatory proteins. Nat. Commun. 2025, 16, 10487. [Google Scholar] [CrossRef] [PubMed]
- Hwu, C.; Havel, V.; Westergaard, X.; Mendieta, A.M.; Serrano, I.C.; Hwu, J.; Walther, D.; Lankri, D.; Selinger, T.L.; He, K.; et al. Deciphering Ibogaine’s Matrix Pharmacology: Multiple Transporter Modulation at Serotonin Synapses. J. Am. Chem. Soc. 2025. [Google Scholar] [CrossRef] [PubMed]
- Nithianantharajah, J.; Komiyama, N.H.; McKechanie, A.; Johnstone, M.; Blackwood, D.H.; St Clair, D.; Emes, R.D.; van de Lagemaat, L.N.; Saksida, L.M.; Bussey, T.J.; et al. Synaptic scaffold evolution generated components of vertebrate cognitive complexity. Nat. Neurosci. 2013, 16, 16–24. [Google Scholar] [CrossRef]
- Orvis, J.; Albertin, C.B.; Shrestha, P.; Chen, S.; Zheng, M.; Rodriguez, C.J.; Tallon, L.J.; Mahurkar, A.; Zimin, A.V.; Kim, M.; et al. The evolution of synaptic and cognitive capacity: Insights from the nervous system transcriptome of Aplysia. Proc. Natl. Acad. Sci. USA 2022, 119, e2122301119. [Google Scholar] [CrossRef]
- Kaizuka, T.; Takumi, T. Postsynaptic density proteins and their involvement in neurodevelopmental disorders. J. Biochem. 2018, 163, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Grant, S.G.N. Synapse diversity and synaptome architecture in human genetic disorders. Hum. Mol. Genet. 2019, 28, R219–R225. [Google Scholar] [CrossRef]
- Nishimune, H. Molecular mechanism of active zone organization at vertebrate neuromuscular junctions. Mol. Neurobiol. 2012, 45, 1–16. [Google Scholar] [CrossRef]
- Ackermann, F.; Waites, C.L.; Garner, C.C. Presynaptic active zones in invertebrates and vertebrates. EMBO Rep. 2015, 16, 923–938. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Cousin, M.A. Synaptic Vesicle Recycling at the Developing Presynapse. J. Neurochem. 2025, 169, e70206. [Google Scholar] [CrossRef] [PubMed]
- Clarke, G.L.; Chen, J.; Nishimune, H. Presynaptic Active Zone Density during Development and Synaptic Plasticity. Front. Mol. Neurosci. 2012, 5, 12. [Google Scholar] [CrossRef]
- Ivanova, D.; Dirks, A.; Fejtova, A. Bassoon and piccolo regulate ubiquitination and link presynaptic molecular dynamics with activity-regulated gene expression. J. Physiol. 2016, 594, 5441–5448. [Google Scholar] [CrossRef]
- Gundelfinger, E.D.; Reissner, C.; Garner, C.C. Role of Bassoon and Piccolo in Assembly and Molecular Organization of the Active Zone. Front. Synaptic Neurosci. 2015, 7, 19. [Google Scholar] [CrossRef]
- Takao-Rikitsu, E.; Mochida, S.; Inoue, E.; Deguchi-Tawarada, M.; Inoue, M.; Ohtsuka, T.; Takai, Y. Physical and functional interaction of the active zone proteins, CAST, RIM1, and Bassoon, in neurotransmitter release. J. Cell Biol. 2004, 164, 301–311. [Google Scholar] [CrossRef]
- Chua, J.J. Macromolecular complexes at active zones: Integrated nano-machineries for neurotransmitter release. Cell. Mol. Life Sci. 2014, 71, 3903–3916. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hu, B.; Zieba, A.; Neumann, N.G.; Kasper-Sonnenberg, M.; Honsbein, A.; Hultqvist, G.; Conze, T.; Witt, W.; Limbach, C.; et al. A protein interaction node at the neurotransmitter release site: Domains of Aczonin/Piccolo, Bassoon, CAST, and rim converge on the N-terminal domain of Munc13-1. J. Neurosci. 2009, 29, 12584–12596. [Google Scholar] [CrossRef]
- Kuo, C.S.; Ruckmani, V.M.; Wang, M.C.; Khawaja, M.S.; Bayansan, O.; Barmaver, S.N.; Bhan, P.; Wagner, O.I. A Mutation in Vesicular Acetylcholine Transporter Increases Tubulin Acetylation Compromising Synaptic Vesicle Transport. J. Neurochem. 2025, 169, e70322. [Google Scholar] [CrossRef]
- Murphy, F.H.; Abramian, A.; Klaassen, R.V.; Koopmans, F.; Persoon, C.M.; Smit, A.B.; Toonen, R.F.; Verhage, M. RIM and MUNC13 membrane-binding domains are essential for neuropeptide secretion. J. Cell Biol. 2025, 224, e202409196. [Google Scholar] [CrossRef]
- Rizo, J. Mechanism of neurotransmitter release coming into focus. Protein Sci. 2018, 27, 1364–1391. [Google Scholar] [CrossRef]
- Rizzoli, S.O. Synaptic vesicle recycling: Steps and principles. EMBO J. 2014, 33, 788–822. [Google Scholar] [CrossRef]
- Toulme, E.; Salazar Lazaro, A.; Trimbuch, T.; Rizo, J.; Rosenmund, C. Neurotransmitter release is triggered by a calcium-induced rearrangement in the Synaptotagmin-1/SNARE complex primary interface. Proc. Natl. Acad. Sci. USA 2024, 121, e2409636121. [Google Scholar] [CrossRef] [PubMed]
- Kaeser, P.S.; Regehr, W.G. Molecular mechanisms for synchronous, asynchronous, and spontaneous neurotransmitter release. Annu. Rev. Physiol. 2014, 76, 333–363. [Google Scholar] [CrossRef]
- Voleti, R.; Jaczynska, K.; Rizo, J. Ca2+-dependent release of synaptotagmin-1 from the SNARE complex on phosphatidylinositol 4,5-bisphosphate-containing membranes. Elife 2020, 9, e57154. [Google Scholar] [CrossRef] [PubMed]
- Jaczynska, K.; Esser, V.; Xu, J.; Sari, L.; Lin, M.M.; Rosenmund, C.; Rizo, J. A lever hypothesis for Synaptotagmin-1 action in neurotransmitter release. Proc. Natl. Acad. Sci. USA 2025, 122, e2417941121. [Google Scholar] [CrossRef]
- Bandura, J.; Feng, Z.P. Dynamin independent mechanism of exo-endocytosis coupling. J. Physiol. 2025, 603, 5869–5870. [Google Scholar] [CrossRef] [PubMed]
- Mochida, S. Mechanisms of Synaptic Vesicle Exo- and Endocytosis. Biomedicines 2022, 10, 1593. [Google Scholar] [CrossRef]
- Saheki, Y.; De Camilli, P. Synaptic vesicle endocytosis. Cold Spring Harb. Perspect. Biol. 2012, 4, a005645. [Google Scholar] [CrossRef]
- Imoto, Y.; Watanabe, S. Beyond Clathrin: Decoding the Mechanism of Ultrafast Endocytosis. Physiology 2025, 40, 454–469. [Google Scholar] [CrossRef]
- Watanabe, S.; Rost, B.R.; Camacho-Perez, M.; Davis, M.W.; Sohl-Kielczynski, B.; Rosenmund, C.; Jorgensen, E.M. Ultrafast endocytosis at mouse hippocampal synapses. Nature 2013, 504, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S. Synaptic Vesicle Recycling Through the Lens of Ultrafast Endocytosis. Annu. Rev. Neurosci. 2025, 48, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Ogunmowo, T.H.; Jing, H.; Raychaudhuri, S.; Kusick, G.F.; Imoto, Y.; Li, S.; Itoh, K.; Ma, Y.; Jafri, H.; Dalva, M.B.; et al. Membrane compression by synaptic vesicle exocytosis triggers ultrafast endocytosis. Nat. Commun. 2023, 14, 2888. [Google Scholar] [CrossRef]
- Gan, Q.; Watanabe, S. Synaptic Vesicle Endocytosis in Different Model Systems. Front. Cell Neurosci. 2018, 12, 171. [Google Scholar] [CrossRef]
- Sun, R.; Allen, J.P.; Mao, Z.; Wilson, L.; Haider, M.; Alten, B.; Zhou, Z.; Wang, X.; Zhou, Q. The postsynaptic density in excitatory synapses is composed of clustered, heterogeneous nanoblocks. J. Cell Biol. 2025, 224, e202406133. [Google Scholar] [CrossRef]
- Harris, K.M.; Kuwajima, M.; Flores, J.C.; Zito, K. Synapse-specific structural plasticity that protects and refines local circuits during LTP and LTD. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2024, 379, 20230224. [Google Scholar] [CrossRef]
- Suzuki, T.; Fujii, T.; Kametani, K.; Li, W.; Tabuchi, K. Tubulin and GTP Are Crucial Elements for Postsynaptic Density Construction and Aggregation. J. Neurochem. 2025, 169, e70085. [Google Scholar] [CrossRef] [PubMed]
- Dosemeci, A.; Weinberg, R.J.; Reese, T.S.; Tao-Cheng, J.H. The Postsynaptic Density: There Is More than Meets the Eye. Front. Synaptic Neurosci. 2016, 8, 23. [Google Scholar] [CrossRef]
- Elias, G.M.; Elias, L.A.; Apostolides, P.F.; Kriegstein, A.R.; Nicoll, R.A. Differential trafficking of AMPA and NMDA receptors by SAP102 and PSD-95 underlies synapse development. Proc. Natl. Acad. Sci. USA 2008, 105, 20953–20958. [Google Scholar] [CrossRef]
- Jewett, B.E.; Thapa, B. Physiology, NMDA Receptor. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Hayashi, M.K.; Tang, C.; Verpelli, C.; Narayanan, R.; Stearns, M.H.; Xu, R.M.; Li, H.; Sala, C.; Hayashi, Y. The postsynaptic density proteins Homer and Shank form a polymeric network structure. Cell 2009, 137, 159–171. [Google Scholar] [CrossRef]
- Tao-Cheng, J.H.; Thein, S.; Yang, Y.; Reese, T.S.; Gallant, P.E. Homer is concentrated at the postsynaptic density and does not redistribute after acute synaptic stimulation. Neuroscience 2014, 266, 80–90. [Google Scholar] [CrossRef]
- Aruna, K.; Pal, S.; Khanna, A.; Bhattacharyya, S. Postsynaptic Density Proteins and Their Role in the Trafficking of Group I Metabotropic Glutamate Receptors. J. Membr. Biol. 2024, 257, 257–268. [Google Scholar] [CrossRef] [PubMed]
- de Leon-Lopez, C.A.M.; Carretero-Rey, M.; Khan, Z.U. AMPA Receptors in Synaptic Plasticity, Memory Function, and Brain Diseases. Cell. Mol. Neurobiol. 2025, 45, 14. [Google Scholar] [CrossRef]
- Nandi, S.; de Deus, J.L.; Faborode, O.S.; Nandi, S. Synaptic Pruning by Microglia: Lessons from Genetic Studies in Mice. Dev. Neurosci. 2025, 47, 362–382. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Alonso, J.; Nicoll, R.A. AMPA receptor trafficking and LTP: Carboxy-termini, amino-termini and TARPs. Neuropharmacology 2021, 197, 108710. [Google Scholar] [CrossRef]
- Vieira, M.; Yong, X.L.H.; Roche, K.W.; Anggono, V. Regulation of NMDA glutamate receptor functions by the GluN2 subunits. J. Neurochem. 2020, 154, 121–143. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Luo, L.D.; Feng, I.; Ma, S. Molecular mechanisms of synaptogenesis. Front. Synaptic Neurosci. 2022, 14, 939793. [Google Scholar] [CrossRef]
- Anderson, M.C.; Dharmasri, P.A.; Damenti, M.; Metzbower, S.R.; Laghaei, R.; Blanpied, T.A.; Levy, A.D. Trans-synaptic molecular context of NMDA receptor nanodomains. Nat. Commun. 2025, 16, 7460. [Google Scholar] [CrossRef]
- Lisman, J.; Schulman, H.; Cline, H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat. Rev. Neurosci. 2002, 3, 175–190. [Google Scholar] [CrossRef]
- Lisman, J.; Yasuda, R.; Raghavachari, S. Mechanisms of CaMKII action in long-term potentiation. Nat. Rev. Neurosci. 2012, 13, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Nicoll, R.A.; Schulman, H. Synaptic memory and CaMKII. Physiol. Rev. 2023, 103, 2877–2925. [Google Scholar] [CrossRef]
- Goodell, D.J.; Zaegel, V.; Coultrap, S.J.; Hell, J.W.; Bayer, K.U. DAPK1 Mediates LTD by Making CaMKII/GluN2B Binding LTP Specific. Cell Rep. 2017, 19, 2231–2243. [Google Scholar] [CrossRef]
- Chen, R.J.; Sharma, S. GABA Receptor. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Wheal, H.V.; Chen, Y.; Mitchell, J.; Schachner, M.; Maerz, W.; Wieland, H.; Van Rossum, D.; Kirsch, J. Molecular mechanisms that underlie structural and functional changes at the postsynaptic membrane during synaptic plasticity. Prog. Neurobiol. 1998, 55, 611–640. [Google Scholar] [CrossRef]
- George, S.; Chiou, T.T.; Kanamalla, K.; De Blas, A.L. Recruitment of Plasma Membrane GABA-A Receptors by Submembranous Gephyrin/Collybistin Clusters. Cell. Mol. Neurobiol. 2022, 42, 1585–1604, Erratum in Cell. Mol. Neurobiol. 2022, 42, 1605. https://doi.org/10.1007/s10571-021-01061-y. [Google Scholar] [CrossRef]
- Herweg, J.; Schwarz, G. Splice-specific glycine receptor binding, folding, and phosphorylation of the scaffolding protein gephyrin. J. Biol. Chem. 2012, 287, 12645–12656. [Google Scholar] [CrossRef] [PubMed]
- Pizzarelli, R.; Griguoli, M.; Zacchi, P.; Petrini, E.M.; Barberis, A.; Cattaneo, A.; Cherubini, E. Tuning GABAergic Inhibition: Gephyrin Molecular Organization and Functions. Neuroscience 2020, 439, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Zacchi, P.; Antonelli, R.; Cherubini, E. Gephyrin phosphorylation in the functional organization and plasticity of GABAergic synapses. Front. Cell Neurosci. 2014, 8, 103. [Google Scholar] [CrossRef]
- Del Pino, I.; Koch, D.; Schemm, R.; Qualmann, B.; Betz, H.; Paarmann, I. Proteomic analysis of glycine receptor beta subunit (GlyRbeta)-interacting proteins: Evidence for syndapin I regulating synaptic glycine receptors. J. Biol. Chem. 2014, 289, 11396–11409. [Google Scholar] [CrossRef]
- Fraser, S.D.; Klaassen, R.V.; Villmann, C.; Smit, A.B.; Harvey, R.J. Milestone Review: Unlocking the Proteomics of Glycine Receptor Complexes. J. Neurochem. 2025, 169, e70061. [Google Scholar] [CrossRef]
- Sanhueza, M.; Lisman, J. The CaMKII/NMDAR complex as a molecular memory. Mol. Brain 2013, 6, 10. [Google Scholar] [CrossRef]
- Tullis, J.E.; Larsen, M.E.; Rumian, N.L.; Freund, R.K.; Boxer, E.E.; Brown, C.N.; Coultrap, S.J.; Schulman, H.; Aoto, J.; Dell’Acqua, M.L.; et al. LTP induction by structural rather than enzymatic functions of CaMKII. Nature 2023, 621, 146–153. [Google Scholar] [CrossRef]
- Blundon, J.A.; Zakharenko, S.S. Dissecting the components of long-term potentiation. Neuroscientist 2008, 14, 598–608. [Google Scholar] [CrossRef] [PubMed]
- Byth, L.A. Ca2+- and CaMKII-mediated processes in early LTP. Ann. Neurosci. 2014, 21, 151–153. [Google Scholar] [CrossRef] [PubMed]
- Sando, R.; Sudhof, T.C. Latrophilin GPCR signaling mediates synapse formation. Elife 2021, 10, e65717. [Google Scholar] [CrossRef]
- Sateesh, S.; Abraham, W.C. Differential Astrocyte-Supplied NMDAR Co-Agonist for CA1 Versus Dentate Gyrus Long-Term Potentiation. Hippocampus 2025, 35, e70029. [Google Scholar] [CrossRef]
- Coultrap, S.J.; Freund, R.K.; O’Leary, H.; Sanderson, J.L.; Roche, K.W.; Dell’Acqua, M.L.; Bayer, K.U. Autonomous CaMKII mediates both LTP and LTD using a mechanism for differential substrate site selection. Cell Rep. 2014, 6, 431–437. [Google Scholar] [CrossRef]
- Camus, C.; Leval, L.; Villicana-Munoz, V.; Jelinkova, S.; Compans, B.; Gambino, F.; Herzog, E.; Choquet, D.; Hosy, E. Synaptic pruning following NMDAR-dependent LTD preferentially affects isolated synapses. iScience 2025, 28, 113093. [Google Scholar] [CrossRef]
- Blackwell, K.T.; Jedrzejewska-Szmek, J. Molecular mechanisms underlying neuronal synaptic plasticity: Systems biology meets computational neuroscience in the wilds of synaptic plasticity. Wiley Interdiscip. Rev. Syst. Biol. Med. 2013, 5, 717–731. [Google Scholar] [CrossRef]
- Pfeiffer, B.E.; Huber, K.M. Current advances in local protein synthesis and synaptic plasticity. J. Neurosci. 2006, 26, 7147–7150. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Gutu, A.; Kim, N.; O’Shea, E.K. Activity-dependent synapse elimination requires caspase-3 activation. Elife 2025, 13, RP101779. [Google Scholar] [CrossRef]
- Frankel, E.B.; Kurshan, P.T. Principles of synaptogenesis: Insights from Caenorhabditiselegans. Curr. Opin. Neurobiol. 2025, 93, 103056. [Google Scholar] [CrossRef]
- Giagtzoglou, N.; Ly, C.V.; Bellen, H.J. Cell adhesion, the backbone of the synapse: “vertebrate” and “invertebrate” perspectives. Cold Spring Harb. Perspect. Biol. 2009, 1, a003079. [Google Scholar] [CrossRef]
- Yamagata, M.; Sanes, J.R.; Weiner, J.A. Synaptic adhesion molecules. Curr. Opin. Cell Biol. 2003, 15, 621–632. [Google Scholar] [CrossRef]
- Oliver, D.B.; Ramachandran, S.; Biswas, K.; Benard, C.Y.; Doitsidou, M.; McKillop, H.; Genao, N.; Lemons, M.L.; Francis, M.M. Genome-wide analysis reveals pathways important for the development and maturation of excitatory synaptic connections to GABAergic neurons. bioRxiv 2025. [Google Scholar] [CrossRef] [PubMed]
- Dalva, M.B.; McClelland, A.C.; Kayser, M.S. Cell adhesion molecules: Signalling functions at the synapse. Nat. Rev. Neurosci. 2007, 8, 206–220. [Google Scholar] [CrossRef] [PubMed]
- Munno, D.W.; Syed, N.I. Synaptogenesis in the CNS: An odyssey from wiring together to firing together. J. Physiol. 2003, 552, 1–11. [Google Scholar] [CrossRef]
- Yamagishi, S.; Bando, Y.; Sato, K. Involvement of Netrins and Their Receptors in Neuronal Migration in the Cerebral Cortex. Front. Cell Dev. Biol. 2020, 8, 590009. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Jiang, M.; Chen, Z.; Li, C.; Yin, X.; Zhang, X.; Wu, M. The Role of the Complement System in Synaptic Pruning after Stroke. Aging Dis. 2024, 16, 1452–1470. [Google Scholar] [CrossRef]
- Cornell, J.; Salinas, S.; Huang, H.Y.; Zhou, M. Microglia regulation of synaptic plasticity and learning and memory. Neural Regen. Res. 2022, 17, 705–716. [Google Scholar] [CrossRef]
- Imai, T. Activity-dependent synaptic competition and dendrite pruning in developing mitral cells. Front. Neural Circuits 2025, 19, 1541926. [Google Scholar] [CrossRef] [PubMed]
- Duran Laforet, V.; Schafer, D.P. Microglia: Activity-dependent regulators of neural circuits. Ann. N. Y. Acad. Sci. 2024, 1533, 38–50. [Google Scholar] [CrossRef]
- Verpelli, C.; Schmeisser, M.J.; Sala, C.; Boeckers, T.M. Scaffold proteins at the postsynaptic density. Adv. Exp. Med. Biol. 2012, 970, 29–61. [Google Scholar] [CrossRef]
- Wang, M.; Fan, J.; Shao, Z. Cellular and Molecular Mechanisms Underlying Synaptic Subcellular Specificity. Brain Sci. 2024, 14, 155. [Google Scholar] [CrossRef]
- Crowl, S.; Coleman, M.B.; Chaphiv, A.; Jordan, B.T.; Naegle, K.M. Systematic analysis of the effects of splicing on the diversity of post-translational modifications in protein isoforms using PTM-POSE. Cell Syst. 2025, 16, 101318. [Google Scholar] [CrossRef] [PubMed]
- Irimia, M.; Denuc, A.; Burguera, D.; Somorjai, I.; Martin-Duran, J.M.; Genikhovich, G.; Jimenez-Delgado, S.; Technau, U.; Roy, S.W.; Marfany, G.; et al. Stepwise assembly of the Nova-regulated alternative splicing network in the vertebrate brain. Proc. Natl. Acad. Sci. USA 2011, 108, 5319–5324. [Google Scholar] [CrossRef]
- Washbourne, P.; Dityatev, A.; Scheiffele, P.; Biederer, T.; Weiner, J.A.; Christopherson, K.S.; El-Husseini, A. Cell adhesion molecules in synapse formation. J. Neurosci. 2004, 24, 9244–9249. [Google Scholar] [CrossRef] [PubMed]
- Tahmasian, N.; Beckett, T.; Cao, K.; Hill, M.; Mandrozos, M.; Wang, C.; McLaurin, J. Drug Development. Alzheimers Dement. 2025, 21, e106608. [Google Scholar] [CrossRef]
- Weng, J.E.M.; Noristani, H.N.; Schuldt, C.E.; Lacor, P.N. Biomarkers. Alzheimers Dement. 2025, 21, e103597. [Google Scholar] [CrossRef]
- Young, L.; King, D.; Chang, Y.Y.; Holt, K.; Gladstein, S.; Finnema, S.J.; Spires-Jones, T.L. Biomarkers. Alzheimers Dement. 2025, 21, e104271. [Google Scholar] [CrossRef]
- Lemoine, L.; Kulagowska, L.; Bonsall, D.; King, D.; Gladstein, S.; Bevis, A.; Sabandal, M.; Wells, L.; Finnema, S.J.; Spires-Jones, T.L.; et al. Biomarkers. Alzheimers Dement. 2025, 21, e103316. [Google Scholar] [CrossRef]
- He, K.; Wang, J.; Wu, J.; Chen, X.; Chen, H.; Li, J.; Guo, Q.; Huang, Y.H.; Guan, Y.; Zhao, J.; et al. Impaired glymphatic function is associated with synaptic loss in cognitive impairment. Eur. J. Nucl. Med. Mol. Imaging 2025. [Google Scholar] [CrossRef] [PubMed]
- Le, H.; Raymond, R.; Vasdev, N.; Ni, R.; Zheng, C. Biomarkers. Alzheimers Dement. 2025, 21, e102328. [Google Scholar] [CrossRef]
- Marin-Castaneda, L.A.; Pacheco Aispuro, G.; Gonzalez-Garibay, G.; Martinez Zamora, C.A.; Romo-Parra, H.; Rubio-Osornio, M.; Rubio, C. Interplay of epilepsy and long-term potentiation: Implications for memory. Front. Neurosci. 2024, 18, 1451740. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Wang, F.; Song, X.; Yang, H.; Gao, X.; Zhu, Y.; Fu, B.; She, X.; Cui, B. Impaired postsynaptic function and disruption of intermediate filaments in GLAST-knockout-induced hidden hearing loss. FEBS J. 2025. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Ramírez-Expósito, M.J.; Cueto-Ureña, C.; Martínez-Martos, J.M. Molecular Physiology of the Neuronal Synapse. Curr. Issues Mol. Biol. 2026, 48, 88. https://doi.org/10.3390/cimb48010088
Ramírez-Expósito MJ, Cueto-Ureña C, Martínez-Martos JM. Molecular Physiology of the Neuronal Synapse. Current Issues in Molecular Biology. 2026; 48(1):88. https://doi.org/10.3390/cimb48010088
Chicago/Turabian StyleRamírez-Expósito, María Jesús, Cristina Cueto-Ureña, and José Manuel Martínez-Martos. 2026. "Molecular Physiology of the Neuronal Synapse" Current Issues in Molecular Biology 48, no. 1: 88. https://doi.org/10.3390/cimb48010088
APA StyleRamírez-Expósito, M. J., Cueto-Ureña, C., & Martínez-Martos, J. M. (2026). Molecular Physiology of the Neuronal Synapse. Current Issues in Molecular Biology, 48(1), 88. https://doi.org/10.3390/cimb48010088







