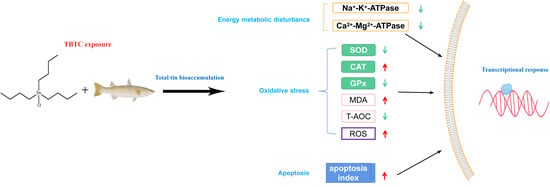
Toxicity Responses from Tributyltin Chloride on Haarder (Planiliza haematocheila) Livers: Oxidative Stress, Energy Metabolism Dysfunction, and Apoptosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Chemicals
2.2. Tributyltin Chloride (TBTC) Exposure Experiments
2.3. Total Tin Content in Haarder
2.4. Growth Indices’ Measurement
2.5. Antioxidant Capacity in Livers
2.6. Total Blood Cell Count and Intracellular Reactive Oxygen Species (ROS) Analyses
2.7. Histological and Apoptosis Detection
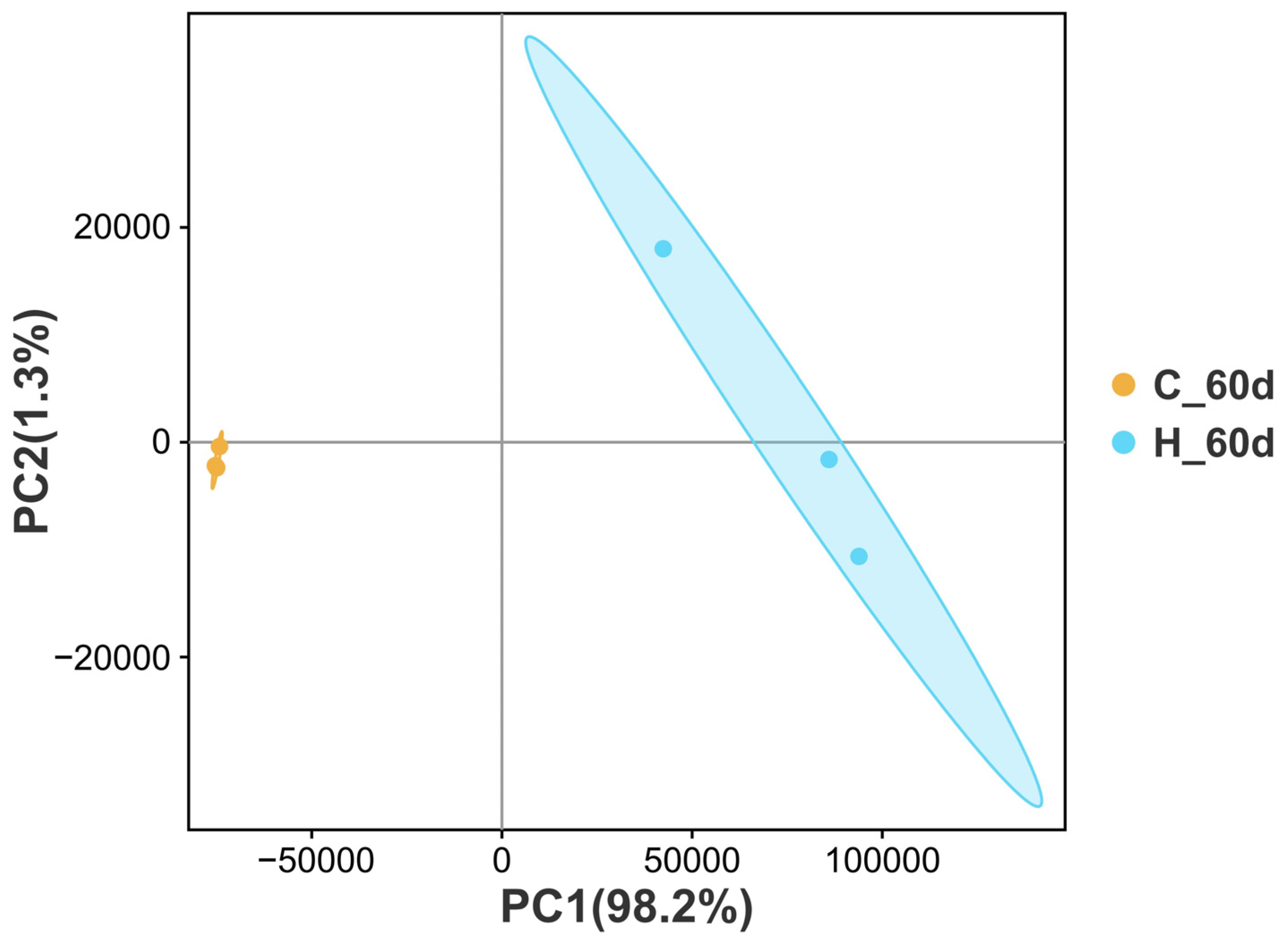
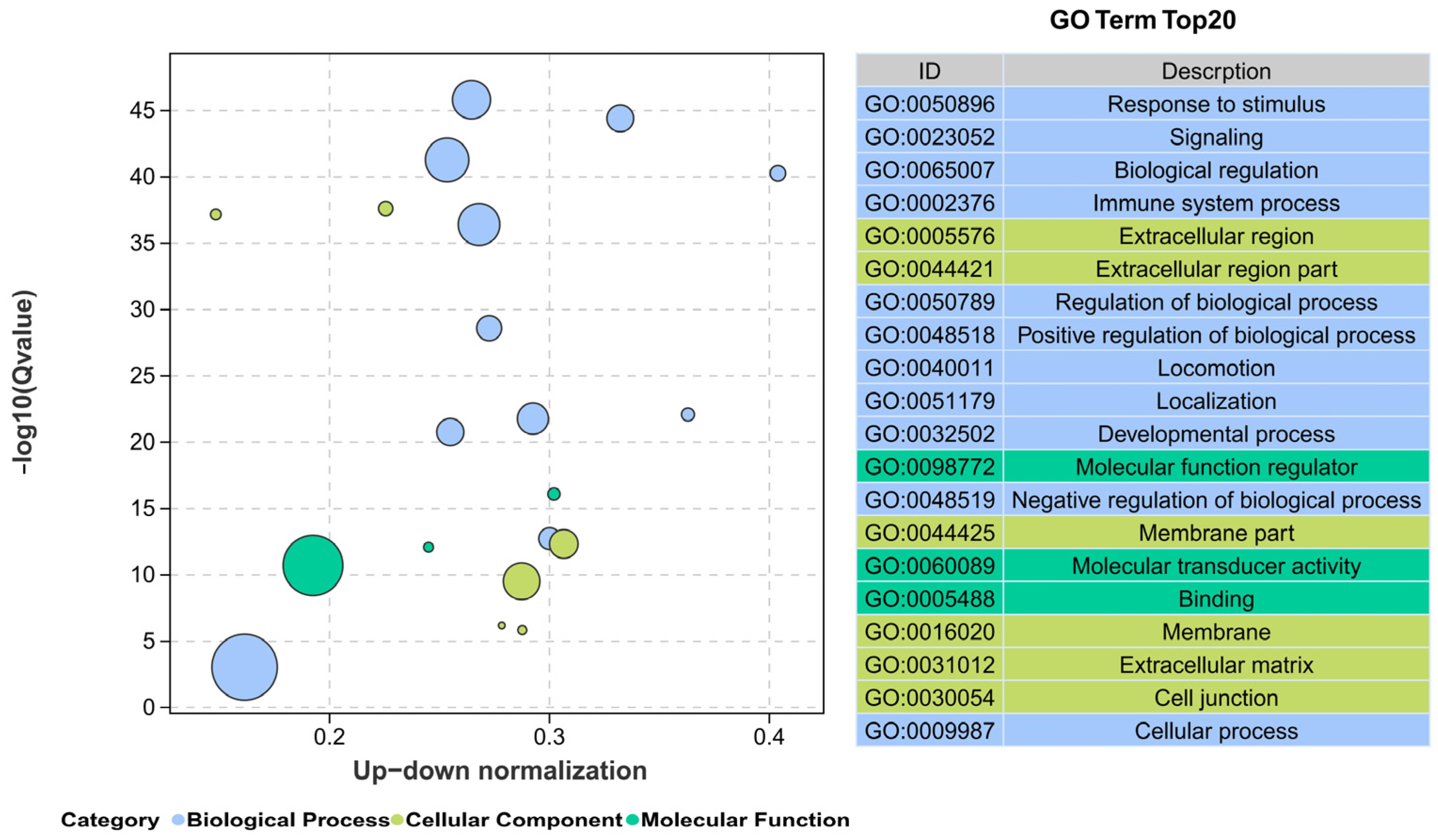
2.8. RNA-Seq and Bioinformatics Analysis
2.9. Quantitative Real-Time PCR (qRT-PCR) Detection of Differentially Expressed Genes (DEGs)
2.10. Statistical Analysis
3. Results
3.1. Total Tin Contents in Livers
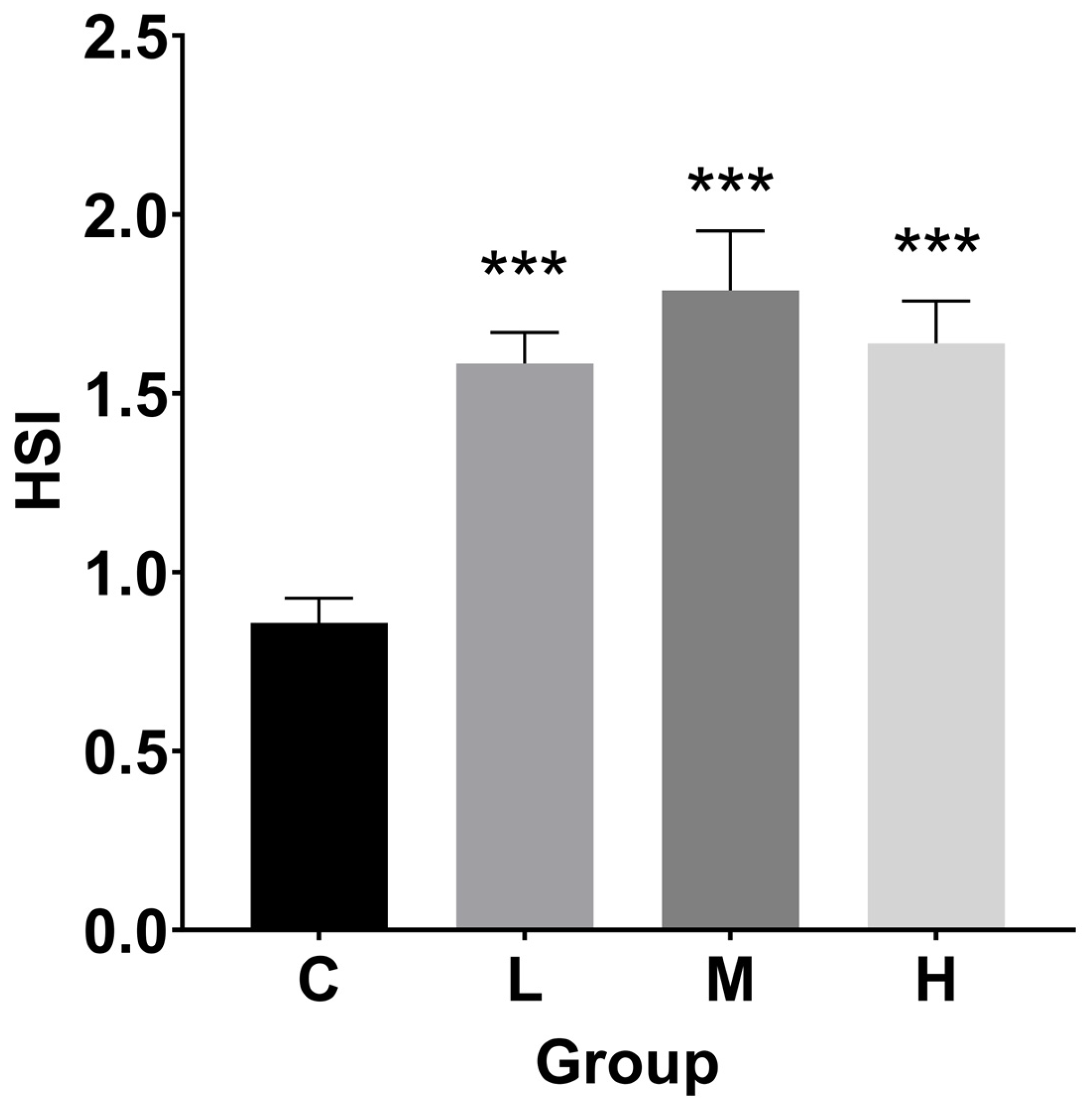
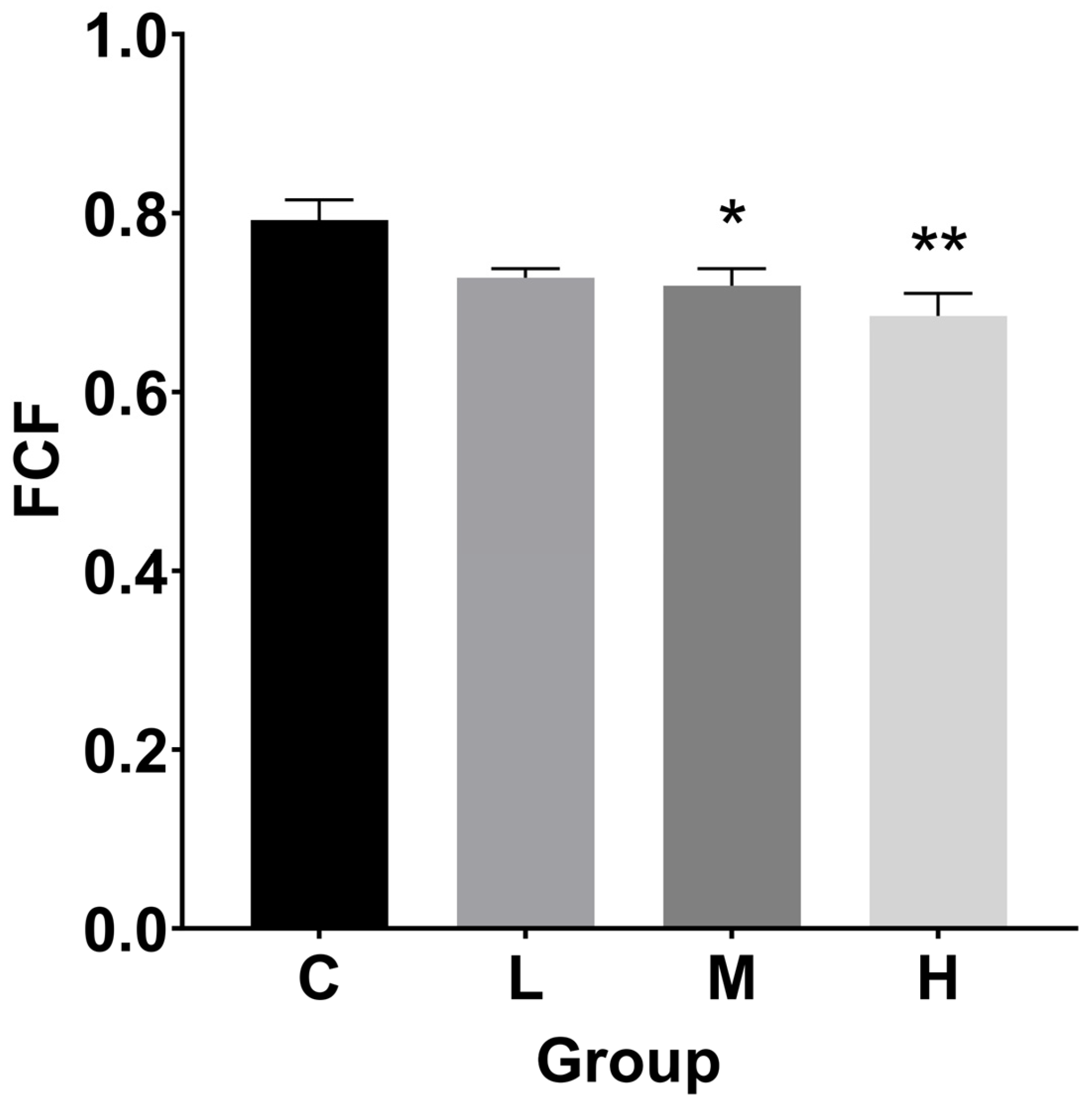
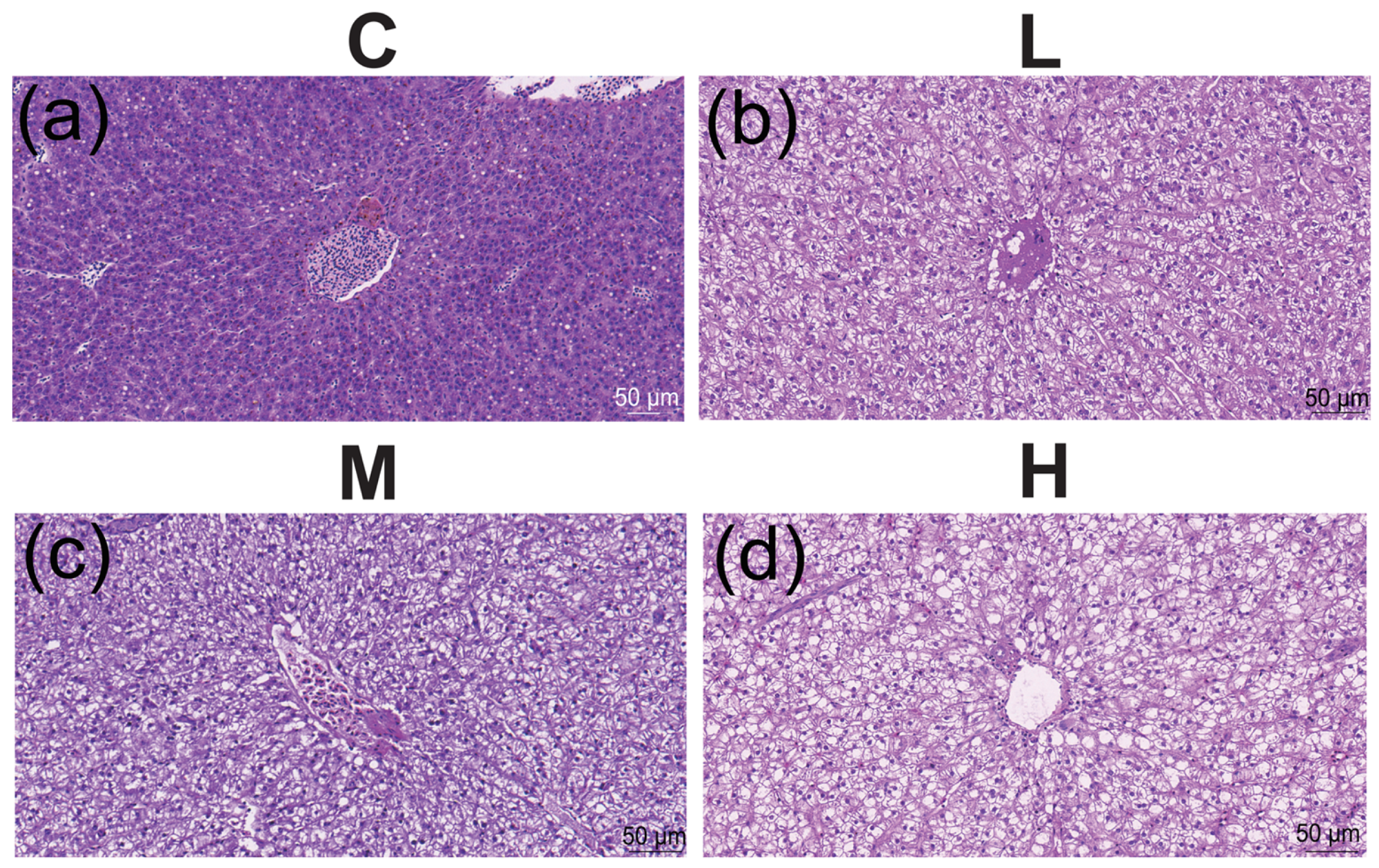
3.2. Hepatosomatic Index (HSI), Fulton’s Condition Factor (FCF), and Histological Index
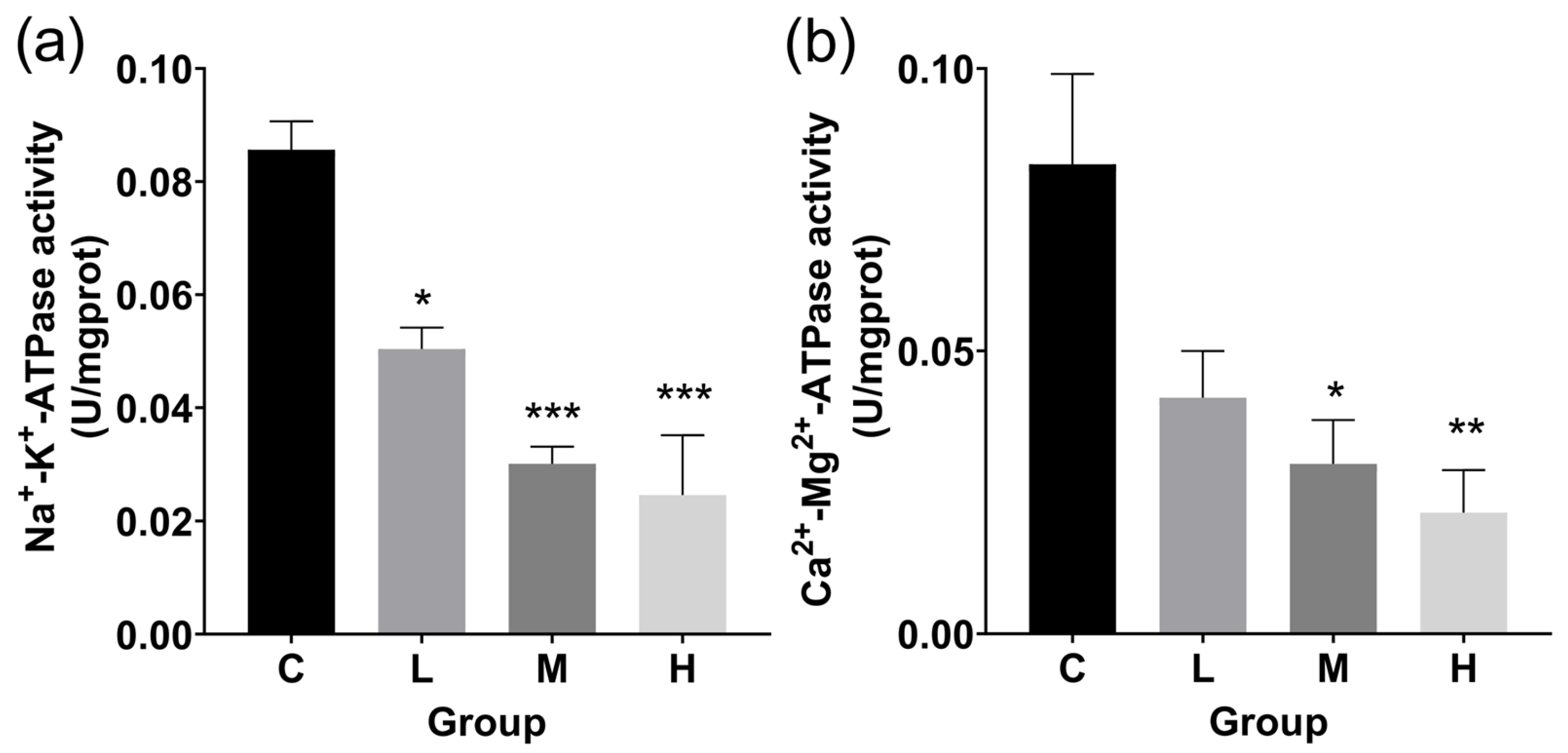
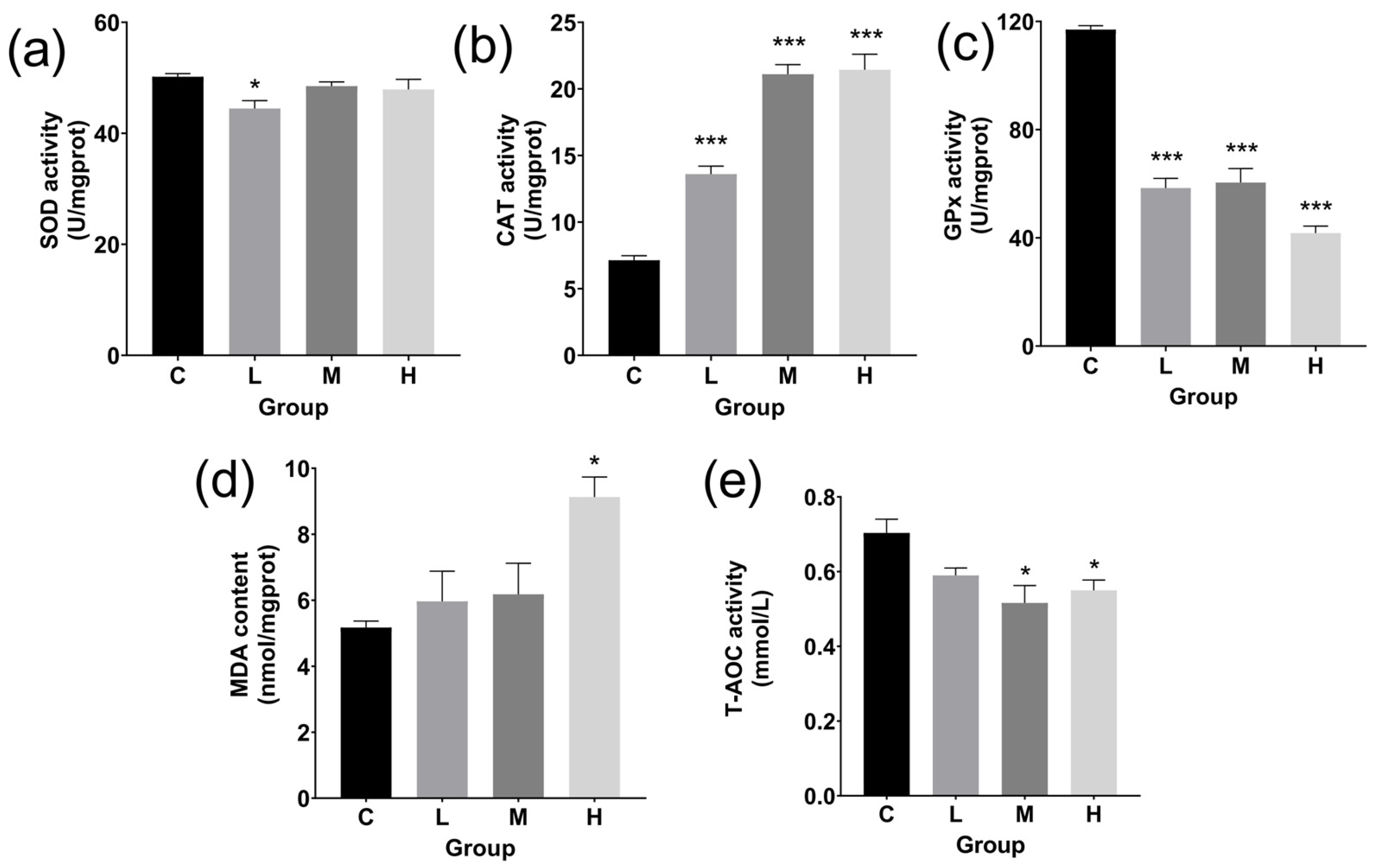
3.3. ATPase Activity and Antioxidant Levels in Livers
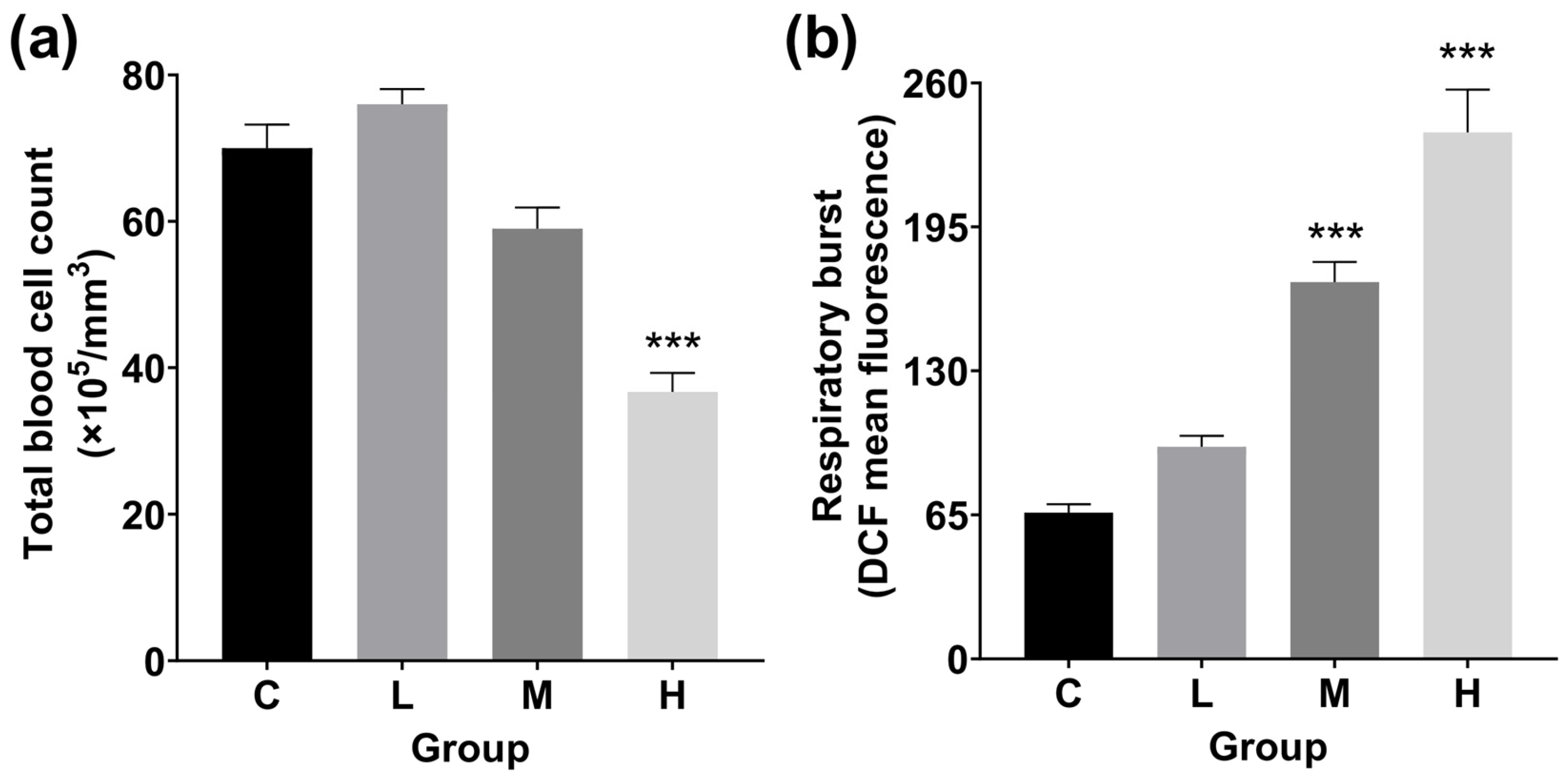
3.4. Total Blood Cell Count and RB Detection
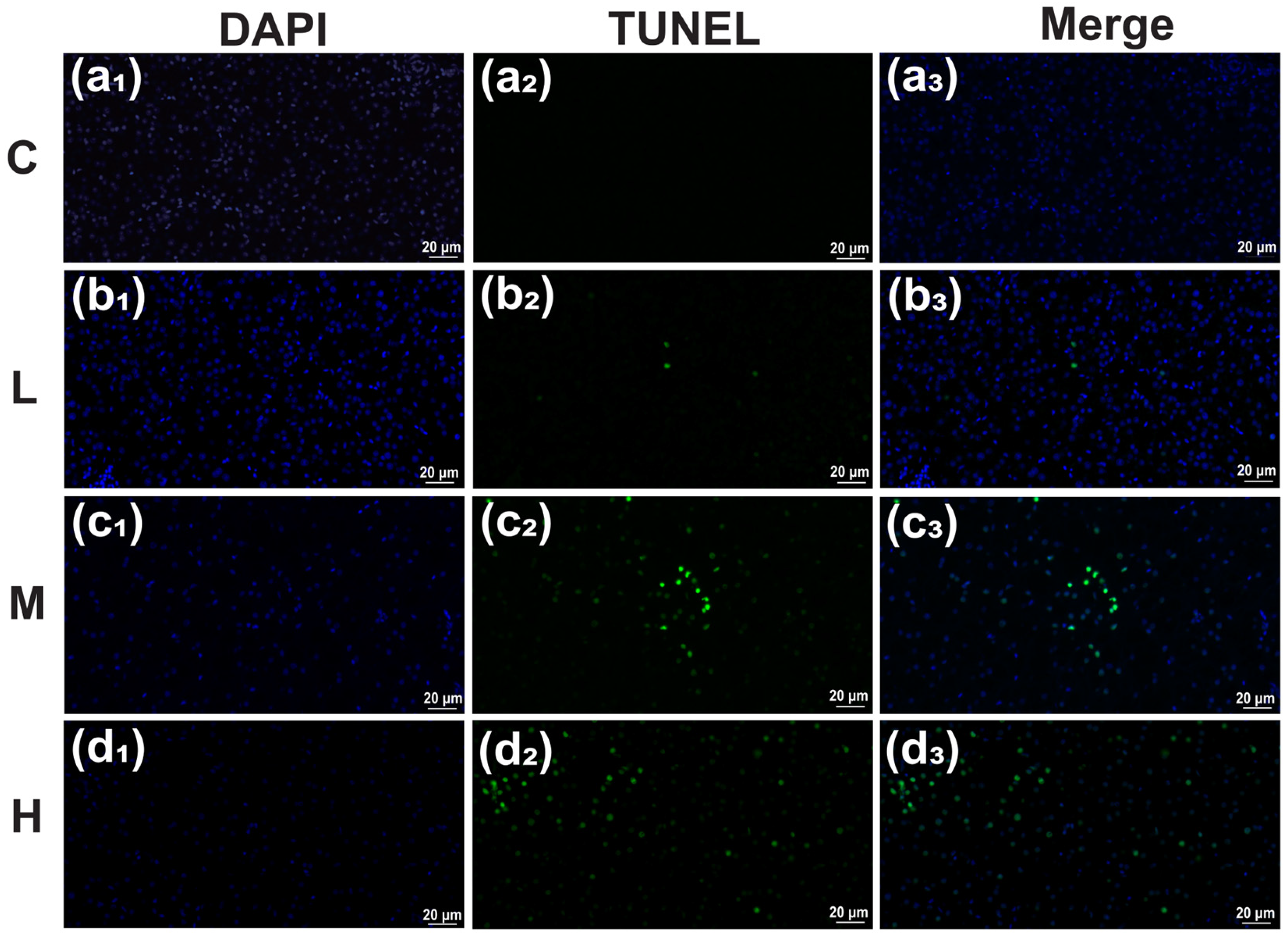
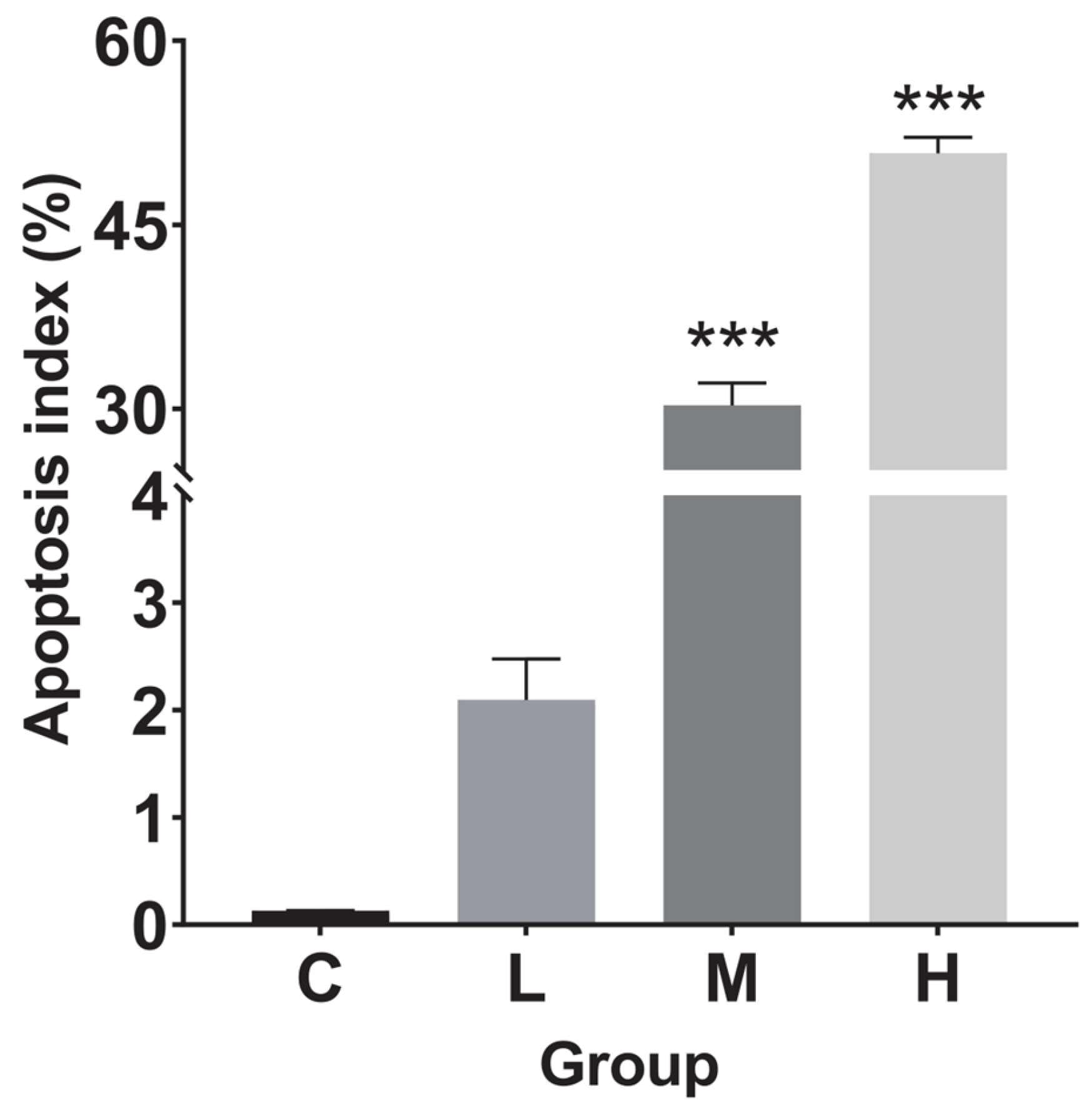
3.5. Terminal-Deoxynucleotidyl Transferase (TdT)-Mediated dUTP Nick End Labeling (TUNEL) Detection
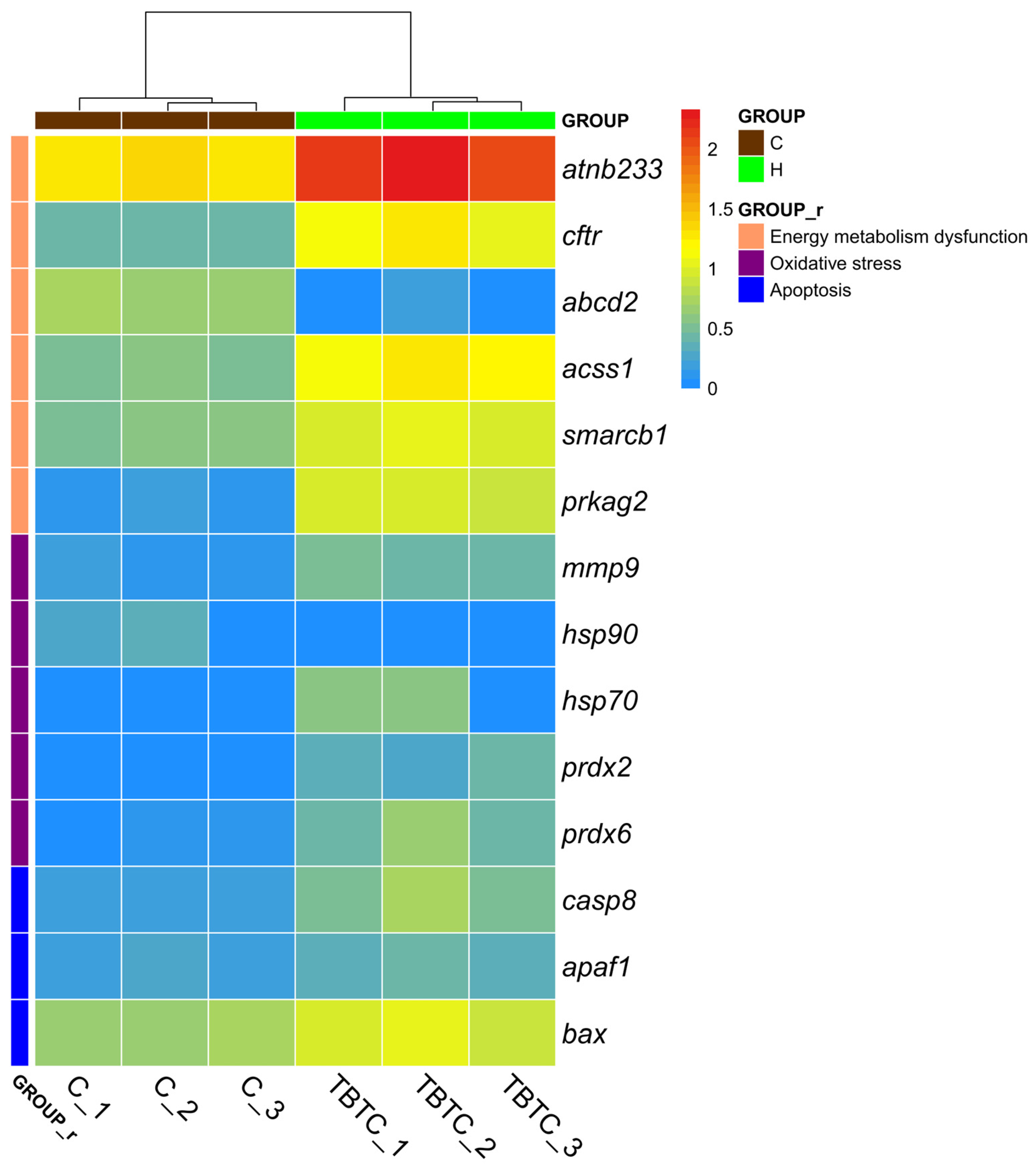
3.6. Transcriptional Levels in the Livers of TBTC-Treated Haarder
4. Discussion
4.1. The Effect of TBTC on Haarder Growth and Energy Metabolism
4.2. Antioxidant Response of Haarder to TBTC
4.3. Apoptosis Reaction of Haarder to TBTC
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Hoch, M. Organotin compounds in the environment—An overview. Appl. Geochem. 2001, 16, 719–743. [Google Scholar] [CrossRef]
- Burton, E.D.; Phillips, I.R.; Hawker, D.W. Sorption and desorption behavior of tributyltin with natural sediments. Environ. Sci. Technol. 2004, 38, 6694. [Google Scholar] [CrossRef] [PubMed]
- Luan, T.G.; Jin, J.; Chan, S.M.N.; Wong, Y.S.; Tam, N.F.Y. Biosorption and biodegradation of tributyltin (TBT) by alginate immobilized Chlorella vulgaris beads in several treatment cycles. Process Biochem. 2006, 41, 1560–1565. [Google Scholar] [CrossRef]
- Gadd, G.M. Microbial interactions with tributyltin compounds: Detoxification, accumulation, and environmental fate. Sci. Total Environ. 2000, 258, 119–127. [Google Scholar] [CrossRef]
- Wang, X.H.; Fang, C.; Hong, H.S.; Wang, W.X. Gender differences in TBT accumulation and transformation in Thais clavigera after aqueous and dietary exposure. Aquat. Toxicol. 2010, 99, 413–422. [Google Scholar] [CrossRef]
- Anastasiou, T.I.; Chatzinikolaou, E.; Mandalakis, M.; Arvanitidis, C. Imposex and organotin compounds in ports of the Mediterranean and the Atlantic: Is the story over? Sci. Total Environ. 2016, 569–570, 1315–1329. [Google Scholar] [CrossRef]
- Batista, R.M.; Castro, I.B.; Fillmann, G. Imposex and butyltin contamination still evident in Chile after TBT global ban. Sci. Total Environ. 2016, 566–567, 446–453. [Google Scholar] [CrossRef]
- Okoro, H.K.; Fatoki, O.S.; Adekola, F.A.; Ximba, B.J.; Snyman, R.G. Spatio-temporal variation of organotin compounds in seawater and sediments from Cape Town harbour, South Africa using gas chromatography with flame photometric detector (GC-FPD). Arab. J. Chem. 2016, 9, 95–104. [Google Scholar] [CrossRef]
- Briant, N.; Bancon-Montigny, C.; Elbaz-Poulichet, F.; Freydier, R.; Delpoux, S.; Cossa, D. Trace elements in the sediments of a large Mediterranean marina (Port Camargue, France): Levels and contamination history. Mar. Pollut. Bull. 2013, 73, 78–85. [Google Scholar] [CrossRef]
- Fung, W.H.; Can, S.Z.; Tse, K.C.W.; Tsoi, Y.T.; Lee, W.W.; Arı, B.; Tunç, M.; Merrick, J.; de Sena, R.C.; Nadeau, K.; et al. CCQM-K155: Elements and tributyltin in seawater. Metrologia 2025, 62, 08007. [Google Scholar] [CrossRef]
- Morris, J.J.; Rose, A.L.; Lu, Z. Reactive oxygen species in the world ocean and their impacts on marine ecosystems. Redox biol. 2022, 52, 102285. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, D.R.; Mitchelmore, C.L.; O’Hara, S.C.M.; Lemaire, P.; Sturve, J.; Förlin, L. Increased potential for NAD(P)H-dependent reactive oxygen species production of hepatic subcellular fractions of fish species with in vivo exposure to contaminants. Mar. Environ. Res. 2000, 50, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Aceves, M.A.; Cohen, A.; Smith, Y.; Faggio, C. Estrogen regulation of gene expression in the teleost fish immune system. Fish Shellfish. Immun. 2016, 58, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Lushchak, V.I. Environmentally induced oxidative stress in aquatic animals. Aquat. Toxicol. 2011, 101, 13–30. [Google Scholar] [CrossRef]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef]
- Zhou, J.; Zhu, X.; Cai, Z. Tributyltin toxicity in abalone (Haliotis diversicolor supertexta) assessed by antioxidant enzyme activity, metabolic response, and histopathology. J. Hazard. Mater. 2010, 183, 428–433. [Google Scholar] [CrossRef]
- Almeida, E.A.; Bainy, A.C.D.; Dafre, A.L.; Gomes, O.F.; Medeiros, M.H.G.; Di Mascio, P. Oxidative stress in digestive gland and gill of the brown mussel (Perna perna) exposed to air and re-submersed. J. Exp. Mar. Biol. Ecol. 2005, 318, 21–30. [Google Scholar] [CrossRef]
- Sun, H.J.; Zhao, W.J.; Teng, X.Q.; Shu, S.P.; Li, S.W.; Hong, H.C.; Guan, D.X. Antioxidant responses and pathological changes in the gill of zebrafish (Danio rerio) after chronic exposure to arsenite at its reference dose. Ecotoxicol. Environ. Saf. 2020, 200, 110743. [Google Scholar] [CrossRef]
- Ventrella, V.; Nesci, S.; Trombetti, F.; Bandiera, P.; Pirini, M.; Borgatti, A.R.; Pagliarani, A. Tributyltin inhibits the oligomycin-sensitive Mg-ATPase activity in Mytilus galloprovincialis digestive gland mitochondria. Comp. Biochem. Phys. C 2011, 153, 75–81. [Google Scholar] [CrossRef]
- Suzuki, N.; Honda, M.; Sato, M.; Yoshitake, S.; Kawabe, K.; Tabuchi, Y.; Omote, T.; Sekiguchi, T.; Furusawa, Y.; Toriba, A.; et al. Hydroxylated benzo[c]phenanthrene metabolites cause osteoblast apoptosis and skeletal abnormalities in fish. Ecotoxicol. Environ. Saf. 2022, 234, 113401. [Google Scholar] [CrossRef]
- Wang, J.X.; Gao, X.Z.; Liu, F.X.; Fang, W.; Dong, J.Q.; Zhao, P.P. Difenoconazole causes cardiotoxicity in common carp (Cyprinus carpio): Involvement of oxidative stress, inflammation, apoptosis and autophagy. Chemosphere 2022, 306, 135562. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.R.; Zhao, F.Q.; Gai, X.X.; Cai, J.Z.; Zhang, X.T.; Chen, X.M.; Zhu, Y.; Zhang, Z.W. Astilbin attenuates apoptosis induced by cadmium through oxidative stress in carp (Cyprinus carpio L.) head kidney lymphocyte. Fish Shellfish. Immun. 2022, 125, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.L.; Song, M.; Xiao, B.N.; Liu, M.L.; Liu, P.L.; Han, Y.F.; Shao, B.; Li, Y.F. ROS-mediated mitophagy and apoptosis are involved in aluminum-induced femoral impairment in mice. Chem. Biol. Interac. 2021, 349, 109663. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Shi, X.; Guan, Y.L.; Li, X.J. Deoxynivalenol induces carp neutrophil apoptosis and necroptosis via CYP450s/ROS/PI3K/AKT pathway. Aquaculture 2021, 545, 737182. [Google Scholar] [CrossRef]
- Jia, Z.L.; Cen, J.; Wang, J.B.; Zhang, F.; Xia, Q.; Wang, X.; Chen, X.Q.; Wang, R.C.; Hsiao, C.D.; Liu, K.C.; et al. Mechanism of isoniazid-induced hepatotoxicity in zebrafish larvae: Activation of ROS-mediated ERS, apoptosis and the Nrf2 pathway. Chemosphere 2019, 227, 541–550. [Google Scholar] [CrossRef]
- Meng, W.; Gao, T.X.; Zheng, B. Genetic analysis of four populations of redlip mullet (Chelon haematocheilus) collected in China seas. J. Ocean. Univ. China 2007, 6, 72–75. [Google Scholar] [CrossRef]
- Kang, B.; Xian, W.W.; Liu, R.Y. Transporting nutrients from sediments: Nitrogen and carbon as indicators to measure scavenging ability of a detritivorous fish, Liza haematocheila. Environ. Biol. Fish 2005, 74, 283–290. [Google Scholar] [CrossRef]
- Xu, D.P.; Fang, D.A.; Zhao, C.S.; Jiang, S.L.; Hu, H.Y. Effect of tributyltin chloride (TBT-Cl) exposure on expression of HSP90β1 in the river pufferfish (Takifugu obscurus): Evidences for its immunologic function involving in exploring process. Gene 2018, 666, 9–17. [Google Scholar] [CrossRef]
- GB 5009.268-2016; Food Safety National Standard—Determination of Multiple Elements in Food. National Health and Family Planning Commission of the People’s Republic of China and the State Food and Drug Administration: Beijing, China, 2016.
- Maddock, D.M.; Burton, M.P.M. Gross and histological observations of ovarian development and related condition changes in American plaice. J. Fish Biol. 1999, 53, 928–944. [Google Scholar] [CrossRef]
- Mao, G.X.; Zheng, L.D.; Cao, Y.B.; Chen, Z.M.; Lv, Y.D.; Wang, Y.Z.; Hu, X.L.; Wang, G.F.; Yan, J. Antiaging effect of pine pollen in human diploid fibroblasts and in a mouse model induced by D-galactose. Oxid. Med. Cell. Longev. 2012, 2012, 750963. [Google Scholar] [CrossRef]
- Zhang, J.Q.; Shen, M.; Zhu, C.C.; Yu, F.X.; Liu, Z.Q.; Ally, N.; Sun, S.C.; Li, K.; Liu, H.L. 3-Nitropropionic acid induces ovarian oxidative stress and impairs follicle in mouse. PLoS ONE 2014, 9, e86589. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Wang, W.; Yu, P.F.; Xi, Z.J.; Xu, L.J.; Li, X.L.; He, N.Y. Comparison of taurine, GABA, Glu, and Asp as scavengers of malondialdehyde in vitro and in vivo. Nanoscale Res. Lett. 2013, 8, 190. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.M.; Liu, X.Y.; Yang, P.; Wu, R.Z.; Wang, S.Y.; He, S.N.; Zhou, Q.H. Potassium application promote cotton acclimation to soil waterlogging stress by regulating endogenous protective enzymes activities and hormones contents. Plant Physiol. Bioch. 2022, 185, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Li, X.L.; Lin, T.; He, D.W.; Wei, G.H.; Liu, J.H.; Li, L.S. The cyclophosphamide metabolite, acrolein, induces cytoskeletal changes and oxidative stress in Sertoli cells. Mol. Biol. Rep. 2012, 39, 493–500. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Xian, J.A.; Wang, A.L.; Tian, J.X.; Huang, J.W.; Ye, C.X.; Wang, W.N.; Sun, R.Y. Morphologic, physiological and immunological changes of haemocytes from Litopenaeus vannamei treated by lipopolysaccharide. Aquaculture 2009, 298, 139–145. [Google Scholar] [CrossRef]
- Stein, M.; Lin, H.; Jeyamohan, C.; Dvorzhinski, D.; Gounder, M.; Bray, K.; Eddy, S.; Goodin, S.; White, E.; DiPaola, R.S. Targeting tumor metabolism with 2-deoxyglucose in patients with castrate-resistant prostate cancer and advanced malignancies. Prostate 2010, 70, 1388–1394. [Google Scholar] [CrossRef]
- Chen, S.F.; Zhou, Y.Q.; Chen, Y.R.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, 884. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef]
- Ran, M.X.; Huang, H.X.; Hu, B.; Hu, S.Q.; Hu, J.W.; Li, L.; He, H.; Liu, H.H.; Wang, J.W. Comparative analysis of testicular histology and lncRNA-mRNA expression patterns between Landes geese (Anser anser) and Sichuan White geese (Anser cygnoides). Front. Genet. 2021, 12, 627384. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Arnot, J.A.; Gobas, F.A.P.C. A review of bioconcentration factor (BCF) and bioaccumulation factor (BAF) assessments for organic chemicals in aquatic organisms. Environ. Rev. 2006, 14, 257–297. [Google Scholar] [CrossRef]
- van den Dungen, M.W.; Murk, A.J.; Kok, D.E.; Steegenga, W.T. Persistent organic pollutants alter DNA methylation during human adipocyte differentiation. Toxicol. Vitr. 2017, 40, 79–87. [Google Scholar] [CrossRef]
- Berto, D.; Giani, M.; Boscolo, R.; Covelli, S.; Giovanardi, O.; Massironi, M.; Grassia, L. Organotins (TBT and DBT) in water, sediments, and gastropods of the southern Venice lagoon (Italy). Mar. Pollut. Bull. 2007, 55, 425–435. [Google Scholar] [CrossRef]
- Zhang, J.L.; Sun, P.; Kong, T.; Yang, F.; Guan, W.C. Tributyltin promoted hepatic steatosis in zebrafish (Danio rerio) and the molecular pathogenesis involved. Aquat. Toxicol. 2016, 170, 208–215. [Google Scholar] [CrossRef]
- Tang, L.; Zhang, Y.H.; Wang, X.; Zhang, C.C.; Qin, G.; Lin, Q. Effects of chronic exposure to environmental levels of tributyltin on the lined seahorse (Hippocampus erectus) liver: Analysis of bioaccumulation, antioxidant defense, and immune gene expression. Sci. Total Environ. 2021, 801, 149646. [Google Scholar] [CrossRef]
- Maisano, M.; Cappello, T.; Oliva, S.; Natalotto, A.; Giannetto, A.; Parrino, V.; Battaglia, P.; Romeo, T.; Salvo, A.; Spanò, N.; et al. PCB and OCP accumulation and evidence of hepatic alteration in the Atlantic bluefin tuna, T. thynnus, from the Mediterranean Sea. Mar. Environ. Res. 2016, 121, 40–48. [Google Scholar] [CrossRef]
- Forrester, G.E.; Fredericks, B.I.; Gerdeman, D.; Evans, B.; Steele, M.A.; Zayed, K.; Schweitzer, L.E.; Suffet, I.H.; Vance, R.R.; Ambrose, R.F. Growth of estuarine fish is associated with the combined concentration of sediment contaminants and shows no adaptation or acclimation to past conditions. Mar. Environ. Res. 2003, 56, 423–442. [Google Scholar] [CrossRef]
- Hauser-Davis, R.A.; Bordon, I.C.; Kannan, K.; Moreira, I.; Quinete, N. Perfluoroalkyl substances associations with morphometric health indices in three fish species from differentially contaminated water bodies in Southeastern Brazil. Environ. Technol. Innov. 2021, 21, 101198. [Google Scholar] [CrossRef]
- Lyssimachou, A.; Santos, J.G.; André, A.; Soares, J.; Lima, D.; Guimarães, L.; Almeida, C.M.R.; Teixeira, C.; Castro, L.F.C.; Santos, M.M. The mammalian "obesogen" tributyltin targets hepatic triglyceride accumulation and the transcriptional regulation of lipid metabolism in the liver and brain of zebrafish. PLoS ONE 2015, 10, e0143911. [Google Scholar] [CrossRef] [PubMed]
- Musharraf, M.; Khan, M.A. Dietary potassium requirement of fingerling Indian major carp, Labeo rohita (Hamilton), based on growth parameters, gill Na+-K+ ATPase activity, tissue mineralization and antioxidant activities. Aquacult. Nutr. 2019, 25, 271–280. [Google Scholar] [CrossRef]
- Li, Z.H.; Li, P.; Shi, Z.C. Chronic effects of tributyltin on multiple biomarkers responses in juvenile common carp, Cyprinus carpio. Environ. Toxicol. 2016, 31, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.H.; Chen, C.; Li, M.; Liu, L.; Dong, K.J.; Chen, K.; Qiu, X.C. Oral exposure to tributyltin induced behavioral abnormality and oxidative stress in the eyes and brains of juvenile Japanese medaka (Oryzias latipes). Antioxidants 2021, 10, 1647. [Google Scholar] [CrossRef]
- Major, K.M.; DeCourten, B.M.; Li, J.; Britton, M.; Settles, M.L.; Mehinto, A.C.; Connon, R.E.; Brander, S.M. Early life exposure to environmentally relevant levels of endocrine disruptors drive multigenerational and transgenerational epigenetic changes in a fish model. Front. Mar. Sci. 2020, 7, 471. [Google Scholar] [CrossRef]
- Gollob, M.H.; Green, M.S.; Tang, A.S.-L.; Gollob, T.; Karibe, A.; Hassan, A.-S.; Ahmad, F.; Lozado, R.; Shah, G.; Fananapazir, L.; et al. Identification of a gene responsible for familial Wolff–Parkinson–White syndrome. N. Engl. J. Med. 2001, 344, 1823–1831. [Google Scholar] [CrossRef]
- Bossus, M.C.; Bollinger, R.J.; Reed, P.J.; Tipsmark, C.K. Prolactin and cortisol regulate branchial claudin expression in Japanese medaka. Gen. Comp. Endocr. 2017, 240, 77–83. [Google Scholar] [CrossRef]
- Bhagat, J.; Zang, L.Q.; Nishimura, N.; Shimada, Y. Application of omics approaches for assessing microplastic and nanoplastic toxicity in fish and seafood species. TrAC Trend Anal. Chem. 2022, 154, 116674. [Google Scholar] [CrossRef]
- de Souza, S.S.; da Silva Castro, J.; Campos, D.F.; Pereira, R.S.; Bataglion, G.A.; da Silva, G.S.; de Almeida-Val, V.M. Temporal exposure to malathion: Biochemical changes in the Amazonian fish tambaqui, Colossoma macropomum. Aquat. Toxicol. 2021, 241, 105997. [Google Scholar] [CrossRef]
- Calhoun, S.; Duan, L.; Maki, C.G. Acetyl-CoA synthetases ACSS1 and ACSS2 are 4-hydroxytamoxifen responsive factors that promote survival in tamoxifen treated and estrogen deprived cells. Transl. Oncol. 2022, 19, 101386. [Google Scholar] [CrossRef]
- Kalimuthu, S.N.; Chetty, R. Gene of the month: SMARCB1. J. Clin. Pathol. 2016, 69, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Emmanouil, C.; Green, R.M.; Willey, F.R.; Chipman, J.K. Oxidative damage in gill of Mytilus edulis from Merseyside, UK, and reversibility after depuration. Environ. Pollut. 2008, 151, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Glorieux, C.; Calderon, P.B. Catalase, a remarkable enzyme: Targeting the oldest antioxidant enzyme to find a new cancer treatment approach. Biol. Chem. 2017, 398, 1095–1108. [Google Scholar] [CrossRef] [PubMed]
- Margis, R.; Dunand, C.; Teixeira, F.K.; Margis-Pinheiro, M. Glutathione peroxidase family—An evolutionary overview. FEBS J. 2008, 275, 3959–3970. [Google Scholar] [CrossRef]
- McRdj, M.; Fridovich, I. Superoxide dismutase: An enzyme function for erychrocuprein. J. Biol. Chem. 1969, 244, 6049. [Google Scholar]
- Chen, H.G.; Jia, X.P.; Cai, W.G.; Lin, Q.; Ma, S.W. Antioxidant responses and bioaccumulation in green-lipped mussels (Perna Viridis) under acute tributyltin chloride exposure. Bull. Environ. Contam. Tox. 2011, 87, 506. [Google Scholar] [CrossRef]
- Zhang, C.N.; Zhang, J.L.; Ren, H.T.; Zhou, B.H.; Wu, Q.J.; Sun, P. Effect of tributyltin on antioxidant ability and immune responses of zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2017, 138, 1–8. [Google Scholar] [CrossRef]
- Rundle, A.; Robertson, A.B.; Blay, A.M.; Butler, K.M.A.; Callaghan, N.I.; Dieni, C.A.; MacCormack, T.J. Cerium oxide nanoparticles exhibit minimal cardiac and cytotoxicity in the freshwater fish Catostomus commersonii. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2016, 181–182, 19–26. [Google Scholar] [CrossRef]
- Li, Z.H.; Li, P.; Shi, Z.C. Molecular responses in digestive tract of juvenile common carp after chronic exposure to sublethal tributyltin. Ecotoxicol. Environ. Saf. 2014, 109, 10–14. [Google Scholar] [CrossRef]
- Wang, Y.X.; Wu, P.P.; Shen, H.Y. Effect of ampicillin wastewater on the T-AOC and LDH activities of zebrafish. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Bristol, UK, 7–9 June 2019; Volume 295, p. 012018. [Google Scholar]
- Mu, W.N.; Li, Z.H.; Zhong, L.Q.; Wu, Y.H. Effect of tributyltin, cadmium, and their combination on physiological responses in juvenile grass carp. J. Aquat. Anim. Health 2016, 28, 181–186. [Google Scholar] [CrossRef]
- Kamada, H.; Yu, F.; Nito, C.; Chan, P.H. Influence of hyperglycemia on oxidative stress and matrix metalloproteinase-9 activation after focal cerebral ischemia/reperfusion in rats. Stroke 2007, 38, 1044–1049. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, H.; Wang, D.; Zhang, L.; Ma, T. Peroxiredoxin2 (prdx2) reduces oxidative stress and apoptosis of myocardial cells induced by acute myocardial infarction by inhibiting the TLR4/nuclear factor kappa B (NF-κB) signaling pathway. Med. Sci. Monit. 2020, 26, e926281. [Google Scholar] [CrossRef] [PubMed]
- Cavalloni, G.; Peraldo-Neia, C.; Massa, A.; Bergamini, C.; Trentini, A.; De Rosa, G.; Daniele, L.; Ciccosanti, F.; Cervellati, C.; Leone, F.; et al. Proteomic analysis identifies deregulated metabolic and oxidative-associated proteins in Italian intrahepatic cholangiocarcinoma patients. BMC Cancer 2021, 21, 865. [Google Scholar] [CrossRef] [PubMed]
- Fatma, N.; Kubo, E.; Toris, C.B.; Stamer, W.D.; Camras, C.B.; Singh, D.P. PRDX6 attenuates oxidative stress- and TGFβ-induced abnormalities of human trabecular meshwork cells. Free. Radic. Res. 2009, 43, 783–795. [Google Scholar] [CrossRef]
- Salo, D.C.; Donovan, C.M.; Davies, K.J.A. HSP70 and other possible heat shock or oxidative stress proteins are induced in skeletal muscle, heart, and liver during exercise. Free Radic. Biol. Med. 1991, 11, 239–246. [Google Scholar] [CrossRef]
- MacPherson, G.; Healey, C.S.; Teare, M.D.; Balasubramanian, S.P.; Reed, M.W.; Pharoah, P.D.P.; Ponder, B.A.J.; Meuth, M.; Bhattacharyya, N.P.; Cox, A. Association of a common variant of the CASP8 gene with reduced risk of breast cancer. J. Natl. Cancer Inst. 2004, 96, 1866–1869. [Google Scholar] [CrossRef]
- Wolter, K.G.; Hsu, Y.-T.; Smith, C.L.; Nechushtan, A.; Xi, X.-G.; Youle, R.J. Movement of bax from the cytosol to mitochondria during apoptosis. J. Cell Biol. 1997, 139, 1281–1292. [Google Scholar] [CrossRef]
- Gao, J.M.; Wu, L.; Chen, Y.P.; Zhou, B.; Guo, J.S.; Zhang, K.; Ouyang, W.J. Spatiotemporal distribution and risk assessment of organotins in the surface water of the Three Gorges Reservoir Region, China. Chemosphere 2017, 171, 405–414. [Google Scholar] [CrossRef]
- Hai Le, L.T.; Takahashi, S.; Saeki, K.; Nakatani, N.; Tanabe, S.; Miyazaki, N.; Fujise, Y. High percentage of butyltin residues in total tin in the livers of cetaceans from Japanese coastal waters. Environ. Sci. Technol. 1999, 33, 1781–1786. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, C.; Suo, A.; Ding, D.; Song, W. Toxicity Responses from Tributyltin Chloride on Haarder (Planiliza haematocheila) Livers: Oxidative Stress, Energy Metabolism Dysfunction, and Apoptosis. Curr. Issues Mol. Biol. 2025, 47, 526. https://doi.org/10.3390/cimb47070526
Zhao C, Suo A, Ding D, Song W. Toxicity Responses from Tributyltin Chloride on Haarder (Planiliza haematocheila) Livers: Oxidative Stress, Energy Metabolism Dysfunction, and Apoptosis. Current Issues in Molecular Biology. 2025; 47(7):526. https://doi.org/10.3390/cimb47070526
Chicago/Turabian StyleZhao, Changsheng, Anning Suo, Dewen Ding, and Wencheng Song. 2025. "Toxicity Responses from Tributyltin Chloride on Haarder (Planiliza haematocheila) Livers: Oxidative Stress, Energy Metabolism Dysfunction, and Apoptosis" Current Issues in Molecular Biology 47, no. 7: 526. https://doi.org/10.3390/cimb47070526
APA StyleZhao, C., Suo, A., Ding, D., & Song, W. (2025). Toxicity Responses from Tributyltin Chloride on Haarder (Planiliza haematocheila) Livers: Oxidative Stress, Energy Metabolism Dysfunction, and Apoptosis. Current Issues in Molecular Biology, 47(7), 526. https://doi.org/10.3390/cimb47070526