1. Introduction
Prostate cancer (PCa) is the most frequent type of cancer diagnosed among men in the EU, accounting for about 25% of all annual cancer diagnoses. Even though most PCa forms start as androgen-dependent hyperplasia, due to mutations and selective pressure, the dependence on androgen receptor (AR) stimulation is gradually lost and evolves into androgen-independent prostate cancer, which is far more aggressive and is correlated with poor outcomes [
1]. Prostate adenocarcinoma can evolve into metastatic (or advanced) prostate cancer, which is a severe form of malignancy that may affect the lymph nodes, lungs, or other organs and has a very poor prognosis [
2]. Hormone therapy in the form of testosterone suppression is employed to shrink tumors and extend patient survival; however, once the cancer cells have been desensitized to AR stimulation and the cancer has evolved into metastatic castrate-resistant prostate cancer (mCRPC), hormone therapy is ineffective [
2]. mCRPC affects about 10–20% of PCa patients within five years of follow-up after their initial therapy, and it is the most advanced and fatal form of PCa [
3]. Current pharmaceutical approaches have limited efficacy against mCRPC and are often accompanied by a set of unbearable collateral effects that significantly impact treated patients. Many substances are screened as potential treatments, among which lies Bortezomib, a proteasome inhibitor (PI) that has saved thousands of lives from multiple myeloma, mantle cell lymphoma, and many other hematological malignancies [
4]. The drug (either alone or in combination with other agents) has been tested against solid tumors (prostate cancer being one of them); however, the results were not satisfying enough to support PIs as a treatment [
5]. Many articles attempted to provide a mechanistic explanation; nonetheless, up to this day, no definite answer has been given [
6,
7,
8,
9,
10,
11]. Nonetheless, molecular characterization of tumors and in vitro assays of cancer cells have revealed significant insight into PI mechanistic actions. Recently, Bortezomib has drawn renowned attention as a potential therapy for mCRPC patients with
PTEN deleterious mutations [
12].
PIs target the catalytic sites of the 26S proteasome, a protein multi-catalytic complex that actively participates in the cell’s homeostatic mechanisms by selectively degrading polyubiquitinated polypeptides In cancer cells, the proteasome is of paramount importance, and its increased activity and subunit accumulation are well-documented in various cancer types. It is believed that cancer cells, due to increased biosynthetic rates and miss-regulated control mechanisms, produce more miss-folded polypeptides that need to be recycled [
13,
14]. Additionally, proteasomal degradation regulates the turnover rates of proteins mediating cell cycle progression/arrest, mitochondrial function, and gene expression [
15,
16]. Cancer cells have been found to rely on the proteasome to “tune” this system, and this mechanism, namely the ubiquitin-proteasome system (UPS), is a very important pathway that, once targeted and sabotaged, can induce apoptosis and lead to cell death [
17]. The activity of the proteasome inside the cell is of high importance for cell survival and, thus, is regulated at several levels, as summarized by Livneh et al. in 2016 [
18]. Given its important role in cell homeostasis, many regulating molecules have been investigated as pharmaceutical targets over the years for cancer as a means to deregulate them, make the malignant cells more susceptible to stress-caused damage and apoptotic signals, and eventually lead to their apoptotic death. Among them, PIs constitute a drug class of proteasome subunits inhibitors, and have entered clinical practice as a therapy against the aforementioned hematological malignancies. Since their discovery, PIs have been tested on various cell lines, animal models, and even clinical studies; however, besides a group of hematological cancers, they are not very efficient against solid tumors as resistance develops [
6,
8,
9,
19,
20,
21,
22,
23,
24].
The first PI with documented tumoricidal actions was Bortezomib, which acts by binding to β5 subunits, the main catalytic subunits of the 20S core particle that exhibit chymotrypsin-like (ChT-L) activity [
25]. Patients who were treated with Bortezomib and relapsed due to resistance were found to have mutations in the
PSMB5 gene, the one coding for the β5 subunit, that significantly reduced the drug binding ability or caused increased expression [
26,
27,
28,
29,
30,
31]. However, mutations are not unique in resistance emergence and driving, since most of the relapsed patients did not have alterations regarding β5 structure or expression. The current hypothesis is that, besides genetic mutations affecting the subunits’ abundance and structure (thus rendering Bortezomib insufficient), changes in the signaling cascades that regulate apoptosis, autophagy, and oxidative stress also play a huge part in resistant cells [
11,
32,
33,
34,
35,
36]. Nf-κB was one of the first molecules to be found active in Bortezomib-resistant cells, implying a role in the emergence of that aggressive phenotype [
37]. The STAT family is another class of transcription factors that have been found active in resistant clones, as well as the ERK1/2 signaling pathway that mostly regulates cell survival and proliferation [
38,
39,
40,
41,
42,
43]. Recent advances in Bortezomib resistance, focused on prostate cancer, report the activation of autophagy in the resistant cells and describe a regulation mechanism that substitutes the impaired UPS [
11,
44,
45,
46,
47]. Additionally, key signaling molecules and oncogenes like Elk1, cJun, cSrc, and epithelial to mesenchymal (EMT) markers have not been adequately investigated up to this day. Therefore, given the complexity of resistance development and the multiple aspects of resistant phenotypes, the need for a new approach is of paramount importance.
The first PI with documented tumoricidal actions was Bortezomib, which acts by binding to β5 subunits, the main catalytic subunits of the 20S core particle that exhibit chymotrypsin-like (ChT-L) activity [
25]. Patients who were treated with Bortezomib and relapsed due to resistance were found to have mutations in the
PSMB5 gene, the one coding for the β5 subunit, that significantly reduced the drug binding ability or caused increased expression [
26,
27,
28,
29,
30,
31]. However, mutations are not unique in resistance emergence and driving, since most of the relapsed patients did not have alterations regarding β5 structure or expression. The current hypothesis is that, besides genetic mutations affecting the subunits’ abundance and structure (thus rendering Bortezomib insufficient), changes in the signaling cascades that regulate apoptosis, autophagy, and oxidative stress also play a huge part in resistant cells [
11,
32,
33,
34,
35,
36]. Nf-κB was one of the first molecules to be found active in Bortezomib-resistant cells, implying a role in the emergence of that aggressive phenotype [
37]. The STAT family is another class of transcription factors that have been found active in resistant clones, as well as the ERK1/2 signaling pathway that mostly regulates cell survival and proliferation [
38,
39,
40,
41,
42,
43]. Recent advances in Bortezomib resistance, focused on prostate cancer, report the activation of autophagy in the resistant cells and describe a regulation mechanism that substitutes the impaired UPS [
11,
44,
45,
46,
47]. Additionally, key signaling molecules and oncogenes like Elk1, cJun, cSrc, and epithelial to mesenchymal (EMT) markers have not been adequately investigated up to this day. Therefore, given the complexity of resistance development and the multiple aspects of resistant phenotypes, the need for a new approach is of paramount importance.
The purpose of this study is to focus on a model of androgen-independent aggressive PCa and thoroughly study cell functions, signaling cascades, and transcription factors to come up with novel data regarding resistance development against PIs. For this purpose, PC-3 cells are used as a model of resistance-emergence, selected for their very specific set of characteristics [
48]. PC-3 cells are derived from bone metastasis of a prostate adenocarcinoma patient and are considered a well-established model for PCa, known for their high metastatic potential (higher than those of other models, like DU-145 cells) [
49]. Additionally, their abolition of androgen-dependence corresponds to aggressive types of cancer observed in advanced-stage patients. Previous data from our laboratory encouraged research on a new model that would have a more aggressive, poorly differentiated phenotype, as it would better simulate in vivo conditions. Moreover, PC-3 cells are homozygous for a deleterious
PTEN mutation and express characteristics aligned with progenitor cells, exhibiting stemness markers [
50,
51]. PC-3 cells can allow for a safer extrapolation of conclusions concerning mechanisms exploited by various cancer types. Given the recent advances in drug resistance research and the better understanding of the signaling pathways we now have, we believe that focusing on the new molecular players in this chess game, unraveling and finally targeting their regulation mechanisms, could renew interest in proteasome inhibitor therapy, thus better arming us against aggressive cancer types and improving patient life quality.
4. Discussion
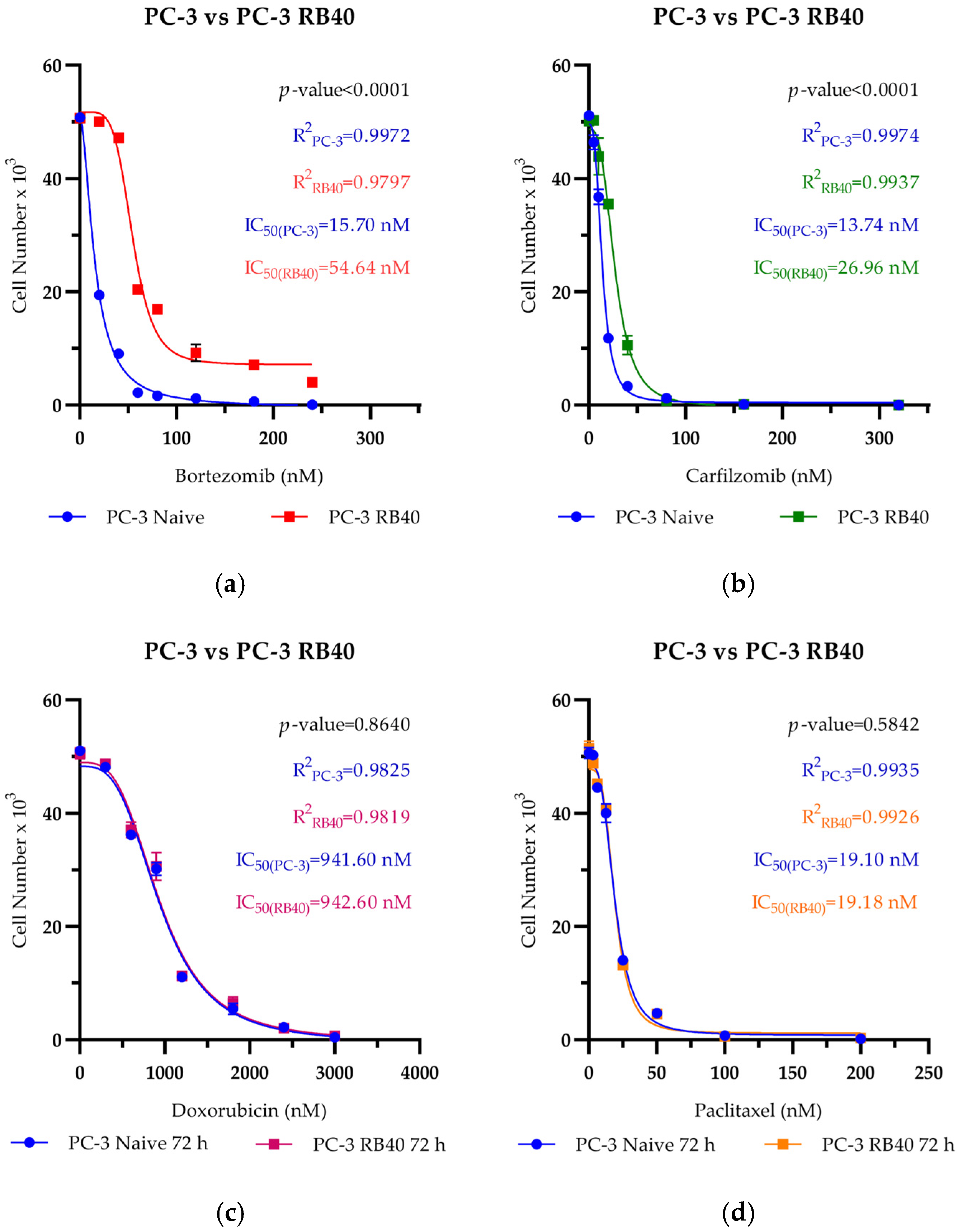
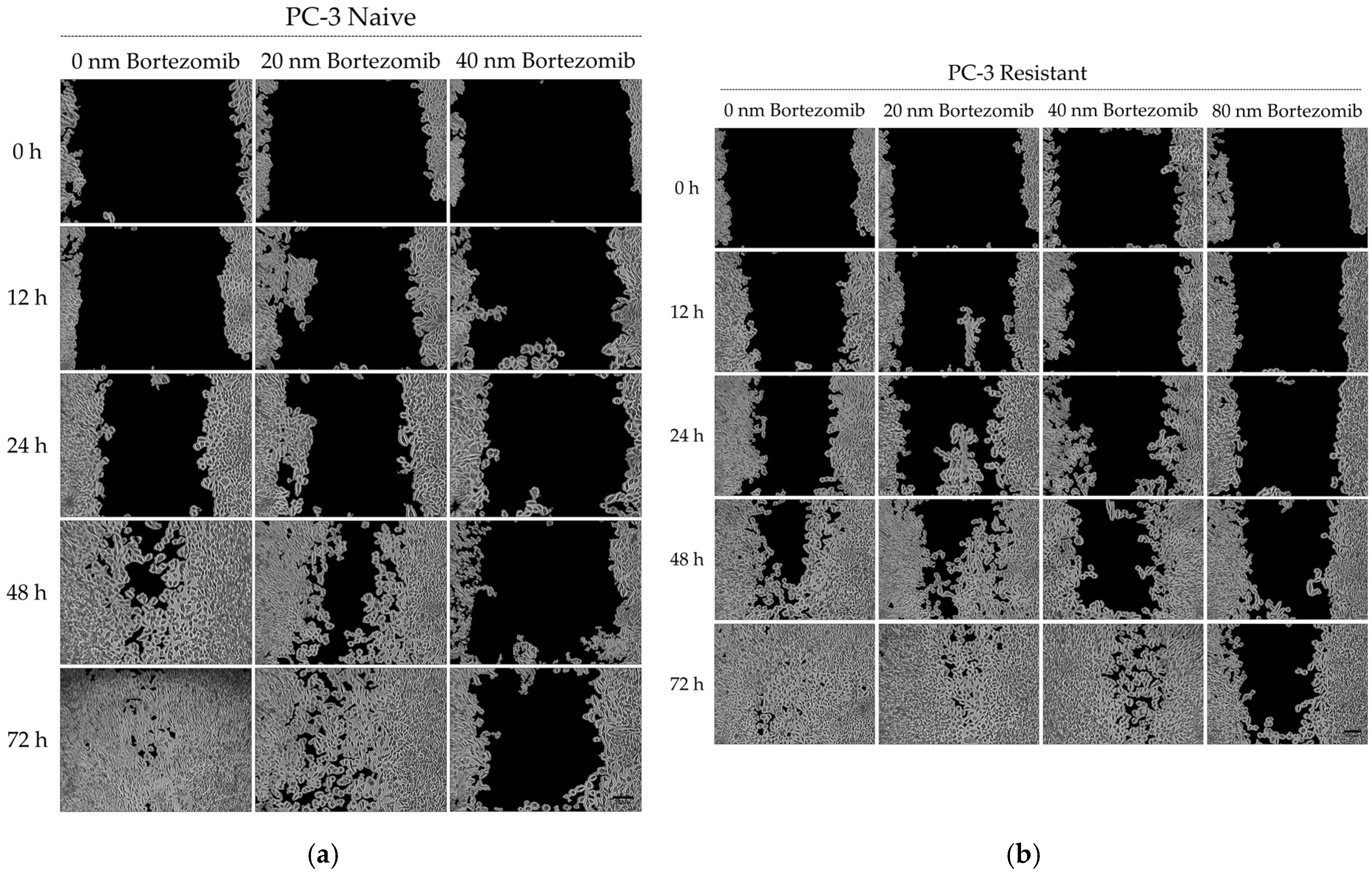
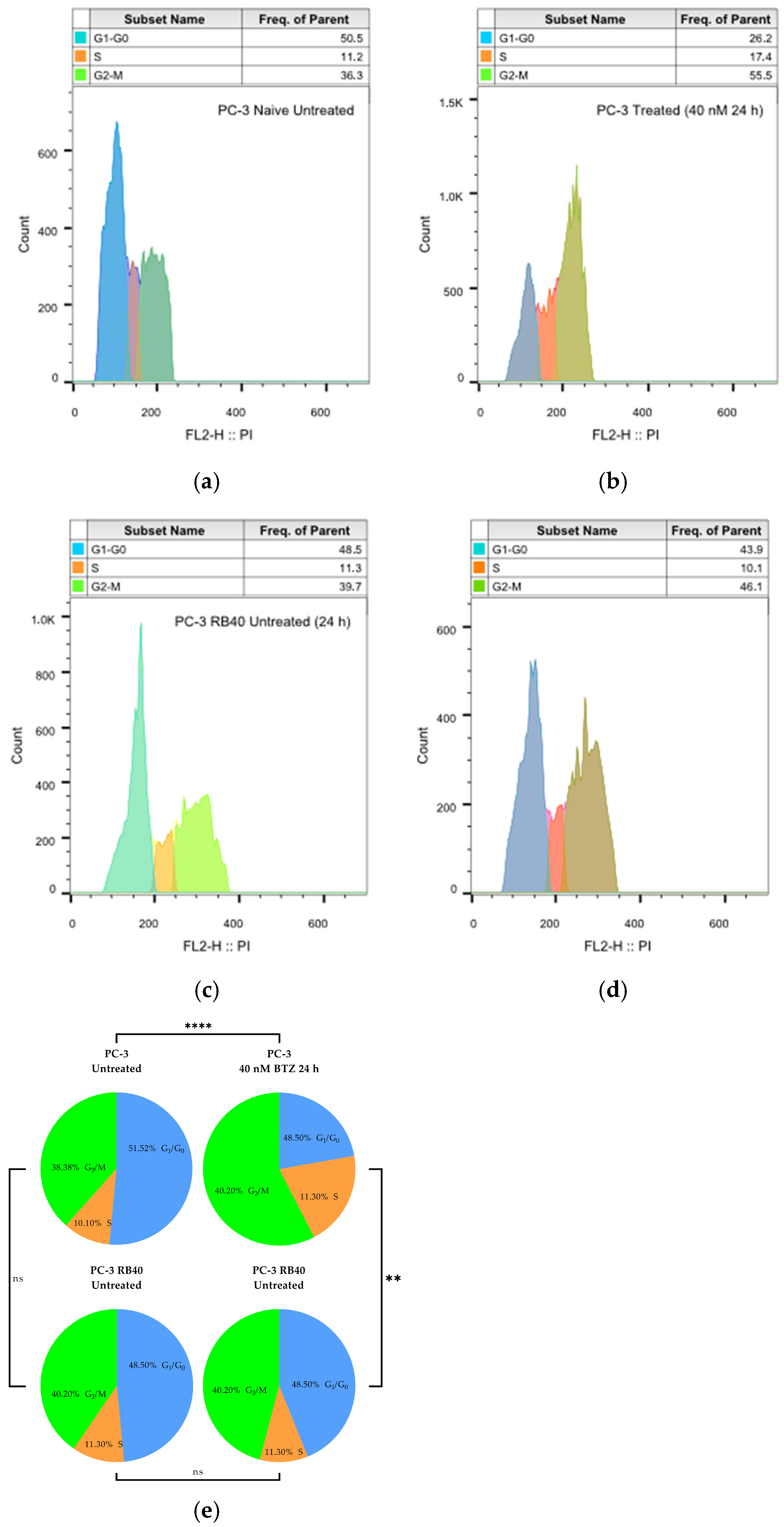
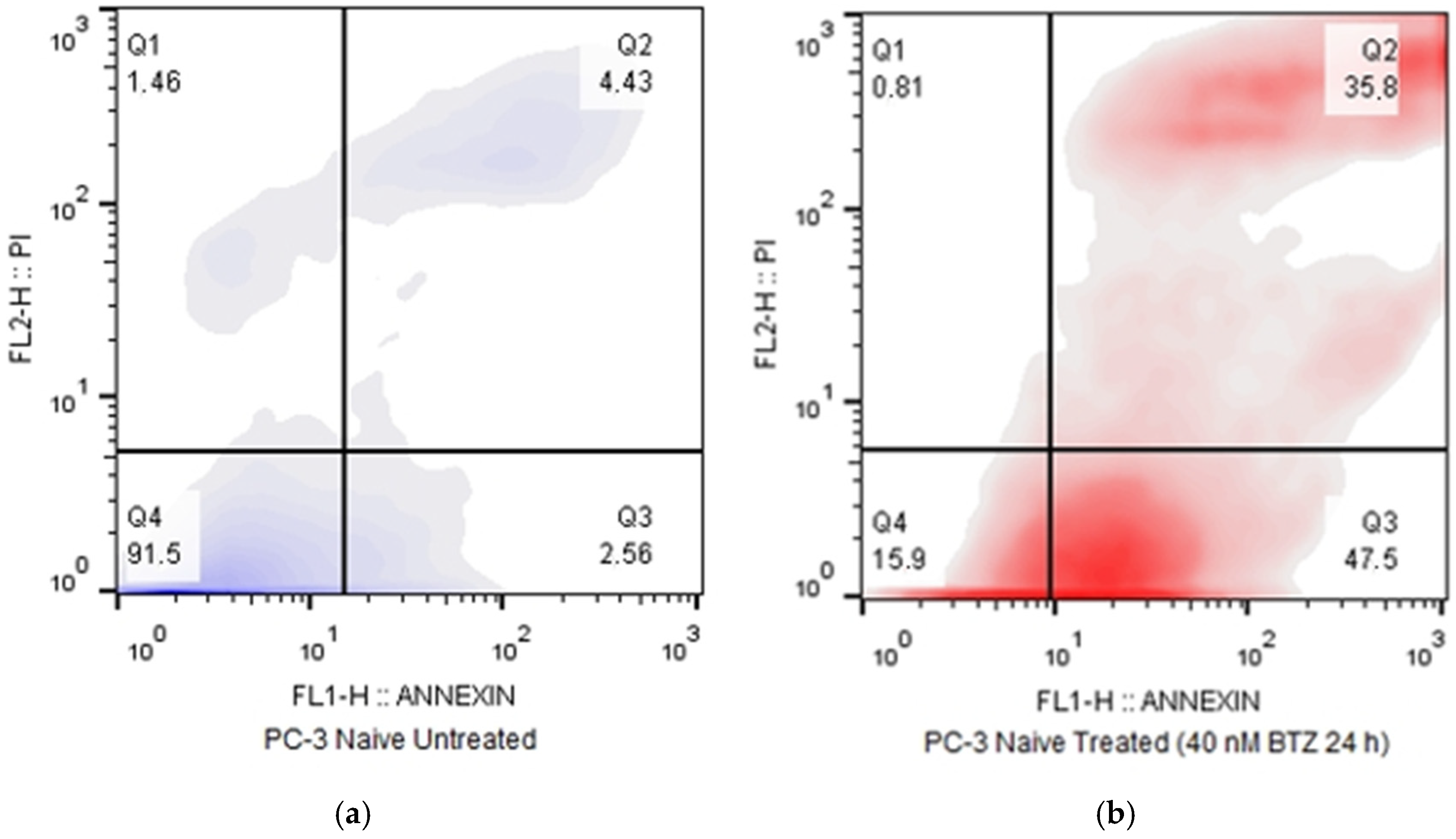
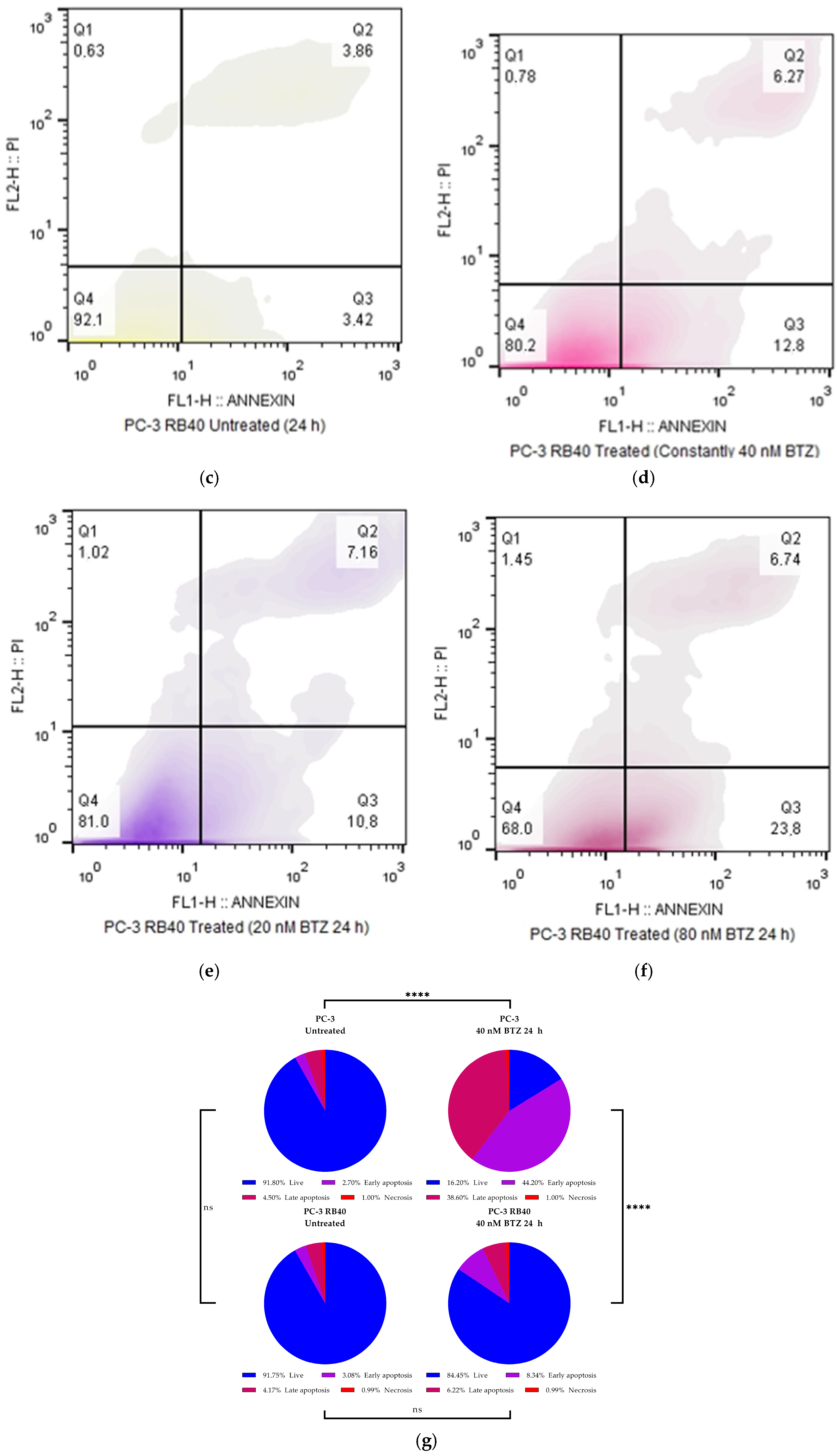
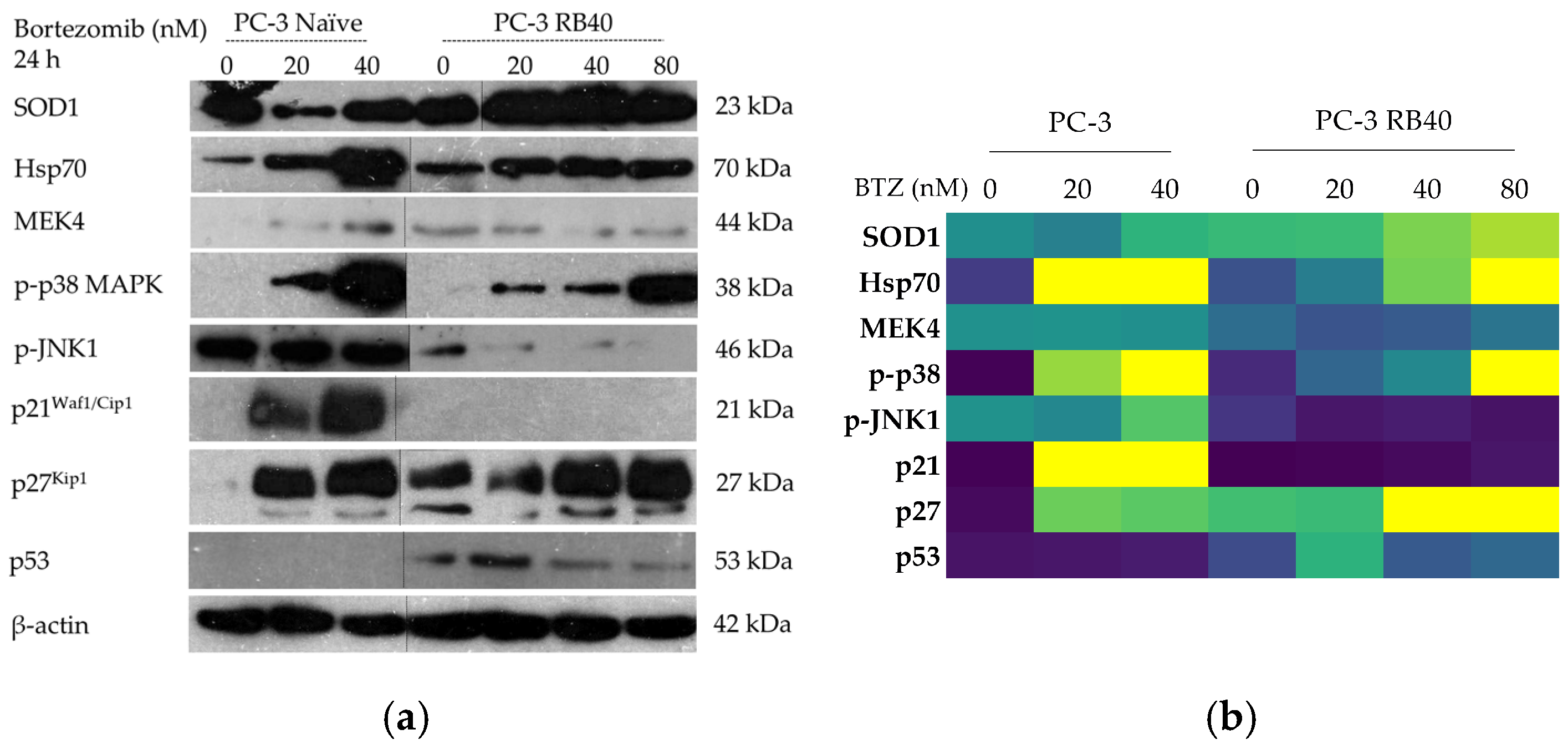
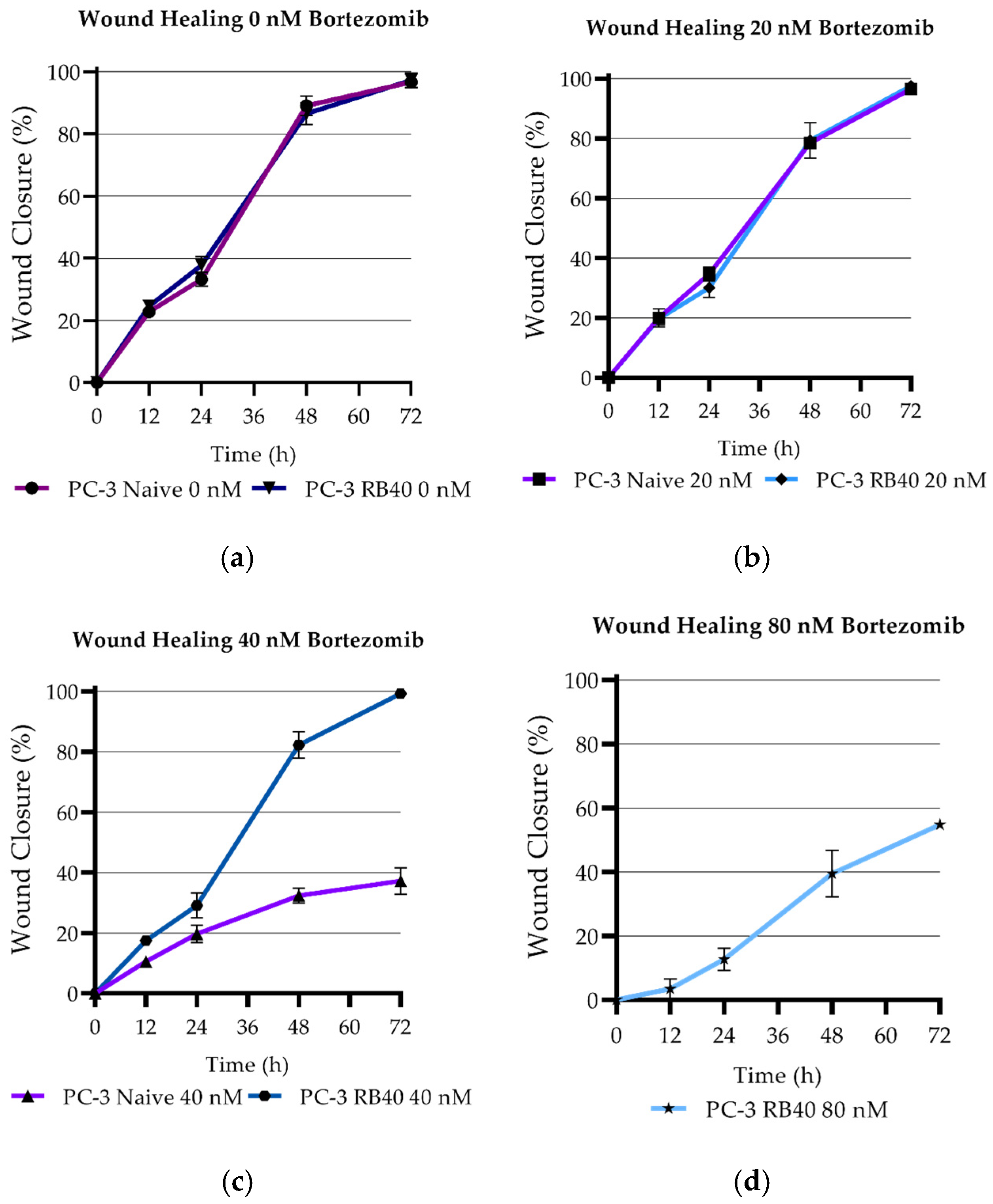
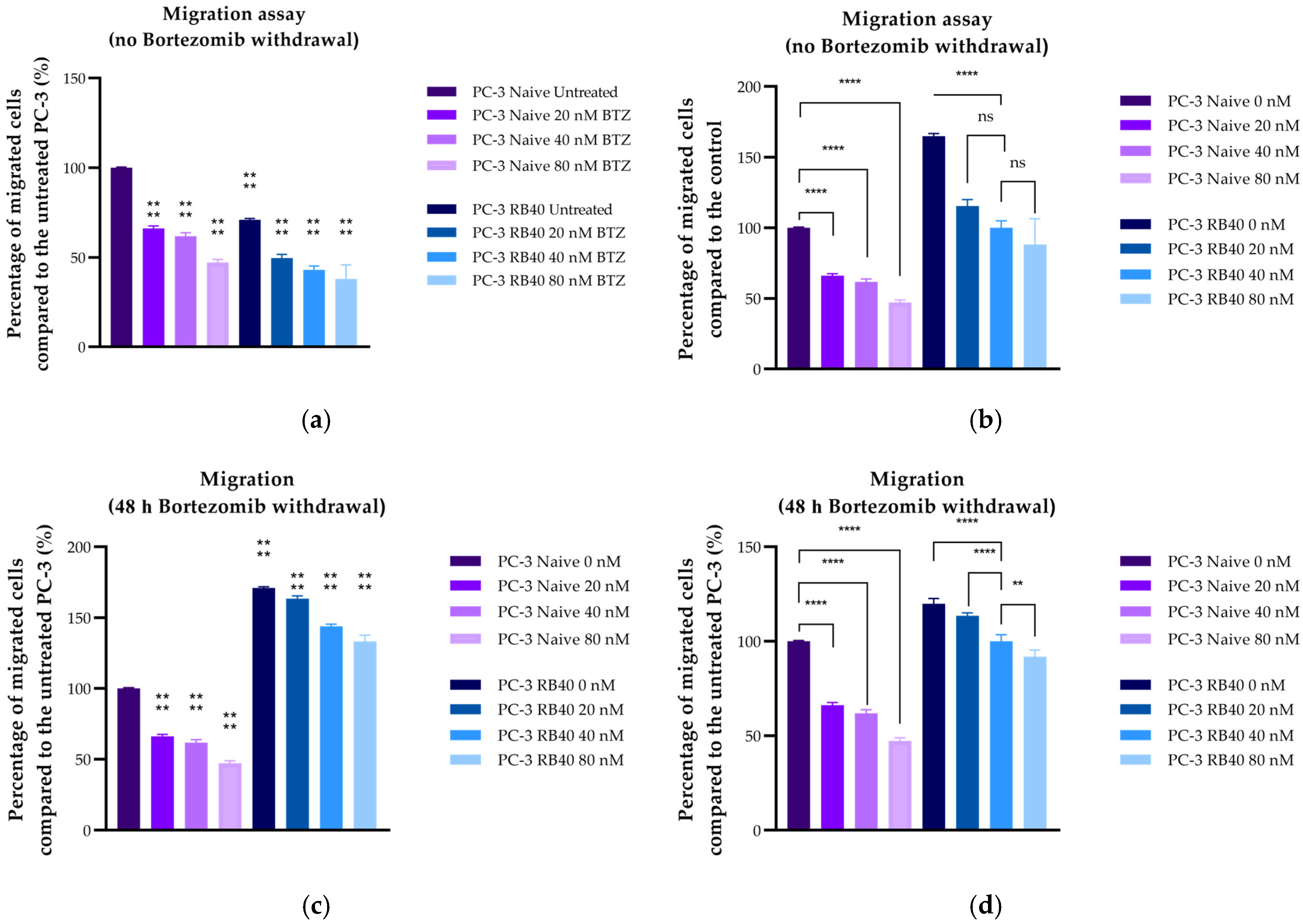
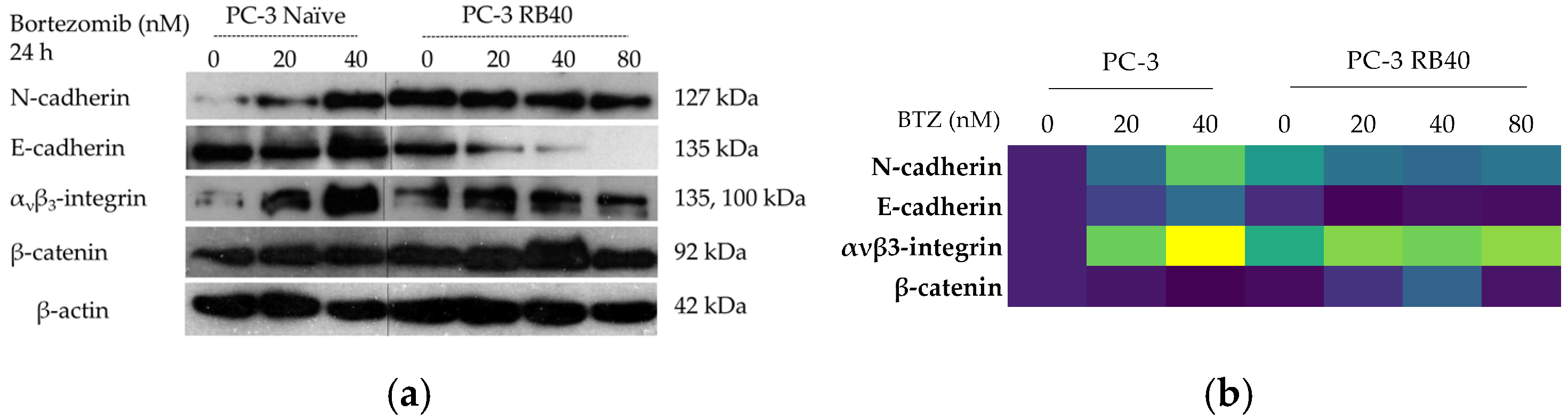
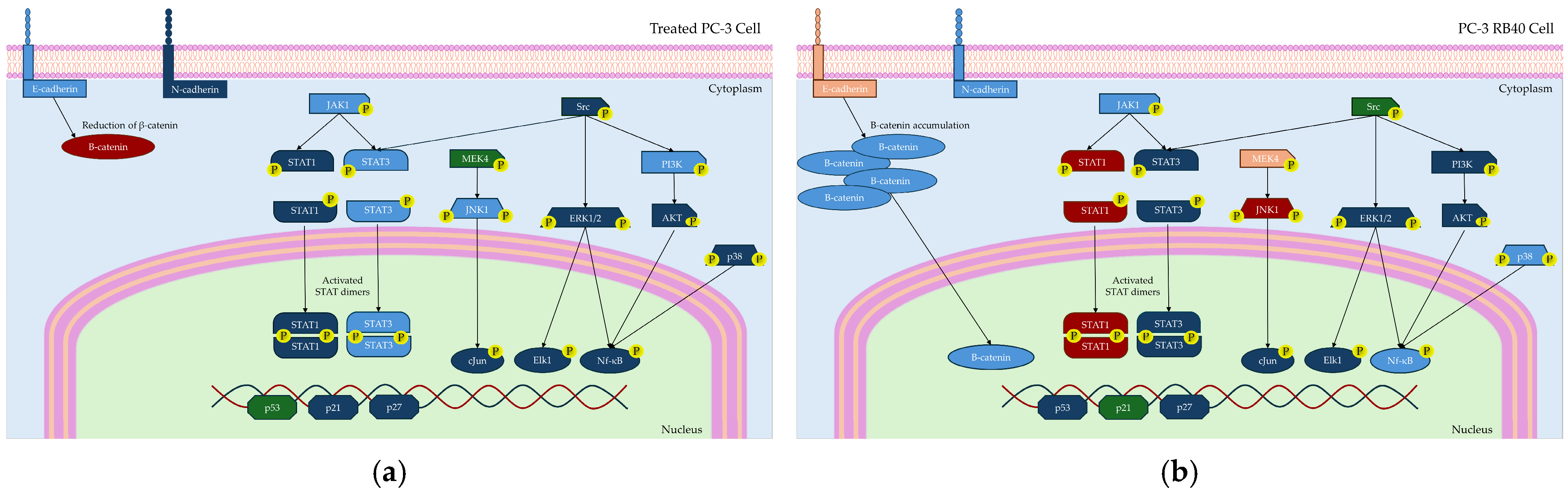
In this study, a Bortezomib-resistant prostate cancer cell line was created, and a broad-spectrum signaling investigation was performed by examining main signal transduction pathways, transcription factors, and stress levels. Our model, the PC-3 cell line, has already been used as a Bortezomib-resistant cell line [
10,
110], as it can acquire resistance to proteasome inhibitors after prolonged exposure. Given the limited efficacy of Bortezomib against solid tumors (including PCa), an attempt to gather evidence on signaling modulations as a way to improve our targeting against drug-resistant tumors and expand our biomarker repertoire. The resulting cell line was assessed for the restoration of its basic biological actions like cell viability, cell proliferation rate, the ability to surpass cell cycle arrest, and changes regarding the cell’s motility and metastatic potential. In addition, the resistant cells were documented to exhibit several EMT characteristics, with the most evident being cadherin switch and β-catenin upregulation. This observation explains the increased migratory potential of resistant cells, as well as the reinforced signal transduction through pathways correlated to cell adhesion signaling (MAPK, Akt, and Wnt signaling). The resistant cells were able to downregulate the accumulation of p21, override the inhibitory actions of p27, and avoid the cell cycle arrest caused by p53. All three proteins are regulated through ubiquitination, and their turnover rate is heavily impaired in treated naïve cells, leading to significant cell cycle arrest in the G
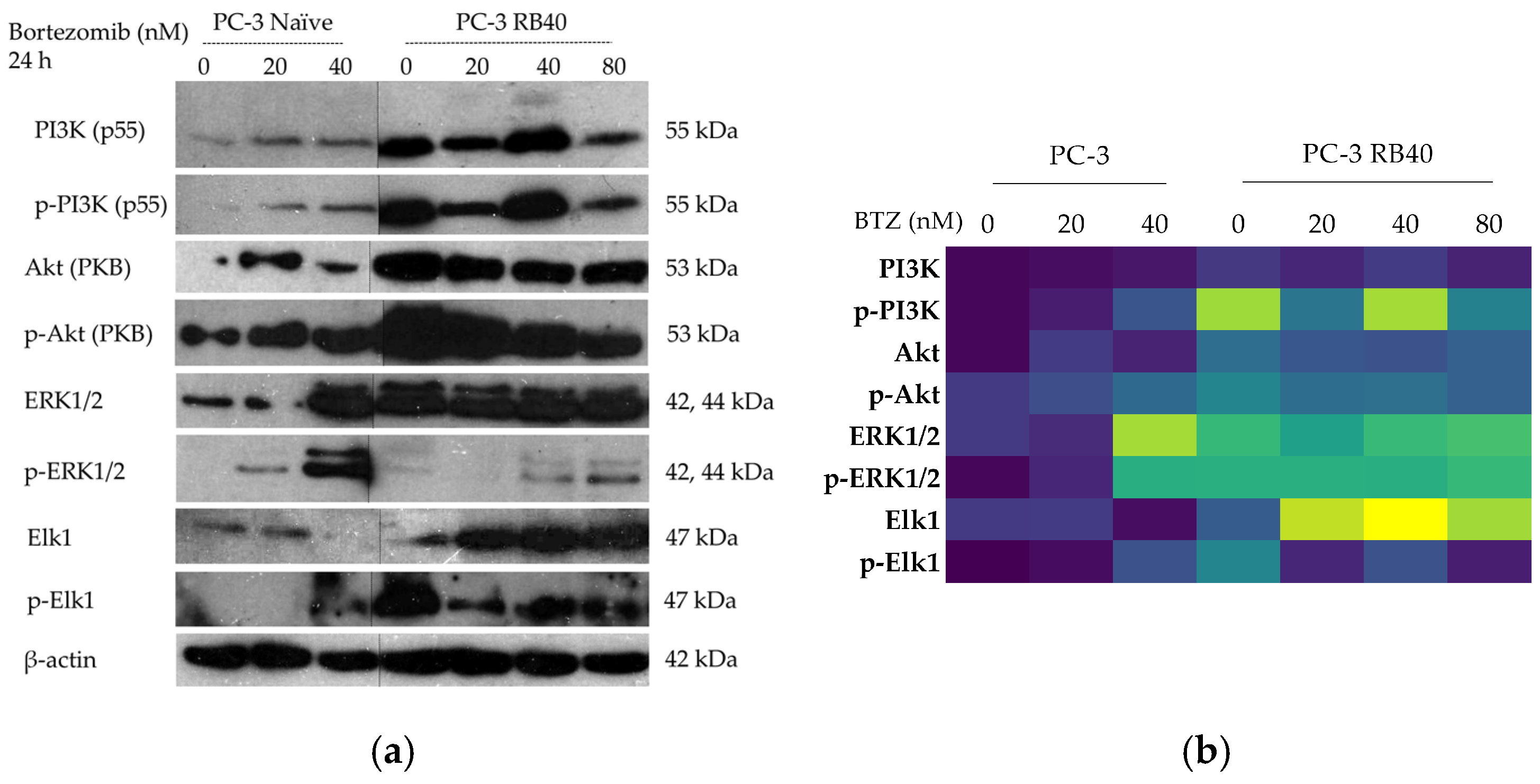
1 phase. However, the resistant cells were able to bypass those checkpoints and not exit the cell cycle, an effect that was considered the consequence of viability pathways’ activation. More specifically, the PI3K-Akt pathway is the main survival pathway of the cells (able to overcome cytostatic signals), and in the resistant clone, it was found to be significantly activated, thus explaining successful cell cycle progression (
Figure 13).
The activation of Akt can be catalyzed by PI3K, which was found to be both overexpressed and activated in the resistant clone, as well as through crosstalk with other kinases. Akt per se is known to be able to override cell cycle arrest signals as well as enhance cytoprotective functions like autophagy [
111,
112,
113]. The same actions are believed to be mediated by the NF-κB pathway, which was also found activated in the resistant cells [
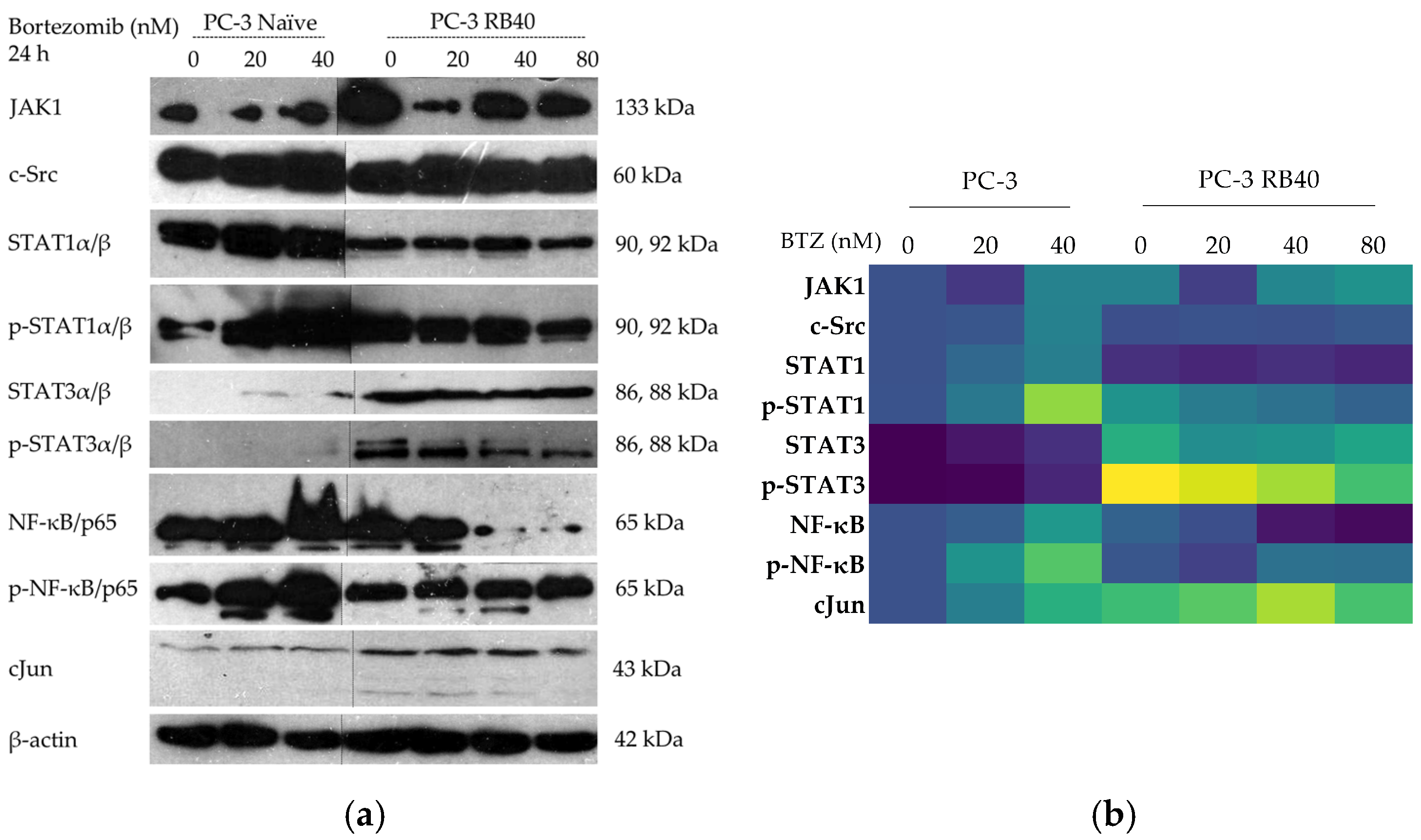
114]. NF-κB signaling is one of the most typical signaling modulation mechanisms, found in Bortezomib-resistant cells. It was initially observed in hematological malignancies where NF-κB is inherently active due to its participation in inflammatory reactions [
37,
98,
100,
115]. In our case, the PCa Bortezomib-resistant cell line PC-3 RB40 exhibited significant NF-κB activation and accumulation compared to the initial lower-level expression and activation of the pathway.
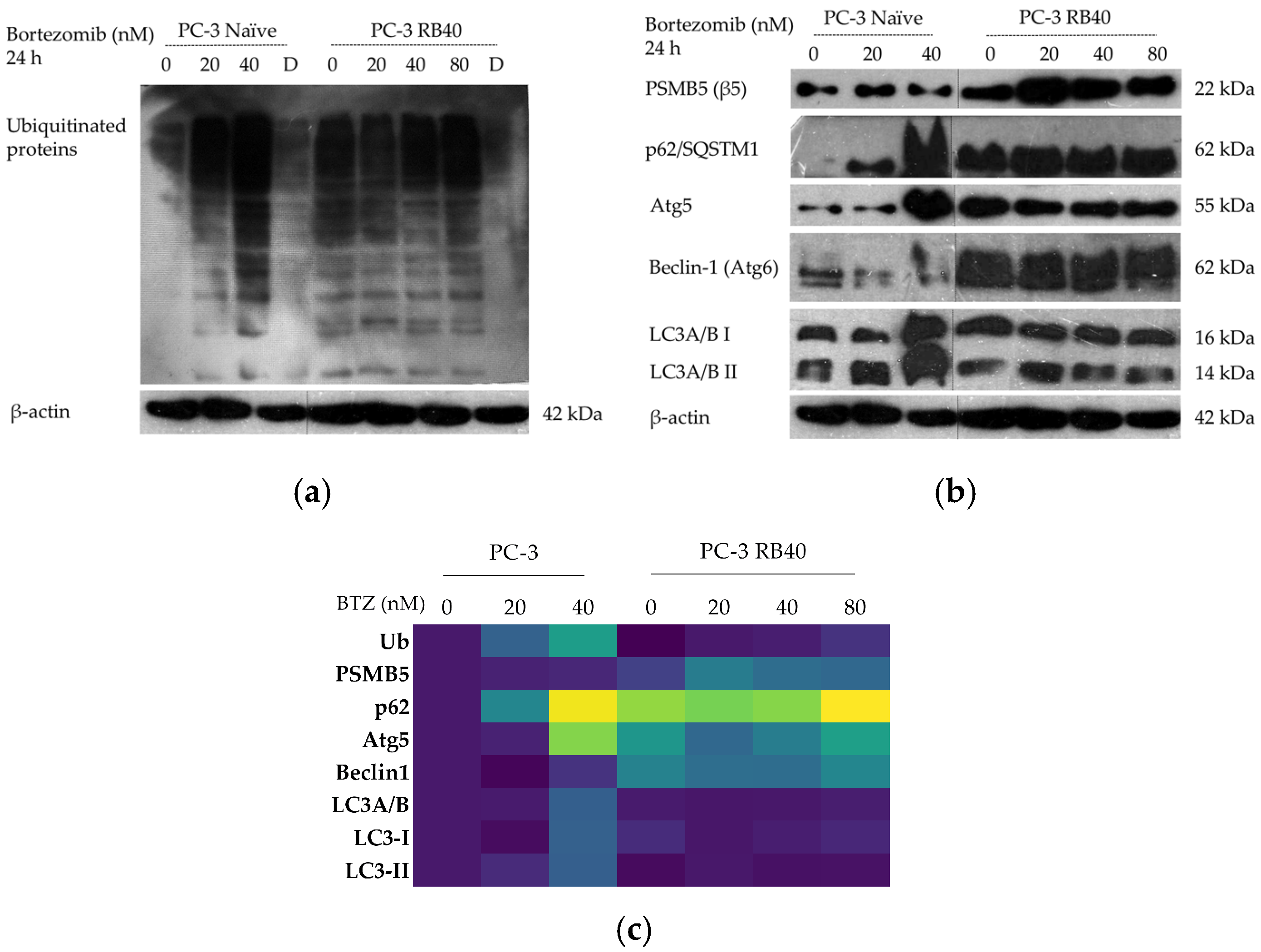
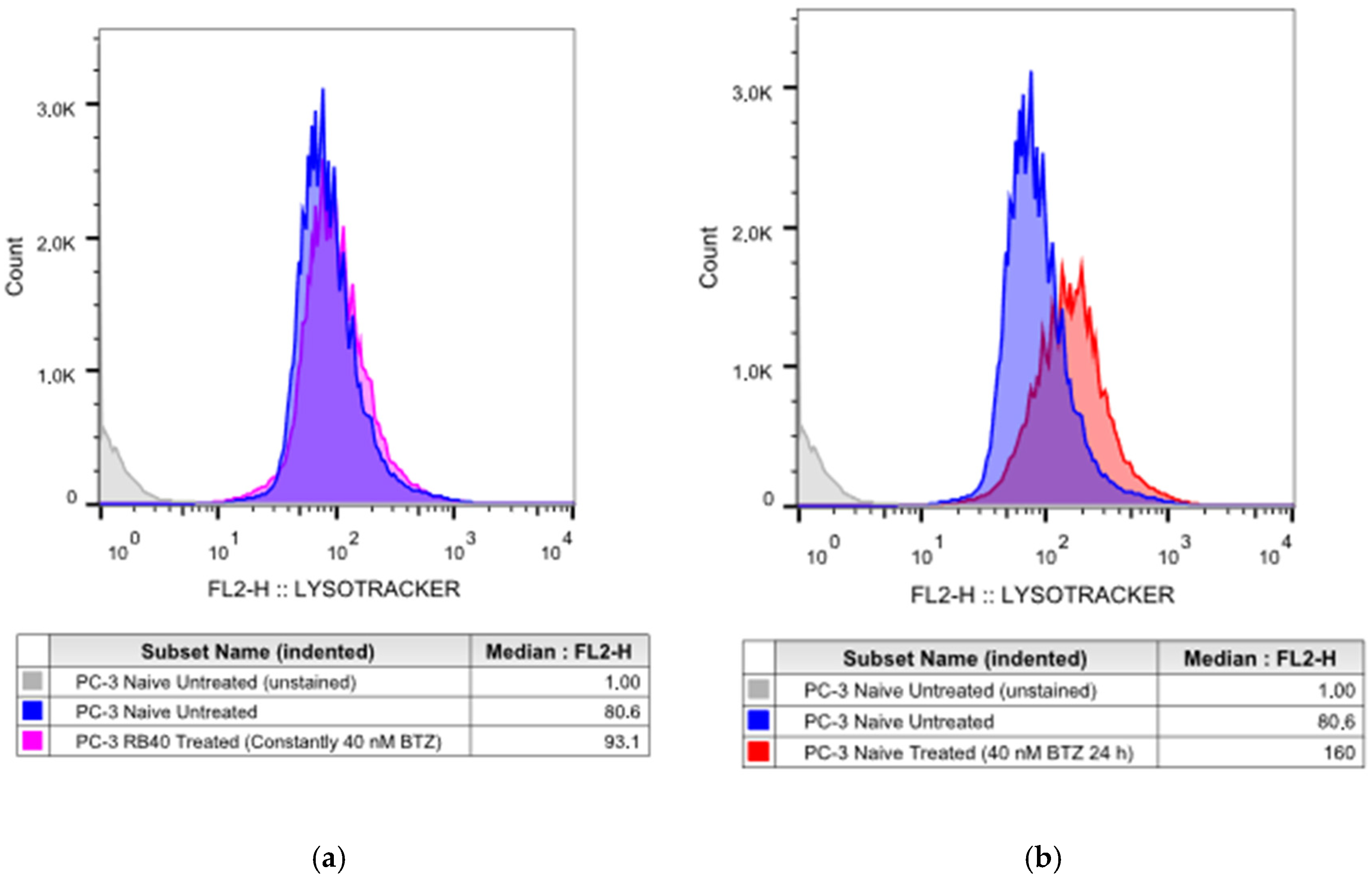
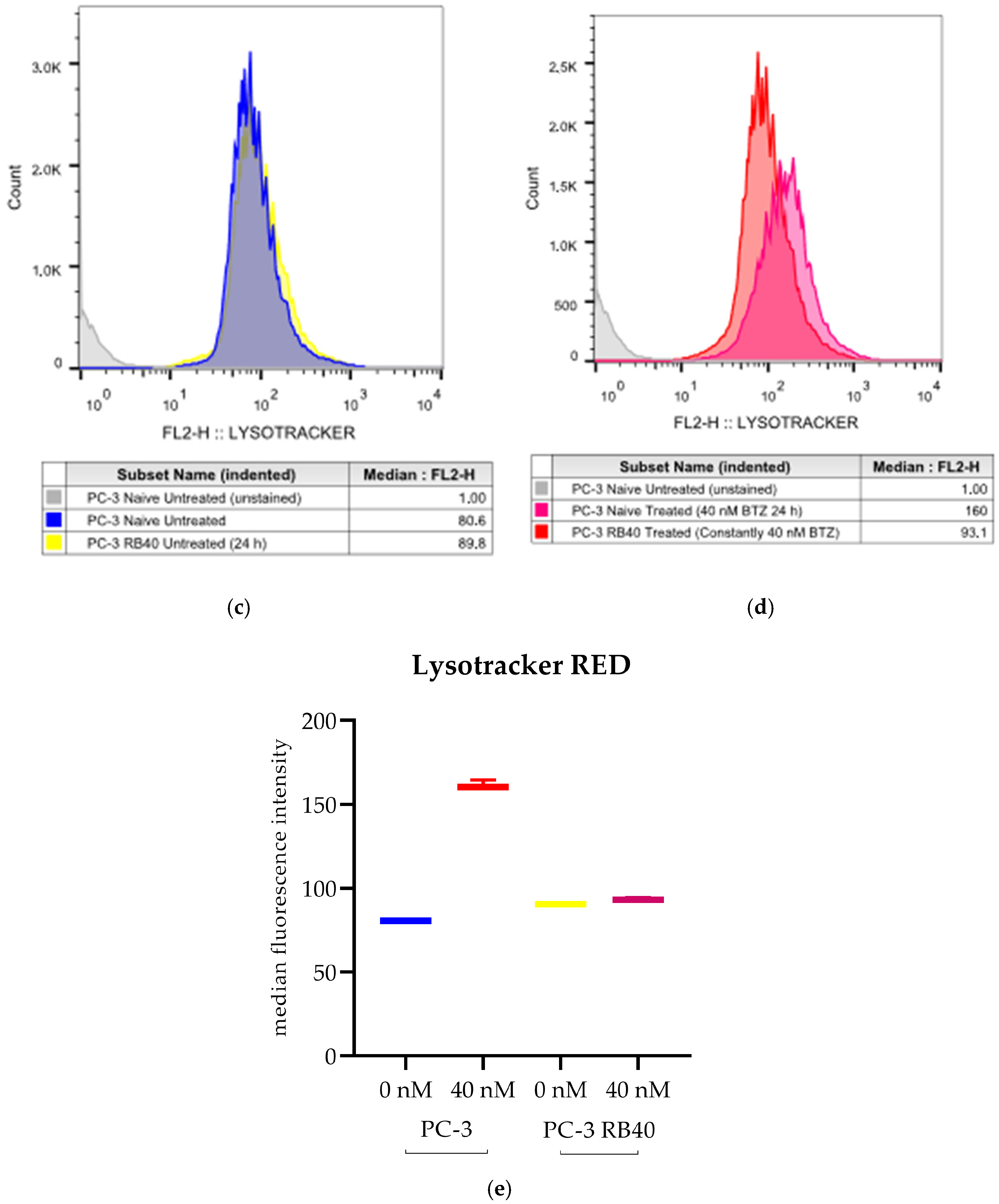
Our results indicate that the resistant clone created kept raising its tolerance against the drug the longer it was cultured in a Bortezomib-rich medium; however, no multidrug-resistant phenotype emerged as no cross-resistance to anthracyclines or paclitaxel was observed. Key milestones of Bortezomib resistance in the PC-3 RB40 cells were the upregulation of PSMB5 synthesis, the utilization of autophagy as the main proteolytic pathway, and the suppression of oxidative stress levels to levels inferior to those observed in non-resistant cells. This observation was also made by our research using a Bortezomib-resistant DU-145 cell line [
11]. The resistant clone was able to downregulate the levels of polyubiquitinated proteins, an observation also made by using hepatocellular carcinoma and prostate cancer cell lines [
10,
28]. In contrast to Yerlikaya and Okur (2019), in which a Bortezomib-resistant cell line had also been created, we observed the mature from of PSMB5 overexpressed, and no accumulation of its precursor, indicting a different pathway of resistance [
10]. Recently, the role of p62 in proteasome inhibitor resistance has gained interest, given the protein’s role as a connection link between ubiquitin-proteasome degradation and ubiquitin-dependent microautophagy [
35,
64,
68]. Resistant cells were found to overexpress p62, confirming data from previous studies that all the autophagy markers assessed indicated a strong indication of the pathway during resistance [
11,
35,
64,
116]. Autophagy modulation as an alternative proteolytic mechanism could explain the cells’ ability to control the turnover of several proteins. Normally, various kinases and cell cycle inhibitors are regulated by the UPS since they would otherwise accumulate and lead the cell to apoptotic death. An important role in this process is attributed to Beclin-1, which was also overexpressed in resistant cells. Aside from the regulation of autophagy, it has been found to directly interact with the Bcl-2 anti-apoptotic protein family, thus acting cytoprotectively [
44,
73,
74]. The modulation of signaling molecules with known cytoprotective roles was evident in the resistant clone, mainly focusing on Hsp70 and the stress-related MAPKs p38 and JNK1/SAPK [
78,
79,
81,
110]. All three proteins are known to be induced by stress conditions, an observation also evident in naïve cells following treatment with Bortezomib (
Figure 11a) [
23,
80,
82]. The accumulation of MEK4 was also observed to be significantly downregulated in the resistant cells, and given the role of MEK4 in phosphorylating both MAPKs [
117,
118], it could potentially act as a target for cell sensitization to Bortezomib (
Figure 13a). The downregulation of key stress markers directed us to oxidative stress levels, which were found to be suppressed in resistant cells. Oxidative stress is believed to rise inside a cell as a result of metabolism modulation, the accumulation of free radicals due to antioxidant defense failure, and specific chemotherapies that can dysregulate these cell functions. Despite Bortezomib being a known oxidative-stress-inducing molecule [
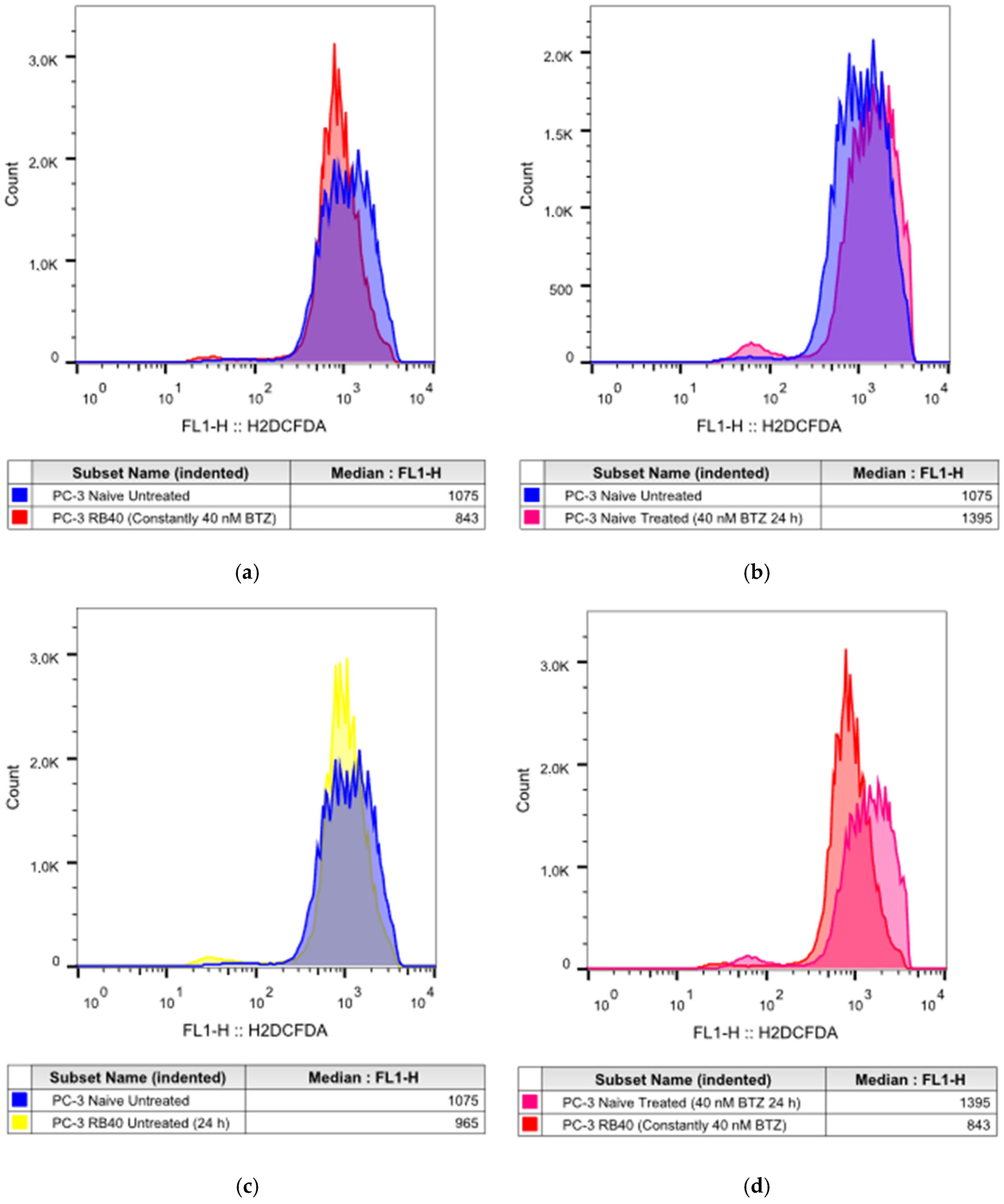
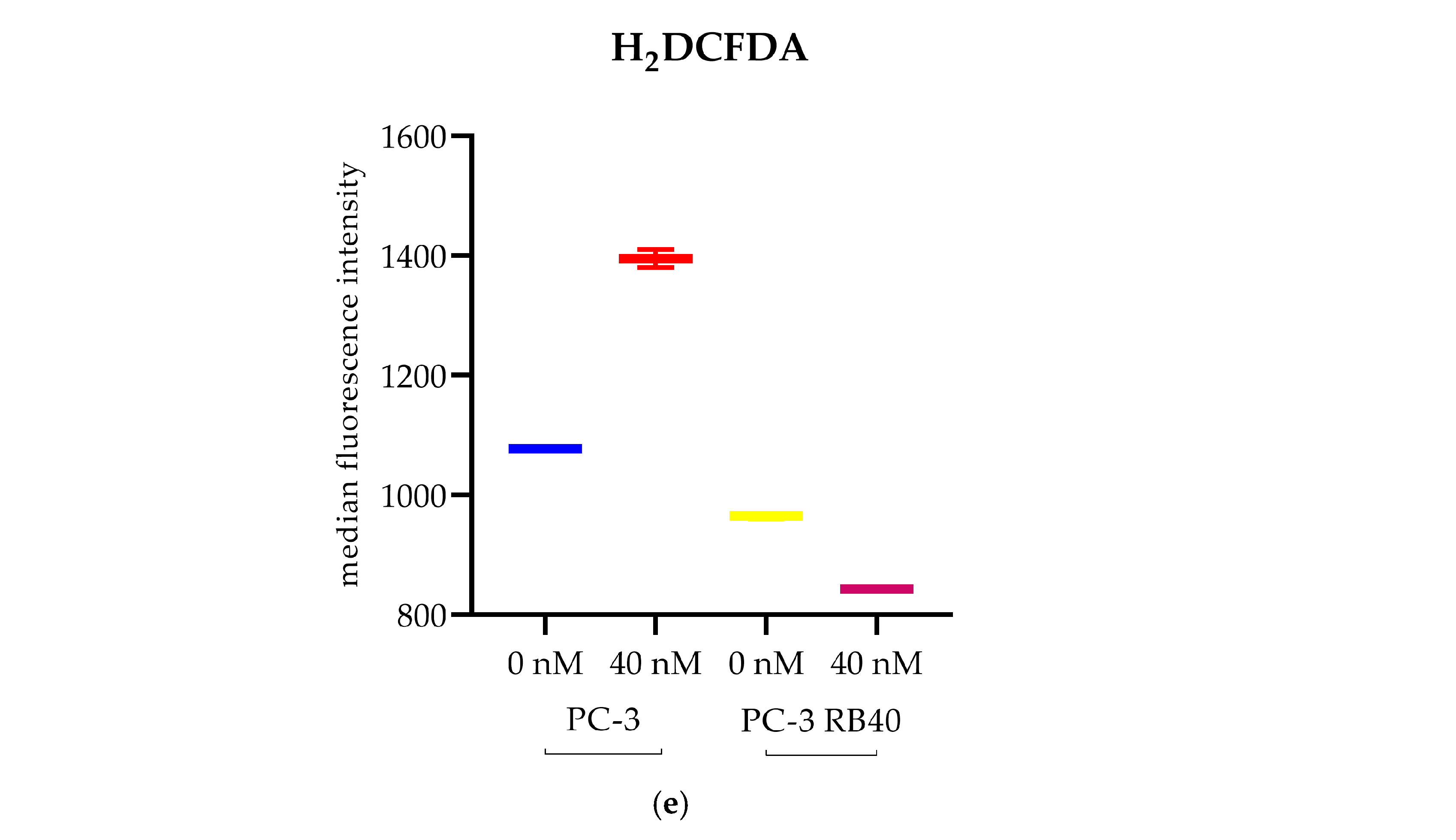
21], this was not observed in PC-3 RB40 cells, indicating changes regarding metabolism- and antioxidant-related protein expression. The fortification of the resistant cells’ antioxidant defense systems was verified by assessing SOD1 levels, which were found to be overexpressed in the resistant clones. The reactive oxygen species inside the resistant cells, as measured using H
2DCFDA, were lower even than those of untreated naïve cells, indicating the new redox equilibrium the resistant cells maintain independently of drug presence or absence. This had also been observed in the DU-145 cell line in a previous publication by our research team [
11].
Given the importance of the signal transduction pathways in regulating these parameters, we focused on the main signaling pathways and examined the accumulation and phosphorylation of the main kinases that participate in survival, proliferation, and anti-apoptotic gene expression, namely, the JAK-STAT, Ras-Raf-MEK-ERK, and PI3K-Akt pathways (
Figure 13). JAK kinases, mainly JAK1 and JAK2, have been found to participate in the pathogenesis of multiple myeloma, a malignancy susceptible to Bortezomib that can develop resistance as well [
119]. JAK silencing has been found to increase cell susceptibility to NK cell-induced cell death in multiple myeloma because both kinases transmit survival signals to the nucleus through the phosphorylation of STATs [
120]. Indeed, the resistant cells exhibited an increased accumulation of JAK1, indicating a way to multiply pro-survival signals (
Figure 13b). JAK proteins are also known activators of the Ras-Raf-MEK-ERK and PI3K-Akt pathways, and the overexpression of JAK we detected in the resistant clone could contribute to these pathways’ activation [
121,
122,
123,
124]. The transcription factors STAT1 and STAT3, downstream of JAK1, were also assessed to monitor the signal transduction route. STAT1, with known pro-apoptotic roles [
42,
96], was found to be downregulated in the resistant clone both in terms of total STAT1 as well as phosphorylated STAT1. The opposite effect was observed regarding STAT3, which was significantly upregulated in resistant cells. Vangala et al. (2014) documented a correlation between STAT3 phosphorylation and PSMB5 protein levels, indicating STAT3 as a transcription factor that controls proteasome function [
102]. STAT3 inhibition downregulated the expression of proteasome subunits, thus increasing the pro-apoptotic effects of Bortezomib. Yuan et al. (2023) also proposed STAT3 inhibition as a way to overcome Bortezomib resistance [
39]. Both studies in multiple myeloma reported high STAT3 expression and phosphorylation, which was also observed in the resistant PC-3 RB40 (
Figure 13b). This result was also verified by Zafeiropoulou et al. (2024), where the same phenomenon was documented in a Bortezomib-resistant DU-145 prostate cancer cell line [
11]. STAT3 has also been reported to be activated by Src in human pancreatic adenocarcinoma cells (independently from JAK activation). This mechanism could shed light on the fact that naïve PC-3 enhanced c-Src accumulation following incubation with a high Bortezomib dose [
125]. Hence, this could be an amplification mechanism of the
PSMB5 gene expression to produce more subunits to replace PI-bound proteasomes. Since c-Src was not found to be significantly overexpressed in the resistant cells, this could indicate that the JAK-independent activation could be a significant parameter regarding the (naïve) cell’s primary response to Bortezomib. However, the resistant cells could also be exploiting other ways to achieve STAT3 phosphorylation and overexpression.
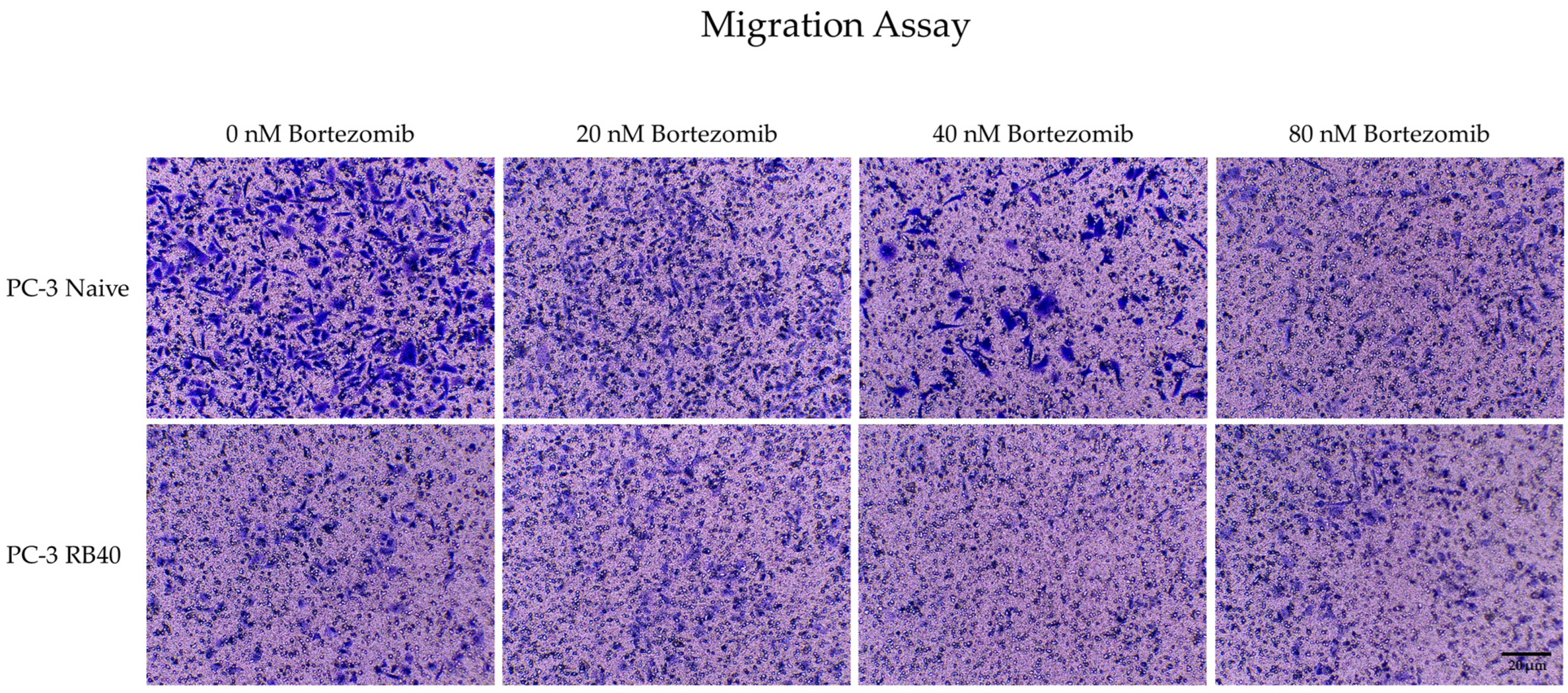
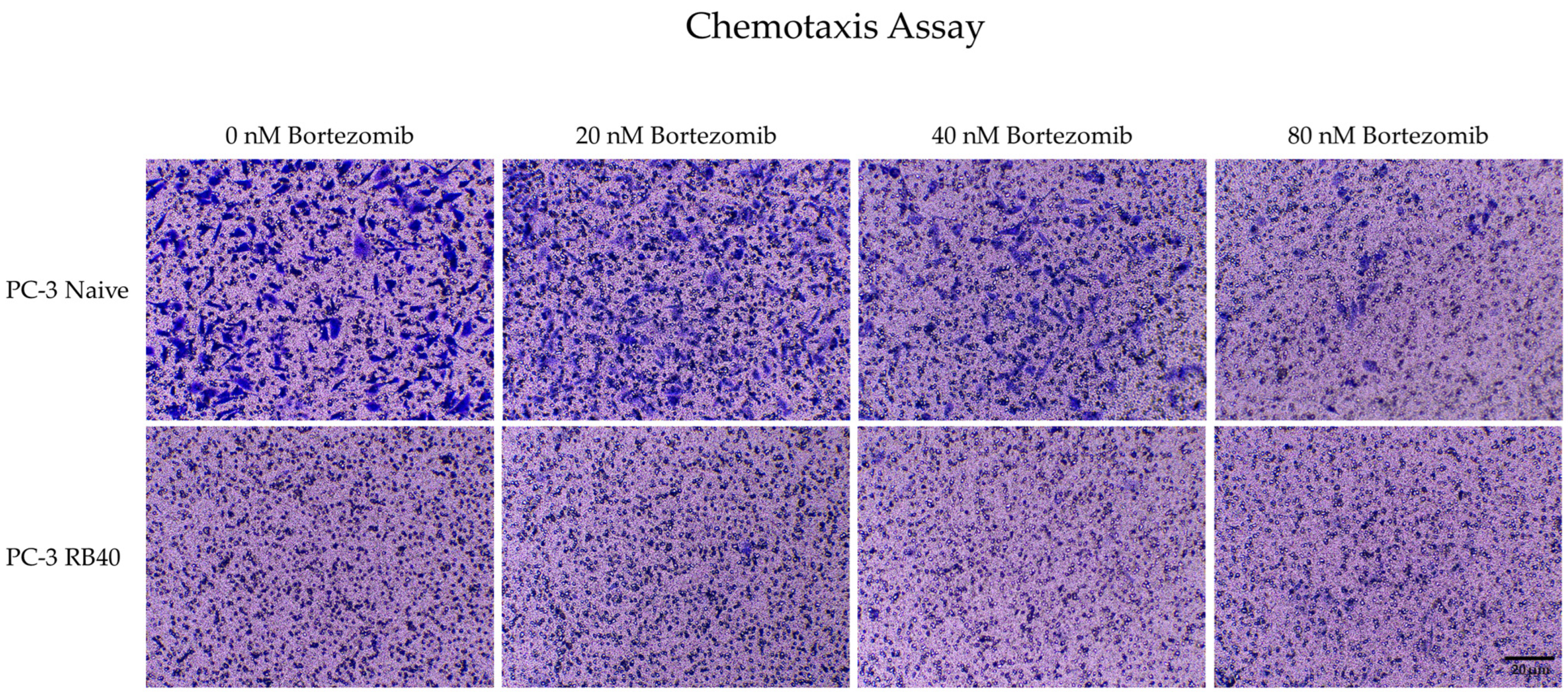
Another major role in Bortezomib resistance is played by the ERK1/2 kinases. Being the foremost downstream molecules of the Ras-Raf-MEK-ERK pathway, they capture and transmit to the nucleus signals from several upstream kinases as well as from other pathways due to crosstalk between them [
117]. ERK1/2 phosphorylation has already been proposed as a molecular target in myelodysplastic syndromes, where the MEK inhibitors U0126 and PD98059 successfully re-sensitized Bortezomib-resistant cells to the drug [
101]. In our experiments, we did not inhibit the signal transduction; however, by assessing the pathway activity, we observed that ERK1/2 was both upregulated and over-phosphorylated in the resistant clones (
Figure 13b). Survival mediated by ERK1/2 in Bortezomib resistance has also been shown in other studies, and prostate cancer is one of them [
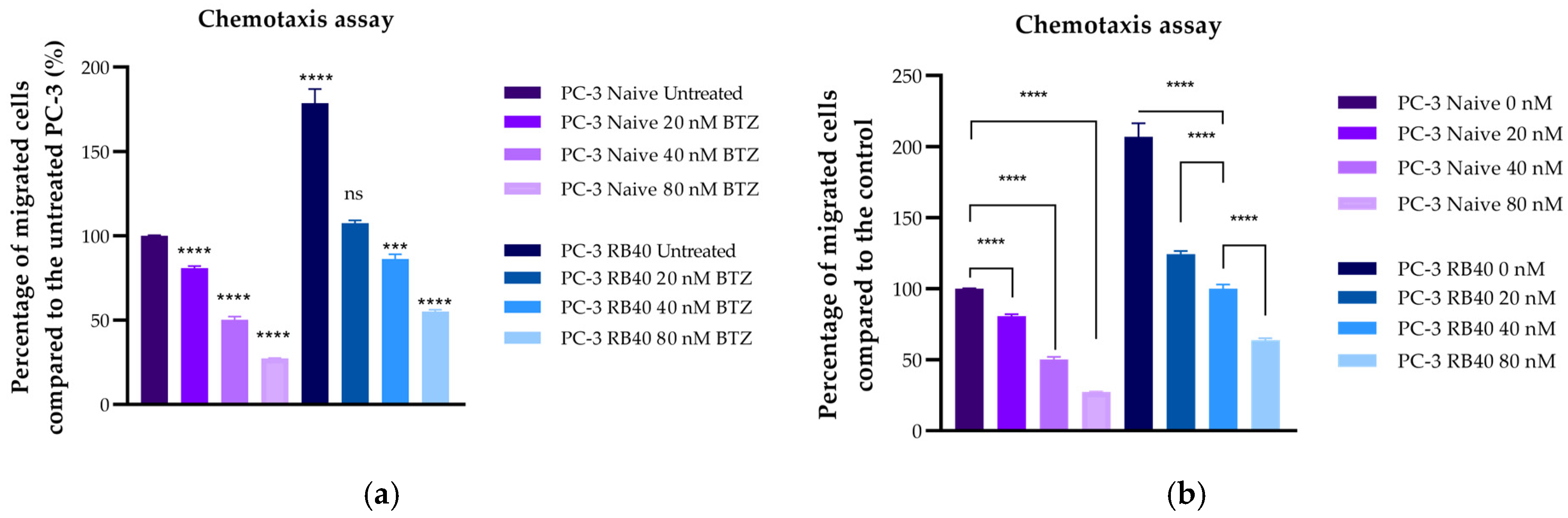
11,
126]. ERK1/2 are believed to regulate many cellular processes, from the transcription of survival-related genes to autophagy and even apoptosis. ERK1/2 could also play a crucial role in restoring the cells’ ability to migrate more effectively, given the kinases’ role in cell-adhesion-related signaling. Even though a functional UPS is needed to degrade inhibitors and maintain the equilibrium between pro-survival/pro-migratory and pro-apoptotic signals, over-expressed and over-phosphorylated MAPKs could counterbalance the loss of UPS. Multiplying signals from external stimuli could be a major contributor to the resulting aggressiveness, as this is supported also by the resistant cells’ response during chemotaxis experiments. Resistant cells were able to bypass the chemorepellent effects Bortezomib has on naïve cells, and successfully migrate towards the FBS chambers, highlighting the strong positive signals received from extracellular growth factors. An important downstream molecule of the ERK1/2 kinases is the transcription factor Elk1, a molecule whose role in Bortezomib resistance has only been poorly studied so far.
Few pieces of evidence regarding Elk1 in proteasome inhibitor resistance have been published to date; however, the interaction between ERK1/2-Elk1 and the proteasome has already been proven [
127]. A study about Carfilzomib sensitivity in mantle cell lymphoma, a hematological malignancy for which proteasome inhibitors are an approved therapy, investigated the role of Elk1 in proteasome capacity and inhibitor resistance [
109]. Elk1 was found to control the proteasome assembling process, regulated by POMP (proteasome maturation protein) expression, which is a molecular chaperone regulating the biosynthesis of proteasome subunits. In that study, the activation of Elk1 through the c-Met/MAPKs led to increased POMP transcription, which in turn increased the assembly of the proteasome and increased both proteasomal activity as well as resistance against Carfilzomib [
109]. In our study, the MAPKs were also found to be activated, and the most interesting fact is that Elk1 was found to be significantly overexpressed and phosphorylated in the resistant PC-3 (RB40) cell line (
Figure 13b). Elk1 has recently been discovered to play important roles in the progression of many cancer types, including the aggressiveness of pancreatic and prostate cancer [
128,
129,
130]. Modulation of Elk1 activity seems to have anti-metastatic effects, as shown by Lai et al. (2023), where Asiatic acid was administered in PC-3 cells, and by interfering with the protein interaction between Elk1 and MZF-1, migration was negatively affected [
131]. Elk1 seems to be an important mediator of resistance by assisting migration towards growth factors and possibly reinforcing the cells’ ability to survive and proliferate, as has also been shown in breast cancer models [
132,
133,
134]. Additionally, Elk1 has been reported to promote survival through autophagy regulation in colorectal cancer cells, a mechanism that could also be present here [
135]. The findings about Elk1 were in complete accordance with the observed phenotype of resistant cells; not only were upstream kinases upregulated, but also the transcription factors’ contribution in aggressiveness was visible via the migration assays. Finally, another interesting finding regarding stress-response signaling pathways was the increased accumulation of cJun in the resistant clones. cJun is the last molecule in the JNK signaling cascade and is overexpressed in aggressive cancer types. cJun overexpression has also been linked to constitutively phosphorylated ERK1/2, a fact that was also observed in the PC-3 RB40 cell clone [
136]. Given the oncogenic functions of cJun, the suppression of cell cycle regulators like p21, and the proliferative and angiogenic effects of its action, it seems to be a key molecule in resistant cell survival. Both Elk1 and cJun are known to respond to stress conditions, and their stable presence in resistant cells possibly indicates that their suppression mechanisms could have been downregulated (or aborted). Their role in prostate cancer, especially in the case of Elk1, has not been fully uncovered; however, it is known that Elk1 and AR signaling have significant interplay [
137]. Given the fact that the cells are immune to external androgen-deprivation therapy, targeting Elk1 as an intracellular component of AR-dependent growth signaling could re-sensitize them by bereaving them of pro-growth signals. Additionally, it is important to mention that Elk1, cJun, and other transcription factors could have even greater translational interest from a biomarker-oriented point of view. Early indices of resistance would assist the clinicians to select the right therapeutic scheme, and non-invasive procedures could further strengthen this notion. Liquid biopsy has recently offered novel biomarkers of high accuracy and significance that can enhance early disease diagnosis and predict prognosis [
138]. Monitoring resistance-specific markers and coupling them with liquid biopsy techniques could offer really robust, cost-effective, and, more importantly, less invasive tools against several cancer types. Therefore, the role, expression patterns, and interaction networks of the aforementioned transcription factor should be further studied, and our understanding expanded, not only in Bortezomib resistance, but also in drug-resistant cells in general.
5. Conclusions
The model of our study, the PC-3 RB40 cell line, developed resistance to Bortezomib, following exposure to gradually increasing doses of the drug for an extended period. Our experiments demonstrated induction of autophagy, downregulation of oxidative stress levels, and increased signal transduction through the ERK1/2, Akt, and JAK kinases that could be the result of significant crosstalk between the main pathways. Following an investigation of various transcription factors downstream of ERK1/2 and Akt, we discovered that STAT1 was significantly downregulated in the resistant clone and STAT3 was both overexpressed and phosphorylated, thus implying a significant reduction in pro-apoptotic signaling and an induction of pro-survival signals. Additionally, alongside the activation of the Νf-κB pathway, which is already extensively studied in cases of Bortezomib resistance, our study elucidated Elk1 and cJun as potential pharmaceutical targets, given their elevated presence in the resistant clone. Elk1 may be an important mediator of PI resistance, regulating the resistant cell’s cell cycle progression, angiogenesis, and migration, thus assisting survival in hostile environments. With the recent attention the MAPK-Elk1 axis has attracted and the evidence supporting STAT3 and cJun as potential therapeutic targets, we propose the three transcription factors as molecules of a significant role in the emergence of Bortezomib resistance, the targeting of which could truly improve the management of prostate cancer cases by expanding our drug repertoire and disease understanding. Our findings are based on a PI-resistant prostate cancer cell line; however, this does not mean that they are exclusively applicable to prostate cancer. PC-3 cells are known for their stemness, the expression of EMT markers, and the aggressiveness of typical advanced-stage tumor cells, thus allowing extrapolation. Further research is warranted to dissect the molecular pathways that underlie the studied phenomena, and attention should be directed towards the crosstalk between the main survival/proliferation pathways (MAPKs, JAK-STAT, Akt). Targeting of autophagy, ERK1/2 signaling, cJun, and Elk1 could provide insight on drug resistance, not only in PCa but in cases of PI-resistance in general. Even though Bortezomib as a therapy for PCa was assessed in the past and resistance led to a reduced interest, the idea was not abolished since its application as a therapy against mCRPC is still being investigated in clinical trials. Understanding how resistance emerges and develops could encourage further clinical trials and assist clinicians and pharmacologists in compiling novel targeted therapies. Therefore, future studies should expand our research by assessing whether PI therapy could be a part of regimens that would effectively block the aforementioned transcription factors, kinases, or cell functions. It is noteworthy that novel approaches or repurposing of current drugs (by adjusting combinations and doses) could enhance our ability to treat resistant tumors and improve the quality of life of thousands of patients.