Caprylic Acid Restores Branched-Chain Amino Acid Metabolism in a Mouse Cachexia Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. In Vitro Cachexia Model
2.3. Animals
2.4. Dietary Interventions
2.5. Protein Extraction
2.6. Western Blotting
2.7. Enzyme-Linked Immunosorbent Assay (ELISA) and Fluorometric Analysis
2.8. Mitochondrial Stress Test (Seahorse Assay)
2.9. Glycolytic Stress Test
2.10. Reverse Transcription–Polymerase Chain Reaction (RT-PCR)
2.11. Statistical Analysis
3. Results
3.1. Effect of BCAAs on Cancer Sarcopenia
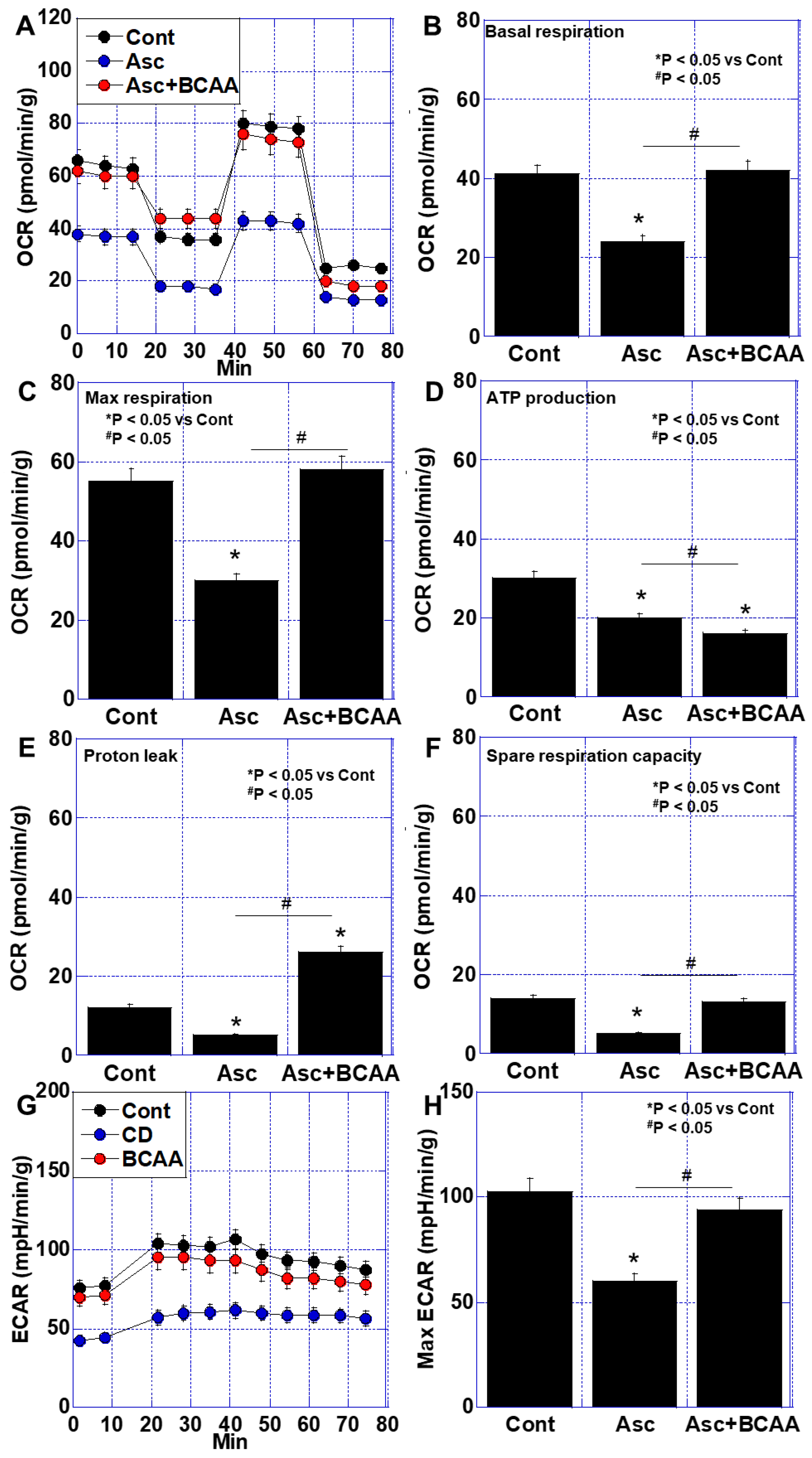
3.2. Effects of BCAAs on Cancer-Related Impairment of Energy Metabolism
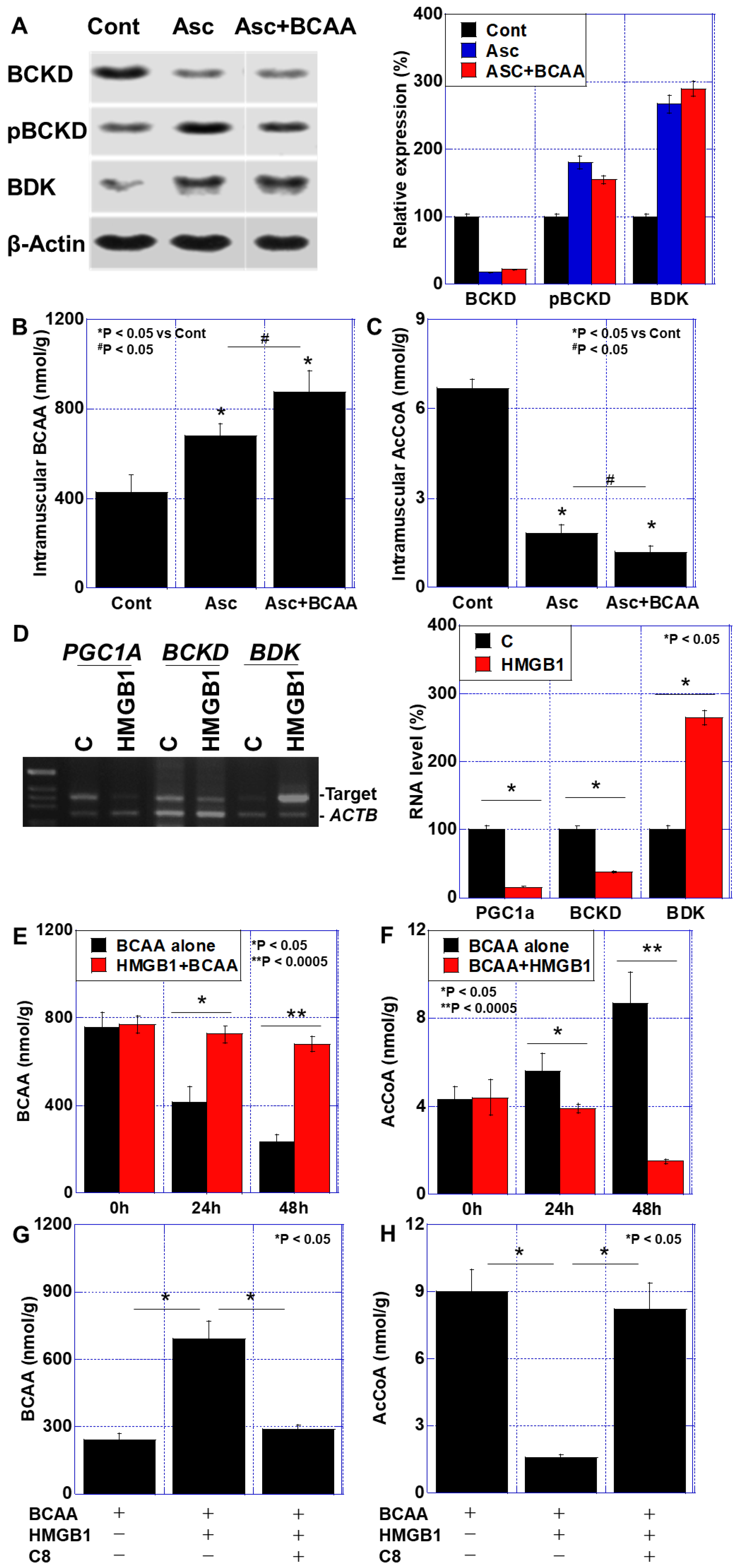
3.3. BCAA Metabolism in the Cachexia Models
3.4. Effects of C8 on HMGB1-Induced Skeletal Muscle Impairment
3.5. Effect of BCAAs When Combined with 5FU Treatment in a Mouse Cachexia Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BCAA | branched-chain amino acid |
| BCKD | branched-chain α-ketoacid dehydrogenase |
| HMGB1 | high-mobility group box-1 |
| QCM | quadriceps femoris muscle |
| SDS-MYL1 | sodium dodecyl sulfate-soluble myosin light chain-1 |
| 4HNE | 4-hydroxynonenal |
| TNF | tumor necrosis factor |
| BDK | BCKD kinase |
| AcCoA | acetyl coenzyme A |
| PGC1α | peroxisome proliferator-activated receptor-γ coactivator-1α |
| C8 | caprylic acid |
References
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, M.; Anthony, T.G.; Ayres, J.S.; Biffi, G.; Brown, J.C.; Caan, B.J.; Cespedes Feliciano, E.M.; Coll, A.P.; Dunne, R.F.; Goncalves, M.D.; et al. Cachexia: A systemic consequence of progressive, unresolved disease. Cell 2023, 186, 1824–1845. [Google Scholar] [CrossRef] [PubMed]
- Bozzetti, F. Screening the nutritional status in oncology: A preliminary report on 1000 outpatients. Support. Care Cancer 2009, 17, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Morena da Silva, F.; Lim, S.; Cabrera, A.R.; Schrems, E.R.; Jones, R.G.; Rosa-Caldwell, M.E.; Washington, T.A.; Murach, K.A.; Greene, N.P. The time-course of cancer cachexia onset reveals biphasic transcriptional disruptions in female skeletal muscle distinct from males. BMC Genom. 2023, 24, 374. [Google Scholar] [CrossRef]
- Blum, D.; Stene, G.B.; Solheim, T.S.; Fayers, P.; Hjermstad, M.J.; Baracos, V.E.; Fearon, K.; Strasser, F.; Kaasa, S. Validation of the Consensus-Definition for Cancer Cachexia and evaluation of a classification model—A study based on data from an international multicentre project (EPCRC-CSA). Ann. Oncol. 2014, 25, 1635–1642. [Google Scholar] [CrossRef]
- Paillaud, E.; Caillet, P.; Campillo, B.; Bories, P.N. Increased risk of alteration of nutritional status in hospitalized elderly patients with advanced cancer. J. Nutr. Health Aging 2006, 10, 91–95. [Google Scholar]
- Evans, W.J.; Morley, J.E.; Argiles, J.; Bales, C.; Baracos, V.; Guttridge, D.; Jatoi, A.; Kalantar-Zadeh, K.; Lochs, H.; Mantovani, G.; et al. Cachexia: A new definition. Clin. Nutr. 2008, 27, 793–799. [Google Scholar] [CrossRef]
- Fearon, K.C.; Voss, A.C.; Hustead, D.S. Definition of cancer cachexia: Effect of weight loss, reduced food intake, and systemic inflammation on functional status and prognosis. Am. J. Clin. Nutr. 2006, 83, 1345–1350. [Google Scholar] [CrossRef]
- Fearon, K.C. Cancer cachexia: Developing multimodal therapy for a multidimensional problem. Eur. J. Cancer 2008, 44, 1124–1132. [Google Scholar] [CrossRef]
- Davis, M.P.; Panikkar, R. Sarcopenia associated with chemotherapy and targeted agents for cancer therapy. Ann. Palliat. Med. 2019, 8, 86–101. [Google Scholar] [CrossRef]
- Fearon, K.; Arends, J.; Baracos, V. Understanding the mechanisms and treatment options in cancer cachexia. Nat. Rev. Clin. Oncol. 2013, 10, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Shachar, S.S.; Williams, G.R.; Muss, H.B.; Nishijima, T.F. Prognostic value of sarcopenia in adults with solid tumours: A meta-analysis and systematic review. Eur. J. Cancer 2016, 57, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Ohmori, H.; Kawahara, I.; Mori, T.; Nukaga, S.; Luo, Y.; Kishi, S.; Fujiwara-Tani, R.; Mori, S.; Goto, K.; Sasaki, T.; et al. Evaluation of Parameters for Cancer-Induced Sarcopenia in Patients Autopsied after Death from Colorectal Cancer. Pathobiology 2019, 86, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Yoneda, J.; Ohmori, H.; Sasaki, T.; Shimbo, K.; Eto, S.; Kato, Y.; Miyano, H.; Kobayashi, T.; Sasahira, T.; et al. Cancer usurps skeletal muscle as an energy repository. Cancer Res. 2014, 74, 330–340. [Google Scholar] [CrossRef]
- Martin, A.; Gallot, Y.S.; Freyssenet, D. Molecular mechanisms of cancer cachexia-related loss of skeletal muscle mass: Data analysis from preclinical and clinical studies. J. Cachexia Sarcopenia Muscle 2023, 14, 1150–1167. [Google Scholar] [CrossRef]
- Shyh-Chang, N. Metabolic Changes During Cancer Cachexia Pathogenesis. Adv. Exp. Med. Biol. 2017, 1026, 233–249. [Google Scholar]
- Luo, Y.; Fujiwara-Tani, R.; Kawahara, I.; Goto, K.; Nukaga, S.; Nishida, R.; Nakashima, C.; Sasaki, T.; Miyagawa, Y.; Ogata, R.; et al. Cancerous Conditions Accelerate the Aging of Skeletal Muscle via Mitochondrial DNA Damage. Int. J. Mol. Sci. 2024, 25, 7060. [Google Scholar] [CrossRef]
- Prado, C.M.; Purcell, S.A.; Laviano, A. Nutrition interventions to treat low muscle mass in cancer. J. Cachexia Sarcopenia Muscle 2020, 11, 366–380. [Google Scholar] [CrossRef]
- Nukaga, S.; Fujiwara-Tani, R.; Mori, T.; Kawahara, I.; Nishida, R.; Miyagawa, Y.; Goto, K.; Ohmori, H.; Fujii, K.; Sasaki, T.; et al. Effects of Antioxidant Amino Acids on Cancer Sarcopenia. Int. J. Mol. Sci. 2024, 26, 272. [Google Scholar] [CrossRef]
- Kamei, Y.; Hatazawa, Y.; Uchitomi, R.; Yoshimura, R.; Miura, S. Regulation of Skeletal Muscle Function by Amino Acids. Nutrients 2020, 12, 261. [Google Scholar] [CrossRef]
- Zaromskyte, G.; Prokopidis, K.; Ioannidis, T.; Tipton, K.D.; Witard, O.C. Evaluating the Leucine Trigger Hypothesis to Explain the Post-prandial Regulation of Muscle Protein Synthesis in Young and Older Adults: A Systematic Review. Front. Nutr. 2021, 8, 685165. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, A.; Selgas, R.; Diéz, J.J.; Bajo, M.A.; Codoceo, R.; Alvarez, V. Anorexia in end-stage renal disease: Pathophysiology and treatment. Expert. Opin. Pharmacother. 2001, 2, 1825–1838. [Google Scholar] [PubMed]
- Cochet, C.; Belloni, G.; Buondonno, I.; Chiara, F.; D’Amelio, P. The Role of Nutrition in the Treatment of Sarcopenia in Old Patients: From Restoration of Mitochondrial Activity to Improvement of Muscle Performance, a Systematic Review. Nutrients 2023, 15, 3703. [Google Scholar] [CrossRef] [PubMed]
- Le Couteur, D.G.; Solon-Biet, S.M.; Cogger, V.C.; Ribeiro, R.; de Cabo, R.; Raubenheimer, D.; Cooney, G.J.; Simpson, S.J. Branched chain amino acids, aging and age-related health. Ageing Res. Rev. 2020, 64, 101198. [Google Scholar] [CrossRef]
- Laviano, A.; Muscaritoli, M.; Cascino, A.; Preziosa, I.; Inui, A.; Mantovani, G.; Rossi-Fanelli, F. Branched-chain amino acids: The best compromise to achieve anabolism? Curr. Opin. Clin. Nutr. Metab. Care 2005, 8, 408–414. [Google Scholar] [CrossRef]
- Nukaga, S.; Mori, T.; Miyagawa, Y.; Fujiwara-Tani, R.; Sasaki, T.; Fujii, K.; Mori, S.; Goto, K.; Kishi, S.; Nakashima, C.; et al. Combined administration of lauric acid and glucose improved cancer-derived cardiac atrophy in a mouse cachexia model. Cancer Sci. 2020, 111, 4605–4615. [Google Scholar] [CrossRef]
- Mori, T.; Ohmori, H.; Luo, Y.; Mori, S.; Miyagawa, Y.; Nukaga, S.; Goto, K.; Fujiwara-Tani, R.; Kishi, S.; Sasaki, T.; et al. Giving combined medium-chain fatty acids and glucose protects against cancer-associated skeletal muscle atrophy. Cancer Sci. 2019, 110, 3391–3399. [Google Scholar] [CrossRef]
- Nishida, R.; Nukaga, S.; Kawahara, I.; Miyagawa, Y.; Goto, K.; Nakashima, C.; Luo, Y.; Sasaki, T.; Fujii, K.; Ohmori, H.; et al. Differential Effects of Three Medium-Chain Fatty Acids on Mitochondrial Quality Control and Skeletal Muscle Maturation. Antioxidants 2024, 13, 821. [Google Scholar] [CrossRef]
- Liu, S.; Li, L.; Lou, P.; Zhao, M.; Wang, Y.; Tang, M.; Gong, M.; Liao, G.; Yuan, Y.; Li, L.; et al. Elevated branched-chain α-keto acids exacerbate macrophage oxidative stress and chronic inflammatory damage in type 2 diabetes mellitus. Free Radic. Biol. Med. 2021, 175, 141–154. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, H.; Chi, M.; Wang, Y.; Zhu, X.; Han, L.; Xin, B.; Gan, R.; Tu, Y.; Sun, X.; et al. Bckdk-Mediated Branch Chain Amino Acid Metabolism Reprogramming Contributes to Muscle Atrophy during Cancer Cachexia. Mol. Nutr. Food Res. 2024, 68, e2300577. [Google Scholar] [CrossRef]
- Paxton, R.; Harris, R.A. Regulation of branched-chain α-ketoacid dehydrogenase kinase. Arch. Biochem. Biophys. 1984, 231, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, Y.; Murakami, T.; Nakai, N.; Nagasaki, M.; Harris, R.A. Exercise promotes BCAA catabolism: Effects of BCAA supplementation on skeletal muscle during exercise. J. Nutr. 2004, 134 (Suppl. 6), 1583S–1587S. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, S.; Kim, H.K.; Hirooka, R.; Tanaka, M.; Shimoda, T.; Chijiki, H.; Kojima, S.; Sasaki, K.; Takahashi, K.; Makino, S.; et al. Distribution of dietary protein intake in daily meals influences skeletal muscle hypertrophy via the muscle clock. Cell Rep. 2021, 36, 109336. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, D.L.; Delcastillo, K.; Van Every, D.W.; Tipton, K.D.; Aragon, A.A.; Schoenfeld, B.J. Isolated Leucine and Branched-Chain Amino Acid Supplementation for Enhancing Muscular Strength and Hypertrophy: A Narrative Review. Int. J. Sport. Nutr. Exerc. Metab. 2021, 31, 292–301. [Google Scholar] [CrossRef]
- Rieu, I.; Balage, M.; Sornet, C.; Giraudet, C.; Pujos, E.; Grizard, J.; Mosoni, L.; Dardevet, D. Leucine supplementation improves muscle protein synthesis in elderly men independently of hyperaminoacidaemia. J. Physiol. 2006, 575 Pt 1, 305–315. [Google Scholar] [CrossRef]
- Liu, X.; Wu, J.; Tang, J.; Xu, Z.; Zhou, B.; Liu, Y.; Hu, F.; Zhang, G.; Cheng, R.; Xia, X.; et al. Prevotella copri alleviates sarcopenia via attenuating muscle mass loss and function decline. J. Cachexia Sarcopenia Muscle 2023, 14, 2275–2288. [Google Scholar] [CrossRef]
- Tanada, Y.; Shioi, T.; Kato, T.; Kawamoto, A.; Okuda, J.; Kimura, T. Branched-chain amino acids ameliorate heart failure with cardiac cachexia in rats. Life Sci. 2015, 137, 20–27. [Google Scholar] [CrossRef]
- Holeček, M. Branched-chain amino acids in health and disease: Metabolism, alterations in blood plasma, and as supplements. Nutr. Metab. 2018, 15, 33. [Google Scholar] [CrossRef]
- Argiles, J.; Costelli, P.; Carbo, N.; Lopezsoriano, F. Branched-chain amino acid catabolism and cancer cachexia (review). Oncol. Rep. 1996, 3, 687–690. [Google Scholar] [CrossRef]
- Harris, R.A.; Kobayashi, R.; Murakami, T.; Shimomura, Y. Regulation of branched-chain α-keto acid dehydrogenase kinase expression in rat liver. J. Nutr. 2001, 131, 841S–845S. [Google Scholar] [CrossRef]
- Shimi, G.; Zand, H.; Pourvali, K.; Ghorbani, A. Colorectal cancer causes alteration of thyroid hormone profile in newly diagnosed patients. Expert. Rev. Endocrinol. Metab. 2021, 16, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Petranović Ovčariček, P.; Deandreis, D.; Giovanella, L. Thyroid dysfunctions induced by molecular cancer therapies: A synopsis for nuclear medicine thyroidologists. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3355–3360. [Google Scholar] [CrossRef] [PubMed]
- Ohmori, H.; Luo, Y.; Kuniyasu, H. Non-histone nuclear factor HMGB1 as a therapeutic target in colorectal cancer. Expert. Opin. Ther. Targets 2011, 15, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Bloomgarden, Z. Diabetes and branched-chain amino acids: What is the link? J. Diabetes 2018, 10, 350–352. [Google Scholar] [CrossRef]
- Yu, J.Y.; Cao, N.; Rau, C.D.; Lee, R.P.; Yang, J.; Flach, R.J.R.; Petersen, L.; Zhu, C.; Pak, Y.L.; Miller, R.A.; et al. Cell-autonomous effect of cardiomyocyte branched-chain amino acid catabolism in heart failure in mice. Acta Pharmacol. Sin. 2023, 44, 1380–1390. [Google Scholar] [CrossRef]
- Blackburn, P.R.; Gass, J.M.; Vairo, F.P.E.; Farnham, K.M.; Atwal, H.K.; Macklin, S.; Klee, E.W.; Atwal, P.S. Maple syrup urine disease: Mechanisms and management. Appl. Clin. Genet. 2017, 10, 57–66. [Google Scholar] [CrossRef]
- Bhat, S.M.; Massey, N.; Shrestha, D.; Karriker, L.A.; Jelesijević, T.; Wang, C.; Charavaryamath, C. Transcriptomic and ultrastructural evidence indicate that anti-HMGB1 antibodies rescue organic dust-induced mitochondrial dysfunction. Cell Tissue Res. 2022, 388, 373–398. [Google Scholar] [CrossRef]
- Hasegawa, A.; Iwasaka, H.; Hagiwara, S.; Asai, N.; Nishida, T.; Noguchi, T. Alternate day calorie restriction improves systemic inflammation in a mouse model of sepsis induced by cecal ligation and puncture. J. Surg. Res. 2012, 174, 136–141. [Google Scholar] [CrossRef]
- Tohme, S.; Yazdani, H.O.; Liu, Y.; Loughran, P.; van der Windt, D.J.; Huang, H.; Simmons, R.L.; Shiva, S.; Tai, S.; Tsung, A. Hypoxia mediates mitochondrial biogenesis in hepatocellular carcinoma to promote tumor growth through HMGB1 and TLR9 interaction. Hepatology 2017, 66, 182–197. [Google Scholar] [CrossRef]
- Narasimhulu, C.A.; Singla, D.K. BMP-7 Attenuates Sarcopenia and Adverse Muscle Remodeling in Diabetic Mice via Alleviation of Lipids, Inflammation, HMGB1, and Pyroptosis. Antioxidants 2023, 12, 331. [Google Scholar] [CrossRef]
- Ho, T.L.; Tang, C.H.; Chang, S.L.; Tsai, C.H.; Chen, H.T.; Su, C.M. HMGB1 Promotes In Vitro and In Vivo Skeletal Muscle Atrophy through an IL-18-Dependent Mechanism. Cells 2022, 11, 3936. [Google Scholar] [CrossRef] [PubMed]
- Nukaga, S.; Fujiwara-Tani, R.; Nishida, R.; Miyagawa, Y.; Goto, K.; Kawahara, I.; Nakashima, C.; Fujii, K.; Ogata, R.; Ohmori, H.; et al. Caprylic Acid Inhibits High Mobility Group Box-1-Induced Mitochondrial Damage in Myocardial Tubes. Int. J. Mol. Sci. 2024, 25, 8081. [Google Scholar] [CrossRef] [PubMed]
- White, P.J.; McGarrah, R.W.; Grimsrud, P.A.; Tso, S.C.; Yang, W.H.; Haldeman, J.M.; Grenier-Larouche, T.; An, J.; Lapworth, A.L.; Astapova, I.; et al. The BCKDH Kinase and Phosphatase Integrate BCAA and Lipid Metabolism via Regulation of ATP-Citrate Lyase. Cell Metab. 2018, 27, 1281–1293.e7. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Price, S.R. Differential regulation of branched-chain α-ketoacid dehydrogenase kinase expression by glucocorticoids and acidification in LLC-PK1-GR101 cells. Am. J. Physiol. Renal Physiol. 2004, 286, F504–F508. [Google Scholar] [CrossRef][Green Version]
- Ferguson, D.; Eichler, S.J.; Yiew, N.K.H.; Colca, J.R.; Cho, K.; Patti, G.J.; Shew, T.M.; Lutkewitte, A.J.; Mukherjee, S.; McCommis, K.S.; et al. Mitochondrial pyruvate carrier inhibition initiates metabolic crosstalk to stimulate branched chain amino acid catabolism. Mol. Metab. 2023, 70, 101694. [Google Scholar] [CrossRef]
- Arp, N.L.; Seim, G.L.; Votava, J.A.; Josephson, J.; Fan, J. Reactive nitrogen species inhibit branched chain α-ketoacid dehydrogenase complex and impact muscle cell metabolism. J. Biol. Chem. 2023, 299, 105333. [Google Scholar] [CrossRef]
- Li, Y.; Xiong, Z.; Yn, W.; Gao, E.; Cheng, H.; Wu, G.; Liu, Y.; Zhang, L.; Li, C.; Wang, S.; et al. Branched chain amino acids exacerbate myocardial ischemia/reperfusion vulnerability via enhancing GCN2/ATF6/PPAR-α pathway-dependent fatty acid oxidation. Theranostics 2020, 10, 5623–5640. [Google Scholar] [CrossRef]




| Component | Medium | ||
|---|---|---|---|
| D-MEM | Ascites Added 1) | CM Added 2) | |
| Glucose (mg/dL) | 450 ± 2 | 361 ± 8 | 378 ± 6 |
| Pyruvate (mg/dL) | 11 ± 0.1 | 9 ± 1 | 9 ± 1 |
| Glutamine (mg/dL) | 58 ± 0.2 | 49 ± 4 | 50 ± 3 |
| Lactate (pmol) | 0 | 7.4 ± 1.4 | 1.2 ± 0.2 |
| HMGB1 (μg/mL) | ND | 18 ± 0.9 | ND |
| TNFα (pg/mL) | ND | 12 ± 0.2 | ND |
| Ingredient | Control Diet | BCAA Diet | BCAA + C8 Diet |
|---|---|---|---|
| Moisture (%) | 8.83 | 8.57 | 8.66 |
| Crude protein (%) | 25.13 | 24.38 | 24.65 |
| Crude fat (%) | 4.92 | 4.77 | 4.65 |
| Crude fiber (%) | 4.42 | 4.28 | 4.21 |
| Crude ash (%) | 6.86 | 6.65 | 6.54 |
| NFE (%) | 49.84 | 48.34 | 47.35 |
| Valine (%) | - | 1 | 1 |
| Laucine (%) | - | 1 | 1 |
| Isoleucine (%) | - | 1 | 1 |
| Energy (kcal) | 334.2 | 345.874 | 371.85 |
| Gene | Accession No. | Upper Primer | Lower Primer |
|---|---|---|---|
| PGC1A | BC156323.1 | aaggatgcgctctcgttcaa | ttcgtttgacctgcgcaaag |
| BDK | CR542093.1 | ctcggtacctgcagcaagaa | tggcatagggatgaagggga |
| ACTB | NM_007393.5 | acaatgagctgcgtgtggcc | agggacagcacagcctggat |
| Target | Cat. No. | Company | Adderess |
| Antibodies | |||
| BCKD | ab126173 | Abcam | Cambridge, MA, USA |
| phospho-BCKD, pS293 | ab200577 | Abcam | Cambridge, MA, USA |
| BDK | ab128935 | Abcam | Cambridge, MA, USA |
| β-Actin | sc-47778 | Santa-Cruz | Dallas, TX, USA |
| ELISA kit | |||
| MYL1 | CSB-EL015305MO | Cusabio Biotech | Houston, TX, USA |
| HMGB1 | 326078738 | Shino Test | Sagamihara, Japan |
| Mouse TNF-α | MTA00B | R&D Systems | Minneapolis, MN, USA |
| GSH/GSSG | CB-P050-K | Creative Biolabs | Shirley, NY, USA |
| 4HNE | STA-838 | Cell Biolabs | San Diego, CA, USA |
| BCAA | MET-5056 | Cell Biolabs | San Diego, CA, USA |
| AcCoA | RE10014 | Reed Biotech | Wuhan, China |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawahara, I.; Fujiwara-Tani, R.; Mori, T.; Nukaga, S.; Nishida, R.; Miyagawa, Y.; Goto, K.; Ohmori, H.; Fujii, K.; Luo, Y.; et al. Caprylic Acid Restores Branched-Chain Amino Acid Metabolism in a Mouse Cachexia Model. Curr. Issues Mol. Biol. 2025, 47, 325. https://doi.org/10.3390/cimb47050325
Kawahara I, Fujiwara-Tani R, Mori T, Nukaga S, Nishida R, Miyagawa Y, Goto K, Ohmori H, Fujii K, Luo Y, et al. Caprylic Acid Restores Branched-Chain Amino Acid Metabolism in a Mouse Cachexia Model. Current Issues in Molecular Biology. 2025; 47(5):325. https://doi.org/10.3390/cimb47050325
Chicago/Turabian StyleKawahara, Isao, Rina Fujiwara-Tani, Takuya Mori, Shota Nukaga, Ryoichi Nishida, Yoshihiro Miyagawa, Kei Goto, Hitoshi Ohmori, Kiyomu Fujii, Yi Luo, and et al. 2025. "Caprylic Acid Restores Branched-Chain Amino Acid Metabolism in a Mouse Cachexia Model" Current Issues in Molecular Biology 47, no. 5: 325. https://doi.org/10.3390/cimb47050325
APA StyleKawahara, I., Fujiwara-Tani, R., Mori, T., Nukaga, S., Nishida, R., Miyagawa, Y., Goto, K., Ohmori, H., Fujii, K., Luo, Y., Sasaki, T., Nakashima, C., Ogata, R., & Kuniyasu, H. (2025). Caprylic Acid Restores Branched-Chain Amino Acid Metabolism in a Mouse Cachexia Model. Current Issues in Molecular Biology, 47(5), 325. https://doi.org/10.3390/cimb47050325





