Abstract
Sorafenib is currently the first-line therapeutic agent for advanced hepatocellular carcinoma (HCC). However, sorafenib resistance remains a major clinical challenge. Studies have reported that 1,25-dihydroxyvitamin D3 (1,25(OH)2D3) can synergize with multiple chemotherapeutic drugs to enhance their antitumor efficacy, but the combinatorial effect between 1,25(OH)2D3 and sorafenib has not yet been investigated. This study aimed to investigate the potential molecular mechanism by which 1,25(OH)2D3 reverses sorafenib resistance in hepatocellular carcinoma using network pharmacology, molecular docking, and experimental validation. We predicted a web-based pharmacological approach to predict potential targets of 1,25(OH)2D3 and its derivatives, as well as sorafenib resistance genes in hepatocellular carcinoma from public databases. We then constructed 1,25(OH)2D3 chemo-sensitizing expression profiles through intersection analysis. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses were employed to predict the potential pathways involved in 1,25(OH)2D3 chemosensitization, followed by molecular docking analysis and analysis of molecular dynamics simulations. Finally, experimental validation were conducted to elucidate the potential mechanisms by which 1,25(OH)2D3 enhances the sensitivity of HCC to sorafenib. Compound and target screening identified 730 predicted targets of 1,25(OH)2D3 and its derivatives, 1144 genes associated with sorafenib resistance in hepatocellular carcinoma, and 56 potential chemosensitization targets from the intersection analysis. KEGG analysis suggested that the chemosensitization effect of 1,25(OH)2D3 might be mediated by the FoxO signaling pathway. Molecular docking showed that both 1,25(OH)2D3 and its derivatives could stably bind to FOXO3A, a key gene in the FoxO family, and molecular dynamics simulation analysis further indicated that the two bind well together. In vitro experiments demonstrated the synergistic effects of 1,25(OH)2D3 and sorafenib, significantly inhibiting the viability and colony formation rate of sorafenib-resistant hepatocellular carcinoma cells. Additionally, the combination treatment promoted apoptosis and inhibited autophagy. Furthermore, the combination modulated the FOXO3A/FOXM1 signaling axis. This study reveals that 1,25(OH)2D3 enhances the chemosensitivity of hepatocellular carcinoma (HCC) to sorafenib, with underlying mechanisms potentially involving the targeted modulation of the FOXO3A/FOXM1 signaling axis and the reversal of sorafenib-resistant phenotypes through the regulation of apoptotic and autophagic pathways.
1. Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors in the world, with a high mortality rate, posing a serious threat to human health globally [1]. Because the symptoms and signs of early HCC are atypical and difficult to detect, more than 50% of HCC patients are diagnosed at the middle or late stages of the disease [1,2]. Sorafenib is a multi-targeted tyrosine kinase inhibitor that promotes apoptosis, attenuates angiogenesis, and inhibits tumor cell proliferation, potentially improving overall survival in patients with intermediate and advanced HCC [3]. However, liver cancer cells can develop resistance to sorafenib through various mechanisms, resulting in limited long-term benefits. Typically, patients become resistant to sorafenib treatment within six months [4].
1,25-dihydroxyvitamin D3 (1,25(OH)2D3, calcitriol) is the active form of vitamin D3, widely recognized for its role in maintaining calcium and phosphate homeostasis [5]. In addition to its classical effects on bones, kidneys, and the intestinal tract, 1,25(OH)2D3 and its analogs have been extensively studied for their antitumor effects on various types of cancer [6,7]. Research conducted in the United States has shown that 1,25(OH)2D3 and its analogs can enhance the inhibitory effects of drugs in cancer treatment when used in combination with other agents [8]. For instance, Ma et al. demonstrated that 1,25(OH)2D3 sensitizes squamous cell carcinoma cells to cisplatin-induced cytotoxicity and apoptosis using both in vivo and in vitro models [9]. Hershberger et al., in vitro, showed that pretreatment with 1,25(OH)2D3 enhances the growth-inhibitory effects of paclitaxe [10]. Additionally, independent studies have reported that combining 1,25(OH)2D3 with various tyrosine kinase inhibitors (TKIs) such as gefitinib/erlotinib (EGFR inhibitors) and sunitinib (a multi-targeted TKI) induces the differentiation of AML cells [11]. Our previous studies have demonstrated that 1,25(OH)2D3 inhibits the proliferation of hepatocellular carcinoma cells. However, no research has yet reported the relationship between 1,25(OH)2D3 and sorafenib resistance in liver cancer. Therefore, this study combined 1,25(OH)2D3 with sorafenib to investigate whether 1,25(OH)2D3 could enhance the chemosensitivity of sorafenib in hepatocellular carcinoma treatment.
Network pharmacology allows for the exploration of the mechanisms by which compounds treat diseases [12]. Integrated network pharmacology approaches combined with Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis and bioinformatics interrogation demonstrated that 1,25(OH)2D3 may potentiate sorafenib chemosensitivity in HCC through the modulation of the FoxO signaling pathway, with FOXO3A identified as a pivotal regulatory mediator, a key protein in the FoxO signaling pathway and a member of the Forkhead family of transcription factors; it plays a crucial role in liver cancer, breast cancer, colon cancer, and other cancers [13]. Located in the nucleus, FOXO3A actively mediates various cellular processes such as apoptosis induction through the transcription of its target genes in their non-phosphorylated form [14], as well as processes related to proliferation [15] and the cell cycle [16]. In addition, FOXO3A plays a crucial role in various aspects of cancer progression and drug resistance. Recent studies have indicated that dysregulation of FOXO3A promotes drug resistance through multiple mechanisms, including the regulation of oncogenic signaling pathways, evasion of apoptosis, and induction of autophagy. Therefore, we hypothesize that FOXO3A dysregulation may contribute to sorafenib resistance in hepatocellular carcinoma by influencing apoptosis and autophagy processes.
An integrated methodology combining network pharmacology, molecular docking, and in vitro experimental validation was implemented to preliminarily investigate the molecular targets and mechanistic basis underlying the 1,25(OH)2D3-mediated enhancement of sorafenib chemosensitivity in hepatocellular carcinoma.
2. Materials and Methods
2.1. GEO Microarray and Acquisition of Sorafenib-Resistant Targets in Hepatocellular Carcinoma
The Gene Expression Omnibus (GEO) database, curated by the National Center for Biotechnology Information (NCBI), serves as a comprehensive repository for high-throughput omics data, housing extensive datasets pertinent to tumor drug resistance mechanisms. The keyword “liver cancer sorafenib resistance” was utilized in the GEO database [17]. The dataset GSE94550 was downloaded from http://www.ncbi.nlm.nih.gov/geo/ (accessed on 20 January 2024) and analyzed using GEO2R to identify differentially expressed genes. A p-value threshold of less than 0.05 and |logFC| greater than 1 were employed as criteria for the up- and down-regulation of the identified genes. The results of differentially expressed genes were visualized through the Xiantao Online website and presented in volcano plots.
2.2. Target Prediction for 1,25(OH)2D3 and Its Derivatives
1,25(OH)2D3 and its derivatives were obtained in SMILE format from the PubChem database [18] (https://pubchem.ncbi.nlm.nih.gov (accessed on 20 January 2024). Swiss Target Prediction [19] was used to predict targets for 1,25(OH)2D3 and its derivatives. Targets with a prediction probability > 0.1 in the Swiss Target Prediction Database were selected, and duplicates were removed to obtain the final targets.
2.3. Prediction of Potential Targets for 1,25(OH)2D3 Chemosensitization
Venn analysis was performed using the Xanadu Online website to analyze the predicted targets of 1,25(OH)2D3 and its derivatives along with genes associated with sorafenib resistance in hepatocellular carcinoma. The intersection of these sets was considered a potential target for reversing sorafenib resistance by 1,25(OH)2D3 and its derivatives.
2.4. Biofunctional Analysis
The identified potential targets were analyzed through Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) using the DAVID database (https://david.ncifcrf.gov/ (accessed on 21 January 2024), an online bioinformatics platform. This analysis aimed to acquire comprehensive functional annotation details for the targets. Annotations were considered to be significantly enriched when they met a significance threshold of p < 0.05. To present the enrichment results in a more intuitive way, the bioinformatics online analysis website https://www.bioinformatics.com.cn (accessed on 21 January 2024) was employed for visualization.
2.5. Construction of “Compound–Target” and “Compound–Target–Pathway” Network Diagrams
The data, comprising 1,25(OH)2D3, its derivatives, predicted targets, and enriched pathways, were imported into Cytoscape v3.7.2 for network visualization. We constructed two interactive networks: a compound–target interaction network and a compound–target–pathway association network. In the graphical representation, each constituent, target gene and pathway was described by node, and the interactions were encoded by edges.
2.6. Molecular Docking
The 3D structure of FOXO3A was retrieved from the PDB database https://www.rcsb.org/ (accessed on 2 March 2024), and the target proteins were pre-treated by desolvation and the removal of small organic molecules using PyMOL software. The 2D structures of 1,25(OH)2D3 and sorafenib were obtained from the PubChem database https://pubchem.ncbi.nlm.nih.gov/ (accessed on 2 March 2024), and these small-molecule structures were converted into 3D structures with energy minimization using Chem3D Ultra 14.0 software. AutoDock 4.2.6 software [20] was employed to add hydrogen atoms to both proteins and small molecules, and molecular docking was conducted after setting rotatable bonds for the small molecules. Binding free energy (ΔG) serves as the central quantitative metric for evaluating the stability of ligand–receptor complexes, a process where more negative values correlate with stronger binding affinity and enhanced complex stabilization [21]. A binding energy ≤ −7 kcal/mol [22,23,24] was generally used as a screening criterion to evaluate the binding stability of FOXO3A with 1,25(OH)2D3 and sorafenib, and the lowest binding energy model was selected for the visualization and analysis by PyMOL 2.4.0 software.
2.7. Molecular Dynamics Simulations
We performed 100 ns MD simulations of the complexes using Gromacs 2023 software. The CHARMM 36 [25] force field parameters were used for the protein and the ligand topology was constructed from the GAFF2 force field parameters. Periodic boundary conditions were used and the protein–ligand complexes were placed in cubic boxes. The TIP3P water model [26] was used to fill the box with water molecules. Electrostatic interactions were treated using the Particle Mesh Ewald (PME) and Verlet algorithms, respectively. Subsequently, 100,000 steps of isothermal isovolumic systematic equilibrium and isothermal isobaric systematic equilibrium were performed with a coupling constant of 0.1 ps and a duration of 100 ps simulation. Both van der Waals and Coulomb interactions were calculated using a cut-off value of 1.0 nm. Finally, the system was subjected to molecular dynamics simulations using Gromacs 2023 at constant temperature (300 K) and constant pressure (1 bar) for a total duration of 100 ns.
2.8. Cell Culture
Hepatocellular carcinoma parental cells, Huh7, and sorafenib-resistant hepatocellular carcinoma cells, Huh7/s, were obtained from the Cell Bank Type Culture Preservation Centre of OLU Bioscience and Technology Co., Ltd. (Shanghai, China). The cells were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum and 1% penicillin–streptomycin solution (100×). They were incubated under standard conditions (37 °C, 5% CO2, 95% humidity), and experiments were conducted when the cells reached 80–90% confluence. The drug resistance of Huh7/s cells was maintained by the addition of 4.3 μM sorafenib. Sorafenib (HY-10201) and 1,25(OH)2D3 (calcitriol, HY-10002) were procured from MedChemExpress (Monmouth Junction, NJ, USA).
2.9. CCK8 Assay and Combination Index Calculations
Cell proliferation assays were performed using the CCK-8 assay [27]. Huh7/s cells were seeded into 96-well plates at a density of 1 × 105 cells per well. After cell attachment, Huh7/s cells were treated with sorafenib or 1,25(OH)2D3 individually or in combination for 48 h. Following the addition of 10 μL of CCK-8 reagent (HY-K0301, MedChemExpress) to each well, plates were further incubated for 2 h. Optical density (OD) values of each well were measured at 450 nm using a spectrophotometer. The half-maximal inhibitory concentration (IC50) of sorafenib or 1,25(OH)2D3 was calculated using GraphPad 9.5 Software (San Diego, CA, USA), and cell proliferation curves were generated. The synergistic index of the drugs (represented by the combination index q-value) was calculated using Kim’s formula [28,29,30,31], where EA and EB denote the inhibition rates of proliferation when drugs A and B are used individually, and E(A + B) denotes the inhibition rate when drugs A and B are used in combination. A q-value > 1.15 indicates synergism, q = 0.85~1.15 indicates an additive effect, and q < 0.85 indicates antagonism.
2.10. Cell Clone Formation Experiments
Huh7/s cells were seeded into 6-well plates at a density of 5 × 102 cells per well. After 4 days of culture, the cells were treated with 4.3 μM sorafenib, 5 μM 1,25(OH)2D3, or their combination for 48 h. Subsequently, the medium was replaced with complete fresh medium and incubated for 12 days. Cells were washed twice with phosphate-buffered saline (PBS), fixed with 4% paraformaldehyde for 30 min, and stained with crystal violet solution after two additional PBS washes. Plates were air-dried and photographed for colony visualization, and the number of visible colonies was quantified using ImageJ 2 software (National Institutes of Health, Bethesda, MD, USA).
2.11. Apoptosis and Cell Cycle Analysis
Huh7/s cells were treated with 4.3 μM sorafenib and 5 μM 1,25(OH)2D3, either alone or in combination, for 48 h. Following treatment, the cells were harvested, washed with PBS, and subjected to Annexin V-FITC/PI staining according to the manufacturer’s instructions. Labeled cells were analyzed by flow cytometry to assess apoptotic changes in Huh7/s cells. This experiment was independently repeated three times.
Additionally, Huh7/s cells were treated with 4.3 μM sorafenib and 5 μM 1,25(OH)2D3 alone or in combination for 48 h. Cells were collected and washed with PBS, then resuspended in pre-cooled 70% ethanol and fixed at −20 °C overnight. After centrifugation to remove the ethanol residue and a subsequent wash with PBS, the cells were stained with propidium iodide (PI) and incubated at 4 °C for 30 min in the dark. Cell cycle phase distribution was assessed by flow cytometry, with three independent experiments conducted.
2.12. Monodansylcadaverine (MDC) Staining
Huh7/s cells were seeded into 12-well plates and treated with 4.3 μM sorafenib, 5 μM 1,25(OH)2D3, or their combination for 48 h. Following treatment, the medium was aspirated, and cells were washed once with 1× wash buffer. An appropriate volume of monodansyl cadaverine (MDC) staining solution was then added, and cells were incubated at room temperature for 15–45 min in the dark. After staining, cells were washed twice with 1× wash buffer and immediately observed under a fluorescence microscope (excitation filter: 355 nm; emission filter: 512 nm) for image acquisition.
2.13. Transmission Electron Microscopy
After the treatment of Huh7/s cells with 4.3 μM sorafenib and 5 μM 1,25(OH)2D3 alone or in combination, respectively, for 48 h, the cells were fixed in 2.5% glutaraldehyde solution and refixed in 1% osmium tetroxide. They were then dehydrated in 30%, 50%, 70%, 80%, 90%, 95% and 100% acetone. Embedding was carried out using Epon-812 pure embedding agent. Ultrathin sections of 60~90 nm in size were made in an ultrathin sectioning machine and fished onto a copper mesh and stained, firstly with uranyl acetate for 10~15 min, and then with lead citrate for 1~2 min at room temperature. Finally, the sections were observed under an electron microscope (HITACHI).
2.14. Western Blot
After 48 h of treatment with sorafenib and 1,25(OH)2D3, either alone or in combination, the cells were lysed with a radioimmunoprecipitation assay (RIPA) lysis buffer, and the supernatant was collected following centrifugation. The BCA protein assay kit, obtained from Biyuntian Biotechnology Research Institute (Shanghai, China), was used to estimate the protein concentration of the cells. Subsequently, SDS-PAGE electrophoresis was performed, and the proteins were transferred to a PVDF membrane, which was then blocked on a shaker at room temperature with 5% skimmed milk powder for 2 h. After blocking, the membrane was washed and incubated overnight at 4 °C with antibodies against key target proteins, diluted according to the manufacturer’s instructions. The primary antibodies used included anti-FOXO3A (ab109629, Abcam, Cambridge, UK), anti-p-FOXO3A (ab154786, Abcam, Cambridge, UK), anti-LC3B (2775S, CST, Danvers, MA, USA), anti-FOXM1 (13147-1-AP, Proteintech, Wuhan, China), anti-Bcl-2 (60178-1-IG, Proteintech, Wuhan, China), anti-Bax (60267-1-IG, Proteintech, Wuhan, China), anti-P62 (18420-1-AP, Proteintech, Wuhan, China), and anti-Beclin-1 (11306-1-AP, Proteintech, Wuhan, China). On the following day, the membrane was washed again, and HRP-labeled goat anti-rabbit IgG secondary antibody was added, followed by incubation at room temperature for 1 h. ECL detection was performed, and the results were analyzed using ImageJ software.
2.15. Statistical Methods
For the statistical examinations, the GraphPad software was employed. The data are showcased in the format of mean ± standard deviation (X ± SD). When it came to comparing the differences between two groups, the t-test was utilized. In contrast, for evaluating the differences among multiple groups, a one-way ANOVA was carried out. Statistical significance was determined at a significance level of p < 0.05.
3. Results
3.1. 1,25(OH)2D3 Target Prediction and Screening of Sorafenib Resistance Genes in Hepatocellular Carcinoma
The chemical structure of 1,25(OH)2D3 is depicted in Figure 1A, and its derivative structures are detailed in Table S1. Three target prediction databases—Pharm Mapper, Swiss Target Prediction, and SEA—were employed to analyze 1,25(OH)2D3 and its derivatives. After eliminating duplicates, 730 predicted targets were identified (Table S2). Network analysis and visualization of these targets were conducted using Cytoscape v3.7.2, resulting in the construction of a compound–target network diagram (Figure 1C). The dataset GSE94550 was retrieved from the GEO database and analyzed using GEO2R to identify differentially expressed genes. A total of 1167 liver cancer sorafenib resistance genes were identified based on criteria (p < 0.05, |logFC| > 1), comprising 551 up-regulated genes and 616 down-regulated genes (Table S3). Visualization of these differential genes was performed, and a gene volcano plot is illustrated in Figure 1B.
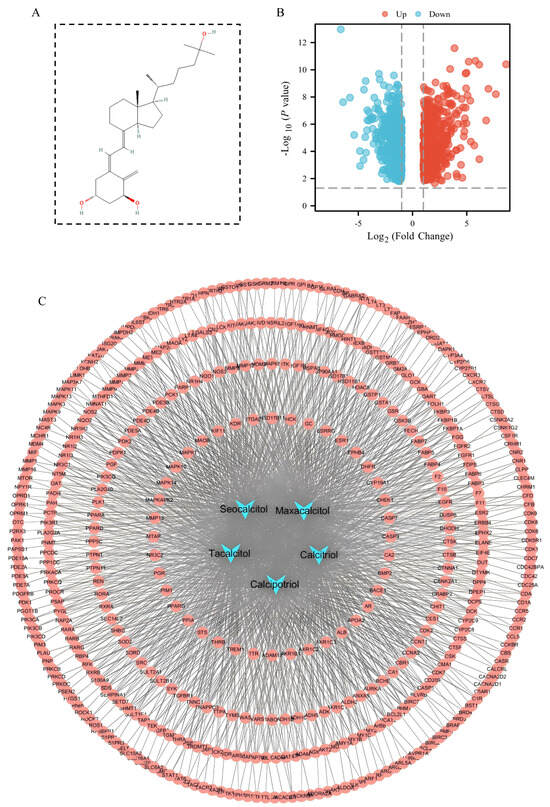
Figure 1.
1,25(OH)2D3 target prediction and screening of sorafenib resistance genes in hepatocellular carcinoma. (A) Chemical structure of 1,25(OH)2D3; (B) volcano diagram of sorafenib resistance differential genes in hepatocellular carcinoma; (C) compound–target network diagram. Blue nodes represent compounds, red nodes represent compound-predicted targets, and connecting lines represent interactions between compounds and predicted targets.
3.2. Identification of Potential Targets for Sorafenib Resistance in 1,25(OH)2D3-Treated HCC
To identify potential targets through which 1,25(OH)2D3 may act on sorafenib resistance in hepatocellular carcinoma (HCC), we analyzed the overlap between predicted targets of 1,25(OH)2D3 and its derivatives, and sorafenib resistance targets in HCC using Venn diagrams. Figure 2 illustrates that a total of 56 targets were found to overlap between the predicted targets of 1,25(OH)2D3 and its derivatives, and sorafenib-resistant targets in HCC.

Figure 2.
Venn analysis of 1,25(OH)2D3 and its derivatives’ predictive targets and sorafenib resistance genes in hepatocellular carcinoma.
3.3. GO and KEGG Analysis
In order to thoroughly explore the molecular mechanism of 1,25(OH)2D3 key targets acting on sorafenib resistance in hepatocellular carcinoma, GO analysis and KEGG analysis were performed in this study. The top 20 GO terms of BP, CC and MF (p < 0.05) are shown in Figure 3A; BP was mainly involved in phosphorus metabolism, protein modification and the regulation of molecular function, while CC was mainly related to the membrane region, plasma membrane region, cytoplasm, etc. MF was mainly involved in macromolecular complexes, metal ion binding and other biological processes. Meanwhile, KEGG pathway enrichment analysis showed (Figure 3B) that these targets were mainly related to signaling pathways such as pathways in cancer, the FoxO signaling pathway (p < 0.05, FDR < 0.25), etc. In addition, a compound–target–pathway network was constructed in this study (Figure 3C), and the results showed that these targets play a role in the drug resistance process of hepatocellular carcinoma through multiple pathways. By integrating literature studies and enrichment results, we focused on the FoxO signaling pathway. Activation of the FoxO signaling pathway plays a crucial role in tumorigenesis, chemotherapy resistance, and metabolic regulation. Additionally, FoxO is a significant class of transcription factors that participates in various essential biological processes, including the cell cycle, cell proliferation, apoptosis, and the response to oxidative stress [13].
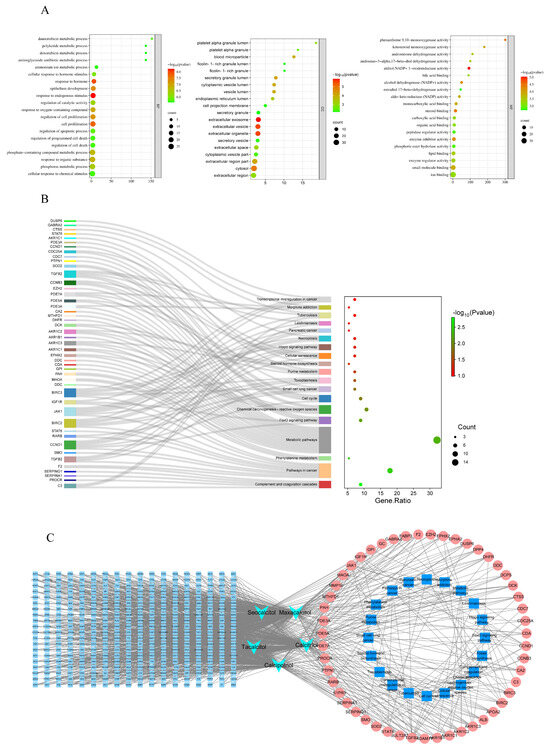
Figure 3.
GO and KEGG enrichment analysis of 56 key targets. (A). Biological process (bp), cellular component (cc) and molecular function (MF) enrichment analysis diagram; (B). KEGG analysis of 56 key targets. (C). Construction of compound–target–pathway network diagram.
3.4. Molecular Docking of Small Molecule Models with FOXO3A
A molecular docking model of 1,25(OH)2D3 and its derivatives (Maxacalcitol, Calcipotriol, Seocalcitol, Tacalcitol) with FOXO3A was constructed using AutoDock software. The results indicated that 1,25(OH)2D3 and its derivatives interacted with amino acid residues at the binding site of FOXO3A through hydrogen bonding interactions (Figure 4). Furthermore, the binding energies of FOXO3A with 1,25(OH)2D3 and its derivatives were all below −7 kcal/mol (Table 1), suggesting a high probability of stable binding between FOXO3A and these small molecule models.

Figure 4.
Molecular docking models of 1,25(OH)2D3 and its derivatives with FOXO3A. (A) Molecular docking model of 1,25(OH)2D3 with FOXO3A; (B) molecular docking model of Maxacalcitol with FOXO3A; (C) molecular docking model of Calcipotriol with FOXO3A; (D) molecular docking model of Seocalcitol with FOXO3A; (E) molecular docking model of Tacalcitol with FOXO3A (Green represents small molecule compounds, while red and blue represent proteins).

Table 1.
Binding energies of 1,25(OH)2D3 and FOXO3A.
3.5. Molecular Dynamics Simulations
To further verify the reliability of molecular docking, we performed 100 ns molecular dynamics simulations for the docking results with the highest binding energies. The root mean square deviation (RMSD) is a good indicator of the conformational stability of proteins and ligands, as well as a measure of the degree of deviation of the atomic positions from their starting positions. The smaller the deviation, the better the conformational stability. Therefore, the ligand–protein complexes were evaluated using RMSD over a 100 ns simulation period. As shown in Figure 5A, the complex systems all reached equilibrium after 80 ns and eventually fluctuated up and down at 2.7 Å. Thus, 1,25(OH)2D3 exhibited high stability when bound to the target protein FOXO3A. Further analysis revealed that the radius of gyration (Rg) value and solvent accessible surface area (SASA) of the complex system showed a slight fluctuation during the movement. It indicated that the target protein FOXO3A underwent a conformational change due to the binding of 1,25(OH)2D3 (Figure 5B,C). In addition, stable hydrogen bonds were formed between 1,25(OH)2D3 and the target protein during the kinetic process, and the number fluctuated between 0 and 4 (Figure 5D), which indicated that 1,25(OH)2D3 had a good hydrogen bonding interaction with the target protein. Root mean square fluctuation (RMSF) can indicate the flexible size of amino acid residues in proteins. As shown in Figure 5E, the RMSF values of complex systems are relatively low (mostly below 6 Å), so they are less flexible and more stable. In summary, the complex systems are stable in binding and the complexes have good hydrogen bonding. Therefore, 1,25(OH)2D3 binds well to the target protein FOXO3A.
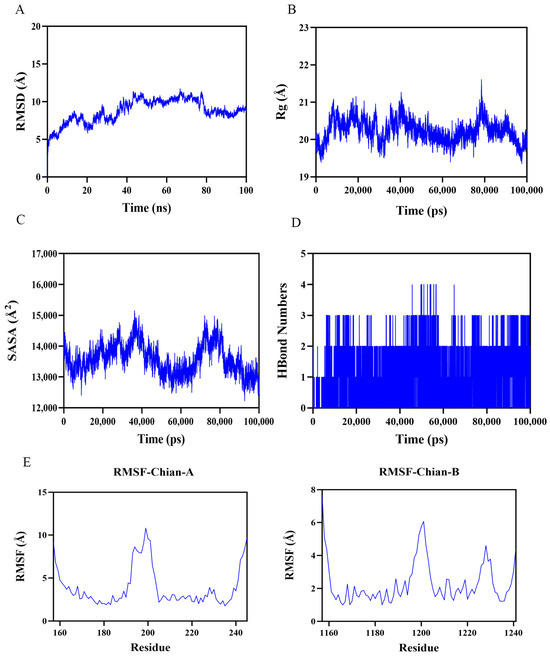
Figure 5.
Molecular dynamics simulation results showing the stability and conformational changes of the ligand–protein complexes in 100 ns simulations. (A). RMSD curves for the stability of the 1,25(OH)2D3–FOXO3A complex; (B) Rg changes in the 1,25(OH)2D3–OXO3A complex; (C) 1,25(OH)2D3–FOXO3A complex SASA variation; (D) hydrogen bonding number in 1,25(OH)2D3–FOXO3A complex; (E) PMSF curves of 1,25(OH)2D3–FOXO3A complexes.
3.6. 1,25(OH)2D3 in Combination with Sorafenib Inhibits the Proliferation of Huh7/s Cells
The cell viability data for parental Huh7 and sorafenib-resistant Huh7/s cells are presented in Figure 6A. The resistance indices of Huh7/s cells at 24, 48, and 72 h were 4.22, 4.6, and 5.68 times higher than those of Huh7, respectively, demonstrating the sorafenib resistance of Huh7/s cells. Subsequently, Huh7/s cells were treated with varying concentrations of 1,25(OH)2D3 (1.25, 2.5, 5, 10, 20 μM) for 48 h. The results shown in Figure 6B indicate that 1,25(OH)2D3 significantly inhibited the proliferation of Huh7/s cells, with all differences being statistically significant (p < 0.05). The IC50 value of 1,25(OH)2D3 was determined to be 10.38 μM (Figure S1). Further investigations involved combining 5 μM 1,25(OH)2D3 with different concentrations of sorafenib (4.3, 8.6, 17.2, 34.4, 68.8 μM). As depicted in Figure 6C, the cell viability in the combination group was markedly reduced compared to that in the sorafenib-only group. Additionally, q-values were calculated for the combination of 5 μM 1,25(OH)2D3 with different sorafenib concentrations (Table 2). The q-value analysis identified that the combination of 5 μM 1,25(OH)2D3 with 4.3 μM Sorafenib exhibited the highest efficacy; thus, this concentration combination was selected for subsequent experiments. To further evaluate the inhibitory effects of this combination on Huh7/s cells, a colony formation assay was conducted. The results depicted in Figure 6D demonstrate a significant reduction in the number of cell colonies in the combination treatment group compared to the sorafenib-only group. These findings strongly suggest that 1,25(OH)2D3 holds promise as a potential candidate for enhancing the chemosensitivity of sorafenib in hepatocellular carcinoma.
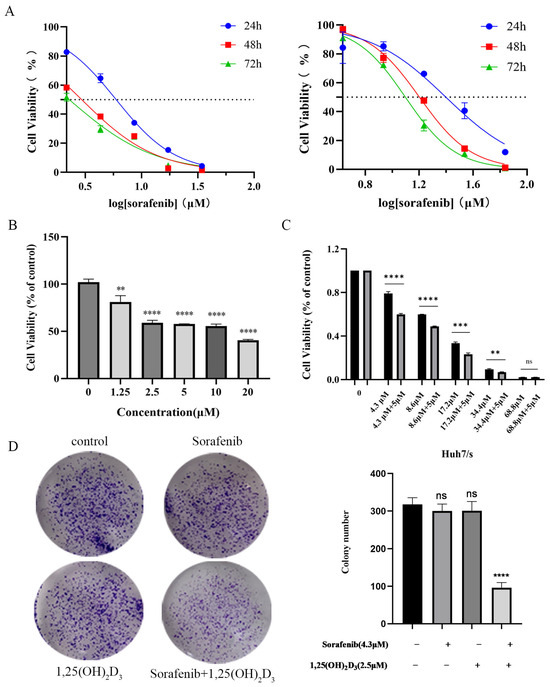
Figure 6.
1,25(OH)2D3 combined with sorafenib inhibited the proliferation of Huh7/s cells. (A) Effect of sorafenib on proliferation of Huh7 and sorafenib-resistant Huh7/s (Huh7/s) cells; (B) 1,25(OH)2D3 inhibition of the proliferation of Huh7/s cells; (C) inhibition of the proliferation of Huh7/s cells by 1,25(OH)2D3 combined with sorafenib; (D) inhibition of the clone-forming ability of Huh7/s cells by 1,25(OH)2D3 combined with sorafenib. ** p < 0.01, *** p < 0.001, and **** p < 0.0001, ns, not significant.

Table 2.
q-values of 1,25(OH)2D3 in combination with different concentrations of sorafenib.
3.7. 1,25(OH)2D3 in Combination with Sorafenib Promotes Apoptosis and Cycle Blockade in Huh7/s Cells
Flow cytometry was employed to observe the impact of 1,25(OH)2D3 combined with sorafenib on apoptosis in Huh7/s cells. The results indicated a significant increase in the apoptotic subpopulation of Huh7/s cells when treated with the combination compared to sorafenib alone (Figure 7A). Subsequently, we assessed the levels of apoptosis-related proteins via Western blot analysis. Figure 7B demonstrates a significant upregulation of the pro-apoptotic protein Bax and a notable downregulation of the apoptosis-inhibiting protein Bcl-2 in response to the combination treatment. Moreover, sorafenib is known to specifically block the G1/G0 phase of the cell cycle. Therefore, we also evaluated the effect of 1,25(OH)2D3 in combination with sorafenib on the cell cycle distribution of Huh7/s cells. Flow cytometry analysis revealed an increased G1/G0 subpopulation upon treatment with the combination (Figure 7C). Taken together, these findings suggest that 1,25(OH)2D3 may enhance chemosensitivity by promoting apoptosis and cell cycle arrest in Huh7/s cells.
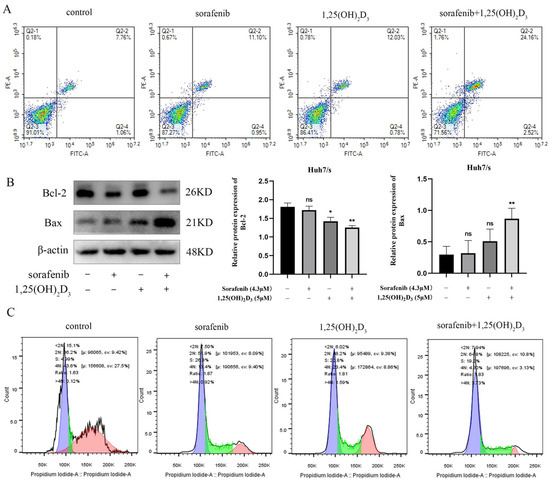
Figure 7.
Effects of 1,25(OH)2D3 combined with sorafenib on the proliferation and cycle of Huh7/s cells. (A) Flow cytometry to detect the effects of 1,25(OH)2D3 on the apoptosis of Huh7/s cells; (B) changes in apoptotic protein levels of Huh7/s cells treated with 1,25(OH)2D3 combined with sorafenib for 48 h detected by Western blot; (C) flow cytometry for detecting the effect of 1,25(OH)2D3 combined with sorafenib on the Huh7/s cell cycle. * p < 0.05, ** p < 0.01, ns, not significant.
3.8. 1,25(OH)2D3 Combined with Sorafenib Inhibits Autophagy in Huh7/s Cells
Autophagy plays a crucial role in chemoresistance, and its inhibition represents a strategy to mitigate sorafenib resistance. To investigate this, we assessed autophagy-related proteins using Western blot analysis. As depicted in Figure 8A, the expression of the autophagy marker P62 increased, while the levels of Beclin-1 and LC3B decreased compared to the control group, suggesting that the combination treatment is more effective in suppressing autophagy than single-agent therapy. Staining of autophagic vesicles using monodansyl cadaverine (MDC) (Figure 8B) and observation of autophagy using transmission electron microscopy (Figure 8C) further confirmed that the association could inhibit autophagy.
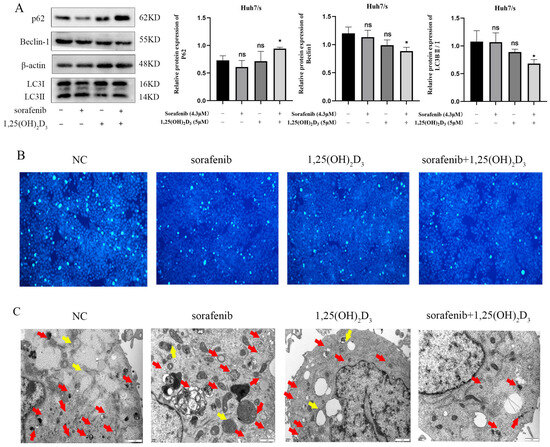
Figure 8.
Effect of 1,25(OH)2D3 in combination with sorafenib on autophagy in Huh7/s cells. (A) Changes in autophagy protein levels detected by Western blot after treatment of Huh7/s cells for 48 h with 1,25(OH)2D3 in combination with sorafenib; quantitative bar graphs were obtained by normalization using Image J. (B) 1,25(OH)2D3 in combination with sorafenib after treatment of Huh7/s cells for 48 h; autophagic vesicles were stained by the monodansyl cadaverine (MDC) method (×100, scale is 100 μm). (C) Treatment of Huh7/s cells for 48 h, with 1,25(OH)2D3 combined with sorafenib, by transmission electron microscopy (scale bar: 1 μm). (Red arrows indicate autophagosomes; yellow arrows indicate autolysosomes). * p < 0.05, ns, not significant.
3.9. Effect of 1,25(OH)2D3 Combined with Sorafenib on FOXO3A/FOXM1 Axis
FOXO3A has been implicated in tumor development and chemoresistance, which is closely linked to apoptosis and autophagy. Network pharmacology and molecular docking results highlighted significant enrichment of the FoxO signaling pathway. Moreover, FOXO3A exhibited strong binding affinity with both 1,25(OH)2D3 and sorafenib. We found that the level of FOXO3A phosphorylation was significantly reduced in the co-treated group compared with the control group by a Western blot assay (Figure 9A). FOXM1, a downstream target of FOXO3A closely associated with chemotherapy resistance, also showed reduced expression upon drug combination, as indicated by Western blotting (Figure 9B). These findings suggest that 1,25(OH)2D3 enhances sorafenib chemosensitivity in hepatocellular carcinoma by deactivating the FOXO3A/FOXM1 axis. The general mechanism diagram of this study is shown in the Figure 10.
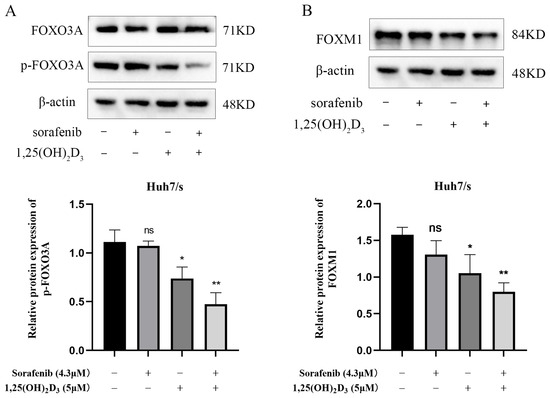
Figure 9.
Effect of 1,25(OH)2D3 combined with sorafenib on FOXO3A/FOXM1 axis. (A,B). Changes in p-FOXO3A and FOXM1 detected by Western blot after treatment of Huh7/s cells for 48 h with 1,25(OH)2D3 combined with sorafenib. * p < 0.05, ** p < 0.01, ns, not significant.

Figure 10.
Molecular mechanism diagram.
4. Discussion
The development of chemotherapy resistance has long been a significant challenge in cancer treatment. Approximately 90% of cancer-related deaths are attributed to primary or acquired drug resistance and subsequent metastasis [32]. Despite this, the molecular mechanisms underlying tumor drug resistance remain largely unclear. How to effectively improve the sensitivity of tumors to chemotherapeutic drugs or avoid drug resistance has become an urgent problem worldwide.
In recent years, combination therapy to overcome resistance to single chemotherapeutic agents has garnered increasing attention. Numerous studies have demonstrated that osteotriol (1,25(OH)2D3), a biologically active metabolite of vitamin D synthesized through two-step metabolism in the liver and kidney, plays pivotal roles in multiple signaling pathways governing proliferation, apoptosis, differentiation, inflammation, invasion, angiogenesis, and metastasis. Consequently, it possesses the potential to influence tumorigenesis and growth [33]. Additionally, studies have demonstrated that 1,25(OH)2D3 interacts with antimetabolites (e.g., 5-fluorouracil, gemcitabine) and various other drugs, significantly enhancing the therapeutic efficacy of individual chemotherapeutic agents. These include antimetabolites (e.g., 5-fluorouracil, gemcitabine), platinum compounds (e.g., cisplatin, oxaliplatin, carboplatin), paclitaxel analogs (e.g., paclitaxel, docetaxel), and tyrosine kinase inhibitors (e.g., gefitinib, erlotinib) [8]. Chronic inflammation has been well documented as a critical driver in the pathogenesis and progression of numerous malignancies [34]. Emerging evidence demonstrates that 1,25(OH)2D3 exerts potent anti-inflammatory properties, positioning it as a promising chemopreventive agent for diverse cancer types [6,35,36,37]. Maintenance of optimal vitamin D status through supplementation in deficient individuals may confer chemopreventive benefits and reduce oncogenic risks. Therapeutic administration of 1,25(OH)2D3, either as monotherapy or in combination with active anticancer agents, could potentially inhibit carcinogenesis and/or decelerate tumor progression. For instance, the results obtained by Ye Li et al. [38], utilizing data from breast cancer patients, human tamoxifen (TAM)-resistant breast cancer cell models, and animal models, collectively demonstrate that vitamin D (VitD) can suppress TAM-induced pro-survival autophagy and restore the sensitivity of TAM-resistant breast cancer cells to TAM therapy, while also inhibiting the development of murine breast cancer in in vivo models. Furthermore, higher vitamin D receptor (VDR) levels correlate with improved prognosis in TAM-treated patients, suggesting that VitD may prevent or reverse TAM resistance in breast cancer patients. Studies by Zhirong Jia et al. [39] revealed that the combination of 1,25(OH)2D3 and gefitinib significantly reduces cell viability, proliferation, and tumor progression in both PC-9/GR cells and PC-9/GR xenograft tumor models compared to monotherapy, offering a promising strategy to enhance gefitinib’s cytotoxicity. Current evidence also highlights vitamin D’s potential dual role in treating and preventing glioblastoma [40,41,42,43], supporting the clinical application of vitamin D or VDR as novel biomarkers in this context. Consequently, early-stage intervention with 1,25(OH)2D3 therapy may improve sorafenib’s cytotoxic effects, prevent drug resistance, and prolong chemotherapy duration. Notably, 1,25(OH)2D3 exhibits superior safety and tolerability profiles compared to regorafenib, a second-line therapeutic agent administered following sorafenib treatment progression. While regorafenib demonstrates modest survival benefits, its clinical utility is limited by significant toxicities, including hand–foot skin reactions, hypertension, gastrointestinal disturbances, and hepatotoxicity [44], which may compromise patient quality of life and treatment adherence. Clinical studies have shown that super-physiological doses of 1,25(OH)2D3 mainly induce dose-dependent hypercalcemia, but this adverse reaction can be effectively controlled through strict serum monitoring and intermittent administration [45]. Furthermore, novel 1,25(OH)2D3 analogs have been engineered to minimize calcitropic side effects while preserving or enhancing the compound’s therapeutic efficacy. Some of the existing analogues have tissue-specific effects and low calcification side effects, and can be administered at higher doses compared with the parent compounds [46,47]. Seocalcitol had potent antiproliferative effects in vitro and significantly decreased tumor growth in vivo in animal models of head and neck squamous cell carcinoma [48]. Inecalcitol is a sidechain analog of calcitriol and has shown more potently decreased tumor growth in various cancer models, including breast [49], squamous cell [50], and prostate [51] cancer models, compared with calcitriol. Furthermore, in mouse experiments, inecalcitol induced tumor regression without significantly affecting serum calcium levels. Reasonable utilization of these structural derivatives may thus optimize pharmacokinetic stability and safety profiles without compromising the antitumor benefits inherent to 1,25(OH)2D3 signaling pathways. In our study, 1,25(OH)2D3 combined with sorafenib exhibited antiproliferative and pro-apoptotic effects on hepatocellular carcinoma sorafenib-resistant cells (Huh7/s), suggesting that 1,25(OH)2D3 has the potential to enhance the sensitization of Huh7/s to sorafenib, and may serve as a potential agent to improve the response to chemotherapy. Therefore, the potential mechanism of 1,25(OH)2D3 in the sensitization of HCC chemotherapy needs to be further investigated.
In our work, the action target of 1,25(OH)2D3 was analyzed using Wayne’s analysis against genes associated with hepatocellular carcinoma sorafenib resistance, identifying intersecting genes as potential targets of 1,25(OH)2D3 for sensitizing hepatocellular carcinoma to sorafenib chemotherapy. Subsequently, GO and KEGG enrichment analyses were conducted on these potential sensitization targets to identify pathways through which 1,25(OH)2D3 may enhance chemotherapy. Finally, a chemo–target–pathway network was constructed. Furthermore, the underlying molecular mechanisms of 1,25(OH)2D3 in enhancing chemosensitivity were preliminarily validated through in vitro experiments. KEGG pathway analysis revealed that 1,25(OH)2D3-mediated chemosensitization involves the Hippo signaling pathway, FoxO signaling pathway, and JAK-STAT signaling pathway, all crucial in tumor development [52]. FOXO3A belongs to the FOXO subfamily of forkhead transcription factors, which plays a crucial role in cancer progression, drug resistance, etc. FOXO3A is considered a tumor suppressor, but it is frequently inactivated in cancer cell lines by the nuclear translocation of the FOXO3A protein. FOXO3A is phosphorylated by Akt, ERK, SGK, IKKβ, and IKBKE, among others. Dysregulation of these kinases is frequently observed in different types of cancer, promoting nucleoplasmic translocation and/or the ubiquitin/proteasome-dependent degradation of FOXO3A, thereby promoting cancer progression [53]. In this study, we evaluated the interaction of 1,25(OH)2D3 and its derivatives with FOXO3A using a molecular docking approach. Molecular dynamics simulation analysis was used to further show that 1,25(OH)2D3 binds significantly to the target protein FOXO3A. The results indicated that both compounds exhibited strong docking activity with FOXO3A, suggesting that they can bind stably to the protein. Since the transcriptional activity of FOXO3A is regulated by its nuclear translocation, which can be inhibited by kinase-mediated phosphorylation, we examined the level of phosphorylation FOXO3A. Compared to treatment with either drug alone, combined treatment of 1,25(OH)2D3 and sorafenib significantly inhibited the level of phosphorylated FOXO3A protein. Studies have demonstrated that the FOXO3A/FOXM1 axis plays a critical role in tumor progression and chemoresistance. FOXM1 is the direct transcription target of FOXO3A [54,55,56]. Reactivation of FOXO3A or suppression of FOXM1 in cancer cells reduces DNA repair capacity and cell survival rates while augmenting cell death and enhancing the efficacy of DNA-damaging anticancer therapies [57,58]. For instance, Junnan Li et al. [59] established a novel and promising strategy to overcome acquired resistance to erlotinib by inducing cell cycle arrest at the G1/S phase via modulation of the FOXO3A/FOXM1 axis. Findings from Hao Liu et al. [60] further revealed the pivotal role of the DNMT1/FOXO3a/FOXM1/SOX2 signaling cascade in regulating breast cancer stem cell (BCSC) properties, providing a rationale for developing therapeutics targeting this pathway to suppress BCSC-driven drug resistance. Additional studies report that the combination of trametinib and the glutamine transporter inhibitor V-9302 increases pyroptosis and cell cycle arrest by modulating the FOXO3A/FOXM1 axis and autophagy [54]. Therefore, we also examined FOXM1 expression and found that the drug combination reduced FOXM1 levels. These findings suggest that 1,25(OH)2D3 may influence sorafenib resistance in hepatocellular carcinoma by modulating the FOXO3A/FOXM1 axis, indicating that targeting FOXO3A and FOXM1 could be a promising molecular therapeutic strategy.
Additionally, dysregulation of FOXO3A has been shown to promote the development of drug resistance through multiple mechanisms, including the regulation of oncogenic signaling pathways, evasion of apoptosis, increased drug efflux, and induction of autophagy [61]. Cellular autophagy and apoptosis are crucial processes contributing to sorafenib resistance in HCC [62]. During apoptosis, members of the B-cell lymphoma-2 (Bcl-2) family play significant roles in regulating the mitochondrial apoptotic pathway and are classified into two main groups based on their effects: anti-apoptotic and pro-apoptotic proteins [63]. Current studies indicate that Bcl-2 proteins are linked to acquired sorafenib resistance and HCC proliferation [64,65]. Therefore, targeting the overexpression of Bcl-2 family proteins in HCC may provide an effective therapeutic strategy [66]. Multiple studies have demonstrated that FOXO3A regulates apoptosis in cancer cells by modulating the expression of apoptosis-related proteins [67,68,69,70]. For instance, Zhebin Dong et al. [71] revealed that miRNA-124-3p.1 regulates FOXO3A phosphorylation and deacetylation by targeting AKT2 and SIRT1, thereby sensitizing HCC cells to sorafenib-induced apoptosis. Additionally, Zhang et al. [67] reported that butein can activate FOXO3A, leading to the downregulation of Bcl-2 and upregulation of Bax, which enhances the sensitivity of cervical cancer cells to cisplatin (CDDP). Chemotherapy-induced autophagy has been identified as a pro-survival mechanism that contributes to drug resistance. Targeting autophagy is thus considered a promising therapeutic approach for cancer patients facing drug resistance. Studies have also highlighted that sorafenib-induced autophagy serves as a pro-survival response [72,73,74,75,76,77]. Furthermore, multiple studies have demonstrated that FOXO3A-mediated autophagy serves as a crucial mechanism underlying sorafenib resistance in HCC. For instance, the investigation by Chao Liang et al. [78] revealed that hypoxia-induced autophagy functions as a primary mechanism through which HCC cells develop sorafenib resistance, with FOXO3A playing a pivotal regulatory role in this hypoxia-triggered autophagic process both in vitro and in vivo. Research by Ziyou Lin et al. [79] has demonstrated that RNA strand m6A methylation modulates sorafenib resistance in HCC via FOXO3-mediated autophagy. Consequently, we examined apoptosis and autophagy in drug-resistant cells following combination drug treatment. As demonstrated by Western blot and flow cytometry experiments, the combination of 1,25(OH)2D3 and sorafenib induced apoptosis in Huh7/s cells by upregulating Bax and downregulating Bcl-2 expression. The combination’s ability to inhibit autophagy in Huh7/s cells was further confirmed by transmission electron microscopy and the detection of autophagy-related markers. These results suggest that the primary mechanism by which 1,25(OH)2D3 enhances the chemosensitivity of HCC to sorafenib may involve the FOXO3A/FOXM1 axis, regulating the proliferation and apoptosis of drug-resistant cells and the onset of cellular autophagy, thereby playing a role in reversing resistance to sorafenib in HCC.
Notably, a complex interplay exists between autophagy and apoptosis. Autophagy can promote or inhibit apoptosis, and apoptosis can also promote or inhibit autophagy [80,81,82,83]. Crucially, the dysregulation of this homeostatic crosstalk has been implicated in tumorigenesis [84]. In cytotoxicity studies, differential cellular responses are observed across cell types depending on pharmacological variables including dosage, concentration, and exposure duration. Our experimental findings demonstrate that co-administration of 1,25(OH)2D3 with sorafenib synergistically promotes apoptosis while suppressing autophagic flux in drug-resistant cells, effectively inhibiting proliferation and restoring chemosensitivity to sorafenib. This phenomenon may be attributed to autophagy impairment-triggered apoptotic activation in resistant cells, thereby curtailing neoplastic growth. However, as a tightly regulated catabolic process, autophagy exhibits dual oncogenic roles—acting as either a tumor suppressor or promoter depending on cellular context [85]. Therefore, systematic investigation into the apoptosis–autophagy interactions under combination therapy remains imperative for elucidating resistance mechanisms in malignant cells.
This study has several limitations that warrant consideration and future investigation. Primarily, it is imperative to systematically determine the optimal drug combination ratio to substantially enhance the clinical translational potential of this therapeutic regimen. Further validation using HCC drug-resistant cell models, coupled with rigorous preclinical validation in animal models and subsequent phase clinical trials, is essential to corroborate these findings and establish clinical applicability. Secondly, the mechanism by which 1,25(OH)2D3 enhances sorafenib chemosensitivity in hepatocellular carcinoma involves multiple targets and pathways. Although several key pathways have been identified in our experiments, these findings remain preliminary. Therefore, additional in-depth pharmacological investigations are warranted to fully elucidate these intricate mechanisms.
5. Conclusions
In summary, our study demonstrates that 1,25(OH)2D3 enhances the chemosensitivity of hepatocellular carcinoma to sorafenib and preliminarily elucidates the mechanisms underlying its chemosensitizing effects. This discovery provides critical insights for the potential clinical application of 1,25(OH)2D3 in overcoming drug resistance during liver cancer therapy.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/cimb47050319/s1.
Author Contributions
Conceptualization, Z.L. and X.W.; methodology, Z.L.; software, Z.L. and X.W.; validation, Z.L. and X.W.; formal analysis, J.H. and S.Z.; investigation, T.L. and X.C.; resources, J.H. and S.Z.; data curation, Z.L. and X.W.; writing—original draft preparation, Z.L.; writing—review and editing, Z.L. and X.W.; visualization, Z.L. and X.W.; supervision, J.H. and S.Z.; project administration, J.H. and S.Z.; funding acquisition, J.H. and S.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81860723, 81960589), the science and Technology Foundation of Guizhou Provincial Health and Family Planning Commission (No. gzwjkj2018-1-073), High-level Innovative Talents in Guizhou Province (gzwjrs2022-014), Guizhou Science and Technology Plan Project (Guizhou Science Support [2021] No. 097), and 2023 Subject Excellent Reserve Talent program of Affiliated Hospital of Guizhou Medical University (No. gyfyxkrc-2023-12); grant from Key Lab for Chronic Disease Biomarkers of Guizhou Medical University (No. 2024fy004).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available on request.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Laube, R.; Sabih, A.H.; Strasser, S.I.; Lim, L.; Cigolini, M.; Liu, K. Palliative care in hepatocellular carcinoma. J. Gastroenterol. Hepatol. 2021, 36, 618–628. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Chen, Z.; Zhang, W.; Cheng, Y.; Zhang, B.; Wu, F.; Wang, Q.; Wang, S.; Rong, D.; Reiter, F.P.; et al. The mechanisms of sorafenib resistance in hepatocellular carcinoma: Theoretical basis and therapeutic aspects. Signal Transduct. Target. Ther. 2020, 5, 87. [Google Scholar] [CrossRef] [PubMed]
- Zhai, B.; Sun, X.Y. Mechanisms of resistance to sorafenib and the corresponding strategies in hepatocellular carcinoma. World J. Hepatol. 2013, 5, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Ma, Z.; Li, W.; Ma, Q.; Guo, J.; Hu, A.; Li, R.; Wang, F.; Han, S. The mechanism of calcitriol in cancer prevention and treatment. Curr. Med. Chem. 2013, 20, 4121–4130. [Google Scholar] [CrossRef]
- Vanoirbeek, E.; Krishnan, A.; Eelen, G.; Verlinden, L.; Bouillon, R.; Feldman, D.; Verstuyf, A. The anti-cancer and anti-inflammatory actions of 1,25(OH)2D3. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 593–604. [Google Scholar] [CrossRef]
- Deeb, K.K.; Trump, D.L.; Johnson, C.S. Vitamin D signalling pathways in cancer: Potential for anticancer therapeutics. Nat. Rev. Cancer 2007, 7, 684–700. [Google Scholar] [CrossRef]
- Abu El Maaty, M.A.; Wölfl, S. Effects of 1,25(OH)2D3 on Cancer Cells and Potential Applications in Combination with Established and Putative Anti-Cancer Agents. Nutrients 2017, 9, 87. [Google Scholar] [CrossRef]
- Ma, Y.; Yu, W.D.; Hershberger, P.A.; Flynn, G.; Kong, R.X.; Trump, D.L.; Johnson, C.S. 1alpha,25-Dihydroxyvitamin D3 potentiates cisplatin antitumor activity by p73 induction in a squamous cell carcinoma model. Mol. Cancer Ther. 2008, 7, 3047–3055. [Google Scholar] [CrossRef]
- Hershberger, P.A.; Yu, W.D.; Modzelewski, R.A.; Rueger, R.M.; Johnson, C.S.; Trump, D.L. Calcitriol (1,25-dihydroxycholecalciferol) enhances paclitaxel antitumor activity in vitro and in vivo and accelerates paclitaxel-induced apoptosis. Clin. Cancer Res. 2001, 7, 1043–1051. [Google Scholar]
- Nishioka, C.; Ikezoe, T.; Yang, J.; Yokoyama, A. Sunitinib, an orally available receptor tyrosine kinase inhibitor, induces monocytic differentiation of acute myelogenous leukemia cells that is enhanced by 1,25-dihydroxyvitamin D3. Leukemia 2009, 23, 2171–2173. [Google Scholar] [CrossRef]
- Luo, T.T.; Lu, Y.; Yan, S.K.; Xiao, X.; Rong, X.L.; Guo, J. Network Pharmacology in Research of Chinese Medicine Formula: Methodology, Application and Prospective. Chin. J. Integr. Med. 2020, 26, 72–80. [Google Scholar] [CrossRef]
- Liu, Y.; Ao, X.; Ding, W.; Ponnusamy, M.; Wu, W.; Hao, X.; Yu, W.; Wang, Y.; Li, P.; Wang, J. Critical role of FOXO3a in carcinogenesis. Mol. Cancer 2018, 17, 104. [Google Scholar] [CrossRef]
- Chen, Y.F.; Pandey, S.; Day, C.H.; Chen, Y.F.; Jiang, A.Z.; Ho, T.J.; Chen, R.J.; Padma, V.V.; Kuo, W.W.; Huang, C.Y. Synergistic effect of HIF-1α and FoxO3a trigger cardiomyocyte apoptosis under hyperglycemic ischemia condition. J. Cell Physiol. 2018, 233, 3660–3671. [Google Scholar] [CrossRef] [PubMed]
- McClelland Descalzo, D.L.; Satoorian, T.S.; Walker, L.M.; Sparks, N.R.; Pulyanina, P.Y.; Zur Nieden, N.I. Glucose-Induced Oxidative Stress Reduces Proliferation in Embryonic Stem Cells via FOXO3A/β-Catenin-Dependent Transcription of p21cip1. Stem Cell Rep. 2016, 7, 55–68. [Google Scholar] [CrossRef]
- McGowan, S.E.; McCoy, D.M. Platelet-derived growth factor-A regulates lung fibroblast S-phase entry through p27kip1 and FoxO3a. Respir. Res. 2013, 14, 68. [Google Scholar] [CrossRef] [PubMed]
- Barrett, T.; Wilhite, S.E.; Ledoux, P.; Evangelista, C.; Kim, I.F.; Tomashevsky, M.; Marshall, K.A.; Phillippy, K.H.; Sherman, P.M.; Holko, M.; et al. NCBI GEO: Archive for functional genomics data sets—Update. Nucleic Acids Res. 2013, 41, D991–D995. [Google Scholar] [CrossRef]
- Kim, S. Getting the most out of PubChem for virtual screening. Expert Opin. Drug Discov. 2016, 11, 843–855. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef]
- Forli, S.; Huey, R.; Pique, M.E.; Sanner, M.F.; Goodsell, D.S.; Olson, A.J. Computational protein-ligand docking and virtual drug screening with the AutoDock suite. Nat. Protoc. 2016, 11, 905–919. [Google Scholar] [CrossRef]
- Murmu, S.; Aravinthkumar, A.; Singh, M.K.; Sharma, S.; Das, R.; Jha, G.K.; Prakash, G.; Rana, V.S.; Kaushik, P.; Farooqi, M.S. Identification of potent phytochemicals against Magnaporthe oryzae through machine learning aided-virtual screening and molecular dynamics simulation approach. Comput. Biol. Med. 2025, 188, 109862. [Google Scholar] [CrossRef] [PubMed]
- Muhetaer, H.; Li, H.; Wang, B.; Cai, X.; Zhang, Y.; Li, Y.; Li, C.; Wu, B. Exploring the Effects and Mechanisms of Valerian Volatile Oil in Treating Insomnia Using Network Pharmacology, Molecular Docking, and Molecular Dynamics Simulation-Based Approaches. Int. J. Mol. Sci. 2025, 26, 1726. [Google Scholar] [CrossRef]
- Li, Y.; Mu, Y.; Chen, X.; Zhao, Y.; Ji, C.; Xu, R.; Jiang, R.; Liu, F.; Wang, M.; Sun, L. Deoxyshikonin from Arnebiae Radix promotes hair growth by targeting the Wnt/β-catenin signaling pathway. Phytomedicine 2025, 140, 156590. [Google Scholar] [CrossRef]
- Jin, L.; Guan, Y.; Li, X.; Wang, M.; Shen, Y.; Wang, N.; He, Z. Combining Network Pharmacology, Molecular Docking and Experimental Validation to Explore the Effects and Mechanisms of Indirubin on Acute Lymphoblastic Leukemia. Drug Des. Devel. Ther. 2025, 19, 1083–1103. [Google Scholar] [CrossRef]
- Jo, S.; Kim, T.; Iyer, V.G.; Im, W. CHARMM-GUI: A web-based graphical user interface for CHARMM. J. Comput. Chem. 2008, 29, 1859–1865. [Google Scholar] [CrossRef]
- Mark, P.; Nilsson, L. Structure and Dynamics of the TIP3P, SPC, and SPC/E Water Models at 298 K. J. Phys. Chem. A 2001, 105, 9954–9960. [Google Scholar] [CrossRef]
- Cai, L.; Qin, X.; Xu, Z.; Song, Y.; Jiang, H.; Wu, Y.; Ruan, H.; Chen, J. Comparison of Cytotoxicity Evaluation of Anticancer Drugs between Real-Time Cell Analysis and CCK-8 Method. ACS Omega J. 2019, 4, 12036–12042. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yan, J.; Huang, J.; Wu, X.; Yuan, Y.; Yuan, Y.; Zhang, S.; Mo, F. Exploring the mechanism by which quercetin re-sensitizes breast cancer to paclitaxel: Network pharmacology, molecular docking, and experimental verification. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2023, 396, 3045–3059. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dong, M.; Qin, H.; An, G.; Cen, L.; Deng, L.; Cui, H. Mulberrin suppresses gastric cancer progression and enhances chemosensitivity to oxaliplatin through HSP90AA1/PI3K/AKT axis. Phytomedicine 2025, 139, 156441. [Google Scholar] [CrossRef]
- Li, Y.; Su, Y.; Zhao, Y.; Hu, X.; Zhao, G.; He, J.; Wan, S.; Lü, M.; Cui, H. Demethylzeylasteral inhibits proliferation, migration, and invasion through FBXW7/c-Myc axis in gastric cancer. MedComm 2021, 2, 467–480. [Google Scholar] [CrossRef]
- Ji, H.; Zhang, K.; Pan, G.; Li, C.; Li, C.; Hu, X.; Yang, L.; Cui, H. Deoxyelephantopin Induces Apoptosis and Enhances Chemosensitivity of Colon Cancer via miR-205/Bcl2 Axis. Int. J. Mol. Sci. 2022, 23, 5051. [Google Scholar] [CrossRef] [PubMed]
- Assaraf, Y.G.; Brozovic, A.; Gonçalves, A.C.; Jurkovicova, D.; Linē, A.; Machuqueiro, M.; Saponara, S.; Sarmento-Ribeiro, A.B.; Xavier, C.P.R.; Vasconcelos, M.H. The multi-factorial nature of clinical multidrug resistance in cancer. Drug Resist. Updates 2019, 46, 100645. [Google Scholar] [CrossRef]
- Abu El Maaty, M.A.; Wölfl, S. Vitamin D as a Novel Regulator of Tumor Metabolism: Insights on Potential Mechanisms and Implications for Anti-Cancer Therapy. Int. J. Mol. Sci. 2017, 18, 2184. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef]
- Banach-Petrosky, W.; Ouyang, X.; Gao, H.; Nader, K.; Ji, Y.; Suh, N.; DiPaola, R.S.; Abate-Shen, C. Vitamin D inhibits the formation of prostatic intraepithelial neoplasia in Nkx3.1;Pten mutant mice. Clin. Cancer Res. 2006, 12, 5895–5901. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, A.V.; Feldman, D. Mechanisms of the anti-cancer and anti-inflammatory actions of vitamin D. Annu. Rev. Pharmacol. Toxicol. 2011, 51, 311–336. [Google Scholar] [CrossRef]
- Krishnan, A.V.; Trump, D.L.; Johnson, C.S.; Feldman, D. The role of vitamin D in cancer prevention and treatment. Endocrinol. Metab. Clin. N. Am. 2010, 39, 401–418, table of contents. [Google Scholar] [CrossRef]
- Li, Y.; Cook, K.L.; Yu, W.; Jin, L.; Bouker, K.B.; Clarke, R.; Hilakivi-Clarke, L. Inhibition of Antiestrogen-Promoted Pro-Survival Autophagy and Tamoxifen Resistance in Breast Cancer through Vitamin D Receptor. Nutrients 2021, 13, 1715. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Zhang, Y.; Yan, A.; Wang, M.; Han, Q.; Wang, K.; Wang, J.; Qiao, C.; Pan, Z.; Chen, C.; et al. 1,25-dihydroxyvitamin D3 signaling-induced decreases in IRX4 inhibits NANOG-mediated cancer stem-like properties and gefitinib resistance in NSCLC cells. Cell Death Dis. 2020, 11, 670. [Google Scholar] [CrossRef]
- Davoust, N.; Wion, D.; Chevalier, G.; Garabedian, M.; Brachet, P.; Couez, D. Vitamin D receptor stable transfection restores the susceptibility to 1,25-dihydroxyvitamin D3 cytotoxicity in a rat glioma resistant clone. J. Neurosci. Res. 1998, 52, 210–219. [Google Scholar] [CrossRef]
- Zigmont, V.; Garrett, A.; Peng, J.; Seweryn, M.; Rempala, G.A.; Harris, R.; Holloman, C.; Gundersen, T.E.; Ahlbom, A.; Feychting, M.; et al. Association Between Prediagnostic Serum 25-Hydroxyvitamin D Concentration and Glioma. Nutr. Cancer 2015, 67, 1120–1130. [Google Scholar] [CrossRef]
- Bak, D.H.; Kang, S.H.; Choi, D.R.; Gil, M.N.; Yu, K.S.; Jeong, J.H.; Lee, N.S.; Lee, J.H.; Jeong, Y.G.; Kim, D.K.; et al. Autophagy enhancement contributes to the synergistic effect of vitamin D in temozolomide-based glioblastoma chemotherapy. Exp. Ther. Med. 2016, 11, 2153–2162. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.S.; Kiang, K.M.; Leung, G.K. Anti-tumor effects of vitamin D in glioblastoma: Mechanism and therapeutic implications. Lab. Investig. 2022, 102, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.M.; Dumas, J.; Adnane, L.; Lynch, M.; Carter, C.A.; Schütz, G.; Thierauch, K.H.; Zopf, D. Regorafenib (BAY 73-4506): A new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int. J. Cancer 2011, 129, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Woloszynska-Read, A.; Johnson, C.S.; Trump, D.L. Vitamin D and cancer: Clinical aspects. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 605–615. [Google Scholar] [CrossRef]
- Leyssens, C.; Verlinden, L.; Verstuyf, A. The future of vitamin D analogs. Front. Physiol. 2014, 5, 122. [Google Scholar] [CrossRef]
- Yee, Y.K.; Chintalacharuvu, S.R.; Lu, J.; Nagpal, S. Vitamin D receptor modulators for inflammation and cancer. Mini-Rev. Med. Chem. 2005, 5, 761–778. [Google Scholar] [CrossRef] [PubMed]
- Prudencio, J.; Akutsu, N.; Benlimame, N.; Wang, T.; Bastien, Y.; Lin, R.; Black, M.J.; Alaoui-Jamali, M.A.; White, J.H. Action of low calcemic 1alpha,25-dihydroxyvitamin D3 analogue EB1089 in head and neck squamous cell carcinoma. J. Natl. Cancer Inst. 2001, 93, 745–753. [Google Scholar] [CrossRef]
- Verlinden, L.; Verstuyf, A.; Van Camp, M.; Marcelis, S.; Sabbe, K.; Zhao, X.Y.; De Clercq, P.; Vandewalle, M.; Bouillon, R. Two novel 14-Epi-analogues of 1,25-dihydroxyvitamin D3 inhibit the growth of human breast cancer cells in vitro and in vivo. Cancer Res. 2000, 60, 2673–2679. [Google Scholar]
- Ma, Y.; Yu, W.D.; Hidalgo, A.A.; Luo, W.; Delansorne, R.; Johnson, C.S.; Trump, D.L. Inecalcitol, an analog of 1,25D3, displays enhanced antitumor activity through the induction of apoptosis in a squamous cell carcinoma model system. Cell Cycle 2013, 12, 743–752. [Google Scholar] [CrossRef]
- Okamoto, R.; Delansorne, R.; Wakimoto, N.; Doan, N.B.; Akagi, T.; Shen, M.; Ho, Q.H.; Said, J.W.; Koeffler, H.P. Inecalcitol, an analog of 1α,25(OH)2 D3, induces growth arrest of androgen-dependent prostate cancer cells. Int. J. Cancer 2012, 130, 2464–2473. [Google Scholar] [CrossRef]
- Farhan, M.; Wang, H.; Gaur, U.; Little, P.J.; Xu, J.; Zheng, W. FOXO Signaling Pathways as Therapeutic Targets in Cancer. Int. J. Biol. Sci. 2017, 13, 815–827. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Q.; Cao, Q.; Wang, F.; Huang, L.Y.; Sang, T.T.; Liu, F.; Chen, S.Y. SIRT1 Protects Against Oxidative Stress-Induced Endothelial Progenitor Cells Apoptosis by Inhibiting FOXO3a via FOXO3a Ubiquitination and Degradation. J. Cell. Physiol. 2015, 230, 2098–2107. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Hu, J.; Li, X.; Gao, H.; Kong, D.; Jin, M. Glutamine transporter inhibitor enhances the sensitivity of NSCLC to trametinib through GSDME-dependent pyroptosis. Biochem. Pharmacol. 2025, 233, 116796. [Google Scholar] [CrossRef]
- Tang, Q.; Li, J.; Zhang, L.; Zeng, S.; Bao, Q.; Hu, W.; He, L.; Huang, G.; Wang, L.; Liu, Y.; et al. Orlistat facilitates immunotherapy via AKT-FOXO3a-FOXM1-mediated PD-L1 suppression. J. Immunother. Cancer 2025, 13, e008923. [Google Scholar] [CrossRef]
- Zhong, M.; Fang, Z.; Zou, J.; Chen, X.; Qiu, Z.; Zhou, L.; Le, Y.; Chen, Z.; Liao, Y.; Nie, F.; et al. SPIN1 accelerates tumorigenesis and confers radioresistance in non-small cell lung cancer by orchestrating the FOXO3a/FOXM1 axis. Cell Death Dis. 2024, 15, 832. [Google Scholar] [CrossRef] [PubMed]
- Nestal de Moraes, G.; Bella, L.; Zona, S.; Burton, M.J.; Lam, E.W. Insights into a Critical Role of the FOXO3a-FOXM1 Axis in DNA Damage Response and Genotoxic Drug Resistance. Curr. Drug Targets 2016, 17, 164–177. [Google Scholar] [CrossRef]
- Yao, S.; Fan, L.Y.; Lam, E.W. The FOXO3-FOXM1 axis: A key cancer drug target and a modulator of cancer drug resistance. Semin. Cancer. Biol. 2018, 50, 77–89. [Google Scholar] [CrossRef]
- Li, J.; Chen, P.; Wu, Q.; Guo, L.; Leong, K.W.; Chan, K.I.; Kwok, H.F. A novel combination treatment of antiADAM17 antibody and erlotinib to overcome acquired drug resistance in non-small cell lung cancer through the FOXO3a/FOXM1 axis. Cell. Mol. Life Sci. 2022, 79, 614. [Google Scholar] [CrossRef]
- Liu, H.; Song, Y.; Qiu, H.; Liu, Y.; Luo, K.; Yi, Y.; Jiang, G.; Lu, M.; Zhang, Z.; Yin, J.; et al. Downregulation of FOXO3a by DNMT1 promotes breast cancer stem cell properties and tumorigenesis. Cell Death Differ. 2020, 27, 966–983. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Li, X.; Jia, Y.; Wang, J.; Ao, X. FOXO3a in cancer drug resistance. Cancer Lett. 2022, 540, 215724. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y. Mechanisms of sorafenib resistance in hepatocellular carcinoma. Clin. Res. Hepatol. Gastroenterol. 2024, 48, 102434. [Google Scholar] [CrossRef]
- Hafezi, S.; Rahmani, M. Targeting BCL-2 in Cancer: Advances, Challenges, and Perspectives. Cancers 2021, 13, 1292. [Google Scholar] [CrossRef] [PubMed]
- Tutusaus, A.; Stefanovic, M.; Boix, L.; Cucarull, B.; Zamora, A.; Blasco, L.; de Frutos, P.G.; Reig, M.; Fernandez-Checa, J.C.; Marí, M.; et al. Antiapoptotic BCL-2 proteins determine sorafenib/regorafenib resistance and BH3-mimetic efficacy in hepatocellular carcinoma. Oncotarget 2018, 9, 16701–16717. [Google Scholar] [CrossRef]
- Niu, L.; Liu, L.; Yang, S.; Ren, J.; Lai, P.B.S.; Chen, G.G. New insights into sorafenib resistance in hepatocellular carcinoma: Responsible mechanisms and promising strategies. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2017, 1868, 564–570. [Google Scholar] [CrossRef] [PubMed]
- de Melo Silva, A.J.; de Melo Gama, J.E.; de Oliveira, S.A. The Role of Bcl-2 Family Proteins and Sorafenib Resistance in Hepatocellular Carcinoma. Int. J. Cell Biol. 2024, 2024, 4972523. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, X.; Li, X.; Li, C.; Zhao, L.; Zhou, Y.; Hou, H. Butein sensitizes HeLa cells to cisplatin through the AKT and ERK/p38 MAPK pathways by targeting FoxO3a. Int. J. Mol. Med. 2015, 36, 957–966. [Google Scholar] [CrossRef]
- Ding, Q.; Chen, Y.; Zhang, Q.; Guo, Y.; Huang, Z.; Dai, L.; Cao, S. 8-bromo-7-methoxychrysin induces apoptosis by regulating Akt/FOXO3a pathway in cisplatin-sensitive and resistant ovarian cancer cells. Mol. Med. Rep. 2015, 12, 5100–5108. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, J.; Tang, J.; Liu, X.; Zhong, Q.; Wang, F.; Hu, W.; Yuan, Z.; Nie, C.; Wei, Y. JNK- and Akt-mediated Puma expression in the apoptosis of cisplatin-resistant ovarian cancer cells. Biochem. J. 2012, 444, 291–301. [Google Scholar] [CrossRef]
- Shoeb, M.; Ramana, K.V.; Srivastava, S.K. Aldose reductase inhibition enhances TRAIL-induced human colon cancer cell apoptosis through AKT/FOXO3a-dependent upregulation of death receptors. Free Radic. Biol. Med. 2013, 63, 280–290. [Google Scholar] [CrossRef]
- Dong, Z.B.; Wu, H.M.; He, Y.C.; Huang, Z.T.; Weng, Y.H.; Li, H.; Liang, C.; Yu, W.M.; Chen, W. MiRNA-124-3p.1 sensitizes hepatocellular carcinoma cells to sorafenib by regulating FOXO3a by targeting AKT2 and SIRT1. Cell Death Dis. 2022, 13, 35. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.H.; Ding, Z.B.; Zhou, J.; Hui, B.; Shi, G.M.; Ke, A.W.; Wang, X.Y.; Dai, Z.; Peng, Y.F.; Gu, C.Y.; et al. Targeting autophagy enhances sorafenib lethality for hepatocellular carcinoma via ER stress-related apoptosis. Autophagy 2011, 7, 1159–1172. [Google Scholar] [CrossRef]
- Zhai, B.; Hu, F.; Jiang, X.; Xu, J.; Zhao, D.; Liu, B.; Pan, S.; Dong, X.; Tan, G.; Wei, Z.; et al. Inhibition of Akt reverses the acquired resistance to sorafenib by switching protective autophagy to autophagic cell death in hepatocellular carcinoma. Mol. Cancer Ther. 2014, 13, 1589–1598. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, S.; Takehara, T.; Hikita, H.; Kodama, T.; Tsunematsu, H.; Miyagi, T.; Hosui, A.; Ishida, H.; Tatsumi, T.; Kanto, T.; et al. Inhibition of autophagy potentiates the antitumor effect of the multikinase inhibitor sorafenib in hepatocellular carcinoma. Int. J. Cancer 2012, 131, 548–557. [Google Scholar] [CrossRef]
- Yuan, H.; Li, A.J.; Ma, S.L.; Cui, L.J.; Wu, B.; Yin, L.; Wu, M.C. Inhibition of autophagy significantly enhances combination therapy with sorafenib and HDAC inhibitors for human hepatoma cells. World J. Gastroenterol. 2014, 20, 4953–4962. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Fu, J.; Xu, A.; Su, B.; Ren, Y.; Li, N.; Zhu, J.; Zhao, X.; Dai, R.; Cao, J.; et al. PSMD10/gankyrin induces autophagy to promote tumor progression through cytoplasmic interaction with ATG7 and nuclear transactivation of ATG7 expression. Autophagy 2016, 12, 1355–1371. [Google Scholar] [CrossRef]
- Ling, S.; Song, L.; Fan, N.; Feng, T.; Liu, L.; Yang, X.; Wang, M.; Li, Y.; Tian, Y.; Zhao, F.; et al. Combination of metformin and sorafenib suppresses proliferation and induces autophagy of hepatocellular carcinoma via targeting the mTOR pathway. Int. J. Oncol. 2017, 50, 297–309. [Google Scholar] [CrossRef]
- Liang, C.; Dong, Z.; Cai, X.; Shen, J.; Xu, Y.; Zhang, M.; Li, H.; Yu, W.; Chen, W. Hypoxia induces sorafenib resistance mediated by autophagy via activating FOXO3a in hepatocellular carcinoma. Cell Death Dis. 2020, 11, 1017. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Niu, Y.; Wan, A.; Chen, D.; Liang, H.; Chen, X.; Sun, L.; Zhan, S.; Chen, L.; Cheng, C.; et al. RNA m6A methylation regulates sorafenib resistance in liver cancer through FOXO3-mediated autophagy. EMBO J. 2020, 39, e103181. [Google Scholar] [CrossRef]
- Xi, H.; Wang, S.; Wang, B.; Hong, X.; Liu, X.; Li, M.; Shen, R.; Dong, Q. The role of interaction between autophagy and apoptosis in tumorigenesis (Review). Oncol. Rep. 2022, 48, 208. [Google Scholar] [CrossRef]
- Rubinstein, A.D.; Eisenstein, M.; Ber, Y.; Bialik, S.; Kimchi, A. The autophagy protein Atg12 associates with antiapoptotic Bcl-2 family members to promote mitochondrial apoptosis. Mol. Cell 2011, 44, 698–709. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Lin, Y.; Gao, S.; Wang, Y.; Pan, H.; Wang, Z.; Pozzolini, M.; Yang, F.; Zhang, H.; Yang, Y.; et al. Luteolin inhibits triple-negative breast cancer by inducing apoptosis and autophagy through SGK1-FOXO3a-BNIP3 signaling. Front. Pharmacol. 2023, 14, 1200843. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Pan, P.; Zhao, Q.; Liu, W.; Sun, Y.; Wang, J.; Liu, C.; Wang, C. Overcoming Basal Autophagy, Kangai Injection Enhances Cisplatin Cytotoxicity by Regulating FOXO3a-Dependent Autophagic Cell Death and Apoptosis in Human Lung Adenocarcinoma A549/DDP Cells. BioMed Res. Int. 2022, 2022, 6022981. [Google Scholar] [CrossRef]
- Lee, I.H.; Kawai, Y.; Fergusson, M.M.; Rovira, I.I., II; Bishop, A.J.; Motoyama, N.; Cao, L.; Finkel, T. Atg7 modulates p53 activity to regulate cell cycle and survival during metabolic stress. Science 2012, 336, 225–228. [Google Scholar] [CrossRef]
- Lim, S.M.; Mohamad Hanif, E.A.; Chin, S.F. Is targeting autophagy mechanism in cancer a good approach? The possible double-edge sword effect. Cell Biosci. 2021, 11, 56. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).