Albumin: A Review of Market Trends, Purification Methods, and Biomedical Innovations
Abstract
1. Introduction
2. Types of Albumin
3. Metabolite of Albumin
4. Functions of Albumin
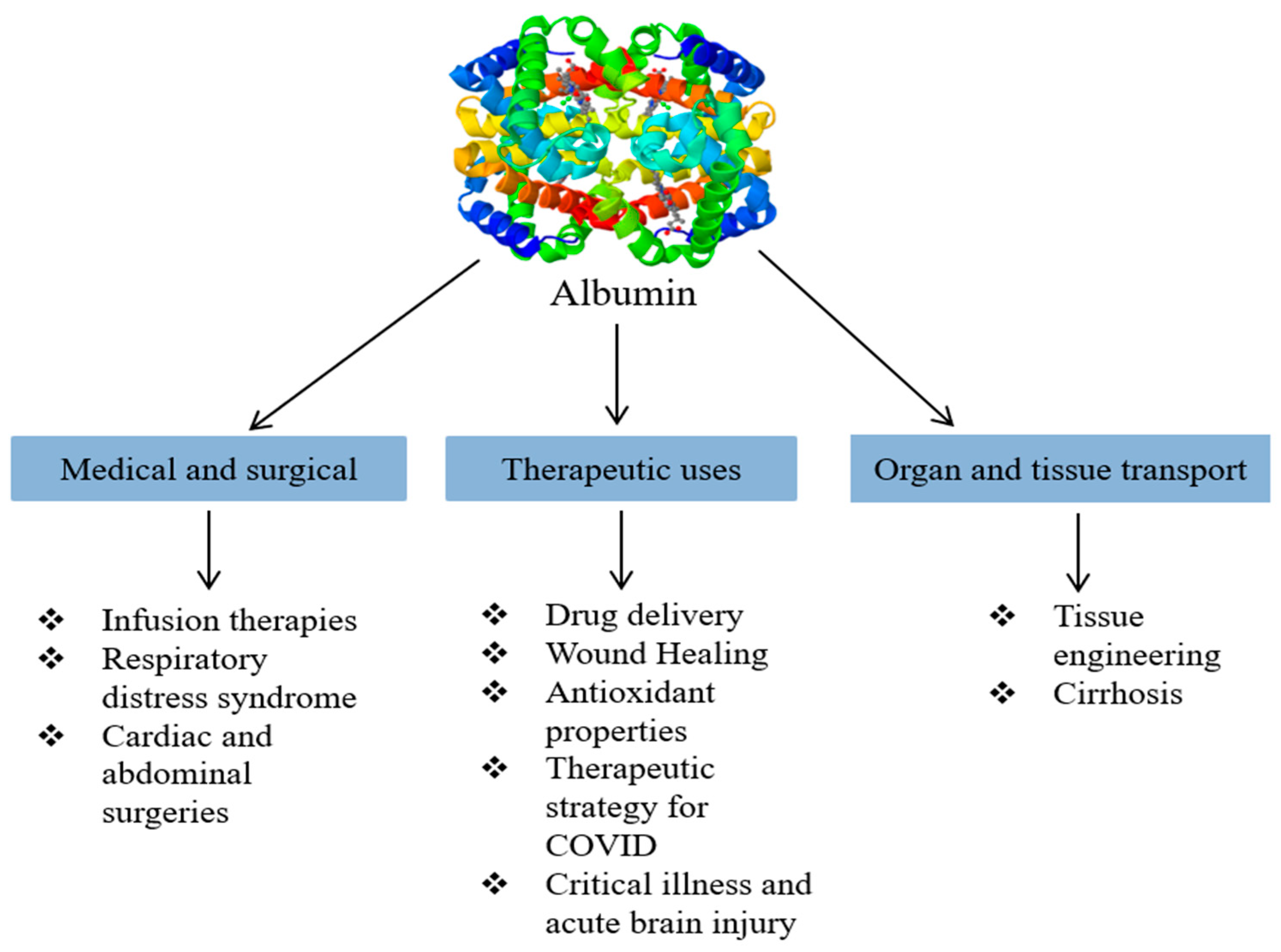
5. Novel Applications of Albumin
5.1. Drug Delivery
5.2. Wound Healing
5.3. Antioxidant
5.4. Infusion Therapy
5.5. Therapeutic Strategy for COVID-19 Infection
5.6. Tissue Engineering
5.7. Critical Illness
5.8. Drug Carrier
5.9. Respiratory Distress Syndrome
5.10. Abdominal and Cardiac Surgeries
5.11. Acute Brain Injury
5.12. Cirrhosis
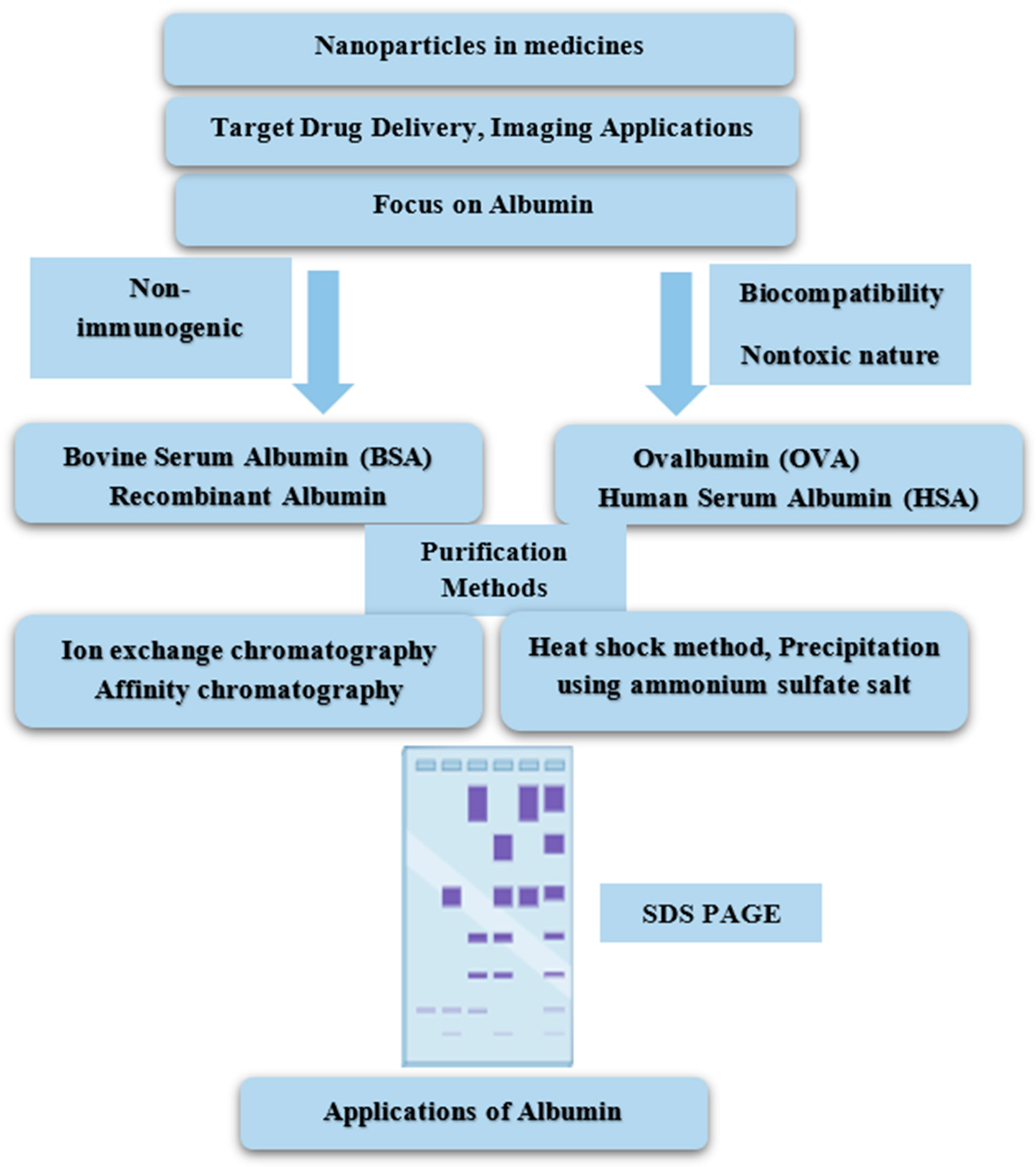
6. Albumin Purification
6.1. Heat-Shock Method
6.2. Precipitation Using Ammonium Sulfate Salt
6.3. Ion Exchange Chromatography (IEC)
6.4. Albumin Purification Using Gel Filtration Chromatography
6.5. Affinity Chromatography
6.6. Electrophoresis Analysis
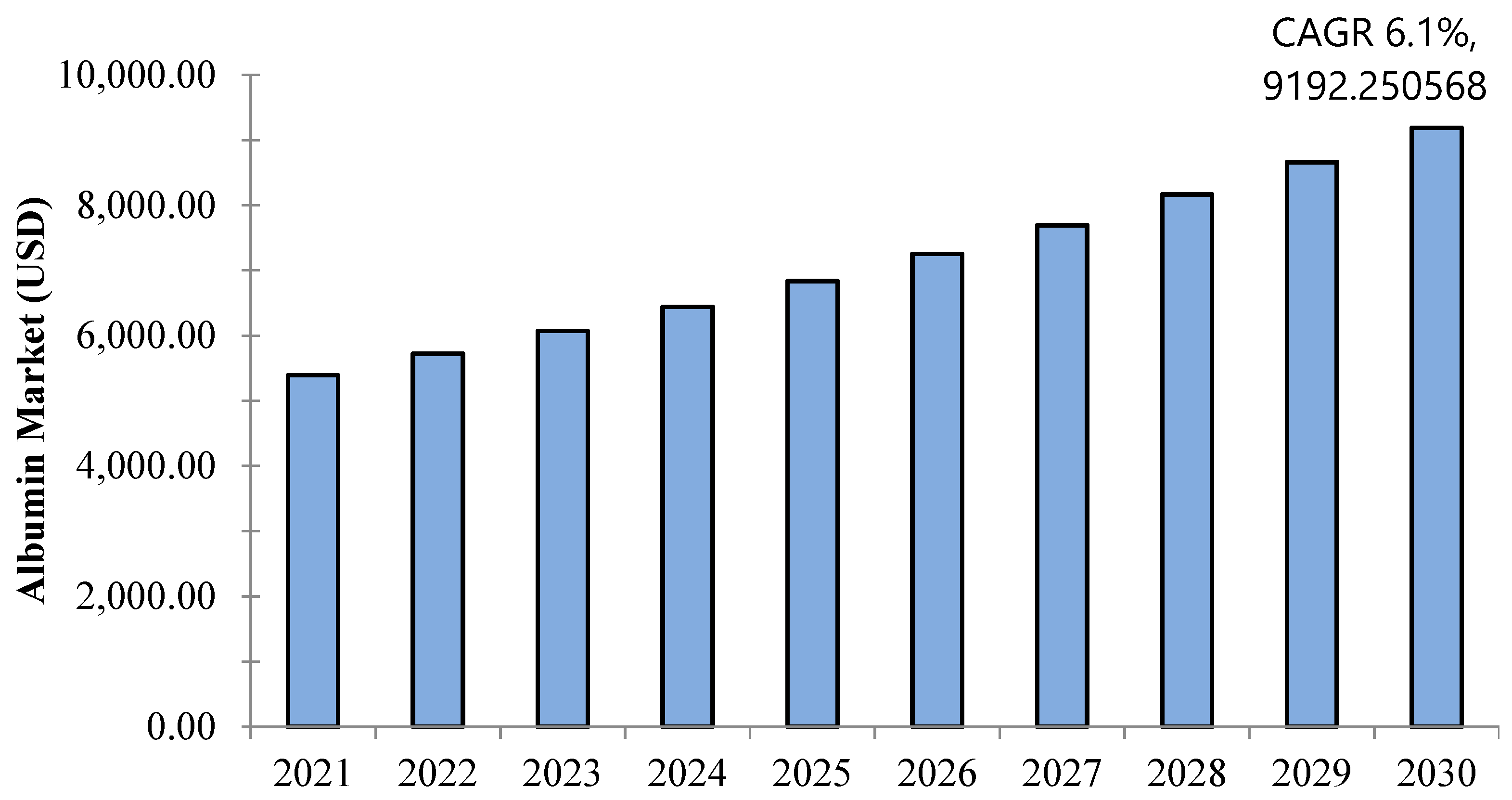
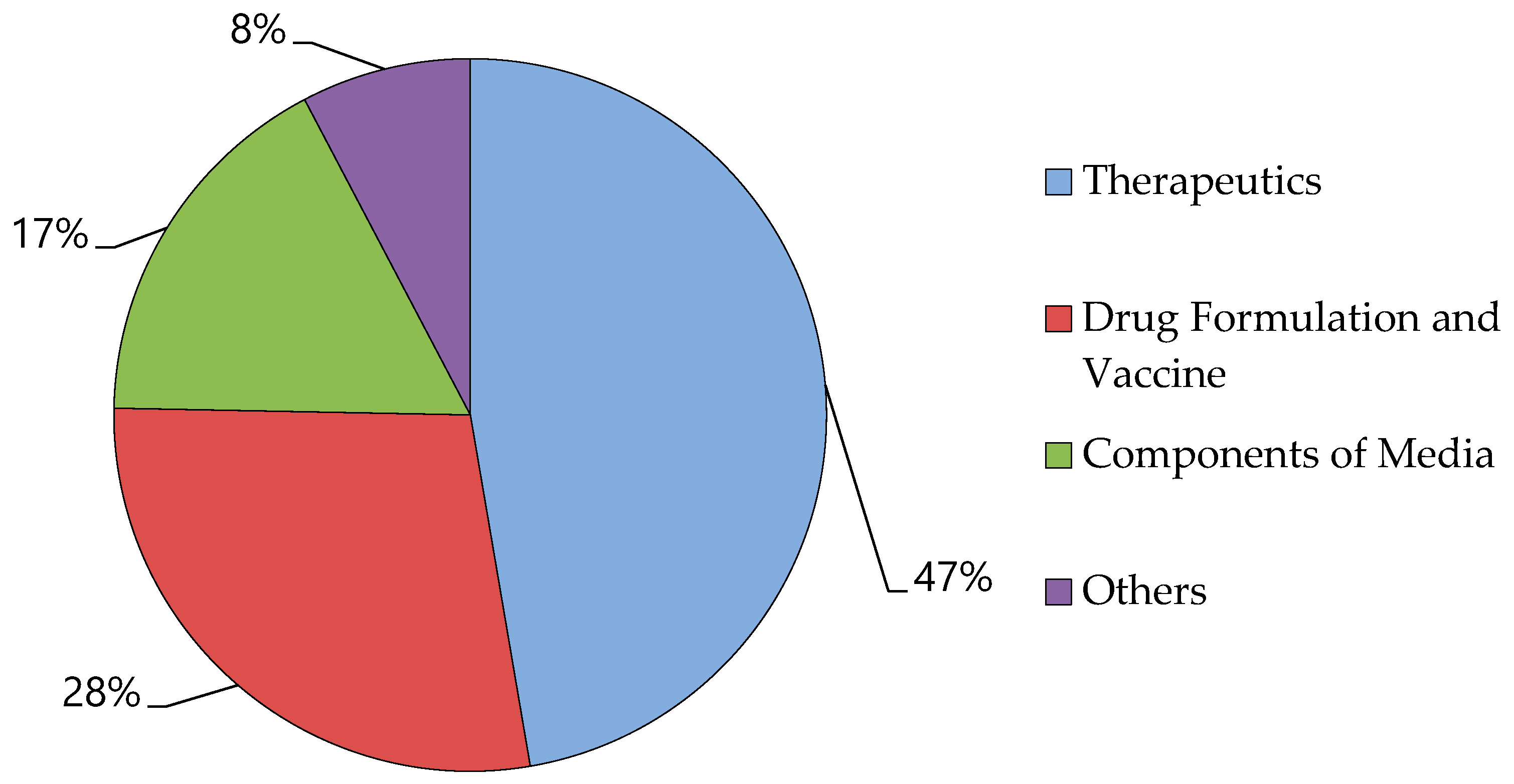
7. Albumin Market
8. Challenges and Solutions
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ömür, A. Investigation of BSA adsorption performances of metal ion attached mineral particles embedded cryogel discs. MANAS J. Eng. 2021, 9, 65–71. [Google Scholar]
- Alacabey, İ.; Acet, Ö.; Önal, B.; Dikici, E.; Karakoç, V.; Gürbüz, F.; Alkan, H.; Odabaşı, M. Pumice particle interface: A case study for immunoglobulin G purification. Polym. Bull. 2021, 78, 5593–5607. [Google Scholar] [CrossRef]
- Acet, Ö.; Önal, B.; Sanz, R.; Sanz-Perez, E.S.; Erdönmez, D.; Odabaşi, M. Preparation of a new chromatographic media and assessment of some kinetic and interaction parameters for lysozyme. J. Mol. Liq. 2019, 276, 480–487. [Google Scholar] [CrossRef]
- Baran, N.Y.; Acet, Ö.; Odabaşı, M. Efficient adsorption of hemoglobin from aqueous solutions by hybrid monolithic cryogel column. Mater. Sci. Eng. C 2017, 73, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Acet, Ö.; Baran, T.; Erdönmez, D.; Aksoy, N.H.; Alacabey, İ.; Menteş, A.; Odabaşi, M. O-carboxymethyl chitosan Schiff base complexes as affinity ligands for immobilized metal-ion affinity chromatography of lysozyme. J. Chromatogr. A 2018, 1550, 21–27. [Google Scholar] [CrossRef]
- Wang, J.; Li, B.; Qiu, L.; Qiao, X.; Yang, H. Dendrimer-based drug delivery systems: History, challenges, and latest developments. J. Biol. Eng. 2022, 16, 18. [Google Scholar] [CrossRef]
- Debnath, S.K.; Srivastava, R. Drug delivery with carbon-based nanomaterials as versatile nanocarriers: Progress and prospects. Front. Nanotechnol. 2021, 3, 644564. [Google Scholar] [CrossRef]
- Dewangan, H.K. Albumin as natural versatile drug carrier for various diseases treatment. In Sustainable Agriculture Reviews 43: Pharmaceutical Technology for Natural Products Delivery Vol. 1 Fundamentals and Applications; Springer: Cham, Switzerland, 2020; pp. 239–268. [Google Scholar]
- Shen, X.; Liu, X.; Li, T.; Chen, Y.; Chen, Y.; Wang, P.; Zheng, L.; Yang, H.; Wu, C.; Deng, S. Recent advancements in serum albumin-based nanovehicles toward potential cancer diagnosis and therapy. Front. Chem. 2021, 9, 746646. [Google Scholar] [CrossRef]
- Fasano, M.; Curry, S.; Terreno, E.; Galliano, M.; Fanali, G.; Narciso, P.; Notari, S.; Ascenzi, P. The extraordinary ligand binding properties of human serum albumin. IUBMB Life 2005, 57, 787–796. [Google Scholar] [CrossRef]
- Quinlan, G.J.; Martin, G.S.; Evans, T.W. Albumin: Biochemical properties and therapeutic potential. Hepatology 2005, 41, 1211–1219. [Google Scholar] [CrossRef]
- Tullis, J.L. Albumin: 1. Background and use. JAMA 1977, 237, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Tullis, J.L. Albumin: 2. Guidelines for clinical use. JAMA 1977, 237, 460–463. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S.; Shiels, J.; Taggart, C.C.; Dalton, J.P.; Weldon, S. Fasciola hepatica-derived molecules as regulators of the host immune response. Front. Immunol. 2020, 11, 2182. [Google Scholar] [CrossRef] [PubMed]
- Bakheet Elsadek, F.K. Impact of albumin on drug delivery—New applications on the horizon. J. Control. Release 2012, 157, 4–28. [Google Scholar] [CrossRef]
- Oakenfull, D.; Pearce, J.; Burley, R.; Damodaran, S.; Paraf, A. Food Proteins and Their Applications. Damodaran, S., Paraf, A., Eds.; CRC Press: Boca Raton, FL, USA, 1997; pp. 111–142. [Google Scholar]
- Hu, Y.-J.; Liu, Y.; Sun, T.-Q.; Bai, A.-M.; Lü, J.-Q.; Pi, Z.-B. Binding of anti-inflammatory drug cromolyn sodium to bovine serum albumin. Int. J. Biol. Macromol. 2006, 39, 280–285. [Google Scholar] [CrossRef]
- Tantra, R.; Tompkins, J.; Quincey, P. Characterisation of the de-agglomeration effects of bovine serum albumin on nanoparticles in aqueous suspension. Colloids Surf. B Biointerfaces 2010, 75, 275–281. [Google Scholar] [CrossRef]
- Hirose, M.; Tachibana, A.; Tanabe, T. Recombinant human serum albumin hydrogel as a novel drug delivery vehicle. Mater. Sci. Eng. C 2010, 30, 664–669. [Google Scholar] [CrossRef]
- Sugimoto, Y.; Sanuki, S.; Ohsako, S.; Higashimoto, Y.; Kondo, M.; Kurawaki, J.; Ibrahim, H.R.; Aoki, T.; Kusakabe, T.; Koga, K. Ovalbumin in developing chicken eggs migrates from egg white to embryonic organs while changing its conformation and thermal stability. J. Biol. Chem. 1999, 274, 11030–11037. [Google Scholar] [CrossRef]
- Al-Harthi, S.; Lachowicz, J.I.; Nowakowski, M.E.; Jaremko, M.; Jaremko, Ł. Towards the functional high-resolution coordination chemistry of blood plasma human serum albumin. J. Inorg. Biochem. 2019, 198, 110716. [Google Scholar] [CrossRef]
- Howes, J.; Hentges Jr, J.; Feaster, J. Blood volume of Brahman and Hereford cattle as measured by injected radioiodinated bovine serum albumin. J. Anim. Sci. 1963, 22, 183–187. [Google Scholar] [CrossRef]
- Blanco, E.; Ruso, J.M.; Prieto, G.; Sarmiento, F. On relationships between surfactant type and globular proteins interactions in solution. J. Colloid Interface Sci. 2007, 316, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Leggio, C.; Galantini, L.; Pavel, N.V. About the albumin structure in solution: Cigar Expanded form versus heart Normal shape. Phys. Chem. Chem. Phys. 2008, 10, 6741–6750. [Google Scholar] [CrossRef] [PubMed]
- Sitar, M.E.; Aydin, S.; Cakatay, U. Human serum albumin and its relation with oxidative stress. Clin. Lab. 2013, 59, 945–952. [Google Scholar] [CrossRef]
- Raoufinia, R.; Mota, A.; Keyhanvar, N.; Safari, F.; Shamekhi, S.; Abdolalizadeh, J. Overview of albumin and its purification methods. Adv. Pharm. Bull. 2016, 6, 495. [Google Scholar] [CrossRef]
- Benedé, S.; López-Expósito, I.; Molina, E.; López-Fandiño, R. Egg proteins as allergens and the effects of the food matrix and processing. Food Funct. 2015, 6, 694–713. [Google Scholar] [CrossRef]
- Curry, S. Plasma albumin as a fatty acid carrier. Adv. Mol. Cell Biol. 2003, 33, 29–46. [Google Scholar]
- Sleep, D. Albumin and its application in drug delivery. Expert Opin. Drug Deliv. 2015, 12, 793–812. [Google Scholar] [CrossRef]
- Verhoeckx, K.C.; Vissers, Y.M.; Baumert, J.L.; Faludi, R.; Feys, M.; Flanagan, S.; Herouet-Guicheney, C.; Holzhauser, T.; Shimojo, R.; van der Bolt, N. Food processing and allergenicity. Food Chem. Toxicol. 2015, 80, 223–240. [Google Scholar] [CrossRef]
- Nakae, H.; Tomida, K.; Kikuya, Y.; Okuyama, M.; Igarashi, T. Comparison of quality of human serum albumin preparations in two pharmaceutical products. Acute Med. Surg. 2017, 4, 251–254. [Google Scholar] [CrossRef]
- Tabata, F.; Wada, Y.; Kawakami, S.; Miyaji, K. Serum Albumin Redox States: More Than Oxidative Stress Biomarker. Antioxidants 2021, 10, 503. [Google Scholar] [CrossRef]
- Carvalho, J.R.; Machado, M.V. New insights about albumin and liver disease. Ann. Hepatol. 2018, 17, 547–560. [Google Scholar] [CrossRef]
- Gburek, J.; Konopska, B.; Gołąb, K. Renal handling of albumin—From early findings to current concepts. Int. J. Mol. Sci. 2021, 22, 5809. [Google Scholar] [CrossRef] [PubMed]
- Moman, R.N.; Gupta, N.; Varacallo, M. Physiology, Albumin. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2017. [Google Scholar]
- Hummelgaard, S.; Weyer, K. Megalin-Mediated Endocytosis in the Kidney Proximal Tubule: Relevance to Regulation of the Renal Renin-Angiotensin System. Nephron 2023, 147, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Long, K.R.; Rbaibi, Y.; Kashlan, O.B.; Weisz, O.A. Receptor-associated protein impairs ligand binding to megalin and megalin-dependent endocytic flux in proximal tubule cells. Am. J. Physiol.-Ren. Physiol. 2023, 325, F457–F464. [Google Scholar] [CrossRef] [PubMed]
- Macošek, J.; Mas, G.; Hiller, S. Redefining molecular chaperones as chaotropes. Front. Mol. Biosci. 2021, 8, 683132. [Google Scholar] [CrossRef]
- Edkins, A.L.; Boshoff, A. General structural and functional features of molecular chaperones. In Heat Shock Proteins of Malaria; Springer: Cham, Switzerland, 2021; pp. 11–73. [Google Scholar] [CrossRef]
- De Simone, G.; di Masi, A.; Ascenzi, P. Serum albumin: A multifaced enzyme. Int. J. Mol. Sci. 2021, 22, 10086. [Google Scholar] [CrossRef]
- Kragh-Hansen, U.; Watanabe, H.; Nakajou, K.; Iwao, Y.; Otagiri, M. Chain length-dependent binding of fatty acid anions to human serum albumin studied by site-directed mutagenesis. J. Mol. Biol. 2006, 363, 702–712. [Google Scholar] [CrossRef]
- Dugaiczyk, A.; Law, S.W.; Dennison, O.E. Nucleotide sequence and the encoded amino acids of human serum albumin mRNA. Proc. Natl. Acad. Sci. USA 1982, 79, 71–75. [Google Scholar] [CrossRef]
- Gerety, R.; Aronson, D. Plasma derivatives and viral hepatitis. Obstet. Gynecol. Surv. 1983, 38, 418–419. [Google Scholar] [CrossRef]
- Fanali, G.; Ascenzi, P.; Fasano, M. Effect of prototypic drugs ibuprofen and warfarin on global chaotropic unfolding of human serum heme-albumin: A fast-field-cycling 1H-NMR relaxometric study. Biophys. Chem. 2007, 129, 29–35. [Google Scholar] [CrossRef]
- L’Heureux, M.; Sternberg, M.; Brath, L.; Turlington, J.; Kashiouris, M.G. Sepsis-induced cardiomyopathy: A comprehensive review. Curr. Cardiol. Rep. 2020, 22, 35. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, S.; Khanadeev, V.A.; Khlebtsov, B.N.; Khlebtsov, N.G.; Deore, M.S.; Packirisamy, G. Albumin-Based Nanocarriers for the Simultaneous Delivery of Antioxidant Gene and Phytochemical to Combat Oxidative Stress. Front. Cell Dev. Biol. 2022, 10, 846175. [Google Scholar] [CrossRef]
- Yao, Y.; Zhou, Y.; Liu, L.; Xu, Y.; Chen, Q.; Wang, Y.; Wu, S.; Deng, Y.; Zhang, J.; Shao, A. Nanoparticle-based drug delivery in cancer therapy and its role in overcoming drug resistance. Front. Mol. Biosci. 2020, 7, 193. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Xie, Q.; Sun, Y. Advances in nanomaterial-based targeted drug delivery systems. Front. Bioeng. Biotechnol. 2023, 11, 1177151. [Google Scholar] [CrossRef] [PubMed]
- Abd Elkodous, M.; Olojede, S.; Morsi, M.; El-Sayyad, G.S. Nanomaterial-based drug delivery systems as promising carriers for patients with COVID-19. RSC Adv. 2021, 11, 26463–26480. [Google Scholar] [CrossRef]
- Nile, S.H.; Baskar, V.; Selvaraj, D.; Nile, A.; Xiao, J.; Kai, G. Nanotechnologies in food science: Applications, recent trends, and future perspectives. Nano-Micro Lett. 2020, 12, 45. [Google Scholar] [CrossRef]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. In Nano-Enabled Medical Applications; Jenny Stanford Publishing: New York, NY, USA, 2020; pp. 61–91. [Google Scholar]
- Sahoo, S.K.; Jain, T.K.; Reddy, M.K.; Labhasetwar, V. Nano-sized carriers for drug delivery. In NanoBioTechnology: BioInspired Devices and Materials of the Future; Humana Press: Totowa, NJ, USA, 2008; pp. 329–348. [Google Scholar] [CrossRef]
- Plucinski, A.; Lyu, Z.; Schmidt, B.V. Polysaccharide nanoparticles: From fabrication to applications. J. Mater. Chem. B 2021, 9, 7030–7062. [Google Scholar] [CrossRef]
- Bhatia, S.; Bhatia, S. Nanoparticles types, classification, characterization, fabrication methods and drug delivery applications. In Natural Polymer Drug Delivery Systems: Nanoparticles, Plants, and Algae; Springer: Cham, Switzerland, 2016; pp. 33–93. [Google Scholar] [CrossRef]
- Kaushik, S. Polymeric and ceramic nanoparticles: Possible role in biomedical applications. In Handbook of Polymer and Ceramic Nanotechnology; Springer: Berlin, Germany, 2021; pp. 1293–1308. [Google Scholar]
- Teame, T.; Wang, A.; Xie, M.; Zhang, Z.; Yang, Y.; Ding, Q.; Gao, C.; Olsen, R.E.; Ran, C.; Zhou, Z. Paraprobiotics and postbiotics of probiotic Lactobacilli, their positive effects on the host and action mechanisms: A review. Front. Nutr. 2020, 7, 570344. [Google Scholar] [CrossRef]
- Raveschot, C.; Cudennec, B.; Coutte, F.; Flahaut, C.; Fremont, M.; Drider, D.; Dhulster, P. Production of bioactive peptides by Lactobacillus species: From gene to application. Front. Microbiol. 2018, 9, 2354. [Google Scholar] [CrossRef]
- Ahmed, I.; Asgher, M.; Sher, F.; Hussain, S.M.; Nazish, N.; Joshi, N.; Sharma, A.; Parra-Saldívar, R.; Bilal, M.; Iqbal, H.M. Exploring marine as a rich source of bioactive peptides: Challenges and opportunities from marine pharmacology. Mar. Drugs 2022, 20, 208. [Google Scholar] [CrossRef]
- Qin, M.; Deng, Y.; Maharjan, S.; Wang, Z.; Huang, D. Engineered tissues using bioactive hydrogels. Front. Bioeng. Biotechnol. 2022, 10, 975907. [Google Scholar] [CrossRef] [PubMed]
- Handing, K.B.; Shabalin, I.G.; Szlachta, K.; Majorek, K.A.; Minor, W. Crystal structure of equine serum albumin in complex with cetirizine reveals a novel drug binding site. Mol. Immunol. 2016, 71, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Caraceni, P.; Tufoni, M.; Bonavita, M.E. Clinical use of albumin. Blood Transfus. 2013, 11 (Suppl. 4), s18. [Google Scholar] [PubMed]
- Aguilera-Garrido, A.; del Castillo-Santaella, T.; Yang, Y.; Galisteo-Gonzalez, F.; Gálvez-Ruiz, M.J.; Molina-Bolívar, J.A.; Holgado-Terriza, J.A.; Cabrerizo-Vílchez, M.Á.; Maldonado-Valderrama, J. Applications of serum albumins in delivery systems: Differences in interfacial behaviour and interacting abilities with polysaccharides. Adv. Colloid Interface Sci. 2021, 290, 102365. [Google Scholar] [CrossRef]
- Feuser, P.E.; Guindani, C.; Possato, J.C.; Guessi, J.P.; Cordeiro, A.P.; Machado-de-Ávila, R.A.; Sayer, C.; de Araújo, P.H.H. Bovine Serum Albumin Conjugation in Superparamagnetic/Poly(methyl methacrylate) Nanoparticles as an Alternative for Magnetic Enzyme-Linked Immunosorbent Assays. J. Nanosci. Nanotechnol. 2021, 21, 5493–5498. [Google Scholar] [CrossRef]
- Qasim, M.; Asghar, K.; Das, D. Preparation and characterization of CoFe2O4 and CoFe2O4@ Albumen nanoparticles for biomedical applications. Ceram. Int. 2019, 45, 24971–24981. [Google Scholar] [CrossRef]
- Mustafa, A.; Widodo, M.A.; Kristianto, Y. Albumin and zinc content of snakehead fish (Channa striata) extract and its role in health. IEESE Int. J. Sci. Technol. 2012, 1, 1–8. [Google Scholar]
- Liu, W.; Sun, J.; Sun, Y.; Xiang, Y.; Yan, Y.; Han, Z.; Bi, W.; Yang, F.; Zhou, Q.; Wang, L. Multifunctional injectable protein-based hydrogel for bone regeneration. Chem. Eng. J. 2020, 394, 124875. [Google Scholar] [CrossRef]
- Yang, S.-P.; Wang, T.-J.; Huang, C.-C.; Chang, S.-C.; Liang, S.-Y.; Yu, C.-H. Influence of albumin and physical activity on postoperative recovery in patients with colorectal cancer: An observational study. Eur. J. Oncol. Nurs. 2021, 54, 102027. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, J.; Li, B.; Li, D.; Meng, Z.; Sun, S.-K. Biocompatible therapeutic albumin/genipin bioglue for postoperative wound adhesion and residual tumor ablation. Biomaterials 2021, 279, 121179. [Google Scholar] [CrossRef]
- Mushtaq, S.; Naqvi, Z.A.; Siddiqui, A.A.; Ahmed, N. Albumin precursor and Hsp70 modulate corneal wound healing in an organ culture model. Acta Histochem. 2011, 113, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Qi, X.; Wu, J.; Guo, C.; Wu, X. Therapeutic contact lenses fabricated by hyaluronic acid and silver incorporated bovine serum albumin porous films for the treatment of alkali-burned corneal wound. Int. J. Biol. Macromol. 2021, 184, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Homaeigohar, S.; Monavari, M.; Koenen, B.; Boccaccini, A.R. Biomimetic biohybrid nanofibers containing bovine serum albumin as a bioactive moiety for wound dressing. Mater. Sci. Eng. C 2021, 123, 111965. [Google Scholar] [CrossRef]
- Zhang, F.; Yang, H.; Yang, Y.; Wang, H.; Li, X.; Wu, X. Stretchable and biocompatible bovine serum albumin fibrous films supported silver for accelerated bacteria-infected wound healing. Chem. Eng. J. 2021, 417, 129145. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Tsai, T.-Y.; Zarie, E.S.; Elbahri, M.; Young, T.-H.; Boccaccini, A.R. Bovine Serum Albumin (BSA)/polyacrylonitrile (PAN) biohybrid nanofibers coated with a biomineralized calcium deficient hydroxyapatite (HA) shell for wound dressing. Mater. Sci. Eng. C 2020, 116, 111248. [Google Scholar] [CrossRef]
- Wu, S.; Wang, X.; Bao, Y.; Zhang, C.; Liu, H.; Li, Z.; Chen, M.; Wang, C.; Guo, Q.; Peng, X. Molecular insight on the binding of monascin to bovine serum albumin (BSA) and its effect on antioxidant characteristics of monascin. Food Chem. 2020, 315, 126228. [Google Scholar] [CrossRef] [PubMed]
- Montero, G.; Arriagada, F.; Günther, G.; Bollo, S.; Mura, F.; Berríos, E.; Morales, J. Phytoestrogen coumestrol: Antioxidant capacity and its loading in albumin nanoparticles. Int. J. Pharm. 2019, 562, 86–95. [Google Scholar] [CrossRef]
- Huo, J.; Luo, X.; Huang, M.; Wu, J.; Zhang, J.; Liu, X.; Li, H.; Sun, X. Identification and antioxidant activity of a novel peptide from Baijiu. Int. J. Pept. Res. Ther. 2020, 26, 1199–1210. [Google Scholar] [CrossRef]
- Huo, X.-z.; Wang, X.; Yang, R.; Qu, L.-b.; Zeng, H.-j. Studies on the effect of a Fupenzi glycoprotein on the fibrillation of bovine serum albumin and its antioxidant activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 237, 118387. [Google Scholar] [CrossRef]
- Ramadori, G. Albumin infusion in critically ill COVID-19 patients: Hemodilution and anticoagulation. Int. J. Mol. Sci. 2021, 22, 7126. [Google Scholar] [CrossRef]
- Nurdiansyah, R.; Rifa’i, M. A comparative analysis of serum albumin from different species to determine a natural source of albumin that might be useful for human therapy. J. Taibah Univ. Med. Sci. 2016, 11, 243–249. [Google Scholar] [CrossRef][Green Version]
- Nathasia, T.S. Albumin, Important Therapy & When to Use It in Ten Patients (Adult & Child): Case Report. J. Dermatol. Res. Ther. 2020, 6, 3–7. [Google Scholar]
- Violi, F.; Ceccarelli, G.; Loffredo, L.; Alessandri, F.; Cipollone, F.; D’ardes, D.; D’Ettorre, G.; Pignatelli, P.; Venditti, M.; Mastroianni, C.M. Albumin supplementation dampens hypercoagulability in COVID-19: A preliminary report. Thromb. Haemost. 2021, 121, 102–105. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y.; Zhang, C.; Huang, F.; Wang, F.; Yuan, J.; Wang, Z.; Li, J.; Li, J.; Feng, C. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci. China Life Sci. 2020, 63, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Suh, S.H.; Ma, S.K.; Kim, S.W.; Bae, E.H. Angiotensin-converting enzyme 2 and kidney diseases in the era of coronavirus disease 2019. Korean J. Intern. Med. 2021, 36, 247. [Google Scholar] [CrossRef]
- Chen, D.-Y.; Shien, J.-H.; Tiley, L.; Chiou, S.-S.; Wang, S.-Y.; Chang, T.-J.; Lee, Y.-J.; Chan, K.-W.; Hsu, W.-L. Curcumin inhibits influenza virus infection and haemagglutination activity. Food Chem. 2010, 119, 1346–1351. [Google Scholar] [CrossRef]
- Li, J.; Song, D.; Wang, S.; Dai, Y.; Zhou, J.; Gu, J. Antiviral effect of epigallocatechin gallate via impairing porcine circovirus type 2 attachment to host cell receptor. Viruses 2020, 12, 176. [Google Scholar] [CrossRef]
- Chowdhury, R.; Boorla, V.S.; Maranas, C.D. Computational biophysical characterization of the SARS-CoV-2 spike protein binding with the ACE2 receptor and implications for infectivity. Comput. Struct. Biotechnol. J. 2020, 18, 2573–2582. [Google Scholar] [CrossRef]
- Vaduganathan, M.; Vardeny, O.; Michel, T.; McMurray, J.J.; Pfeffer, M.A.; Solomon, S.D. Renin–angiotensin–aldosterone system inhibitors in patients with Covid-19. N. Engl. J. Med. 2020, 382, 1653–1659. [Google Scholar] [CrossRef]
- Patel, V.B.; Zhong, J.-C.; Grant, M.B.; Oudit, G.Y. Role of the ACE2/angiotensin 1–7 axis of the renin–angiotensin system in heart failure. Circ. Res. 2016, 118, 1313–1326. [Google Scholar] [CrossRef]
- Ramchand, J.; Patel, S.K.; Srivastava, P.M.; Farouque, O.; Burrell, L.M. Elevated plasma angiotensin converting enzyme 2 activity is an independent predictor of major adverse cardiac events in patients with obstructive coronary artery disease. PLoS ONE 2018, 13, e0198144. [Google Scholar] [CrossRef] [PubMed]
- Bader, M. ACE2, angiotensin-(1–7), and Mas: The other side of the coin. Pflüg. Arch.-Eur. J. Physiol. 2013, 465, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-C.; Serio, A.; Amdursky, N.; Besnard, C.; Stevens, M.M. Fabrication of hemin-doped serum albumin-based fibrous scaffolds for neural tissue engineering applications. ACS Appl. Mater. Interfaces 2018, 10, 5305–5317. [Google Scholar] [CrossRef] [PubMed]
- Cometta, S.; Bock, N.; Suresh, S.; Dargaville, T.R.; Hutmacher, D.W. Antibacterial albumin-tannic acid coatings for scaffold-guided breast reconstruction. Front. Bioeng. Biotechnol. 2021, 9, 638577. [Google Scholar] [CrossRef]
- Tao, C.; Zhu, W.; Iqbal, J.; Xu, C.; Wang, D.-A. Stabilized albumin coatings on engineered xenografts for attenuation of acute immune and inflammatory responses. J. Mater. Chem. B 2020, 8, 6080–6091. [Google Scholar] [CrossRef]
- Investigators, S.S. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N. Engl. J. Med. 2004, 350, 2247–2256. [Google Scholar]
- Investigators, S.; Finfer, S.; McEvoy, S.; Bellomo, R.; McArthur, C.; Myburgh, J. Impact of albumin compared to saline on organ function and mortality of patients with severe sepsis. Intensive Care Med. 2011, 37, 86–96. [Google Scholar] [CrossRef]
- Caironi, P.; Tognoni, G.; Masson, S.; Fumagalli, R.; Pesenti, A.; Romero, M.; Fanizza, C.; Caspani, L.; Faenza, S.; Grasselli, G. Albumin replacement in patients with severe sepsis or septic shock. N. Engl. J. Med. 2014, 370, 1412–1421. [Google Scholar] [CrossRef]
- Park, C.H.L.; de Almeida, J.P.; de Oliveira, G.Q.; Rizk, S.I.; Fukushima, J.T.; Nakamura, R.E.; Mourão, M.M.; Galas, F.R.B.G.; Abdala, E.; Freire, M.P. Lactated Ringer’s versus 4% albumin on lactated Ringer’s in early sepsis therapy in cancer patients: A pilot single-center randomized trial. Crit. Care Med. 2019, 47, e798–e805. [Google Scholar] [CrossRef]
- Delaney, A.P.; Dan, A.; McCaffrey, J.; Finfer, S. The role of albumin as a resuscitation fluid for patients with sepsis: A systematic review and meta-analysis. Crit. Care Med. 2011, 39, 386–391. [Google Scholar] [CrossRef]
- Haase, N.; Perner, A.; Hennings, L.I.; Siegemund, M.; Lauridsen, B.; Wetterslev, M.; Wetterslev, J. Hydroxyethyl starch 130/0.38-0.45 versus crystalloid or albumin in patients with sepsis: Systematic review with meta-analysis and trial sequential analysis. Bmj 2013, 346, f839. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Crit. Care Med. 2021, 49, e1063–e1143. [Google Scholar] [CrossRef]
- Philips, C.A.; Maiwall, R.; Sharma, M.K.; Jindal, A.; Choudhury, A.K.; Kumar, G.; Bhardwaj, A.; Mitra, L.G.; Agarwal, P.M.; Sarin, S.K. Comparison of 5% human albumin and normal saline for fluid resuscitation in sepsis induced hypotension among patients with cirrhosis (FRISC study): A randomized controlled trial. Hepatol. Int. 2021, 15, 983–994. [Google Scholar] [CrossRef]
- Karimi, M.; Bahrami, S.; Ravari, S.B.; Zangabad, P.S.; Mirshekari, H.; Bozorgomid, M.; Shahreza, S.; Sori, M.; Hamblin, M.R. Albumin nanostructures as advanced drug delivery systems. Expert Opin. Drug Deliv. 2016, 13, 1609–1623. [Google Scholar] [CrossRef]
- Elzoghby, A.O.; Samy, W.M.; Elgindy, N.A. Albumin-based nanoparticles as potential controlled release drug delivery systems. J. Control. Release 2012, 157, 168–182. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Jeon, S.I.; Ahn, C.-H.; Shim, M.K.; Kim, K. Emerging albumin-binding anticancer drugs for tumor-targeted drug delivery: Current understandings and clinical translation. Pharmaceutics 2022, 14, 728. [Google Scholar] [CrossRef] [PubMed]
- Rahimizadeh, P.; Yang, S.; Lim, S.I. Albumin: An emerging opportunity in drug delivery. Biotechnol. Bioprocess Eng. 2020, 25, 985–995. [Google Scholar] [CrossRef]
- Carter, D.C.; Ho, J.X. Structure of serum albumin. Adv. Protein Chem. 1994, 45, 153–203. [Google Scholar]
- Schnitzer, J.E. gp60 is an albumin-binding glycoprotein expressed by continuous endothelium involved in albumin transcytosis. Am. J. Physiol.-Heart Circ. Physiol. 1992, 262, H246–H254. [Google Scholar] [CrossRef]
- Pyzik, M.; Rath, T.; Lencer, W.I.; Baker, K.; Blumberg, R.S. FcRn: The architect behind the immune and nonimmune functions of IgG and albumin. J. Immunol. 2015, 194, 4595–4603. [Google Scholar] [CrossRef]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release 2000, 65, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Desai, N. Nanoparticle albumin-bound paclitaxel (Abraxane®). In Albumin in Medicine: Pathological and Clinical Applications; Springer: Berlin, Germany, 2016; pp. 101–119. [Google Scholar]
- Sur, S.; Rathore, A.; Dave, V.; Reddy, K.R.; Chouhan, R.S.; Sadhu, V. Recent developments in functionalized polymer nanoparticles for efficient drug delivery system. Nano-Struct. Nano-Objects 2019, 20, 100397. [Google Scholar] [CrossRef]
- Viegas, C.; Patrício, A.B.; Prata, J.; Fonseca, L.; Macedo, A.S.; Duarte, S.O.; Fonte, P. Advances in Pancreatic Cancer treatment by Nano-Based drug delivery systems. Pharmaceutics 2023, 15, 2363. [Google Scholar] [CrossRef]
- Kratz, F. Albumin as a drug carrier: Design of prodrugs, drug conjugates and nanoparticles. J. Control. Release 2008, 132, 171–183. [Google Scholar] [CrossRef]
- Vaglio, S.; Calizzani, G.; Lanzoni, M.; Candura, F.; Profili, S.; Catalano, L.; Cannata, L.; Liumbruno, G.M.; Grazzini, G. The demand for human albumin in Italy. Blood Transfus. 2013, 11 (Suppl. 4), s26. [Google Scholar] [PubMed]
- Martin, G.S.; Moss, M.; Wheeler, A.P.; Mealer, M.; Morris, J.A.; Bernard, G.R. A randomized, controlled trial of furosemide with or without albumin in hypoproteinemic patients with acute lung injury. Crit. Care Med. 2005, 33, 1681–1687. [Google Scholar] [CrossRef]
- Uhlig, C.; Silva, P.L.; Deckert, S.; Schmitt, J.; de Abreu, M.G. Albumin versus crystalloid solutions in patients with the acute respiratory distress syndrome: A systematic review and meta-analysis. Crit. Care 2014, 18, R10. [Google Scholar] [CrossRef]
- Cordemans, C.; De Laet, I.; Van Regenmortel, N.; Schoonheydt, K.; Dits, H.; Martin, G.; Huber, W.; Malbrain, M.L. Aiming for a negative fluid balance in patients with acute lung injury and increased intra-abdominal pressure: A pilot study looking at the effects of PAL-treatment. Ann. Intensive Care 2012, 2, S15. [Google Scholar] [CrossRef]
- Labgaa, I.; Joliat, G.-R.; Kefleyesus, A.; Mantziari, S.; Schäfer, M.; Demartines, N.; Hübner, M. Is postoperative decrease of serum albumin an early predictor of complications after major abdominal surgery? A prospective cohort study in a European centre. BMJ Open 2017, 7, e013966. [Google Scholar] [CrossRef]
- Sang, B.-H.; Bang, J.-Y.; Song, J.-G.; Hwang, G.-S. Hypoalbuminemia within two postoperative days is an independent risk factor for acute kidney injury following living donor liver transplantation: A propensity score analysis of 998 consecutive patients. Crit. Care Med. 2015, 43, 2552–2561. [Google Scholar] [CrossRef]
- Haynes, G.; Navickis, R.; Wilkes, M. Albumin administration–what is the evidence of clinical benefit? A systematic review of randomized controlled trials. Eur. J. Anaesthesiol. 2003, 20, 771–793. [Google Scholar] [CrossRef]
- Fitzgerald, D.C.; Holmes, S.D.; Onge, J.R.S.; Ioanou, C.; Martin, L.M.; Ad, N. Systemic inflammatory response during cardiac surgery: A pilot study. Innovations 2015, 10, 125–132. [Google Scholar] [PubMed]
- Skhirtladze, K.; Base, E.; Lassnigg, A.; Kaider, A.; Linke, S.; Dworschak, M.; Hiesmayr, M. Comparison of the effects of albumin 5%, hydroxyethyl starch 130/0.4 6%, and Ringer’s lactate on blood loss and coagulation after cardiac surgery. Br. J. Anaesth. 2014, 112, 255–264. [Google Scholar] [CrossRef]
- Navickis, R.J.; Haynes, G.R.; Wilkes, M.M. Effect of hydroxyethyl starch on bleeding after cardiopulmonary bypass: A meta-analysis of randomized trials. J. Thorac. Cardiovasc. Surg. 2012, 144, 223–230.e5. [Google Scholar] [CrossRef] [PubMed]
- Kingeter, A.J.; Kingeter, M.A.; Shaw, A.D. Fluids and organ dysfunction: A narrative review of the literature and discussion of 5 controversial topics. J. Cardiothorac. Vasc. Anesth. 2018, 32, 2054–2066. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.-H.; Kim, W.-J.; Kim, J.-Y.; Chin, J.-H.; Choi, D.-K.; Sim, J.-Y.; Choo, S.-J.; Chung, C.-H.; Lee, J.-W.; Choi, I.-C. Effect of exogenous albumin on the incidence of postoperative acute kidney injury in patients undergoing off-pump coronary artery bypass surgery with a preoperative albumin level of less than 4.0 g/dl. Surv. Anesthesiol. 2016, 60, 227–228. [Google Scholar] [CrossRef]
- Nadim, M.K.; Forni, L.G.; Bihorac, A.; Hobson, C.; Koyner, J.L.; Shaw, A.; Arnaoutakis, G.J.; Ding, X.; Engelman, D.T.; Gasparovic, H. Cardiac and vascular surgery–associated acute kidney injury: The 20th international consensus conference of the ADQI (acute disease quality initiative) group. J. Am. Heart Assoc. 2018, 7, e008834. [Google Scholar] [CrossRef]
- Feigin, V.L.; Nichols, E.; Alam, T.; Bannick, M.S.; Beghi, E.; Blake, N.; Culpepper, W.J.; Dorsey, E.R.; Elbaz, A.; Ellenbogen, R.G. Global, regional, and national burden of neurological disorders, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 459–480. [Google Scholar] [CrossRef]
- Ibrahim, G.M.; Macdonald, R.L. The effects of fluid balance and colloid administration on outcomes in patients with aneurysmal subarachnoid hemorrhage: A propensity score-matched analysis. Neurocrit. Care 2013, 19, 140–149. [Google Scholar] [CrossRef]
- Martin, R.H.; Yeatts, S.D.; Hill, M.D.; Moy, C.S.; Ginsberg, M.D.; Palesch, Y.Y. ALIAS (Albumin in Acute Ischemic Stroke) trials: Analysis of the combined data from parts 1 and 2. Stroke 2016, 47, 2355–2359. [Google Scholar] [CrossRef]
- Schrader, J.; Lüders, S.; Kulschewski, A.; Berger, J.r.; Zidek, W.; Treib, J.; Einhäupl, K.; Diener, H.C.; Dominiak, P. The ACCESS study: Evaluation of acute candesartan cilexetil therapy in stroke survivors. Stroke 2003, 34, 1699–1703. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.J.; Myburgh, J.; Heritier, S.; Finfer, S.; Bellomo, R.; Billot, L.; Murray, L.; Vallance, C.; the SAFE-TBI Investigators; the Australian and New Zealand Intensive Care Society Clinical Trials Group. Albumin resuscitation for traumatic brain injury: Is intracranial hypertension the cause of increased mortality? J. Neurotrauma 2013, 30, 512–518. [Google Scholar] [CrossRef]
- Huang, Z.; Dong, W.; Yan, Y.; Xiao, Q.; Man, Y. Effects of intravenous human albumin and furosemide on EEG recordings in patients with intracerebral hemorrhage. Clin. Neurophysiol. 2002, 113, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Dong, V.; Karvellas, C.J. Acute-on-chronic liver failure: Objective admission and support criteria in the intensive care unit. JHEP Rep. 2019, 1, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Weil, D.; Levesque, E.; McPhail, M.; Cavallazzi, R.; Theocharidou, E.; Cholongitas, E.; Galbois, A.; Pan, H.C.; Karvellas, C.J.; Sauneuf, B. Prognosis of cirrhotic patients admitted to intensive care unit: A meta-analysis. Ann. Intensive Care 2017, 7, 33. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, S.; Duan, Z.; Hu, K.-Q. Overview on acute-on-chronic liver failure. Front. Med. 2016, 10, 1–17. [Google Scholar] [CrossRef]
- Bernardi, M.; Caraceni, P.; Navickis, R.J.; Wilkes, M.M. Albumin infusion in patients undergoing large-volume paracentesis: A meta-analysis of randomized trials. Hepatology 2012, 55, 1172–1181. [Google Scholar] [CrossRef]
- Caraceni, P.; Riggio, O.; Angeli, P.; Alessandria, C.; Neri, S.; Foschi, F.G.; Levantesi, F.; Airoldi, A.; Boccia, S.; Svegliati-Baroni, G. Long-term albumin administration in decompensated cirrhosis (ANSWER): An open-label randomised trial. Lancet 2018, 391, 2417–2429. [Google Scholar] [CrossRef]
- Best, L.M.; Freeman, S.C.; Sutton, A.J.; Cooper, N.J.; Tng, E.L.; Csenar, M.; Hawkins, N.; Pavlov, C.S.; Davidson, B.R.; Thorburn, D. Treatment for hepatorenal syndrome in people with decompensated liver cirrhosis: A network meta-analysis. Cochrane Database Syst. Rev. 2019. [Google Scholar] [CrossRef]
- Alagna, L.; Meessen, J.M.; Bellani, G.; Albiero, D.; Caironi, P.; Principale, I.; Vivona, L.; Grasselli, G.; Motta, F.; Agnelli, N.M. Higher levels of IgA and IgG at sepsis onset are associated with higher mortality: Results from the Albumin Italian Outcome Sepsis (ALBIOS) trial. Ann. Intensive Care 2021, 11, 161. [Google Scholar] [CrossRef]
- Denizli, A. Plasma fractionation: Conventional and chromatographic methods for albumin purification. Hacet. J. Biol. Chem. 2011, 39, 315–341. [Google Scholar]
- Aghaie, A.; Khorsand Mohammad Pour, H.; Banazadeh, S. Preparation of albumin from human plasma by heat denaturation method in plasma bag. Transfus. Med. 2012, 22, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Raoufinia, R.; Mota, A.; Nozari, S.; Aghebati Maleki, L.; Balkani, S.; Abdolalizadeh, J. A methodological approach for purification and characterization of human serum albumin. J. Immunoass. Immunochem. 2016, 37, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.; Stein, W.H. Column chromatography of peptides and proteins. In Advances in Protein Chemistry; Elsevier: Oxford, UK, 1956; Volume 11, pp. 191–236. [Google Scholar]
- Ward, W.W.; Swiatek, G. Protein purification. Curr. Anal. Chem. 2009, 5, 85–105. [Google Scholar] [CrossRef]
- Altova, E.P.; Hargittai, I. Mikhail S. Tsvet—Pioneer of chromatography—150 years from his birth. Struct. Chem. 2022, 33, 1–3. [Google Scholar] [CrossRef]
- Rufino, A.F.; Almeida, M.R.; Sharma, M.; Coutinho, J.A.; Freire, M.G. Separation of Albumin from Bovine Serum Applying Ionic-Liquid-Based Aqueous Biphasic Systems. Appl. Sci. 2022, 12, 707. [Google Scholar] [CrossRef]
- Curling, J.; Berglöf, J.; Lindquist, L.-O.; Eriksson, S. A chromatographic procedure for the purification of human plasma albumin. Vox Sang. 1977, 33, 97–107. [Google Scholar] [CrossRef]
- Frerick, C.; Kreis, P.; Górak, A.; Tappe, A.; Melzner, D. Simulation of a human serum albumin downstream process incorporating ion-exchange membrane adsorbers. Chem. Eng. Process. Process Intensif. 2008, 47, 1128–1138. [Google Scholar] [CrossRef]
- Boi, C. Membrane adsorbers as purification tools for monoclonal antibody purification. J. Chromatogr. B 2007, 848, 19–27. [Google Scholar] [CrossRef]
- Chen, J.; Yu, B.; Cong, H.; Shen, Y. Recent development and application of membrane chromatography. Anal. Bioanal. Chem. 2023, 415, 45–65. [Google Scholar] [CrossRef]
- Rathore, A.S.; Kumar, D.; Kateja, N. Recent developments in chromatographic purification of biopharmaceuticals. Biotechnol. Lett. 2018, 40, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, S.R.; Mason, B.; Doucette, A.A. Review of membrane separation models and technologies: Processing complex food-based biomolecular fractions. Food Bioprocess Technol. 2021, 14, 415–428. [Google Scholar] [CrossRef]
- Charcosset, C. Membrane processes in biotechnology: An overview. Biotechnol. Adv. 2006, 24, 482–492. [Google Scholar] [CrossRef]
- Low, D.; O’Leary, R.; Pujar, N.S. Future of antibody purification. J. Chromatogr. B 2007, 848, 48–63. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, H.L.; Fahrner, R.L.; Xu, Y.; Norling, L.A.; Blank, G.S. Membrane ion-exchange chromatography for process-scale antibody purification. J. Chromatogr. A 2001, 907, 145–154. [Google Scholar] [CrossRef]
- Stanton, P. Gel filtration chromatography. In HPLC of Peptides and Proteins: Methods and Protocols; Humana Press: Totowa, NJ, USA, 2004; pp. 55–73. [Google Scholar] [CrossRef]
- Raoufinia, R.; Balkani, S.; Keyhanvar, N.; Mahdavipor, B.; Abdolalizadeh, J. Human albumin purification: A modified and concise method. J. Immunoass. Immunochem. 2018, 39, 687–695. [Google Scholar] [CrossRef]
- Cuatrecasas, P.; Wilchek, M.; Anfinsen, C.B. Selective enzyme purification by affinity chromatography. Proc. Natl. Acad. Sci. USA 1968, 61, 636–643. [Google Scholar] [CrossRef]
- Hage, D.S.; Anguizola, J.A.; Bi, C.; Li, R.; Matsuda, R.; Papastavros, E.; Pfaunmiller, E.; Vargas, J.; Zheng, X. Pharmaceutical and biomedical applications of affinity chromatography: Recent trends and developments. J. Pharm. Biomed. Anal. 2012, 69, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Abdolalizadeh, J.; Nouri, M.; Majidi Zolbanin, J.; Barzegari, A.; Baradaran, B.; Barar, J.; Coukos, G.; Omidi, Y. Targeting cytokines: Production and characterization of anti-TNF-α scFvs by phage display technology. Curr. Pharm. Des. 2013, 19, 2839–2847. [Google Scholar] [CrossRef]
- Abdolalizadeh, J.; Zolbanin, J.M.; Nouri, M.; Baradaran, B.; Movassaghpour, A.; Farajnia, S.; Omidi, Y. Affinity purification of tumor necrosis factor-α expressed in raji cells by produced scFv antibody coupled CNBr-activated sepharose. Adv. Pharm. Bull. 2013, 3, 19. [Google Scholar]
- Abdolalizadeh, J.; Nouri, M.; Zolbanin, J.M.; Baradaran, B.; Barzegari, A.; Omidi, Y. Downstream characterization of anti-TNF-α single chain variable fragment antibodies. Hum. Antibodies 2012, 21, 41–48. [Google Scholar] [CrossRef]
- John, W.; Jones, A. Affinity chromatography: A precise method for glycosylated albumin estimation. Ann. Clin. Biochem. 1985, 22, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Iannotti, A.M.; Rabideau, D.A.; Dougherty, J.J. Characterization of purified avian 90,000-Da heat shock protein. Arch. Biochem. Biophys. 1988, 264, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Odunuge, O.O.; Shazhko, A. Ammonium sulfate precipitation combined with liquid chromatography is sufficient for purification of bovine serum albumin that is suitable for most routine laboratory applications. Biochem. Compd. 2013. [Google Scholar] [CrossRef][Green Version]
- Padashi, N.; Arjmand, M.; Rajaei, S.; Dabbagh, A. Purification of human serum albumin by ion exchange chromatography. J. Cell. Mol. Anesth. 2016, 1, e149524. [Google Scholar]
- Neyestani, T.R.; Djalali, M.; Pezeshki, M. Isolation of α-lactalbumin, β-lactoglobulin, and bovine serum albumin from cow’s milk using gel filtration and anion-exchange chromatography including evaluation of their antigenicity. Protein Expr. Purif. 2003, 29, 202–208. [Google Scholar] [CrossRef]
- Wichman, A.; Andersson, L.-O. Purification of human serum albumin by affinity chromatography. Biochim. Biophys. Acta (BBA)-Gen. Subj. 1974, 372, 218–224. [Google Scholar] [CrossRef]
- Brzeski, H.; Katenhusen, R.A.; Sullivan, A.G.; Russell, S.; George, A.; Somiari, R.I.; Shriver, C. Albumin depletion method for improved plasma glycoprotein analysis by two-dimensional difference gel electrophoresis. Biotechniques 2003, 35, 1128–1132. [Google Scholar] [CrossRef]
- Albumin Market Size, Share & Trends Analysis Report by Product (Human Serum, Bovine Serum, Recombinant), by Application (Therapeutics, Drug Formulation), by End-User, by Region, and Segment Forecasts, 2023 to 2030. Available online: https://www.grandviewresearch.com/industry-analysis/albumin-market-report (accessed on 15 April 2025).
- Garg, U.; Jain, N.; Kaul, S.; Nagaich, U. Role of Albumin as a Targeted Drug Carrier in the Management of Rheumatoid Arthritis: A Comprehensive Review. Mol. Pharm. 2023, 20, 5345–5358. [Google Scholar] [CrossRef]
- Moriyama, M.; Koshiba, T.; Ichinohe, T. Influenza A virus M2 protein triggers mitochondrial DNA-mediated antiviral immune responses. Nat. Commun. 2019, 10, 4624. [Google Scholar] [CrossRef]
- Järviö, N.; Parviainen, T.; Maljanen, N.-L.; Kobayashi, Y.; Kujanpää, L.; Ercili-Cura, D.; Landowski, C.P.; Ryynänen, T.; Nordlund, E.; Tuomisto, H.L. Ovalbumin production using Trichoderma reesei culture and low-carbon energy could mitigate the environmental impacts of chicken-egg-derived ovalbumin. Nat. Food 2021, 2, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Ovalbumin Powder Market Size, Share, Growth, and Industry Analysis, By Type (Organic Ovalbumin Powder, Conventional Ovalbumin Powder), Application By Supermarket, Convenience Store, Onlinle Store, Others), and Regional Forecast to 2028; June 2023. Available online: https://industrygrowthinsights.com/report/ovalbumin-powder-market/ (accessed on 21 March 2025).
- Tumpey, T.M.; Alvarez, R.; Swayne, D.E.; Suarez, D.L. Diagnostic approach for differentiating infected from vaccinated poultry on the basis of antibodies to NS1, the nonstructural protein of influenza A virus. J. Clin. Microbiol. 2005, 43, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Choudhry, N.R. Egg Albumin Protein Market By Flavor, Type, Application, Function & Region|Forecast 2022 to 2032. Future Market Insight July 2022. 2022.
- Global Big Data Market Research Report 2018: Insights, Opportunity, Analysis, Market Shares and Forecasts 2016–2023. Eastern Daylight Time; 2018.
- Albumin Market by Type (Human Serum Albumin, Bovine Serum, and, Recombinant Albumin) and Application (Therapeutics, Drug Formulation & Vaccines, Component of Media, and Others): Global Opportunity Analysis and Industry Forecast, 2021–2030; 2022.
- Samuel, H.S.; This, E.E.; Nweke-Maraizou, U.; Yakubu, S. Advancements in green chemistry: Sustainable synthesis and processes. J. Belarusian State Univ. Chem. 2024, 3–16. Available online: https://elib.bsu.by/handle/123456789/322710 (accessed on 15 April 2025).
- Otoni, C.G.; Queiros, M.V.; Sabadini, J.B.; Rojas, O.J.; Loh, W. Charge matters: Electrostatic complexation as a green approach to assemble advanced functional materials. ACS Omega 2020, 5, 1296–1304. [Google Scholar] [CrossRef]
- Mahomoodally, M.F.; Sadeer, N.; Edoo, M.; Venugopala, K.N. The potential application of novel drug delivery systems for phytopharmaceuticals and natural extracts–current status and future perspectives. Mini Rev. Med. Chem. 2021, 21, 2731–2746. [Google Scholar] [CrossRef]
- Fagnan, D.E. Analytics for Financing Drug Development. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 2015. [Google Scholar]
- Caliceti, P.; Veronese, F.M. Pharmacokinetic and biodistribution properties of poly(ethylene glycol)–protein conjugates. Adv. Drug Deliv. Rev. 2003, 55, 1261–1277. [Google Scholar] [CrossRef]
- Lamichhane, S.; Lee, S. Albumin nanoscience: Homing nanotechnology enabling targeted drug delivery and therapy. Arch. Pharmacal Res. 2020, 43, 118–133. [Google Scholar] [CrossRef]
- Schubert, J.; Chanana, M. Coating matters: Review on colloidal stability of nanoparticles with biocompatible coatings in biological media, living cells and organisms. Curr. Med. Chem. 2018, 25, 4553–4586. [Google Scholar] [CrossRef]


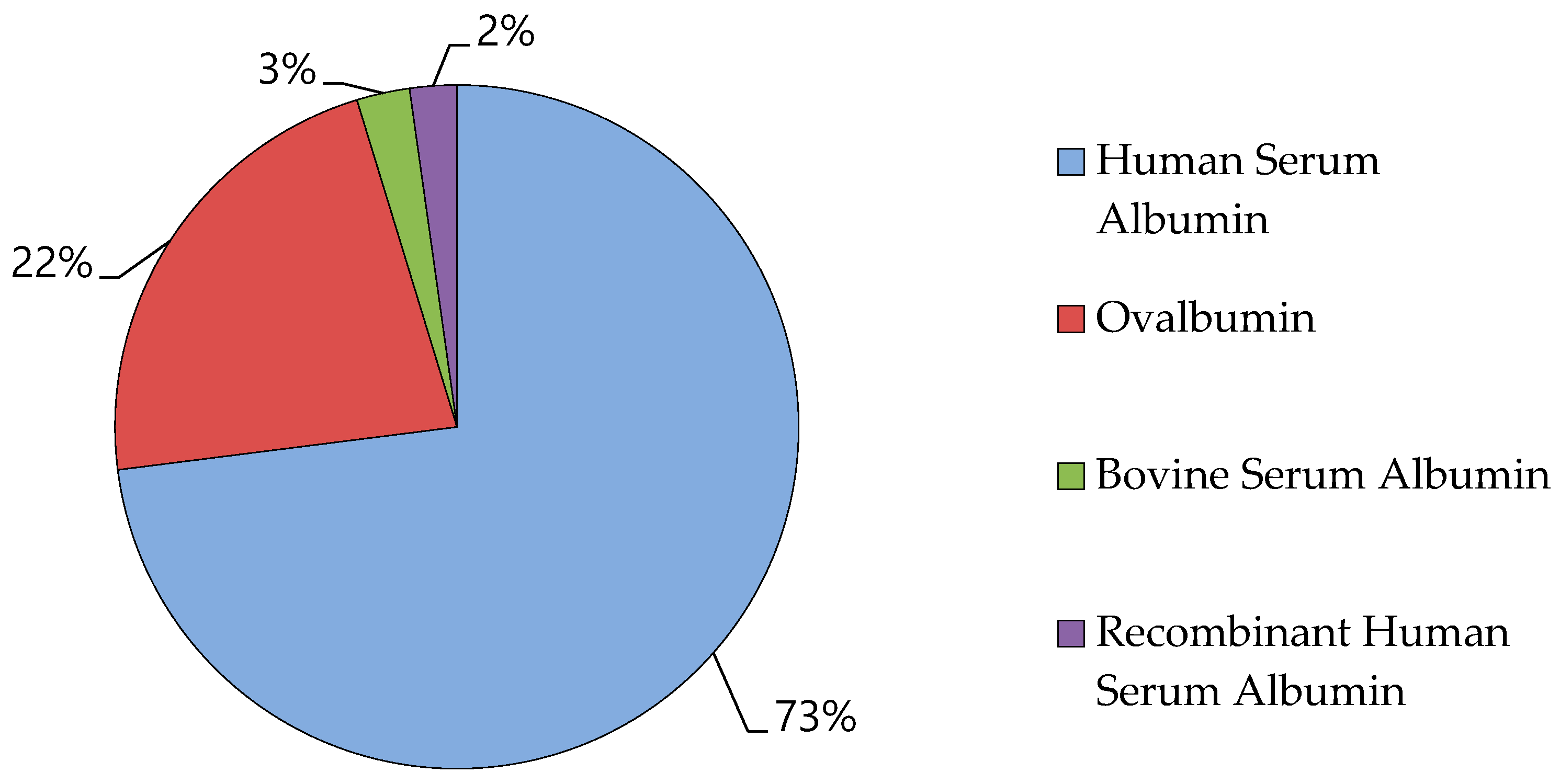
| Properties | Ovalbumin | Human Serum Albumin | Bovine Serum Albumin | References |
|---|---|---|---|---|
| Source | Chicken egg white | Human blood plasma | Cow blood plasma | [20,21,22] |
| Structure | Globular protein, single-chain polypeptide | Globular protein, single-chain polypeptide, heart-shaped structure | Globular protein, single-chain polypeptide, extensively used in labs | [23,24,25] |
| Molecular Weight | ~47 kDa | ~64 kDa | ~69 kDa | [26] |
| Isoelectric Point | ~4.8 | ~5.9 | ~4.7 | [26] |
| Function | Storage protein in eggs as an immunological tool | Maintains osmotic pressure and transports hormones | Carrier protein for hormones and fatty acids | [25,27,28] |
| Applications | Immunology research, vaccine development | Blood volume expander, drug delivery carrier | Research assays, cell culture diagnostics | [29] |
| Disadvantages | Potential allergen in some individuals, limited stability in certain conditions | Limited availability and high cost, potential transmission of bloodborne diseases | Potential source of prion transmission, batch-to-batch variability | [30] |
| Purification Method | Principle | Purity | Application | Disadvantages | Cost | Time Efficiency | Scalability | Reference |
|---|---|---|---|---|---|---|---|---|
| Heat-Shock Method | Non-specific denaturation and precipitation of unwanted proteins. | Moderate to high, may have impurities. | Rapid and simple purification for some heat-stable proteins. | Low specificity and limited to heat-stable proteins. | Low | Quick | Limited | [164] |
| Ammonium Sulfate Precipitation | Solubility differences in proteins at varying salt concentrations. | Moderate to high, depends on conditions. | Initial step for protein concentration, followed by other methods for higher purity. | Non-specific precipitation and challenging to achieve high purity. | Moderate | Moderate | Moderate | [165] |
| Ion Exchange Chromatography | Differential binding to charged groups on the resin. | High, especially with multiple steps. | Separation of proteins with different charges, effective for highly charged proteins. | Can be harsh for sensitive proteins and pH-sensitive. | High | Moderate | High | [166] |
| Gel Filtration Chromatography | Separation based on size; smaller molecules take longer to travel through the gel. | High, as it removes smaller impurities. | Effective for desalting, buffer exchange, and separating proteins of different sizes. | Not suitable for very small proteins, might not achieve high resolution. | Moderate | Moderate | Moderate | [167] |
| Affinity Chromatography | The target protein selectively binds to an immobilized ligand. | High, as it isolates the specific protein. | Excellent for highly specific purification of target proteins. | Ligand selection is critical and can be expensive. | High | Variable | Variable | [168] |
| Gel Electrophoresis | Movement of charged proteins through a gel matrix based on size and charge. | Depends on the gel and conditions used. | Analyzing protein mixtures, assessing purity, and estimating molecular weight. | Limited scale, time-consuming, and may not provide high purity for preparative purposes. | Low | Variable | Limited | [169] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashraf, M.A.; Shen, B.; Raza, M.A.; Yang, Z.; Amjad, M.N.; Din, G.u.; Yue, L.; Kousar, A.; Kanwal, Q.; Hu, Y. Albumin: A Review of Market Trends, Purification Methods, and Biomedical Innovations. Curr. Issues Mol. Biol. 2025, 47, 303. https://doi.org/10.3390/cimb47050303
Ashraf MA, Shen B, Raza MA, Yang Z, Amjad MN, Din Gu, Yue L, Kousar A, Kanwal Q, Hu Y. Albumin: A Review of Market Trends, Purification Methods, and Biomedical Innovations. Current Issues in Molecular Biology. 2025; 47(5):303. https://doi.org/10.3390/cimb47050303
Chicago/Turabian StyleAshraf, Muhammad Awais, Bei Shen, Muhammad Asif Raza, Zhu Yang, Muhammad Nabeel Amjad, Ghayyas ud Din, Lihuan Yue, Afifa Kousar, Qudsia Kanwal, and Yihong Hu. 2025. "Albumin: A Review of Market Trends, Purification Methods, and Biomedical Innovations" Current Issues in Molecular Biology 47, no. 5: 303. https://doi.org/10.3390/cimb47050303
APA StyleAshraf, M. A., Shen, B., Raza, M. A., Yang, Z., Amjad, M. N., Din, G. u., Yue, L., Kousar, A., Kanwal, Q., & Hu, Y. (2025). Albumin: A Review of Market Trends, Purification Methods, and Biomedical Innovations. Current Issues in Molecular Biology, 47(5), 303. https://doi.org/10.3390/cimb47050303







