Laryngeal Squamous Cell Carcinoma Is Characterized by a Stronger Expression of Nectin-4 Compared to Nectin-2
Abstract
1. Introduction
2. Materials and Methods
2.1. Tumor Samples
2.2. Tissue Microarray Construction and Immunohistochemistry
2.3. Evaluation of Staining
2.4. Statistical Analysis
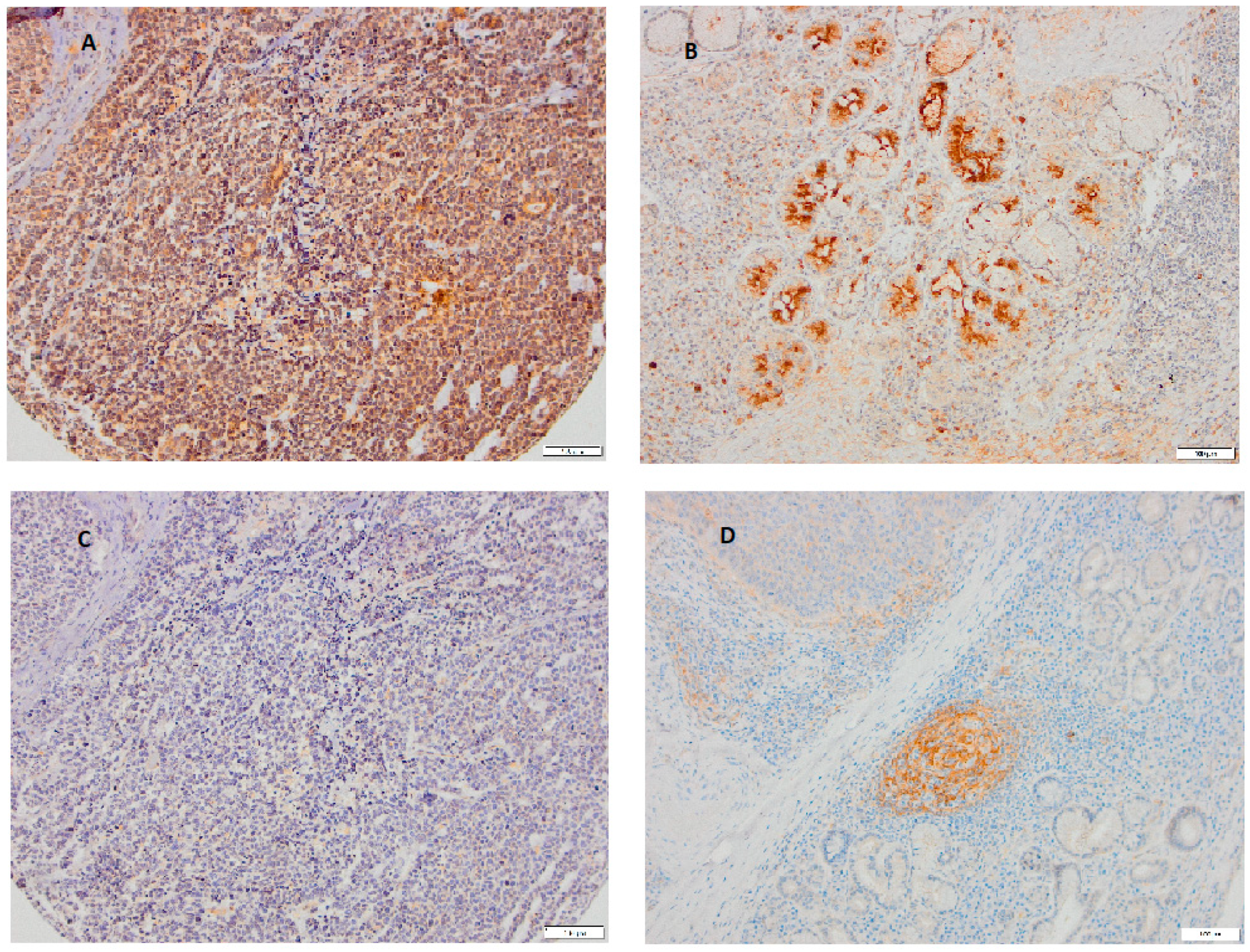
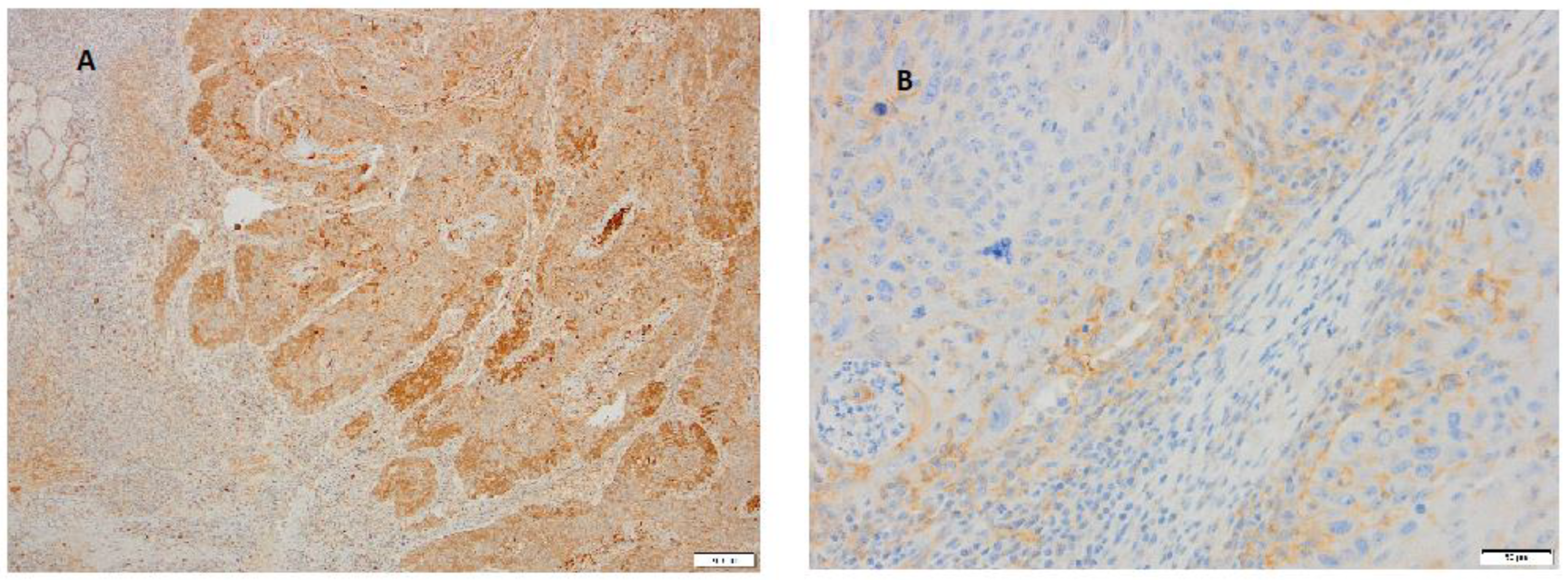
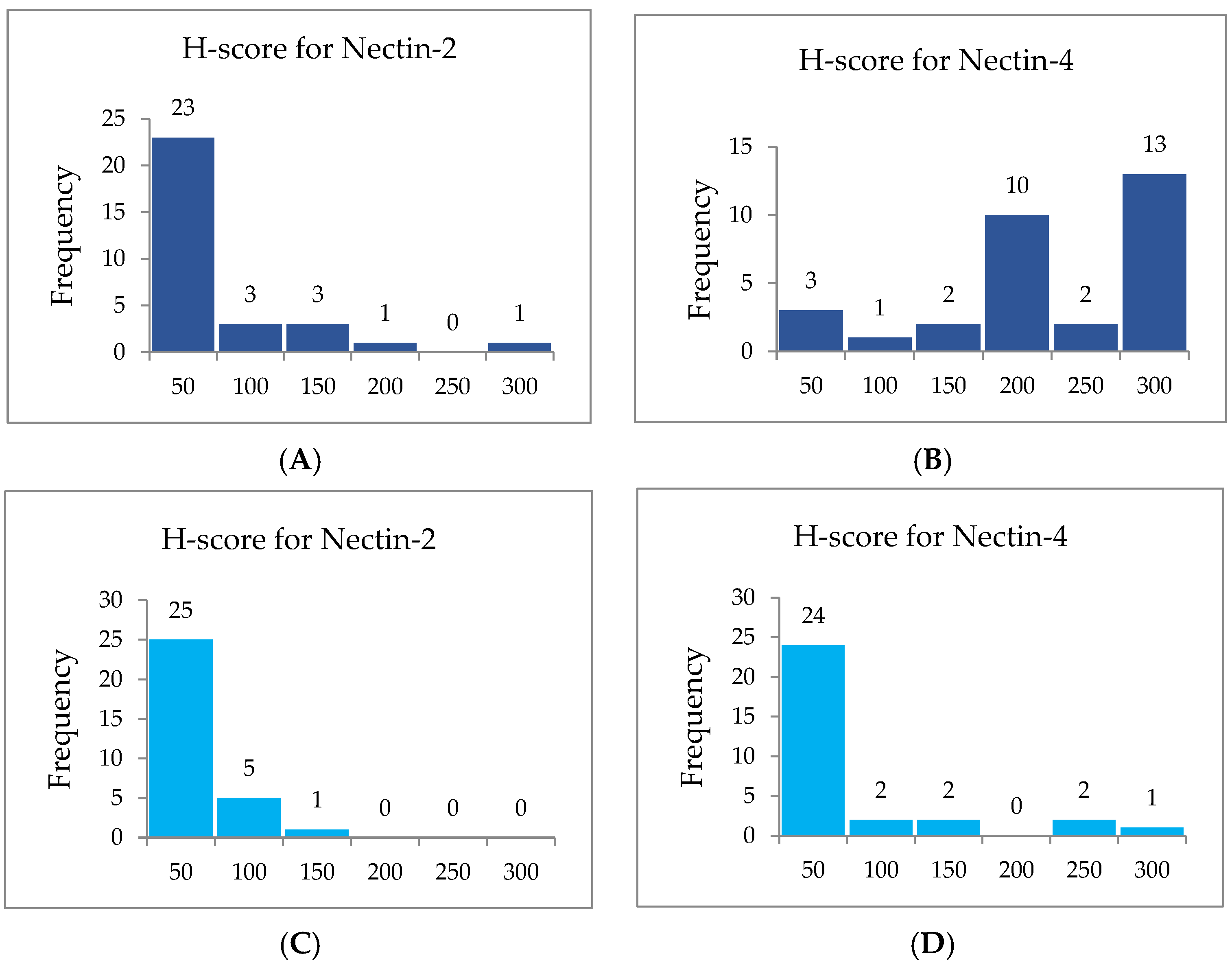
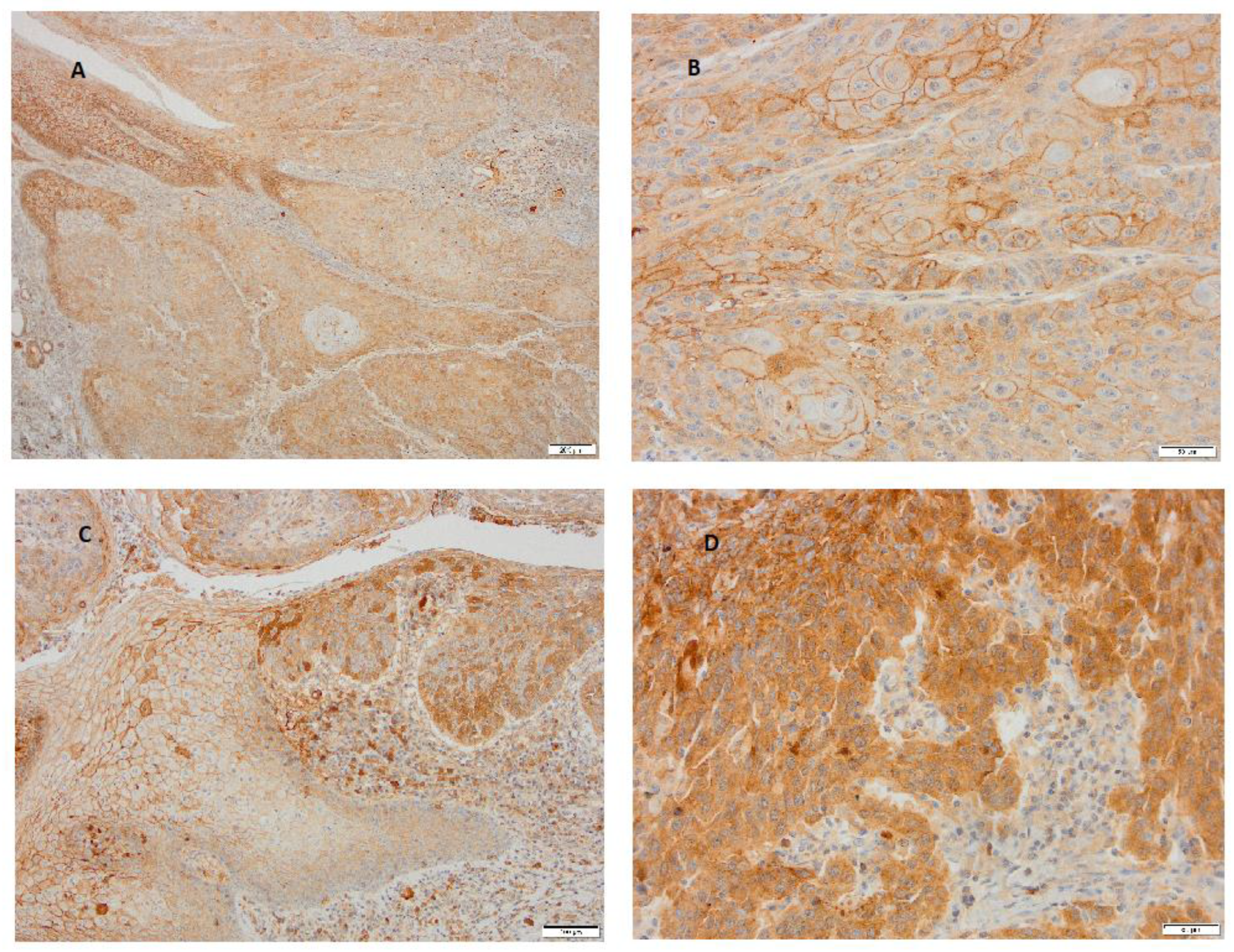
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Abbreviations and Acronyms
| ADC | Antibody–drug conjugate |
| EGFR | epidermal growth factor receptor |
| EV | enfortumab vedotin |
| HER-2 | human epidermal growth factor receptor-2 |
| Histo score | H-score |
| HNSCC | head and neck squamous cell carcinoma |
| IHC | immunohistochemistry |
| LSCC | laryngeal squamous cell carcinoma |
| TMA | tissue microarray |
References
- Shen, Y.; Qi, Y.; Wang, C.; Wu, C.; Zhan, X. Predicting specific mortality from laryngeal cancer based on competing risk model: A retrospective analysis based on the SEER database. Ann. Transl. Med. 2023, 11, 179. [Google Scholar] [CrossRef] [PubMed]
- Nocini, R.; Molteni, G.; Mattiuzzi, C.; Lippi, G. Updates on larynx cancer epidemiology. Chin J. Cancer Res. 2020, 32, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Chan, S.C.; Ko, S.; Lok, V.; Zhang, L.; Lin, X.; Lucero-Prisno, D.E., III; Xu, W.; Zheng, Z.J.; Elcarte, E.; et al. Updated disease distributions, risk factors, and trends of laryngeal cancer: A global analysis of cancer registries. Int. J. Surg. 2023, 110, 810–819. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Liberale, C.; Soloperto, D.; Marchioni, A.; Monzani, D.; Sacchetto, L. Updates on Larynx Cancer: Risk Factors and Oncogenesis. Int. J. Mol. Sci. 2023, 24, 12913. [Google Scholar] [CrossRef]
- Kühn, J.P.; Schmid, W.; Körner, S.; Bochen, F.; Wemmert, S.; Rimbach, H.; Smola, S.; Radosa, J.C.; Wagner, M.; Morris, L.G.; et al. HPV Status as Prognostic Biomarker in Head and Neck Cancer-Which Method Fits the Best for Outcome Prediction? Cancers 2021, 13, 4730. [Google Scholar] [CrossRef]
- Torrente, M.C.; Rodrigo, J.P.; Haigentz, M.; Dikkers, F.G.; Rinaldo, A.; Takes, R.P.; Olofsson, J.; Ferlito, A. Human papillomavirus infections in laryngeal cancer. Head Neck 2011, 33, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.C.; Chuang, H.C.; Lin, Y.T.; Huang, C.C.; Chien, C.Y. Clinical impact of human papillomavirus in laryngeal squamous cell carcinoma: A retrospective study. PeerJ 2017, 5, e3395. [Google Scholar] [CrossRef]
- Panuganti, B.A.; Finegersh, A.; Flagg, M.; Tu, X.; Orosco, R.; Weissbrod, P.A.; Califano, J. Prognostic Significance of HPV Status in Laryngeal Squamous Cell Carcinoma: A Large-Population Database Study. Otolaryngol. Head Neck Surg. 2021, 165, 113–121. [Google Scholar] [CrossRef]
- Kreimer, A.R.; Clifford, G.M.; Boyle, P.; Franceschi, S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: A systematic review. Cancer Epidemiol. Biomark. Prev. 2005, 14, 467–475. [Google Scholar] [CrossRef]
- Wang, S.; Freedman, N.D.; Katki, H.A.; Matthews, C.; Graubard, B.I.; Kahle, L.L.; Abnet, C.C. Gastroesophageal reflux disease: A risk factor for laryngeal squamous cell carcinoma and esophageal squamous cell carcinoma in the NIH-AARP Diet and Health Study cohort. Cancer 2021, 127, 1871–1879. [Google Scholar] [CrossRef]
- Falco, M.; Tammaro, C.; Takeuchi, T.; Cossu, A.M.; Scafuro, G.; Zappavigna, S.; Itro, A.; Addeo, R.; Scrima, M.; Lombardi, A.; et al. Overview on Molecular Biomarkers for Laryngeal Cancer: Looking for New Answers to an Old Problem. Cancers 2022, 14, 1716. [Google Scholar] [CrossRef] [PubMed]
- Reches, A.; Ophir, Y.; Stein, N.; Kol, I.; Isaacson, B.; Charpak-Amikam, Y.; Elnekave, A.; Tsukerman, P.; Kucan Brlic, P.; Lenac, T.; et al. Nectin4 is a novel TIGIT ligand which combines checkpoint inhibition and tumor specificity. J. Immunother. Cancer 2020, 8, e000266. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Ganguly, S. The Nectin family ligands, PVRL2 and PVR, in cancer immunology and immunotherapy. Front. Immunol. 2024, 15, 1441730. [Google Scholar] [CrossRef] [PubMed]
- Kobecki, J.; Gajdzis, P.; Mazur, G.; Chabowski, M. Nectins and Nectin-like Molecules in Colorectal Cancer: Role in Diagnostics, Prognostic Values, and Emerging Treatment Options: A Literature Review. Diagnostics 2022, 12, 3076. [Google Scholar] [CrossRef]
- Johnston, R.J.; Lee, P.S.; Strop, P.; Smyth, M.J. Smyth3 Cancer Immunotherapy and the Nectin Family. Annu. Rev. Cancer Biol. 2021, 5, 203–219. [Google Scholar] [CrossRef]
- Bekes, I.; Löb, S.; Holzheu, I.; Janni, W.; Baumann, L.; Wöckel, A.; Wulff, C. Nectin-2 in ovarian cancer: How is it expressed and what might be its functional role? Cancer Sci. 2019, 110, 1872–1882. [Google Scholar] [CrossRef]
- Oshima, T.; Sato, S.; Kato, J.; Ito, Y.; Watanabe, T.; Tsuji, I.; Hori, A.; Kurokawa, T.; Kokubo, T. Nectin-2 is a potential target for antibody therapy of breast and ovarian cancers. Mol. Cancer 2013, 12, 60. [Google Scholar] [CrossRef]
- Miao, X.; Yang, Z.L.; Xiong, L.; Zou, Q.; Yuan, Y.; Li, J.; Liang, L.; Chen, M.; Chen, S. Nectin-2 and DDX3 are biomarkers for metastasis and poor prognosis of squamous cell/adenosquamous carcinomas and adenocarcinoma of gallbladder. Int. J. Clin. Exp. Pathol. 2013, 6, 179–190. [Google Scholar]
- Li, M.; Qiao, D.; Pu, J.; Wang, W.; Zhu, W.; Liu, H. Elevated Nectin-2 expression is involved in esophageal squamous cell carcinoma by promoting cell migration and invasion. Oncol. Lett. 2018, 15, 4731–4736. [Google Scholar] [CrossRef]
- Luo, H.; Ikenaga, N.; Nakata, K.; Higashijima, N.; Zhong, P.; Kubo, A.; Wu, C.; Tsutsumi, C.; Shimada, Y.; Hayashi, M.; et al. Tumor-associated neutrophils upregulate Nectin2 expression, creating the immunosuppressive microenvironment in pancreatic ductal adenocarcinoma. J. Exp. Clin. Cancer Res. 2024, 43, 258. [Google Scholar] [CrossRef]
- Ando, T.; Ka, M.; Sugiura, Y.; Tokunaga, M.; Nakagawa, N.; Iida, T.; Matsumoto, Y.; Watanabe, K.; Kawakami, M.; Sato, M.; et al. NECTIN2 is a prognostic biomarker and potential therapeutic target in lung adenocarcinoma. Respir. Investig. 2024, 62, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Kobecki, J.; Gajdzis, P.; Mazur, G.; Chabowski, M. Prognostic Potential of Nectin Expressions in Colorectal Cancer: An Exploratory Study. Int. J. Mol. Sci. 2023, 24, 15900. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, G.; Wang, H.; Qi, Q.; Wang, X.; Lu, H. Targeted therapeutic strategies for Nectin-4 in breast cancer: Recent advances and future prospects. Breast 2025, 79, 103838. [Google Scholar] [CrossRef] [PubMed]
- M-Rabet, M.; Cabaud, O.; Josselin, E.; Finetti, P.; Castellano, R.; Farina, A.; Agavnian-Couquiaud, E.; Saviane, G.; Collette, Y.; Viens, P.; et al. Nectin-4: A new prognostic biomarker for efficient therapeutic targeting of primary and metastatic triple-negative breast cancer. Ann. Oncol. 2017, 28, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Bekos, C.; Muqaku, B.; Dekan, S.; Horvat, R.; Polterauer, S.; Gerner, C.; Aust, S.; Pils, D. NECTIN4 (PVRL4) as Putative Therapeutic Target for a Specific Subtype of High Grade Serous Ovarian Cancer-An Integrative Multi-Omics Approach. Cancers 2019, 11, 698. [Google Scholar] [CrossRef]
- Ma, J.; Sheng, Z.; Lv, Y.; Liu, W.; Yao, Q.; Pan, T.; Xu, Z.; Zhang, C.; Xu, G. Expression and clinical significance of Nectin-4 in hepatocellular carcinoma. Onco. Targets Ther. 2016, 9, 183–190. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Zhang, Y.; Zhang, J.; Shen, Q.; Yin, W.; Huang, H.; Liu, Y.; Ni, Q. High expression of Nectin-4 is associated with unfavorable prognosis in gastric cancer. Oncol. Lett. 2018, 15, 8789–8795. [Google Scholar] [CrossRef]
- Nishiwada, S.; Sho, M.; Yasuda, S.; Shimada, K.; Yamato, I.; Akahori, T.; Kinoshita, S.; Nagai, M.; Konishi, N.; Nakajima, Y. Nectin-4 expression contributes to tumor proliferation, angiogenesis and patient prognosis in human pancreatic cancer. J. Exp. Clin. Cancer Res. 2015, 34, 30. [Google Scholar] [CrossRef]
- Tomiyama, E.; Fujita, K.; Pena, M.D.C.R.; Taheri, D.; Banno, E.; Kato, T.; Hatano, K.; Kawashima, A.; Ujike, T.; Uemura, M.; et al. Expression of Nectin-4 and PD-L1 in Upper Tract Urothelial Carcinoma. Int. J. Mol. Sci. 2020, 21, 5390. [Google Scholar] [CrossRef]
- Miyake, M.; Nishimura, N.; Ohnishi, S.; Oda, Y.; Owari, T.; Ohnishi, K.; Morizawa, Y.; Hori, S.; Gotoh, D.; Nakai, Y.; et al. Diagnostic and Prognostic Roles of Urine Nectin-2 and Nectin-4 in Human Bladder Cancer. Cancers 2023, 15, 2565. [Google Scholar] [CrossRef]
- Rodler, S.; Eismann, L.; Schlenker, B.; Casuscelli, J.; Brinkmann, I.; Sendelhofert, A.; Waidelich, R.; Buchner, A.; Stief, C.; Schulz, G.B.; et al. Expression of Nectin-4 in Variant Histologies of Bladder Cancer and Its Prognostic Value-Need for Biomarker Testing in High-Risk Patients? Cancers 2022, 14, 4411. [Google Scholar] [CrossRef]
- Hashimoto, H.; Tanaka, Y.; Murata, M.; Ito, T. Nectin-4: A Novel Therapeutic Target for Skin Cancers. Curr. Treat. Options Oncol. 2022, 23, 578–593. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Murata, M.; Oda, Y.; Furue, M.; Ito, T. Nectin Cell Adhesion Molecule 4 (NECTIN4) Expression in Cutaneous Squamous Cell Carcinoma: A New Therapeutic Target? Biomedicines 2021, 9, 355. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Shi, H.; Chen, L.; Zhou, Y.; Jiang, J. Over-expression of Nectin-4 promotes progression of esophageal cancer and correlates with poor prognosis of the patients. Cancer Cell Int. 2019, 19, 106. [Google Scholar] [CrossRef] [PubMed]
- Nagatani, Y.; Kiyota, N.; Imamura, Y.; Koyama, T.; Kimbara, S.; Funakoshi, Y.; Itoh, T.; Fujiwara, H.; Shimoda, H.; Teshima, M.; et al. 426P Trop-2 and Nectin-4 expression and their relationship with tumor immune microenvironment in salivary gland. Ann. Oncol. 2024, 35, S1562–S1563. [Google Scholar] [CrossRef]
- Dekanić, A.; Babarović, E.; Brlić, P.K.; Knežić, M.; Vuković, A.S.; Mazor, M.; Jonjić, N. The prognostic significance of Nectin-2 and Nectin-4 expression in glial tumors. Pathol. Res. Pract. 2023, 244, 154416. [Google Scholar] [CrossRef]
- Zeindler, J.; Soysal, S.D.; Piscuoglio, S.; Ng, C.K.Y.; Mechera, R.; Isaak, A.; Weber, W.P.; Muenst, S.; Kurzeder, C. Nectin-4 Expression Is an Independent Prognostic Biomarker and Associated with Better Survival in Triple-Negative Breast Cancer. Front. Med. 2019, 6, 200. [Google Scholar] [CrossRef]
- Sanders, C.; Lau, J.-F.; Dietrich, D.; Strieth, S.; Brossart, P.; Kristiansen, G. Nectin-4 is widely expressed in head and neck squamous cell carcinoma. Oncotarget 2022, 13, 1166–1173. [Google Scholar] [CrossRef]
- Vukelic, J.; Dobrila-Dintinjana, R.; Dekanic, A.; Marijic, B.; Cubranic, A.; Braut, T. The Relevance of Assessing the Cell Proliferation Factor Ki-67 in Squamous Cell Carcinoma of the Larynx. BioMed Res. Int. 2019, 2019, 8142572. [Google Scholar] [CrossRef]
- Maržić, D.; Čoklo, M.; Marijić, B.; Hadžisejdić, I.; Dekanić, A.; Krstulja, M.; Šepić, T.; Avirović, M.; Braut, T. The expression of ribonuclear protein IMP3 in laryngeal carcinogenesis. Pathol. Res. Pract. 2020, 216, 152974. [Google Scholar] [CrossRef]
- Marijić, B.; Braut, T.; Babarović, E.; Krstulja, M.; Maržić, D.; Avirović, M.; Kujundžić, M.; Hadžisejdić, I. Nuclear EGFR Expression Is Associated with Poor Survival in Laryngeal Carcinoma. Appl. Immunohistochem. Mol. Morphol. 2021, 29, 576–584. [Google Scholar] [CrossRef]
- Braut, T.; Krstulja, M.; Marijić, B.; Maržić, D.; Kujundžić, M.; Brumini, G.; Vučinić, D.; Oštarijaš, E. Immunohistochemical analysis of vocal cord polyps applying markers of squamous cell carcinogenesis. Pathol. Res. Pract. 2019, 215, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Braut, T.; Krstulja, M.; Rukavina, K.M.; Jonjić, N.; Kujundžić, M.; Manestar, I.D.; Katunarić, M.; Manestar, D. Cytoplasmic EGFR staining and gene amplification in glottic cancer: A better indicator of EGFR-driven signaling? Appl. Immunohistochem. Mol. Morphol. 2014, 22, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Braut, T.; Kujundzić, M.; Vukelić, J.; Manestar, D.; Krstulja, M.; Starcević, R.; Grahovac, B. Gene amplification of epidermal growth factor receptor in atypical glottic hyperplasia. Coll. Antropol. 2012, 36 (Suppl. S2), 87–91. [Google Scholar] [PubMed]
- Braut, T.; Krstulja, M.; Kujundžić, M.; Manestar, D.; Hadžisejdić, I.; Jonjić, N.; Grahovac, B.; Manestar, D. Epidermal growth factor receptor protein expression and gene amplification in normal, hyperplastic, and cancerous glottic tissue: Immunohistochemical and fluorescent in situ hybridization study on tissue microarrays. Croat. Med J. 2009, 50, 370–379. [Google Scholar] [CrossRef]
- WHO. Head and Neck Tumours. In WHO Classification of Tumours Editorial, 5th ed.; WHO Classification of Tumours Editorial Board, Ed.; WHO: Geneva, Switzerland, 2024; Volume 9. [Google Scholar]
- Koroulakis, A.; Agarwal, M. Laryngeal Cancer. In StatPearls [Internet]; Koroulakis, A., Agarwal, M., Eds.; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK526076/ (accessed on 7 February 2025).
- Carvalho, G.B.; Kohler, H.F.; Lira, R.B.; Vartanian, J.G.; Kowalski, L.P. Survival results of 3786 patients with stage I or II laryngeal squamous cell carcinoma: A study based on a propensity score. Braz. J. Otorhinolaryngol. 2022, 88, 337–344. [Google Scholar] [CrossRef]
- Markou, K.; Christoforidou, A.; Karasmanis, I.; Tsiropoulos, G.; Triaridis, S.; Constantinidis, I.; Vital, V.; Nikolaou, A. Laryngeal cancer: Epidemiological data from Νorthern Greece and review of the literature. Hippokratia 2013, 17, 313–318. [Google Scholar]
- Chuang, C.H.; Yuan, C.-T.; Chang, C.-C.; Liu, T.-H.; Shen, Y.-C.; Lu, L.-C.; Ou, D.-L.; Hsu, C.-H.; Cheng, A.L. Exploring Nectin-4 expression in hepatocellular carcinoma. J. Clin. Oncol. 2025, 43. [Google Scholar] [CrossRef]
- Hoffman-Censits, J.; Lombardo, K.; McConkey, D.; Hahn, N.M.; Bashir, B.; Kelly, W.K.; Johnson, B.; Matoso, A. New and topics: Enfortumab vedotin mechanisms of response and resistance in urothelial cancer—What do we understand so far? Urol. Oncol. 2021, 39, 619–622. [Google Scholar] [CrossRef]
- Jindal, T.; Zhang, L.; Deshmukh, P.; Reyes, K.; Chan, E.; Kumar, V.; Zhu, X.; Maldonado, E.; Feng, S.; Johnson, M.; et al. Impact of Squamous Histology on Clinical Outcomes and Molecular Profiling in Metastatic Urothelial Carcinoma Patients Treated with Immune Checkpoint Inhibitors or Enfortumab Vedotin. Clin. Genitourin. Cancer 2023, 21, e394–e404. [Google Scholar] [CrossRef]
- Klekowski, J.; Zielińska, D.; Hofman, A.; Zajdel, N.; Gajdzis, P.; Chabowski, M. Clinical Significance of Nectins in HCC and Other Solid Malignant Tumors: Implications for Prognosis and New Treatment Opportunities-A Systematic Review. Cancers 2023, 15, 15. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bouleftour, W.; Sargos, P.; Magne, N. Nectin-4: A Tumor Cell Target and Status of Inhibitor Development. Curr. Oncol. Rep. 2023, 25, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Khosravanian, M.J.; Mirzaei, Y.; Mer, A.H.; Keyhani-Khankahdani, M.; Abdinia, F.S.; Misamogooe, F.; Amirkhani, Z.; Bagheri, N.; Meyfour, A.; Jahandideh, S.; et al. Nectin-4-directed antibody-drug conjugates (ADCs): Spotlight on preclinical and clinical evidence. Life Sci. 2024, 352, 122910. [Google Scholar] [CrossRef]
- Li, K.; Zhou, Y.; Zang, M.; Jin, X.; Li, X. Therapeutic prospects of nectin-4 in cancer: Applications and value. Front. Oncol. 2024, 14, 1354543. [Google Scholar] [CrossRef]
- Challita-Eid, P.M.; Satpayev, D.; Yang, P.; An, Z.; Morrison, K.; Shostak, Y.; Raitano, A.; Nadell, R.; Liu, W.; Lortie, D.R.; et al. Enfortumab Vedotin Antibody-Drug Conjugate Targeting Nectin-4 Is a Highly Potent Therapeutic Agent in Multiple Preclinical Cancer Models. Cancer Res. 2016, 76, 3003–3013. [Google Scholar] [CrossRef]
- Swiecicki, P.L.; Yilmaz, E.; Rosenberg, A.J.; Fujisawa, T.; Bruce, J.Y.; Meng, C.; Wozniak, M.; Zhao, Y.; Mihm, M.; Kaplan, J.; et al. Phase II Trial of Enfortumab Vedotin in Patients with Previously Treated Advanced Head and Neck Cancer. J. Clin. Oncol. 2025, 43, 578–588. [Google Scholar] [CrossRef]
- Mayer, M.; Nachtsheim, L.; Prinz, J.; Shabli, S.; Suchan, M.; Klußmann, J.P.; Quaas, A.; Arolt, C.; Wolber, P. Nectin-4 is frequently expressed in primary salivary gland cancer and corresponding lymph node metastases and represents an important treatment-related biomarker. Clin. Exp. Metastasis 2023, 40, 395–405. [Google Scholar] [CrossRef]

| Target Protein | Antibody Type Used | Clone | Manufacturer | Dilution | Diluent |
|---|---|---|---|---|---|
| Nectin-2 | rabbit monoclonal (recombinant) antibody | EPR21124/ab233384 | Abcam (Cambridge, UK) | 1:250 | DAKO Antibody Diluent S0809 |
| Nectin-4 | rabbit polyclonalantibody | ab155692 | Abcam | 1:100 | DAKO Antibody Diluent S0809 |
| 0 | Negative (no detectable staining at high magnification) |
| 1 | Weak (faint staining visible only at high magnification) |
| 2 | Moderate (easily visible at low magnification) |
| 3 | Strong (intense staining evident even at low magnification) |
| Varible | LSCC (n = 31) |
|---|---|
| Age | |
| Median age (years) | 66.6 |
| Range | 49.1–87.4 |
| Mean | 66.0 |
| Standard deviation | 8.5 |
| Gender | |
| Male | 30 (96.77%) |
| Female | 1 (3.23%) |
| T-stage | |
| T1 | / |
| T2 | / |
| T3 | 29 (93.55%) |
| T4 | 2 (6.45%) |
| SCC histologic grade | |
| Grade 1 | 6 (19.35%) |
| Grade 2 | 20 (64.52%) |
| Grade 3 | 5 (16.13%) |
| Measure 1 | Measure 2 | W | z | p | Hodges–Lehmann Estimate | Rank– Biserial Correlation | SE Rank– Biserial Correlation |
|---|---|---|---|---|---|---|---|
| H-score Nectin-2 | H-score Nectin-4 | 0.000 | −4.703 | <0.001 | −190.000 | −1.000 | 0.209 |
| Measure 1 | Measure 2 | W | z | p | Hodges–Lehmann Estimate | Rank– Biserial Correlation | SE Rank– Biserial Correlation |
|---|---|---|---|---|---|---|---|
| H-score Nectin-2 | H-score Nectin-4 | 83.500 | −0.463 | 0.658 | −12.480 | −0.121 | 0.256 |
| Cytoplasmic | H-Score for Nectin-2 | H-Score for Nectin-4 | ||
|---|---|---|---|---|
| Range | Frequencies | Percentage | Frequencies | Percentage |
| 0–50 | 23 | 74.19% | 3 | 9.68% |
| 51–100 | 3 | 9.68% | 1 | 3.23% |
| 101–150 | 3 | 9.68% | 2 | 6.45% |
| 151–200 | / | / | 10 | 32.26% |
| 201–250 | 1 | 3.23% | 2 | 6.45% |
| 251–300 | 1 | 3.23% | 13 | 41.94% |
| Total | 31 | 100% | 31 | 100% |
| Membranous | H-Score for Nectin 2 | H-Score for Nectin 4 | ||
|---|---|---|---|---|
| Range | Frequencies | Percentage | Frequencies | Percentage |
| 0–50 | 25 | 80.65% | 24 | 77.42% |
| 51–100 | 5 | 16.13% | 2 | 6.45% |
| 101–150 | 1 | 3.23% | 2 | 6.45% |
| 151–200 | / | / | / | / |
| 201–250 | / | / | 2 | 6.45% |
| 251–300 | / | / | 1 | 3.23% |
| Total | 31 | 100% | 31 | 100% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maršić, M.; Jonjić, N.; Gligora Marković, M.; Janković, S.; Velepič, M.; Vrebac, I.; Batičić, L.; Braut, T. Laryngeal Squamous Cell Carcinoma Is Characterized by a Stronger Expression of Nectin-4 Compared to Nectin-2. Curr. Issues Mol. Biol. 2025, 47, 296. https://doi.org/10.3390/cimb47050296
Maršić M, Jonjić N, Gligora Marković M, Janković S, Velepič M, Vrebac I, Batičić L, Braut T. Laryngeal Squamous Cell Carcinoma Is Characterized by a Stronger Expression of Nectin-4 Compared to Nectin-2. Current Issues in Molecular Biology. 2025; 47(5):296. https://doi.org/10.3390/cimb47050296
Chicago/Turabian StyleMaršić, Matej, Nives Jonjić, Maja Gligora Marković, Svjetlana Janković, Marko Velepič, Ilinko Vrebac, Lara Batičić, and Tamara Braut. 2025. "Laryngeal Squamous Cell Carcinoma Is Characterized by a Stronger Expression of Nectin-4 Compared to Nectin-2" Current Issues in Molecular Biology 47, no. 5: 296. https://doi.org/10.3390/cimb47050296
APA StyleMaršić, M., Jonjić, N., Gligora Marković, M., Janković, S., Velepič, M., Vrebac, I., Batičić, L., & Braut, T. (2025). Laryngeal Squamous Cell Carcinoma Is Characterized by a Stronger Expression of Nectin-4 Compared to Nectin-2. Current Issues in Molecular Biology, 47(5), 296. https://doi.org/10.3390/cimb47050296







