Synergistic Effect of Serratia fonticola and Pseudomonas koreensis on Mitigating Salt Stress in Cucumis sativus L.
Abstract
1. Introduction
2. Materials and Methods
2.1. PGPR Strain Cultures
2.2. Cucumber Seed Sterilization
2.3. Evaluation of the Range of Cucumber Seedling Tolerance to Salinity Stress
2.4. Salt Stress Application, PGPR Interactions, and NaCl Stress
2.5. Determination of Leaf Relative Water Content (LRWC)
2.6. Measuring the Electrolyte Leakage (EL)
2.7. Evaluation of Oxidative Stress Markers
2.8. Enzymatic Antioxidant Activity
2.9. Estimation of Endogenous Abscisic Acid (ABA)
2.10. Gene Expression Analysis Using qRT-PCR
2.11. Statistical Analysis
3. Results
3.1. Effect of PGPRs (S. fonticola and P. koreensis) on Plant Growth Under Salt Stress
3.2. PGPRs’ Role in Leaf Water Potential Under NaCl Stress
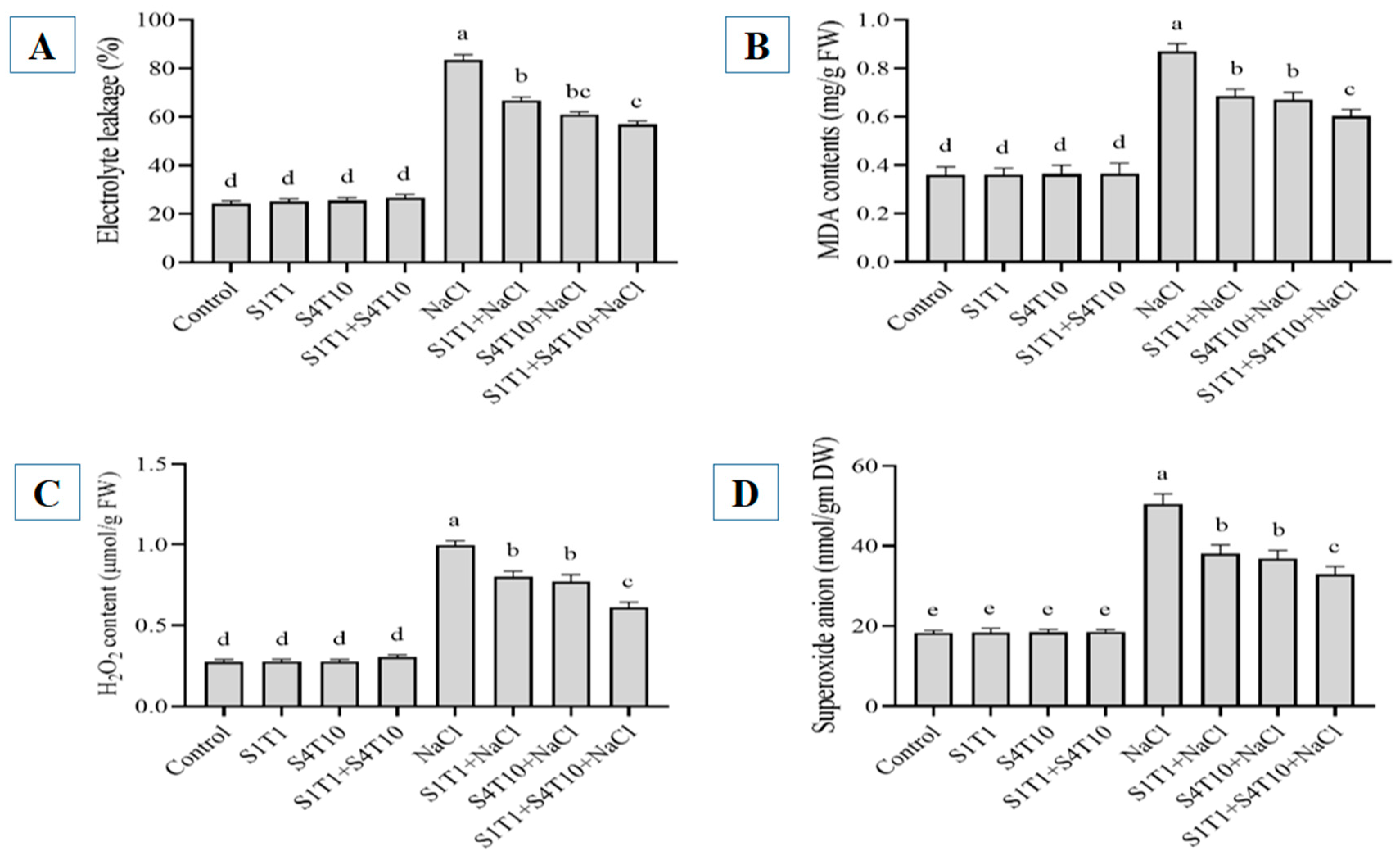
3.3. Effects of PGPRs on Electrolyte Leakage, MDA, H2O2, and SOAs Under NaCl Stress
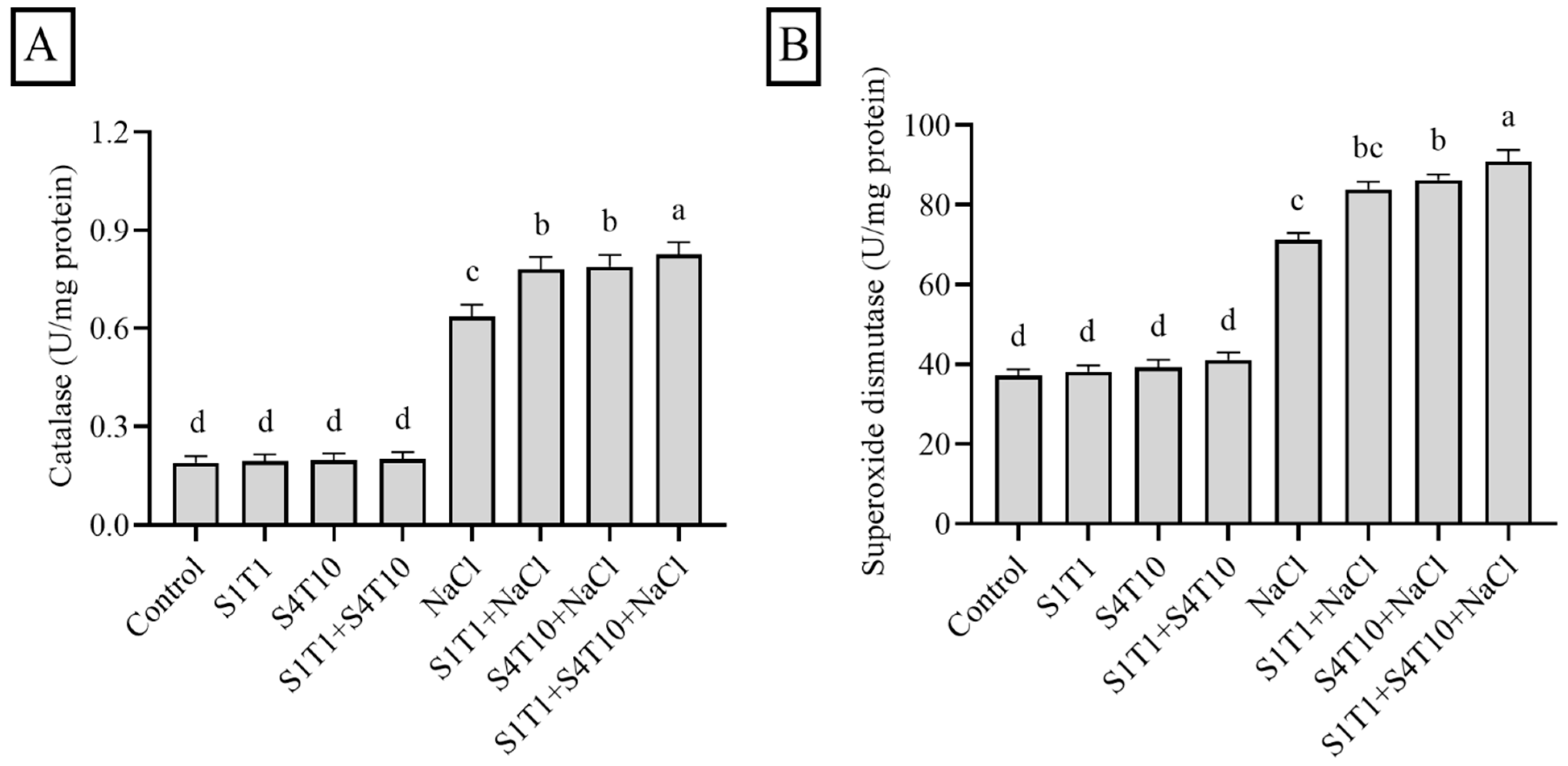
3.4. Effects of PGPRs on Antioxidant Enzymes Under NaCl Stress
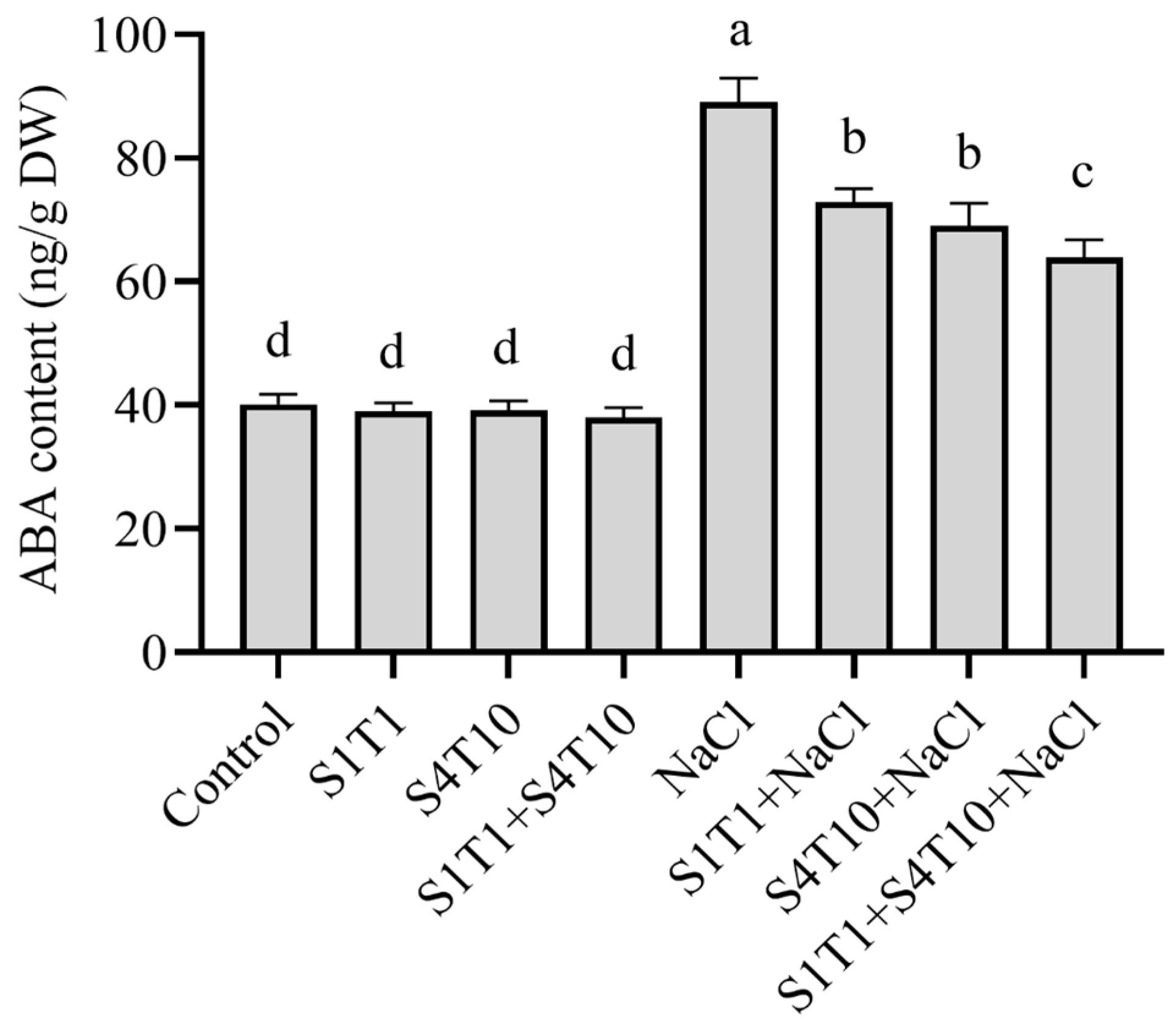
3.5. Regulation of Endogenous ABA by PGPRs Under NaCl Stress
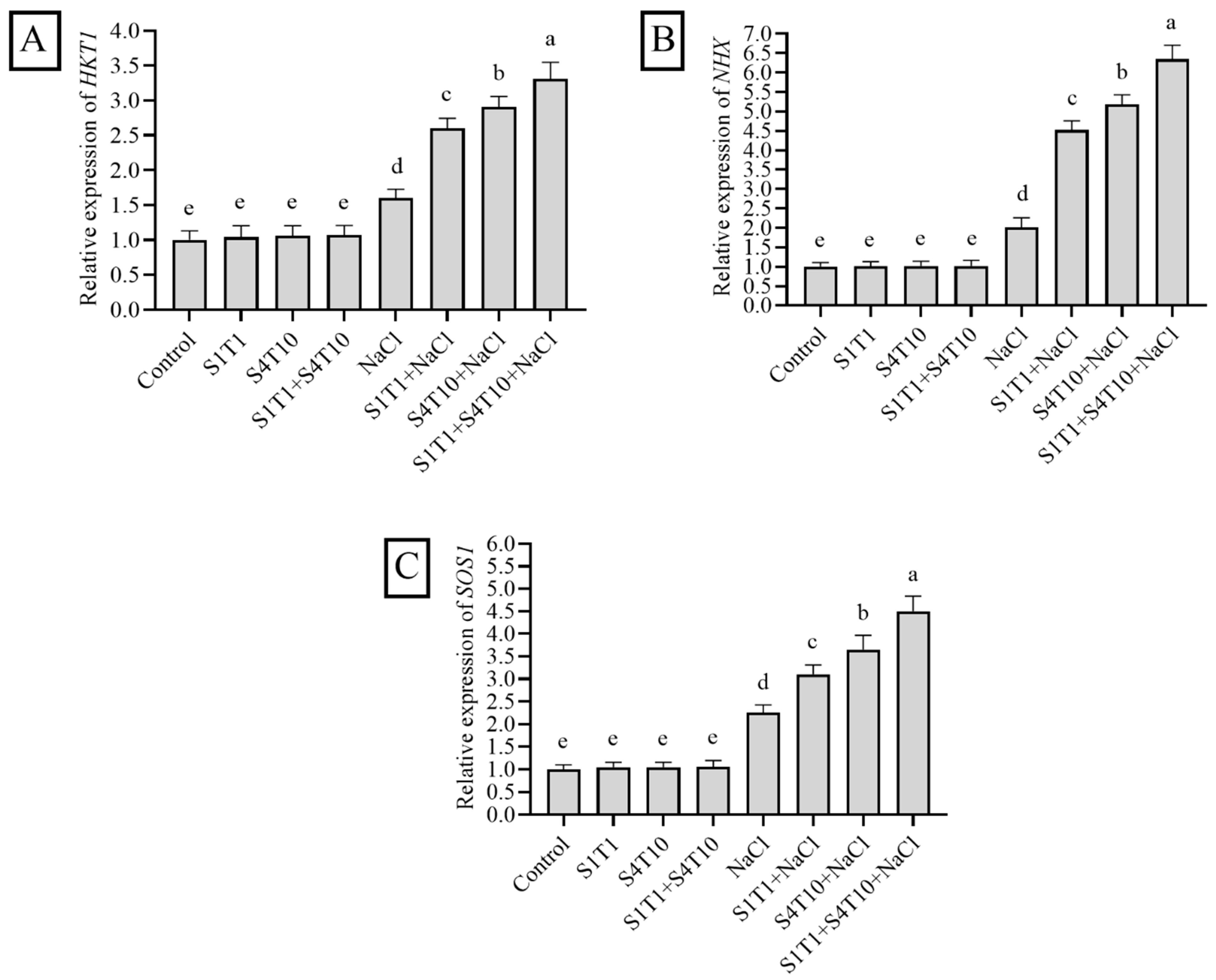
3.6. Expression of NaCl Stress-Related Genes and Effects of PGPRs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, A. Soil salinity: A global threat to sustainable development. Soil Use Manag. 2022, 38, 39–67. [Google Scholar] [CrossRef]
- de Andrade, L.A.; Santos, C.H.B.; Frezarin, E.T.; Sales, L.R.; Rigobelo, E.C. Plant growth-promoting rhizobacteria for sustainable agricultural production. Microorganisms 2023, 11, 1088. [Google Scholar] [CrossRef]
- Etesami, H.; Maheshwari, D.K. Use of plant growth promoting rhizobacteria (PGPRs) with multiple plant growth promoting traits in stress agriculture: Action mechanisms and future prospects. Ecotoxicol. Environ. Saf. 2018, 156, 225–246. [Google Scholar] [CrossRef] [PubMed]
- Sangiorgio, D.; Cellini, A.; Donati, I.; Pastore, C.; Onofrietti, C.; Spinelli, F.J.A. Facing climate change: Application of microbial biostimulants to mitigate stress in horticultural crops. Agronomy 2020, 10, 794. [Google Scholar] [CrossRef]
- Trușcă, M.; Gâdea, Ș.; Vidican, R.; Stoian, V.; Vâtcă, A.; Balint, C.; Stoian, V.A.; Horvat, M.; Vâtcă, S. Exploring the Research Challenges and Perspectives in Ecophysiology of Plants Affected by Salinity Stress. Agriculture 2023, 13, 734. [Google Scholar] [CrossRef]
- Machado, R.M.A.; Serralheiro, R.P. Soil salinity: Effect on vegetable crop growth. Management practices to prevent and mitigate soil salinization. Horticulturae 2017, 3, 30. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, H.; Song, C.; Zhu, J.-K.; Shabala, S. Mechanisms of plant responses and adaptation to soil salinity. Innovation 2020, 1, 100017. [Google Scholar] [CrossRef]
- Ismail, A.; Takeda, S.; Nick, P. Life and death under salt stress: Same players, different timing? J. Exp. Bot. 2014, 65, 2963–2979. [Google Scholar] [CrossRef]
- Shah, A.N.; Tanveer, M.; Abbas, A.; Fahad, S.; Baloch, M.S.; Ahmad, M.I.; Saud, S.; Song, Y. Targeting salt stress coping mechanisms for stress tolerance in Brassica: A research perspective. Plant Physiol. Biochem. 2021, 158, 53–64. [Google Scholar] [CrossRef]
- Giannelli, G.; Potestio, S.; Visioli, G. The contribution of PGPR in salt stress tolerance in crops: Unravelling the molecular mechanisms of cross-talk between plant and bacteria. Plants 2023, 12, 2197. [Google Scholar] [CrossRef]
- Glick, B.R. The enhancement of plant growth by free-living bacteria. Can. J. Microbiol. 1995, 41, 109–117. [Google Scholar] [CrossRef]
- Samain, E.; Ernenwein, C.; Aussenac, T.; Selim, S. Effective and durable systemic wheat-induced resistance by a plant-growth-promoting rhizobacteria consortium of Paenibacillus sp. strain B2 and Arthrobacter spp. strain AA against Zymoseptoria tritici and drought stress. Physiol. Mol. Plant Pathol. 2022, 119, 101830. [Google Scholar] [CrossRef]
- Sivasakthi, S.; Usharani, G.; Saranraj, P. Biocontrol potentiality of plant growth promoting bacteria (PGPR)-Pseudomonas fluorescens and Bacillus subtilis: A review. Afr. J. Agric. Res. 2014, 9, 1265–1277. [Google Scholar]
- Martínez, O.A.; Jorquera, M.A.; Crowley, D.E.; de la Luz Mora, M. Influence of nitrogen fertilisation on pasture culturable rhizobacteria occurrence and the role of environmental factors on their potential PGPR activities. Biol. Fertil. Soils 2011, 47, 875–885. [Google Scholar] [CrossRef]
- Yuan, J.; Cao, H.; Qin, W.; Yang, S.; Zhang, D.; Zhu, L.; Song, H.; Zhang, Q. Genomic and Modern Biotechnological Strategies for Enhancing Salt Tolerance in Crops. New Crops 2024, 2, 100057. [Google Scholar] [CrossRef]
- Gholizadeh, F.; Mirmazloum, I.; Janda, T. Genome-wide identification of HKT gene family in wheat (Triticum aestivum L.): Insights from the expression of multiple genes (HKT, SOS, TVP and NHX) under salt stress. Plant Stress 2024, 13, 100539. [Google Scholar] [CrossRef]
- Moon, Y.-S.; Ali, S. Isolation and identification of multi-trait plant growth–promoting rhizobacteria from coastal sand dune plant species of Pohang beach. Folia Microbiol. 2022, 67, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.-S.; Khan, M.; Khan, M.A.; Ali, S. Ameliorative symbiosis of Serratia fonticola (S1T1) under salt stress condition enhance growth-promoting attributes of Cucumis sativus L. Symbiosis 2023, 89, 283–297. [Google Scholar] [CrossRef]
- Rashmi, I.; Roy, T.; Kartika, K.S.; Pal, R.; Coumar, V.; Kala, S.; Shinoji, K.C. Organic and inorganic fertilizer contaminants in agriculture: Impact on soil and water resources. In Contaminants in Agriculture: Sources, Impacts and Management; Springer: Cham, Switzerland, 2020; pp. 3–41. [Google Scholar]
- Bittencourt, P.P.; Alves, A.F.; Ferreira, M.B.; da Silva Irineu, L.E.S.; Pinto, V.B.; Olivares, F.L. Mechanisms and applications of bacterial inoculants in plant drought stress tolerance. Microorganisms 2023, 11, 502. [Google Scholar] [CrossRef]
- Ofek, M.; Hadar, Y.; Minz, D. Colonization of cucumber seeds by bacteria during germination. Environ. Microbiol. 2011, 13, 2794–2807. [Google Scholar] [CrossRef]
- Jhuma, T.A.; Rafeya, J.; Sultana, S.; Rahman, M.T.; Karim, M.M. Isolation of endophytic salt-tolerant plant growth-promoting rhizobacteria from Oryza sativa and evaluation of their plant growth-promoting traits under salinity stress condition. Front. Sustain. Food Syst. 2021, 5, 687531. [Google Scholar] [CrossRef]
- Liu, D.; Dong, S.; Bo, K.; Miao, H.; Li, C.; Zhang, Y.; Zhang, S.; Gu, X. Identification of QTLs controlling salt tolerance in cucumber (Cucumis sativus L.) seedlings. Plants 2021, 10, 85. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Du, L.; Cao, Q.; Yang, Z.; Liu, Y.; Yang, H.; Duan, X.; Meng, Z. Salt tolerance evaluation of cucumber germplasm under sodium chloride stress. Plants 2023, 12, 2927. [Google Scholar] [CrossRef]
- Barrs, H.D.; Weatherley, P.E. A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust. J. Biol. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef]
- Dellagi, A.; Brisset, M.-N.; Paulin, J.-P.; Expert, D. Dual role of desferrioxamine in Erwinia amylovora pathogenicity. Mol. Plant-Microbe Interact. 1998, 11, 734–742. [Google Scholar] [CrossRef]
- Khan, M.A.; Hamayun, M.; Asaf, S.; Khan, M.; Yun, B.-W.; Kang, S.-M.; Lee, I.-J. Rhizospheric Bacillus spp. rescues plant growth under salinity stress via regulating gene expression, endogenous hormones, and antioxidant system of Oryza sativa L. Front. Plant Sci. 2021, 12, 665590. [Google Scholar] [CrossRef]
- Gajewska, E.; Skłodowska, M. Effect of nickel on ROS content and antioxidative enzyme activities in wheat leaves. Biometals 2007, 20, 27–36. [Google Scholar] [CrossRef]
- Khan, M.A.; Ullah, I.; Waqas, M.; Hamayun, M.; Khan, A.L.; Asaf, S.; Kang, S.-M.; Kim, K.-M.; Jan, R.; Lee, I.-J. Halo-tolerant rhizospheric Arthrobacter woluwensis AK1 mitigates salt stress and induces physio-hormonal changes and expression of GmST1 and GmLAX3 in soybean. Symbiosis 2019, 77, 9–21. [Google Scholar] [CrossRef]
- Park, H.-S.; Kazerooni, E.A.; Kang, S.-M.; Al-Sadi, A.M.; Lee, I.-J. Melatonin enhances the tolerance and recovery mechanisms in Brassica juncea (L.) Czern. under saline conditions. Front. Plant Sci. 2021, 12, 593717. [Google Scholar] [CrossRef]
- Srivastava, S.; Sinha, P.; Sharma, Y.K. Status of photosynthetic pigments, lipid peroxidation and anti-oxidative enzymes in Vigna mungo in presence of arsenic. J. Plant Nutr. 2017, 40, 298–306. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Lee, I.-J. Spermine promotes acclimation to osmotic stress by modifying antioxidant, abscisic acid, and jasmonic acid signals in soybean. J. Plant Growth Regul. 2013, 32, 22–30. [Google Scholar] [CrossRef]
- Marklund, S.; Marklund, G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.; Rose, P.A.; Abrams, G.D.; Taylor, D.C.; Abrams, S.R.; Cutler, A.J. (+)-Abscisic acid metabolism, 3-ketoacyl-coenzyme A synthase gene expression, and very-long-chain monounsaturated fatty acid biosynthesis in Brassica napus embryos. Plant Physiol. 1998, 117, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Cai, G.; Zu, F.; Zhao, Q.; Qasim, M.U.; Hong, Y.; Fan, C.; Zhou, Y. Comparative transcriptome analysis of developing seeds and silique wall reveals dynamic transcription networks for effective oil production in Brassica napus L. Int. J. Mol. Sci. 2019, 20, 1982. [Google Scholar] [CrossRef] [PubMed]
- Niyoifasha, C.J.; Borena, B.M.; Ukob, I.T.; Minh, P.N.; Al Azzawi, T.N.I.; Imran, M.; Ali, S.; Inthavong, A.; Mun, B.-G.; Lee, I.-J. Alleviation of Hg-, Cr-, Cu-, and Zn-Induced Heavy Metals Stress by Exogenous Sodium Nitroprusside in Rice Plants. Plants 2023, 12, 1299. [Google Scholar] [CrossRef] [PubMed]
- Zörb, C.; Geilfus, C.M.; Dietz, K.J. Salinity and crop yield. Plant Biol. 2019, 21, 31–38. [Google Scholar] [CrossRef]
- Ansari, M.; Shekari, F.; Mohammadi, M.H.; Juhos, K.; Végvári, G.; Biró, B. Salt-tolerant plant growth-promoting bacteria enhanced salinity tolerance of salt-tolerant alfalfa (Medicago sativa L.) cultivars at high salinity. Acta Physiol. Plant. 2019, 41, 195. [Google Scholar] [CrossRef]
- Vocciante, M.; Grifoni, M.; Fusini, D.; Petruzzelli, G.; Franchi, E. The role of plant growth-promoting rhizobacteria (PGPR) in mitigating plant’s environmental stresses. Appl. Sci. 2022, 12, 1231. [Google Scholar] [CrossRef]
- Shah, A.; Nazari, M.; Antar, M.; Msimbira, L.A.; Naamala, J.; Lyu, D.; Rabileh, M.; Zajonc, J.; Smith, D.L. PGPR in agriculture: A sustainable approach to increasing climate change resilience. Front. Sustain. Food Syst. 2021, 5, 667546. [Google Scholar] [CrossRef]
- Mohanty, P.; Singh, P.K.; Chakraborty, D.; Mishra, S.; Pattnaik, R. Insight into the role of PGPR in sustainable agriculture and environment. Front. Sustain. Food Syst. 2021, 5, 667150. [Google Scholar] [CrossRef]
- Ali, S.; Moon, Y.-S.; Hamayun, M.; Khan, M.A.; Bibi, K.; Lee, I.-J. Pragmatic role of microbial plant biostimulants in abiotic stress relief in crop plants. J. Plant Interact. 2022, 17, 705–718. [Google Scholar] [CrossRef]
- Nadeem, S.M.; Ahmad, M.; Naveed, M.; Imran, M.; Zahir, Z.A.; Crowley, D.E. Relationship between in vitro characterization and comparative efficacy of plant growth-promoting rhizobacteria for improving cucumber salt tolerance. Arch. Microbiol. 2016, 198, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Sapre, S.; Gontia-Mishra, I.; Tiwari, S. Plant growth-promoting rhizobacteria ameliorates salinity stress in pea (Pisum sativum). J. Plant Growth Regul. 2022, 41, 647–656. [Google Scholar] [CrossRef]
- Shabaan, M.; Asghar, H.N.; Zahir, Z.A.; Zhang, X.; Sardar, M.F.; Li, H. Salt-tolerant PGPR confer salt tolerance to maize through enhanced soil biological health, enzymatic activities, nutrient uptake and antioxidant defense. Front. Microbiol. 2022, 13, 901865. [Google Scholar] [CrossRef]
- Singh, R.; Soni, S.K.; Patel, R.P.; Kalra, A. Technology for improving essential oil yield of Ocimum basilicum L.(sweet basil) by application of bioinoculant colonized seeds under organic field conditions. Ind. Crops Prod. 2013, 45, 335–342. [Google Scholar] [CrossRef]
- Ilyas, N.; Mazhar, R.; Yasmin, H.; Khan, W.; Iqbal, S.; Enshasy, H.E.; Dailin, D.J. Rhizobacteria isolated from saline soil induce systemic tolerance in wheat (Triticum aestivum L.) against salinity stress. Agronomy 2020, 10, 989. [Google Scholar] [CrossRef]
- Yasmeen, R.; Shaheed Siddiqui, Z. Physiological responses of crop plants against Trichoderma harzianum in saline environment. Acta Bot. Croat. 2017, 76, 154–162. [Google Scholar] [CrossRef]
- Bennis, M.; Kaddouri, K.; Badaoui, B.; Bouhnik, O.; Chaddad, Z.; Perez-Tapia, V.; Lamin, H.; Alami, S.; Lamrabet, M.; Abdelmoumen, H. Plant growth promoting activities of Pseudomonas sp. and Enterobacter sp. isolated from the rhizosphere of Vachellia gummifera in Morocco. FEMS Microbiol. Ecol. 2023, 99, fiad114. [Google Scholar] [CrossRef]
- Sumreen, S.; Sharif, M.; Tariq Sultan, D.M.; Khan, A. Effect of isolated plant growth promoting rhizobacteria on growth and nutrients uptake by maize in acidic and alkaline soil conditions. Pak. J. Bot 2025, 57, 37–46. [Google Scholar] [CrossRef]
- Yilmaz, H. Enhancements in morphology, biochemicals, nutrients, and L-Dopa in Faba bean through plant growth promoting rhizobacteria and arbuscular mycorrhizal Fungi. Sci. Rep. 2025, 15, 7390. [Google Scholar] [CrossRef]
- Zareen, S.; Ali, A.; Yun, D.-J. Significance of ABA Biosynthesis in Plant Adaptation to Drought Stress. J. Plant Biol. 2024, 67, 175–184. [Google Scholar] [CrossRef]
- Zulfiqar, B.; Raza, M.A.S.; Saleem, M.F.; Ali, B.; Aslam, M.U.; Al-Ghamdi, A.A.; Elshikh, M.S.; Hassan, M.U.; Toleikienė, M.; Ahmed, J. Abscisic acid improves drought resilience, growth, physio-biochemical and quality attributes in wheat (Triticum aestivum L.) at critical growth stages. Sci. Rep. 2024, 14, 20411. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Medina, M.J.; Steinkellner, S.; Vierheilig, H.; Ocampo Bote, J.A.; Garcia Garrido, J. Abscisic acid determines arbuscule development and functionality in the tomato arbuscular mycorrhiza. New Phytol. 2007, 175, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Evelin, H.; Kapoor, R.; Giri, B. Arbuscular mycorrhizal fungi in alleviation of salt stress: A review. Ann. Bot. 2009, 104, 1263–1280. [Google Scholar] [CrossRef]
- Roy, S.; Chakraborty, A.P.; Chakraborty, R. Understanding the potential of root microbiome influencing salt-tolerance in plants and mechanisms involved at the transcriptional and translational level. Physiol. Plant. 2021, 173, 1657–1681. [Google Scholar] [CrossRef]
- Liu, S.; Tian, Y.; Jia, M.; Lu, X.; Yue, L.; Zhao, X.; Jin, W.; Wang, Y.; Zhang, Y.; Xie, Z. Induction of salt tolerance in Arabidopsis thaliana by volatiles from Bacillus amyloliquefaciens FZB42 via the jasmonic acid signaling pathway. Front. Microbiol. 2020, 11, 562934. [Google Scholar] [CrossRef]


| Symbol of Gene | Forward Primer | Reverse Primer |
|---|---|---|
| SOS1 | 5′-AGGAAGGTTCAAAGCCTAGTG-3′ | 5′-CATGAGTAAATGTGGGGTGCA-3′ |
| HKT1 | 5′-GGACCTCTCCACCTTGTCGT-3′ | 5′-CTGCTACCGTTTGTTTGTCACTCT-3′ |
| NHX1 | 5′-TGCTTTTGCCACCCTTTCA-3′ | 5′-TTCCAACCAGAACCAATCCC-3′ |
| Treatment | Shoot Fresh Weight (gm/plant) | Shoot Dry Weight (gm/plant) | Shoot Length/(cm) | Root Length/(cm) | Chlorophyll Content/(SPAD) |
|---|---|---|---|---|---|
| Control | 23.35 ± 1.32 b | 5.98 ± 1.18 b | 22.41 ± 1.11 b | 21.27 ± 0.14 b | 40.11 ± 1.20 b |
| S1T1-Treated | 27.62 ± 0.24 a | 7.12 ± 0.35 a | 26.86 ± 0.23 a | 27.81 ± 1.81 a | 44.08 ± 1.05 a |
| S4T10-Treated | 28.15 ± 1.21 a | 7.41 ± 0.17 a | 26.90 ± 0.22 a | 27.08 ± 0.35 a | 44.62 ± 0.12 a |
| S1T1+ S4T10 Consortia-Treated | 29.17 ± 0.11 a | 7.60 ± 1.15 a | 28.21 ± 0.41 a | 30.67 ± 0.56 a | 44.98 ± 0.46 a |
| NaCl (200mM) Stress | 14.83 ± 1.41 e | 3.68 ± 0.22 e | 13.47 ± 1.12 e | 15.34 ± 0.41 e | 29.23 ± 0.22 e |
| S1T1+ NaCl Stress | 18.25 ± 0.64 d | 4.28 ± 0.56 d | 15.75 ± 0.32 d | 18.17 ± 0.11 d | 36.41 ± 0.48 d |
| S4T10 + NaCl Stress | 18.44 ± 0.52 d | 4.41 ± 0.47 d | 16.31 ± 0.65 d | 19.05 ± 0.52 d | 36.69 ± 0.34 d |
| Consortia (S1T1+ S4T10) + NaCl Stress | 19.05 ± 1.54 c | 4.58 ± 0.64 c | 17.04 ± 0.41 c | 20.13 ± 0.55 c | 37.05 ± 0.61 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, S.; Khan, M.; Moon, Y.-S. Synergistic Effect of Serratia fonticola and Pseudomonas koreensis on Mitigating Salt Stress in Cucumis sativus L. Curr. Issues Mol. Biol. 2025, 47, 194. https://doi.org/10.3390/cimb47030194
Ali S, Khan M, Moon Y-S. Synergistic Effect of Serratia fonticola and Pseudomonas koreensis on Mitigating Salt Stress in Cucumis sativus L. Current Issues in Molecular Biology. 2025; 47(3):194. https://doi.org/10.3390/cimb47030194
Chicago/Turabian StyleAli, Sajid, Murtaza Khan, and Yong-Sun Moon. 2025. "Synergistic Effect of Serratia fonticola and Pseudomonas koreensis on Mitigating Salt Stress in Cucumis sativus L." Current Issues in Molecular Biology 47, no. 3: 194. https://doi.org/10.3390/cimb47030194
APA StyleAli, S., Khan, M., & Moon, Y.-S. (2025). Synergistic Effect of Serratia fonticola and Pseudomonas koreensis on Mitigating Salt Stress in Cucumis sativus L. Current Issues in Molecular Biology, 47(3), 194. https://doi.org/10.3390/cimb47030194







