Innovation in Osteogenesis Activation: Role of Marine-Derived Materials in Bone Regeneration
Abstract
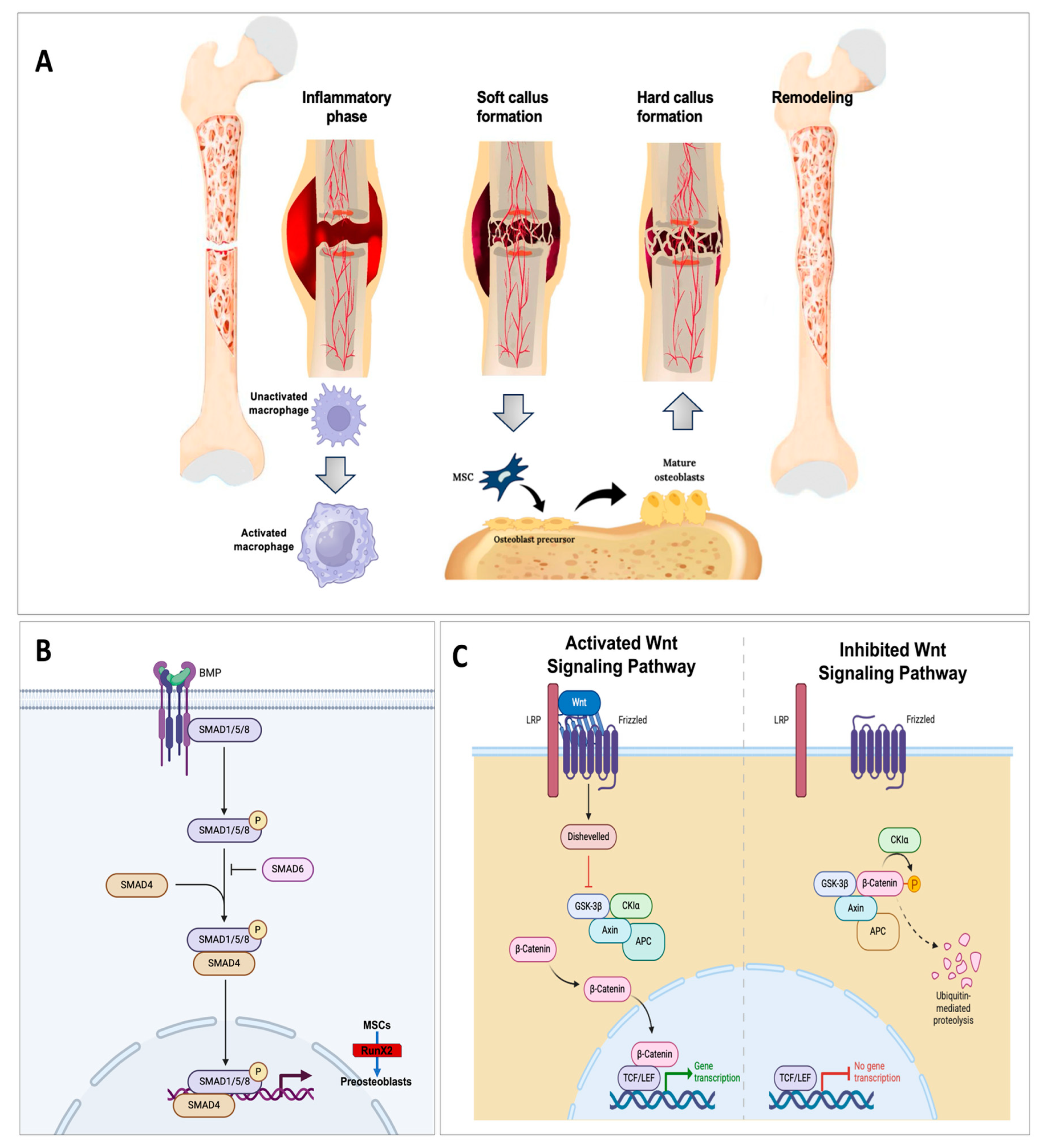
1. Introduction
2. Biological Profile and Activity of Biomaterials for Bone Regeneration
3. Marine-Derived Biomaterials: Chemical Profile and Biological Activity
3.1. Marine-Derived Inorganic Materials
3.1.1. Calcium Carbonate
3.1.2. Magnesium Salts
3.1.3. Silica
3.2. Marine-Derived Organic Materials
3.2.1. Polysaccharides
3.2.2. Proteins and Peptides
3.2.3. Marine-Organism-Derived Cartilage
3.2.4. Lipids
4. Sources of Biomaterials from Marine Environment
4.1. Seashells
4.2. Coral
4.3. Mussels’ Byssus
4.4. Jellyfish
5. Marine Microbial Polymers
5.1. Bacterial Exopolysaccharides
5.2. Microalgae
| Polymer | Microorganism | Composition | Osteogenesis Molecular Target | References |
|---|---|---|---|---|
| HE800 EPS | Vibrio diabolicus | N-acetyl glucosamine and N-acetyl galactosamine | IL-1β induction, the secretion of matrix metalloproteinases, stromelysin 1 | [186] |
| EPS GY785 | Alteromonas infernus | Galacturonic and glucuronic acid) and galactose and glucose | Complement cascade cytokines inhibition of IL-1β and TNF-α | [188] |
| EPS-B315 | Bacillus licheniformis B3-15 | Mannose–glucose | Activating TLR-2 and TLR-4 downregulating IL-8 | [192] |
| Ethanolic extract | Skeletonema costatum | n.d. | Upregulation of genes involved in bone synthesis and remodeling (p7, col1a1a, oc1, and oc2; acp5a | [201] |
| Ethanolic extract | Tetraselmis striata | n.d. | Upregulation of genes involved in bone synthesis and remodeling (p7, col1a1a, oc1, and oc2; acp5a Antioxidant gene (cat, sod1) | [201] |
| Aqueous extract | Arthospira platensis | n.d. | Upregulation of β- catenin in human amniotic mesenchymal stem cells | [202] |
| NOP | Nannochloropsis oculata | Peptide | stimulate human osteoblast (MG-63) upregulate p-Smad1/5/8 | [204] |
| Biomass | Chlamydomonas reinhardtii | n.d. | Scaffolds increase the adhesion and growth of human cells | [196] |
6. Innovative Tools for Bone Tissue Engineering: Phage Display and Marine-Derived Peptides
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Berendsen, A.D.; Olsen, B.R. Bone Development. Bone 2015, 80, 14–18. [Google Scholar] [CrossRef]
- Zaidi, M. Skeletal Remodeling in Health and Disease. Nat. Med. 2007, 13, 791–801. [Google Scholar] [CrossRef]
- Javaheri, B.; Caetano-Silva, S.P.; Kanakis, I.; Bou-Gharios, G.; Pitsillides, A.A. The Chondro-Osseous Continuum: Is It Possible to Unlock the Potential Assigned Within? Front. Bioeng. Biotechnol. 2018, 6, 28. [Google Scholar] [CrossRef]
- Kronenberg, H.M. Developmental Regulation of the Growth Plate. Nature 2003, 423, 332–336. [Google Scholar] [CrossRef]
- Trompet, D.; Melis, S.; Chagin, A.S.; Maes, C. Skeletal Stem and Progenitor Cells in Bone Development and Repair. J. Bone Miner. Res. 2024, 39, 633–654. [Google Scholar] [CrossRef]
- Vermeulen, S.; Tahmasebi Birgani, Z.; Habibovic, P. Biomaterial-Induced Pathway Modulation for Bone Regeneration. Biomaterials 2022, 283, 121431. [Google Scholar] [CrossRef]
- Ashfaq, R.; Kovács, A.; Berkó, S.; Budai-Szűcs, M. Developments in Alloplastic Bone Grafts and Barrier Membrane Biomaterials for Periodontal Guided Tissue and Bone Regeneration Therapy. Int. J. Mol. Sci. 2024, 25, 7746. [Google Scholar] [CrossRef]
- Bauer, T.W.; Muschler, G.F. Bone Graft Materials: An Overview of the Basic Science. Clin. Orthop. Relat. Res.® 2000, 371, 10. [Google Scholar] [CrossRef]
- Alonzo, M.; Alvarez Primo, F.; Anil Kumar, S.; Mudloff, J.A.; Dominguez, E.; Fregoso, G.; Ortiz, N.; Weiss, W.M.; Joddar, B. Bone Tissue Engineering Techniques, Advances, and Scaffolds for Treatment of Bone Defects. Curr. Opin. Biomed. Eng. 2021, 17, 100248. [Google Scholar] [CrossRef]
- Girón, J.; Kerstner, E.; Medeiros, T.; Oliveira, L.; Machado, G.M.; Malfatti, C.F.; Pranke, P. Biomaterials for Bone Regeneration: An Orthopedic and Dentistry Overview. Braz. J. Med. Biol. Res. 2021, 54, e11055. [Google Scholar] [CrossRef]
- Oryan, A.; Alidadi, S.; Moshiri, A.; Maffulli, N. Bone Regenerative Medicine: Classic Options, Novel Strategies, and Future Directions. J. Orthop. Surg. Res. 2014, 9, 18. [Google Scholar] [CrossRef]
- Ho-Shui-Ling, A.; Bolander, J.; Rustom, L.E.; Johnson, A.W.; Luyten, F.P.; Picart, C. Bone Regeneration Strategies: Engineered Scaffolds, Bioactive Molecules and Stem Cells Current Stage and Future Perspectives. Biomaterials 2018, 180, 143–162. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Xuan, M.; Yang, R.; Zhang, J.; Chang, J. Marine Biomaterials for Sustainable Bone Regeneration. Giant 2024, 19, 100298. [Google Scholar] [CrossRef]
- Marsell, R.; Einhorn, T.A. The Biology of Fracture Healing. Injury 2011, 42, 551–555. [Google Scholar] [CrossRef]
- Chen, D.; Zhao, M.; Mundy, G.R. Bone Morphogenetic Proteins. Growth Factors 2004, 22, 233–241. [Google Scholar] [CrossRef]
- Boyce, B.F.; Xing, L. Functions of RANKL/RANK/OPG in Bone Modeling and Remodeling. Arch. Biochem. Biophys. 2008, 473, 139–146. [Google Scholar] [CrossRef]
- Lin, G.L.; Hankenson, K.D. Integration of BMP, Wnt, and Notch Signaling Pathways in Osteoblast Differentiation. J. Cell. Biochem. 2011, 112, 3491–3501. [Google Scholar] [CrossRef]
- Čolnik, M.; Primožič, M.; Knez, Ž.; Leitgeb, M. Use of Non-Conventional Cell Disruption Method for Extraction of Proteins from Black Yeasts. Front. Bioeng. Biotechnol. 2016, 4, 33. [Google Scholar] [CrossRef][Green Version]
- Nauth, A.; Schemitsch, E.; Norris, B.; Nollin, Z.; Watson, J.T. Critical-Size Bone Defects: Is There a Consensus for Diagnosis and Treatment? J. Orthop. Trauma 2018, 32, S7. [Google Scholar] [CrossRef]
- Hutchings, G.; Moncrieff, L.; Dompe, C.; Janowicz, K.; Sibiak, R.; Bryja, A.; Jankowski, M.; Mozdziak, P.; Bukowska, D.; Antosik, P.; et al. Bone Regeneration, Reconstruction and Use of Osteogenic Cells; from Basic Knowledge, Animal Models to Clinical Trials. J. Clin. Med. 2020, 9, 139. [Google Scholar] [CrossRef]
- García-Gareta, E.; Coathup, M.J.; Blunn, G.W. Osteoinduction of Bone Grafting Materials for Bone Repair and Regeneration. Bone 2015, 81, 112–121. [Google Scholar] [CrossRef]
- Veronesi, F.; Maglio, M.; Brogini, S.; Fini, M. In Vivo Studies on Osteoinduction: A Systematic Review on Animal Models, Implant Site, and Type and Postimplantation Investigation. J. Biomed. Mater. Res. Part A 2020, 108, 1834–1866. [Google Scholar] [CrossRef]
- Albrektsson, T.; Johansson, C. Osteoinduction, Osteoconduction and Osseointegration. Eur. Spine J. 2001, 10, S96–S101. [Google Scholar] [CrossRef]
- Solheim, E. Osteoinduction by Demineralised Bone. Int. Orthop. SICOT 1998, 22, 335–342. [Google Scholar] [CrossRef]
- Schmidmaier, G.; Wildemann, B.; Ostapowicz, D.; Kandziora, F.; Stange, R.; Haas, N.P.; Raschke, M. Long-Term Effects of Local Growth Factor (IGF-I and TGF-Β1) Treatment on Fracture Healing: A Safety Study for Using Growth Factors. J. Orthop. Res. 2004, 22, 514–519. [Google Scholar] [CrossRef]
- Barradas, A.M.; Yuan, H.; van Blitterswijk, C.; Habibovic, P. Osteoinductive Biomaterials: Current Knowledge of Properties, Experimental Models and Biological Mechanisms. eCM 2010, 21, 407–429. [Google Scholar] [CrossRef]
- Mehta, M.; Schmidt-Bleek, K.; Duda, G.N.; Mooney, D.J. Biomaterial Delivery of Morphogens to Mimic the Natural Healing Cascade in Bone. Adv. Drug Deliv. Rev. 2012, 64, 1257–1276. [Google Scholar] [CrossRef]
- Kirker-Head, C.A. Potential Applications and Delivery Strategies for Bone Morphogenetic Proteins. Adv. Drug Deliv. Rev. 2000, 43, 65–92. [Google Scholar] [CrossRef]
- Polini, A.; Pisignano, D.; Parodi, M.; Quarto, R.; Scaglione, S. Osteoinduction of Human Mesenchymal Stem Cells by Bioactive Composite Scaffolds without Supplemental Osteogenic Growth Factors. PLoS ONE 2011, 6, e26211. [Google Scholar] [CrossRef]
- Kübler, N.R. Osteoinduction and -reparation. Mund Kiefer Gesichtschir 1997, 1, 2–25. [Google Scholar] [CrossRef]
- Yuan, H.; de Bruijn, J.D.; Zhang, X.; van Blitterswijk, C.A.; de Groot, K. Bone Induction by Porous Glass Ceramic Made from Bioglass® (45S5). J. Biomed. Mater. Res. 2001, 58, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.-Q.; Nakamura, T.; Kawanabe, K.; Nishigochi, S.; Oka, M.; Kokubo, T. Apatite Layer-Coated Titanium for Use as Bone Bonding Implants. Biomaterials 1997, 18, 1185–1190. [Google Scholar] [CrossRef] [PubMed]
- Nygren, H.; Ilver, L.; Malmberg, P. Mineralization at Titanium Surfaces Is a Two-Step Process. J. Funct. Biomater. 2016, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Wróbel, E.; Witkowska-Zimny, M.; Przybylski, J. Biological Mechanisms of Implant Osseointegration. Ortop. Traumatol. Rehabil. 2010, 12, 401–409. [Google Scholar]
- Ragone, V.; Canciani, E.; Arosio, M.; Olimpo, M.; Piras, L.A.; von Degerfeld, M.M.; Augusti, D.; D’Ambrosi, R.; Dellavia, C. In Vivo Osseointegration of a Randomized Trabecular Titanium Structure Obtained by an Additive Manufacturing Technique. J. Mater. Sci. Mater. Med. 2020, 31, 17. [Google Scholar] [CrossRef]
- Fabbro, M.D.; Taschieri, S.; Canciani, E.; Addis, A.; Musto, F.; Weinstein, R.; Dellavia, C. Osseointegration of Titanium Implants With Different Rough Surfaces: A Histologic and Histomorphometric Study in an Adult Minipig Model. Implant Dent. 2017, 26, 357. [Google Scholar] [CrossRef]
- Lagopati, N.; Pippa, N.; Gatou, M.-A.; Papadopoulou-Fermeli, N.; Gorgoulis, V.G.; Gazouli, M.; Pavlatou, E.A. Marine-Originated Materials and Their Potential Use in Biomedicine. Appl. Sci. 2023, 13, 9172. [Google Scholar] [CrossRef]
- Singh, H.; Parida, A.; Debbarma, K.; Ray, D.P.; Banerjee, P. Common Marine Organisms: A Novel Source of Medicinal Compounds. IJBS 2020, 7, 39–49. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L.; Wang, Y.; Wang, X.; Qian, D.; Yan, J.; Sun, Z.; Cui, P.; Yu, L.; Wu, J.; et al. Marine Biomaterials in Biomedical Nano/Micro-Systems. J. Nanobiotechnol. 2023, 21, 408. [Google Scholar] [CrossRef]
- Kiuru, P.; D’Auria, M.V.; Muller, C.D.; Tammela, P.; Vuorela, H.; Yli-Kauhaluoma, J. Exploring Marine Resources for Bioactive Compounds. Planta Medica 2014, 80, 1234–1246. [Google Scholar] [CrossRef]
- Poblete-Castro, I.; Hoffmann, S.-L.; Becker, J.; Wittmann, C. Cascaded Valorization of Seaweed Using Microbial Cell Factories. Curr. Opin. Biotechnol. 2020, 65, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Zeng, Y.; Yuan, X.; Wang, J.K.; Tay, C.Y. Waste-to-Resource: Extraction and Transformation of Aquatic Biomaterials for Regenerative Medicine. Biomater. Adv. 2025, 166, 214023. [Google Scholar] [CrossRef] [PubMed]
- Ben-Nissan, B.; Choi, A.H.; Green, D.W. Marine Derived Biomaterials for Bone Regeneration and Tissue Engineering: Learning from Nature. In Marine-Derived Biomaterials for Tissue Engineering Applications; Choi, A.H., Ben-Nissan, B., Eds.; Springer: Singapore, 2019; pp. 51–78. ISBN 9789811388552. [Google Scholar]
- Foran, E.; Weiner, S.; Fine, M. Biogenic Fish-Gut Calcium Carbonate Is a Stable Amorphous Phase in the Gilt-Head Seabream, Sparus Aurata. Sci. Rep. 2013, 3, 1700. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Zhu, Y.-J.; Lu, B.-Q.; Zhao, X.-Y.; Zhao, J.; Chen, F.; Wu, J. ATP-Stabilized Amorphous Calcium Carbonate Nanospheres and Their Application in Protein Adsorption. Small 2014, 10, 2047–2056. [Google Scholar] [CrossRef]
- Šupová, M. Isolation and Preparation of Nanoscale Bioapatites from Natural Sources: A Review. J. Nanosci. Nanotechnol. 2014, 14, 546–563. [Google Scholar] [CrossRef]
- Bharadwaz, A.; Jayasuriya, A.C. Recent Trends in the Application of Widely Used Natural and Synthetic Polymer Nanocomposites in Bone Tissue Regeneration. Mater. Sci. Eng. C 2020, 110, 110698. [Google Scholar] [CrossRef]
- Akram, M.; Ahmed, R.; Shakir, I.; Ibrahim, W.A.W.; Hussain, R. Extracting Hydroxyapatite and Its Precursors from Natural Resources. J. Mater. Sci. 2014, 49, 1461–1475. [Google Scholar] [CrossRef]
- Sivakumar, M.; Kumar, T.S.S.; Shantha, K.L.; Rao, K.P. Development of Hydroxyapatite Derived from Indian Coral. Biomaterials 1996, 17, 1709–1714. [Google Scholar] [CrossRef]
- Jinawath, S.; Polchai, D.; Yoshimura, M. Low-Temperature, Hydrothermal Transformation of Aragonite to Hydroxyapatite. Mater. Sci. Eng. C 2002, 22, 35–39. [Google Scholar] [CrossRef]
- Walsh, P.J.; Buchanan, F.J.; Dring, M.; Maggs, C.; Bell, S.; Walker, G.M. Low-Pressure Synthesis and Characterisation of Hydroxyapatite Derived from Mineralise Red Algae. Chem. Eng. J. 2008, 137, 173–179. [Google Scholar] [CrossRef]
- Kumar, G.S.; Girija, E.K.; Venkatesh, M.; Karunakaran, G.; Kolesnikov, E.; Kuznetsov, D. One Step Method to Synthesize Flower-like Hydroxyapatite Architecture Using Mussel Shell Bio-Waste as a Calcium Source. Ceram. Int. 2017, 43, 3457–3461. [Google Scholar] [CrossRef]
- Pal, A.; Maity, S.; Chabri, S.; Bera, S.; Chowdhury, A.R.; Das, M.; Sinha, A. Mechanochemical Synthesis of Nanocrystalline Hydroxyapatite from Mercenaria Clam Shells and Phosphoric Acid. Biomed. Phys. Eng. Express 2017, 3, 015010. [Google Scholar] [CrossRef]
- Nandi, S.K.; Kundu, B.; Mukherjee, J.; Mahato, A.; Datta, S.; Balla, V.K. Converted Marine Coral Hydroxyapatite Implants with Growth Factors: In Vivo Bone Regeneration. Mater. Sci. Eng. C 2015, 49, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Lalzawmliana, V.; Anand, A.; Mukherjee, P.; Chaudhuri, S.; Kundu, B.; Nandi, S.K.; Thakur, N.L. Marine Organisms as a Source of Natural Matrix for Bone Tissue Engineering. Ceram. Int. 2019, 45, 1469–1481. [Google Scholar] [CrossRef]
- Neto, A.S.; Ferreira, J.M.F. Synthetic and Marine-Derived Porous Scaffolds for Bone Tissue Engineering. Materials 2018, 11, 1702. [Google Scholar] [CrossRef]
- Klar, R.M.; Duarte, R.; Dix-Peek, T.; Dickens, C.; Ferretti, C.; Ripamonti, U. Calcium Ions and Osteoclastogenesis Initiate the Induction of Bone Formation by Coral-Derived Macroporous Constructs. J. Cell. Mol. Med. 2013, 17, 1444–1457. [Google Scholar] [CrossRef]
- Zhou, H.; Liang, B.; Jiang, H.; Deng, Z.; Yu, K. Magnesium-Based Biomaterials as Emerging Agents for Bone Repair and Regeneration: From Mechanism to Application. J. Magnes. Alloys 2021, 9, 779–804. [Google Scholar] [CrossRef]
- Bavya Devi, K.; Nandi, S.K.; Roy, M. Magnesium Silicate Bioceramics for Bone Regeneration: A Review. J. Indian Inst. Sci. 2019, 99, 261–288. [Google Scholar] [CrossRef]
- Wang, J.-L.; Xu, J.-K.; Hopkins, C.; Chow, D.H.-K.; Qin, L. Biodegradable Magnesium-Based Implants in Orthopedics—A General Review and Perspectives. Adv. Sci. 2020, 7, 1902443. [Google Scholar] [CrossRef]
- Zwanenburg, F.A.; Dzurak, A.S.; Morello, A.; Simmons, M.Y.; Hollenberg, L.C.L.; Klimeck, G.; Rogge, S.; Coppersmith, S.N.; Eriksson, M.A. Silicon Quantum Electronics. Rev. Mod. Phys. 2013, 85, 961–1019. [Google Scholar] [CrossRef]
- Morganti, D.; Leonardi, A.A.; Lo Faro, M.J.; Leonardi, G.; Salvato, G.; Fazio, B.; Musumeci, P.; Livreri, P.; Conoci, S.; Neri, G.; et al. Ultrathin Silicon Nanowires for Optical and Electrical Nitrogen Dioxide Detection. Nanomaterials 2021, 11, 1767. [Google Scholar] [CrossRef] [PubMed]
- Lo Faro, M.J.; Ruello, G.; Leonardi, A.A.; Morganti, D.; Irrera, A.; Priolo, F.; Gigan, S.; Volpe, G.; Fazio, B. Visualization of Directional Beaming of Weakly Localized Raman from a Random Network of Silicon Nanowires. Adv. Sci. 2021, 8, 2100139. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, A.A.; Sciuto, E.L.; Lo Faro, M.J.; Morganti, D.; Midiri, A.; Spinella, C.; Conoci, S.; Irrera, A.; Fazio, B. Molecular Fingerprinting of the Omicron Variant Genome of SARS-CoV-2 by SERS Spectroscopy. Nanomaterials 2022, 12, 2134. [Google Scholar] [CrossRef] [PubMed]
- Harraz, F.A. Porous Silicon Chemical Sensors and Biosensors: A Review. Sens. Actuators B Chem. 2014, 202, 897–912. [Google Scholar] [CrossRef]
- Leonardi, A.A.; Battaglia, R.; Morganti, D.; Faro, M.J.L.; Fazio, B.; De Pascali, C.; Francioso, L.; Palazzo, G.; Mallardi, A.; Purrello, M.; et al. A Novel Silicon Platform for Selective Isolation, Quantification, and Molecular Analysis of Small Extracellular Vesicles. Int. J. Nanomed. 2021, 16, 5153–5165. [Google Scholar] [CrossRef]
- Morganti, D.; Faro, M.J.L.; Leonardi, A.A.; Fazio, B.; Conoci, S.; Irrera, A. Luminescent Silicon Nanowires as Novel Sensor for Environmental Air Quality Control. Sensors 2022, 22, 8755. [Google Scholar] [CrossRef]
- Habibovic, P.; de Groot, K. Osteoinductive Biomaterials—Properties and Relevance in Bone Repair. J. Tissue Eng. Regen. Med. 2007, 1, 25–32. [Google Scholar] [CrossRef]
- Pedraza, A.J.; Fowlkes, J.D.; Guan, Y.-F. Surface Nanostructuring of Silicon. Appl. Phys. A 2003, 77, 277–284. [Google Scholar] [CrossRef]
- Huang, Z.; Geyer, N.; Werner, P.; de Boor, J.; Gösele, U. Metal-Assisted Chemical Etching of Silicon: A Review. Adv. Mater. 2011, 23, 285–308. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; Liu, X.; Huang, Q.; He, W.; Zhang, R.; Feng, Q.; Benayahu, D. The Stimulatory Effect of Silica Nanoparticles on Osteogenic Differentiation of Human Mesenchymal Stem Cells. Biomed. Mater. 2016, 12, 015001. [Google Scholar] [CrossRef]
- Gaharwar, A.K.; Mihaila, S.M.; Swami, A.; Patel, A.; Sant, S.; Reis, R.L.; Marques, A.P.; Gomes, M.E.; Khademhosseini, A. Bioactive Silicate Nanoplatelets for Osteogenic Differentiation of Human Mesenchymal Stem Cells. Adv. Mater. 2013, 25, 3329–3336. [Google Scholar] [CrossRef] [PubMed]
- Aw, M.S.; Simovic, S.; Yu, Y.; Addai-Mensah, J.; Losic, D. Porous Silica Microshells from Diatoms as Biocarrier for Drug Delivery Applications. Powder Technol. 2012, 223, 52–58. [Google Scholar] [CrossRef]
- Le, T.D.H.; Bonani, W.; Speranza, G.; Sglavo, V.; Ceccato, R.; Maniglio, D.; Motta, A.; Migliaresi, C. Processing and Characterization of Diatom Nanoparticles and Microparticles as Potential Source of Silicon for Bone Tissue Engineering. Mater. Sci. Eng. C 2016, 59, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Losic, D.; Mitchell, J.G.; Voelcker, N.H. Diatomaceous Lessons in Nanotechnology and Advanced Materials. Adv. Mater. 2009, 21, 2947–2958. [Google Scholar] [CrossRef]
- Martins, E.; Rapp, H.T.; Xavier, J.R.; Diogo, G.S.; Reis, R.L.; Silva, T.H. Macro and Microstructural Characteristics of North Atlantic Deep-Sea Sponges as Bioinspired Models for Tissue Engineering Scaffolding. Front. Mar. Sci. 2021, 7, 613647. [Google Scholar] [CrossRef]
- Ahmadipour, M.; Mohammadi, H.; Pang, A.L.; Arjmand, M.; Ayode Otitoju, T.; Okoye, P.U.; Rajitha, B. A Review: Silicate Ceramic-Polymer Composite Scaffold for Bone Tissue Engineering. Int. J. Polym. Mater. Polym. Biomater. 2022, 71, 180–195. [Google Scholar] [CrossRef]
- Rozan, H.E.; Wu, G.; Zhou, Z.; Li, Q.; Sharaf, M.; Chen, X. The Complex Hydrogel Based on Diatom Biosilica and Hydroxybutyl Chitosan for Wound Healing. Colloids Surf. B Biointerfaces 2022, 216, 112523. [Google Scholar] [CrossRef]
- Wiens, M.; Wang, X.; Schloßmacher, U.; Lieberwirth, I.; Glasser, G.; Ushijima, H.; Schröder, H.C.; Müller, W.E.G. Osteogenic Potential of Biosilica on Human Osteoblast-Like (SaOS-2) Cells. Calcif. Tissue Int. 2010, 87, 513–524. [Google Scholar] [CrossRef]
- Martins, E.; Diogo, G.S.; Pires, R.; Reis, R.L.; Silva, T.H. 3D Biocomposites Comprising Marine Collagen and Silica-Based Materials Inspired on the Composition of Marine Sponge Skeletons Envisaging Bone Tissue Regeneration. Mar. Drugs 2022, 20, 718. [Google Scholar] [CrossRef]
- Aslam, B.; Augustyniak, A.; Clarke, S.A.; McMahon, H. Development of a Novel Marine-Derived Tricomposite Biomaterial for Bone Regeneration. Mar. Drugs 2023, 21, 473. [Google Scholar] [CrossRef]
- Senni, K.; Pereira, J.; Gueniche, F.; Delbarre-Ladrat, C.; Sinquin, C.; Ratiskol, J.; Godeau, G.; Fischer, A.-M.; Helley, D.; Colliec-Jouault, S. Marine Polysaccharides: A Source of Bioactive Molecules for Cell Therapy and Tissue Engineering. Mar. Drugs 2011, 9, 1664–1681. [Google Scholar] [CrossRef] [PubMed]
- Kolambkar, Y.M.; Dupont, K.M.; Boerckel, J.D.; Huebsch, N.; Mooney, D.J.; Hutmacher, D.W.; Guldberg, R.E. An Alginate-Based Hybrid System for Growth Factor Delivery in the Functional Repair of Large Bone Defects. Biomaterials 2011, 32, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Barre, A.; Naudot, M.; Colin, F.; Sevestre, H.; Collet, L.; Devauchelle, B.; Lack, S.; Marolleau, J.-P.; Le Ricousse, S. An Alginate-Based Hydrogel with a High Angiogenic Capacity and a High Osteogenic Potential. BioRes. Open Access 2020, 9, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Peng, X.-L.; Li, H.-R.; Liu, J.-X.; Cheng, J.-S.-Y.; Qi, X.-Y.; Ye, S.-J.; Gong, H.-L.; Zhao, X.-H.; Yu, J.; et al. Marine-Derived Collagen as Biomaterials for Human Health. Front. Nutr. 2021, 8, 702108. [Google Scholar] [CrossRef]
- Muralidharan, N.; Jeya Shakila, R.; Sukumar, D.; Jeyasekaran, G. Skin, Bone and Muscle Collagen Extraction from the Trash Fish, Leather Jacket (Odonus Niger) and Their Characterization. J. Food Sci. Technol. 2013, 50, 1106–1113. [Google Scholar] [CrossRef]
- Hou, Y.; Shavandi, A.; Carne, A.; Bekhit, A.A.; Ng, T.B.; Cheung, R.C.F.; Bekhit, A.E.A. Marine Shells: Potential Opportunities for Extraction of Functional and Health-Promoting Materials. Crit. Rev. Environ. Sci. Technol. 2016, 46, 1047–1116. [Google Scholar] [CrossRef]
- Meng, K.; Chen, L.; Xia, G.; shen, X. Effects of Zinc Sulfate and Zinc Lactate on the Properties of Tilapia (Oreochromis Niloticus) Skin Collagen Peptide Chelate Zinc. Food Chem. 2021, 347, 129043. [Google Scholar] [CrossRef]
- Witten, P.E.; Huysseune, A.; Hall, B.K. A Practical Approach for the Identification of the Many Cartilaginous Tissues in Teleost Fish. J. Appl. Ichthyol. 2010, 26, 257–262. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Rodríguez-Amado, I.; Montemayor, M.I.; Fraguas, J.; González, M.D.P.; Murado, M.A. Chondroitin Sulfate, Hyaluronic Acid and Chitin/Chitosan Production Using Marine Waste Sources: Characteristics, Applications and Eco-Friendly Processes: A Review. Mar. Drugs 2013, 11, 747–774. [Google Scholar] [CrossRef]
- Knudson, C.B.; Knudson, W. Cartilage Proteoglycans. Semin. Cell Dev. Biol. 2001, 12, 69–78. [Google Scholar] [CrossRef]
- Volpi, N. Chondroitin Sulfate Safety and Quality. Molecules 2019, 24, 1447. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ura, K.; Takagi, Y. Industrial Application of Fish Cartilaginous Tissues. Curr. Res. Food Sci. 2022, 5, 698–709. [Google Scholar] [CrossRef] [PubMed]
- Varghese, S.; Hwang, N.S.; Canver, A.C.; Theprungsirikul, P.; Lin, D.W.; Elisseeff, J. Chondroitin Sulfate Based Niches for Chondrogenic Differentiation of Mesenchymal Stem Cells. Matrix Biol. 2008, 27, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Ashhurst, D.E. The Cartilaginous Skeleton of an Elasmobranch Fish Does Not Heal. Matrix Biol. 2004, 23, 15–22. [Google Scholar] [CrossRef]
- Zamani, A.; Khajavi, M.; Nazarpak, M.H.; Solouk, A.; Atef, M. Preliminary Evaluation of Fish Cartilage as a Promising Biomaterial in Cartilage Tissue Engineering. Ann. Anat.—Anat. Anz. 2024, 253, 152232. [Google Scholar] [CrossRef]
- Seidel, R.; Blumer, M.; Pechriggl, E.-J.; Lyons, K.; Hall, B.K.; Fratzl, P.; Weaver, J.C.; Dean, M.N. Calcified Cartilage or Bone? Collagens in the Tessellated Endoskeletons of Cartilaginous Fish (Sharks and Rays). J. Struct. Biol. 2017, 200, 54–71. [Google Scholar] [CrossRef]
- Dean, M.N.; Summers, A.P. Mineralized Cartilage in the Skeleton of Chondrichthyan Fishes. Zoology 2006, 109, 164–168. [Google Scholar] [CrossRef]
- Aubourg, S.P. Fluorescence Study of the Pro-oxidant Effect of Free Fatty Acids on Marine Lipids. J. Sci. Food Agric. 2001, 81, 385–390. [Google Scholar] [CrossRef]
- Luján-Amoraga, L.; Delgado-Martín, B.; Lourenço-Marques, C.; Gavaia, P.J.; Bravo, J.; Bandarra, N.M.; Dominguez, D.; Izquierdo, M.S.; Pousão-Ferreira, P.; Ribeiro, L. Exploring Omega-3′s Impact on the Expression of Bone-Related Genes in Meagre (Argyrosomus Regius). Biomolecules 2024, 14, 56. [Google Scholar] [CrossRef]
- Chen, Y.; Cao, H.; Sun, D.; Lin, C.; Wang, L.; Huang, M.; Jiang, H.; Zhang, Z.; Jin, D.; Zhang, B.; et al. Endogenous Production of N-3 Polyunsaturated Fatty Acids Promotes Fracture Healing in Mice. J. Healthc. Eng. 2017, 2017, 3571267. [Google Scholar] [CrossRef]
- Banu, J.; Bhattacharya, A.; Rahman, M.; Kang, J.X.; Fernandes, G. Endogenously Produced N-3 Fatty Acids Protect against Ovariectomy Induced Bone Loss in Fat-1 Transgenic Mice. J. Bone Miner. Metab. 2010, 28, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, T.; Kawai, T.; Takei, N.; Kise, T.; Eda, S.; Urist, M.R. Evaluation of Heterotopic Bone Formation Induced by Squalane and Bone Morphogenetic Protein Composite. Clin. Orthop. Relat. Res. (1976–2007) 1997, 337, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Qin, C.; Li, Z.; Lu, L.; Tong, Y.; Yuan, J.; Yin, F.; Cheng, Y.; Wu, C. Diatomite-Incorporated Hierarchical Scaffolds for Osteochondral Regeneration. Bioact. Mater. 2024, 38, 305–320. [Google Scholar] [CrossRef]
- Tamburaci, S.; Kimna, C.; Tihminlioglu, F. Bioactive Diatomite and POSS Silica Cage Reinforced Chitosan/Na-Carboxymethyl Cellulose Polyelectrolyte Scaffolds for Hard Tissue Regeneration. Mater. Sci. Eng. C 2019, 100, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Min, K.H.; Kim, D.H.; Youn, S.; Pack, S.P. Biomimetic Diatom Biosilica and Its Potential for Biomedical Applications and Prospects: A Review. Int. J. Mol. Sci. 2024, 25, 2023. [Google Scholar] [CrossRef]
- Olăreț, E.; Dinescu, S.; Dobranici, A.-E.; Ginghină, R.-E.; Voicu, G.; Mihăilescu, M.; Curti, F.; Banciu, D.D.; Sava, B.; Amarie, S.; et al. Osteoblast Responsive Biosilica-Enriched Gelatin Microfibrillar Microenvironments. Biomater. Adv. 2024, 161, 213894. [Google Scholar] [CrossRef]
- O’Gorman, D.M.; Tierney, C.M.; Brennan, O.; O’Brien, F.J. The Marine-Derived, Multi-Mineral Formula, Aquamin, Enhances Mineralisation of Osteoblast Cells In Vitro. Phytother. Res. 2012, 26, 375–380. [Google Scholar] [CrossRef]
- Widaa, A.; Brennan, O.; O’Gorman, D.M.; O’Brien, F.J. The Osteogenic Potential of the Marine-Derived Multi-Mineral Formula Aquamin Is Enhanced by the Presence of Vitamin D. Phytother. Res. 2014, 28, 678–684. [Google Scholar] [CrossRef]
- Wang, S.; Wang, X.; Draenert, F.G.; Albert, O.; Schröder, H.C.; Mailänder, V.; Mitov, G.; Müller, W.E.G. Bioactive and Biodegradable Silica Biomaterial for Bone Regeneration. Bone 2014, 67, 292–304. [Google Scholar] [CrossRef]
- Wang, X.; Schröder, H.C.; Wiens, M.; Schloßmacher, U.; Müller, W.E.G. Chapter Five—Biosilica: Molecular Biology, Biochemistry and Function in Demosponges as Well as Its Applied Aspects for Tissue Engineering. In Advances in Marine Biology; Becerro, M.A., Uriz, M.J., Maldonado, M., Turon, X., Eds.; Academic Press: Cambridge, MA, USA, 2012; Volume 62, pp. 231–271. ISBN 0065-2881. [Google Scholar]
- Müller, W.E.; Neufurth, M.; Wang, S.; Schröder, H.C.; Wang, X. The Physiological Inorganic Polymers Biosilica and Polyphosphate as Key Drivers for Biomedical Materials in Regenerative Nanomedicine. Int. J. Nanomed. 2024, 19, 1303–1337. [Google Scholar] [CrossRef]
- Sathiyavimal, S.; Vasantharaj, S.; Mattheos, N.; Pugazhendhi, A.; Subbalekha, K. Mussel Shell-Derived Biogenic Hydroxyapatite as Reinforcement on Chitosan-Loaded Gentamicin Composite for Antibacterial Activity and Bone Regeneration. Int. J. Biol. Macromol. 2024, 278, 134143. [Google Scholar] [CrossRef] [PubMed]
- Akilal, N.; Lemaire, F.; Bercu, N.B.; Sayen, S.; Gangloff, S.C.; Khelfaoui, Y.; Rammal, H.; Kerdjoudj, H. Cowries Derived Aragonite as Raw Biomaterials for Bone Regenerative Medicine. Mater. Sci. Eng. C 2019, 94, 894–900. [Google Scholar] [CrossRef] [PubMed]
- Green, D.W.; Ben-Nissan, B.; Yoon, K.S.; Milthorpe, B.; Jung, H.-S. Natural and Synthetic Coral Biomineralization for Human Bone Revitalization. Trends Biotechnol. 2017, 35, 43–54. [Google Scholar] [CrossRef] [PubMed]
- La, W.-G.; Shin, J.-Y.; Bhang, S.H.; Jin, M.; Yoon, H.H.; Noh, S.-S.; Im, G.-I.; Kim, C.-S.; Kim, B.-S. Culture on a 3,4-Dihydroxy-l-Phenylalanine-Coated Surface Promotes the Osteogenic Differentiation of Human Mesenchymal Stem Cells. Tissue Eng. Part A 2013, 19, 1255–1263. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, S.; Sun, Y.; Ru Yie, K.H.; Zhuang, J.; Liu, T.; Si, W.; Zhang, Y.; Liu, Z.; Xiong, L.; et al. Mussel Byssus-Inspired Dual-Functionalization of Zirconia Dental Implants for Improved Bone Integration. Mater. Today Bio 2024, 25, 101007. [Google Scholar] [CrossRef]
- Gao, P.; Zhang, Q.; Sun, Y.; Cheng, H.; Wu, S.; Zhang, Y.; Si, W.; Sun, H.; Sun, N.; Yang, J.; et al. Synergistic Catecholamine and Coordination Chemistry for Enhanced Bioactivity and Secondary Grafting Activity of Zirconia Dental Implants. Colloids Surf. B Biointerfaces 2025, 246, 114361. [Google Scholar] [CrossRef]
- Rigogliuso, S.; Salamone, M.; Barbarino, E.; Barbarino, M.; Nicosia, A.; Ghersi, G. Production of Injectable Marine Collagen-Based Hydrogel for the Maintenance of Differentiated Chondrocytes in Tissue Engineering Applications. Int. J. Mol. Sci. 2020, 21, 5798. [Google Scholar] [CrossRef]
- Flaig, I.; Radenković, M.; Najman, S.; Pröhl, A.; Jung, O.; Barbeck, M. In Vivo Analysis of the Biocompatibility and Immune Response of Jellyfish Collagen Scaffolds and Its Suitability for Bone Regeneration. Int. J. Mol. Sci. 2020, 21, 4518. [Google Scholar] [CrossRef]
- Atlan, G.; Balmain, N.; Berland, S.; Vidal, B.; Lopez, É. Reconstruction of Human Maxillary Defects with Nacre Powder: Histological Evidence for Bone Regeneration. Comptes Rendus L’académie Sci.-Ser. III-Sci. Vie 1997, 320, 253–258. [Google Scholar] [CrossRef]
- Alakpa, E.V.; Burgess, K.E.V.; Chung, P.; Riehle, M.O.; Gadegaard, N.; Dalby, M.J.; Cusack, M. Nacre Topography Produces Higher Crystallinity in Bone than Chemically Induced Osteogenesis. ACS Nano 2017, 11, 6717–6727. [Google Scholar] [CrossRef]
- Sun, F.; Zhou, H.; Lee, J. Various Preparation Methods of Highly Porous Hydroxyapatite/Polymer Nanoscale Biocomposites for Bone Regeneration. Acta Biomater. 2011, 7, 3813–3828. [Google Scholar] [CrossRef] [PubMed]
- Oktar, F.N.; Unal, S.; Gunduz, O.; Nissan, B.B.; Macha, I.J.; Akyol, S.; Duta, L.; Ekren, N.; Altan, E.; Yetmez, M. Marine-Derived Bioceramics for Orthopedic, Reconstructive and Dental Surgery Applications. J. Aust. Ceram. Soc. 2023, 59, 57–81. [Google Scholar] [CrossRef]
- Muntean, F.L.; Olariu, I.; Marian, D.; Olariu, T.; Petrescu, E.L.; Olariu, T.; Drăghici, G.A. Hydroxyapatite from Mollusk Shells: Characteristics, Production, and Potential Applications in Dentistry. Dent. J. 2024, 12, 409. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Penas, R.; Verdugo-Escamilla, C.; Triunfo, C.; Gärtner, S.; D’Urso, A.; Oltolina, F.; Follenzi, A.; Maoloni, G.; Cölfen, H.; Falini, G.; et al. A Sustainable One-Pot Method to Transform Seashell Waste Calcium Carbonate to Osteoinductive Hydroxyapatite Micro-Nanoparticles. J. Mater. Chem. B 2023, 11, 7766–7777. [Google Scholar] [CrossRef]
- Cheng, M.; Liu, M.; Chang, L.; Liu, Q.; Wang, C.; Hu, L.; Zhang, Z.; Ding, W.; Chen, L.; Guo, S.; et al. Overview of Structure, Function and Integrated Utilization of Marine Shell. Sci. Total Environ. 2023, 870, 161950. [Google Scholar] [CrossRef]
- Azarian, M.H.; Sutapun, W. Biogenic Calcium Carbonate Derived from Waste Shells for Advanced Material Applications: A Review. Front. Mater. 2022, 9, 1024977. [Google Scholar] [CrossRef]
- Zhang, X.; Vecchio, K.S. Conversion of Natural Marine Skeletons as Scaffolds for Bone Tissue Engineering. Front. Mater. Sci. 2013, 7, 103–117. [Google Scholar] [CrossRef]
- Coringa, R.; de Sousa, E.M.; Botelho, J.N.; Diniz, R.S.; de Sá, J.C.; da Cruz, M.C.F.N.; Paschoal, M.A.B.; Gonçalves, L.M. Bone Substitute Made from a Brazilian Oyster Shell Functions as a Fast Stimulator for Bone-Forming Cells in an Animal Model. PLoS ONE 2018, 13, e0198697. [Google Scholar] [CrossRef]
- Ho, W.-F.; Lee, M.-H.; Thomas, J.L.; Li, J.-A.; Wu, S.-C.; Hsu, H.-C.; Lin, H.-Y. Porous Biphasic Calcium Phosphate Granules from Oyster Shell Promote the Differentiation of Induced Pluripotent Stem Cells. Int. J. Mol. Sci. 2021, 22, 9444. [Google Scholar] [CrossRef]
- Green, D.W.; Padula, M.; Santos, J.; Chou, J.; Milthorpe, B.; Ben-Nissan, B. A New Role for Marine Skeletal Proteins in Regenerative Orthopaedics. Key Eng. Mater. 2013, 529, 654–659. [Google Scholar] [CrossRef]
- Falini, G.; Fermani, S.; Goffredo, S. Coral Biomineralization: A Focus on Intra-Skeletal Organic Matrix and Calcification. Semin. Cell Dev. Biol. 2015, 46, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Abramovitch-Gottlib, L.; Geresh, S.; Vago, R. Biofabricated Marine Hydrozoan: A Bioactive Crystalline Material Promoting Ossification of Mesenchymal Stem Cells. Tissue Eng. 2006, 12, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Geiger, F.; Lorenz, H.; Xu, W.; Szalay, K.; Kasten, P.; Claes, L.; Augat, P.; Richter, W. VEGF Producing Bone Marrow Stromal Cells (BMSC) Enhance Vascularization and Resorption of a Natural Coral Bone Substitute. Bone 2007, 41, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Green, D.; Walsh, D.; Mann, S.; Oreffo, R.O.C. The Potential of Biomimesis in Bone Tissue Engineering: Lessons from the Design and Synthesis of Invertebrate Skeletons. Bone 2002, 30, 810–815. [Google Scholar] [CrossRef]
- Mygind, T.; Stiehler, M.; Baatrup, A.; Li, H.; Zou, X.; Flyvbjerg, A.; Kassem, M.; Bünger, C. Mesenchymal Stem Cell Ingrowth and Differentiation on Coralline Hydroxyapatite Scaffolds. Biomaterials 2007, 28, 1036–1047. [Google Scholar] [CrossRef]
- Shors, E.C. Coralline Bone Graft Substitutes. Orthop. Clin. 1999, 30, 599–613. [Google Scholar] [CrossRef]
- Wiesmann, H.P.; Joos, U.; Meyer, U. Biological and Biophysical Principles in Extracorporal Bone Tissue Engineering: Part II. Int. J. Oral Maxillofac. Surg. 2004, 33, 523–530. [Google Scholar] [CrossRef]
- Coughlin, M.J.; Grimes, J.S.; Kennedy, M.P. Coralline Hydroxyapatite Bone Graft Substitute in Hindfoot Surgery. Foot Ankle Int. 2006, 27, 19–22. [Google Scholar] [CrossRef]
- Griesshaber, E.; Schmahl, W.W.; Neuser, R.; Pettke, T.; Blüm, M.; Mutterlose, J.; Brand, U. Crystallographic Texture and Microstructure of Terebratulide Brachiopod Shell Calcite: An Optimized Materials Design with Hierarchical Architecture. Am. Mineral. 2007, 92, 722–734. [Google Scholar] [CrossRef]
- Im, K.; Park, J.H.; Kim, K.N.; Kim, K.M.; Choi, S.H.; Kim, C.K.; Lee, Y.K. Organic-Inorganic Hybrids of Hydroxyapatite with Chitosan. Key Eng. Mater. 2005, 284, 729–732. [Google Scholar] [CrossRef]
- Lahaye, M.; Robic, A. Structure and Functional Properties of Ulvan, a Polysaccharide from Green Seaweeds. Biomacromolecules 2007, 8, 1765–1774. [Google Scholar] [CrossRef] [PubMed]
- Martina, M.; Subramanyam, G.; Weaver, J.C.; Hutmacher, D.W.; Morse, D.E.; Valiyaveettil, S. Developing Macroporous Bicontinuous Materials as Scaffolds for Tissue Engineering. Biomaterials 2005, 26, 5609–5616. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-Y.; Lin, A.Y.M.; Lin, Y.-S.; Seki, Y.; Stokes, A.G.; Peyras, J.; Olevsky, E.A.; Meyers, M.A.; McKittrick, J. Structure and Mechanical Properties of Selected Biological Materials. J. Mech. Behav. Biomed. Mater. 2008, 1, 208–226. [Google Scholar] [CrossRef] [PubMed]
- Green, D.; Howard, D.; Yang, X.; Kelly, M.; Oreffo, R. Natural Marine Sponge Fiber Skeleton: A Biomimetic Scaffold for Human Osteoprogenitor Cell Attachment, Growth, and Differentiation. Tissue Eng. 2003, 9, 1159–1166. [Google Scholar] [CrossRef]
- Laza, A.L.; Jaber, M.; Miehé-Brendlé, J.; Demais, H.; Le Deit, H.; Delmotte, L.; Vidal, L. Green Nanocomposites: Synthesis and Characterization. J. Nanosci. Nanotechnol. 2007, 7, 3207–3213. [Google Scholar] [CrossRef]
- Oliveira, J.M.; Grech, J.M.R.; Leonor, I.B.; Mano, J.F.; Reis, R.L. Calcium-Phosphate Derived from Mineralized Algae for Bone Tissue Engineering Applications. Mater. Lett. 2007, 61, 3495–3499. [Google Scholar] [CrossRef][Green Version]
- Bächle, M.; Hübner, U.; Kohal, R.J.; Han, J.S.; Wiedmann-Al-Ahmad, M. Structure and in Vitro Cytocompatibility of the Gastropod Shell of Helix Pomatia. Tissue Cell 2006, 38, 337–344. [Google Scholar] [CrossRef]
- Hou, R.; Chen, F.; Yang, Y.; Cheng, X.; Gao, Z.; Yang, H.O.; Wu, W.; Mao, T. Comparative Study between Coral-Mesenchymal Stem Cells-rhBMP-2 Composite and Auto-Bone-Graft in Rabbit Critical-Sized Cranial Defect Model. J. Biomed. Mater. Res. Part A 2007, 80A, 85–93. [Google Scholar] [CrossRef]
- Kujala, S.; Raatikainen, T.; Ryhänen, J.; Kaarela, O.; Jalovaara, P. Composite Implant of Native Bovine Bone Morphogenetic Protein (BMP) and Biocoral in the Treatment of Scaphoid Nonunions—A Preliminary Study. Scand. J. Surg. 2002, 91, 186–190. [Google Scholar] [CrossRef]
- Doherty, M.J.; Schlag, G.; Schwarz, N.; Mollan, R.A.B.; Nolan, P.C.; Wilson, D.J. Biocompatibility of Xenogeneic Bone, Commercially Available Coral, a Bioceramic and Tissue Sealant for Human Osteoblasts. Biomaterials 1994, 15, 601–608. [Google Scholar] [CrossRef]
- Gravel, M.; Vago, R.; Tabrizian, M. Use of Natural Coralline Biomaterials As Reinforcing and Gas-Forming Agent for Developing Novel Hybrid Biomatrices: Microarchitectural and Mechanical Studies. Tissue Eng. 2006, 12, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Galea, G.; Kopman, D.; Graham, B.J.M. Supply and Demand of Bone Allograft for Revision Hip Surgery in Scotland. J. Bone Jt. Surg. Br. Vol. 1998, 80-B, 595–599. [Google Scholar] [CrossRef]
- Waite, J.H.; Qin, X.-X.; Coyne, K.J. The Peculiar Collagens of Mussel Byssus. Matrix Biol. 1998, 17, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Suhre, M.H.; Gertz, M.; Steegborn, C.; Scheibel, T. Structural and Functional Features of a Collagen-Binding Matrix Protein from the Mussel Byssus. Nat. Commun. 2014, 5, 3392. [Google Scholar] [CrossRef]
- Hagenau, A.; Scheidt, H.A.; Serpell, L.; Huster, D.; Scheibel, T. Structural Analysis of Proteinaceous Components in Byssal Threads of the Mussel Mytilus Galloprovincialis. Macromol. Biosci. 2009, 9, 162–168. [Google Scholar] [CrossRef]
- Babarro, J.M.F.; Reiriz, M.J.F. Secretion of Byssal Threads in Mytilus Galloprovincialis: Quantitative and Qualitative Values after Spawning Stress. J. Comp. Physiol. B 2010, 180, 95–104. [Google Scholar] [CrossRef]
- Impellitteri, F.; Yunko, K.; Calabrese, G.; Porretti, M.; Martyniuk, V.; Gnatyshyna, L.; Nava, V.; Potortì, A.G.; Piccione, G.; Di Bella, G.; et al. Chlorpromazine’s Impact on Mytilus Galloprovincialis: A Multi-Faceted Investigation. Chemosphere 2024, 350, 141079. [Google Scholar] [CrossRef]
- Yin, D.; Komasa, S.; Yoshimine, S.; Sekino, T.; Okazaki, J. Effect of Mussel Adhesive Protein Coating on Osteogenesis in Vitro and Osteointegration in Vivo to Alkali-Treated Titanium with Nanonetwork Structures. Int. J. Nanomed. 2019, 14, 3831–3843. [Google Scholar] [CrossRef]
- Waite, J.H. Mussel Adhesion–Essential Footwork. J. Exp. Biol. 2017, 220, 517–530. [Google Scholar] [CrossRef]
- Hong, J.M.; Kim, B.J.; Shim, J.-H.; Kang, K.S.; Kim, K.-J.; Rhie, J.W.; Cha, H.J.; Cho, D.-W. Enhancement of Bone Regeneration through Facile Surface Functionalization of Solid Freeform Fabrication-Based Three-Dimensional Scaffolds Using Mussel Adhesive Proteins. Acta Biomater. 2012, 8, 2578–2586. [Google Scholar] [CrossRef]
- Mariod, A.A.; Fadul, H. Gelatin, Source, Extraction and Industrial Applications. Acta Sci. Pol. Technol. Aliment. 2013, 12, 135–147. [Google Scholar]
- Lueyot, A.; Rungsardthong, V.; Vatanyoopaisarn, S.; Hutangura, P.; Wonganu, B.; Wongsa-Ngasri, P.; Charoenlappanit, S.; Roytrakul, S.; Thumthanaruk, B. Influence of Collagen and Some Proteins on Gel Properties of Jellyfish Gelatin. PLoS ONE 2021, 16, e0253254. [Google Scholar] [CrossRef] [PubMed]
- Coppola, D.; Oliviero, M.; Vitale, G.A.; Lauritano, C.; D’Ambra, I.; Iannace, S.; de Pascale, D. Marine Collagen from Alternative and Sustainable Sources: Extraction, Processing and Applications. Mar. Drugs 2020, 18, 214. [Google Scholar] [CrossRef] [PubMed]
- Sewing, J.; Klinger, M.; Notbohm, H. Jellyfish Collagen Matrices Conserve the Chondrogenic Phenotype in Two- and Three-Dimensional Collagen Matrices. J. Tissue Eng. Regen. Med. 2017, 11, 916–925. [Google Scholar] [CrossRef]
- Hoyer, B.; Bernhardt, A.; Lode, A.; Heinemann, S.; Sewing, J.; Klinger, M.; Notbohm, H.; Gelinsky, M. Jellyfish Collagen Scaffolds for Cartilage Tissue Engineering. Acta Biomater. 2014, 10, 883–892. [Google Scholar] [CrossRef]
- Pustlauk, W.; Paul, B.; Brueggemeier, S.; Gelinsky, M.; Bernhardt, A. Modulation of Chondrogenic Differentiation of Human Mesenchymal Stem Cells in Jellyfish Collagen Scaffolds by Cell Density and Culture Medium. J. Tissue Eng. Regen. Med. 2017, 11, 1710–1722. [Google Scholar] [CrossRef]
- Pustlauk, W.; Paul, B.; Gelinsky, M.; Bernhardt, A. Jellyfish Collagen and Alginate: Combined Marine Materials for Superior Chondrogenesis of hMSC. Mater. Sci. Eng. C 2016, 64, 190–198. [Google Scholar] [CrossRef]
- Song, E.; Yeon Kim, S.; Chun, T.; Byun, H.-J.; Lee, Y.M. Collagen Scaffolds Derived from a Marine Source and Their Biocompatibility. Biomaterials 2006, 27, 2951–2961. [Google Scholar] [CrossRef]
- Addad, S.; Exposito, J.-Y.; Faye, C.; Ricard-Blum, S.; Lethias, C. Isolation, Characterization and Biological Evaluation of Jellyfish Collagen for Use in Biomedical Applications. Mar. Drugs 2011, 9, 967–983. [Google Scholar] [CrossRef]
- Steward, A.J.; Wagner, D.R.; Kelly, D.J. The Pericellular Environment Regulates Cytoskeletal Development and the Differentiation of Mesenchymal Stem Cells and Determines Their Response to Hydrostatic Pressure. Eur. Cell Mater. 2013, 25, 167–178. [Google Scholar] [CrossRef]
- Mousavi, S.J.; Doweidar, M.H. Role of Mechanical Cues in Cell Differentiation and Proliferation: A 3D Numerical Model. PLoS ONE 2015, 10, e0124529. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Gunn, J.; Chen, M.-H.; Cooper, A.; Zhang, M. On-Site Alginate Gelation for Enhanced Cell Proliferation and Uniform Distribution in Porous Scaffolds. J. Biomed. Mater. Res. Part A 2008, 86A, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Casillo, A.; Lanzetta, R.; Parrilli, M.; Corsaro, M.M. Exopolysaccharides from Marine and Marine Extremophilic Bacteria: Structures, Properties, Ecological Roles and Applications. Mar. Drugs 2018, 16, 69. [Google Scholar] [CrossRef] [PubMed]
- Nwodo, U.U.; Green, E.; Okoh, A.I. Bacterial Exopolysaccharides: Functionality and Prospects. Int. J. Mol. Sci. 2012, 13, 14002–14015. [Google Scholar] [CrossRef]
- Finore, I.; Di Donato, P.; Mastascusa, V.; Nicolaus, B.; Poli, A. Fermentation Technologies for the Optimization of Marine Microbial Exopolysaccharide Production. Mar. Drugs 2014, 12, 3005–3024. [Google Scholar] [CrossRef]
- Freitas, F.; Torres, C.A.V.; Reis, M.A.M. Engineering Aspects of Microbial Exopolysaccharide Production. Bioresour. Technol. 2017, 245, 1674–1683. [Google Scholar] [CrossRef]
- Möckl, L. The Emerging Role of the Mammalian Glycocalyx in Functional Membrane Organization and Immune System Regulation. Front. Cell Dev. Biol. 2020, 8, 253. [Google Scholar] [CrossRef]
- Torgbo, S.; Sukyai, P. Bacterial Cellulose-Based Scaffold Materials for Bone Tissue Engineering. Appl. Mater. Today 2018, 11, 34–49. [Google Scholar] [CrossRef]
- Witzler, M.; Büchner, D.; Shoushrah, S.H.; Babczyk, P.; Baranova, J.; Witzleben, S.; Tobiasch, E.; Schulze, M. Polysaccharide-Based Systems for Targeted Stem Cell Differentiation and Bone Regeneration. Biomolecules 2019, 9, 840. [Google Scholar] [CrossRef]
- Litwiniuk, M.; Krejner, A.; Speyrer, M.S.; Gauto, A.R.; Grzela, T. Hyaluronic Acid in Inflammation and Tissue Regeneration. Wounds 2016, 28, 78–88. [Google Scholar]
- DeVore, D.P.; Hughes, E.; Scott, J.B. Effectiveness of Injectable Filler Materials for Smoothing Wrinkle Lines and Depressed Scars. Med. Prog. Technol. 1994, 20, 243–250. [Google Scholar] [PubMed]
- Shi, Q.; Li, Y.; Sun, J.; Zhang, H.; Chen, L.; Chen, B.; Yang, H.; Wang, Z. The Osteogenesis of Bacterial Cellulose Scaffold Loaded with Bone Morphogenetic Protein-2. Biomaterials 2012, 33, 6644–6649. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y.; Yan, X.J.; Jiang, Y.; Cong, J. Development and Characteristic of Bacterial Cellulose for Antimicrobial Wound Dressing. Adv. Mater. Res. 2011, 152, 978–987. [Google Scholar] [CrossRef]
- Bagnol, R.; Grijpma, D.; Eglin, D.; Moriarty, T.F. The Production and Application of Bacterial Exopolysaccharides as Biomaterials for Bone Regeneration. Carbohydr. Polym. 2022, 291, 119550. [Google Scholar] [CrossRef]
- Zanchetta, P.; Lagarde, N.; Guezennec, J. Systemic Effects on Bone Healing of a New Hyaluronic Acid-Like Bacterial Exopolysaccharide. Calcif. Tissue Int. 2003, 73, 232–236. [Google Scholar] [CrossRef]
- Ruiz Velasco, C.; Baud’Huin, M.; Sinquin, C.; Maillasson, M.; Heymann, D.; Colliec-Jouault, S.; Padrines, M. Effects of a Sulfated Exopolysaccharide Produced by Altermonas Infernus on Bone Biology. Glycobiology 2011, 21, 781–795. [Google Scholar] [CrossRef]
- Caccamo, M.T.; Gugliandolo, C.; Zammuto, V.; Magazù, S. Thermal Properties of an Exopolysaccharide Produced by a Marine Thermotolerant Bacillus Licheniformis by ATR-FTIR Spectroscopy. Int. J. Biol. Macromol. 2020, 145, 77–83. [Google Scholar] [CrossRef]
- Zammuto, V.; Spanò, A.; Agostino, E.; Macrì, A.; De Pasquale, C.; Ferlazzo, G.; Rizzo, M.G.; Nicolò, M.S.; Guglielmino, S.; Gugliandolo, C. Anti-Bacterial Adhesion on Abiotic and Biotic Surfaces of the Exopolysaccharide from the Marine Bacillus Licheniformis B3-15. Mar. Drugs 2023, 21, 313. [Google Scholar] [CrossRef]
- Zammuto, V.; Agostino, E.; Macrì, A.; Spanò, A.; Grillo, E.; Nicolò, M.S.; Gugliandolo, C. Synergistic Antibiofilm Effects of Exopolymers Produced by the Marine, Thermotolerant Bacillus Licheniformis B3-15 and Their Potential Medical Applications. J. Mar. Sci. Eng. 2023, 11, 1660. [Google Scholar] [CrossRef]
- Rizzo, M.G.; Zammuto, V.; Spanò, A.; Gugliandolo, C.; Calabrese, G.; Guglielmino, S. Anti-Inflammatory Effects in LPS-Induced Macrophages and Antibiofilm Activity of the Mannose-Rich Exopolysaccharide Produced by Bacillus Licheniformis B3-15. Heliyon 2024, 10, e38367. [Google Scholar] [CrossRef]
- Shao, B.-M.; Dai, H.; Xu, W.; Lin, Z.-B.; Gao, X.-M. Immune Receptors for Polysaccharides from Ganoderma Lucidum. Biochem. Biophys. Res. Commun. 2004, 323, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Meng, J.; Zhang, Y.; Liu, J.; Nie, X.; Wu, F.; Yang, Y.; Wang, C.; Gu, N.; Xu, H. Macrophage Phenotypic Mechanomodulation of Enhancing Bone Regeneration by Superparamagnetic Scaffold upon Magnetization. Biomaterials 2017, 140, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Raguénès, G.; Peres, A.; Ruimy, R.; Pignet, P.; Christen, R.; Loaec, M.; Rougeaux, H.; Barbier, G.; Guezennec, J. Alteromonas Infernus Sp. Nov., a New Polysaccharide-producing Bacterium Isolated from a Deep-sea Hydrothermal Vent. J. Appl. Microbiol. 1997, 82, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Arrieta Payares, L.M.; Gutiérrez Púa, L.D.C.; Di Mare Pareja, L.A.; Paredes Méndez, S.C.; Paredes Méndez, V.N. Microalgae Applications to Bone Repairing Processes: A Review. ACS Biomater. Sci. Eng. 2023, 9, 2991–3009. [Google Scholar] [CrossRef]
- Raposo, M.F. de J.; De Morais, R.M.S.C.; Bernardo de Morais, A.M.M. Bioactivity and Applications of Sulphated Polysaccharides from Marine Microalgae. Mar. Drugs 2013, 11, 233–252. [Google Scholar] [CrossRef]
- Merlo, S.; Gabarrell Durany, X.; Pedroso Tonon, A.; Rossi, S. Marine Microalgae Contribution to Sustainable Development. Water 2021, 13, 1373. [Google Scholar] [CrossRef]
- Kumar, V.; Kashyap, M.; Gautam, S.; Shukla, P.; Joshi, K.B.; Vinayak, V. Fast Fourier Infrared Spectroscopy to Characterize the Biochemical Composition in Diatoms. J. Biosci. 2018, 43, 717–729. [Google Scholar] [CrossRef]
- Markou, G.; Arapoglou, D.; Eliopoulos, C.; Balafoutis, A.; Taddeo, R.; Panara, A.; Thomaidis, N. Cultivation and Safety Aspects of Arthrospira Platensis (Spirulina) Grown with Struvite Recovered from Anaerobic Digestion Plant as Phosphorus Source. Algal Res. 2019, 44, 101716. [Google Scholar] [CrossRef]
- Carletti, A.; Rosa, J.T.; Pes, K.; Borges, I.; Santos, T.; Barreira, L.; Varela, J.; Pereira, H.; Cancela, M.L.; Gavaia, P.J.; et al. The Osteogenic and Mineralogenic Potential of the Microalgae Skeletonema Costatum and Tetraselmis Striata CTP4 in Fish Models. Cell. Mol. Life Sci. 2023, 80, 310. [Google Scholar] [CrossRef]
- Moradi, F.; Hadavi, M.; Aghamaali, M.R.; Fallah, S.F. Arthrospira Platensis and Gracilaria Gracilis Algae Extracts as Biological Inducers for Human Amniotic Mesenchymal Stem Cells (hAMSC). J. Appl. Phycol. 2024, 36, 2999–3009. [Google Scholar] [CrossRef]
- Yu, H.; Chen, Y.; Zhu, J. Osteogenic Activities of Four Calcium-Chelating Microalgae Peptides. J. Sci. Food Agric. 2022, 102, 6643–6649. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.H.T. Osteoblastic Differentiation Effects of a Novel Peptide from Biodiesel By-Products of Microalgae Nannochloropsis Oculata on MG-63 and D1 Cells. Ph.D. Dissertation, Chosun University, Gwangju, Republic of Korea, 2012. [Google Scholar]
- de Melo, R.G.; de Andrade, A.F.; Bezerra, R.P.; Viana Marques, D.d.A.; da Silva, V.A.; Paz, S.T.; de Lima Filho, J.L.; Porto, A.L.F. Hydrogel-Based Chlorella Vulgaris Extracts: A New Topical Formulation for Wound Healing Treatment. J. Appl. Phycol. 2019, 31, 3653–3663. [Google Scholar] [CrossRef]
- Zambrano-Mila, M.S.; Blacio, K.E.S.; Vispo, N.S. Peptide Phage Display: Molecular Principles and Biomedical Applications. Ther. Innov. Regul. Sci. 2020, 54, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, M.G.; Palermo, N.; D’Amora, U.; Oddo, S.; Guglielmino, S.P.P.; Conoci, S.; Szychlinska, M.A.; Calabrese, G. Multipotential Role of Growth Factor Mimetic Peptides for Osteochondral Tissue Engineering. Int. J. Mol. Sci. 2022, 23, 7388. [Google Scholar] [CrossRef]
- Rizzo, M.G.; Plano, L.M.D.; Palermo, N.; Franco, D.; Nicolò, M.; Sciuto, E.L.; Calabrese, G.; Oddo, S.; Conoci, S.; Guglielmino, S.P.P. A Novel Serum-Based Diagnosis of Alzheimer’s Disease Using an Advanced Phage-Based Biochip. Adv. Sci. 2023, 10, 2301650. [Google Scholar] [CrossRef]
- Rizzo, M.G.; Carnazza, S.; De Plano, L.M.; Franco, D.; Nicolò, M.S.; Zammuto, V.; Petralia, S.; Calabrese, G.; Gugliandolo, C.; Conoci, S.; et al. Rapid Detection of Bacterial Pathogens in Blood through Engineered Phages-Beads and Integrated Real-Time PCR into MicroChip. Sens. Actuators B Chem. 2021, 329, 129227. [Google Scholar] [CrossRef]


| Marine Source | Marine-Derived Biomaterial | Chemical Category | Use/Biological Activity | References |
|---|---|---|---|---|
| Diatom skeleton | Diatomite, natural diatomaceoussediment that can stably release Silicon ions | Mineral | Osteochondral repair scaffold promotes osteogenic differentiation of BMSCs (bone marrow mesenchymal stem cells) and enhances proliferation and maturation of chondrocytes | [74,104,105,106,107] |
| Red algae Lithothamnion corallioides | Aquamin is a natural, multimineral, rich in calcium, magnesium, and 72 other trace minerals | Mineral | Aquamin supplement promotes increased mineralization in osteoblast cell culture | [108,109] |
| Sponge skeleton | Biosilica is abiocompatible, natural inorganic polymer formed by an enzymatic silicatein-mediated reaction in siliceous sponges to build up their inorganic skeleton | Mineral | Microspheres containing β-TCP and silica, and β-TCP and silicatein, induce the mineralization of human osteoblast-like cells (SaOS-2) in vitro;capability to support cell adhesion and proliferation | [80,110,111,112] |
| Seashell | Mussel shell hydroxyapatite(combined with chitosan and gentamicin sulfate antibiotic) | Mineral | Composite with biocompatibility and antibacterial activity (enhanced osteoblast cell proliferation) | [113] |
| Seashell | Aragonite is acowrie-shell-derived powder | Mineral | Biocompatibility with human stem cells; useful carrier/scaffold for bone tissue engineering and raw material for 3D-printed orthopedic devices | [114] |
| Coral skeletons | Corals, mainly the species Acropora sp., Goniopora sp., and Porties sp. | Mineral matrix fraction (peptide, lipid, carbohydrates) | Extraction and use of coral matrix proteins (osteoinduction);laboratory-grown coral materials for bone;framework for hard tissue engineering with new and superior properties; augmented coral skeleton;permeation with synthetic materials biomolecules and mineral ions | [115] |
| Mussel’s byssus | 3,4-dihydroxyphenyl-l-alanine (Dopa) catecholic amino acid in mussel adhesive proteins | Adhesive protein amino acid | In vitro 3,4-dihydroxyphenyl-l-alanine (Dopa)-coated plates enhance osteogenic differentiation of human mesenchymal stem cells’functional coating with an integrin-targeting sequence to improve cell adhesion by mussel byssus-inspired surface chemistry enhancing osteogenic activity in zirconia dental implants | [116,117,118] |
| Jellyfish | Injectable marine hydrogel obtained from collagen native to the jellyfish Rhizostoma pulmo with hydroxyphenyl propionic acid (HPA)–functionalized marine gelatin | Protein lipid | Biocompatible hydrogel able to enzymatically cross-link using horseradish peroxidase (HPR) and H2 O2 favoring the placement of cells inside | [119] |
| Jellyfish | Collagen scaffold | Protein (collagen) | In vivo, jellyfish collagen scaffolds showed a long-term anti-inflammatory macrophage response and an optimal vascularization pattern within their implant beds, showing excellent biocompatibility and (bone) tissue regenerative properties | [120] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizzo, M.G.; Briglia, M.; Zammuto, V.; Morganti, D.; Faggio, C.; Impellitteri, F.; Multisanti, C.R.; Graziano, A.C.E. Innovation in Osteogenesis Activation: Role of Marine-Derived Materials in Bone Regeneration. Curr. Issues Mol. Biol. 2025, 47, 175. https://doi.org/10.3390/cimb47030175
Rizzo MG, Briglia M, Zammuto V, Morganti D, Faggio C, Impellitteri F, Multisanti CR, Graziano ACE. Innovation in Osteogenesis Activation: Role of Marine-Derived Materials in Bone Regeneration. Current Issues in Molecular Biology. 2025; 47(3):175. https://doi.org/10.3390/cimb47030175
Chicago/Turabian StyleRizzo, Maria Giovanna, Marilena Briglia, Vincenzo Zammuto, Dario Morganti, Caterina Faggio, Federica Impellitteri, Cristiana Roberta Multisanti, and Adriana Carol Eleonora Graziano. 2025. "Innovation in Osteogenesis Activation: Role of Marine-Derived Materials in Bone Regeneration" Current Issues in Molecular Biology 47, no. 3: 175. https://doi.org/10.3390/cimb47030175
APA StyleRizzo, M. G., Briglia, M., Zammuto, V., Morganti, D., Faggio, C., Impellitteri, F., Multisanti, C. R., & Graziano, A. C. E. (2025). Innovation in Osteogenesis Activation: Role of Marine-Derived Materials in Bone Regeneration. Current Issues in Molecular Biology, 47(3), 175. https://doi.org/10.3390/cimb47030175











