Development of an Anti-Zearalenone Nanobody Phage Display Library and Preparation of Specific Nanobodies
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Alpaca Immunization and Serum Titer Test
2.3. Construction of the Phage Display Nanobody Library
2.4. Screening and Identification of Anti-ZEN Phage Display Nanobodies
2.5. Molecular Recognition Mechanism of Nanobody and Zearalenone
2.6. Expression and Purification of Anti-ZEN Nanobody
2.7. Determining the Specificity and Binding Affinity of Anti-ZEN Nanobody V44
2.8. Statistical Analysis
3. Results
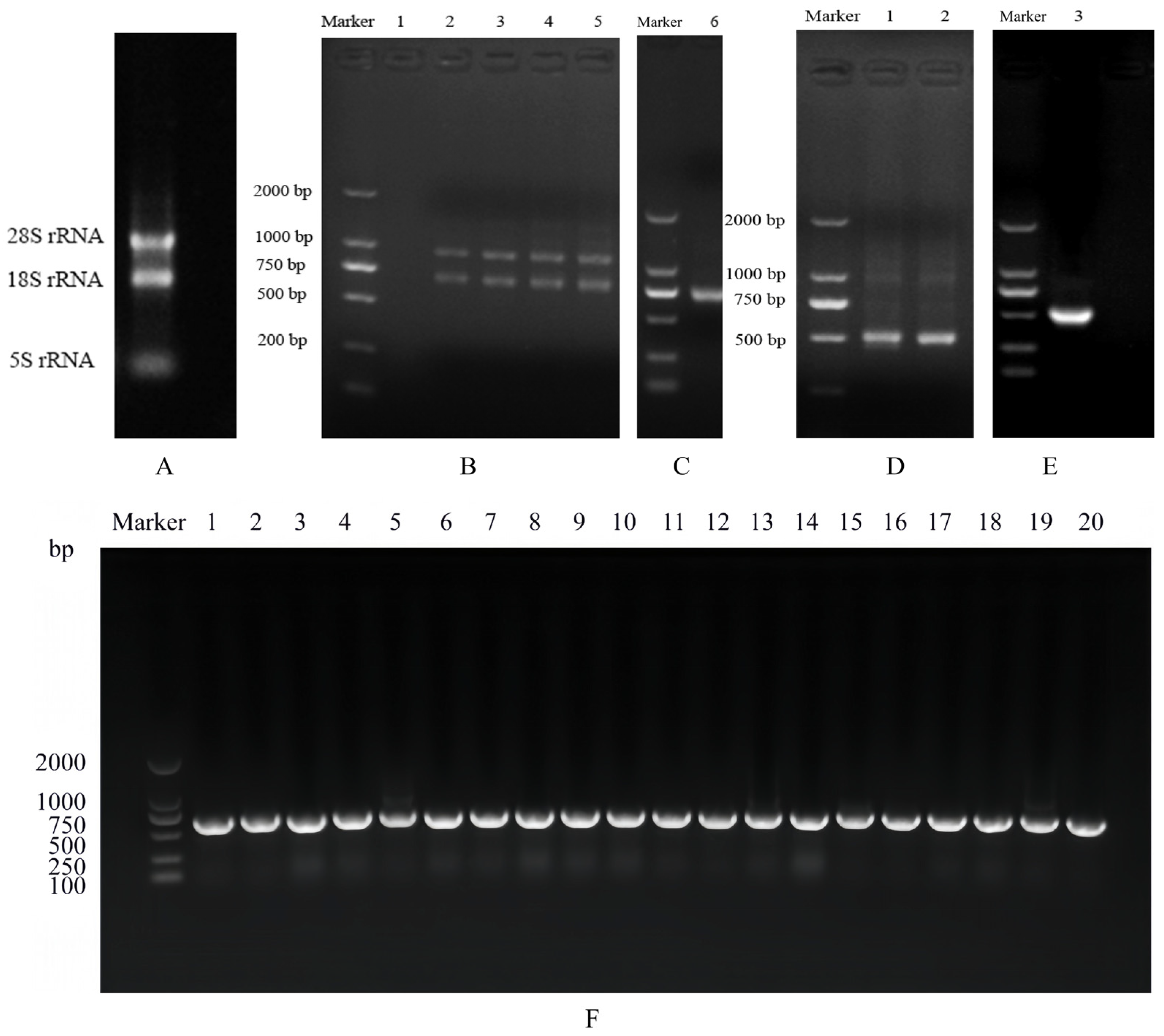
3.1. Serum Antibody Titer Test
3.2. VHH Gene Amplification
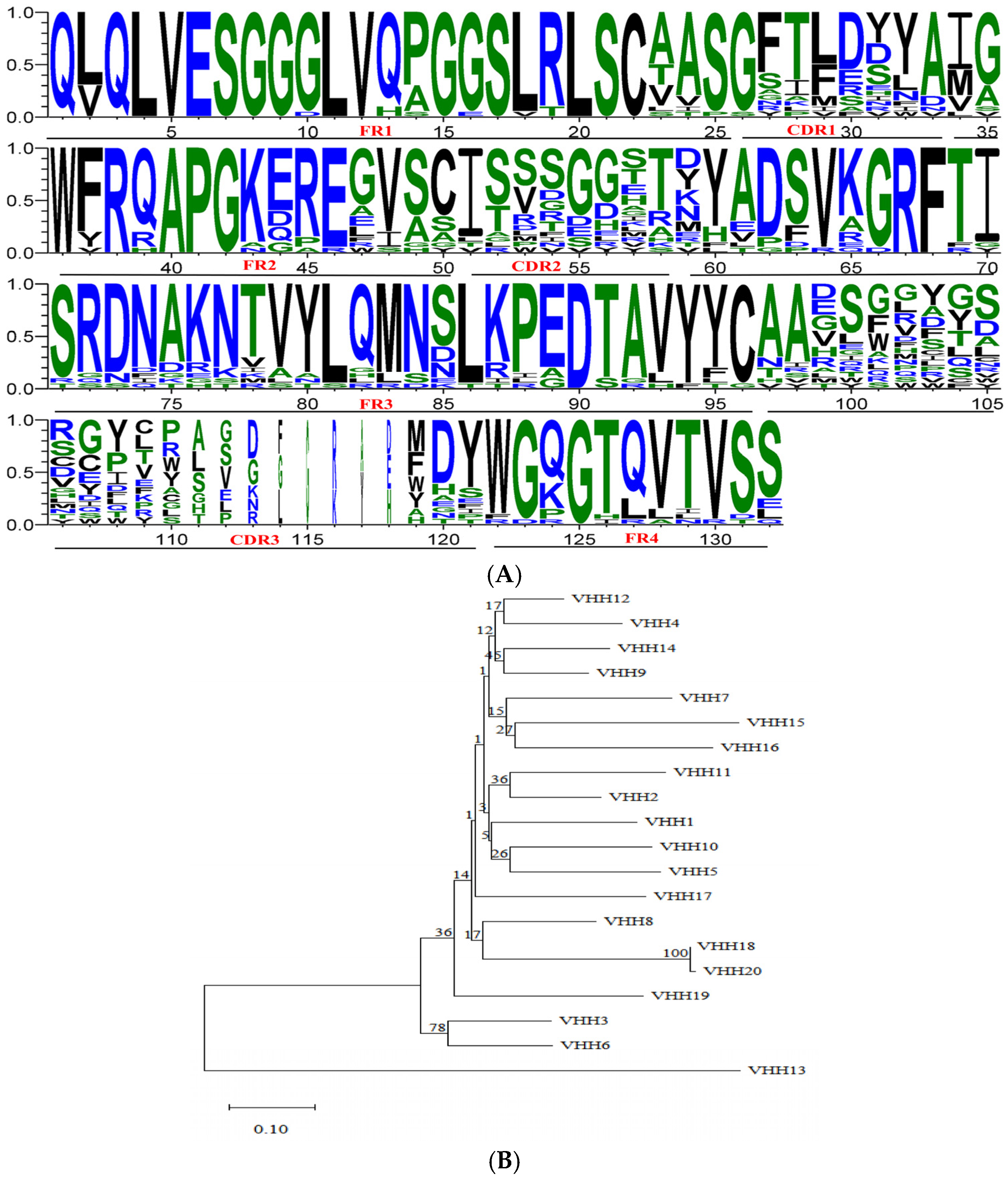
3.3. Identification of Primary Nanobody Library
3.4. Library Biopanning
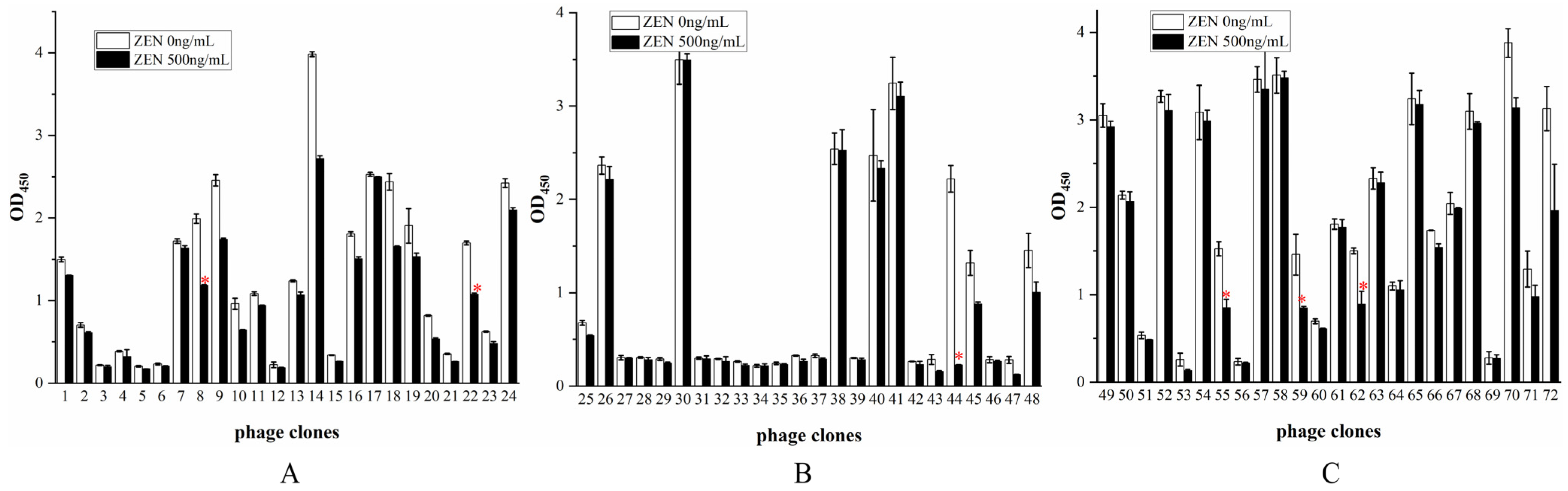
3.5. Identification of Positive Phage Clones
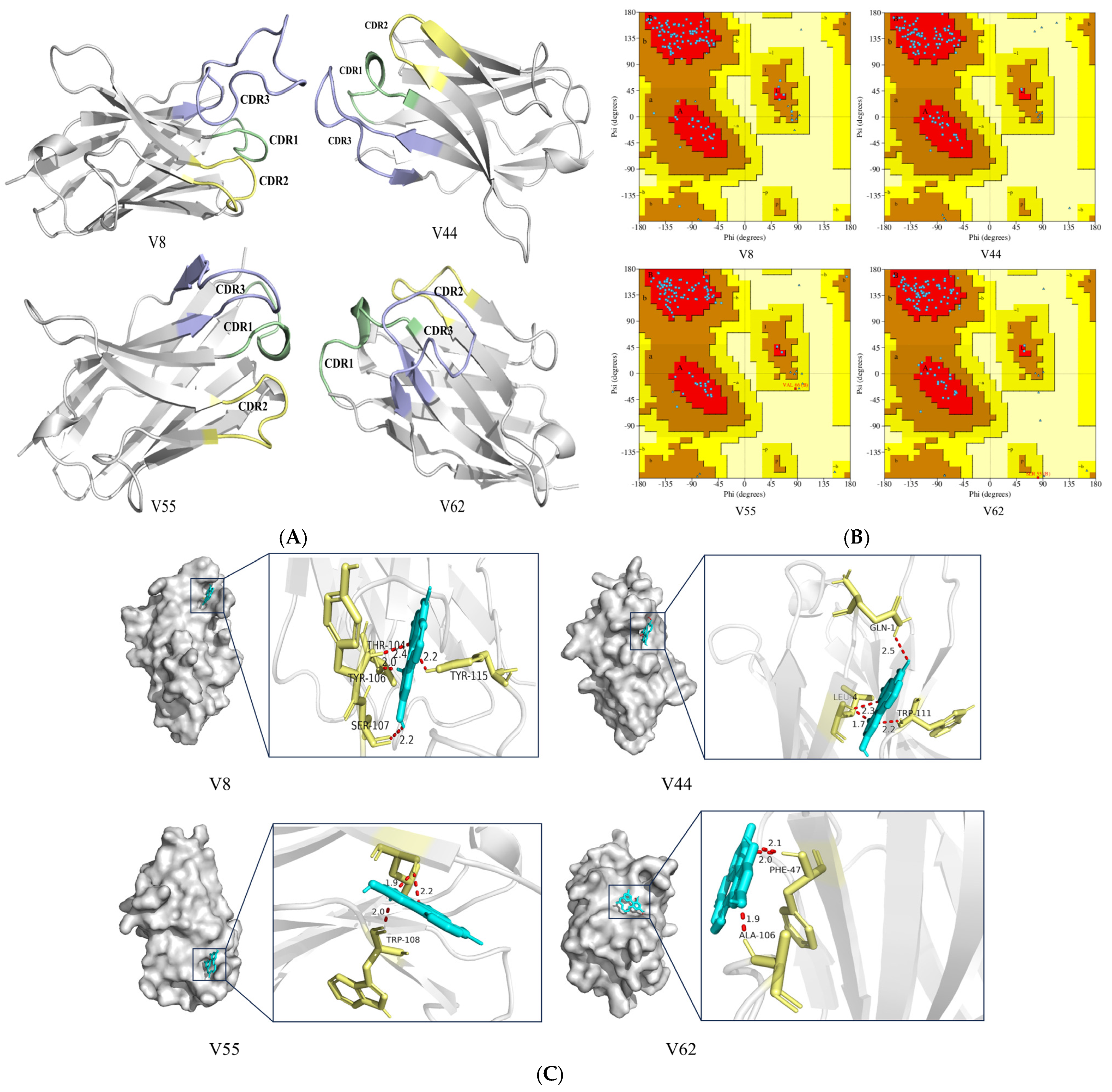
3.6. Homology Modeling and Molecular Docking
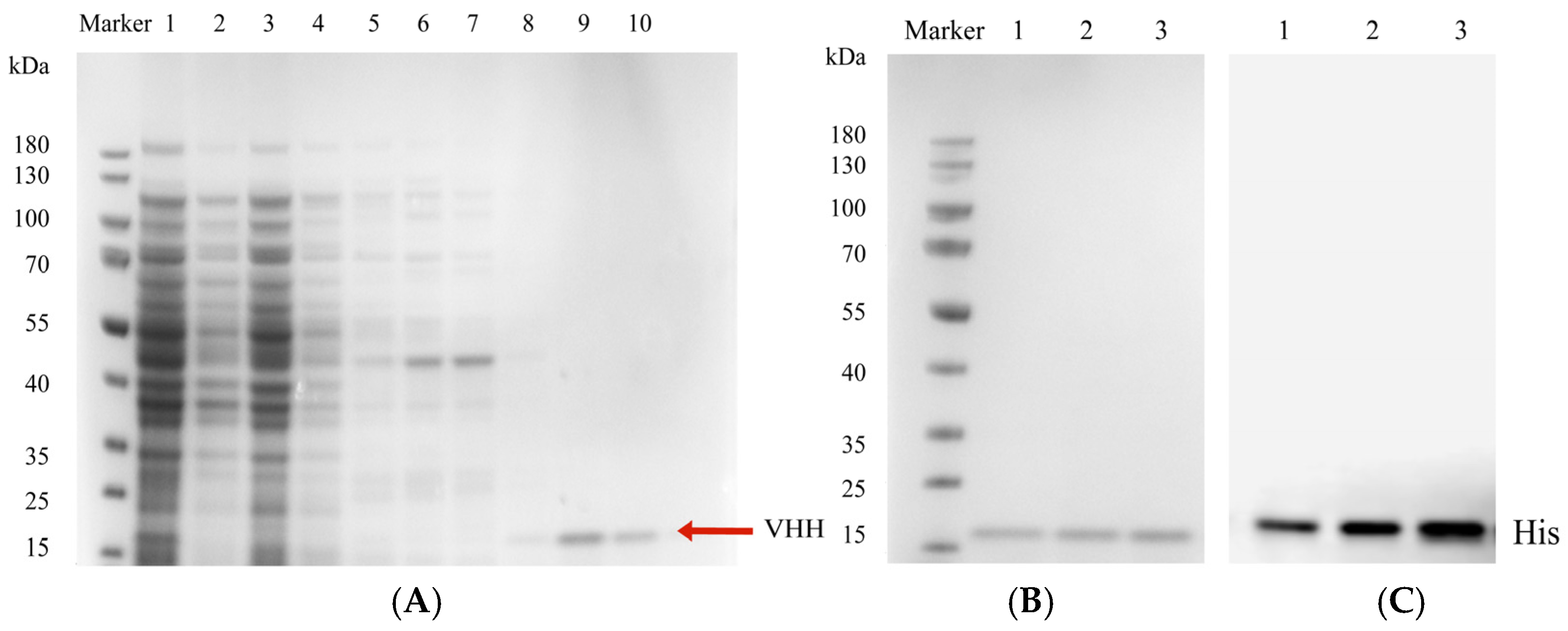
3.7. Purification and Identification of Anti-ZEN VHH
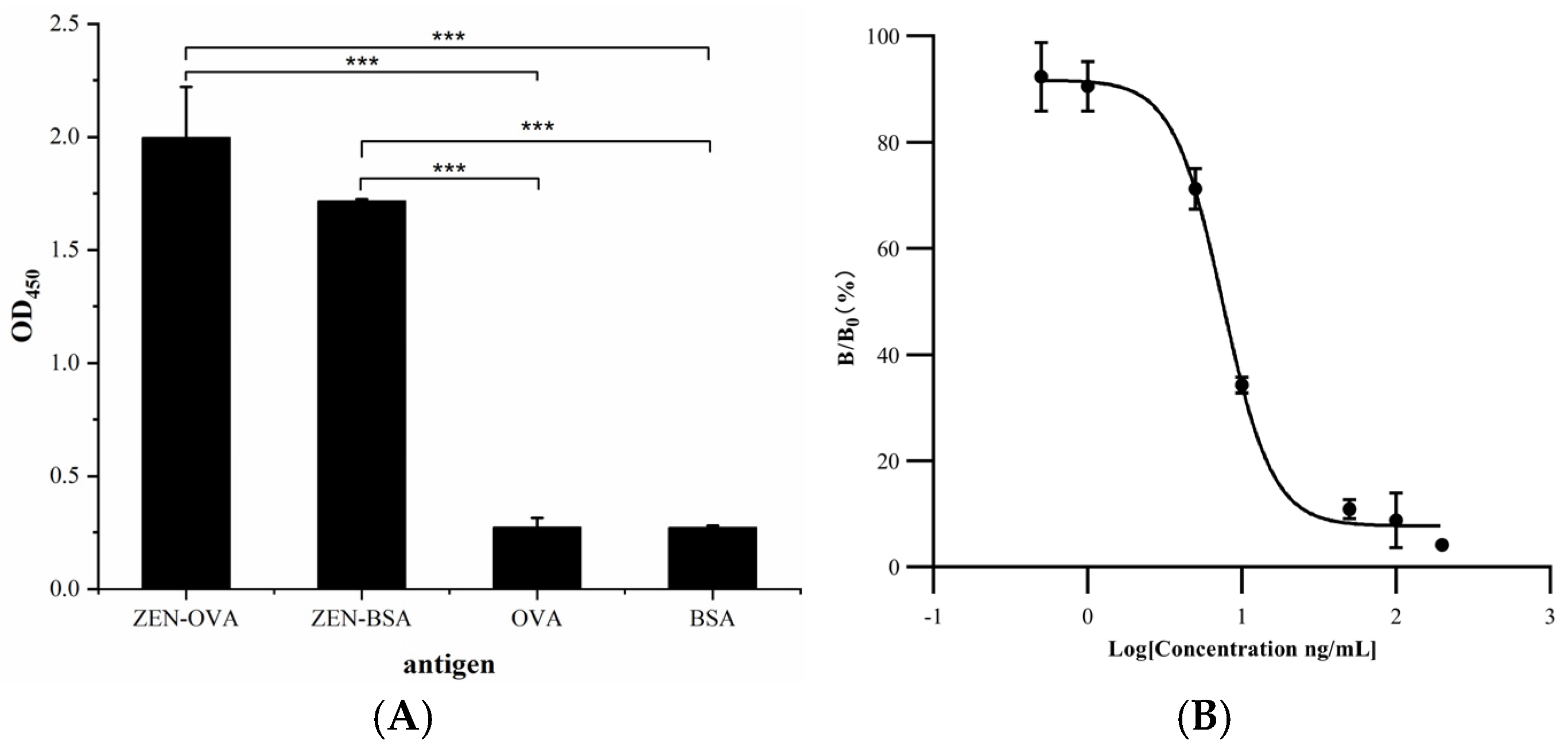
3.8. Validation of Specific Anti-ZEN VHH
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stob, M.; Baldwin, R.S.; Tuite, J.; Andrews, F.N.; Gillette, K.G. Isolation of an anabolic, uterotrophic compound from corn infected with Gibberella zeae. Nature 1962, 196, 1318. [Google Scholar] [CrossRef]
- Bulgaru, C.V.; Marin, D.E.; Pistol, G.C.; Taranu, I. Zearalenone and the Immune Response. Toxins 2021, 13, 248. [Google Scholar] [CrossRef]
- Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; Grasl-Kraupp, B.; et al. Risks for animal health related to the presence of zearalenone and its modified forms in feed. EFSA J. Eur. Food Saf. Auth. 2017, 15, e04851. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, B.; Li, X.; Wang, T.; Zou, H.; Gu, J.; Yuan, Y.; Liu, X.; Bai, J.; Bian, J.; et al. Zearalenone Promotes Cell Proliferation or Causes Cell Death? Toxins 2018, 10, 184. [Google Scholar] [CrossRef]
- Sirot, V.; Fremy, J.M.; Leblanc, J.C. Dietary exposure to mycotoxins and health risk assessment in the second French total diet study. Food Chem. Toxicol. 2013, 52, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.; Das, M.; Tripathi, A. Occurrence and toxicity of a fusarium mycotoxin, zearalenone. Crit. Rev. Food Sci. Nutr. 2020, 60, 2710–2729. [Google Scholar] [CrossRef] [PubMed]
- Ropejko, K.; Twarużek, M. Zearalenone and Its Metabolites-General Overview, Occurrence, and Toxicity. Toxins 2021, 13, 35. [Google Scholar] [CrossRef]
- Nagl, V.; Grenier, B.; Pinton, P.; Ruczizka, U.; Dippel, M.; Bünger, M.; Oswald, I.P.; Soler, L. Exposure to Zearalenone Leads to Metabolic Disruption and Changes in Circulating Adipokines Concentrations in Pigs. Toxins 2021, 13, 790. [Google Scholar] [CrossRef]
- Cai, P.; Feng, Z.; Feng, N.; Zou, H.; Gu, J.; Liu, X.; Liu, Z.; Yuan, Y.; Bian, J. Activated AMPK promoted the decrease of lactate production in rat Sertoli cells exposed to Zearalenone. Ecotoxicol. Environ. Saf. 2021, 220, 112367. [Google Scholar] [CrossRef]
- Kong, L.; Zhao, A.H.; Wang, Q.W.; Feng, Y.Q.; Yan, Z.H.; Li, M.H.; Zhang, F.L.; Wang, H.; Shen, K.Y.; Liu, Y.; et al. Maternal Zearalenone exposure impacted ovarian follicle formation and development of suckled offspring. Sci. Total Environ. 2021, 788, 147792. [Google Scholar] [CrossRef]
- Al-Jaal, B.; Latiff, A.; Salama, S.; Hussain, H.M.; Al-Thani, N.A.; Al-Naimi, N.; Al-Qasmi, N.; Horvatovich, P.; Jaganjac, M. Analysis of Multiple Mycotoxins in the Qatari Population and Their Relation to Markers of Oxidative Stress. Toxins 2021, 13, 267. [Google Scholar] [CrossRef] [PubMed]
- González-Alvarez, M.E.; McGuire, B.C.; Keating, A.F. Obesity alters the ovarian proteomic response to zearalenone exposure. Biol. Reprod. 2021, 105, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Adetunji, M.C.; Aroyeun, S.O.; Osho, M.B.; Sulyok, M.; Krska, R.; Mwanza, M. Fungal metabolite and mycotoxins profile of cashew nut from selected locations in two African countries. Food Addit. Contam. Part. A Chem. Anal. Control Expo. Risk Assess. 2019, 36, 1847–1859. [Google Scholar] [CrossRef]
- Udovicki, B.; Audenaert, K.; De Saeger, S.; Rajkovic, A. Overview on the Mycotoxins Incidence in Serbia in the Period 2004–2016. Toxins 2018, 10, 279. [Google Scholar] [CrossRef]
- Birr, T.; Jensen, T.; Preußke, N.; Sönnichsen, F.D.; De Boevre, M.; De Saeger, S.; Hasler, M.; Verreet, J.A.; Klink, H. Occurrence of Fusarium Mycotoxins and Their Modified Forms in Forage Maize Cultivars. Toxins 2021, 13, 110. [Google Scholar] [CrossRef]
- Awapak, D.; Petchkongkaew, A.; Sulyok, M.; Krska, R. Co-occurrence and toxicological relevance of secondary metabolites in dairy cow feed from Thailand. Food Addit. Contam. Part. A Chem. Anal. Control Expo. Risk Assess. 2021, 38, 1013–1027. [Google Scholar] [CrossRef]
- De Ruyck, K.; Huybrechts, I.; Yang, S.; Arcella, D.; Claeys, L.; Abbeddou, S.; De Keyzer, W.; De Vries, J.; Ocke, M.; Ruprich, J.; et al. Mycotoxin exposure assessments in a multi-center European validation study by 24-hour dietary recall and biological fluid sampling. Environ. Int. 2020, 137, 105539. [Google Scholar] [CrossRef] [PubMed]
- Gambacorta, L.; Olsen, M.; Solfrizzo, M. Pig Urinary Concentration of Mycotoxins and Metabolites Reflects Regional Differences, Mycotoxin Intake and Feed Contaminations. Toxins 2019, 11, 378. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.; Vidal, A.; De Boevre, M.; De Saeger, S.; Nunes, C.; Torres, D.; Goios, A.; Lopes, C.; Assunção, R.; Alvito, P. Exposure assessment of Portuguese population to multiple mycotoxins: The human biomonitoring approach. Int. J. Hyg. Environ. Health 2019, 222, 913–925. [Google Scholar] [CrossRef]
- Schaafsma, A.W.; Nicol, R.W.; Savard, M.E.; Sinha, R.C.; Reid, L.M.; Rottinghaus, G. Analysis of Fusarium toxins in maize and wheat using thin layer chromatography. Mycopathologia 1998, 142, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Liu, Y.; Guo, Q.; Wang, X.; Tian, Y.; Yang, W.; Li, J.; Chen, Y. Determination of Zearalenone and Its Derivatives in Feed by Gas Chromatography-Mass Spectrometry with Immunoaffinity Column Cleanup and Isotope Dilution. Toxins 2022, 14, 764. [Google Scholar] [CrossRef]
- Qiu, T.; Zhang, H.; Lei, H.; Zhang, L.; Zhang, Y.; Shen, X.; Xu, B.; Zhu, J.; Xiao, W.; Zheng, J.; et al. Preparation of Anti-Zearalenone IgY and Development of an Indirect Competitive ELISA Method for the Measurement of Zearalenone in Post-Fermented Tea. Foods 2023, 12, 4478. [Google Scholar] [CrossRef]
- Lee, M.G.; Yuan, Q.P.; Hart, L.P.; Pestka, J.J. Enzyme-linked immunosorbent assays of zearalenone using polyclonal, monoclonal and recombinant antibodies. Methods Mol. Biol. 2001, 157, 159–170. [Google Scholar] [CrossRef]
- Hamers-Casterman, C.; Atarhouch, T.; Muyldermans, S.; Robinson, G.; Hamers, C.; Songa, E.B.; Bendahman, N.; Hamers, R. Naturally occurring antibodies devoid of light chains. Nature 1993, 363, 446–448. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.K.; Hamers, R.; Wyns, L.; Muyldermans, S. Loss of splice consensus signal is responsible for the removal of the entire C(H)1 domain of the functional camel IGG2A heavy-chain antibodies. Mol. Immunol. 1999, 36, 515–524. [Google Scholar] [CrossRef]
- Wolfson, W. Ablynx makes nanobodies from llama bodies. Chem. Biol. 2006, 13, 1243–1244. [Google Scholar] [CrossRef] [PubMed]
- Nambulli, S.; Xiang, Y.; Tilston-Lunel, N.L.; Rennick, L.J.; Sang, Z.; Klimstra, W.B.; Reed, D.S.; Crossland, N.A.; Shi, Y.; Duprex, W.P. Inhalable Nanobody (PiN-21) prevents and treats SARS-CoV-2 infections in Syrian hamsters at ultra-low doses. Sci. Adv. 2021, 7, eabh0319. [Google Scholar] [CrossRef]
- He, T.; Wang, Y.; Li, P.; Zhang, Q.; Lei, J.; Zhang, Z.; Ding, X.; Zhou, H.; Zhang, W. Nanobody-based enzyme immunoassay for aflatoxin in agro-products with high tolerance to cosolvent methanol. Anal. Chem. 2014, 86, 8873–8880. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Qu, G.; Quan, H.; Wang, Y.; Wang, C.; Haque, M.A.; He, C. A Novel Cost-Effective Nanobody against Fumonisin B1 Contaminations: Efficacy Test in Dairy Milk and Chickens. Toxins 2022, 14, 821. [Google Scholar] [CrossRef]
- Tang, X.; Catanante, G.; Huang, X.; Marty, J.L.; Wang, H.; Zhang, Q.; Li, P. Screen-printed electrochemical immunosensor based on a novel nanobody for analyzing aflatoxin M(1) in milk. Food Chem. 2022, 383, 132598. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; He, Q.; Xu, Y.; Liu, X.; Shu, M.; Tu, Z.; Li, Y.; Wang, W.; Cao, D. Anti-idiotypic VHH phage display-mediated immuno-PCR for ultrasensitive determination of mycotoxin zearalenone in cereals. Talanta 2016, 147, 410–415. [Google Scholar] [CrossRef]
- Kumar, R.; Parray, H.A.; Shrivastava, T.; Sinha, S.; Luthra, K. Phage display antibody libraries: A robust approach for generation of recombinant human monoclonal antibodies. Int. J. Biol. Macromol. 2019, 135, 907–918. [Google Scholar] [CrossRef] [PubMed]
- Zanganeh, S.; Rouhani Nejad, H.; Mehrabadi, J.F.; Hosseini, R.; Shahi, B.; Tavassoli, Z.; Aramvash, A. Rapid and Sensitive Detection of Staphylococcal Enterotoxin B by Recombinant Nanobody Using Phage Display Technology. Appl. Biochem. Biotechnol. 2019, 187, 493–505. [Google Scholar] [CrossRef]
- Pardon, E.; Laeremans, T.; Triest, S.; Rasmussen, S.G.; Wohlkönig, A.; Ruf, A.; Muyldermans, S.; Hol, W.G.; Kobilka, B.K.; Steyaert, J. A general protocol for the generation of Nanobodies for structural biology. Nat. Protoc. 2014, 9, 674–693. [Google Scholar] [CrossRef]
- Li, S.; Jiang, K.; Wang, T.; Zhang, W.; Shi, M.; Chen, B.; Hua, Z. Nanobody against PDL1. Biotechnol. Lett. 2020, 42, 727–736. [Google Scholar] [CrossRef]
- Crossley, B.M.; Bai, J.; Glaser, A.; Maes, R.; Porter, E.; Killian, M.L.; Clement, T.; Toohey-Kurth, K. Guidelines for Sanger sequencing and molecular assay monitoring. J. Vet. Diagn. Investig. 2020, 32, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Tang, Q.; Xia, H.; Xu, H.; Bi, F. Identification and characterization of inhibitory nanobody against p38δ. Biochem. Biophys. Res. Commun. 2022, 600, 60–66. [Google Scholar] [CrossRef]
- Meng, N.; Cheng, X.; Sun, M.; Zhang, Y.; Sun, X.; Liu, X.; Chen, J. Screening, Expression and Identification of Nanobody Against Monkeypox Virus A35R. Int. J. Nanomed. 2023, 18, 7173–7181. [Google Scholar] [CrossRef]
- Gasteiger, E.; Gattiker, A.; Hoogland, C.; Ivanyi, I.; Appel, R.D.; Bairoch, A. ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003, 31, 3784–3788. [Google Scholar] [CrossRef]
- Laskowski, R.A.; Chistyakov, V.V.; Thornton, J.M. PDBsum more: New summaries and analyses of the known 3D structures of proteins and nucleic acids. Nucleic Acids Res. 2005, 33, D266–D268. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2023 update. Nucleic Acids Res. 2023, 51, D1373–D1380. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Schrodinger, LLC. The PyMOL Molecular Graphics System, Version 1.8; Schrodinger, Inc.: New York, NY, USA, 2015.
- Li, S.; Zhang, W.; Jiang, K.; Shan, H.; Shi, M.; Chen, B.; Hua, Z. Nanobody against the E7 oncoprotein of human papillomavirus 16. Mol. Immunol. 2019, 109, 12–19. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, Y.; Zhao, Q.; He, Y.; Li, Z.; Chen, A.; Wang, C.; Wang, B.; Jiao, B.; Cui, Y. A sensitive and practical ELISA for analyzing naringenin in pummelo and herb samples. Food Chem. 2021, 362, 130223. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, M.N.; Jian, M.; King, M.T.; Brooks, C.L. Role of a noncanonical disulfide bond in the stability, affinity, and flexibility of a VHH specific for the Listeria virulence factor InlB. Protein Sci. A Publ. Protein Soc. 2020, 29, 1004–1017. [Google Scholar] [CrossRef]
- Mitchell, L.S.; Colwell, L.J. Comparative analysis of nanobody sequence and structure data. Proteins 2018, 86, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, S.; Tegoni, M.; Frenken, L.; van Vliet, C.; Cambillau, C. Lateral recognition of a dye hapten by a llama VHH domain. J. Mol. Biol. 2001, 311, 123–129. [Google Scholar] [CrossRef]
- Bolhassani, A.; Talebi, S.; Anvar, A. Endogenous and Exogenous Natural Adjuvants for Vaccine Development. Mini Rev. Med. Chem. 2017, 17, 1442–1456. [Google Scholar] [CrossRef]
- Rader, C. The pComb3 Phagemid Family of Phage Display Vectors. Cold Spring Harb. Protoc. 2023, 2024, pdb.over107756. [Google Scholar] [CrossRef]
- de Bruin, R.; Spelt, K.; Mol, J.; Koes, R.; Quattrocchio, F. Selection of high-affinity phage antibodies from phage display libraries. Nat. Biotechnol. 1999, 17, 397–399. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Wang, S.; Fotina, H.; Wang, Z. Development of a Highly Sensitive and Specific Monoclonal Antibody Based on Indirect Competitive Enzyme-Linked Immunosorbent Assay for the Determination of Zearalenone in Food and Feed Samples. Toxins 2022, 14, 220. [Google Scholar] [CrossRef]

| Round | ZEN-OVA (μg/mL) | Input (pfu/mL) | Output (pfu/mL) | Recovery Rate | Enrichment |
|---|---|---|---|---|---|
| 1 | 10.00 | 1.00 × 1011 | 5.55 × 104 | 5.55 × 10−7 | / |
| 2 | 5.00 | 8.70 × 1011 | 1.04 × 108 | 1.20 × 10−4 | 216.22 |
| 3 | 2.50 | 1.21 × 1011 | 1.24 × 107 | 1.02 × 10−4 | 0.85 |
| 4 | 1.25 | 7.00 × 1011 | 1.86 × 108 | 2.66 × 10−4 | 2.66 |
| ZEN-OVA | Anti-ZEN VHH | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 200 | 400 | 800 | 1000 | 2000 | ||||||
| 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | |
| 500 | 1.65 | 1.37 | 1.23 | 0.94 | 0.94 | 0.50 | 0.88 | 0.50 | 0.72 | 0.36 |
| 1000 | 1.82 | 1.54 | 1.48 | 1.13 | 1.33 | 0.89 | 1.31 | 0.84 | 1.04 | 0.61 |
| 2000 | 1.70 | 1.44 | 1.43 | 1.06 | 1.22 | 0.80 | 1.22 | 0.90 | 1.03 | 0.49 |
| 4000 | 1.37 | 1.24 | 1.11 | 0.73 | 0.83 | 0.47 | 0.77 | 0.44 | 0.64 | 0.26 |
| 8000 | 1.24 | 1.08 | 1.10 | 0.80 | 1.01 | 0.56 | 0.50 | 0.32 | 0.42 | 0.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, Y.; Hu, Y.; Chen, G.; Feng, Q.; Wang, R.; Zhang, Z.; Chen, J.; Liao, J.; Lin, D.; Zhu, W. Development of an Anti-Zearalenone Nanobody Phage Display Library and Preparation of Specific Nanobodies. Curr. Issues Mol. Biol. 2025, 47, 157. https://doi.org/10.3390/cimb47030157
Zeng Y, Hu Y, Chen G, Feng Q, Wang R, Zhang Z, Chen J, Liao J, Lin D, Zhu W. Development of an Anti-Zearalenone Nanobody Phage Display Library and Preparation of Specific Nanobodies. Current Issues in Molecular Biology. 2025; 47(3):157. https://doi.org/10.3390/cimb47030157
Chicago/Turabian StyleZeng, Ying, Yiying Hu, Ganying Chen, Qingqing Feng, Ruiting Wang, Zhilin Zhang, Jinxian Chen, Junbin Liao, Danrong Lin, and Wei Zhu. 2025. "Development of an Anti-Zearalenone Nanobody Phage Display Library and Preparation of Specific Nanobodies" Current Issues in Molecular Biology 47, no. 3: 157. https://doi.org/10.3390/cimb47030157
APA StyleZeng, Y., Hu, Y., Chen, G., Feng, Q., Wang, R., Zhang, Z., Chen, J., Liao, J., Lin, D., & Zhu, W. (2025). Development of an Anti-Zearalenone Nanobody Phage Display Library and Preparation of Specific Nanobodies. Current Issues in Molecular Biology, 47(3), 157. https://doi.org/10.3390/cimb47030157






