A Study of Variation in the Major Phenolic Acid Components of Dandelions Across Different Regions, and the Potential Molecular Mechanisms of Their Anti-Inflammatory Activity
Abstract
1. Introduction
2. Sampling Area and Sample Information
3. Materials and Methods
3.1. Sampling Design and Sample Collection
3.2. Extraction of Phenolic Acid Components
3.3. Preparation of Mixed Standard Solution
3.4. Determination of Phenolic Acid Content
3.5. Molecular Docking and Molecular Dynamics Simulation
3.5.1. Bioinformatics Platforms and Software
3.5.2. Molecular Docking of Chlorogenic Acid, Caffeic Acid, and Chicoric Acid with the LPS/TLR4/MD-2 Complex Crystal Structure
3.5.3. Molecular Dynamics Simulation
3.6. Data Analysis
4. Results and Analysis
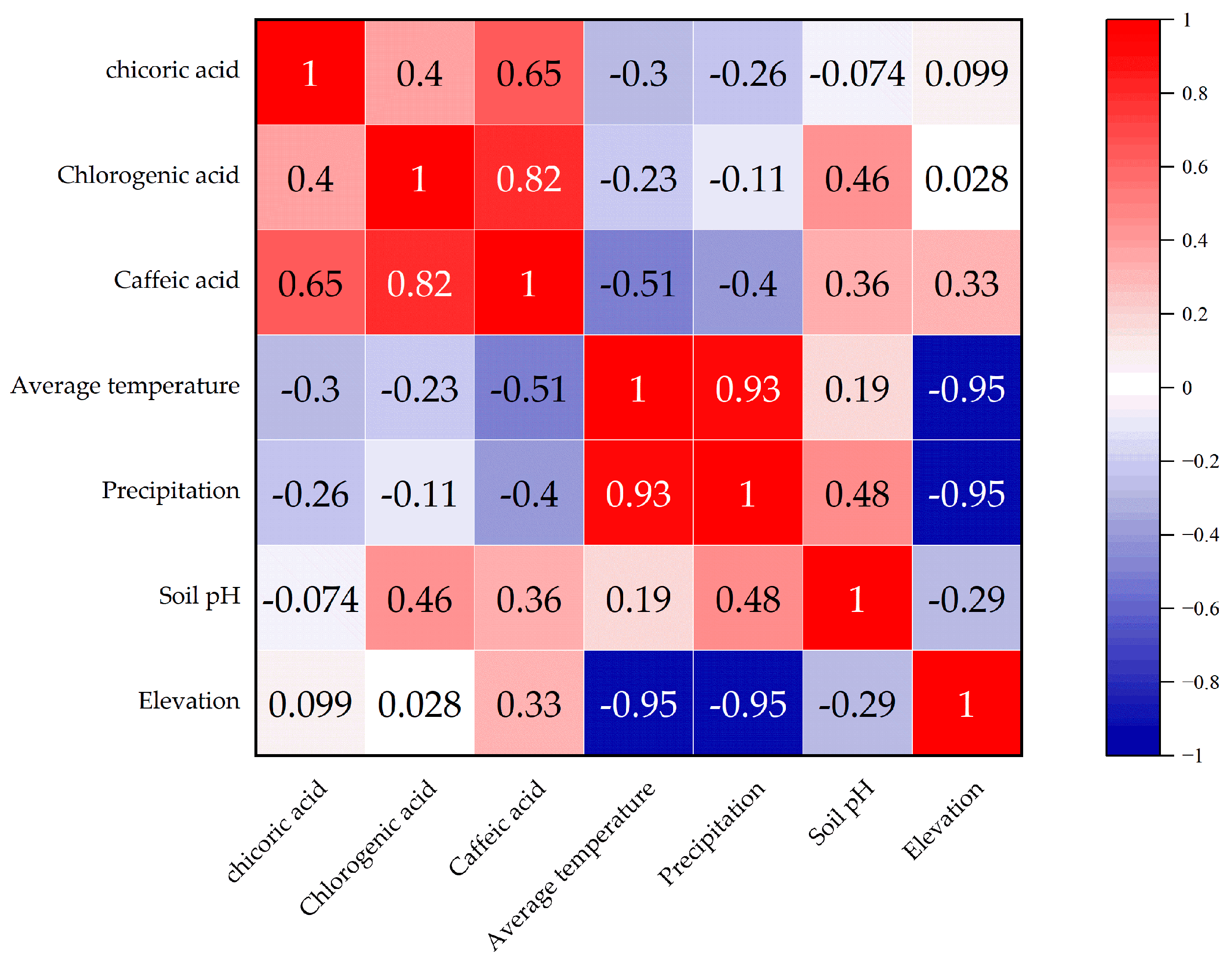
4.1. Correlation Analysis Between Environmental Factors and Phenolic Acid Components
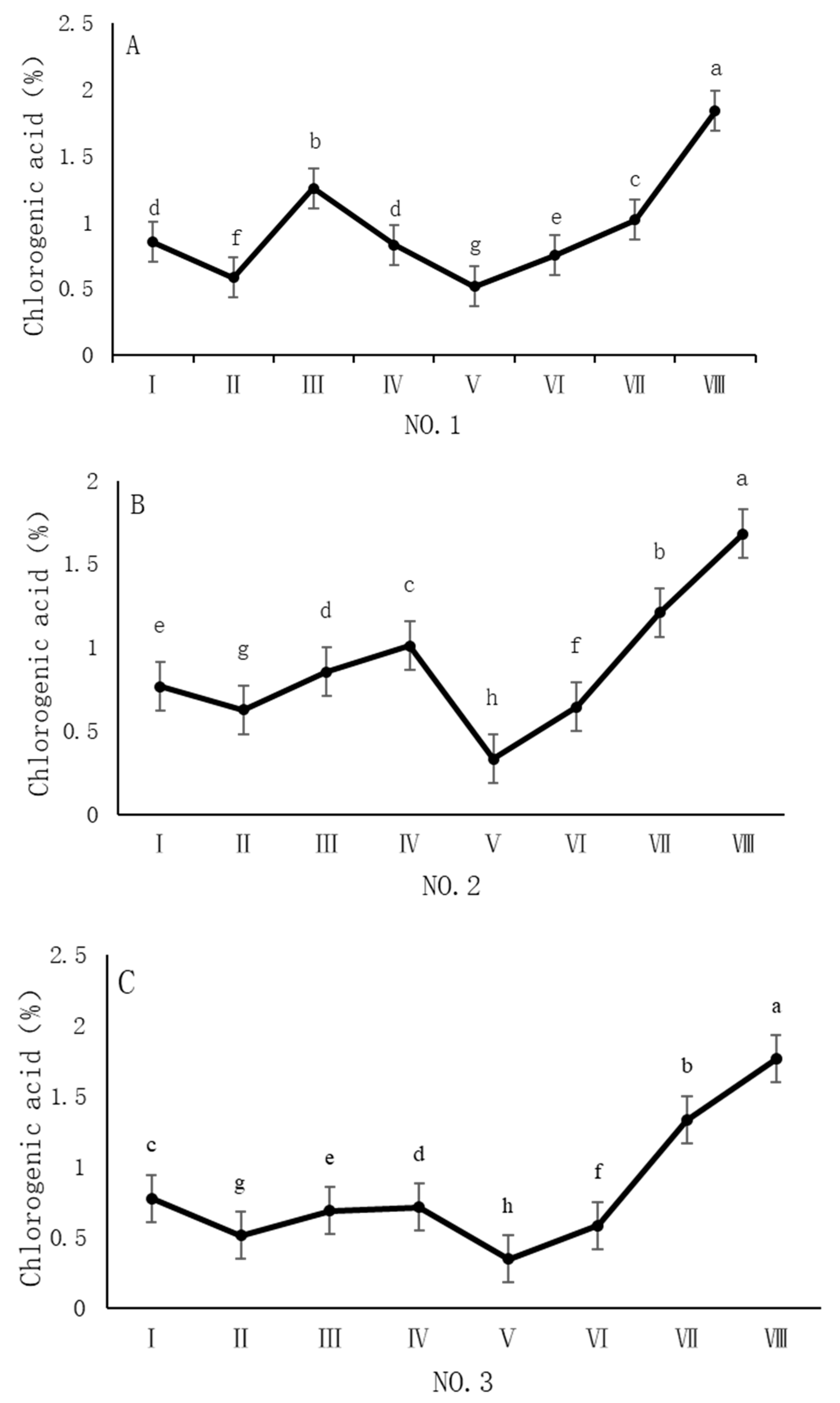
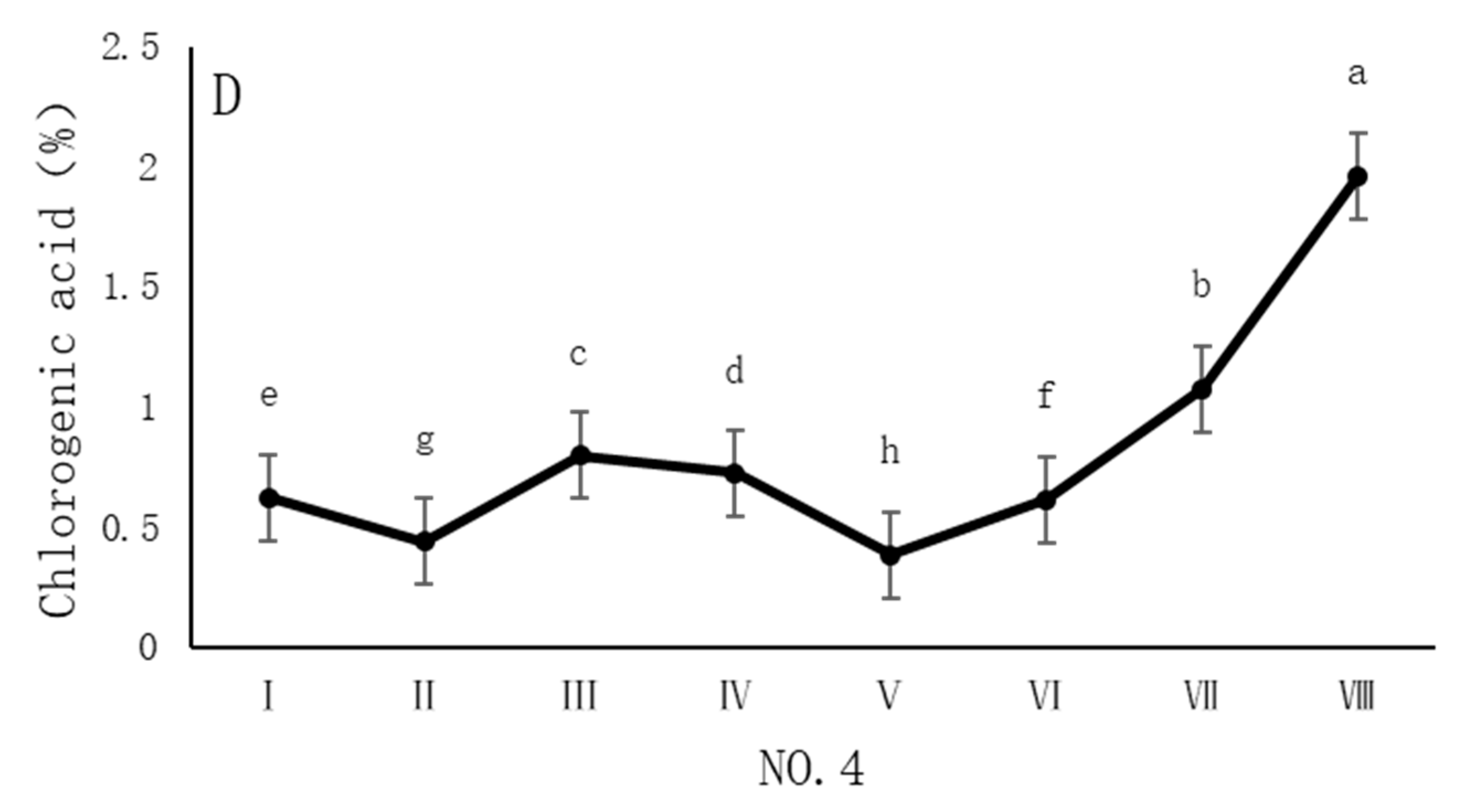
4.2. Variation in Chlorogenic Acid Content of Dandelions Across Different Regions
4.3. Variation in Caffeic Acid Content of Dandelions Across Different Regions
4.4. Variation in Chicoric Acid Content of Dandelions Across Different Regions
4.5. Correlation Analysis of Major Phenolic Acid Components in Dandelions Across Different Regions
4.6. Visualization of the Interaction Between Chlorogenic Acid, Caffeic Acid, Chicoric Acid, LPS, and MD-2
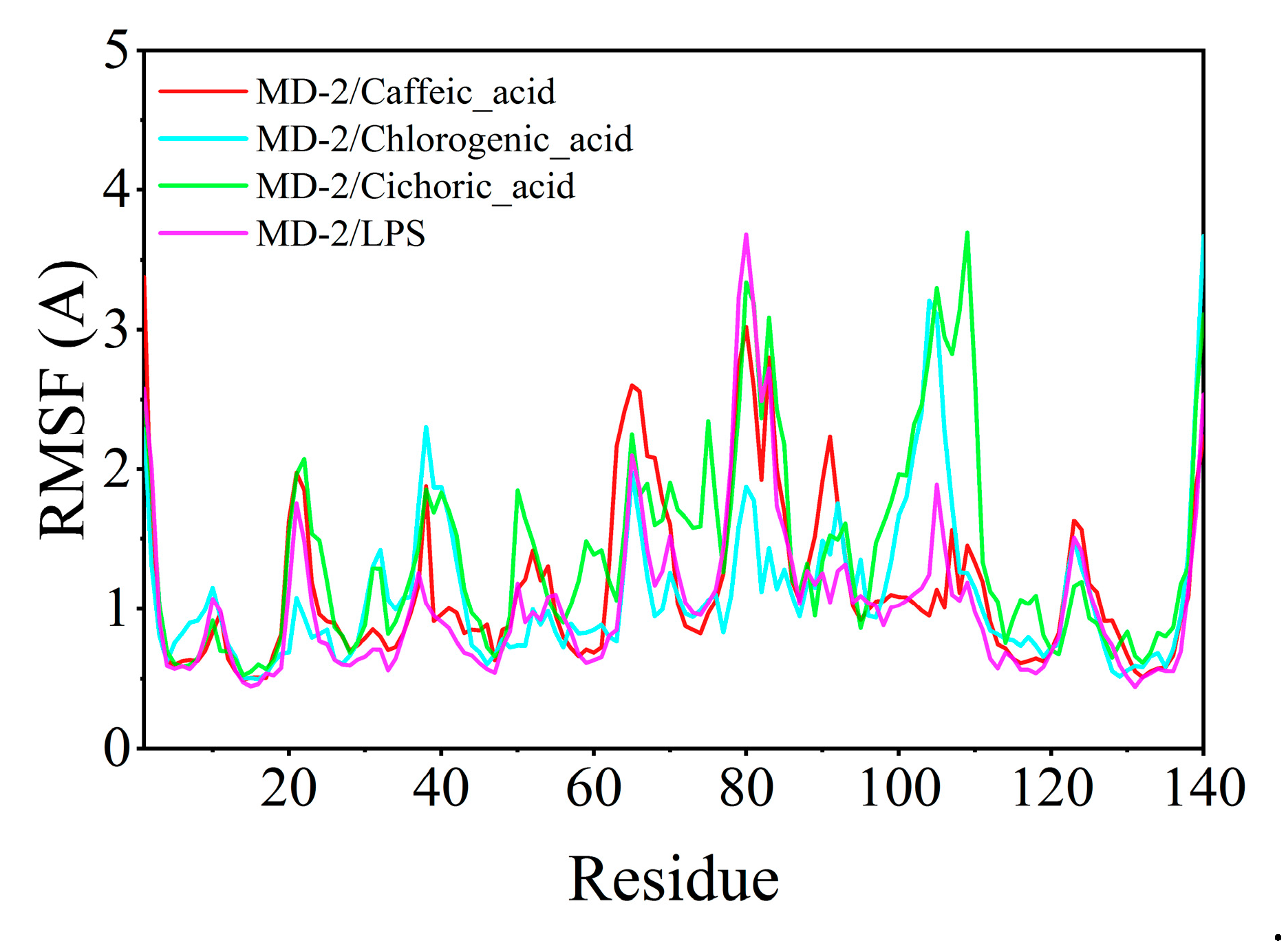
4.7. Molecular Dynamics Simulations of Chlorogenic Acid, Caffeic Acid, Chicoric Acid, LPS, and MD-2
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Resman, N.; Vasl, J.; Oblak, A.; Pristovsek, P.; Gioannini, T.L.; Weiss, J.P.; Jerala, R. Essential roles of hydrophobic residues in both MD-2 and Toll-like receptor 4 in activation by endotoxin. J. Biol. Chem. 2009, 284, 15052–15060. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Yan, D.; Sun, Q.; Tao, J.; Xu, L.; Sun, H.; Zhao, H. Ginsenoside Rg1 attenuates cardiomyocyte apoptosis and inflammation via the TLR4/NF-κB/NLRP3 pathway. J. Cell Biochem. 2020, 121, 2994–3004. [Google Scholar] [CrossRef] [PubMed]
- Leon, C.G.; Tory, R.; Jia, J.; Sivak, O.; Wasan, K.M. Discovery and development of Toll like receptor 4 (TLR4) antagonists: A new paradigm for treating sepsis and other diseases. Pharm. Res. 2008, 25, 1751–1761. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ma, Q.; Wang, Y.; Yu, S.; Bian, H.; Huang, L. Research progress on the pharmacological substance basis of dandelion. Chin. Arch. Tradit. Chin. Med. 2022, 40, 148–152. [Google Scholar]
- Yang, N.; Dong, Z.; Tian, G.; Zhu, M.; Li, C.; Bu, W.; Chen, J.; Hou, X.; Liu, Y.; Wang, G.; et al. Protective effects of organic acid component from Taraxacum mongolicum Hand.-Mazz. against LPS-induced inflammation: Regulating the TLR4/IKK/NF-κB signal pathway. J. Ethnopharmacol. 2016, 194, 395–402. [Google Scholar] [CrossRef]
- Choi, J.; Yoon, K.D.; Kim, J. Chemical constituents from Taraxacum officinale and their α-glucosidase inhibitory activities. Bioorg. Med. Chem. Lett. 2018, 28, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Faria, T.; Nascimento, C.; De Vasconcelos, S.; Stephens, P. Literature review on the biological effects of Taraxacum officinale plant in therapy. Asian. J. Pharm. Res. Dev. 2019, 7, 94–99. [Google Scholar] [CrossRef]
- Wirngo, F.E.; Lambert, M.N.; Jeppesen, P.B. The Physiological effects of dandelion (Taraxacum officinale) in type 2 diabetes. Rev. Diabetic Stud. 2016, 13, 113–131. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.J.; Kang, H.J.; Jung, H.J.; Kang, Y.S.; Lim, C.J.; Kim, Y.M.; Park, E.H. Anti-inflammatory activity of Taraxacum officinale. J. Ethnopharmacol. 2008, 115, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Grauso, L.; Emrick, S.; de Falco, B.; Lanzotti, V.; Bonanomi, G. Common dandelion: A review of its botanical, phytochemical and pharmacological profiles. Phytochem. Rev. 2019, 18, 1115–1132. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [PubMed]
- González, R.; Ballester, I.; López-Posadas, R.; Suárez, M.; Zarzuelo, A.; Martínez-Augustin, O.; Medina, F.S.D. Effects of flavonoids and other polyphenols on inflammation. Crit. Rev. Food Sci. Nutr. 2011, 51, 331–362. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Li, C.; Tian, G.; Zhu, M.; Bu, W.; Chen, J.; Hou, X.; Di, L.; Jia, X.; Dong, Z.; et al. Organic acid component from Taraxacum mongolicum Hand.-Mazz. alleviates inflammatory injury in lipopolysaccharide-induced acute tracheobronchitis of ICR mice through TLR4/NF-κB signaling pathway. Int. Immunopharmacol. 2016, 34, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Zhang, S.; Du, M.; Zhu, M. Dandelion extract suppresses reactive oxidative species and inflammasome in intestinal epithelial cells. J. Funct. Foods. 2017, 29, 10–18. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, Y.; Shen, C.; Xiao, Y.; Wang, Y.; Liu, Z.; Liu, X. Chicoric acid supplementation prevents systemic inflammation-induced memory impairment and amyloidogenesis via inhibition of NF-κB. FASEB J. 2017, 31, 1494–1507. [Google Scholar] [CrossRef]
- Bimbiraitė-Survilienė, K.; Stankevičius, M.; Šuštauskaitė, S.; Gęgotek, A.; Maruška, A.; Skrzydlewska, E.; Barsteigienė, Z.; Akuņeca, I.; Ragažinskienė, O.; Lukošius, A. Evaluation of chemical composition, radical scavenging and antitumor activities of Satureja hortensis L. herb extracts. Antioxidants 2021, 10, 53. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.A.; Al-Omar, M.S.; Mohammed, S.A.; Aly, M.S.; Alsuqub, A.N.; Khan, R.A. Drying induced impact on composition and oil quality of rosemary herb, Rosmarinus officinalis Linn. Molecules 2020, 25, 2830. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Li, Z.; Xue, Z.; Lu, X.; Wang, X. Optimization of extraction technology for determination of caffeic and chlorogenic acid in dandelion. Banat. J. Biotechnol. 2020, 11, 26–37. [Google Scholar] [CrossRef]
- Vogt, T. Phenylpropanoid biosynthesis. Mol. Plant 2010, 3, 2–20. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.A.; Paiva, N.L. Stress-induced phenylpropanoid metabolism. Plant Cell 1995, 7, 1085–1097. [Google Scholar] [CrossRef]
- Jaakola, L.; Hohtola, A. Effect of latitude on flavonoid biosynthesis in plants. Plant Cell Environ. 2010, 33, 1239–1247. [Google Scholar] [CrossRef]
- Marschner, P. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Takushi, H.; Chihiro, K.W.; Masaru, F.; Toshiki, I.; Kentaro, T.; Maki, K.; Hirofumi, U.; Yukifumi, U.; Ichiro, T.; Ko, N. Nitrate Addition Alleviates Ammonium Toxicity Without Lessening Ammonium Accumulation, Organic Acid Depletion and Inorganic Cation Depletion in Arabidopsis thaliana Shoots. Plant Cell Physiol. 2012, 53, 577–591. [Google Scholar]
- Christian, K. The use of ‘altitude’ in ecological research. Trends Ecol. Evol. 2007, 22, 569–574. [Google Scholar]
- Rozema, J.; van de Staaij, J.; Björn, L.O.; Caldwell, M. UV-B as an environmental factor in plant life: Stress and regulation. Trends Ecol. Evol. 1997, 12, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Niggeweg, R.; Michael, A.J.; Martin, C. Engineering plants with increased levels of the antioxidant chlorogenic acid. Nat. Biotechnol. 2004, 22, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.; Simmonds, M.S.J. Rosmarinic acid. Phytochemistry 2003, 62, 121–125. [Google Scholar] [CrossRef]
- Korkina, L.G. Phenylpropanoids as naturally occurring antioxidants: From plant defense to human health. Cell. Mol. Biol. 2007, 53, 15–25. [Google Scholar] [PubMed]
- Lin, L.Z.; Harnly, J.M. Phenolic component profiles of mustard greens, yu choy, and 15 other Brassica vegetables. J. Agric. Food Chem. 2010, 58, 6850–6857. [Google Scholar] [CrossRef] [PubMed]
- Karakaya, S. Bioavailability of phenolic compounds. Crit. Rev. Food Sci. Nutr. 2004, 44, 453–464. [Google Scholar] [CrossRef]
- Miao, X.; Wang, R.; Ye, S. Protective effect of salvianolic acid B on kidney of diabetic nephropathy rats and its possible mechanism. Prog. Anat. Sci. 2022, 28, 17–20. [Google Scholar]
- Yan, L.; Qiu, X.; Kang, J.; Gu, C.; Zhang, S.; Zhang, C.; Wang, Y.; Kong, J.; Luo, G.; Zhang, Y. Study on Effects of Total Phenolic Acids from Saussureae involucratae Herba on Release of Inflammatory Mediators in Lipopolysaccharide-stimulated RAW264.7 Macrophages and Its Mechanism. Mod. Chin. Med. 2023, 25, 2109–2118. [Google Scholar]
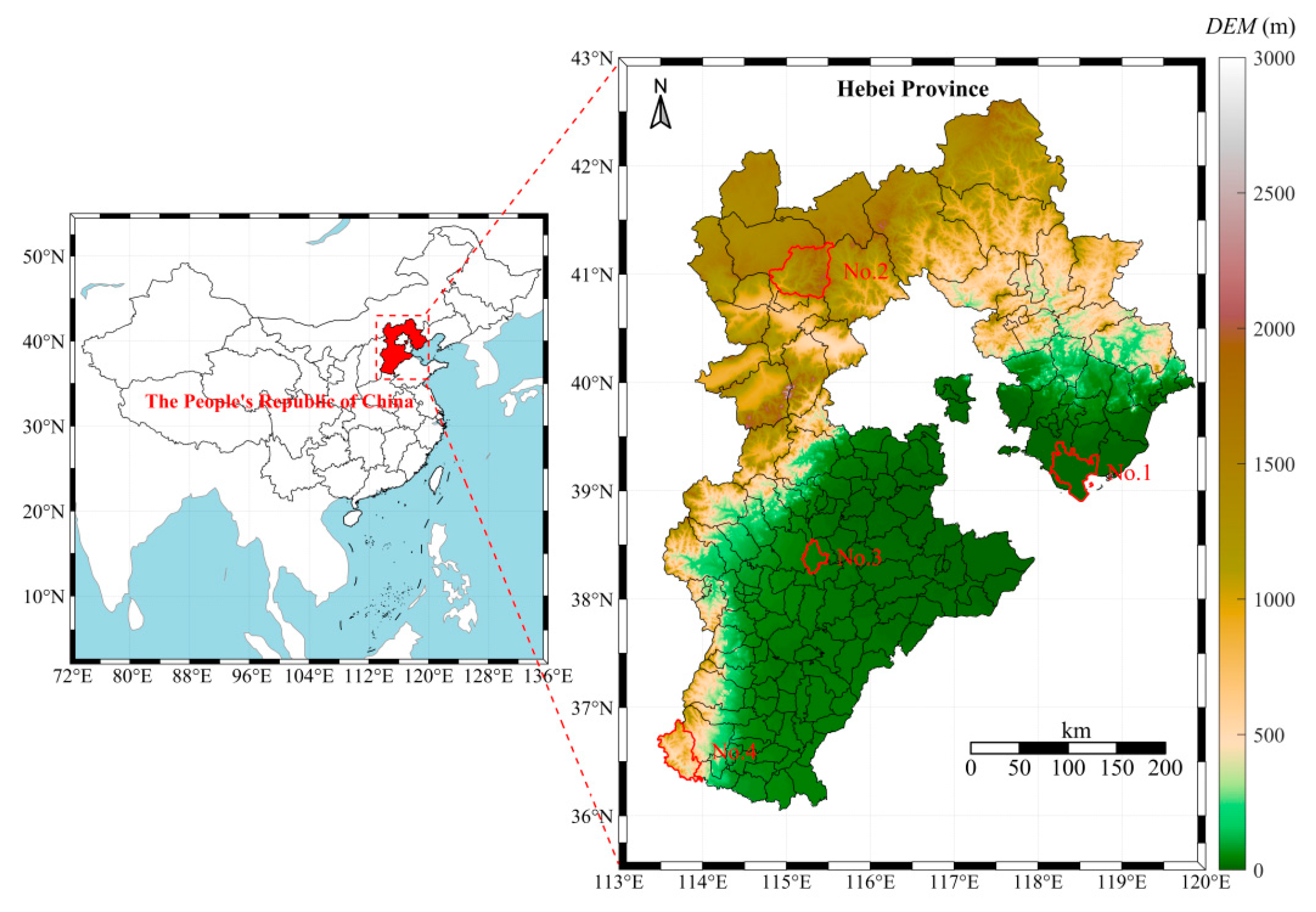
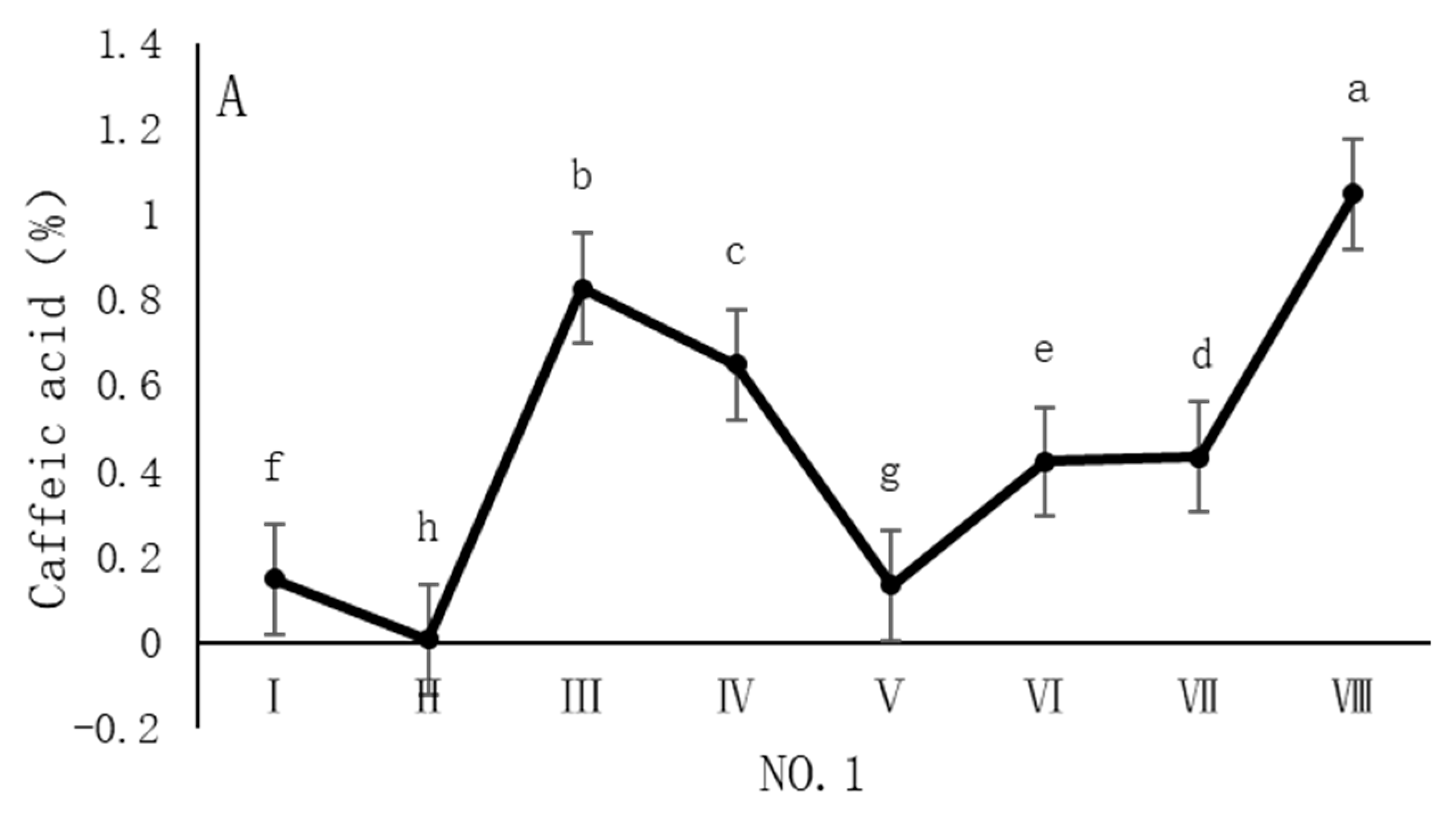
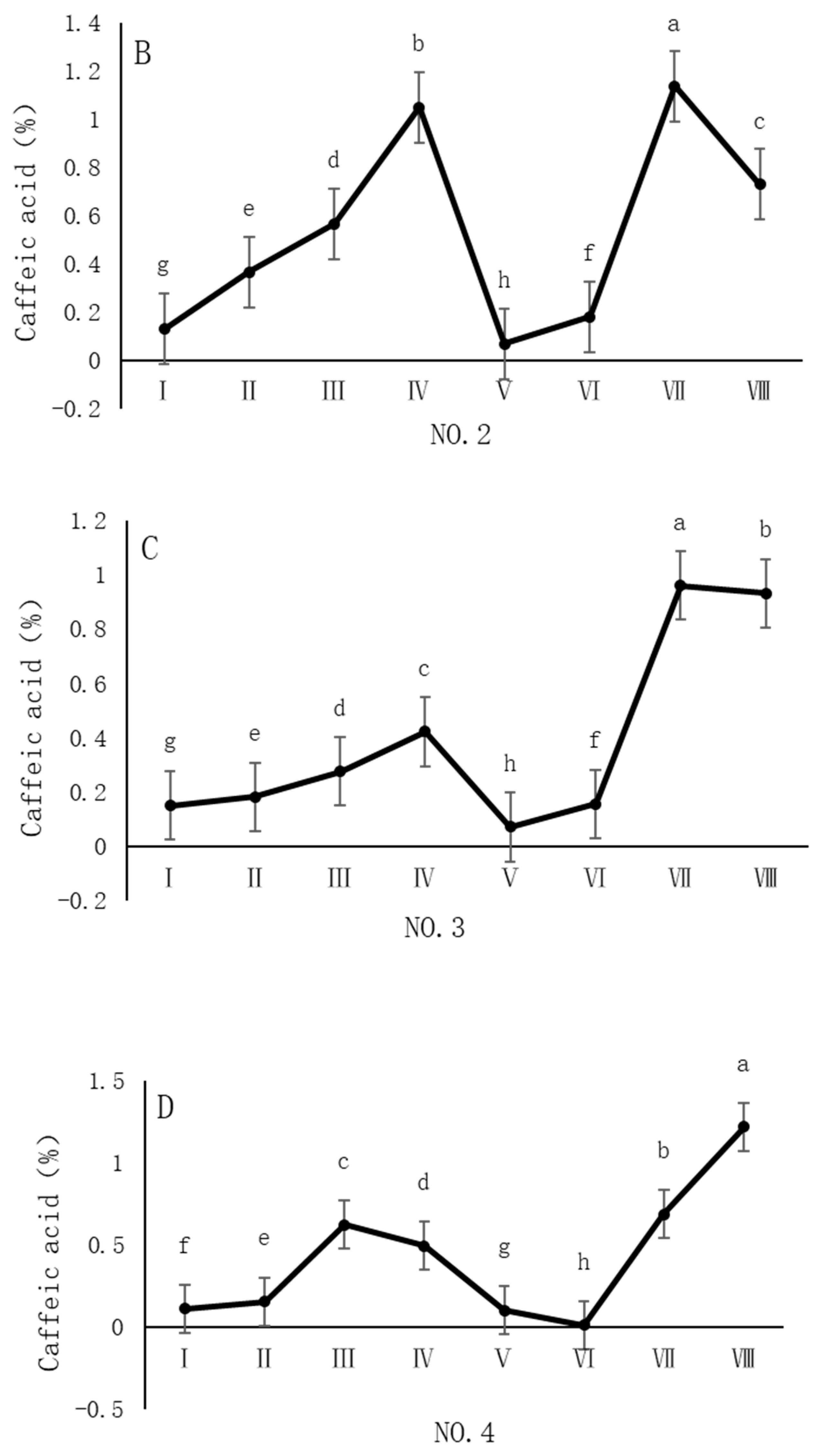
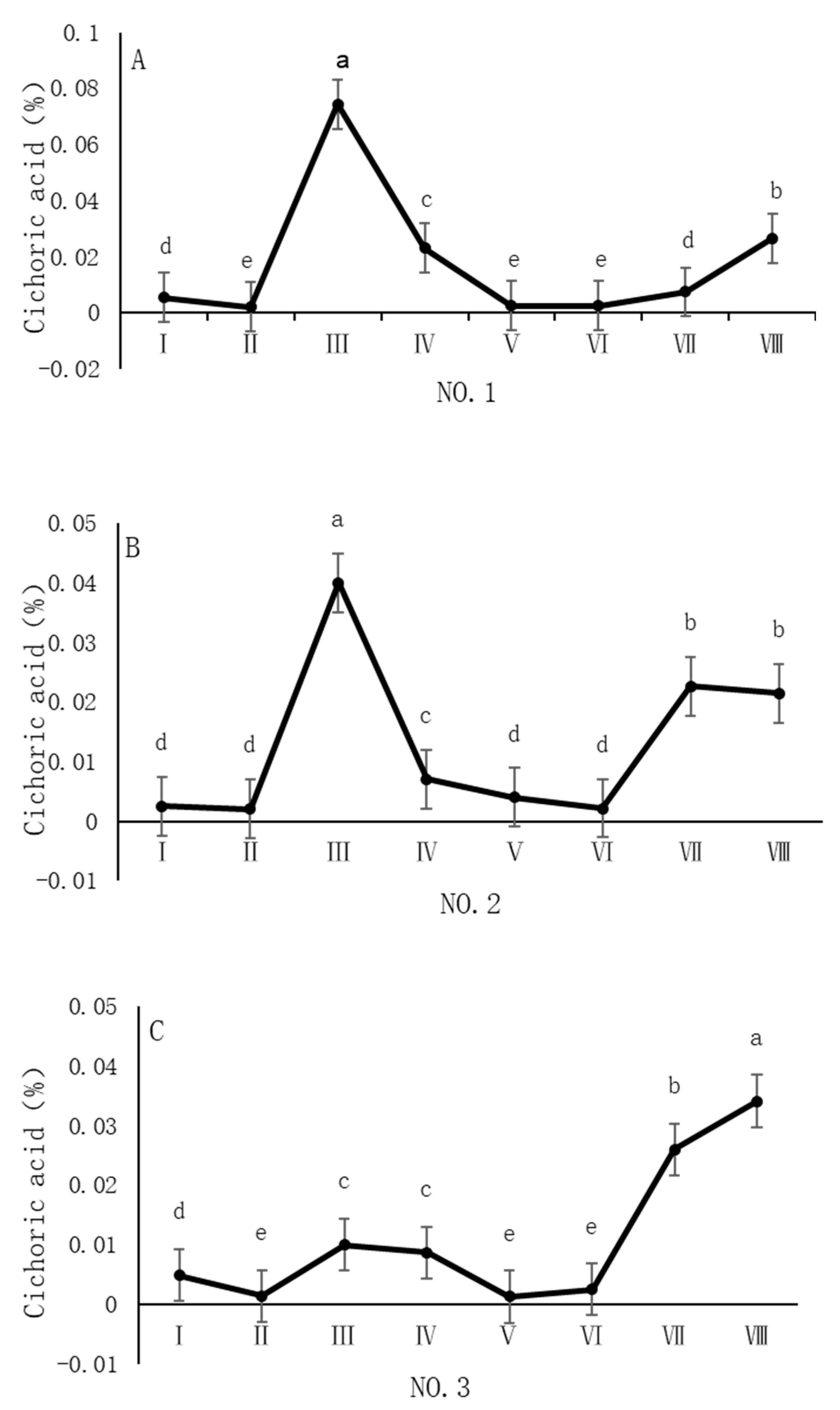
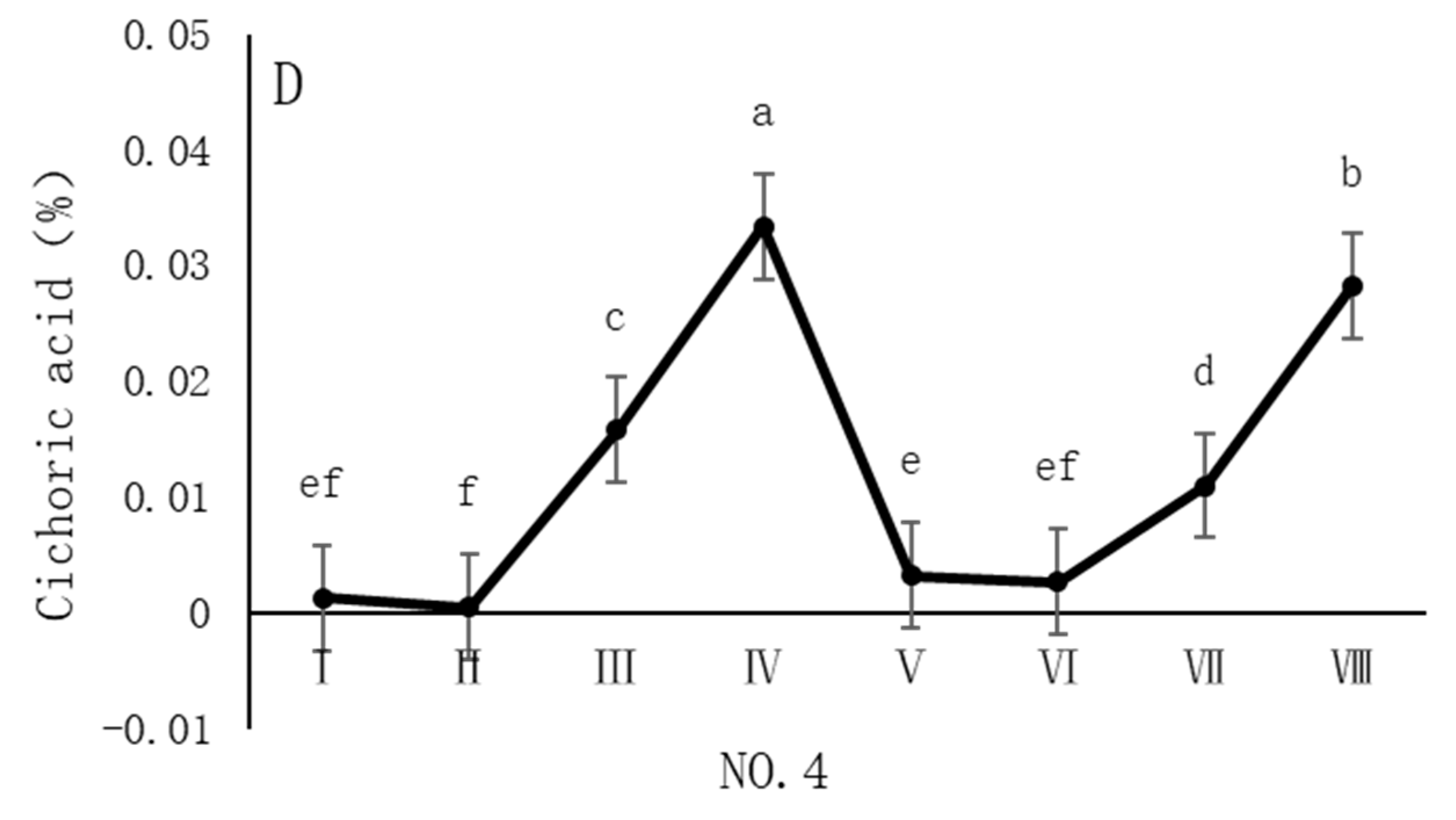
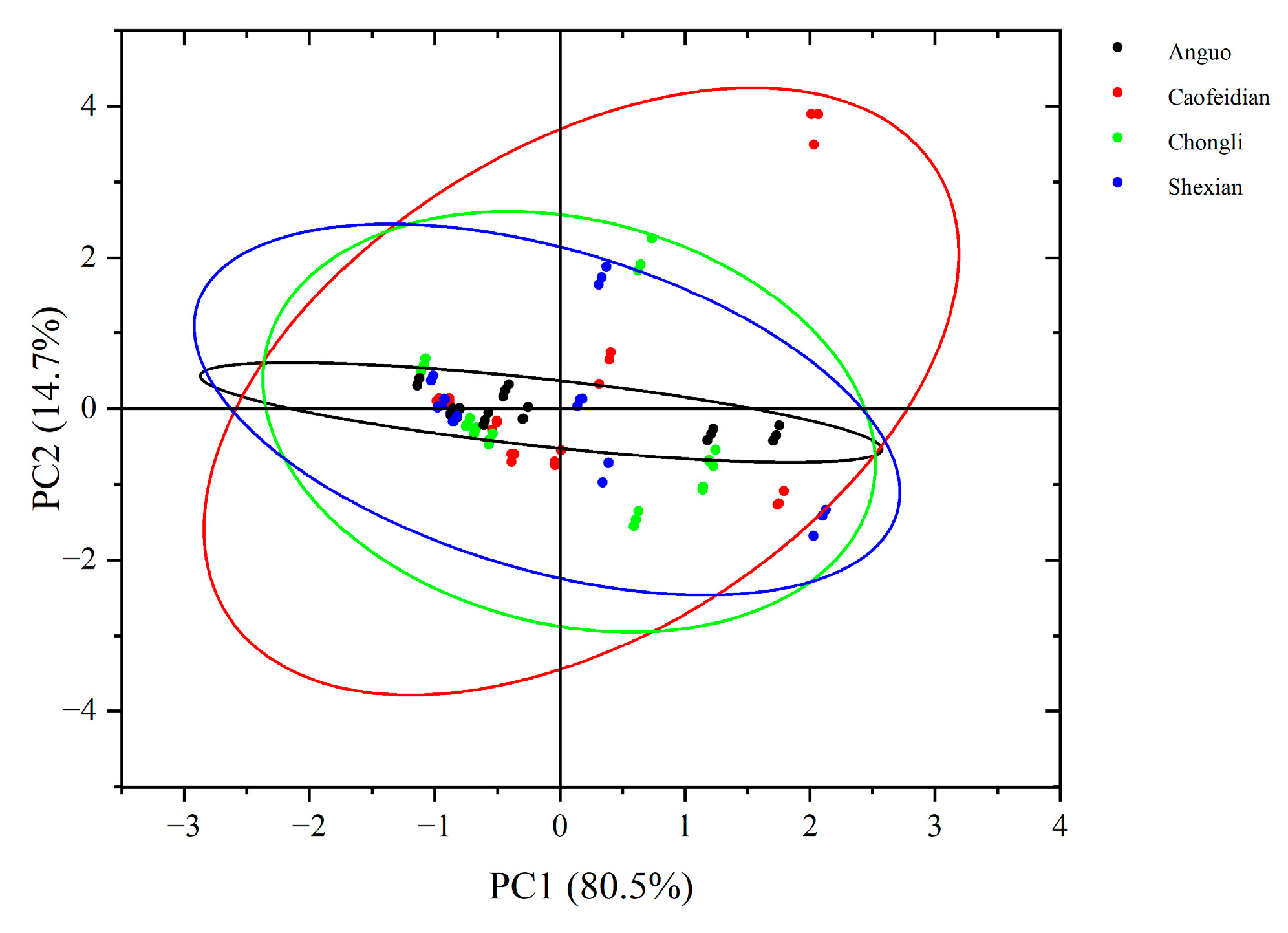
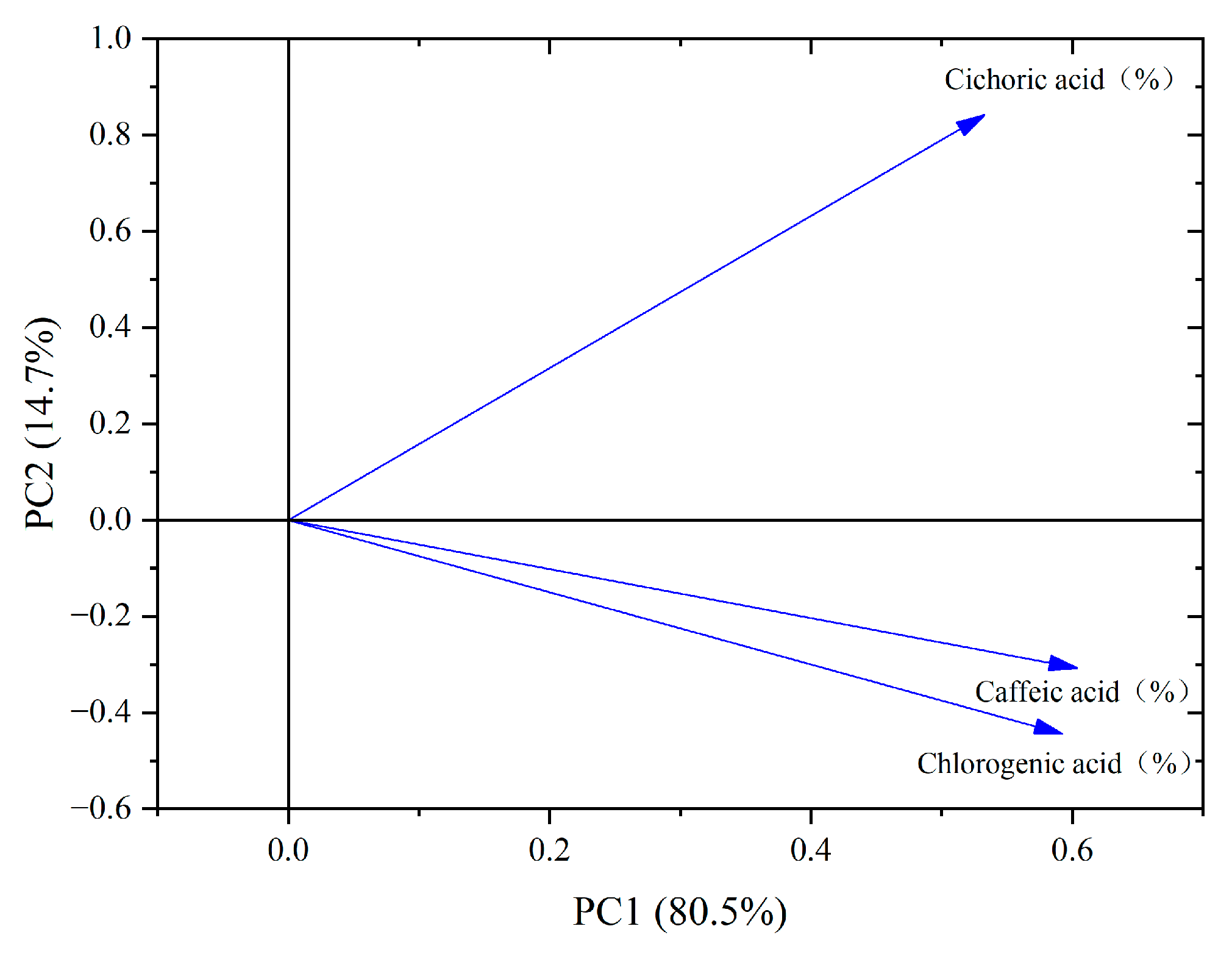
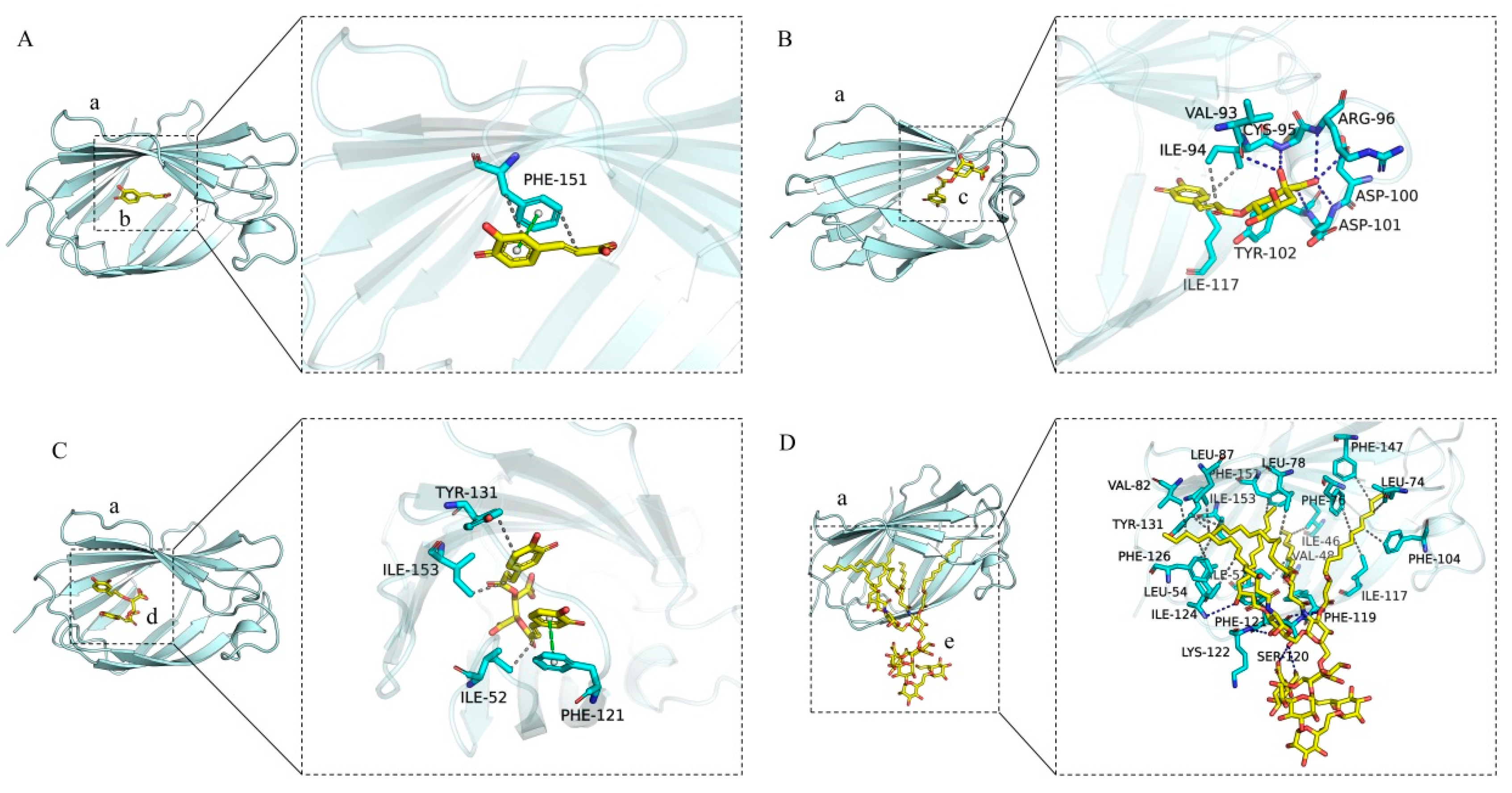
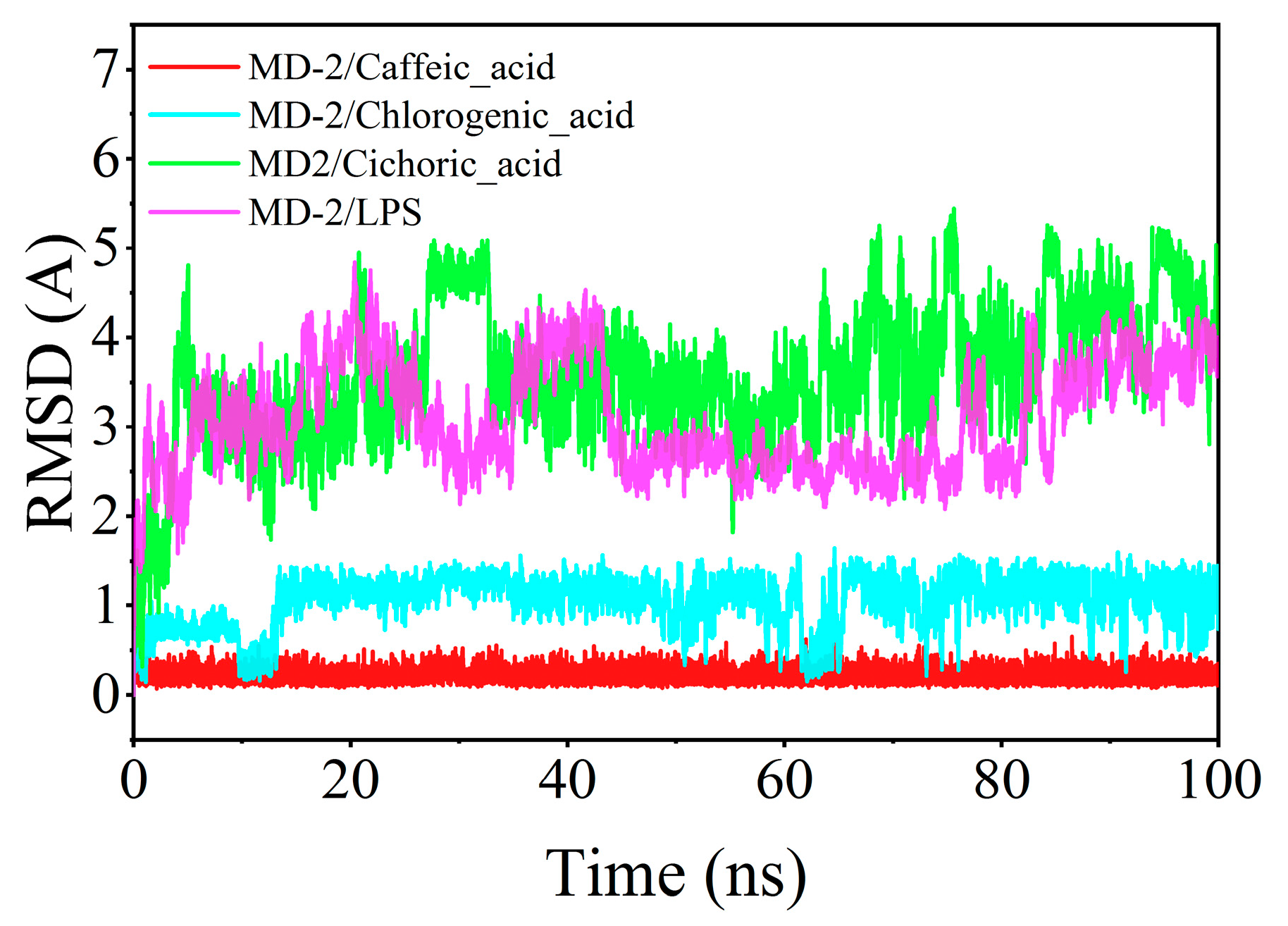
| Region | Average Annual Temperature (°C) | Precipitation (mm) | Soil pH | Altitude (m) |
|---|---|---|---|---|
| Caofeidian | 11.5 | 650 | 8.0 | 5 |
| Chongli | 6.5 | 400 | 6.5 | 1200 |
| Anguo | 12 | 600 | 7.8 | 30 |
| Shexian | 12.3 | 580 | 7.2 | 500 |
| Place of Origin | Number | Place of Origin | Number | Growth Period | Harvesting Time |
|---|---|---|---|---|---|
| (NO. 1) Caofeidian District, Tangshan City, Hebei Province | S1 | (NO. 3) Anguo City, Baoding City, Hebei Province | S17 | I | 15 July 2023 |
| S2 | S18 | II | 30 July 2023 | ||
| S3 | S19 | III | 15 August 2023 | ||
| S4 | S20 | IV | 30 August 2023 | ||
| S5 | S21 | V | 15 September 2023 | ||
| S6 | S22 | VI | 30 September 2023 | ||
| S7 | S23 | VII | 15 October 2023 | ||
| S8 | S24 | VIII | 30 October 2023 | ||
| (NO. 2) Chongli District, Zhangjiakou City, Hebei Province | S9 | (NO. 4) She County, Handan City, Hebei Province | S25 | I | 15 July 2023 |
| S10 | S26 | II | 30 July 2023 | ||
| S11 | S27 | III | 15 August 2023 | ||
| S12 | S28 | IV | 30 August 2023 | ||
| S13 | S29 | V | 15 September 2023 | ||
| S14 | S30 | VI | 30 September 2023 | ||
| S15 | S31 | VII | 15 October 2023 | ||
| S16 | S32 | VIII | 30 October 2023 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Meng, R.; Feng, W.; Wu, Z.; Lu, X.; Wang, X.; Sun, L. A Study of Variation in the Major Phenolic Acid Components of Dandelions Across Different Regions, and the Potential Molecular Mechanisms of Their Anti-Inflammatory Activity. Curr. Issues Mol. Biol. 2025, 47, 145. https://doi.org/10.3390/cimb47030145
Li Z, Meng R, Feng W, Wu Z, Lu X, Wang X, Sun L. A Study of Variation in the Major Phenolic Acid Components of Dandelions Across Different Regions, and the Potential Molecular Mechanisms of Their Anti-Inflammatory Activity. Current Issues in Molecular Biology. 2025; 47(3):145. https://doi.org/10.3390/cimb47030145
Chicago/Turabian StyleLi, Zhaojia, Ran Meng, Wei Feng, Zhe Wu, Xuelin Lu, Xiuping Wang, and Liangdan Sun. 2025. "A Study of Variation in the Major Phenolic Acid Components of Dandelions Across Different Regions, and the Potential Molecular Mechanisms of Their Anti-Inflammatory Activity" Current Issues in Molecular Biology 47, no. 3: 145. https://doi.org/10.3390/cimb47030145
APA StyleLi, Z., Meng, R., Feng, W., Wu, Z., Lu, X., Wang, X., & Sun, L. (2025). A Study of Variation in the Major Phenolic Acid Components of Dandelions Across Different Regions, and the Potential Molecular Mechanisms of Their Anti-Inflammatory Activity. Current Issues in Molecular Biology, 47(3), 145. https://doi.org/10.3390/cimb47030145


_Kim.png)



