Research Progress on Signalling Pathways Related to Sepsis-Associated Acute Kidney Injury in Children
Abstract
1. Introduction
2. Epidemiology and Clinical Risk Factors
3. Pathophysiology and Key Signalling Pathways of SA-AKI
3.1. Inflammatory Signalling Pathways
3.2. Apoptosis and Necrosis-Related Signals
3.3. Metabolic Abnormality Pathways
3.4. Immune Pathways
3.5. Emerging Pathways
3.6. The Influence of Genetics and Epigenetics on SA-AKI Heterogeneity
4. Biomarkers
4.1. Urinary Biomarkers
4.2. Blood Biomarkers
4.3. Risk Stratification Models
4.4. Clinical Applications
5. Treatment Strategies
5.1. Current Treatment Approaches
5.2. Pathway-Targeted Therapies
5.3. Precision Medicine
- Mitochondrial Protection and Metabolism: Given the critical role of mitochondrial dysfunction, Choline Supplementation has been explored for its ability to restore mitochondrial function and has shown improved estimated Glomerular Filtration Rate (eGFR) in pilot studies, directly addressing metabolic defects [95,170]. Similarly, Humanin, a mitochondrial-derived peptide, inhibits apoptosis and inflammation, reducing the injury biomarker NGAL by 20–25% in preclinical models [137,173].
- Cell Death and Inflammasome Inhibition: Emerging pathways of regulated cell death present clear targets. Pyroptosis Inhibitors, which block the NLRP3 inflammasome and caspase-1 activation, have resulted in a 25–35% reduction in AKI severity in animal models, showing strong preclinical promise for pediatric SA-AKI [90,175]. Furthermore, inhibiting the cGAS-STING pathway, which reduces interferon signalling and pyroptosis, has been linked to 30% less tubular damage in sepsis models [118,119,174].
6. Conclusions and Future Directions
6.1. Challenges in Translational Medicine
6.2. Challenges in Translating Animal Findings to Pediatric SA-AKI Clinical Trials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Shankar-Hari, M.; Phillips, G.S.; Levy, M.L.; Seymour, C.W.; Liu, V.X.; Deutschman, C.S.; Angus, D.C.; Rubenfeld, G.D.; Singer, M. Developing a New Definition and Assessing New Clinical Criteria for Septic Shock: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 775–787. [Google Scholar] [CrossRef]
- Mellhammar, L.; Wollter, E.; Dahlberg, J.; Donovan, B.; Olséen, C.J.; Wiking, P.O.; Rose, N.; Schwarzkopf, D.; Friedrich, M.; Fleischmann-Struzek, C.; et al. Estimating Sepsis Incidence Using Administrative Data and Clinical Medical Record Review. JAMA Netw. Open 2023, 6, e2331168. [Google Scholar] [CrossRef]
- Lameire, N.H.; Levin, A.; Kellum, J.A.; Cheung, M.; Jadoul, M.; Winkelmayer, W.C.; Stevens, P.E. Harmonizing acute and chronic kidney disease definition and classification: Report of a Kidney Disease: Improving Global Outcomes (KDIGO) Consensus Conference. Kidney Int. 2021, 100, 516–526. [Google Scholar] [CrossRef]
- Zarbock, A.; Nadim, M.K.; Pickkers, P.; Gomez, H.; Bell, S.; Joannidis, M.; Kashani, K.; Koyner, J.L.; Pannu, N.; Meersch, M.; et al. Sepsis-associated acute kidney injury: Consensus report of the 28th Acute Disease Quality Initiative workgroup. Nat. Rev. Nephrol. 2023, 19, 401–417. [Google Scholar] [CrossRef]
- Peerapornratana, S.; Manrique-Caballero, C.L.; Gómez, H.; Kellum, J.A. Acute kidney injury from sepsis: Current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int. 2019, 96, 1083–1099. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.M.; Jiang, Y.J.; Zheng, X.; Li, W.; Wang, M.P.; Xi, X.M.; Li, W.X. The attributable mortality of sepsis for acute kidney injury: A propensity-matched analysis based on multicenter prospective cohort study. Ren. Fail. 2023, 45, 2162415. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.Q.; Yan, P.; Zhang, N.Y.; Luo, B.; Wang, M.; Deng, Y.H.; Wu, T.; Wu, X.; Liu, Q.; Wang, H.S.; et al. Machine learning for early discrimination between transient and persistent acute kidney injury in critically ill patients with sepsis. Sci. Rep. 2021, 11, 20269. [Google Scholar] [CrossRef]
- Turgut, F.; Awad, A.S.; Abdel-Rahman, E.M. Acute Kidney Injury: Medical Causes and Pathogenesis. J. Clin. Med. 2023, 12, 375. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Wang, M.; Shang, Y.; Zhang, B.; Zhang, S.; Liu, X.; Cao, P.; Fan, Y.; Tan, K. Apoptosis-related prognostic biomarkers and potential targets for acute kidney injury based on machine learning algorithm and in vivo experiments. Apoptosis 2024, 29, 303–320. [Google Scholar] [CrossRef]
- Chang, Y.M.; Chou, Y.T.; Kan, W.C.; Shiao, C.C. Sepsis and Acute Kidney Injury: A Review Focusing on the Bidirectional Interplay. Int. J. Mol. Sci. 2022, 23, 9159. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, J.H.; Lee, D.Y.; Kim, H.Y.; Kim, D.H.; Oh, J.S.; Sin, Y.H.; Kim, J.K.; Hwang, S.D. Allograft dysfunction and parenchymal necrosis associated with renal artery stenosis and perigraft hematoma after kidney transplantation. Korean J. Transpl. 2020, 34, 126–131. [Google Scholar] [CrossRef]
- El-Zayat, S.R.; Sibaii, H.; Mannaa, F.A. Toll-like receptors activation, signaling, and targeting: An overview. Bull. Natl. Res. Cent. 2019, 43, 187. [Google Scholar] [CrossRef]
- Kumar, V. Toll-like receptors in sepsis-associated cytokine storm and their endogenous negative regulators as future immunomodulatory targets. Int. Immunopharmacol. 2020, 89, 107087. [Google Scholar] [CrossRef]
- Toro, J.; Manrique-Caballero, C.L.; Gómez, H. Metabolic Reprogramming and Host Tolerance: A Novel Concept to Understand Sepsis-Associated AKI. J. Clin. Med. 2021, 10, 4184. [Google Scholar] [CrossRef]
- Tang, D.; Kang, R.; Berghe, T.V.; Vandenabeele, P.; Kroemer, G. The molecular machinery of regulated cell death. Cell Res. 2019, 29, 347–364. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Deng, J.; Zhou, H.; Tan, W.; Lin, L.; Yang, J. Programmed Cell Death in Sepsis Associated Acute Kidney Injury. Front. Med. 2022, 9, 883028. [Google Scholar] [CrossRef] [PubMed]
- Bannerman, D.D.; Goldblum, S.E. Mechanisms of bacterial lipopolysaccharide-induced endothelial apoptosis. Am. J. Physiol. Lung Cell Mol. Physiol. 2003, 284, L899–L914. [Google Scholar] [CrossRef] [PubMed]
- Nagata, S.; Segawa, K. Sensing and clearance of apoptotic cells. Curr. Opin. Immunol. 2021, 68, 1–8. [Google Scholar] [CrossRef]
- Galluzzi, L.; Kepp, O.; Chan, F.K.; Kroemer, G. Necroptosis: Mechanisms and Relevance to Disease. Annu. Rev. Pathol. 2017, 12, 103–130. [Google Scholar] [CrossRef]
- Jiao, H.; Wachsmuth, L.; Kumari, S.; Schwarzer, R.; Lin, J.; Eren, R.O.; Fisher, A.; Lane, R.; Young, G.R.; Kassiotis, G.; et al. Z-nucleic-acid sensing triggers ZBP1-dependent necroptosis and inflammation. Nature 2020, 580, 391–395, Correction in Nature 2020, 580, E10. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, I.; Rayamajhi, M.; Miao, E.A. Programmed cell death as a defence against infection. Nat. Rev. Immunol. 2017, 17, 151–164. [Google Scholar] [CrossRef]
- Abu Khweek, A.; Amer, A.O. Pyroptotic and non-pyroptotic effector functions of caspase-11. Immunol. Rev. 2020, 297, 39–52. [Google Scholar] [CrossRef]
- Ram, C.; Gairola, S.; Verma, S.; Mugale, M.N.; Bonam, S.R.; Murty, U.S.; Sahu, B.D. Biochanin A Ameliorates Nephropathy in High-Fat Diet/Streptozotocin-Induced Diabetic Rats: Effects on NF-kB/NLRP3 Axis, Pyroptosis, and Fibrosis. Antioxidants 2023, 12, 1052. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhao, Y.; Wang, K.; Shi, X.; Wang, Y.; Huang, H.; Zhuang, Y.; Cai, T.; Wang, F.; Shao, F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 2015, 526, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Yang, Q.; Zhang, Y.; Xu, H.; Ye, Y.; Li, L.; Yang, Y.; Jin, S. Maresin conjugates in tissue regeneration-1 suppresses ferroptosis in septic acute kidney injury. Cell Biosci. 2021, 11, 221. [Google Scholar] [CrossRef]
- von Krusenstiern, A.N.; Robson, R.N.; Qian, N.; Qiu, B.; Hu, F.; Reznik, E.; Smith, N.; Zandkarimi, F.; Estes, V.M.; Dupont, M.; et al. Identification of essential sites of lipid peroxidation in ferroptosis. Nat. Chem. Biol. 2023, 19, 719–730. [Google Scholar] [CrossRef]
- Stockwell, B.R.; Jiang, X.; Gu, W. Emerging Mechanisms and Disease Relevance of Ferroptosis. Trends Cell Biol. 2020, 30, 478–490. [Google Scholar] [CrossRef]
- Bhatia, D.; Choi, M.E. Autophagy in kidney disease: Advances and therapeutic potential. Prog. Mol. Biol. Transl. Sci. 2020, 172, 107–133. [Google Scholar]
- Wu, Y.; Wang, L.; Meng, L.; Cao, G.K.; Zhao, Y.L.; Zhang, Y. Biological effects of autophagy in mice with sepsis-induced acute kidney injury. Exp. Ther. Med. 2019, 17, 316–322. [Google Scholar] [CrossRef]
- Nguyen, J.A.; Yates, R.M. Better Together: Current Insights Into Phagosome-Lysosome Fusion. Front. Immunol. 2021, 12, 636078. [Google Scholar] [CrossRef]
- Zhao, S.; Liao, J.; Shen, M.; Li, X.; Wu, M. Epigenetic dysregulation of autophagy in sepsis-induced acute kidney injury: The underlying mechanisms for renoprotection. Front. Immunol. 2023, 14, 1180866. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, J.; Tian, J.; Virzì, G.M.; Digvijay, K.; Cueto, L.; Yin, Y.; Rosner, M.H.; Ronco, C. Mitochondria in Sepsis-Induced AKI. J. Am. Soc. Nephrol. 2019, 30, 1151–1161. [Google Scholar] [CrossRef]
- Vargas, J.N.S.; Hamasaki, M.; Kawabata, T.; Youle, R.J.; Yoshimori, T. The mechanisms and roles of selective autophagy in mammals. Nat. Rev. Mol. Cell Biol. 2023, 24, 167–185. [Google Scholar] [CrossRef]
- Levine, B.; Kroemer, G. Biological Functions of Autophagy Genes: A Disease Perspective. Cell 2019, 176, 11–42. [Google Scholar] [CrossRef] [PubMed]
- Doran, A.C.; Yurdagul, A., Jr.; Tabas, I. Efferocytosis in health and disease. Nat. Rev. Immunol. 2020, 20, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, S.; Goggins, E.; Okusa, M.D. The Pathophysiology of Sepsis-Associated AKI. Clin. J. Am. Soc. Nephrol. 2022, 17, 1050–1069. [Google Scholar] [CrossRef]
- Arai, Y.; Yamaoka, Y.; Morioka, S. Sweeping Up Dying Cells during Tissue Injury. Nephron 2022, 146, 249–252. [Google Scholar] [CrossRef]
- Ronco, C.; Bellomo, R.; Kellum, J.A. Acute kidney injury. Lancet 2019, 394, 1949–1964. [Google Scholar] [CrossRef] [PubMed]
- Post, E.H.; Kellum, J.A.; Bellomo, R.; Vincent, J.L. Renal perfusion in sepsis: From macro- to microcirculation. Kidney Int. 2017, 91, 45–60. [Google Scholar] [CrossRef]
- Liu, P.; Cai, X.; Zhang, Y.; Li, Y.; Liu, L. The clinical application of ultrasound for acute kidney injury during sepsis-from macroscopic to microscopic renal perfusion perspectives. Ultrasound Med. Biol. 2023, 49, 2017–2024. [Google Scholar] [CrossRef]
- Deng, J.; Li, L.; Feng, Y.; Yang, J. Comprehensive Management of Blood Pressure in Patients with Septic AKI. J. Clin. Med. 2023, 12, 1018. [Google Scholar] [CrossRef]
- Wang, Z.; Holthoff, J.H.; Seely, K.A.; Pathak, E.; Spencer, H.J., 3rd; Gokden, N.; Mayeux, P.R. Development of oxidative stress in the peritubular capillary microenvironment mediates sepsis-induced renal microcirculatory failure and acute kidney injury. Am. J. Pathol. 2012, 180, 505–516. [Google Scholar] [CrossRef]
- von Groote, T.; Albert, F.; Meersch, M.; Koch, R.; Gerss, J.; Arlt, B.; Sadjadi, M.; Porschen, C.; Pickkers, P.; Zarbock, A. Evaluation of Proenkephalin A 119-159 for liberation from renal replacement therapy: An external, multicenter pilot study in critically ill patients with acute kidney injury. Crit. Care 2023, 27, 276. [Google Scholar] [CrossRef]
- Frigyesi, A.; Boström, L.; Lengquist, M.; Johnsson, P.; Lundberg, O.H.M.; Spångfors, M.; Annborn, M.; Cronberg, T.; Nielsen, N.; Levin, H.; et al. Plasma proenkephalin A 119-159 on intensive care unit admission is a predictor of organ failure and 30-day mortality. Intensive Care Med. Exp. 2021, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Hollinger, A.; Wittebole, X.; François, B.; Pickkers, P.; Antonelli, M.; Gayat, E.; Chousterman, B.G.; Lascarrou, J.B.; Dugernier, T.; Di Somma, S.; et al. Proenkephalin A 119-159 (Penkid) Is an Early Biomarker of Septic Acute Kidney Injury: The Kidney in Sepsis and Septic Shock (Kid-SSS) Study. Kidney Int. Rep. 2018, 3, 1424–1433, Erratum in Kidney Int. Rep. 2019, 4, 187. [Google Scholar] [CrossRef] [PubMed]
- Schaalan, M.; Mohamed, W. Predictive ability of circulating osteoprotegerin as a novel biomarker for early detection of acute kidney injury induced by sepsis. Eur. Cytokine Netw. 2017, 28, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Kim, M.J.; Ko, H.J.; Lee, E.J.; Kim, H.R.; Jeon, J.W.; Ham, Y.R.; Na, K.R.; Lee, K.W.; Lee, S.I.; et al. Diagnostic and Prognostic Roles of C-Reactive Protein, Procalcitonin, and Presepsin in Acute Kidney Injury Patients Initiating Continuous Renal Replacement Therapy. Diagnostics 2023, 13, 777. [Google Scholar] [CrossRef]
- Sun, H.; Jiang, H.; Eliaz, A.; Kellum, J.A.; Peng, Z.; Eliaz, I. Galectin-3 in septic acute kidney injury: A translational study. Crit. Care 2021, 25, 109. [Google Scholar] [CrossRef]
- Wang, F.; Ye, J.; Zhu, W.; Ge, R.; Hu, C.; Qian, Y.; Li, Y.; Peng, Z. Galectin-3 Mediates Endotoxin Internalization and Caspase-4/11 Activation in Tubular Epithelials and Macrophages During Sepsis and Sepsis-Associated Acute Kidney Injury. Inflammation 2024, 47, 454–468. [Google Scholar] [CrossRef]
- Ouyang, X.; Fu, M.; Li, J.; Gao, J.; Xu, L.; Pei, Y.; Jiang, X. Risk factors for occurrence and death of sepsis-associated acute kidney injury in children with sepsis. Int. Immunopharmacol. 2024, 143, 113551. [Google Scholar] [CrossRef]
- Honore, P.M.; Nguyen, H.B.; Gong, M.; Chawla, L.S.; Bagshaw, S.M.; Artigas, A.; Shi, J.; Joannes-Boyau, O.; Vincent, J.L.; Kellum, J.A. Urinary Tissue Inhibitor of Metalloproteinase-2 and Insulin-Like Growth Factor-Binding Protein 7 for Risk Stratification of Acute Kidney Injury in Patients with Sepsis. Crit. Care Med. 2016, 44, 1851–1860. [Google Scholar] [CrossRef]
- Pan, P.; Liu, X.; Wu, L.; Li, X.; Wang, K.; Wang, X.; Zhou, X.; Long, Y.; Liu, D.; Xie, L.; et al. TREM-1 promoted apoptosis and inhibited autophagy in LPS-treated HK-2 cells through the NF-κB pathway. Int. J. Med. Sci. 2021, 18, 8–17. [Google Scholar] [CrossRef]
- Kellum, J.A.; Artigas, A.; Gunnerson, K.J.; Honore, P.M.; Kampf, J.P.; Kwan, T.; McPherson, P.; Nguyen, H.B.; Rimmelé, T.; Shapiro, N.I.; et al. Use of Biomarkers to Identify Acute Kidney Injury to Help Detect Sepsis in Patients With Infection. Crit. Care Med. 2021, 49, e360–e368. [Google Scholar] [CrossRef]
- Kamijo-Ikemori, A.; Ichikawa, D.; Matsui, K.; Yokoyama, T.; Sugaya, T.; Kimura, K. Urinary L-type fatty acid binding protein (L-FABP) as a new urinary biomarker promulgated by the Ministry of Health, Labour and Welfare in Japan. Rinsho Byori 2013, 61, 635–640. [Google Scholar]
- Yang, J.J.; Wu, B.B.; Han, F.; Chen, J.H.; Yang, Y. Gene expression profiling of sepsis-associated acute kidney injury. Exp. Ther. Med. 2020, 20, 34. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Fan, Y.; Cheng, J.; Deng, Y.; Zhang, X.; Chen, Y.; Jing, H.; Li, W.; Liu, P.; Xie, J.; et al. AFM negatively regulates the infiltration of monocytes to mediate sepsis-associated acute kidney injury. Front. Immunol. 2023, 14, 1049536. [Google Scholar] [CrossRef]
- Liu, B.; Ao, S.; Tan, F.; Ma, W.; Liu, H.; Liang, H.; Yang, X.; Chi, X. Transcriptomic analysis and laboratory experiments reveal potential critical genes and regulatory mechanisms in sepsis-associated acute kidney injury. Ann. Transl. Med. 2022, 10, 737. [Google Scholar] [CrossRef]
- Ma, W.; Miao, X.; Xia, F.; Ruan, C.; Tao, D.; Li, B. The Potential of miR-370-3p and miR-495-3p Serving as Biomarkers for Sepsis-Associated Acute Kidney Injury. Comput. Math. Methods Med. 2022, 2022, 2439509. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Wu, J.; Chen, Z. Regulatory networks of circRNA- centred ceRNAs in sepsis-induced acute kidney injury. Epigenetics 2023, 18, 2278960. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, J.; Li, Y.; Shi, X.; Zhu, H.; Chen, L. Multidimensional Landscape of SA-AKI Revealed by Integrated Proteomics and Metabolomics Analysis. Biomolecules 2023, 13, 1329. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Cui, L. Multi-Omics Techniques Make it Possible to Analyze Sepsis-Associated Acute Kidney Injury Comprehensively. Front. Immunol. 2022, 13, 905601. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.Q.; Yan, P.; Duan, S.B.; Kang, Y.X.; Deng, Y.H.; Liu, Q.; Wu, T.; Wu, X. Development and Validation of Machine Learning Models for Real-Time Mortality Prediction in Critically Ill Patients With Sepsis-Associated Acute Kidney Injury. Front. Med. 2022, 9, 853102. [Google Scholar] [CrossRef]
- Li, X.; Wu, R.; Zhao, W.; Shi, R.; Zhu, Y.; Wang, Z.; Pan, H.; Wang, D. Machine learning algorithm to predict mortality in critically ill patients with sepsis-associated acute kidney injury. Sci. Rep. 2023, 13, 5223. [Google Scholar] [CrossRef]
- Yang, S.; Su, T.; Huang, L.; Feng, L.H.; Liao, T. A novel risk-predicted nomogram for sepsis associated-acute kidney injury among critically ill patients. BMC Nephrol. 2021, 22, 173. [Google Scholar] [CrossRef]
- White, K.C.; Serpa-Neto, A.; Hurford, R.; Clement, P.; Laupland, K.B.; See, E.; McCullough, J.; White, H.; Shekar, K.; Tabah, A.; et al. Sepsis-associated acute kidney injury in the intensive care unit: Incidence, patient characteristics, timing, trajectory, treatment, and associated outcomes. A multicenter, observational study. Intensive Care Med. 2023, 49, 1079–1089. [Google Scholar] [CrossRef]
- Eskesen, T.G.; Wetterslev, M.; Perner, A. Systematic review including re-analyses of 1148 individual data sets of central venous pressure as a predictor of fluid responsiveness. Intensive Care Med. 2016, 42, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Garzotto, F.; Ostermann, M.; Martín-Langerwerf, D.; Sánchez-Sánchez, M.; Teng, J.; Robert, R.; Marinho, A.; Herrera-Gutierrez, M.E.; Mao, H.J.; Benavente, D.; et al. The Dose Response Multicentre Investigation on Fluid Assessment (DoReMIFA) in critically ill patients. Crit. Care 2016, 20, 196. [Google Scholar] [CrossRef]
- Zampieri, F.G.; Bagshaw, S.M.; Semler, M.W. Fluid Therapy for Critically Ill Adults With Sepsis: A Review. JAMA 2023, 329, 1967–1980. [Google Scholar] [CrossRef]
- Sakr, Y.; Bauer, M.; Nierhaus, A.; Kluge, S.; Schumacher, U.; Putensen, C.; Fichtner, F.; Petros, S.; Scheer, C.; Jaschinski, U.; et al. Randomized controlled multicentre study of albumin replacement therapy in septic shock (ARISS): Protocol for a randomized controlled trial. Trials 2020, 21, 1002. [Google Scholar] [CrossRef]
- Lai, C.F.; Liu, J.H.; Tseng, L.J.; Tsao, C.H.; Chou, N.K.; Lin, S.L.; Chen, Y.M.; Wu, V.C. Unsupervised clustering identifies sub-phenotypes and reveals novel outcome predictors in patients with dialysis-requiring sepsis-associated acute kidney injury. Ann. Med. 2023, 55, 2197290, Correction in Ann. Med. 2024, 55, 2288985. [Google Scholar] [CrossRef]
- Peng, J.; Tang, R.; Yu, Q.; Wang, D.; Qi, D. No sex differences in the incidence, risk factors and clinical impact of acute kidney injury in critically ill patients with sepsis. Front. Immunol. 2022, 13, 895018. [Google Scholar] [CrossRef] [PubMed]
- Monard, C.; Bianchi, N.; Kelevina, T.; Altarelli, M.; Schneider, A. Epidemiology and outcomes of early versus late septic acute kidney injury in critically ill patients: A retrospective cohort study. Anaesth. Crit. Care Pain. Med. 2024, 43, 101332. [Google Scholar] [CrossRef]
- Wang, M.; Wang, X.; Zhu, B.; Li, W.; Jiang, Q.; Zuo, Y.; Wen, J.; He, Y.; Xi, X.; Jiang, L. The effects of timing onset and progression of AKI on the clinical outcomes in AKI patients with sepsis: A prospective multicenter cohort study. Ren. Fail. 2023, 45, 2138433. [Google Scholar] [CrossRef]
- Fan, Y.; Chen, L.; Jiang, S.; Huang, Y.; Leng, Y.; Gao, C. Timely renal replacement therapy linked to better outcome in patients with sepsis-associated acute kidney injury. J. Intensive Med. 2022, 2, 173–182. [Google Scholar] [CrossRef]
- Agapito Fonseca, J.; Gameiro, J.; Marques, F.; Lopes, J.A. Timing of Initiation of Renal Replacement Therapy in Sepsis-Associated Acute Kidney Injury. J. Clin. Med. 2020, 9, 1413. [Google Scholar] [CrossRef]
- Lee, W.Y.; Kim, H.J.; Kim, E.Y. Impact of polymyxin B hemoperfusion therapy on high endotoxin activity level patients after successful infection source control: A prospective cohort study. Sci. Rep. 2021, 11, 24132. [Google Scholar] [CrossRef] [PubMed]
- Iba, T.; Klein, D.J. The wind changed direction and the big river still flows: From EUPHRATES to TIGRIS. J. Intensive Care 2019, 7, 31. [Google Scholar] [CrossRef] [PubMed]
- Ronco, C.; Bellomo, R. Hemoperfusion: Technical aspects and state of the art. Crit. Care 2022, 26, 135. [Google Scholar] [CrossRef]
- König, C.; Röhr, A.C.; Frey, O.R.; Brinkmann, A.; Roberts, J.A.; Wichmann, D.; Braune, S.; Kluge, S.; Nierhaus, A. In vitro removal of anti-infective agents by a novel cytokine adsorbent system. Int. J. Artif. Organs 2019, 42, 57–64. [Google Scholar] [CrossRef]
- Rhee, H.; Jang, G.S.; Han, M.; Park, I.S.; Kim, I.Y.; Song, S.H.; Seong, E.Y.; Lee, D.W.; Lee, S.B.; Kwak, I.S. The role of the specialized team in the operation of continuous renal replacement therapy: A single-center experience. BMC Nephrol. 2017, 18, 332. [Google Scholar] [CrossRef]
- Joannes-Boyau, O.; Velly, L.; Ichai, C. Optimizing continuous renal replacement therapy in the ICU: A team strategy. Curr. Opin. Crit. Care 2018, 24, 476–482. [Google Scholar] [CrossRef]
- Neyra, J.A.; Goldstein, S.L. Optimizing renal replacement therapy deliverables through multidisciplinary work in the intensive care unit. Clin. Nephrol. 2018, 90, 1–5. [Google Scholar] [CrossRef]
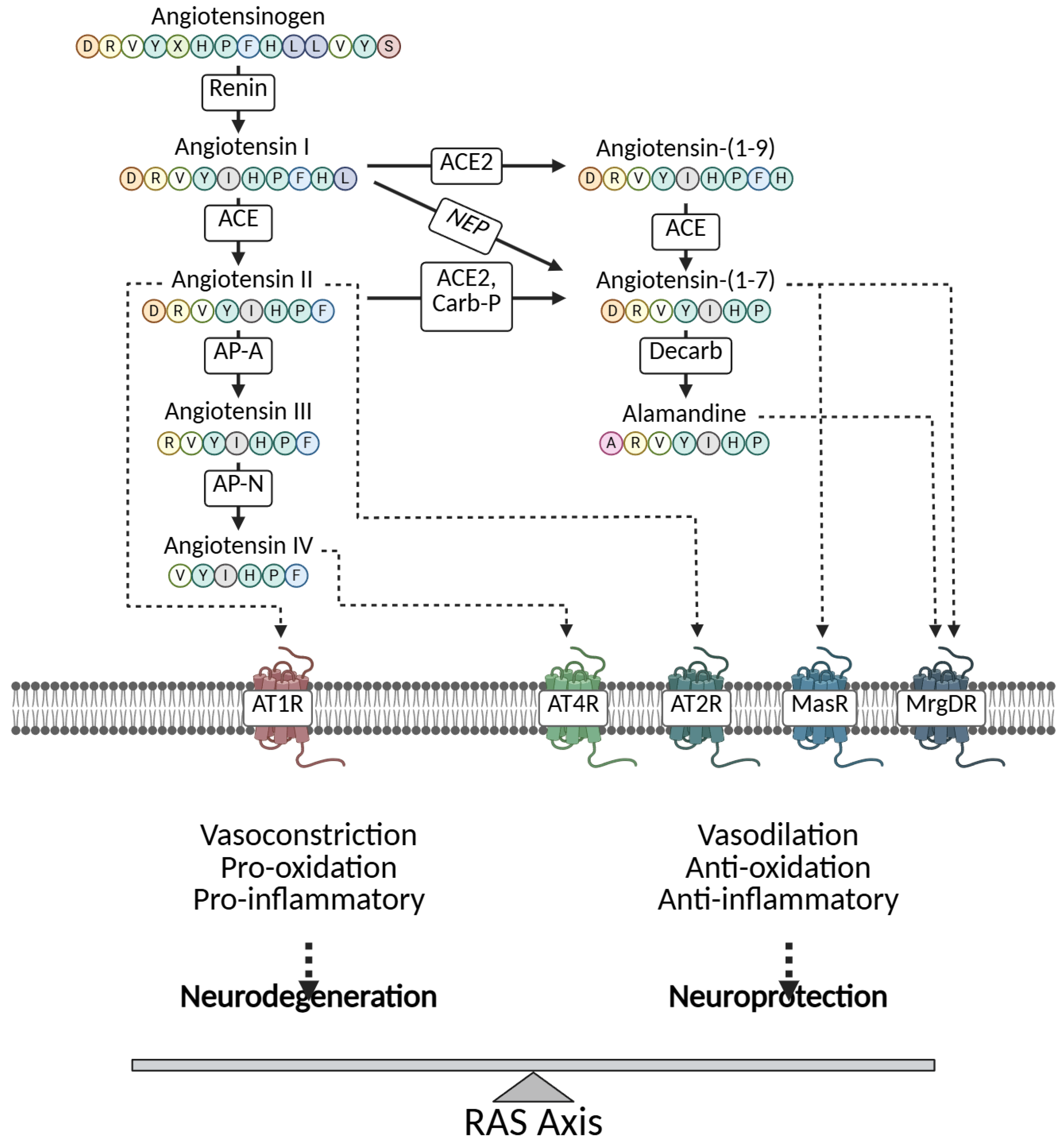
- Garcia, B.; Zarbock, A.; Bellomo, R.; Legrand, M. The role of renin-angiotensin system in sepsis-associated acute kidney injury: Mechanisms and therapeutic implications. Curr. Opin. Crit. Care 2023, 29, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Garcia, B.; Zarbock, A.; Bellomo, R.; Legrand, M. The alternative renin-angiotensin system in critically ill patients: Pathophysiology and therapeutic implications. Crit. Care 2023, 27, 453. [Google Scholar] [CrossRef] [PubMed]
- Leisman, D.E.; Fernandes, T.D.; Bijol, V.; Abraham, M.N.; Lehman, J.R.; Taylor, M.D.; Capone, C.; Yaipan, O.; Bellomo, R.; Deutschman, C.S. Impaired angiotensin II type 1 receptor signaling contributes to sepsis-induced acute kidney injury. Kidney Int. 2021, 99, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Dai, X.; Li, Y.; Li, G.; Lin, X.; Ai, C.; Cao, Y.; Li, T.; Lin, B. Role of Parkin-mediated mitophagy in the protective effect of polydatin in sepsis-induced acute kidney injury. J. Transl. Med. 2020, 18, 114. [Google Scholar] [CrossRef]
- Chen, Z.D.; Hu, B.C.; Shao, X.P.; Hong, J.; Zheng, Y.; Zhang, R.; Shao, Z.Q.; Liu, J.Q.; Yang, X.H.; Sun, R.H.; et al. Ascorbate uptake enables tubular mitophagy to prevent septic AKI by PINK1-PARK2 axis. Biochem. Biophys. Res. Commun. 2021, 554, 158–165. [Google Scholar] [CrossRef]
- Jia, J.; Gong, X.; Zhao, Y.; Yang, Z.; Ji, K.; Luan, T.; Zang, B.; Li, G. Autophagy Enhancing Contributes to the Organ Protective Effect of Alpha-Lipoic Acid in Septic Rats. Front. Immunol. 2019, 10, 1491. [Google Scholar] [CrossRef]
- Guo, G.; Wang, Y.; Kou, W.; Gan, H. Identifying the molecular mechanisms of sepsis-associated acute kidney injury and predicting potential drugs. Front. Genet. 2022, 13, 1062293. [Google Scholar] [CrossRef]
- Cheng, Y.C.; Chen, C.A.; Chang, J.M.; Chen, H.C. Albumin overload down-regulates integrin-β1 through reactive oxygen species-endoplasmic reticulum stress pathway in podocytes. J. Biochem. 2015, 158, 101–108. [Google Scholar] [CrossRef]
- Charlson Comorbidity Index Calculator. Available online: http://omnicalculator.com (accessed on 16 January 2024).
- Elias, E.E.; Lyons, B.; Muruve, D.A. Gasdermins and pyroptosis in the kidney. Nat. Rev. Nephrol. 2023, 19, 337–350. [Google Scholar] [CrossRef]
- Howell, G.M.; Gomez, H.; Collage, R.D.; Loughran, P.; Zhang, X.; Escobar, D.A.; Billiar, T.R.; Zuckerbraun, B.S.; Rosengart, M.R. Augmenting autophagy to treat acute kidney injury during endotoxemia in mice. PLoS ONE 2013, 8, e69520. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Hao, Y.; Tang, S.; Han, X.; Li, R.; Zhou, X. Energy metabolic reprogramming regulates programmed cell death of renal tubular epithelial cells and might serve as a new therapeutic target for acute kidney injury. Front. Cell Dev. Biol. 2023, 11, 1276217. [Google Scholar] [CrossRef]
- Bayır, H.; Dixon, S.J.; Tyurina, Y.Y.; Kellum, J.A.; Kagan, V.E. Ferroptotic mechanisms and therapeutic targeting of iron metabolism and lipid peroxidation in the kidney. Nat. Rev. Nephrol. 2023, 19, 315–336. [Google Scholar] [CrossRef]
- Ji, Y.-M.; Li, T.; Qin, Y.-H.; Xiao, S.-Y.; Lv, Y.-H.; Dong, Y.; Cui, X.-R.; Hu, Y. Neutrophil Extracellular Traps (NETs) in Sterile Inflammatory Diseases. J. Inflamm. Res. 2025, ume 18, 7989–8004. [Google Scholar] [CrossRef]
- Sunahara, S.; Watanabe, E.; Hatano, M.; Swanson, P.E.; Oami, T.; Fujimura, L.; Teratake, Y.; Shimazui, T.; Lee, C.; Oda, S. Influence of autophagy on acute kidney injury in a murine cecal ligation and puncture sepsis model. Sci. Rep. 2018, 8, 1050. [Google Scholar] [CrossRef]
- Yang, T.; Feng, X.; Zhao, Y.; Zhang, H.; Cui, H.; Wei, M.; Yang, H.; Fan, H. Dexmedetomidine Enhances Autophagy via α2-AR/AMPK/mTOR Pathway to Inhibit the Activation of NLRP3 Inflammasome and Subsequently Alleviates Lipopolysaccharide-Induced Acute Kidney Injury. Front. Pharmacol. 2020, 11, 790. [Google Scholar] [CrossRef]
- Zhao, Y.; Feng, X.; Li, B.; Sha, J.; Wang, C.; Yang, T.; Cui, H.; Fan, H. Dexmedetomidine Protects Against Lipopolysaccharide-Induced Acute Kidney Injury by Enhancing Autophagy Through Inhibition of the PI3K/AKT/mTOR Pathway. Front. Pharmacol. 2020, 11, 128. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wang, R.; Liu, D. Bone Marrow-Derived Mesenchymal Stem Cells Ameliorate Sepsis-Induced Acute Kidney Injury by Promoting Mitophagy of Renal Tubular Epithelial Cells via the SIRT1/Parkin Axis. Front. Endocrinol. 2021, 12, 639165. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Gu, J.; Li, T.; Chen, H.; Liu, K.; Liu, M.; Zhang, H.; Xiao, X. Inhibition of aerobic glycolysis alleviates sepsis-induced acute kidney injury by promoting lactate/Sirtuin 3/AMPK-regulated autophagy. Int. J. Mol. Med. 2021, 47, 4852. [Google Scholar] [CrossRef]
- Li, T.; Zhao, J.; Miao, S.; Chen, Y.; Xu, Y.; Liu, Y. Protective effect of H(2)S on LPS-induced AKI by promoting autophagy. Mol. Med. Rep. 2022, 25, 12612. [Google Scholar] [CrossRef]
- Li, T.; Ji, X.; Liu, J.; Guo, X.; Pang, R.; Zhuang, H.; Dong, L.; Duan, M.; Li, A. Ulinastatin Improves Renal Microcirculation by Protecting Endothelial Cells and Inhibiting Autophagy in a Septic Rat Model. Kidney Blood Press. Res. 2022, 47, 256–269. [Google Scholar] [CrossRef]
- Yu, Y.Y.; Li, X.Q.; Hu, W.P.; Cu, S.C.; Dai, J.J.; Gao, Y.N.; Zhang, Y.T.; Bai, X.Y.; Shi, D.Y. Self-developed NF-κB inhibitor 270 protects against LPS-induced acute kidney injury and lung injury through improving inflammation. Biomed. Pharmacother. 2022, 147, 112615. [Google Scholar] [CrossRef]
- Luo, C.; Luo, F.; Che, L.; Zhang, H.; Zhao, L.; Zhang, W.; Man, X.; Bu, Q.; Luan, H.; Zhou, B.; et al. Mesenchymal stem cells protect against sepsis-associated acute kidney injury by inducing Gal-9/Tim-3 to remodel immune homeostasis. Ren. Fail. 2023, 45, 2187229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Rao, J.; Liu, X.; Wang, X.; Wang, C.; Fu, S.; Xiao, J. Attenuation of Sepsis-Induced Acute Kidney Injury by Exogenous H(2)S via Inhibition of Ferroptosis. Molecules 2023, 28, 4770. [Google Scholar] [CrossRef]
- Luo, C.; Luo, F.; Man, X.; Liu, X.; Zhao, L.; Che, L.; Zhang, W.; Guo, J.; Cai, S.; Wang, D.; et al. Mesenchymal Stem Cells Attenuate Sepsis-associated Acute Kidney Injury by Changing the Balance of Th17 cells/Tregs via Gal-9/Tim-3. Curr. Stem Cell Res. Ther. 2023, 18, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xu, J.; Tu, J.; Sun, Y.; Zhang, C.; Qiu, Z.; Xiao, H. Polygonum cuspidatum Sieb. et Zucc. Extracts improve sepsis-associated acute kidney injury by inhibiting NF-κB-mediated inflammation and pyroptosis. J. Ethnopharmacol. 2024, 319, 117101. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, A.; Kazi, F. Updates in sepsis management. Lancet Infect. Dis. 2022, 22, 24. [Google Scholar] [CrossRef]
- Cecconi, M.; Evans, L.; Levy, M.; Rhodes, A. Sepsis and septic shock. Lancet 2018, 392, 75–87. [Google Scholar] [CrossRef]
- Patel, S.; Puri, N.; Dellinger, R.P. Sepsis management for the nephrologist. Clin. J. Am. Soc. Nephrol. 2022, 17, 880–889. [Google Scholar] [CrossRef]
- Donaldson, L.H.; Vlok, R.; Sakurai, K.; Burrows, M.; McDonald, G.; Venkatesh, K.; Bagshaw, S.M.; Bellomo, R.; Delaney, A.; Myburgh, J.; et al. Quantifying the impact of alternative definitions of sepsis-associated acute kidney injury on its incidence and outcomes: A systematic review and meta-analysis. Crit. Care Med. 2024, 52, 1264–1274. [Google Scholar] [CrossRef]
- Stenson, E.K.; Kendrick, J.; Dixon, B.; Thurman, J.M. The complement system in pediatric acute kidney injury. Pediatr. Nephrol. 2023, 38, 1411–1425. [Google Scholar] [CrossRef] [PubMed]
- Poston, J.T.; Koyner, J.L. Sepsis associated acute kidney injury. BMJ 2019, 364, k4891. [Google Scholar] [CrossRef] [PubMed]
- Legrand, M.; Bagshaw, S.M.; Bhatraju, P.K.; Bihorac, A.; Caniglia, E.; Khanna, A.K.; Kellum, J.A.; Koyner, J.; Harhay, M.O.; Zampieri, F.G.; et al. Sepsis-associated acute kidney injury: Recent advances in enrichment strategies, sub-phenotyping and clinical trials. Crit. Care 2024, 28, 92. [Google Scholar] [CrossRef]
- Sun, Y.; Yuan, Z.; Li, W.; Lin, X.; Yang, C.; Yang, Y.; Miao, H.; Wang, S.; Cui, Z.; Bao, Z.; et al. Activation of the cGAS-STING pathway in monocytes exacerbates pulmonary fibrosis induced by paraquat poisoning. Toxicol. Appl. Pharmacol. 2025, 504, 117502. [Google Scholar] [CrossRef] [PubMed]
- Kounatidis, D.; Vallianou, N.G.; Psallida, S.; Panagopoulos, F.; Margellou, E.; Tsilingiris, D.; Karampela, I.; Stratigou, T.; Dalamaga, M. Sepsis-associated acute kidney injury: Where are we now? Medicina 2024, 60, 434. [Google Scholar] [CrossRef] [PubMed]
- Walle, L.V.; Lamkanfi, M. Pyroptosis. Curr. Biol. 2016, 26, R568–R572. [Google Scholar] [CrossRef]
- Bergsbaken, T.; Fink, S.L.; Cookson, B.T. Pyroptosis: Host cell death and inflammation. Nat. Rev. Microbiol. 2009, 7, 99–109. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Yang, A.; Xia, F. Downregulation of IRF2 alleviates sepsis-related acute kidney injury in vitro and in vivo. Drug Design Dev. Therapy 2021, 15, 5123–5132. [Google Scholar] [CrossRef]
- Broz, P. Caspase target drives pyroptosis. Nature 2015, 526, 642–643. [Google Scholar] [CrossRef] [PubMed]
- Bertheloot, D.; Latz, E.; Franklin, B.S. Necroptosis, pyroptosis and apoptosis: An intricate game of cell death. Cell. Mol. Immunol. 2021, 18, 1106–1121. [Google Scholar] [CrossRef]
- Yu, P.; Zhang, X.; Liu, N.; Tang, L.; Peng, C.; Chen, X. Pyroptosis: Mechanisms and diseases. Signal Transduct. Target. Ther. 2021, 6, 128. [Google Scholar] [CrossRef]
- Burdette, B.E.; Esparza, A.N.; Zhu, H.; Wang, S. Gasdermin D in pyroptosis. Acta Pharm. Sin. B 2021, 11, 2768–2782. [Google Scholar] [CrossRef]
- Lieberman, J.; Wu, H.; Kagan, J.C. Gasdermin D Activity in inflammation and host defense. Sci. Immunol. 2019, 4, eaav1447. [Google Scholar] [CrossRef]
- Man, S.M.; Kanneganti T-DGasdermin, D. The long-awaited executioner of pyroptosis. Cell Res. 2015, 25, 1183–1184. [Google Scholar] [CrossRef]
- Stanski, N.L.; Stenson, E.K.; Cvijanovich, N.Z.; Weiss, S.L.; Fitzgerald, J.C.; Bigham, M.T.; Jain, P.N.; Schwarz, A.; Lutfi, R.; Nowak, J.; et al. PERSEVERE Biomarkers Predict Severe Acute Kidney Injury and Renal Recovery in Pediatric Septic Shock. Am. J. Respir. Crit. Care Med. 2020, 201, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Meena, J.; Thomas, C.C.; Kumar, J.; Mathew, G.; Bagga, A. Biomarkers for prediction of acute kidney injury in pediatric patients: A systematic review and meta-analysis of diagnostic test accuracy studies. Pediatr. Nephrol. 2023, 38, 3241–3251. [Google Scholar] [CrossRef]
- Xie, Y.; Huang, P.; Zhang, J.; Tian, R.; Jin, W.; Xie, H.; Du, J.; Zhou, Z.; Wang, R. Biomarkers for the diagnosis of sepsis-associated acute kidney injury: Systematic review and meta-analysis. Ann. Palliat. Med. 2021, 10, 4159–4173. [Google Scholar] [CrossRef]
- Al-Ismaili, Z.; Palijan, A.; Zappitelli, M. Biomarkers of acute kidney injury in children: Discovery, evaluation, and clinical application. Pediatr. Nephrol. 2011, 26, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Odum, J.D.; Wong, H.R.; Stanski, N.L. A Precision Medicine Approach to Biomarker Utilization in Pediatric Sepsis-Associated Acute Kidney Injury. Front. Pediatr. 2021, 9, 632248. [Google Scholar] [CrossRef]
- Bazargani, B.; Moghtaderi, M. New Biomarkers in Early Diagnosis of Acute Kidney Injury in Children. Avicenna J. Med. Biotechnol. 2022, 14, 264–269. [Google Scholar] [CrossRef]
- Saleh, N.Y.; Abo El Fotoh, W.M.M.; El-Hawy, M.A. Serum Neutrophil Gelatinase-Associated Lipocalin: A Diagnostic Marker in Pediatric Sepsis. Pediatr. Crit. Care Med. 2017, 18, e245–e252. [Google Scholar] [CrossRef]
- Deng, L.-T.; Wang, Q.-L.; Yu, C.; Gao, M. lncRNA PVT1 modulates NLRP3-mediated pyroptosis in septic acute kidney injury by targeting miR-20a-5p. Mol. Med. Rep. 2021, 23, 1. [Google Scholar] [CrossRef]
- Li, C.; Wang, W.; Xie, S.-S.; Ma, W.-X.; Fan, Q.-W.; Chen, Y.; He, Y.; Wang, J.-N.; Yang, Q.; Li, H.-D. The programmed cell death of macrophages, endothelial cells, and tubular epithelial cells in sepsis-AKI. Front. Med. 2021, 8, 796724. [Google Scholar] [CrossRef]
- DeWolf, S.E.; Kasimsetty, S.G.; Hawkes, A.A.; Stocks, L.M.; Kurian, S.M.; McKay, D.B. DAMPs released from injured renal tubular epithelial cells activate innate immune signals in healthy renal tubular epithelial cells. Transplantation 2022, 106, 1589–1599. [Google Scholar] [CrossRef]
- Nadella, V.; Kanneganti, T.D. Inflammasomes and their role in PANoptosomes. Curr. Opin. Immunol. 2024, 91, 102489. [Google Scholar] [CrossRef] [PubMed]
- Pandeya, A.; Kanneganti, T.D. Therapeutic potential of PANoptosis: Innate sensors, inflammasomes, and RIPKs in PANoptosomes. Trends Mol. Med. 2024, 30, 74–88. [Google Scholar] [CrossRef]
- Yang, M.; Shen, P.; Xu, L.; Kong, M.; Yu, C.; Shi, Y. Theacrine alleviates sepsis-induced acute kidney injury by repressing the activation of NLRP3/Caspase-1 inflammasome. PeerJ 2022, 10, e14109. [Google Scholar] [CrossRef] [PubMed]
- Hamie, L.; Daoud, G.; Nemer, G.; Nammour, T.; El Chediak, A.; Uthman, I.W.; Kibbi, A.G.; Eid, A.; Kurban, M. SuPAR, an emerging biomarker in kidney and inflammatory diseases. Postgrad. Med. J. 2018, 94, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Duan, H.; Fu, Y.; Cheng, Z. Renal tissue damage induced by acute kidney injury in sepsis rat model is inhibited by cynaropicrin via IL-1β and TNF-α down-regulation. Dokl. Biochem. Biophys. 2021, 497, 151–157. [Google Scholar] [CrossRef]
- Xu, D.; Jiang, J.; Liu, Y.; Pang, J.; Suo, J.; Li, Y.; Peng, Z. TIMP2 protects against sepsis-associated acute kidney injury by cAMP/NLRP3 axis-mediated pyroptosis. Am. J. Physiol. Cell Physiol. 2024, 326, C1353–C1366. [Google Scholar] [CrossRef]
- Lu, J.; Hou, Y.; Liu, S.X.; Jin, B.; Liu, J.; Li, N.; Zhu, Y.; Zhang, Q.Y.; Wan, C.; Feng, Y.; et al. Acetyl-CoA synthetase 2 induces pyroptosis and inflammation of renal epithelial tubular cells in sepsis-induced acute kidney injury by upregulating the KLF5/NF-κB pathway. Cell Commun. Signal. 2024, 22, 187. [Google Scholar] [CrossRef]
- Xu, J.; Chen, J.; Li, M.; Jiang, Z.; Fang, F.; Hu, J.; Zhou, Y.; Li, H.; Bai, Z.; Li, X.; et al. Utility of plasma suPAR to identify AKI and sepsis associated AKI in critically ill children. iScience 2024, 27, 111247. [Google Scholar] [CrossRef]
- Ye, Z.; Zhang, L.; Li, R.; Dong, W.; Liu, S.; Li, Z.; Liang, H.; Wang, L.; Shi, W.; Malik, A.B.; et al. Caspase-11 mediates pyroptosis of tubular epithelial cells and septic acute kidney injury. Kidney Blood Press. Res. 2019, 44, 465–478. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; Wang, Y. Clinical characteristics and prognosis of acute kidney injury in elderly patients with sepsis. Chin. Crit. Care Med. 2019, 31, 837–841. [Google Scholar] [CrossRef]
- Tilley, S.L.; Coffman, T.M.; Koller, B.H. Mixed messages: Modulation of inflammation and immune responses by prostaglandins and thromboxanes. J. Clin. Investig. 2001, 108, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Zwiers, A.J.; de Wildt, S.N.; van Rosmalen, J.; de Rijke, Y.B.; Buijs, E.A.; Tibboel, D.; Cransberg, K. Urinary neutrophil gelatinase-associated lipocalin identifies critically ill young children with acute kidney injury following intensive care admission: A prospective cohort study. Crit. Care 2015, 19, 181. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Ge, X.; Wang, Y.; Niu, L.; Tang, L.; Pan, S. USF2 knockdown downregulates THBS1 to inhibit the TGF-β signaling pathway and reduce pyroptosis in sepsis-induced acute kidney injury. Pharmacol. Res. 2022, 176, 105962. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yao, L.; Zeng, X.; Hu, B.; Zhang, X.; Wang, J.; Zhu, R.; Yu, Q. miR-30c-5p alleviated pyroptosis during sepsis-induced acute kidney injury via targeting TXNIP. Inflammation 2021, 44, 217–228. [Google Scholar] [CrossRef]
- Sun, M.; Wang, F.; Li, H.; Li, M.; Wang, Y.; Wang, C.; Zhang, Y.; Zhang, D.; Li, J.; Yao, S. Maresin-1 attenuates sepsis-associated acute kidney injury via suppressing inflammation, endoplasmic reticulum stress and pyroptosis by activating the AMPK/SIRT3 pathway. J. Inflamm. Res. 2024, 17, 1349–1364. [Google Scholar] [CrossRef]
- Guo, J.; Yuan, Z.; Wang, R. Zn(2+) improves sepsis-induced acute kidney injury by upregulating SIRT7-mediated Parkin acetylation. Am. J. Physiol. Renal. Physiol. 2024, 327, F184–F197. [Google Scholar] [CrossRef]
- Zhang, J.N.; Gong, R.; Wang, Y.Q.; Chong, Y.; Gu, Q.K.; Zhao, M.B.; Huang, P.; Qi, Y.C.; Meng, X.L.; Zhao, M.Y. Critical role of S100A9 in sepsis-associated acute kidney injury: Mechanistic insights through pyroptosis pathway modulation. Inflammation 2024, 48, 1879–1899. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, C.; Zhao, W.; Tian, X.; Liu, P.; Wei, L.; Zhu, Z.; Liu, M.; Fu, R.; Jia, L. WIP1-mediated regulation of p38 MAPK signaling attenuates pyroptosis in sepsis-associated acute kidney injury. Immunobiology 2024, 229, 152832. [Google Scholar] [CrossRef]
- Zhang, M.; Zhi, D.; Lin, J.; Liu, P.; Wang, Y.; Duan, M. miR-181a-5p inhibits pyroptosis in sepsis-induced acute kidney injury through downregulation of NEK7. J. Immunol. Res. 2022, 2022, 1825490. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Chen, X.; Zhu, Z.; Luo, Z.; Hao, Y.; Yang, X.; Feng, J.; Zhang, Z.; Hu, J.; Jian, Y.; et al. STING contributes to lipopolysaccharide-induced tubular cell inflammation and pyroptosis by activating endoplasmic reticulum stress in acute kidney injury. Cell Death Dis. 2024, 15, 217. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Huang, H.; Alam, A.; Chen, Q.; Suen, K.C.; Cui, J.; Sun, Q.; Ologunde, R.; Zhang, W.; Lian, Q.; et al. VEGF mitigates histone-induced pyroptosis in the remote liver injury associated with renal allograft ischemia-reperfusion injury in rats. Am. J. Transplant. 2018, 18, 1890–1903. [Google Scholar] [CrossRef] [PubMed]
- Atreya, M.R.; Wong, H.R. Precision medicine in pediatric sepsis. Curr. Opin. Pediatr. 2019, 31, 322–327. [Google Scholar] [CrossRef]
- Rathinam, V.A.K.; Zhao, Y.; Shao, F. Innate immunity to intracellular LPS. Nat. Immunol. 2019, 20, 527–533. [Google Scholar] [CrossRef]
- Barnett, K.C.; Li, S.; Liang, K.; Ting, J.P. A 360° view of the inflammasome: Mechanisms of activation, cell death, and diseases. Cell 2023, 186, 2288–2312. [Google Scholar] [CrossRef]
- Fu, J.; Schroder, K.; Wu, H. Mechanistic insights from inflammasome structures. Nat. Rev. Immunol. 2024, 24, 518–535. [Google Scholar] [CrossRef]
- Dubyak, G.R.; Miller, B.A.; Pearlman, E. Pyroptosis in neutrophils: Multimodal integration of inflammasome and regulated cell death signaling pathways. Immunol. Rev. 2023, 314, 229–249. [Google Scholar] [CrossRef]
- Shi, X.; Sun, Q.; Hou, Y.; Zeng, H.; Cao, Y.; Dong, M.; Ding, J.; Shao, F. Recognition and maturation of IL-18 by caspase-4 noncanonical inflammasome. Nature 2023, 624, 442–450. [Google Scholar] [CrossRef]
- Cui, X.; Li, Y.; Yuan, S.; Huang, Y.; Chen, X.; Han, Y.; Liu, Z.; Li, Z.; Xiao, Y.; Wang, Y.; et al. Alpha-kinase1 promotes tubular injury and interstitial inflammation in diabetic nephropathy by canonical pyroptosis pathway. Biol. Res. 2023, 56, 5. [Google Scholar] [CrossRef] [PubMed]
- You, R.; He, X.; Zeng, Z.; Zhan, Y.; Xiao, Y.; Xiao, R. Pyroptosis and its role in autoimmune disease: A potential therapeutic target. Front. Immunol. 2022, 13, 841732. [Google Scholar] [CrossRef]
- Sethi, S.K.; Bansal, S.B.; Khare, A.; Dhaliwal, M.; Wadhwani, N.; Nandwani, A.; Yadav, D.K.; Mahapatra, A.K.; Raina, R.; Raghunathan, V. Heparin free dialysis in critically sick children using sustained low efficiency dialysis (SLEDD-f): A new hybrid therapy for dialysis in developing world. PLoS ONE 2018, 13, e0195536. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Gao, W.; Shao, F. Pyroptosis: Gasdermin-mediated programmed necrotic cell death. Trends Biochem. Sci. 2017, 42, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.J.; He, L.Y.; Pan, S.Y.; Cheng, R.J.; Zhang, Q.P.; Liu, Y. Disease Severity Determines Timing of Initiating Continuous Renal Replacement Therapies: A Systematic Review and Meta-Analysis. Front. Med. 2021, 8, 580144. [Google Scholar] [CrossRef]
- Silva, C.M.S.; Wanderley, C.W.S.; Veras, F.P.; Sonego, F.; Nascimento, D.C.; Gonçalves, A.V.; Martins, T.V.; Cólon, D.F.; Borges, V.F.; Brauer, V.S.; et al. Gasdermin D inhibition prevents multiple organ dysfunction during sepsis by blocking NET formation. Blood 2021, 138, 2702–2713. [Google Scholar] [CrossRef]
- Zhai, B.; Ma, L.S.; Shen, R.Q.; Yu, J.; Tao, Y.N.; Xu, A.P.; Shao, D.C. Caspase-1/-11 participates in LPS-induced sepsis-associated acute kidney injury by cleaving GSDMD. Sheng Li Xue Bao 2023, 75, 10–16. [Google Scholar]
- Chancharoenthana, W.; Tiranathanagul, K.; Srisawat, N.; Susantitaphong, P.; Leelahavanichkul, A.; Praditpornsilpa, K.; Tungsanga, K.; Eiam-Ong, S. Enhanced Vascular Endothelial Growth Factor and Inflammatory Cytokine Removal with Online Hemodiafiltration Over High-Flux Hemodialysis in Sepsis-Related Acute Kidney Injury Patients. Ther. Apher. Dial. 2013, 17, 557–563. [Google Scholar] [CrossRef]
- Deng, J.; Tan, W.; Luo, Q.; Lin, L.; Zheng, L.; Yang, J. Long Non-coding RNA MEG3 promotes renal tubular epithelial cell pyroptosis by regulating the miR-18a-3p/GSDMD pathway in lipopolysaccharide-induced acute kidney injury. Front. Physiol. 2021, 12, 663216. [Google Scholar] [CrossRef]
- Lian, N.; Chen, Y.; Chen, S.; Zhang, Y.; Chen, H.; Yang, Y.; Gu, H.; Chen, Q.; Li, M.; Chen, X. Gasdermin D-mediated keratinocyte pyroptosis as a key step in psoriasis pathogenesis. Cell Death Dis. 2023, 14, 595. [Google Scholar] [CrossRef]
- Sanz, A.B.; Sanchez-Niño, M.D.; Ramos, A.M.; Ortiz, A. Regulated cell death pathways in kidney disease. Nat. Rev. Nephrol. 2023, 19, 281–299. [Google Scholar] [CrossRef]
- Fujimura, K.; Karasawa, T.; Komada, T.; Yamada, N.; Mizushina, Y.; Baatarjav, C.; Matsumura, T.; Otsu, K.; Takeda, N.; Mizukami, H.; et al. NLRP3 inflammasome-driven IL-1β and IL-18 contribute to lipopolysaccharide-induced septic cardiomyopathy. J. Mol. Cell. Cardiol. 2023, 180, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Hong, Z.; Huang, L.S.; Tsukasaki, Y.; Nepal, S.; Di, A.; Zhong, M.; Wu, W.; Ye, Z.; Gao, X.; et al. IL-1β suppression of VE-cadherin transcription underlies sepsis-induced inflammatory lung injury. J. Clin. Investig. 2020, 130, 3684–3698, Erratum in J. Clin. Investig. 2023, 133, e169500. [Google Scholar] [CrossRef]
- Wynn, J.L.; Wilson, C.S.; Hawiger, J.; Scumpia, P.O.; Marshall, A.F.; Liu, J.H.; Zharkikh, I.; Wong, H.R.; Lahni, P.; Benjamin, J.T.; et al. Targeting IL-17A attenuates neonatal sepsis mortality induced by IL-18. Proc. Natl. Acad. Sci. USA 2016, 113, E2627–E2635. [Google Scholar] [CrossRef]
- Exconde, P.M.; Hernandez-Chavez, C.; Bourne, C.M.; Richards, R.M.; Bray, M.B.; Lopez, J.L.; Srivastava, T.; Egan, M.S.; Zhang, J.; Yoo, W. The tetrapeptide sequence of IL-18 and IL-1β regulates their recruitment and activation by inflammatory caspases. Cell Rep. 2023, 42, 12113581. [Google Scholar] [CrossRef] [PubMed]
- He, W.T.; Wan, H.; Hu, L.; Chen, P.; Wang, X.; Huang, Z.; Yang, Z.H.; Zhong, C.Q.; Han, J. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res. 2015, 25, 1285–1298. [Google Scholar] [CrossRef] [PubMed]

| Category | Risk Factor | Description | References |
|---|---|---|---|
| Patient-Specific | Older Age (Adolescents) | Higher susceptibility due to physiological changes; OR > 1.5 | [50] |
| Lower Baseline eGFR | Indicates reduced renal reserve; an independent predictor | [50] | |
| Obesity | Increases early SA-AKI risk via inflammation | [55] | |
| Sepsis-Related | Septic Shock | Hypoperfusion and cytokine storm; most common trigger | [49] |
| Infection/Sepsis Severity | Direct endothelial damage; 46.5% of AKI cases | [39,40] | |
| Iatrogenic | Mechanical Ventilation | Alters renal hemodynamics; risk in 76.9% | [51] |
| Nephrotoxic Drugs | Antibiotics/vasoactives; 45–46.8% attribution | [52,53] | |
| Hypovolemic Shock | Volume depletion is common in dehydration | [54] | |
| Underlying Conditions | Hypertension/Cardiac Disease | Vascular instability amplifies risks | [54] |
| Glomerulonephritis | Inflammatory overlap; interstitial damage | [54] |
| Pathway | Mechanism | Evidence | Pediatric Relevance | References |
|---|---|---|---|---|
| Inflammatory (TLR/NF-κB) | PAMP recognition, cytokine transcription | Elevated IL-6/TNF-α in septic children | High in neonates due to immune immaturity | [72,73,74,75,76,77,78,79,80,81,82] |
| Apoptosis/Necrosis/Pyroptosis | Bcl-2 MOMP, RIPK3 necroptosis, NLRP3 GSDMD | OLFM4 promotes apoptosis; pyroptosis via NF-κB | Reduced in OLFM4-null models | [83,84,85,86,87,88,89,90,91,92,93,94,95,96,97] |
| Metabolic (Choline/Nrf2/Mitophagy) | Energy disruption, antioxidant activation, mitochondrial clearance | Choline supplementation attenuates injury | Metabolic shifts in pediatric sepsis | [98,99,100,101,102,103,104,105,106] |
| Immune (Complement/PTMs) | C3/C5 activation, phosphorylation/ubiquitination | Urinary complement correlates with severity | Complement inhibitors for children | [39,107,108,109,110,111,112,113,114,115] |
| Emerging (cGAS-STING/TNF-α/STING-PERK) | DNA sensing, fibrosis, ER stress | STING inhibition reduces inflammation | Potential for pediatric fibrosis prevention | [116,117,118,119,120,121,122,123,124,125,126,127] |
| Biomarker | Type | Sensitivity (%) | Specificity (%) | Clinical Utility | Recent Studies |
|---|---|---|---|---|---|
| NGAL | Urinary | 70–85 | 65–80 | Early detection, AKI severity | [128,129,130] |
| KIM-1 | Urinary | 65–80 | 60–75 | Tubular injury, hospital stay | [131,132] |
| DKK3 | Urinary | 60–75 | 80–90 | AKI progression, severe AKI | [133,134] |
| OLFM4 | Urinary | 55–70 | 65–80 | Apoptosis, sepsis severity | [79,88] |
| ApoA5 | Blood | 60–75 | 60–70 | Microvascular dysfunction | [135,136] |
| Humanin | Blood | 65–80 | 60–75 | Mitochondrial stress | [137,138] |
| Alanine | Blood | 55–70 | 55–65 | Metabolic reprogramming | [139] |
| suPAR | Blood | 75–85 | 85–92 | Immune Activation, AKI specificity | [140,141] |
| Penkid | Blood | 80–88 | 75–85 | Early AKI, neonatal diagnosis | [142,143] |
| PERSEVERE-II | Risk Model | 70–85 | 80–90 | Risk stratification, mortality | [144,145,146,147] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Sheng, M.; Bao, Y.; Tang, C. Research Progress on Signalling Pathways Related to Sepsis-Associated Acute Kidney Injury in Children. Curr. Issues Mol. Biol. 2025, 47, 888. https://doi.org/10.3390/cimb47110888
Zhang Z, Sheng M, Bao Y, Tang C. Research Progress on Signalling Pathways Related to Sepsis-Associated Acute Kidney Injury in Children. Current Issues in Molecular Biology. 2025; 47(11):888. https://doi.org/10.3390/cimb47110888
Chicago/Turabian StyleZhang, Zhenkun, Meijun Sheng, Yiyao Bao, and Chao Tang. 2025. "Research Progress on Signalling Pathways Related to Sepsis-Associated Acute Kidney Injury in Children" Current Issues in Molecular Biology 47, no. 11: 888. https://doi.org/10.3390/cimb47110888
APA StyleZhang, Z., Sheng, M., Bao, Y., & Tang, C. (2025). Research Progress on Signalling Pathways Related to Sepsis-Associated Acute Kidney Injury in Children. Current Issues in Molecular Biology, 47(11), 888. https://doi.org/10.3390/cimb47110888





