Development and Evaluation of Six Novel Recombinant GRA Proteins in Serodiagnosis of Human Toxoplasmosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Serum Samples
2.2. Construction of Recombinant Plasmids
2.3. Expression and Purification of Recombinant Proteins
2.4. Preparation of T. gondii Tachyzoite Lysate Antigen (TLA)
2.5. IgG ELISA
2.6. Statistical Analysis
3. Results
3.1. Construction of Recombinant Plasmids, Expression and Purification of Recombinant Proteins
3.2. IgG ELISA
3.2.1. Preliminary Evaluation of Diagnostic Performance
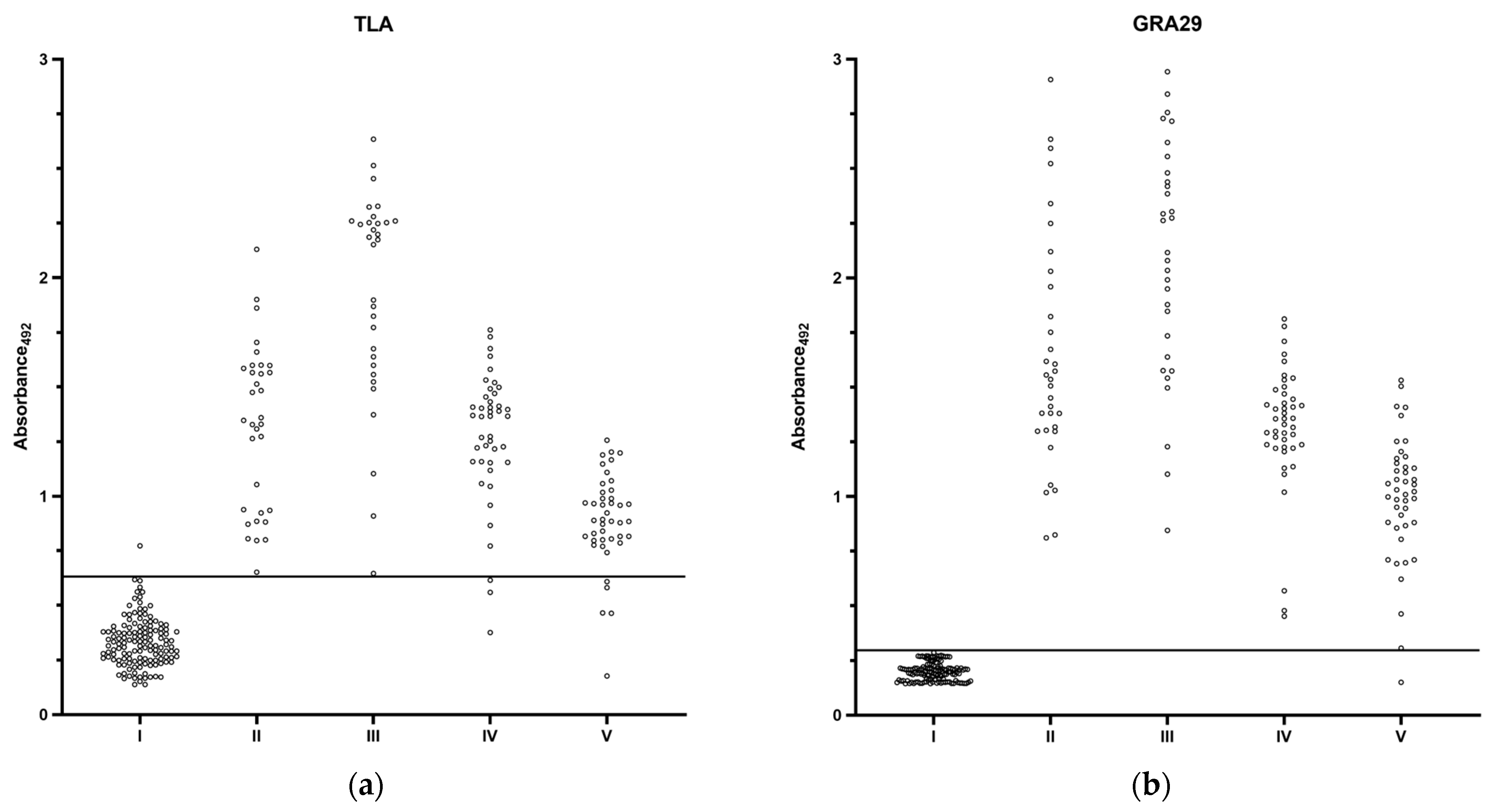
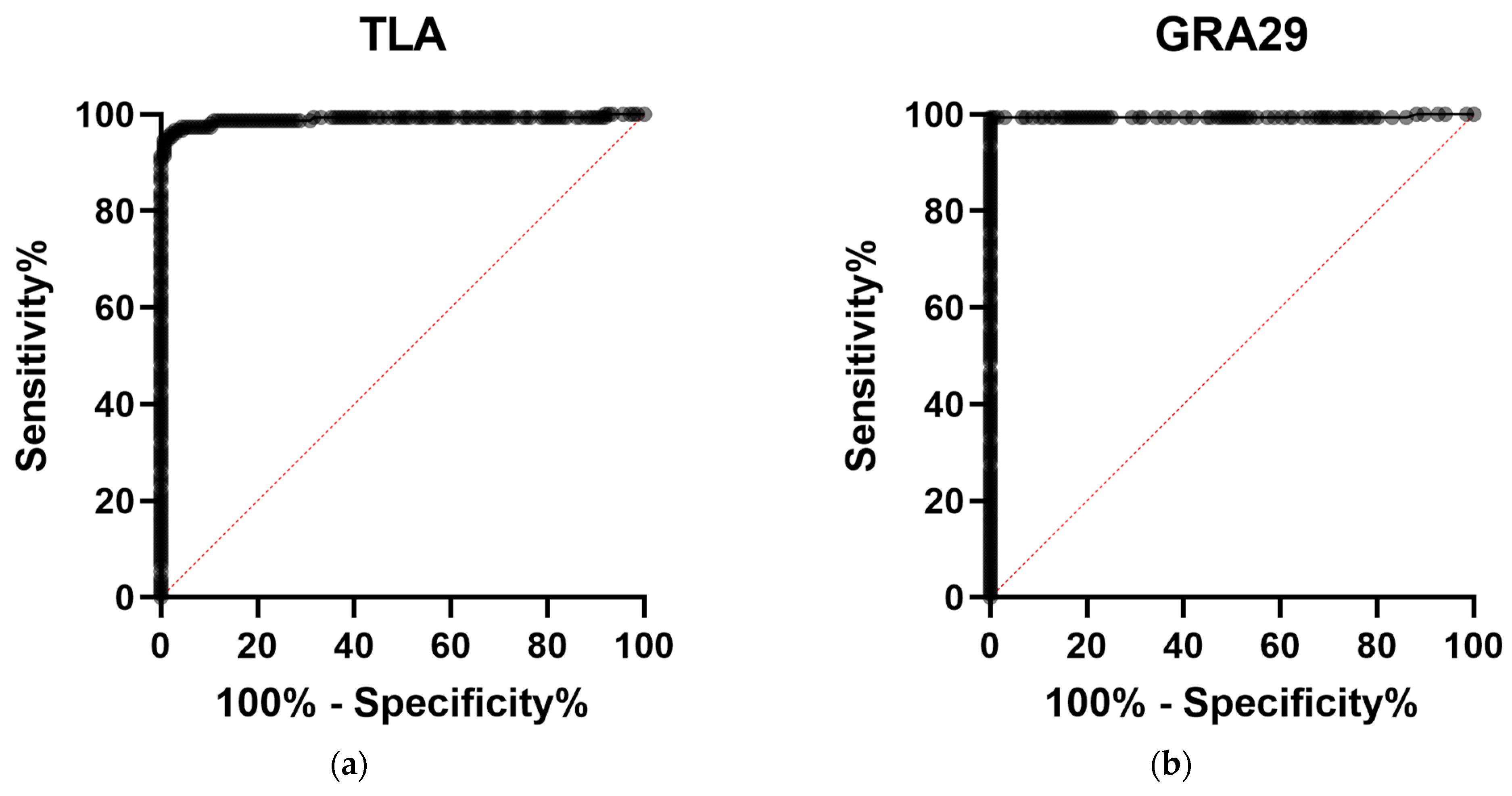
3.2.2. Extended Evaluation of GRA29-Based IgG ELISA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TLA | Tachyzoite lysate antigen |
| ESA | Excretory–secretory antigens |
| GRA | Dense granule proteins |
| PV | Parasitophorous vacuole |
| PVM | Parasitophorous vacuole membrane |
| IVN | Intravacuolar network |
| ELISA | Enzyme-linked immunosorbent assay |
| OD | Optical density |
| PPV | Positive predictive value |
| NPV | Negative predictive value |
| AUC | Area under the curve |
| ROC | Receiver operating characteristic |
References
- Montoya, J.G.; Liesenfeld, O. Toxoplasmosis. Lancet 2004, 363, 1965–1976. [Google Scholar] [CrossRef] [PubMed]
- Warschkau, D.; Seeber, F. Advances towards the Complete in Vitro Life Cycle of Toxoplasma gondii. Fac. Rev. 2023, 12, 1. [Google Scholar] [CrossRef]
- Florence, R.-G.; Marie-Laure, D. Epidemiology of and Diagnostic Strategies for Toxoplasmosis. Clin. Microbiol. Rev. 2012, 25, 264–296. [Google Scholar] [CrossRef]
- Akins, G.K.H.; Furtado, J.M.; Smith, J.R. Diseases Caused by and Behaviors Associated with Toxoplasma gondii Infection. Pathogens 2024, 13, 968. [Google Scholar] [CrossRef]
- Cerisola, A.; Francia, M.; Gesuele, J.P. Congenital Toxoplasmosis. Semin. Pediatr. Neurol. 2025, 54, 101203. [Google Scholar] [CrossRef] [PubMed]
- Bollani, L.; Auriti, C.; Achille, C.; Garofoli, F.; De Rose, D.U.; Meroni, V.; Salvatori, G.; Tzialla, C. Congenital Toxoplasmosis: The State of the Art. Front. Pediatr. 2022, 10, 894573. [Google Scholar] [CrossRef]
- Garweg, J.G.; Kieffer, F.; Mandelbrot, L.; Peyron, F.; Wallon, M. Long-Term Outcomes in Children with Congenital Toxoplasmosis-A Systematic Review. Pathogens 2022, 11, 1187. [Google Scholar] [CrossRef]
- Kim, M.-J.; Park, S.J.; Park, H. Trend in Serological and Molecular Diagnostic Methods for Toxoplasma gondii Infection. Eur. J. Med. Res. 2024, 29, 520. [Google Scholar] [CrossRef]
- Dupont, D.; Fricker-Hidalgo, H.; Brenier-Pinchart, M.-P.; Garnaud, C.; Wallon, M.; Pelloux, H. Serology for Toxoplasma in Immunocompromised Patients: Still Useful? Trends Parasitol. 2021, 37, 205–213. [Google Scholar] [CrossRef]
- de Oliveira Azevedo, C.T.; do Brasil, P.E.A.A.; Guida, L.; Lopes Moreira, M.E. Performance of Polymerase Chain Reaction Analysis of the Amniotic Fluid of Pregnant Women for Diagnosis of Congenital Toxoplasmosis: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0149938. [Google Scholar] [CrossRef] [PubMed]
- Ybañez, R.H.D.; Ybañez, A.P.; Nishikawa, Y. Review on the Current Trends of Toxoplasmosis Serodiagnosis in Humans. Front. Cell. Infect. Microbiol. 2020, 10, 204. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, Z.-D.; Huang, S.-Y.; Zhu, X.-Q. Diagnosis of Toxoplasmosis and Typing of Toxoplasma gondii. Parasit. Vectors 2015, 8, 292. [Google Scholar] [CrossRef] [PubMed]
- Holec-Gasior, L. Toxoplasma gondii Recombinant Antigens as Tools for Serodiagnosis of Human Toxoplasmosis: Current Status of Studies. Clin. Vaccine Immunol. 2013, 20, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Darcy, F.; Deslee, D.; Santoro, F.; Charif, H.; Auriault, C.; Decoster, A.; Duquesne, V.; Capron, A. Induction of a Protective Antibody-Dependent Response against Toxoplasmosis by in Vitro Excreted/Secreted Antigens from Tachyzoites of Toxoplasma gondii. Parasite Immunol. 1988, 10, 553–567. [Google Scholar] [CrossRef]
- Cesbron-Delauw, M.F.; Capron, A. Excreted/Secreted Antigens of Toxoplasma gondii—Their Origin and Role in the Host-Parasite Interaction. Res. Immunol. 1993, 144, 41–44. [Google Scholar] [CrossRef]
- Rezaei, F.; Sharif, M.; Sarvi, S.; Hejazi, S.H.; Aghayan, S.; Pagheh, A.S.; Dodangeh, S.; Daryani, A. A Systematic Review on the Role of GRA Proteins of Toxoplasma gondii in Host Immunization. J. Microbiol. Methods 2019, 165, 105696. [Google Scholar] [CrossRef]
- Schwab, J.C.; Beckers, C.J.; Joiner, K.A. The Parasitophorous Vacuole Membrane Surrounding Intracellular Toxoplasma gondii Functions as a Molecular Sieve. Proc. Natl. Acad. Sci. USA 1994, 91, 509–513. [Google Scholar] [CrossRef]
- Griffith, M.B.; Pearce, C.S.; Heaslip, A.T. Dense Granule Biogenesis, Secretion, and Function in Toxoplasma gondii. J. Eukaryot. Microbiol. 2022, 69, e12904. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, D.; Lu, S.; Zheng, B. Toxoplasmosis Vaccines: What We Have and Where to Go? npj Vaccines 2022, 7, 131. [Google Scholar] [CrossRef]
- Mamaghani, A.J.; Fathollahi, A.; Arab-Mazar, Z.; Kohansal, K.; Fathollahi, M.; Spotin, A.; Bashiri, H.; Bozorgomid, A. Toxoplasma gondii Vaccine Candidates: A Concise Review. Ir. J. Med. Sci. 2023, 192, 231–261. [Google Scholar] [CrossRef]
- Holec, L.; Gasior, A.; Brillowska-Dabrowska, A.; Kur, J. Toxoplasma gondii: Enzyme-Linked Immunosorbent Assay Using Different Fragments of Recombinant Microneme Protein 1 (MIC1) for Detection of Immunoglobulin G Antibodies. Exp. Parasitol. 2008, 119, 1–6. [Google Scholar] [CrossRef]
- Dabrowski, S.; Kur, J. Cloning, Overexpression, and Purification of the Recombinant His-Tagged SSB Protein of Escherichia coli and Use in Polymerase Chain Reaction Amplification. Protein Expr. Purif. 1999, 16, 96–102. [Google Scholar] [CrossRef]
- Holec-Gasior, L.; Kur, J. Toxoplasma gondii: Recombinant GRA5 Antigen for Detection of Immunoglobulin G Antibodies Using Enzyme-Linked Immunosorbent Assay. Exp. Parasitol. 2010, 124, 272–278. [Google Scholar] [CrossRef]
- Rota, M.; Antolini, L. Finding the Optimal Cut-Point for Gaussian and Gamma Distributed Biomarkers. Comput. Stat. Data Anal. 2014, 69, 1–14. [Google Scholar] [CrossRef]
- Lecordier, L.; Fourmaux, M.P.; Mercier, C.; Dehecq, E.; Masy, E.; Cesbron-Delauw, M.F. Enzyme-Linked Immunosorbent Assays Using the Recombinant Dense Granule Antigens GRA6 and GRA1 of Toxoplasma gondii for Detection of Immunoglobulin G Antibodies. Clin. Diagn. Lab. Immunol. 2000, 7, 607–611. [Google Scholar] [CrossRef] [PubMed]
- Pietkiewicz, H.; Hiszczyńska-Sawicka, E.; Kur, J.; Petersen, E.; Nielsen, H.V.; Stankiewicz, M.; Andrzejewska, I.; Myjak, P. Usefulness of Toxoplasma gondii-Specific Recombinant Antigens in Serodiagnosis of Human Toxoplasmosis. J. Clin. Microbiol. 2004, 42, 1779–1781. [Google Scholar] [CrossRef]
- Aubert, D.; Maine, G.T.; Villena, I.; Hunt, J.C.; Howard, L.; Sheu, M.; Brojanac, S.; Chovan, L.E.; Nowlan, S.F.; Pinon, J.M. Recombinant Antigens to Detect Toxoplasma gondii-Specific Immunoglobulin G and Immunoglobulin M in Human Sera by Enzyme Immunoassay. J. Clin. Microbiol. 2000, 38, 1144–1150. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Wan, J.; Tang, Z.; Shen, S. Identification of Novel Antigens for Serum IgG Diagnosis of Human Toxoplasmosis. Exp. Parasitol. 2019, 204, 107722. [Google Scholar] [CrossRef]
- Holec-Gasior, L.; Kur, J.; Hiszczyńska-Sawicka, E. GRA2 and ROP1 Recombinant Antigens as Potential Markers for Detection of Toxoplasma gondii-Specific Immunoglobulin G in Humans with Acute Toxoplasmosis. Clin. Vaccine Immunol. 2009, 16, 510–514. [Google Scholar] [CrossRef]
- Golkar, M.; Rafati, S.; Abdel-Latif, M.S.; Brenier-Pinchart, M.-P.; Fricker-Hidalgo, H.; Sima, B.K.; Babaie, J.; Pelloux, H.; Cesbron-Delauw, M.-F.; Mercier, C. The Dense Granule Protein GRA2, a New Marker for the Serodiagnosis of Acute Toxoplasma Infection: Comparison of Sera Collected in Both France and Iran from Pregnant Women. Diagn. Microbiol. Infect. Dis. 2007, 58, 419–426. [Google Scholar] [CrossRef]
- Nigro, M.; Gutierrez, A.; Hoffer, A.M.; Clemente, M.; Kaufer, F.; Carral, L.; Martin, V.; Guarnera, E.A.; Angel, S.O. Evaluation of Toxoplasma gondii Recombinant Proteins for the Diagnosis of Recently Acquired Toxoplasmosis by an Immunoglobulin G Analysis. Diagn. Microbiol. Infect. Dis. 2003, 47, 609–613. [Google Scholar] [CrossRef]
- Golkar, M.; Azadmanesh, K.; Khalili, G.; Khoshkholgh-Sima, B.; Babaie, J.; Mercier, C.; Brenier-Pinchart, M.-P.; Fricker-Hidalgo, H.; Pelloux, H.; Cesbron-Delauw, M.-F. Serodiagnosis of Recently Acquired Toxoplasma gondii Infection in Pregnant Women Using Enzyme-Linked Immunosorbent Assays with a Recombinant Dense Granule GRA6 Protein. Diagn. Microbiol. Infect. Dis. 2008, 61, 31–39. [Google Scholar] [CrossRef]
- Redlich, A.; Müller, W.A. Serodiagnosis of Acute Toxoplasmosis Using a Recombinant Form of the Dense Granule Antigen GRA6 in an Enzyme-Linked Immunosorbent Assay. Parasitol. Res. 1998, 84, 700–706. [Google Scholar] [CrossRef]
- Ybañez, R.H.; Nishikawa, Y. Comparative Performance of Recombinant GRA6, GRA7, and GRA14 for the Serodetection of T. gondii Infection and Analysis of IgG Subclasses in Human Sera from the Philippines. Pathogens 2022, 11, 277. [Google Scholar] [CrossRef]
- Arab-Mazar, Z.; Fallahi, S.; Koochaki, A.; Haghighi, A.; Seyyed Tabaei, S.J. Immunodiagnosis and Molecular Validation of Toxoplasma gondii-Recombinant Dense Granular (GRA) 7 Protein for the Detection of Toxoplasmosis in Patients with Cancer. Microbiol. Res. 2016, 183, 53–59. [Google Scholar] [CrossRef]
- Beghetto, E.; Spadoni, A.; Bruno, L.; Buffolano, W.; Gargano, N. Chimeric Antigens of Toxoplasma gondii: Toward Standardization of Toxoplasmosis Serodiagnosis Using Recombinant Products. J. Clin. Microbiol. 2006, 44, 2133–2140. [Google Scholar] [CrossRef] [PubMed]
- Ferra, B.T.; Holec-Gąsior, L.; Gatkowska, J.; Dziadek, B.; Dzitko, K.; Grąźlewska, W.; Lautenbach, D. The First Study on the Usefulness of Recombinant Tetravalent Chimeric Proteins Containing Fragments of SAG2, GRA1, ROP1 and AMA1 Antigens in the Detection of Specific Anti-Toxoplasma gondii Antibodies in Mouse and Human Sera. PLoS ONE 2019, 14, e0217866. [Google Scholar] [CrossRef] [PubMed]
- Ferra, B.; Holec-Gąsior, L.; Kur, J. A New Toxoplasma gondii Chimeric Antigen Containing Fragments of SAG2, GRA1, and ROP1 Proteins—Impact of Immunodominant Sequences Size on Its Diagnostic Usefulness. Parasitol. Res. 2015, 114, 3291–3299. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Young, J.C.; Broncel, M.; Teague, H.; Russell, M.R.G.; McGovern, O.L.; Renshaw, M.; Frith, D.; Snijders, A.P.; Collinson, L.; Carruthers, V.B.; et al. Phosphorylation of Toxoplasma gondii Secreted Proteins during Acute and Chronic Stages of Infection. mSphere 2020, 5, e00792-20. [Google Scholar] [CrossRef]
- Young, J.; Dominicus, C.; Wagener, J.; Butterworth, S.; Ye, X.; Kelly, G.; Ordan, M.; Saunders, B.; Instrell, R.; Howell, M.; et al. A CRISPR Platform for Targeted in Vivo Screens Identifies Toxoplasma gondii Virulence Factors in Mice. Nat. Commun. 2019, 10, 3963. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cirelli, K.M.; Barros, P.D.C.; Sangaré, L.O.; Butty, V.; Hassan, M.A.; Pesavento, P.; Mete, A.; Saeij, J.P.J. Three Toxoplasma gondii Dense Granule Proteins Are Required for Induction of Lewis Rat Macrophage Pyroptosis. MBio 2019, 10, e02388-18. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hollingsworth, L.R.; Sangaré, L.O.; Paredes-Santos, T.C.; Krishnamurthy, S.; Penn, B.H.; Wu, H.; Saeij, J.P.J. Host E3 Ubiquitin Ligase ITCH Mediates Toxoplasma gondii Effector GRA35-Triggered NLRP1 Inflammasome Activation and Cell-Autonomous Immunity. MBio 2024, 15, e0330223. [Google Scholar] [CrossRef]
- Li, D.; Han, M.; Cao, Y.; Du, J.; An, R. Protective Effect against Toxoplasmosis in BALB/C Mice Vaccinated with Recombinant Toxoplasma gondii CDPK3, GRA35, and ROP46 Protein Cocktail Vaccine. Vaccine 2024, 42, 1342–1351. [Google Scholar] [CrossRef]
- Shi, Y.; Li, J.; Yang, W.; Chen, J. Protective Immunity Induced by DNA Vaccine Containing TgGRA35, TgGRA42, and TgGRA43 against Toxoplasma gondii Infection in Kunming Mice. Front. Cell. Infect. Microbiol. 2023, 13, 1236130. [Google Scholar] [CrossRef]
- Cygan, A.M.; Theisen, T.C.; Mendoza, A.G.; Marino, N.D.; Panas, M.W.; Boothroyd, J.C. Coimmunoprecipitation with MYR1 Identifies Three Additional Proteins within the Toxoplasma gondii Parasitophorous Vacuole Required for Translocation of Dense Granule Effectors into Host Cells. mSphere 2020, 5, e00858-19. [Google Scholar] [CrossRef]
- Coffey, M.J.; Dagley, L.F.; Seizova, S.; Kapp, E.A.; Infusini, G.; Roos, D.S.; Boddey, J.A.; Webb, A.I.; Tonkin, C.J. Aspartyl Protease 5 Matures Dense Granule Proteins That Reside at the Host-Parasite Interface in Toxoplasma gondii. MBio 2018, 9, e01796-18. [Google Scholar] [CrossRef]
- Wang, Y.; Sangaré, L.O.; Paredes-Santos, T.C.; Hassan, M.A.; Krishnamurthy, S.; Furuta, A.M.; Markus, B.M.; Lourido, S.; Saeij, J.P.J. Genome-Wide Screens Identify Toxoplasma gondii Determinants of Parasite Fitness in IFNγ-Activated Murine Macrophages. Nat. Commun. 2020, 11, 5258. [Google Scholar] [CrossRef]
- Tachibana, Y.; Hashizaki, E.; Sasai, M.; Yamamoto, M. Host Genetics Highlights IFN-γ-Dependent Toxoplasma Genes Encoding Secreted and Non-Secreted Virulence Factors in In Vivo CRISPR Screens. Cell Rep. 2023, 42, 112592. [Google Scholar] [CrossRef]
- Mayoral, J.; Guevara, R.B.; Rivera-Cuevas, Y.; Tu, V.; Tomita, T.; Romano, J.D.; Gunther-Cummins, L.; Sidoli, S.; Coppens, I.; Carruthers, V.B.; et al. Dense Granule Protein GRA64 Interacts with Host Cell ESCRT Proteins during Toxoplasma gondii Infection. MBio 2022, 13, e01442-22. [Google Scholar] [CrossRef] [PubMed]
- Sołowińska, K.; Holec-Gąsior, L. Single Cell Expression Systems for the Production of Recombinant Proteins for Immunodiagnosis and Immunoprophylaxis of Toxoplasmosis. Microorganisms 2024, 12, 1731. [Google Scholar] [CrossRef] [PubMed]
- Babaie, J.; Miri, M.; Sadeghiani, G.; Zare, M.; Khalili, G.; Golkar, M. Expression and Single-Step Purification of GRA8 Antigen of Toxoplasma gondii in Escherichia Coli. Avicenna J. Med. Biotechnol. 2011, 3, 67–77. [Google Scholar] [PubMed]
- Murray, A.; Mercier, C.; Decoster, A.; Lecordier, L.; Capron, A.; Cesbron-Delauw, M.F. Multiple B-Cell Epitopes in a Recombinant GRA2 Secreted Antigen of Toxoplasma gondii. Appl. Parasitol. 1993, 34, 235–244. [Google Scholar] [PubMed]


| Primer Name | Primer Sequence | Amplicon Size [bp] |
|---|---|---|
| gra29FOR | agcccagatctgggtCAAATCTTGGCTCTGCTTC | 1354 |
| gra29REV | gacggagctcgaattACGTGTCCCTCTTCCC | |
| gra35FOR | agcccagatctgggtGCGAAGCGTCAATATGAG | 742 |
| gra35REV | gacggagctcgaattAGTCTGTTTCGGCTCCG | |
| gra36FOR | agcccagatctgggtTATCGGCGCTATCGTGAT | 760 |
| gra36REV | gacggagctcgaattCGTACGCTGTGCCC | |
| gra45FOR | gacggagctcgaattCGTACGCTGTGCCC | 1117 |
| gra45REV | gacggagctcgaattCGTACGCTGTGCCC | |
| gra54FOR | agcccagatctgggtGGTGCAACACGAGGAC | 931 |
| gra54REV | gacggagctcgaattTTGTGCCCTCACACCAG | |
| gra64FOR | gacggagctcgaattCGTCTTAAACCCCTTCAAGC | 763 |
| gra64REV | agcccagatctgggtGCAAACTACGATTTCTTTGTTCTT |
| Protein Name | E. coli Expression Strain | Post-Induction Temperature [°C] | Post-Induction Incubation Time [h] |
|---|---|---|---|
| GRA29 | BL21(DE3)pLacI | 37 | 18 |
| GRA35 | BL21(DE3)pLacI | 25 | 18 |
| GRA36 | Rosetta(DE3)pLysS | 25 | 18 |
| GRA45 | BL21(DE3)pLysS | 30 | 6 |
| GRA54 | BL21(DE3)pLacI | 25 | 18 |
| GRA64 | BL21(DE3)pLacI | 37 | 18 |
| Protein Name | Molecular Weight [kDa] | Isoelectric Point |
|---|---|---|
| GRA29 | 52.0 | 8.03 |
| GRA35 | 33.7 | 6.14 |
| GRA36 | 34.5 | 6.43 |
| GRA45 | 47.7 | 5.17 |
| GRA54 | 40.1 | 9.64 |
| GRA64 | 32.5 | 7.95 |
| Protein Name | Yield [mg] |
|---|---|
| GRA29 | 28.3 |
| GRA35 | 164.8 |
| GRA36 | 138.0 |
| GRA45 | 123.0 |
| GRA54 | 18.1 |
| GRA64 | 38.0 |
| Antigen | AUC 1 (95% CI) | Cut-Off | Sensitivity [%] (95% CI) | Specificity [%] (95% CI) | Fisher’s Exact p-Value |
|---|---|---|---|---|---|
| GRA29 | 0.9983 (0.9925–1.000) | 0.3740 | 95.83 (79.76–99.79) | 100.00 (86.20–100.00) | <0.0001 |
| GRA35 | 0.5269 (0.3600–0.6938) | 1.193 | 12.50 (4.34–31.00) | 91.67 (74.15–98.52) | ns 2 |
| GRA36 | 0.5260 (0.3544–0.6977) | 0.9945 | 79.17 (59.53–90.76) | 41.67 (24.47–61.17) | ns |
| GRA45 | 0.8507 (0.3544–0.6977) | 0.4415 | 79.17 (59.53–90.76) | 87.50 (69.00–95.66) | <0.0001 |
| GRA54 | 0.9323 (0.8464–1.000) | 0.4090 | 70.83 (50.83–85.09) | 95.83 (79.76–99.79) | <0.0001 |
| GRA64 | 0.5503 (0.3848–0.7159) | 0.8390 | 41.67 (24.47–61.17) | 75.00 (55.10–88.00) | ns |
| TLA | 0.9948 (0.9822–1.000) | 0.6355 | 95.83 (79.76–99.79) | 95.83 (79.76–99.79) | <0.0001 |
| Antigen | AUC 1 (95% CI) | Cut-Off | Sensitivity [%] (95% CI) | Specificity [%] (95% CI) | PPV 2 [%] | NPV 3 [%] |
|---|---|---|---|---|---|---|
| TLA | 0.9894 (0.9765–1.000) | 0.6320 | 99.30 (96.15–99.96) | 94.41 (89.35–97.14) | 94.67 (89.83–97.27) | 99.26 (95.95–99.96) |
| GRA29 | 0.9942 (0.9828–1.000) | 0.2965 | 100.00 (97.49–100.00) | 99.27 (95.98–99.96) | 99.33 (96.32–99.97) | 100.00 (97.25–100.00) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sołowińska, K.; Holec-Gąsior, L. Development and Evaluation of Six Novel Recombinant GRA Proteins in Serodiagnosis of Human Toxoplasmosis. Curr. Issues Mol. Biol. 2025, 47, 879. https://doi.org/10.3390/cimb47110879
Sołowińska K, Holec-Gąsior L. Development and Evaluation of Six Novel Recombinant GRA Proteins in Serodiagnosis of Human Toxoplasmosis. Current Issues in Molecular Biology. 2025; 47(11):879. https://doi.org/10.3390/cimb47110879
Chicago/Turabian StyleSołowińska, Karolina, and Lucyna Holec-Gąsior. 2025. "Development and Evaluation of Six Novel Recombinant GRA Proteins in Serodiagnosis of Human Toxoplasmosis" Current Issues in Molecular Biology 47, no. 11: 879. https://doi.org/10.3390/cimb47110879
APA StyleSołowińska, K., & Holec-Gąsior, L. (2025). Development and Evaluation of Six Novel Recombinant GRA Proteins in Serodiagnosis of Human Toxoplasmosis. Current Issues in Molecular Biology, 47(11), 879. https://doi.org/10.3390/cimb47110879






