Recombinant Oncolytic Viruses: Hexagonal Warriors in the Field of Solid Tumor Immunotherapy
Abstract
1. Introduction
2. Representative Agents of Recombinant Oncolytic Viruses
2.1. H101
2.2. T-VEC
2.3. JX-594
2.4. G47Δ
2.5. RP1
2.6. CG0070
2.7. OH2
2.8. PVSRIPO
2.9. Olvi-Vec
2.10. REOLYSIN
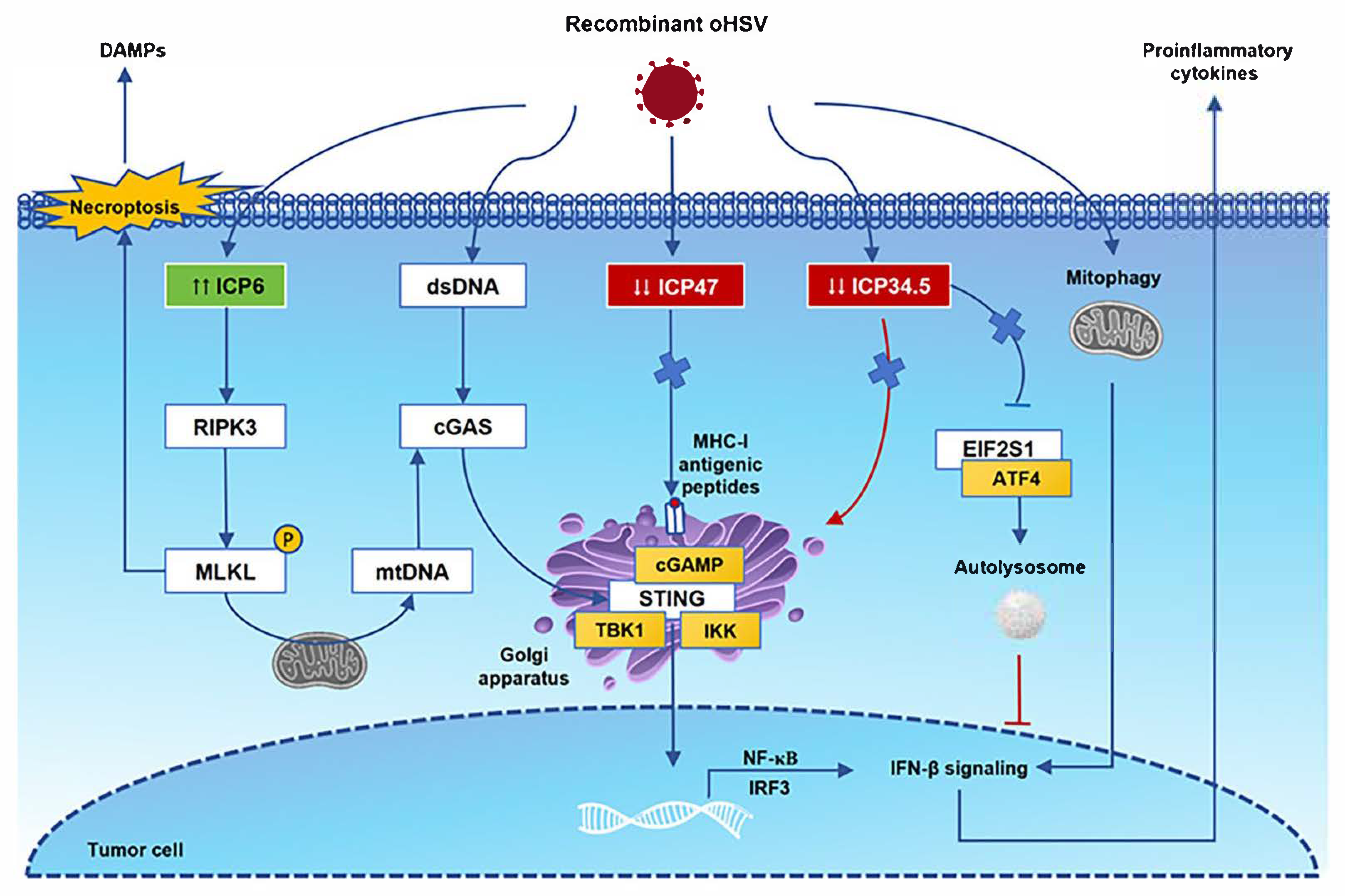
3. The Safety and Immunomodulatory Mechanisms of Oncolytic Viruses
4. The Effectiveness and Systemic Delivery Dilemmas of Oncolytic Viruses
5. Conclusions and Future Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AE | Adverse event |
| ATF4 | Activating transcription factor 4 |
| CDC | Complement-dependent cytotoxicity |
| cGAS-STING | Cyclic GMP-AMP synthase–stimulator of interferon genes |
| CLEC10A | C-type lectin domain-containing 10A |
| CRC | Colorectal cancer |
| CTL | Cytotoxic T lymphocyte |
| DAMPs | Damage-associated molecular patterns |
| DC | Dendritic cell |
| EGFR | Epidermal growth factor receptor |
| EIF2S1 | Eukaryotic translation initiation factor 2 subunit alpha |
| GM-CSF | Granulocyte–macrophage colony-stimulating factor |
| GZMK | Granzyme K |
| HCC | Hepatocellular carcinoma |
| HSV | Herpes simplex virus |
| HVR | Hexon hypervariable region |
| ICI | Immune checkpoint inhibitor |
| IFNγ | Interferon-γ |
| IL-12 | Interleukin-12 |
| IRF3 | Interferon regulatory factor 3 |
| MDSC | Myeloid-derived suppressor cell |
| MHC-I | Major histocompatibility complex class I |
| MPA | Metastatic pancreatic cancer |
| NAb | Neutralizing antibody |
| NK cells | Natural killer cells |
| NMIBC | Non-muscle-invasive bladder cancer |
| ORR | Objective response rate |
| OV | Oncolytic virus |
| PDAC | Pancreatic ductal adenocarcinoma |
| S100A1 | S100 calcium-binding protein A1 |
| STING | Stimulator of interferon gene |
| TAM | Tumor-associated macrophage |
| TAP | Transporter-associated peptide |
| TBK1 | TANK-binding kinase 1 |
| TIME | Tumor immune microenvironment |
| Treg cell | Regulatory T cell |
| TME | Tumor microenvironment |
References
- Chouljenko, D.V.; Ding, J.; Lee, I.F.; Murad, Y.M.; Bu, X.; Liu, G.; Delwar, Z.; Sun, Y.; Yu, S.; Samudio, I.; et al. Induction of Durable Antitumor Response by a Novel Oncolytic Herpesvirus Expressing Multiple Immunomodulatory Transgenes. Biomedicines 2020, 8, 484. [Google Scholar] [CrossRef]
- Foltz, J.A.; Tran, J.; Wong, P.; Fan, C.; Schmidt, E.; Fisk, B.; Becker-Hapak, M.; Russler-Germain, D.A.; Johnson, J.; Marin, N.D.; et al. Cytokines drive the formation of memory-like NK cell subsets via epigenetic rewiring and transcriptional regulation. Sci. Immunol. 2024, 9, eadk4893. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Song, W.; Lin, D.; Zhang, X.; Wang, M.; Li, Y.; Yang, Z.; Guo, S.; Wang, Z.; Sheng, J.; et al. VG161 activates systemic antitumor immunity in pancreatic cancer models as a novel oncolytic herpesvirus expressing multiple immunomodulatory transgenes. J. Med. Virol. 2023, 95, e28108. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Bai, X.; Zhang, Q.; Liang, X.; Jin, X.; Zhao, Z.; Song, W.; Tan, Q.; Zhao, R.; Jia, W.; et al. Oncolytic virus VG161 in refractory hepatocellular carcinoma. Nature 2025, 641, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Shen, Y.; Yi, M.; Zhang, C.; Zhao, B.; Zhong, G.; Lou, W.; Xue, D.; Leng, Q.; Ding, J.; et al. Combination of novel oncolytic herpesvirus with paclitaxel as an efficient strategy for breast cancer therapy. J. Med. Virol. 2023, 95, e28768. [Google Scholar] [CrossRef]
- Zhong, L.; Gan, L.; Wang, B.; Wu, T.; Yao, F.; Gong, W.; Peng, H.; Deng, Z.; Xiao, G.; Liu, X.; et al. Hyperacute rejection-engineered oncolytic virus for interventional clinical trial in refractory cancer patients. Cell 2025, 188, 1119–1136.e3. [Google Scholar] [CrossRef]
- Zhong, L.; Huang, Y.; He, J.; Yang, N.; Xu, B.; Ma, Y.; Liu, J.; Tang, C.; Luo, C.; Wu, P.; et al. Generation of in situ CRISPR-mediated primary and metastatic cancer from monkey liver. Signal Transduct. Target. Ther. 2021, 6, 411. [Google Scholar] [CrossRef]
- Xia, Z.J.; Chang, J.H.; Zhang, L.; Jiang, W.Q.; Guan, Z.Z.; Liu, J.W.; Zhang, Y.; Hu, X.H.; Wu, G.H.; Wang, H.Q.; et al. Phase III randomized clinical trial of intratumoral injection of E1B gene-deleted adenovirus (H101) combined with cisplatin-based chemotherapy in treating squamous cell cancer of head and neck or esophagus. Ai Zheng = Aizheng = Chin. J. Cancer 2004, 23, 1666–1670. [Google Scholar]
- Yuan, Z.Y.; Zhang, L.; Li, S.; Qian, X.Z.; Guan, Z.Z. Safety of an E1B deleted adenovirus administered intratumorally to patients with cancer. Ai Zheng = Aizheng = Chin. J. Cancer 2003, 22, 310–313. [Google Scholar]
- Andtbacka, R.H.; Kaufman, H.L.; Collichio, F.; Amatruda, T.; Senzer, N.; Chesney, J.; Delman, K.A.; Spitler, L.E.; Puzanov, I.; Agarwala, S.S.; et al. Talimogene Laherparepvec Improves Durable Response Rate in Patients With Advanced Melanoma. J. Clin. Oncol. 2015, 33, 2780–2788. [Google Scholar] [CrossRef]
- Hidalgo, P.; Ip, W.H.; Dobner, T.; Gonzalez, R.A. The biology of the adenovirus E1B 55K protein. FEBS Lett. 2019, 593, 3504–3517. [Google Scholar] [CrossRef]
- Wang, Z.M.; Li, M.K.; Yang, Q.L.; Duan, S.X.; Lou, X.Y.; Yang, X.Y.; Liu, Y.; Zhong, Y.W.; Qiao, Y.; Wang, Z.S.; et al. Recombinant human adenovirus type 5 promotes anti-tumor immunity via inducing pyroptosis in tumor endothelial cells. Acta Pharmacol. Sin. 2024, 45, 2646–2656. [Google Scholar] [CrossRef]
- Yi, L.; Ning, Z.; Xu, L.; Shen, Y.; Zhu, X.; Yu, W.; Xie, J.; Meng, Z. The combination treatment of oncolytic adenovirus H101 with nivolumab for refractory advanced hepatocellular carcinoma: An open-label, single-arm, pilot study. ESMO Open 2024, 9, 102239. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhao, H.; Shan, M.; Chen, H.; Xu, B.; He, Y.; Zhao, Y.; Liu, Z.; Chen, J.; Xu, Q. Oncolytic adenovirus H101 ameliorate the efficacy of anti-PD-1 monotherapy in colorectal cancer. Cancer Med. 2022, 11, 4575–4587. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, J.; Liu, Z.; Wang, J.; Wang, F.; Wang, T.; Shi, F.; Su, J.; Zhao, Y. Recombinant Human Adenovirus Type 5 (H101) Intra-Tumor Therapy in Patients with Persistent, Recurrent, or Metastatic Cervical Cancer: Genomic Profiling Relating to Clinical Efficacy. Drug Des. Dev. Ther. 2023, 17, 3507–3522. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Lv, X.; Wang, F.; Zhou, Q.; Zhang, F.; Zhang, M.; Chen, J. Intratumoral injection of oncolytic virus (H101) in combination with concurrent chemoradiotherapy for locally advanced cervical cancer. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2023, 33, 1051–1056. [Google Scholar] [CrossRef]
- Xi, P.; Zeng, D.; Chen, M.; Jiang, L.; Zhang, Y.; Qin, D.; Yao, Z.; He, C. Enhancing pancreatic cancer treatment: The role of H101 oncolytic virus in irreversible electroporation. Front. Immunol. 2025, 16, 1546242. [Google Scholar] [CrossRef]
- Zhang, Y.; Qian, L.; Chen, K.; Gu, S.; Wang, J.; Meng, Z.; Li, Y.; Wang, P. Intraperitoneal oncolytic virotherapy for patients with malignant ascites: Characterization of clinical efficacy and antitumor immune response. Mol. Ther. Oncolytics 2022, 25, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qian, L.; Chen, K.; Gu, S.; Meng, Z.; Wang, J.; Li, Y.; Wang, P. Oncolytic adenovirus in treating malignant ascites: A phase II trial and longitudinal single-cell study. Mol. Ther. J. Am. Soc. Gene Ther. 2024, 32, 2000–2020. [Google Scholar] [CrossRef]
- Chen, Z.; Deng, J.; Cao, J.; Wu, H.; Feng, G.; Zhang, R.; Ran, B.; Hu, K.; Cao, H.; Zhu, X.; et al. Nano-hydroxyapatite-evoked immune response synchronized with controllable immune adjuvant release for strengthening melanoma-specific growth inhibition. Acta Biomater. 2022, 145, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Dougan, M.; Dranoff, G.; Dougan, S.K. GM-CSF, IL-3, and IL-5 Family of Cytokines: Regulators of Inflammation. Immunity 2019, 50, 796–811. [Google Scholar] [CrossRef] [PubMed]
- Ruffolo, L.I.; Jackson, K.M.; Kuhlers, P.C.; Dale, B.S.; Figueroa Guilliani, N.M.; Ullman, N.A.; Burchard, P.R.; Qin, S.S.; Juviler, P.G.; Keilson, J.M.; et al. GM-CSF drives myelopoiesis, recruitment and polarisation of tumour-associated macrophages in cholangiocarcinoma and systemic blockade facilitates antitumour immunity. Gut 2022, 71, 1386–1398. [Google Scholar] [CrossRef]
- van Akkooi, A.C.J.; Haferkamp, S.; Papa, S.; Franke, V.; Pinter, A.; Weishaupt, C.; Huber, M.A.; Loquai, C.; Richtig, E.; Gokani, P.; et al. A Retrospective Chart Review Study of Real-World Use of Talimogene Laherparepvec in Unresectable Stage IIIB-IVM1a Melanoma in Four European Countries. Adv. Ther. 2021, 38, 1245–1262. [Google Scholar] [CrossRef] [PubMed]
- Shalhout, S.Z.; Miller, D.M.; Emerick, K.S.; Kaufman, H.L. Therapy with oncolytic viruses: Progress and challenges. Nat. Rev. Clin. Oncol. 2023, 20, 160–177. [Google Scholar] [CrossRef] [PubMed]
- Dummer, R.; Gyorki, D.E.; Hyngstrom, J.R.; Ning, M.; Lawrence, T.; Ross, M.I. Final 5-Year Follow-Up Results Evaluating Neoadjuvant Talimogene Laherparepvec Plus Surgery in Advanced Melanoma: A Randomized Clinical Trial. JAMA Oncol. 2023, 9, 1457–1459. [Google Scholar] [CrossRef]
- Chesney, J.A.; Puzanov, I.; Collichio, F.A.; Singh, P.; Milhem, M.M.; Glaspy, J.; Hamid, O.; Ross, M.; Friedlander, P.; Garbe, C.; et al. Talimogene laherparepvec in combination with ipilimumab versus ipilimumab alone for advanced melanoma: 5-year final analysis of a multicenter, randomized, open-label, phase II trial. J. Immunother. Cancer 2023, 11, e006270. [Google Scholar] [CrossRef]
- Chesney, J.A.; Ribas, A.; Long, G.V.; Kirkwood, J.M.; Dummer, R.; Puzanov, I.; Hoeller, C.; Gajewski, T.F.; Gutzmer, R.; Rutkowski, P.; et al. Randomized, Double-Blind, Placebo-Controlled, Global Phase III Trial of Talimogene Laherparepvec Combined With Pembrolizumab for Advanced Melanoma. J. Clin. Oncol. 2023, 41, 528–540. [Google Scholar] [CrossRef]
- Ressler, J.M.; Plaschka, M.; Silmbrod, R.; Bachmayr, V.; Shaw, L.E.; Silly, T.; Zila, N.; Stepan, A.; Kusienicka, A.; Tschandl, P.; et al. Efficacy and tolerability of neoadjuvant therapy with Talimogene laherparepvec in cutaneous basal cell carcinoma: A phase II trial (NeoBCC trial). Nat. Cancer 2025, 6, 51–66. [Google Scholar] [CrossRef]
- Parato, K.A.; Breitbach, C.J.; Le Boeuf, F.; Wang, J.; Storbeck, C.; Ilkow, C.; Diallo, J.S.; Falls, T.; Burns, J.; Garcia, V.; et al. The oncolytic poxvirus JX-594 selectively replicates in and destroys cancer cells driven by genetic pathways commonly activated in cancers. Mol. Ther. J. Am. Soc. Gene Ther. 2012, 20, 749–758. [Google Scholar] [CrossRef]
- Kim, M.K.; Breitbach, C.J.; Moon, A.; Heo, J.; Lee, Y.K.; Cho, M.; Lee, J.W.; Kim, S.G.; Kang, D.H.; Bell, J.C.; et al. Oncolytic and immunotherapeutic vaccinia induces antibody-mediated complement-dependent cancer cell lysis in humans. Sci. Transl. Med. 2013, 5, 185ra163. [Google Scholar] [CrossRef]
- Breitbach, C.J.; Burke, J.; Jonker, D.; Stephenson, J.; Haas, A.R.; Chow, L.Q.; Nieva, J.; Hwang, T.H.; Moon, A.; Patt, R.; et al. Intravenous delivery of a multi-mechanistic cancer-targeted oncolytic poxvirus in humans. Nature 2011, 477, 99–102. [Google Scholar] [CrossRef]
- Breitbach, C.J.; Arulanandam, R.; De Silva, N.; Thorne, S.H.; Patt, R.; Daneshmand, M.; Moon, A.; Ilkow, C.; Burke, J.; Hwang, T.H.; et al. Oncolytic vaccinia virus disrupts tumor-associated vasculature in humans. Cancer Res. 2013, 73, 1265–1275. [Google Scholar] [CrossRef] [PubMed]
- Park, B.H.; Hwang, T.; Liu, T.C.; Sze, D.Y.; Kim, J.S.; Kwon, H.C.; Oh, S.Y.; Han, S.Y.; Yoon, J.H.; Hong, S.H.; et al. Use of a targeted oncolytic poxvirus, JX-594, in patients with refractory primary or metastatic liver cancer: A phase I trial. Lancet Oncol. 2008, 9, 533–542. [Google Scholar] [CrossRef]
- Samson, A.; West, E.J.; Carmichael, J.; Scott, K.J.; Turnbull, S.; Kuszlewicz, B.; Dave, R.V.; Peckham-Cooper, A.; Tidswell, E.; Kingston, J.; et al. Neoadjuvant Intravenous Oncolytic Vaccinia Virus Therapy Promotes Anticancer Immunity in Patients. Cancer Immunol. Res. 2022, 10, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Toulmonde, M.; Guegan, J.P.; Spalato-Ceruso, M.; Peyraud, F.; Kind, M.; Vanhersecke, L.; Le Loarer, F.; Perret, R.; Cantarel, C.; Bellera, C.; et al. Reshaping the tumor microenvironment of cold soft-tissue sarcomas with oncolytic viral therapy: A phase 2 trial of intratumoral JX-594 combined with avelumab and low-dose cyclophosphamide. Mol. Cancer 2024, 23, 38. [Google Scholar] [CrossRef]
- Toulmonde, M.; Cousin, S.; Kind, M.; Guegan, J.P.; Bessede, A.; Le Loarer, F.; Perret, R.; Cantarel, C.; Bellera, C.; Italiano, A. Randomized phase 2 trial of intravenous oncolytic virus JX-594 combined with low-dose cyclophosphamide in patients with advanced soft-tissue sarcoma. J. Hematol. Oncol. 2022, 15, 149. [Google Scholar] [CrossRef]
- Cousin, S.; Toulmonde, M.; Kind, M.; Guegan, J.P.; Bessede, A.; Cantarel, C.; Bellera, C.; Italiano, A. Phase 2 trial of intravenous oncolytic virus JX-594 combined with low-dose cyclophosphamide in patients with advanced breast cancer. Exp. Hematol. Oncol. 2022, 11, 104. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Lee, M.E.; Kim, J.; Oh, K.; Lee, N.; Jung, M.; Jang, W.S.; Ham, W.S. PD-1 inhibitor plus oncolytic vaccinia virus is a safe and effective treatment option for metastatic renal cell carcinoma. Cancer Cell Int. 2024, 24, 50. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Galle, P.R.; Chao, Y.; Erinjeri, J.; Heo, J.; Borad, M.J.; Luca, A.; Burke, J.; Pelusio, A.; Agathon, D.; et al. PHOCUS: A Phase 3, Randomized, Open-Label Study of Sequential Treatment with Pexa-Vec (JX-594) and Sorafenib in Patients with Advanced Hepatocellular Carcinoma. Liver Cancer 2024, 13, 248–264. [Google Scholar] [CrossRef]
- Frampton, J.E. Teserpaturev/G47Δ: First Approval. BioDrugs Clin. Immunother. Biopharm. Gene Ther. 2022, 36, 667–672. [Google Scholar] [CrossRef]
- Fukuhara, H.; Takeshima, Y.; Todo, T. Triple-mutated oncolytic herpes virus for treating both fast- and slow-growing tumors. Cancer Sci. 2021, 112, 3293–3301. [Google Scholar] [CrossRef] [PubMed]
- Fukuhara, H.; Ino, Y.; Todo, T. Oncolytic virus therapy: A new era of cancer treatment at dawn. Cancer Sci. 2016, 107, 1373–1379. [Google Scholar] [CrossRef]
- Todo, T.; Ino, Y.; Ohtsu, H.; Shibahara, J.; Tanaka, M. A phase I/II study of triple-mutated oncolytic herpes virus G47∆ in patients with progressive glioblastoma. Nat. Commun. 2022, 13, 4119. [Google Scholar] [CrossRef]
- Todo, T.; Ito, H.; Ino, Y.; Ohtsu, H.; Ota, Y.; Shibahara, J.; Tanaka, M. Intratumoral oncolytic herpes virus G47∆ for residual or recurrent glioblastoma: A phase 2 trial. Nat. Med. 2022, 28, 1630–1639. [Google Scholar] [CrossRef]
- Thomas, S.; Kuncheria, L.; Roulstone, V.; Kyula, J.N.; Mansfield, D.; Bommareddy, P.K.; Smith, H.; Kaufman, H.L.; Harrington, K.J.; Coffin, R.S. Development of a new fusion-enhanced oncolytic immunotherapy platform based on herpes simplex virus type 1. J. Immunother. Cancer 2019, 7, 214. [Google Scholar] [CrossRef]
- Wong, M.K.; Milhem, M.M.; Sacco, J.J.; Michels, J.; In, G.K.; Couselo, E.M.; Schadendorf, D.; Beasley, G.M.; Niu, J.; Chmielowski, B.; et al. RP1 Combined With Nivolumab in Advanced Anti-PD-1-Failed Melanoma (IGNYTE). J. Clin. Oncol. 2025, JCO2501346. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, N.; Ge, Y.; Ennist, D.L.; Zhu, M.; Mina, M.; Ganesh, S.; Reddy, P.S.; Yu, D.C. CG0070, a conditionally replicating granulocyte macrophage colony-stimulating factor—armed oncolytic adenovirus for the treatment of bladder cancer. Clin. Cancer Res. An. Off. J. Am. Assoc. Cancer Res. 2006, 12, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Burke, J.M.; Lamm, D.L.; Meng, M.V.; Nemunaitis, J.J.; Stephenson, J.J.; Arseneau, J.C.; Aimi, J.; Lerner, S.; Yeung, A.W.; Kazarian, T.; et al. A first in human phase 1 study of CG0070, a GM-CSF expressing oncolytic adenovirus, for the treatment of nonmuscle invasive bladder cancer. J. Urol. 2012, 188, 2391–2397. [Google Scholar] [CrossRef]
- Packiam, V.T.; Lamm, D.L.; Barocas, D.A.; Trainer, A.; Fand, B.; Davis, R.L.; Clark, W.; Kroeger, M.; Dumbadze, I.; Chamie, K.; et al. An open label, single-arm, phase II multicenter study of the safety and efficacy of CG0070 oncolytic vector regimen in patients with BCG-unresponsive non-muscle-invasive bladder cancer: Interim results. Urol. Oncol. 2018, 36, 440–447. [Google Scholar] [CrossRef]
- Li, R.; Villa, N.Y.; Yu, X.; Johnson, J.O.; Borjas, G.; Dhillon, J.; Moran-Segura, C.M.; Kim, Y.; Francis, N.; Dorman, D.; et al. Oncolytic immunotherapy with nivolumab in muscle-invasive bladder cancer: A phase 1b trial. Nat. Med. 2025, 31, 176–188. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, J.; Wu, Z.; Hu, S.; Hu, H.; Ning, Z.; Li, Y.; Dong, Y.; Zou, J.; Mao, Z.; et al. Stability and anti-tumor effect of oncolytic herpes simplex virus type 2. Oncotarget 2018, 9, 24672–24683. [Google Scholar] [CrossRef]
- Wu, Q.; Hu, X.; Zhang, X.; Kong, D.; Yang, Z.; Li, G.; Gu, Z.; Zhang, Q.; Wan, D.; Cheng, S.; et al. Single-cell transcriptomics of peripheral blood reveals anti-tumor systemic immunity induced by oncolytic virotherapy. Theranostics 2022, 12, 7371–7389. [Google Scholar] [CrossRef]
- Cao, Y.; Xiong, F.; Kan, X.; Guo, X.; Ouyang, T.; Wang, R.; Zheng, C. Transarterial viroembolization improves the therapeutic efficacy of im-mune-excluded liver cancer: Three birds with one stone. Pharmacol. Res. 2023, 187, 106581. [Google Scholar] [CrossRef]
- Wang, X.; Tian, H.; Chi, Z.; Si, L.; Sheng, X.; Hu, H.; Gu, X.; Li, S.; Li, C.; Lian, B.; et al. Oncolytic virus OH2 extends survival in patients with PD-1 pretreated melanoma: Phase Ia/Ib trial results and biomarker insights. J. Immunother. Cancer 2025, 13, e010662. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, X.; Ji, Q.; Fang, A.; Song, L.; Xu, X.; Lin, Y.; Peng, Y.; Yu, J.; Xie, L.; et al. OH2 oncolytic virus: A novel approach to glioblastoma intervention through direct targeting of tumor cells and augmentation of anti-tumor immune responses. Cancer Lett. 2024, 589, 216834. [Google Scholar] [CrossRef]
- Zhang, B.; Huang, J.; Tang, J.; Hu, S.; Luo, S.; Luo, Z.; Zhou, F.; Tan, S.; Ying, J.; Chang, Q.; et al. Intratumoral OH2, an oncolytic herpes simplex virus 2, in patients with advanced solid tumors: A multicenter, phase I/II clinical trial. J. Immunother. Cancer 2021, 9, e002224. [Google Scholar] [CrossRef]
- Tan, Z.; Wu, Y.; Fan, Z.; Gao, T.; Ding, S.; Han, L.; Luo, S.; Fan, Q.; Shi, J.; Bai, C.; et al. Intratumoral oncolytic virus OH2 injection in patients with locally advanced or metastatic sarcoma: A phase 1/2 trial. J. Immunother. Cancer 2025, 13, e010543. [Google Scholar] [CrossRef]
- Brown, M.C.; Gromeier, M. Cytotoxic and immunogenic mechanisms of recombinant oncolytic poliovirus. Curr. Opin. Virol. 2015, 13, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Walton, R.W.; Brown, M.C.; Sacco, M.T.; Gromeier, M. Engineered Oncolytic Poliovirus PVSRIPO Subverts MDA5-Dependent Innate Immune Responses in Cancer Cells. J. Virol. 2018, 92, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.C.; Holl, E.K.; Boczkowski, D.; Dobrikova, E.; Mosaheb, M.; Chandramohan, V.; Bigner, D.D.; Gromeier, M.; Nair, S.K. Cancer immunotherapy with recombinant poliovirus induces IFN-dominant activation of dendritic cells and tumor antigen-specific CTLs. Sci. Transl. Med. 2017, 9, eaan4220. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Brown, M.C.; Zhang, G.; Stevenson, K.; Mohme, M.; Kornahrens, R.; Bigner, D.D.; Ashley, D.M.; López, G.Y.; Gromeier, M. Polio virotherapy targets the malignant glioma myeloid infiltrate with diffuse microglia activation engulfing the CNS. Neuro-Oncol. 2023, 25, 1631–1643. [Google Scholar] [CrossRef]
- Ochiai, H.; Campbell, S.A.; Archer, G.E.; Chewning, T.A.; Dragunsky, E.; Ivanov, A.; Gromeier, M.; Sampson, J.H. Targeted therapy for glioblastoma multiforme neoplastic meningitis with intrathecal delivery of an oncolytic recombinant poliovirus. Clin. Cancer Res. An. Off. J. Am. Assoc. Cancer Res. 2006, 12, 1349–1354. [Google Scholar] [CrossRef]
- Beasley, G.M.; Nair, S.K.; Farrow, N.E.; Landa, K.; Selim, M.A.; Wiggs, C.A.; Jung, S.H.; Bigner, D.D.; True Kelly, A.; Gromeier, M.; et al. Phase I trial of intratumoral PVSRIPO in patients with unresectable, treatment-refractory melanoma. J. Immunother. Cancer 2021, 9, e002203. [Google Scholar] [CrossRef]
- Desjardins, A.; Gromeier, M.; Herndon, J.E.; Beaubier, N.; Bolognesi, D.P.; Friedman, A.H.; Friedman, H.S.; McSherry, F.; Muscat, A.M.; Nair, S.; et al. Recurrent Glioblastoma Treated with Recombinant Poliovirus. N. Engl. J. Med. 2018, 379, 150–161. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, Y.A.; Wang, E.; Chen, N.; Danner, R.L.; Munson, P.J.; Marincola, F.M.; Szalay, A.A. Eradication of solid human breast tumors in nude mice with an intravenously injected light-emitting oncolytic vaccinia virus. Cancer Res. 2007, 67, 10038–10046. [Google Scholar] [CrossRef] [PubMed]
- Ascierto, M.L.; Worschech, A.; Yu, Z.; Adams, S.; Reinboth, J.; Chen, N.G.; Pos, Z.; Roychoudhuri, R.; Di Pasquale, G.; Bedognetti, D.; et al. Permissivity of the NCI-60 cancer cell lines to oncolytic Vaccinia Virus GLV-1h68. BMC Cancer 2011, 11, 451. [Google Scholar] [CrossRef] [PubMed]
- Chintala, N.K.; Choe, J.K.; McGee, E.; Bellis, R.; Saini, J.K.; Banerjee, S.; Moreira, A.L.; Zauderer, M.G.; Adusumilli, P.S.; Rusch, V.W. Correlative analysis from a phase I clinical trial of intrapleural administration of oncolytic vaccinia virus (Olvi-vec) in patients with malignant pleural mesothelioma. Front. Immunol. 2023, 14, 1112960. [Google Scholar] [CrossRef]
- Holloway, R.W.; Mendivil, A.A.; Kendrick, J.E.; Abaid, L.N.; Brown, J.V.; LeBlanc, J.; McKenzie, N.D.; Mori, K.M.; Ahmad, S. Clinical Activity of Olvimulogene Nanivacirepvec-Primed Immunochemotherapy in Heavily Pretreated Patients With Platinum-Resistant or Platinum-Refractory Ovarian Cancer: The Nonrandomized Phase 2 VIRO-15 Clinical Trial. JAMA Oncol. 2023, 9, 903–908. [Google Scholar] [CrossRef]
- Carew, J.S.; Espitia, C.M.; Zhao, W.; Kelly, K.R.; Coffey, M.; Freeman, J.W.; Nawrocki, S.T. Reolysin is a novel reovirus-based agent that induces endoplasmic reticular stress-mediated apoptosis in pancreatic cancer. Cell Death Dis. 2013, 4, e728. [Google Scholar] [CrossRef]
- Noonan, A.M.; Farren, M.R.; Geyer, S.M.; Huang, Y.; Tahiri, S.; Ahn, D.; Mikhail, S.; Ciombor, K.K.; Pant, S.; Aparo, S.; et al. Randomized Phase 2 Trial of the Oncolytic Virus Pelareorep (Reolysin) in Upfront Treatment of Metastatic Pancreatic Adenocarcinoma. Mol. Ther. J. Am. Soc. Gene Ther. 2016, 24, 1150–1158. [Google Scholar] [CrossRef] [PubMed]
- Mahalingam, D.; Goel, S.; Aparo, S.; Patel Arora, S.; Noronha, N.; Tran, H.; Chakrabarty, R.; Selvaggi, G.; Gutierrez, A.; Coffey, M.; et al. A Phase II Study of Pelareorep (REOLYSIN®) in Combination with Gemcitabine for Patients with Advanced Pancreatic Adenocarcinoma. Cancers 2018, 10, 160. [Google Scholar] [CrossRef]
- Mahalingam, D.; Wilkinson, G.A.; Eng, K.H.; Fields, P.; Raber, P.; Moseley, J.L.; Cheetham, K.; Coffey, M.; Nuovo, G.; Kalinski, P.; et al. Pembrolizumab in Combination with the Oncolytic Virus Pelareorep and Chemotherapy in Patients with Advanced Pancreatic Adenocarcinoma: A Phase Ib Study. Clin. Cancer Res. An. Off. J. Am. Assoc. Cancer Res. 2020, 26, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, V.; Ellard, S.L.; Dent, S.F.; Tu, D.; Mates, M.; Dhesy-Thind, S.K.; Panasci, L.; Gelmon, K.A.; Salim, M.; Song, X.; et al. A randomized phase II study of weekly paclitaxel with or without pelareorep in patients with metastatic breast cancer: Final analysis of Canadian Cancer Trials Group IND.213. Breast Cancer Res. Treat. 2018, 167, 485–493. [Google Scholar] [CrossRef]
- Clark, A.S.; Zhao, F.; Klein, P.; Montero, A.J.; Falkson, C.; Krill-Jackson, E.; Rowland, K.; Sardesai, S.; Incorvati, J.; Dillon, P.; et al. A Phase II Randomized Study of Paclitaxel Alone or Combined with Pelareorep with or without Avelumab in Metastatic Hormone Receptor-Positive Breast Cancer: The BRACELET-01/PrE0113 Study. Clin. Cancer Res. An. Off. J. Am. Assoc. Cancer Res. 2025, 31, 2655–2662. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhang, H.; Xue, M.; Zheng, C.; Chen, Q. HSV-1 as a gene delivery platform for cancer gene therapy. Trends Pharmacol. Sci. 2025, 46, 324–336. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, Y.; Zhuang, J.; Liu, R.; Sun, C. Demystifying the cGAS-STING pathway: Precision regulation in the tumor immune microenvironment. Mol. Cancer 2025, 24, 178. [Google Scholar] [CrossRef]
- Zhou, C.; Chen, X.; Planells-Cases, R.; Chu, J.; Wang, L.; Cao, L.; Li, Z.; López-Cayuqueo, K.I.; Xie, Y.; Ye, S.; et al. Transfer of cGAMP into Bystander Cells via LRRC8 Volume-Regulated Anion Channels Augments STING-Mediated Interferon Responses and Anti-viral Immunity. Immunity 2020, 52, 767–781.e6. [Google Scholar] [CrossRef]
- Pan, S.; Liu, X.; Ma, Y.; Cao, Y.; He, B. Herpes Simplex Virus 1 γ(1)34.5 Protein Inhibits STING Activation That Restricts Viral Replication. J. Virol. 2018, 92, e01015-18. [Google Scholar] [CrossRef]
- Song, X.; Wang, Y.; Zou, W.; Wang, Z.; Cao, W.; Liang, M.; Li, F.; Zeng, Q.; Ren, Z.; Wang, Y.; et al. Inhibition of mitophagy via the EIF2S1-ATF4-PRKN pathway contributes to viral encephalitis. J. Adv. Res. 2025, 73, 199–217. [Google Scholar] [CrossRef] [PubMed]
- York, I.A.; Roop, C.; Andrews, D.W.; Riddell, S.R.; Graham, F.L.; Johnson, D.C. A cytosolic herpes simplex virus protein inhibits antigen presentation to CD8+ T lymphocytes. Cell 1994, 77, 525–535. [Google Scholar] [CrossRef]
- Pourchet, A.; Fuhrmann, S.R.; Pilones, K.A.; Demaria, S.; Frey, A.B.; Mulvey, M.; Mohr, I. CD8(+) T-cell Immune Evasion Enables Oncolytic Virus Immunotherapy. EBioMedicine 2016, 5, 59–67. [Google Scholar] [CrossRef]
- Chai, H.H.; Kim, T.H.; Kim, Y.R.; Lim, D. Structure and function of the porcine TAP protein and its inhibition by the viral immune evasion protein ICP47. Int. J. Biol. Macromol. 2021, 178, 514–526. [Google Scholar] [CrossRef]
- Huang, Z.; Wu, S.Q.; Liang, Y.; Zhou, X.; Chen, W.; Li, L.; Wu, J.; Zhuang, Q.; Chen, C.; Li, J.; et al. RIP1/RIP3 binding to HSV-1 ICP6 initiates necroptosis to restrict virus propagation in mice. Cell Host Microbe 2015, 17, 229–242. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Liu, S.; Yu, X.; Li, L.; Shi, C.; He, W.; Li, J.; Xu, L.; Hu, Z.; et al. Direct activation of RIP3/MLKL-dependent necrosis by herpes simplex virus 1 (HSV-1) protein ICP6 triggers host antiviral defense. Proc. Natl. Acad. Sci. USA 2014, 111, 15438–15443. [Google Scholar] [CrossRef]
- Ding, Z.; Wang, R.; Li, Y.; Wang, X. MLKL activates the cGAS-STING pathway by releasing mitochondrial DNA upon necroptosis induction. Mol. Cell 2025, 85, 2610–2625.e5. [Google Scholar] [CrossRef]
- Macedo, N.; Miller, D.M.; Haq, R.; Kaufman, H.L. Clinical landscape of oncolytic virus research in 2020. J. Immunother Cancer 2020, 8, e001486. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Shen, Y.; Liang, T. Oncolytic virotherapy: Basic principles, recent advances and future directions. Signal Transduct. Target. Ther. 2023, 8, 156. [Google Scholar] [CrossRef]
- Becher, B.; Tugues, S.; Greter, M. GM-CSF: From Growth Factor to Central Mediator of Tissue Inflammation. Immunity 2016, 45, 963–973. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wan, R.; Duan, J.; Yuan, L.; Wang, Z.; Zhong, J.; Zhang, X.; Ma, Z.; Bai, H.; Wang, J. Targeting tumor-intrinsic S100 calcium-binding protein A1 augments antitumor immunity and potentiates immunotherapy efficacy. Signal Transduct. Target. Ther. 2025, 10, 99. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Mansurov, A.; Kurtanich, T.; Chun, H.R.; Slezak, A.J.; Volpatti, L.R.; Chang, K.; Wang, T.; Alpar, A.T.; Refvik, K.C.; et al. Engineered GM-CSF polarizes protumorigenic tumor-associated macrophages to an antitumorigenic phenotype and potently synergizes with IL-12 immunotherapy. J. Immunother. Cancer 2024, 12, e009541. [Google Scholar] [CrossRef]
- Liu, M.; Hu, S.; Yan, N.; Popowski, K.D.; Cheng, K. Inhalable extracellular vesicle delivery of IL-12 mRNA to treat lung cancer and promote systemic immunity. Nat. Nanotechnol. 2024, 19, 565–575. [Google Scholar] [CrossRef]
- Azar, F.; Deforges, J.; Demeusoit, C.; Kleinpeter, P.; Remy, C.; Silvestre, N.; Foloppe, J.; Fend, L.; Spring-Giusti, C.; Quéméneur, E.; et al. TG6050, an oncolytic vaccinia virus encoding interleukin-12 and anti-CTLA-4 antibody, favors tumor regression via profound immune remodeling of the tumor microenvironment. J. Immunother. Cancer 2024, 12, e009302. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Le, H.; Zhang, X.; Dai, Y.; Guo, F.; Ran, X.; Hu, G.; Xie, Q.; Wang, D.; Cai, Y. Identification of restrictive molecules involved in oncolytic virotherapy using genome-wide CRISPR screening. J. Hematol. Oncol. 2024, 17, 36. [Google Scholar] [CrossRef]
- Fu, R.; Qi, R.; Xiong, H.; Lei, X.; Jiang, Y.; He, J.; Chen, F.; Zhang, L.; Qiu, D.; Chen, Y.; et al. Combination therapy with oncolytic virus and T cells or mRNA vaccine amplifies antitumor effects. Signal Transduct. Target. Ther. 2024, 9, 118. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, J.; Luo, J.; Wu, W.; Luo, H.; Wei, W.; Lyu, H.; Wang, Y.; Yi, H.; Zhang, Y.; et al. Lactobacillus acidophilus potentiates oncolytic virotherapy through modulating gut microbiota homeostasis in hepatocellular carcinoma. Nat. Commun. 2025, 16, 3315. [Google Scholar] [CrossRef]
- Wedge, M.E.; Jennings, V.A.; Crupi, M.J.F.; Poutou, J.; Jamieson, T.; Pelin, A.; Pugliese, G.; de Souza, C.T.; Petryk, J.; Laight, B.J.; et al. Virally programmed extracellular vesicles sensitize cancer cells to oncolytic virus and small molecule therapy. Nat. Commun. 2022, 13, 1898. [Google Scholar] [CrossRef]
- Tian, L.; Xu, B.; Chen, Y.; Li, Z.; Wang, J.; Zhang, J.; Ma, R.; Cao, S.; Hu, W.; Chiocca, E.A.; et al. Specific targeting of glioblastoma with an oncolytic virus expressing a cetuximab-CCL5 fusion protein via innate and adaptive immunity. Nat. Cancer 2022, 3, 1318–1335. [Google Scholar] [CrossRef] [PubMed]
- Groeneveldt, C.; van den Ende, J.; van Montfoort, N. Preexisting immunity: Barrier or bridge to effective oncolytic virus therapy? Cytokine Growth Factor Rev. 2023, 70, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Burton, D.R. Antiviral neutralizing antibodies: From in vitro to in vivo activity. Nat. Rev. Immunol. 2023, 23, 720–734. [Google Scholar] [CrossRef]
- Shin, D.H.; Jiang, H.; Gillard, A.G.; Kim, D.; Fan, X.; Singh, S.K.; Nguyen, T.T.; Sohoni, S.S.; Lopez-Rivas, A.R.; Parthasarathy, A.; et al. Chimeric oncolytic adenovirus evades neutralizing antibodies from human patients and exhibits enhanced anti-glioma efficacy in immunized mice. Mol. Ther. J. Am. Soc. Gene Ther. 2024, 32, 722–733. [Google Scholar] [CrossRef]
- Dai, Z.; Si, Y.; Xiong, S.; Li, Y.; Ye, J.; Gao, Q.; Ma, D.; Jin, X.; Li, F. Chimeric Ad5/35 oncolytic adenovirus overcome preexisting neutralizing antibodies and enhance tumor targeting efficiency. Cancer Gene Ther. 2025, 32, 418–436. [Google Scholar] [CrossRef] [PubMed]
- Mato-Berciano, A.; Morgado, S.; Maliandi, M.V.; Farrera-Sal, M.; Gimenez-Alejandre, M.; Ginestà, M.M.; Moreno, R.; Torres-Manjon, S.; Moreno, P.; Arias-Badia, M.; et al. Oncolytic adenovirus with hyaluronidase activity that evades neutralizing antibodies: VCN-11. J. Control. Release Off. J. Control. Release Soc. 2021, 332, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Jeon, Y.H.; Yoo, J.; Shin, S.K.; Lee, S.; Park, M.J.; Jung, B.J.; Hong, Y.K.; Lee, D.S.; Oh, K. Generation of novel oncolytic vaccinia virus with improved intravenous efficacy through protection against complement-mediated lysis and evasion of neutralization by vaccinia virus-specific antibodies. J. Immunother. Cancer 2023, 11, e006024. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Wang, B.; Chen, Q.; Fu, X.; Jiang, C.; Lin, Z.; Zhuang, Q.; Zeng, Y.; Liu, X.; Zhang, D. Systemic delivery of glycosylated-PEG-masked oncolytic virus enhances targeting of antitumor immuno-virotherapy and modulates T and NK cell infiltration. Theranostics 2023, 13, 5452–5468. [Google Scholar] [CrossRef]
- Huang, H.; Liu, M.; Sun, M.; Duan, S.; Pan, S.; Liu, P.; Cheng, Z.; Ergonul, O.; Can, F.; Wang, Z.; et al. Virus-Protein Corona Replacement Strategy to Improve the Antitumor Efficacy of Intravenously Injected Oncolytic Adenovirus. ACS Nano 2023, 17, 14461–14474. [Google Scholar] [CrossRef]
- Groeneveldt, C.; Kinderman, P.; Griffioen, L.; Rensing, O.; Labrie, C.; van den Wollenberg, D.J.M.; Hoeben, R.C.; Coffey, M.; Loghmani, H.; Verdegaal, E.M.E.; et al. Neutralizing Antibodies Impair the Oncolytic Efficacy of Reovirus but Permit Effective Combination with T cell-Based Immunotherapies. Cancer Immunol. Res. 2024, 12, 334–349. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, X.; Bao, W.; Liu, G.; Wei, W.; Ping, Y. An oncolytic virus-T cell chimera for cancer immunotherapy. Nat. Biotechnol. 2024, 42, 1876–1887. [Google Scholar] [CrossRef]
- Pakola, S.A.; Peltola, K.J.; Clubb, J.H.A.; Jirovec, E.; Haybout, L.; Kudling, T.V.; Alanko, T.; Korpisaari, R.; Juteau, S.; Jaakkola, M.; et al. Safety, Efficacy, and Biological Data of T-Cell-Enabling Oncolytic Adenovirus TILT-123 in Advanced Solid Cancers from the TUNIMO Monotherapy Phase I Trial. Clin. Cancer Res. An. Off. J. Am. Assoc. Cancer Res. 2024, 30, 3715–3725. [Google Scholar] [CrossRef]
- Block, M.S.; Clubb, J.H.A.; Mäenpää, J.; Pakola, S.; Quixabeira, D.C.A.; Kudling, T.; Jirovec, E.; Haybout, L.; van der Heijden, M.; Zahraoui, S.; et al. The oncolytic adenovirus TILT-123 with pembrolizumab in platinum resistant or refractory ovarian cancer: The phase 1a PROTA trial. Nat. Commun. 2025, 16, 1381. [Google Scholar] [CrossRef]
- Havunen, R.; Siurala, M.; Sorsa, S.; Grönberg-Vähä-Koskela, S.; Behr, M.; Tähtinen, S.; Santos, J.M.; Karell, P.; Rusanen, J.; Nettelbeck, D.M.; et al. Oncolytic Adenoviruses Armed with Tumor Necrosis Factor Alpha and Interleukin-2 Enable Successful Adoptive Cell Therapy. Mol. Ther. Oncolytics 2017, 4, 77–86. [Google Scholar] [CrossRef]
- Jirovec, E.; Quixabeira, D.C.A.; Clubb, J.H.A.; Pakola, S.A.; Kudling, T.; Arias, V.; Haybout, L.; Jalkanen, K.; Alanko, T.; Monberg, T.; et al. Single intravenous administration of oncolytic adenovirus TILT-123 results in systemic tumor transduction and immune response in patients with advanced solid tumors. J. Exp. Clin. Cancer Res. CR 2024, 43, 297. [Google Scholar] [CrossRef]
- Ayele, K.; Wakimoto, H.; Saha, D. An oncolytic adenovirus co-expressing a bi-specific T cell engager and IL-2 for the treatment of ovarian cancer. Mol. Ther. 2024, 32, 2810–2813. [Google Scholar] [CrossRef]
- Tian, L.; Xu, B.; Teng, K.Y.; Song, M.; Zhu, Z.; Chen, Y.; Wang, J.; Zhang, J.; Feng, M.; Kaur, B.; et al. Targeting Fc Receptor-Mediated Effects and the “Don’t Eat Me” Signal with an Oncolytic Virus Expressing an Anti-CD47 Antibody to Treat Metastatic Ovarian Cancer. Clin. Cancer Res. An. Off. J. Am. Assoc. Cancer Res. 2022, 28, 201–214. [Google Scholar] [CrossRef]
- Howard, F.H.N.; Al-Janabi, H.; Patel, P.; Cox, K.; Smith, E.; Vadakekolathu, J.; Pockley, A.G.; Conner, J.; Nohl, J.F.; Allwood, D.A.; et al. Nanobugs as Drugs: Bacterial Derived Nanomagnets Enhance Tumor Targeting and Oncolytic Activity of HSV-1 Virus. Small 2022, 18, e2104763. [Google Scholar] [CrossRef]
- Lowenstein, P.R.; Varela, M.L.; Castro, M.G. The discrete charm of oncolytic viruses: Toward the finish line. Cancer Cell 2025, 43, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, T.E.; Knol, L.I.; Haas, F.V.; Hartley, A.; Pernickel, S.C.S.; Jády, A.; Finkbeiner, M.S.C.; Achberger, J.; Arelaki, S.; Modic, Ž.; et al. Biomarker screen for efficacy of oncolytic virotherapy in patient-derived pancreatic cancer cultures. EBioMedicine 2024, 105, 105219. [Google Scholar] [CrossRef]
- Dai, W.; Tian, R.; Yu, L.; Bian, S.; Chen, Y.; Yin, B.; Luan, Y.; Chen, S.; Fan, Z.; Yan, R.; et al. Overcoming therapeutic resistance in oncolytic herpes virotherapy by targeting IGF2BP3-induced NETosis in malignant glioma. Nat. Commun. 2024, 15, 131. [Google Scholar] [CrossRef]
- Chen, X.; Liu, J.; Li, Y.; Zeng, Y.; Wang, F.; Cheng, Z.; Duan, H.; Pan, G.; Yang, S.; Chen, Y.; et al. IDH1 mutation impairs antiviral response and potentiates oncolytic virotherapy in glioma. Nat. Commun. 2023, 14, 6781. [Google Scholar] [CrossRef]
- Jarnagin, W.R.; Zager, J.S.; Klimstra, D.; Delman, K.A.; Malhotra, S.; Ebright, M.; Little, S.; DeRubertis, B.; Stanziale, S.F.; Hezel, M.; et al. Neoadjuvant treatment of hepatic malignancy: An oncolytic herpes simplex virus expressing IL-12 effectively treats the parent tumor and protects against recurrence-after resection. Cancer Gene Ther. 2003, 10, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, X.; Li, Z.; Gao, S.; Mao, L.; Dai, J.; Li, C.; Cui, C.; Chi, Z.; Sheng, X.; et al. Neoadjuvant oncolytic virus orienx010 and toripalimab in resectable acral melanoma: A phase Ib trial. Signal Transduct. Target. Ther. 2024, 9, 318. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois-Daigneault, M.C.; Roy, D.G.; Aitken, A.S.; El Sayes, N.; Martin, N.T.; Varette, O.; Falls, T.; St-Germain, L.E.; Pelin, A.; Lichty, B.D.; et al. Neoadjuvant oncolytic virotherapy before surgery sensitizes triple-negative breast cancer to immune checkpoint therapy. Sci. Transl. Med. 2018, 10, eaao1641. [Google Scholar] [CrossRef] [PubMed]

| Agent | Virus | Construction Scheme | Indication | Route of Administration * |
|---|---|---|---|---|
| H101 | Adenovirus | Deleted E1B-55kD | HNSCC | * |
| T-VEC | HSV-1 | Expressing GM-CSF | Melanoma | * |
| JX-594 | Poxvirus | Deleted TK, expressing GM-CSF | HCC | Intravenous injection |
| G47Δ | HSV-1 | Deleted ICP34.5 and ICP47, inactivated ICP6 | GBM | * |
| RP1 | HSV-1 | Expressing GALV-GP-R- and GM-CSF | Melanoma | * |
| CG0070 | Adenovirus | Deleted E1A, expressing GM-CSF | NMIBC | Intravesical injection |
| OH2 | HSV-2 | Deleted ICP34.5 and ICP47, expressing GM-CSF | Melanoma | * |
| PVSRIPO | Polio–rhinovirus chimera | Polio–rhinovirus chimera, expressing CD155 | Melanoma and GBM | Intratumoral and intrathecal injection |
| Olvi-Vec | Vaccinia virus | Replacing the TK, hemagglutinin, and F145L genes with three expression cassettes encoding β-galactosidase, β-glucuronidase, and RLuc-GFP fusion proteins | OV and lung cancer | Intraperitoneal injection and intrathoracic injection |
| Reolysin | Reovirus | A type 3 oncolytic reovirus hijacking the Ras signaling pathway | Breast cancer and MPA | Intravenous injection |
| VG161 | HSV-1 | Deleted ICP34.5, expressing IL-12, IL-15, IL-15Rα, and PD-1/PD-L1 | HCC | * |
| NDV-GT | Newcastle disease virus | Expressing porcine α1,3 GT gene | HCC | Intravenous injection |
| Agents | Indications | NCT Number | Enrollment | Phase | Arms | ORR | OS | TRAEs * | PMID |
|---|---|---|---|---|---|---|---|---|---|
| H101 | Head and neck or esophagus squamous cell cancer | - | 160 | 3 | A: H101 + PF/AF B: PF or AF | H101 + PF = 78.8% PF = 39.6% H101 + AF = 50.0% AF = 50.0% | Unknown | None | 15601557 |
| H101 | Refractory malignant ascites | NCT04771676 | 25 | 2 | H101 | Unknown | Increased median time to repeat paracentesis of 45 days from 13 days. | 8.0% | 38659226 |
| T-VEC | Unresected stage IIIB to IV melanoma | NCT00769704 | 436 | 3 | A: T-VEC B: GM-CSF | The ORR was 26.4% in arm A. | The median OS was 23.3 months with T-VEC and 18.9 months with GM-CSF. | A: 13.4% B: 7.1% | 26014293 |
| T-VEC | Unresected stage IIIB to IVM1c melanoma | NCT02263508 (Terminated) | 692 | 3 | A: T-VEC + Pembrolizumab B: Placebo + Pembrolizumab | The ORR was 48.6% in arm A and 41.3% in arm B. | T-VEC-pembrolizumab did not significantly improve PFS or OS compared with arm B. | A: 20.7% B: 19.5% | 35998300 |
| T-VEC | Unresected stage IIIB to IVM1c melanoma | NCT02211131 | 150 | 2 | A: Neoadjuvant T-VEC + surgery B: Surgery alone | Pathological CR was 17.1% in arm A. | The 2-year OS was 88.9% for arm A and 77.4% for arm B. | 5.5% | 34608333 |
| T-VEC | Unresected stage IIIB to IV melanoma | NCT01740297 | 198 | 2 | A: T-VEC + Ipilimumab B: Ipilimumab alone | The ORR was improved from 16.0% in arm B to 35.7% in arm A. | The estimated 5-year OS increased from 48.4% in arm B to 54.7% in arm A. | A: 46.3% B: 43.2% | 37142291 |
| JX-594 | Advanced hepatocellular carcinoma | NCT01721772 (Terminated) | 459 | 3 | A: JX-594 + Sorafenib B: Sorafenib alone | The ORR was 19.2% in arm A and 20.9% in arm B. | The median OS was 12.7 months in arm A and 14.0 months in arm B. | A: 53.7% B: 35.5% | 38756145 |
| RP1 | Anti-PD-1-failed melanoma | NCT03767348 | 140 | 2 | RP1 + Nivolumab | The ORR was 32.9% | Overall survival rates at 1 and 2 years were 75.3% and 63.3%, respectively. | 12.9% | 40627813 |
| CG0070 | BCG-unresponsive non-muscle-invasive bladder cancer | NCT02365818 | 45 | 2 | CG0070 | The overall 6-month CR was 47.0%. | Unknown | 4.5% | 28755959 |
| PVSRIPO | Recurrent supratentorial glioblastoma | NCT04479241 | 25 | 2 | PVSRIPO + Pembrolizumab | Unknown | The median OS was 10.2 months. | 32.0% | Unpublished |
| PVSRIPO | Recurrent WHO grade IV malignant glioma | NCT02986178 | 121 | 2 | A: PVSRIPO B: PVSRIPO + Lomustine | The ORR was 5.4% in arm A and 7.4% in arm B. | The median 5-year OS was 7.0 months in arm A and 7.1 months in arm B. | A: 18.1% B: 30.8% | Unpublished |
| Olvi-Vec | Platinum-resistant or platinum-refractory ovarian cancer | NCT02759588 | 27 | 2 | Olvi-Vec + platinum-based chemotherapy with or without bevacizumab | The ORR was 54.0%. | The median OS was 15.7 months in all patients. | 11.1% | 37227734 |
| Reolysin | Hormone receptor+, HER2-metastatic breast cancer | NCT04215146 | 48 | 2 | A: Paclitaxel B: Paclitaxel + Reolysin C: Paclitaxel + Reolysin + avelumab | The 16-week ORR was 20.0% (arm A), 31.0% (arm B), and 14.0% (arm C). | The median PFS was 6.4 (arm A), 12.1 (arm B), and 5.8 months (arm C). | A: 16.7% B: 87.5% C: 148.0% | 40300087 |
| Indications | Trial Identifier | Agent | Estimated Enrollment | Arms | Estimated Study Completion Date |
|---|---|---|---|---|---|
| Patients with unresectable or metastatic melanoma who have failed at least second-line standard therapy | NCT05868707 | OH2 | 340 | A: OH2 B: Salvage chemotherapy or best supportive care | March 2027 |
| Patients with unresectable stage IIIb-IV cutaneous melanoma whose disease progressed on an anti-PD-1- and anti-CTLA-4-containing regimen or who are not candidates for treatment with an anti-CTLA-4 therapy | NCT06264180 | RP1 | 400 | A: RP1 + Nivolumab B: Nivolumab + Relatlimab (as Opdualag) C: Anti-PD-1 monotherapy (nivolumab or pembrolizumab) D: Single-agent chemotherapy (dacarbazine, temozolomide, or paclitaxel/albumin-bound paclitaxel) | August 2034 |
| Intermediate risk non-muscle-invasive bladder cancer following transurethral resection of bladder tumor | NCT06111235 | CG0070 | 364 | A: CG0070 B: Surveillance | January 2030 |
| Platinum-resistant/refractory ovarian cancer, fallopian tube cancer, and primary peritoneal cancer | NCT05281471 | Olvi-Vec | 186 | A: Olvi-Vec + Platinum-doublet and bevacizumab B: Chemotherapy and bevacizumab | October 2026 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Sun, Q. Recombinant Oncolytic Viruses: Hexagonal Warriors in the Field of Solid Tumor Immunotherapy. Curr. Issues Mol. Biol. 2025, 47, 878. https://doi.org/10.3390/cimb47110878
Zhang C, Sun Q. Recombinant Oncolytic Viruses: Hexagonal Warriors in the Field of Solid Tumor Immunotherapy. Current Issues in Molecular Biology. 2025; 47(11):878. https://doi.org/10.3390/cimb47110878
Chicago/Turabian StyleZhang, Cong, and Qian Sun. 2025. "Recombinant Oncolytic Viruses: Hexagonal Warriors in the Field of Solid Tumor Immunotherapy" Current Issues in Molecular Biology 47, no. 11: 878. https://doi.org/10.3390/cimb47110878
APA StyleZhang, C., & Sun, Q. (2025). Recombinant Oncolytic Viruses: Hexagonal Warriors in the Field of Solid Tumor Immunotherapy. Current Issues in Molecular Biology, 47(11), 878. https://doi.org/10.3390/cimb47110878







