Study on Autophagy Death of Alpha TC1 Clone 6 (αTC1-6) Cells Induced by Trametenolic Acid Through PI3K/AKT Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Cell Culture
2.3. Cell Viability Assay
2.4. Detection of Glucagon Secretion
2.5. Monodansylcadaverine (MDC) Staining
2.6. Western Blot Analysis
2.7. Statistical Analysis
3. Results
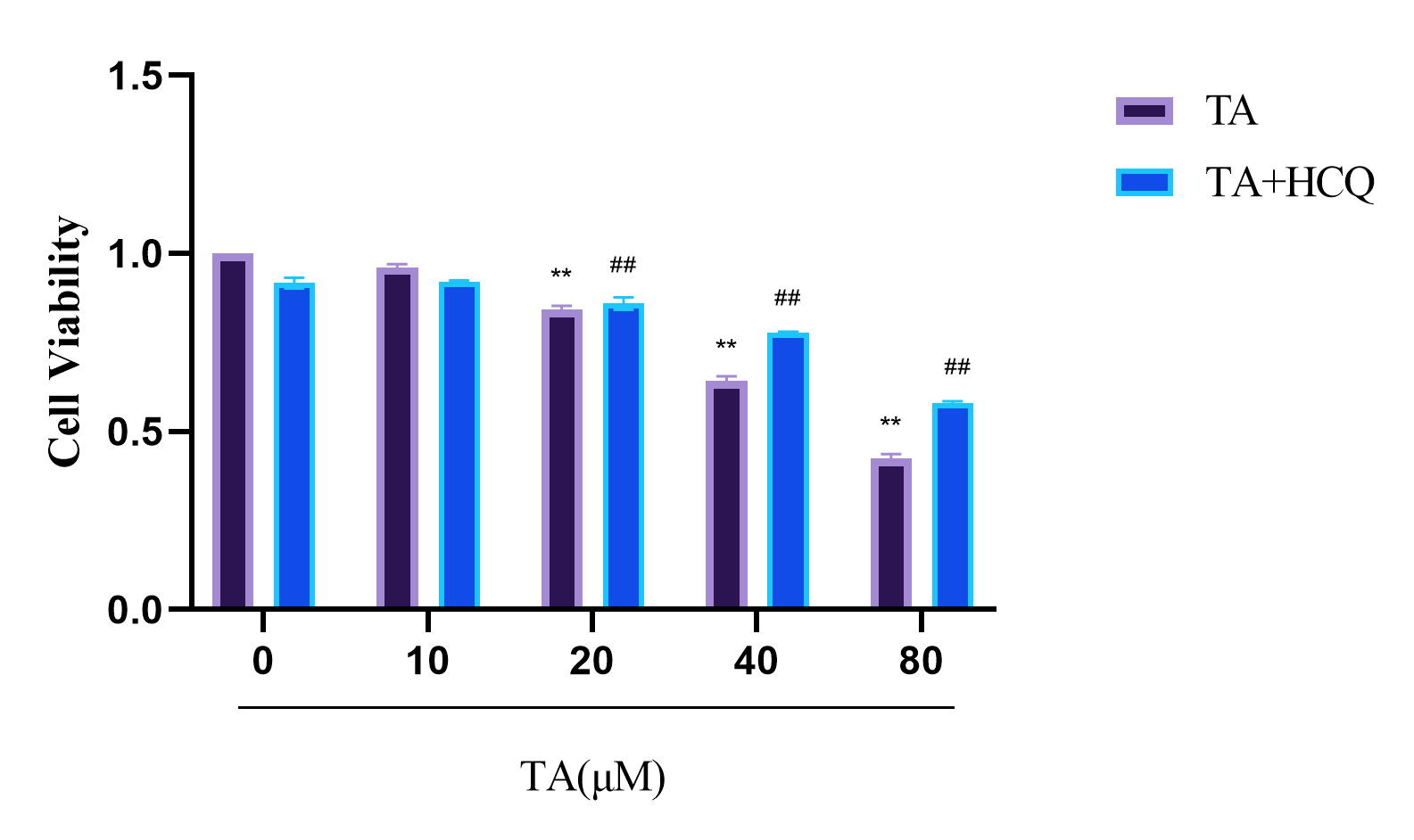
3.1. TA Suppressed the Proliferation of αTC1-6 Cells
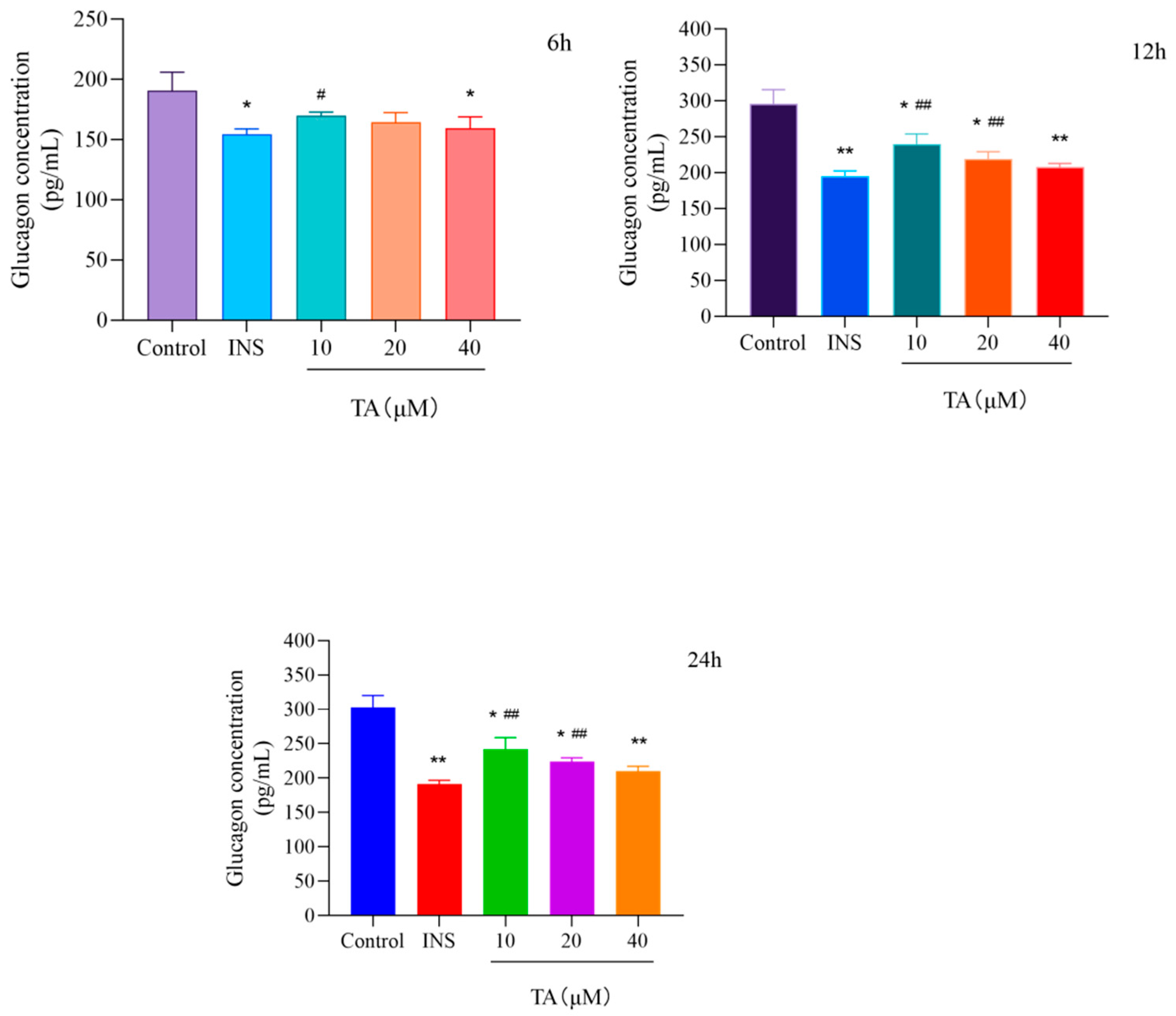
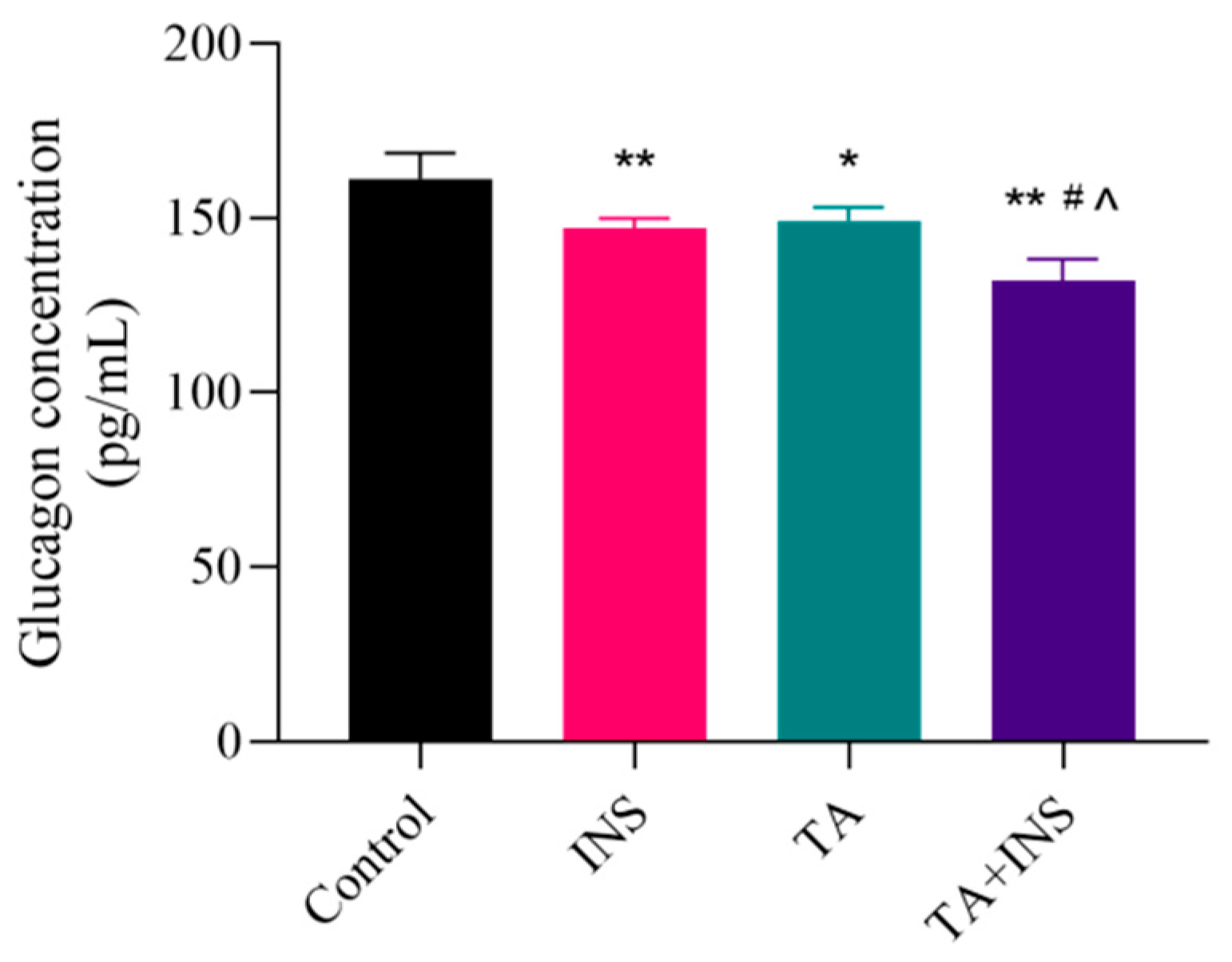
3.2. TA Inhibited Glucagon Secretion in αTC1-6 Cells
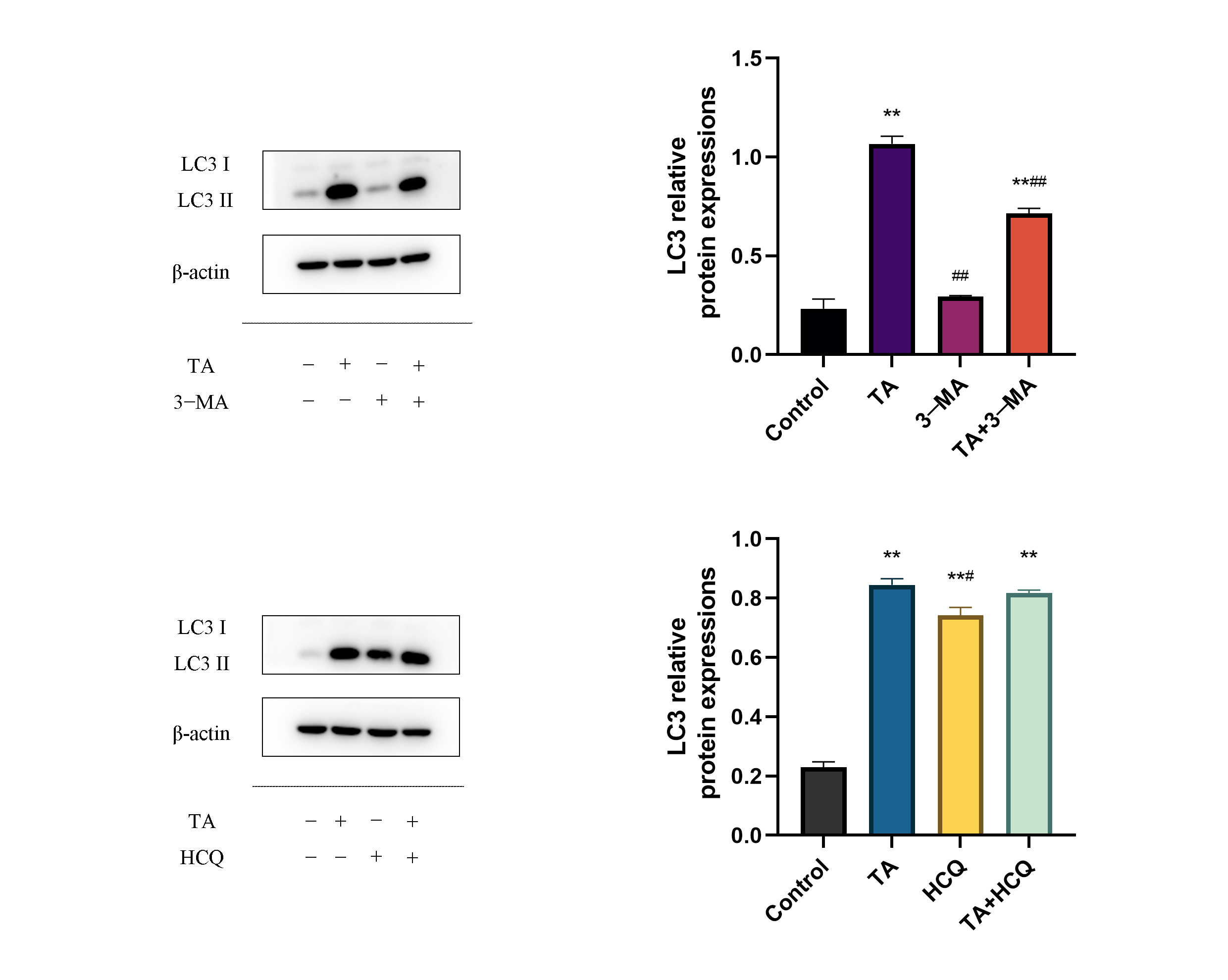
3.3. Effect of TA on the Autophagy of αTC1-6 Cells
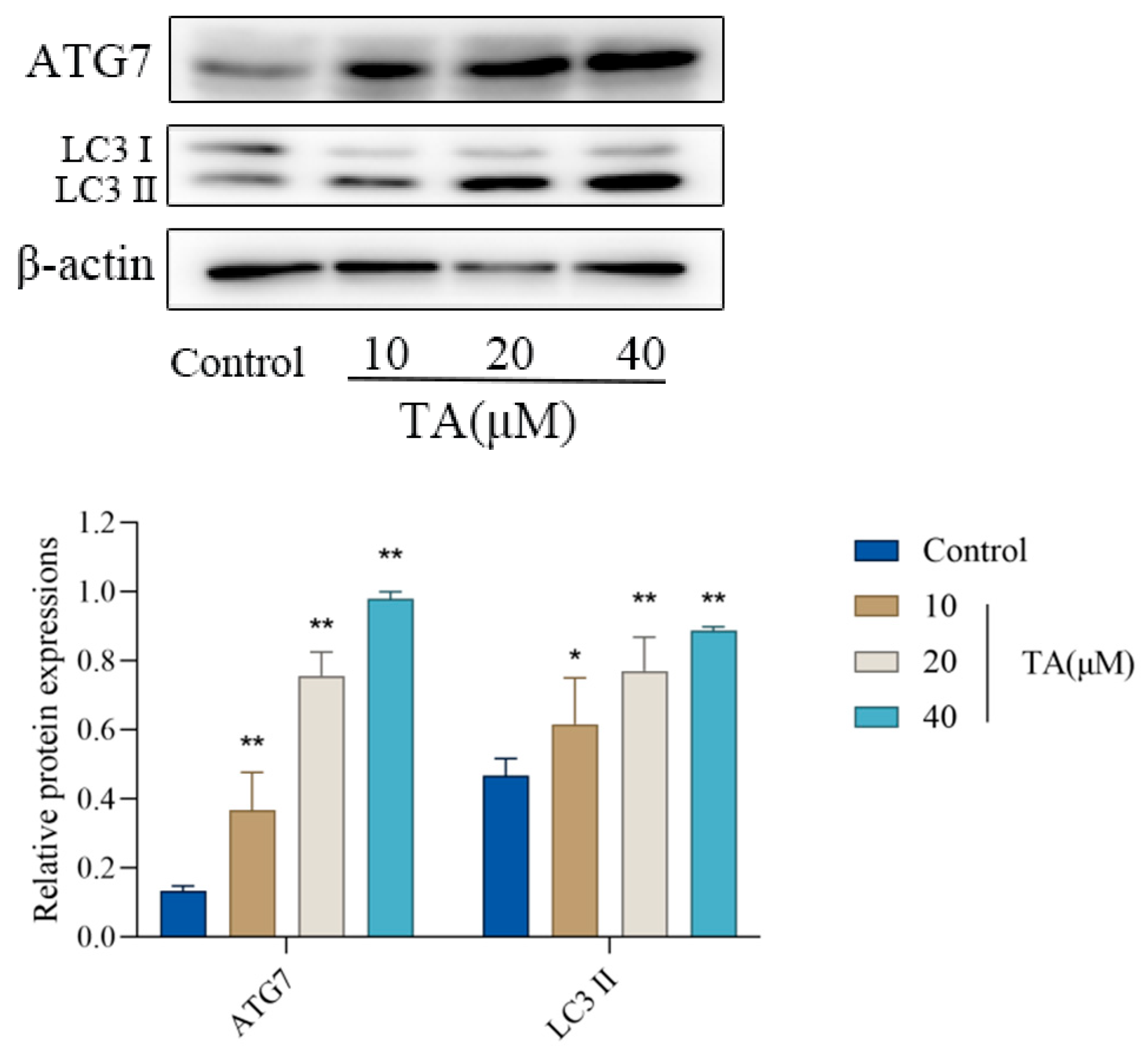
3.4. Effect of TA on the Expression of Autophagy-Related Proteins in αTC1-6 Cells
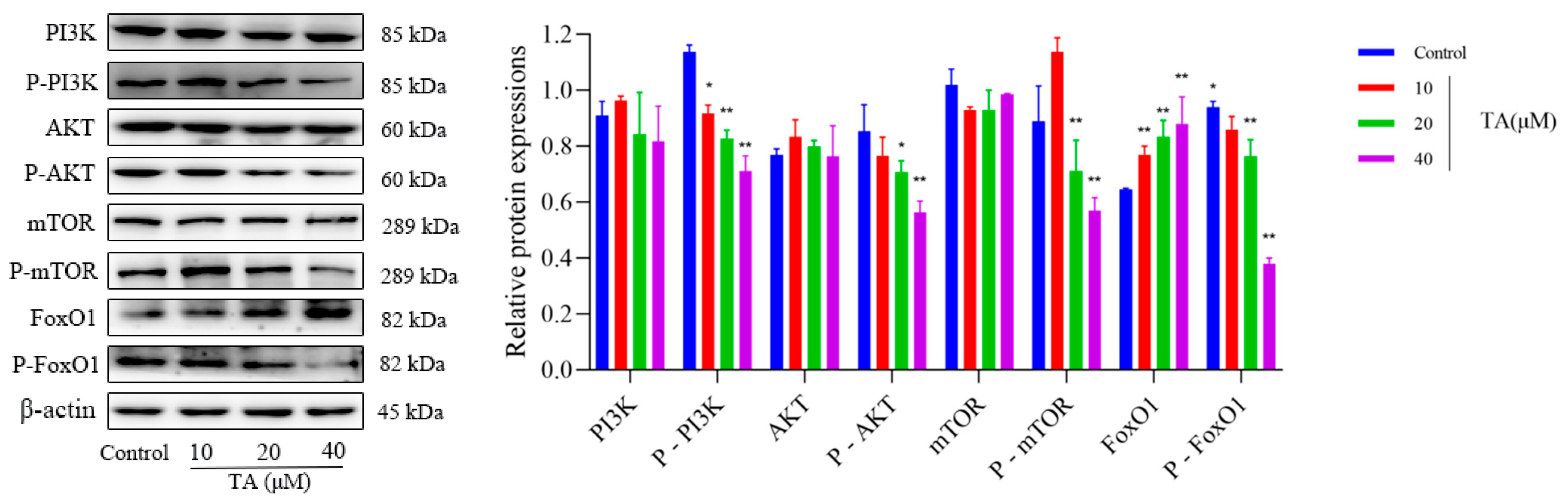
3.5. Effect of TA on PI3K/AKT Pathway Related Protein Expression in αTC1-6 Cells
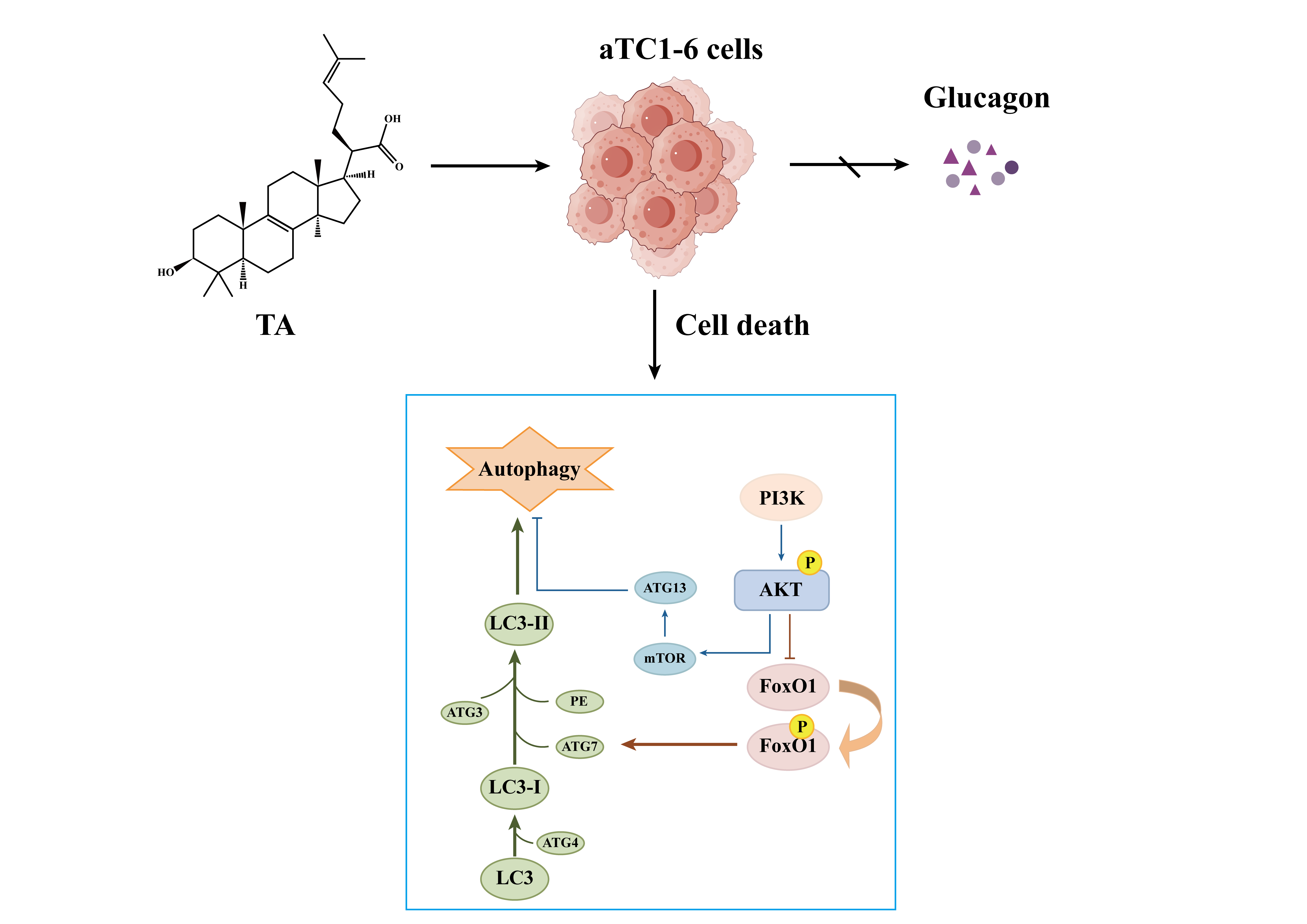
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TA | Trametenolic Acid |
| αTC1-6 | alpha TC1 clone 6 |
| MDC | Monodansylcadaverine |
| GCGN | Glucagonoma |
| INS | Insulin |
References
- Boujan, N.; Géraud, C. Neuropsychiatric symptoms, skin disease, and weight loss: Necrolytic migratory erythema and a glucagonoma. Lancet 2020, 395, 985. [Google Scholar] [CrossRef]
- Perrier, M.; Brugel, M.; Gérard, L.; Goichot, B.; Lièvre, A.; Lepage, C.; Hautefeuille, V.; Cao, C.D.; Smith, D.; Thuillier, P.; et al. Characteristics and treatment options of glucagonomas: A national study from the French Group of Endocrine Tumors and ENDOCAN-RENATEN network. Eur. J. Endocrinol. 2023, 189, 575–583. [Google Scholar] [CrossRef]
- Marini, F.; Giusti, F.; Brandi, M.L. Genetic disorders and insulinoma/glucagonoma. Endocr. Relat. Cancer 2024, 31, e230245. [Google Scholar] [CrossRef] [PubMed]
- Onyango, A.N. Mechanisms of the Regulation and Dysregulation of Glucagon Secretion. Oxidative Med. Cell. Longev. 2020, 2020, 3089139. [Google Scholar] [CrossRef]
- Merkhassine, M.; Coch, R.W.; Frederick, C.E.; Bennett, L.L.; A Peng, S.; Morse, B.; Cummings, B.P.; Loftus, J.P. Glucagon infusion alters the circulating metabolome and urine amino acid excretion in dogs. J. Endocrinol. 2024, 262, e240051. [Google Scholar] [CrossRef]
- Stacpoole, P.W. The glucagonoma syndrome: Clinical features, diagnosis, and treatment. Endocr. Rev. 1981, 2, 347–361. [Google Scholar] [CrossRef] [PubMed]
- Popoviciu, M.S.; Paduraru, L.; Nutas, R.M.; Ujoc, A.M.; Yahya, G.; Metwally, K.; Cavalu, S. Diabetes Mellitus Secondary to Endocrine Diseases: An Update of Diagnostic and Treatment Particularities. Int. J. Mol. Sci. 2023, 24, 12676. [Google Scholar] [CrossRef] [PubMed]
- Andersen, K.Ø.; Detlefsen, S.; Brusgaard, K.; Christesen, H.T. Well-differentiated G1 and G2 pancreatic neuroendocrine tumors: A meta-analysis of published expanded DNA sequencing data. Front. Endocrinol. 2024, 15, 1351624. [Google Scholar] [CrossRef]
- Radny, P.; Eigentler, T.K.; Soennichsen, K.; Overkamp, D.; Raab, H.-R.; Viebahn, R.; Mueller-Horvart, C.; Sotlar, K.; Rassner, G. Metastatic glucagonoma: Treatment with liver transplantation. J. Am. Acad. Dermatol. 2006, 54, 344–347. [Google Scholar] [CrossRef]
- Ye, H.; Ma, S.; Qiu, Z.; Huang, S.; Deng, G.; Li, Y.; Xu, S.; Yang, M.; Shi, H.; Wu, C.; et al. Poria cocos polysaccharides rescue pyroptosis-driven gut vascular barrier disruption in order to alleviates non-alcoholic steatohepatitis. J. Ethnopharmacol. 2022, 296, 115457. [Google Scholar] [CrossRef]
- Wan, J.; Wang, F.; Xiao, Y.; Cheng, Y.; Zheng, S.; Jiang, Q.; Tan, B.; Li, X.; Chen, J.; Liao, S. Poria cocos polysaccharide alleviates dextran sulphate sodium-induced ulcerative colitis in mice by modulating intestinal inflammatory responses and microbial dysbiosis. Int. J. Biol. Macromol. 2024, 283 Pt 2, 137450. [Google Scholar] [CrossRef]
- Zheng, G.; Liu, X.; Abuduwufuer, A.; Yu, H.; He, S.; Ji, W. Poria cocos inhibits the invasion, migration, and epithelial-mesenchymal transition of gastric cancer cells by inducing ferroptosis in cells. Eur. J. Med. Res. 2024, 29, 531. [Google Scholar] [CrossRef]
- Xu, T.; Zhang, H.; Wang, S.; Xiang, Z.; Kong, H.; Xue, Q.; He, M.; Yu, X.; Li, Y.; Sun, D.; et al. A review on the advances in the extraction methods and structure elucidation of Poria cocos polysaccharide and its pharmacological activities and drug carrier applications. Int. J. Biol. Macromol. 2022, 217, 536–551. [Google Scholar] [CrossRef]
- Li, X.; He, Y.; Zeng, P.; Liu, Y.; Zhang, M.; Hao, C.; Wang, H.; Lv, Z.; Zhang, L. Molecular basis for Poria cocos mushroom polysaccharide used as an antitumour drug in China. J. Cell Mol. Med. 2019, 23, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhao, S.; Chen, Q.; Luo, P.; Li, X.; Song, Y.; Pan, S.; Wu, Q.; Zhang, Y.; Shen, X.; et al. Poria cocos polysaccharides ameliorate AOM/DSS-induced colorectal cancer in mice by remodeling intestinal microbiota composition and enhancing intestinal barrier function. Int. J. Biol. Macromol. 2025, 315 Pt 2, 144477. [Google Scholar] [CrossRef] [PubMed]
- Nie, A.; Chao, Y.; Zhang, X.; Jia, W.; Zhou, Z.; Zhu, C. Phytochemistry and Pharmacological Activities of Wolfiporia cocos (F.A. Wolf) Ryvarden & Gilb. Front. Pharmacol. 2020, 11, 505249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, J.; He, H.; Liu, H.; Yan, X.; Zou, K. Trametenolic acid B reverses multidrug resistance in breast cancer cells through regulating the expression level of P-glycoprotein. Phytother. Res. 2014, 28, 1037–1044. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Wang, D.; Xu, Y.; Dong, R.; Yang, Y.; Lv, Q.; Chen, X.; Zhang, Z. The Upstream Pathway of mTOR-Mediated Autophagy in Liver Diseases. Cells 2019, 8, 1597. [Google Scholar] [CrossRef]
- Wei, Z.; Xie, B.; Meng, X.; Zhang, K.; Wei, H.; Gao, Y.; Liang, C.; Chen, H. HSC70 Promotes Breast Cancer Progression via PTEN Autophagic Degradation and PI3K/AKT/mTOR Activation. Mol. Carcinog. 2025, 64, 1287–1301. [Google Scholar] [CrossRef]
- Wu, G.; Yao, W.; Cheng, L.; Wang, X.; Chen, T. SIRT1 rescues autophagic flux via PI3K/AKT/mTOR inactivation to suppress DOX-induced senescence in MCF-7 cells. Exp. Cell Res. 2025, 452, 114754. [Google Scholar] [CrossRef]
- Villalobos-Labra, R.; Subiabre, M.; Toledo, F.; Pardo, F.; Sobrevia, L. Endoplasmic reticulum stress and development of insulin resistance in adipose, skeletal, liver, and foetoplacental tissue in diabesity. Mol. Asp. Med. 2019, 66, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Pires, K.M.; Ferhat, M.; Chaurasia, B.; Buffolo, M.A.; Smalling, R.; Sargsyan, A.; Atkinson, D.L.; Summers, S.A.; Graham, T.E.; et al. Autophagy Ablation in Adipocytes Induces Insulin Resistance and Reveals Roles for Lipid Peroxide and Nrf2 Signaling in Adipose-Liver Crosstalk. Cell Rep. 2018, 25, 1708–1717.e5. [Google Scholar] [CrossRef]
- Wendt, A.; Eliasson, L. Pancreatic α-cells–The unsung heroes in islet function. Semin. Cell Dev. Biol. 2020, 103, 41–50. [Google Scholar] [CrossRef]
- Qu, S.; Li, H.; Liu, Z. A Rare Cause of Rash. Gastroenterology 2021, 160, 1943–1946. [Google Scholar] [CrossRef]
- Anelli, S.; Mazzilli, R.; Zamponi, V.; Giorgini, B.; Golisano, B.; Mancini, C.; Russo, F.; Panzuto, F.; Faggiano, A. Glucagonoma and Glucagonoma Syndrome: An Updated Review. Clin. Endocrinol. 2025, 103, 417–426. [Google Scholar] [CrossRef]
- McGlone, E.R.; Bloom, S.R.; Tan, T.M. Glucagon resistance and metabolic-associated steatotic liver disease: A review of the evidence. J. Endocrinol. 2024, 261, e230365. [Google Scholar] [CrossRef]
- Ishida, H.; Lam, A.K. Pancreatic neuroendocrine neoplasms: The latest surgical and medical treatment strategies based on the current World Health Organization classification. Crit. Rev. Oncol. Hematol. 2020, 145, 102835. [Google Scholar] [CrossRef]
- Bevere, M.; Gkountakos, A.; Martelli, F.M.; Scarpa, A.; Luchini, C.; Simbolo, M. An Insight on Functioning Pancreatic Neuroendocrine Neoplasms. Biomedicines 2023, 11, 303. [Google Scholar] [CrossRef]
- Giri, S.; Sahoo, J. Advancements in medical treatment for pancreatic neuroendocrine tumors: A beacon of hope. World J. Gastroenterol. 2024, 30, 1670–1675. [Google Scholar] [CrossRef] [PubMed]
- Melhorn, P.; Raderer, M.; Mazal, P.; Berchtold, L.; Beer, L.; Kiesewetter, B. Liver metastases in high-grade neuroendocrine neoplasms: A comparative study of hepatic tumor volume and biochemical findings in NET G3 versus NEC. J. Neuroendocrinol. 2024, 36, e13454. [Google Scholar] [CrossRef] [PubMed]
- Lamarca, A.; Cives, M.; de Mestier, L.; Crona, J.; Spada, F.; Öberg, K.; Pavel, M.; Alonso-Gordoa, T. Advanced small-bowel well-differentiated neuroendocrine tumours: An international survey of practice on 3rd-line treatment. World J. Gastroenterol. 2021, 27, 976–989. [Google Scholar] [CrossRef]
- Rogoza, O.; Megnis, K.; Kudrjavceva, M.; Gerina-Berzina, A.; Rovite, V. Role of Somatostatin Signalling in Neuroendocrine Tumours. Int. J. Mol. Sci. 2022, 23, 1447. [Google Scholar] [CrossRef]
- Mavi, M.E.; Tuncel, M. Treatment of Glucagonoma-Related Necrolytic Migratory Erythema With Peptide Receptor Radionuclide Therapy. Clin. Nucl. Med. 2021, 46, 1002–1003. [Google Scholar] [CrossRef]
- Liu, R.; Qiu, M.; Deng, X.; Zhang, M.; Gao, Z.; Wang, Y.; Mei, H.; Zhai, M.; Zhang, Q.; Hao, J.; et al. Erianin inhibits the progression of pancreatic cancer by directly targeting AKT and ASK1. Cancer Cell Int. 2024, 24, 348. [Google Scholar] [CrossRef]
- Lepareur, N.; Ramée, B.; Mougin-Degraef, M.; Bourgeois, M. Clinical Advances and Perspectives in Targeted Radionuclide Therapy. Pharmaceutics 2023, 15, 1733. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros-Álvarez, J.; Andersen, J.K. mTORC2: The other mTOR in autophagy regulation. Aging Cell 2021, 20, e13431. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Huang, S.; Yu, Y.; Lian, Q.; Liu, Y.; Dai, W.; Liu, Q.; Pan, Y.; Liu, G.; Li, K.; et al. Human adipose and synovial-derived MSCs synergistically attenuate osteoarthritis by promoting chondrocyte autophagy through FoxO1 signaling. Stem Cell Res. Ther. 2024, 15, 261. [Google Scholar] [CrossRef]
- Xing, Y.Q.; Li, A.; Yang, Y.; Li, X.X.; Zhang, L.N.; Guo, H.C. The regulation of FOXO1 and its role in disease progression. Life Sci. 2018, 193, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ge, S.; He, K.; Zhao, X.; Wu, Y.; Shao, Y.; Wu, X. FoxO1 inhibits autophagosome-lysosome fusion leading to endothelial autophagic-apoptosis in diabetes. Cardiovasc. Res. 2019, 115, 2008–2020. [Google Scholar] [CrossRef]
- Liang, R.; Liu, N.; Cao, J.; Liu, T.; Sun, P.; Cai, X.; Zhang, L.; Liu, Y.; Zou, J.; Wang, L.; et al. HIF-1α/FOXO1 axis regulated autophagy is protective for β cell survival under hypoxia in human islets. Biochim. Biophys. Acta Mol. Basis Dis. 2022, 1868, 166356. [Google Scholar] [CrossRef]
- Wang, P.; Li, J.; Li, C.G.; Zhou, X.; Chen, X.; Zhu, M.; Wang, H. Restoring Autophagy by Exercise Ameliorates Insulin Resistance Partly via Calcineurin-Driven TFEB Nuclear Translocation. Clin. Exp. Pharmacol. Physiol. 2025, 52, e70010. [Google Scholar] [CrossRef]
- Yue, J.; Aobulikasimu, A.; Sun, W.; Liu, S.; Xie, W.; Sun, W. Targeted regulation of FoxO1 in chondrocytes prevents age-related osteoarthritis via autophagy mechanism. J. Cell Mol. Med. 2022, 26, 3075–3082. [Google Scholar] [CrossRef]
- Ye, S.; Cheng, Z.; Zhuo, D.; Liu, S. Different Types of Cell Death in Diabetic Neuropathy: A Focus on Mechanisms and Therapeutic Strategies. Int. J. Mol. Sci. 2024, 25, 8126. [Google Scholar] [CrossRef] [PubMed]
- Meijer, A.J.; Codogno, P. Autophagy: Regulation and role in disease. Crit. Rev. Clin. Lab. Sci. 2009, 46, 210–240. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Bie, L.Y.; Pang, D.R.; Li, X.; Yang, L.-F.; Chen, D.-D.; Wang, Y.-R.; Gao, Y. The role of autophagy in the treatment of type II diabetes and its complications: A review. Front. Endocrinol. 2023, 14, 1228045. [Google Scholar] [CrossRef] [PubMed]
- Boortz, K.A.; Syring, K.E.; Pound, L.D.; Wang, Y.; Oeser, J.K.; O’Brien, R.M. Functional Analysis of Mouse G6pc1 Mutations Using a Novel In Situ Assay for Glucose-6-Phosphatase Activity and the Effect of Mutations in Conserved Human G6PC1/G6PC2 Amino Acids on G6PC2 Protein Expression. PLoS ONE 2016, 11, e0162439. [Google Scholar] [CrossRef]
- Hao, C.; Wei, Y.; Meng, W.; Zhang, J.; Yang, X. PI3K/AKT/mTOR inhibitors for hormone receptor-positive advanced breast cancer. Cancer Treat. Rev. 2025, 132, 102861. [Google Scholar] [CrossRef]
- Huang, S. mTOR Signaling in Metabolism and Cancer. Cells 2020, 9, 2278. [Google Scholar] [CrossRef]
- Deleyto-Seldas, N.; Efeyan, A. The mTOR-Autophagy Axis and the Control of Metabolism. Front. Cell Dev. Biol. 2021, 9, 655731. [Google Scholar] [CrossRef]
- Liu, G.Y.; Sabatini, D.M. mTOR at the nexus of nutrition, growth, ageing and disease. Nat. Rev. Mol. Cell Biol. 2020, 21, 183–203. [Google Scholar] [CrossRef]
- Xu, Z.; Han, X.; Ou, D.; Liu, T.; Li, Z.; Jiang, G.; Liu, J.; Zhang, J. Targeting PI3K/AKT/mTOR-mediated autophagy for tumor therapy. Appl. Microbiol. Biotechnol. 2020, 104, 575–587. [Google Scholar] [CrossRef]
- Tang, W.; Chen, Z.; Zhang, W.; Cheng, Y.; Zhang, B.; Wu, F.; Wang, Q.; Wang, S.; Rong, D.; Reiter, F.P.; et al. The mechanisms of sorafenib resistance in hepatocellular carcinoma: Theoretical basis and therapeutic aspects. Signal Transduct. Target. Ther. 2020, 5, 87. [Google Scholar] [CrossRef]
- Hua, H.; Zhang, H.; Chen, J.; Wang, J.; Liu, J.; Jiang, Y. Targeting Akt in cancer for precision therapy. J. Hematol. Oncol. 2021, 14, 128. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, A.S. PI3K/Akt/mTOR inhibitors in cancer: At the bench and bedside. Semin. Cancer Biol. 2019, 59, 125–132. [Google Scholar] [CrossRef]
- Martorana, F.; Motta, G.; Pavone, G.; Motta, L.; Stella, S.; Vitale, S.R.; Manzella, L.; Vigneri, P. AKT Inhibitors: New Weapons in the Fight Against Breast Cancer? Front. Pharmacol. 2021, 12, 662232. [Google Scholar] [CrossRef]
- Sabbah, D.A.; Hajjo, R.; Bardaweel, S.K.; Zhong, H.A. Targeting the PI3K/AKT signaling pathway in anticancer research: A recent update on inhibitor design and clinical trials (2020–2023). Expert Opin. Ther. Pat. 2024, 34, 141–158. [Google Scholar] [CrossRef]
- Zhang, W.Y.; Wang, M.H.; Xie, C. Potential of traditional Chinese medicine in the treatment of nonalcoholic fatty liver disease: A promising future. World J. Gastroenterol. 2024, 30, 4597–4601. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.A.; Xue, W.; Harrison, D.J.; Hawkins, D.S.; Dasgupta, R.; Wolden, S.; Shulkin, B.; Qumseya, A.; Routh, J.C.; MacDonald, T.; et al. Addition of temsirolimus to chemotherapy in children, adolescents, and young adults with intermediate-risk rhabdomyosarcoma (ARST1431): A randomised, open-label, phase 3 trial from the Children’s Oncology Group. Lancet Oncol. 2024, 25, 912–921. [Google Scholar] [CrossRef] [PubMed]
- Abuzakhm, S.M.; Sukrithan, V.; Fruth, B.; Qin, R.; Strosberg, J.; Hobday, T.J.; Semrad, T.; Reidy-Lagunes, D.; Kindler, H.L.; Kim, G.P.; et al. A phase II study of bevacizumab and temsirolimus in advanced extra-pancreatic neuroendocrine tumors. Endocr. Relat. Cancer 2023, 30, e220301. [Google Scholar] [CrossRef]
- Pungsrinont, T.; Kallenbach, J.; Baniahmad, A. Role of PI3K-AKT-mTOR Pathway as a Pro-Survival Signaling and Resistance-Mediating Mechanism to Therapy of Prostate Cancer. Int. J. Mol. Sci. 2021, 22, 11088. [Google Scholar] [CrossRef]
- Xu, H.; Wang, S.; Jiang, Y.; Wu, J.; Chen, L.; Ding, Y.; Zhou, Y.; Deng, L.; Chen, X. Poria cocos Polysaccharide Ameliorated Antibiotic-Associated Diarrhea in Mice via Regulating the Homeostasis of the Gut Microbiota and Intestinal Mucosal Barrier. Int. J. Mol. Sci. 2023, 24, 1423. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.Y.J.; Lai, N.P.Y.; Ng, W.M.; Siah, K.T.H.; Gan, R.Y.; Zhong, L.L.D. Chemical structures, extraction and analysis technologies, and bioactivities of edible fungal polysaccharides from Poria cocos: An updated review. Int. J. Biol. Macromol. 2024, 261 Pt 1, 129555. [Google Scholar] [CrossRef] [PubMed]
- Lan, K.; Yang, H.; Zheng, J.; Hu, H.; Zhu, T.; Zou, X.; Hu, B.; Liu, H. Poria cocos oligosaccharides ameliorate dextran sodium sulfate-induced colitis mice by regulating gut microbiota dysbiosis. Food Funct. 2023, 14, 857–873. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhang, Y.; Wang, X.; Huang, X.; Fei, Y.; Yu, Y.; Shou, D. Antioxidant property of water-soluble polysaccharides from Poria cocos Wolf using different extraction methods. Int. J. Biol. Macromol. 2016, 83, 103–110. [Google Scholar] [CrossRef]
- Zhang, D.D.; Li, H.J.; Zhang, H.R.; Ye, X.C. Poria cocos water-soluble polysaccharide modulates anxiety-like behavior induced by sleep deprivation by regulating the gut dysbiosis, metabolic disorders and TNF-α/NF-κB signaling pathway. Food Funct. 2022, 13, 6648–6664. [Google Scholar] [CrossRef]


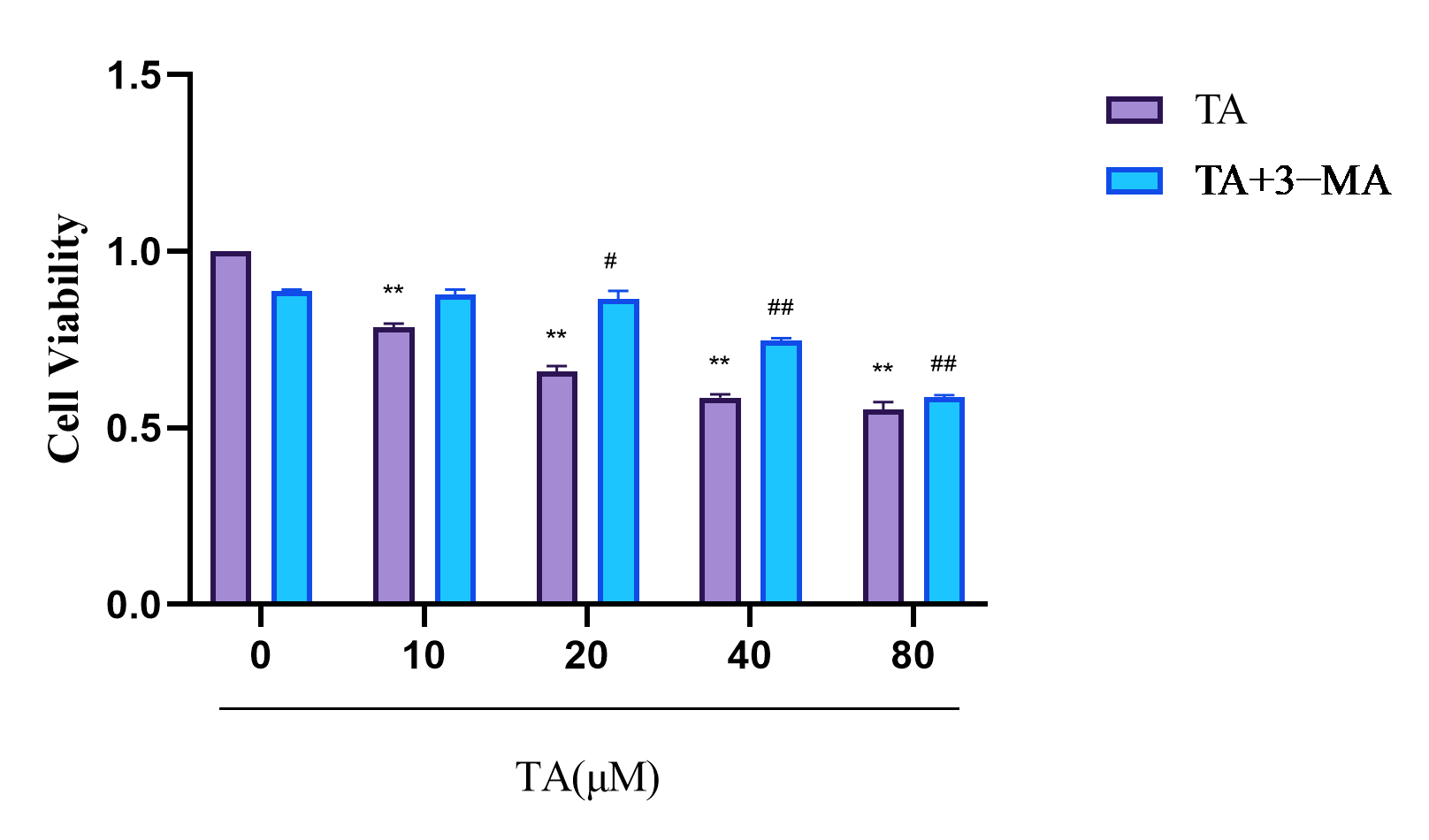
| Name | Molecular Weight (kDa) | Dilution Rate | Art. No. | Company |
|---|---|---|---|---|
| LC3A/B | 14/16 | 1:1000 | 12741S | Cell Signaling Technology |
| ATG7 | 78 | 1:1000 | 8558S | Cell Signaling Technology |
| β-actin | 45 | 1:1000 | 5125S | Cell Signaling Technology |
| PI3K | 85 | 1:1000 | 4249S | Cell Signaling Technology |
| p-PI3K | 85 | 1:1000 | 17366S | Cell Signaling Technology |
| AKT | 60 | 1:1000 | 4691S | Cell Signaling Technology |
| p-AKT | 60 | 1:1000 | 4060S | Cell Signaling Technology |
| mTOR | 289 | 1:1000 | 2983S | Cell Signaling Technology |
| p-mTOR | 289 | 1:1000 | 5536S | Cell Signaling Technology |
| FoxO1 | 82 | 1:1000 | 2880S | Cell Signaling Technology |
| p-FoxO1 | 82 | 1:1000 | 9461S | Cell Signaling Technology |
| Anti-rabbit IgG | - | 1:5000 | 7074S | Cell Signaling Technology |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, W.; Pan, S.; Zhang, H.; Zhang, X.; Wang, J. Study on Autophagy Death of Alpha TC1 Clone 6 (αTC1-6) Cells Induced by Trametenolic Acid Through PI3K/AKT Pathway. Curr. Issues Mol. Biol. 2025, 47, 871. https://doi.org/10.3390/cimb47100871
Ye W, Pan S, Zhang H, Zhang X, Wang J. Study on Autophagy Death of Alpha TC1 Clone 6 (αTC1-6) Cells Induced by Trametenolic Acid Through PI3K/AKT Pathway. Current Issues in Molecular Biology. 2025; 47(10):871. https://doi.org/10.3390/cimb47100871
Chicago/Turabian StyleYe, Wangyang, Shangling Pan, Hongqi Zhang, Xiaolan Zhang, and Junzhi Wang. 2025. "Study on Autophagy Death of Alpha TC1 Clone 6 (αTC1-6) Cells Induced by Trametenolic Acid Through PI3K/AKT Pathway" Current Issues in Molecular Biology 47, no. 10: 871. https://doi.org/10.3390/cimb47100871
APA StyleYe, W., Pan, S., Zhang, H., Zhang, X., & Wang, J. (2025). Study on Autophagy Death of Alpha TC1 Clone 6 (αTC1-6) Cells Induced by Trametenolic Acid Through PI3K/AKT Pathway. Current Issues in Molecular Biology, 47(10), 871. https://doi.org/10.3390/cimb47100871







