Pro-Angiogenic Bioactive Molecules in Vascular Morphogenesis: Integrating Endothelial Cell Dynamics
Abstract
1. Introduction
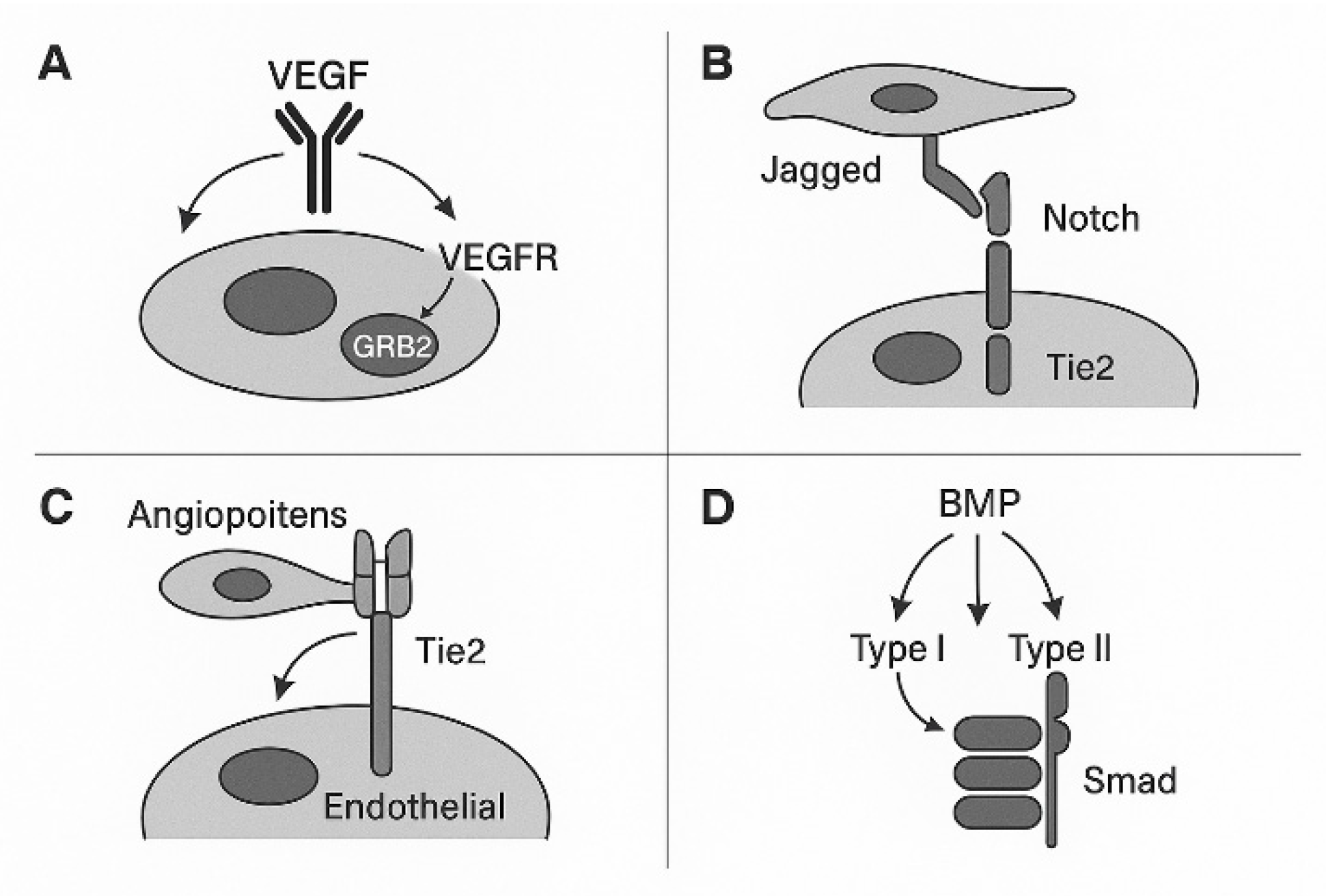
2. Molecular Regulators of Vascular Morphogenesis
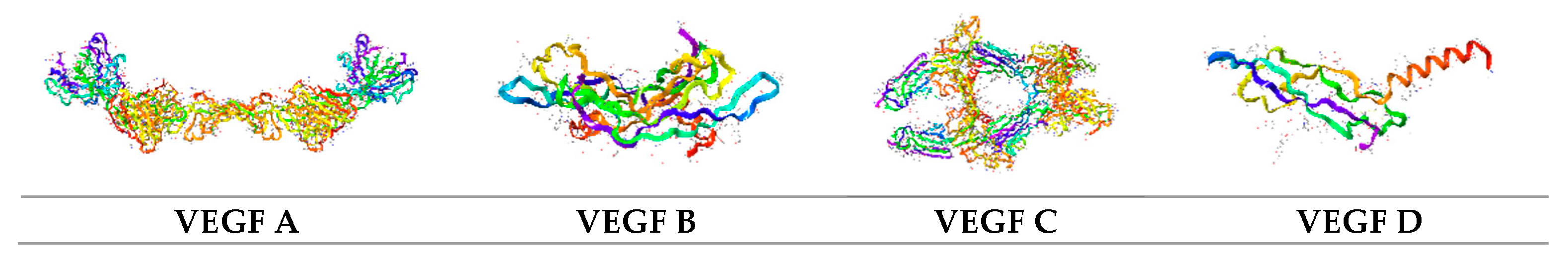
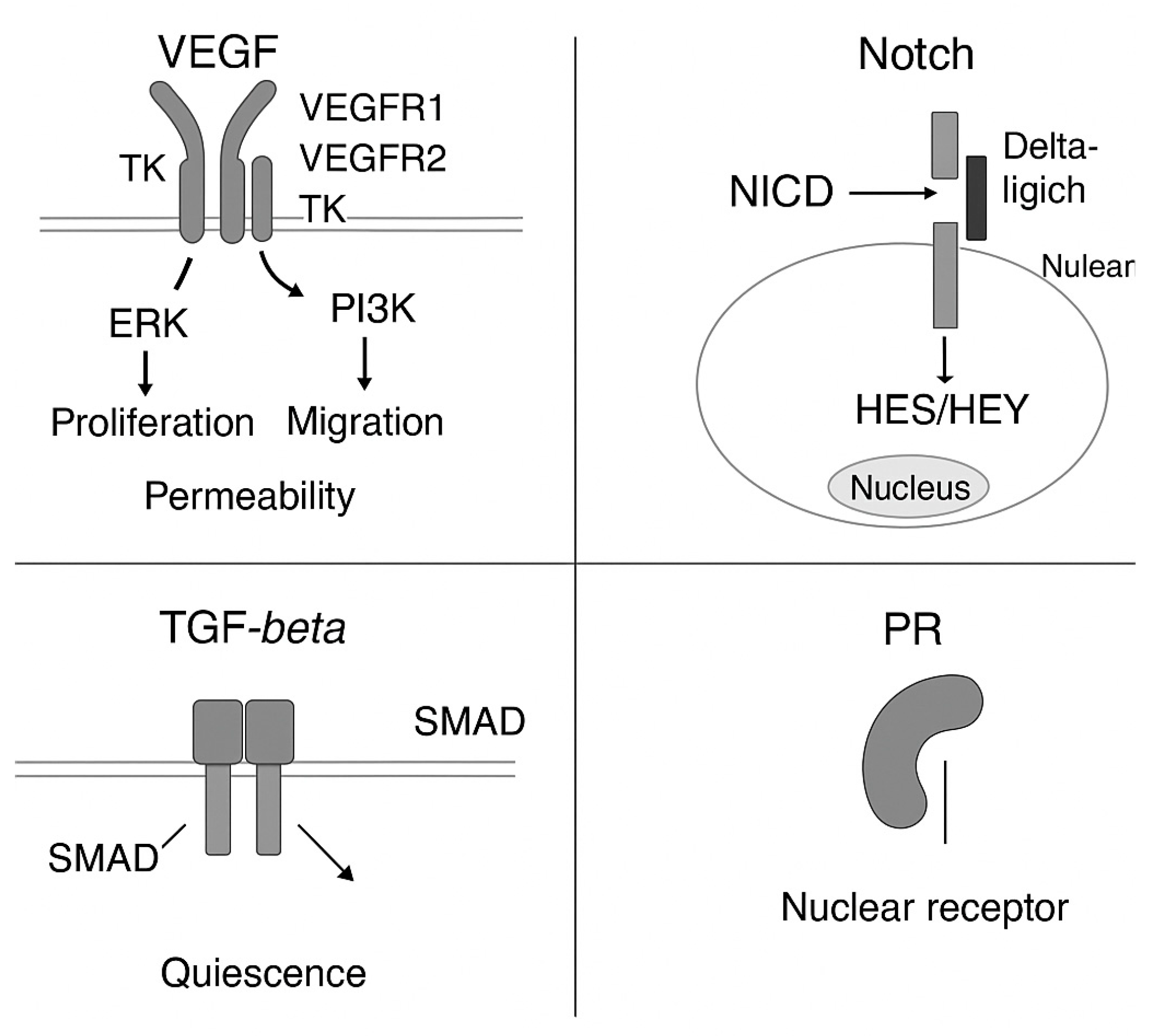
2.1. VEGF–VEGFR Signaling Axis. VEGFR Signaling Pathway in Vascular Morphogenesis
2.2. VEGFR1
2.3. VEGFR2
2.4. VEGFR3
- VEGFR2 is the principal mediator of endothelial proliferation and sprouting angiogenesis.
- VEGFR1 modulates angiogenesis through decoy activity and inflammatory recruitment.
- VEGFR3 primarily governs lymphangiogenesis and stabilizes VEGFR2 signaling.
- Crosstalk and ligand competition among VEGFRs fine-tune angiogenic outcomes.
2.5. HIF-1α Gene
2.6. Notch Signaling in Vascular Morphogenesis
2.7. VEGF Mimetics
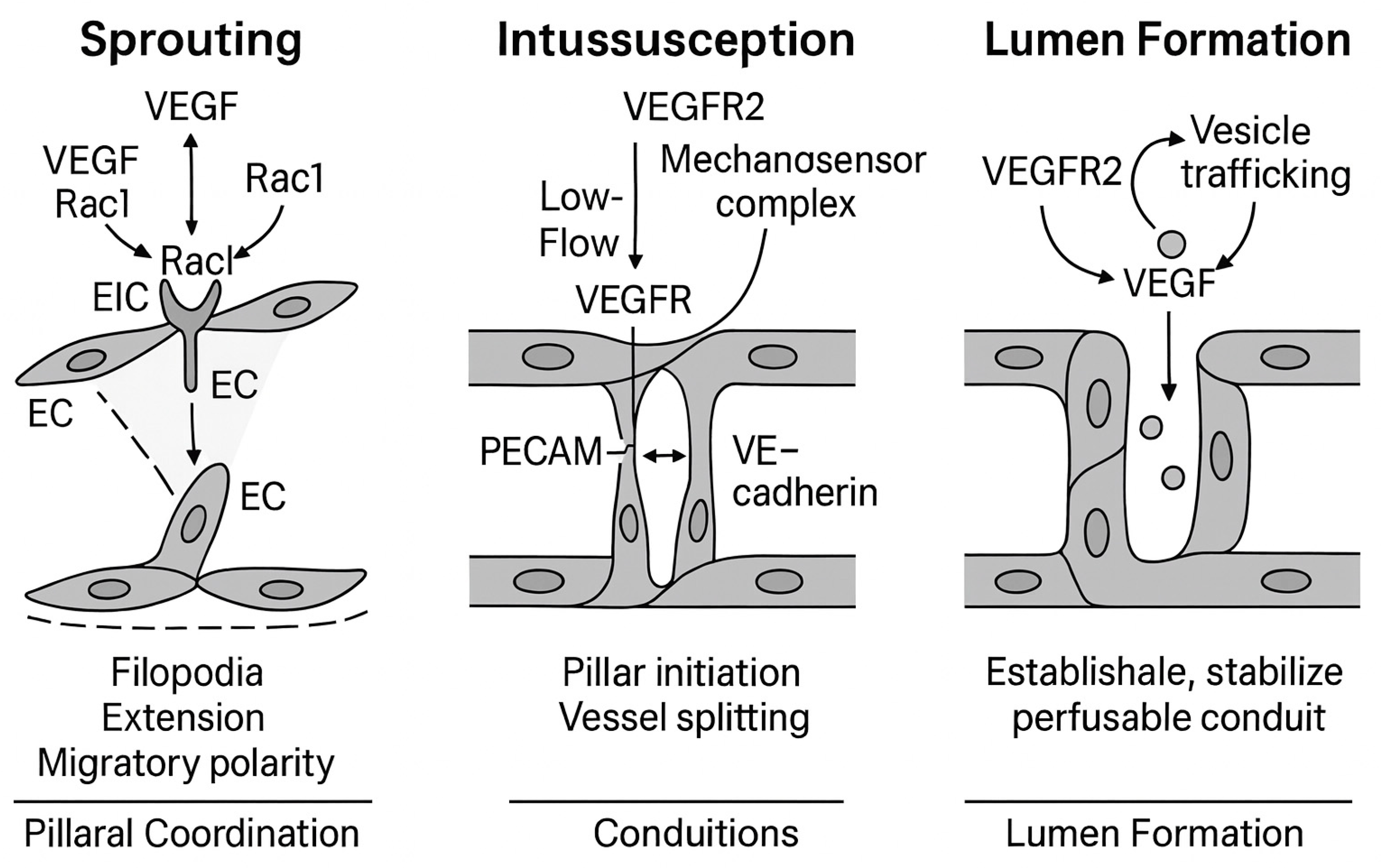
3. Structural Mechanisms of Vascular Morphogenesis Mechanistic Basis of Angiogenic Morphogenesis: Integration of VEGFR Signaling in Sprouting, Intussusception, and Lumen Formation
3.1. Sprouting Angiogenesis: Tip/Stalk Cell Selection and Invasion
3.2. Intussusception: Vascular Splitting by Pillar Insertion
3.3. Lumen Formation: Cell Hollowing and Cord Hollowing
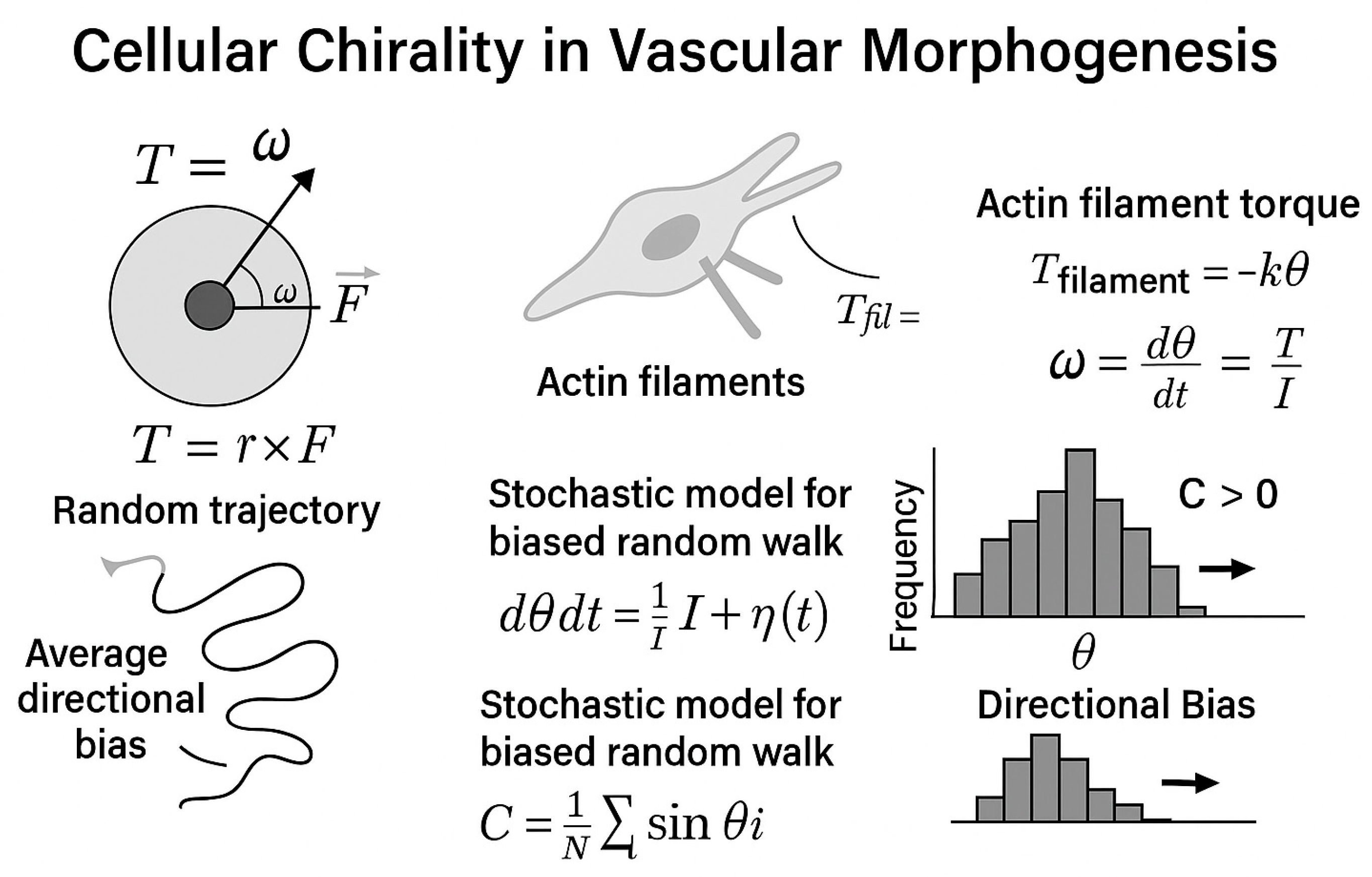
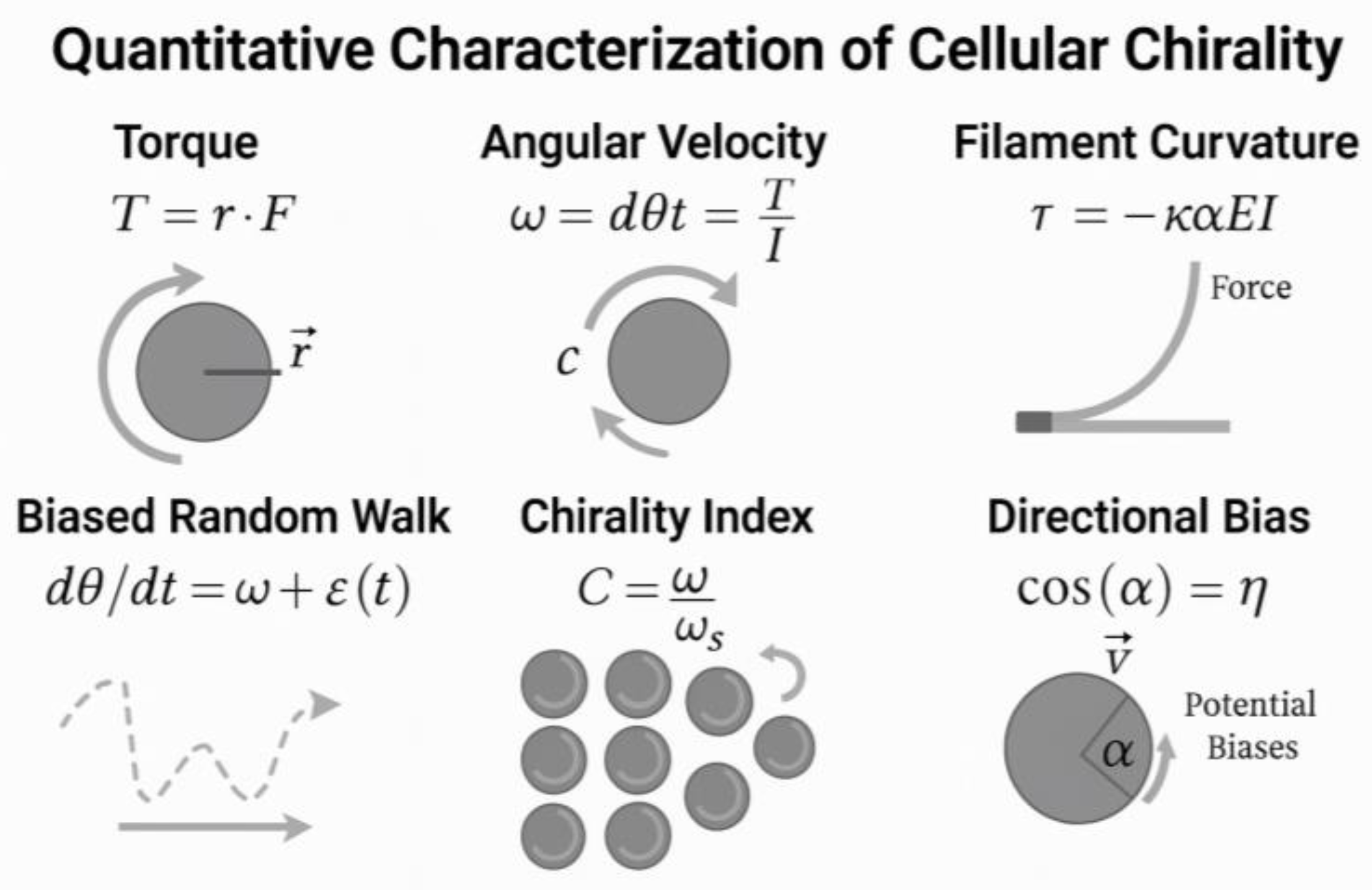
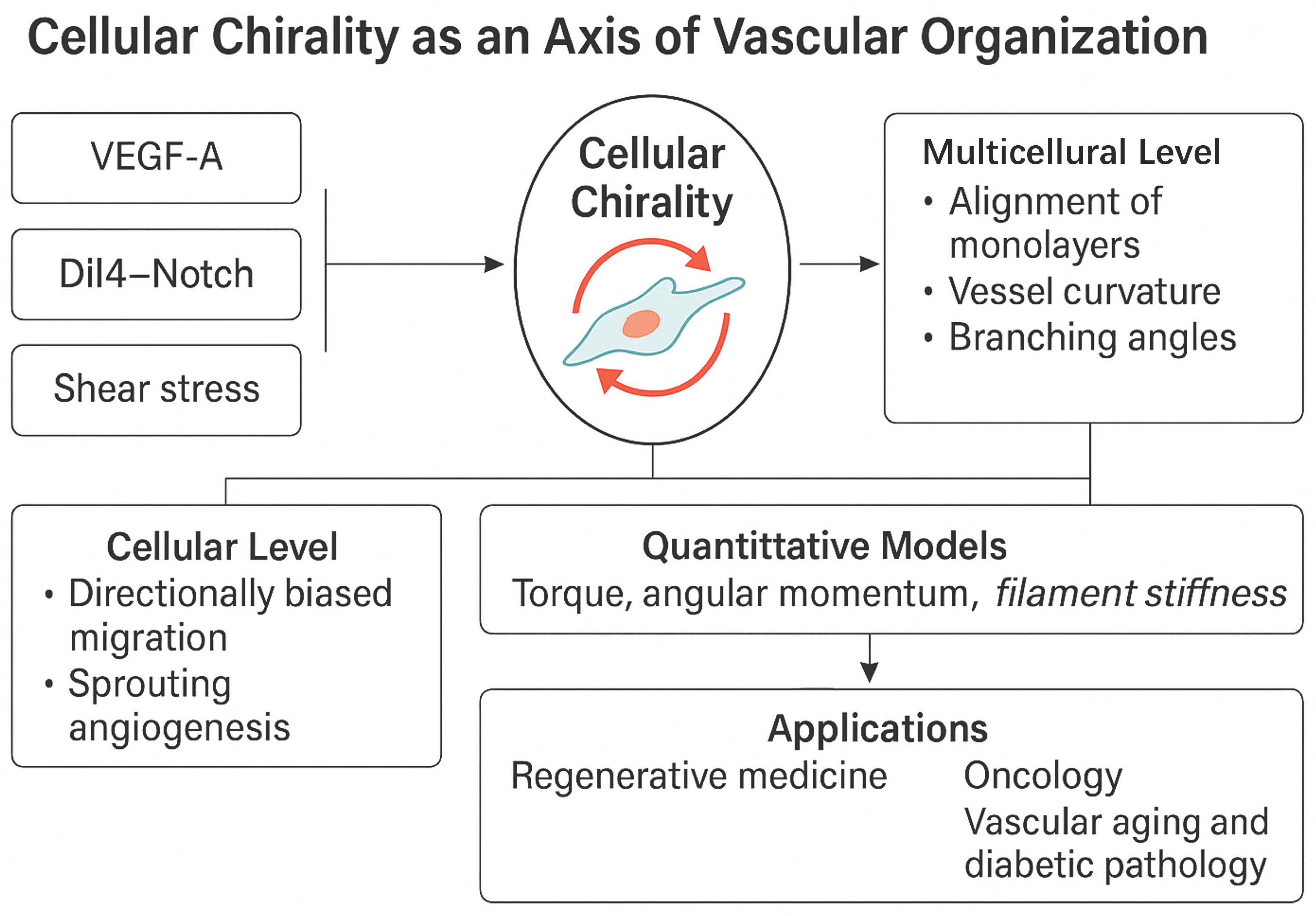
4. Emerging and Integrative Concepts in Vascular Morphogenesis—Cellular Chirality in Vascular Morphogenesis: Context, Mechanisms, and Functional Consequences
4.1. Cell Chirality and Vasculogenesis
4.1.1. Experimental and Phenotypic Evidence
4.1.2. Molecular Mechanisms and Crosstalk
- Cdc42 orchestrates the establishment of cell polarity by activating the PAR6–aPKC complex, which positions the centrosome and Golgi apparatus in alignment with the migration front.
- Rac1 modulates lamellipodia extension and controls junctional stability by activating PAKs (p21-activated kinases) downstream.
- RhoA, through ROCK1/2, regulates actomyosin contractility and stress fiber formation, thereby maintaining cortical stiffness and defining the direction of cytoskeletal torque.
- VEGF signaling strongly interfaces with these pathways. Upon VEGF-A binding to VEGFR2, several downstream cascades become activated:
- PI3K–Akt supports cell survival and maintains cell polarity.
- PLCγ–IP3–Ca2+ signaling elevates intracellular calcium, which activates PKC, a known modulator of chirality.
4.1.3. ECM, Integrins, and Mechanical Cues
4.1.4. Functional Outcomes and Pathophysiological Implications
- Impaired lumen continuity
- Increased vascular permeability due to VE-cadherin mislocalization
- Defective tip/stalk cell organization
- Abnormal branching angles and disorganized vasculature [226].
4.2. VEGF-VEGFR Signaling and Cellular Chirality
4.3. Angiogenesis Shaping Using Cellular/Tissular Indices
- Cellular chirality refers to the intrinsic left-right bias in cell shape, migration, and alignment.
- It influences vascular geometry, lumen formation, and sprout organization.
- Chirality operates in concert with VEGF gradients and mechanical forces, adding a spatial layer of morphogenetic control.
4.4. VEGF Signaling and Vascular Specification
4.5. Tip-Stalk Patterning and Notch Feedback
4.6. Cellular Chirality: A New Axis in Vascular Morphogenesis
4.7. Future Directions
4.7.1. Gene Therapy
4.7.2. Precise Modulation of Chirality, Flow, Signaling Balance) in Therapeutic Strategies
- Right-handed tube helicity in 3D endothelial cultures
- Disruption of chiral bias upon inhibition of PKC, mDia1, or Cdc42
- Flipping of vessel orientation with exogenous signaling or cytoskeletal interference
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/ (accessed on 5 April 2025).
- Marziano, C.; Genet, G.; Hirschi, K.K. Vascular endothelial cell specification in health and disease. Angiogenesis 2021, 24, 213–236. [Google Scholar] [CrossRef]
- Kim, J.; Kim, Y.H.; Kim, J.; Park, D.Y.; Bae, H.; Lee, D.-H.; Kim, K.H.; Hong, S.P.; Jang, S.P.; Kubota, Y.; et al. YAP/TAZ regulates sprouting angiogenesis and vascular barrier maturation. J. Clin. Investig. 2017, 127, 3441–3461. [Google Scholar] [CrossRef] [PubMed]
- Zarkada, G.; Howard, J.P.; Xiao, X.; Park, H.; Bizou, M.; Leclerc, S.; Künzel, S.E.; Boisseau, B.; Li, J.; Cagnone, G.; et al. Specialized endothelial tip cells guide neuroretina vascularization and blood-retina-barrier formation. Dev. Cell 2021, 56, 2237–2251.e6. [Google Scholar] [CrossRef]
- Dang, L.T.H.; Lawson, N.D.; Fish, J.E. MicroRNA control of vascular endothelial growth factor signaling output during vascular development. Arter. Thromb. Vasc. Biol. 2013, 33, 193–200. [Google Scholar] [CrossRef]
- Mabeta, P.; Steenkamp, V. The VEGF/VEGFR Axis Revisited: Implications for Cancer Therapy. Int. J. Mol. Sci. 2022, 23, 15585. [Google Scholar] [CrossRef]
- Liu, Z.L.; Chen, H.H.; Zheng, L.L.; Sun, L.P.; Shi, L. Angiogenic signaling pathways and antiangiogenic therapy for cancer. Signal Transduct. Target. Ther. 2023, 8, 198. [Google Scholar] [CrossRef]
- Apte, R.S.; Chen, D.S.; Ferrara, N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 2019, 176, 1248–1264. [Google Scholar] [CrossRef]
- Zeng, L.; Xiao, Q.; Chen, M.; Margariti, A.; Martin, D.; Ivetic, A.; Xu, H.; Mason, J.; Wang, W.; Cockerill, G.; et al. Vascular endothelial cell growth-activated XBP1 splicing in endothelial cells is crucial for angiogenesis. Circulation 2013, 127, 1712–1722. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Guo, M.; Li, C.; Shao, Y.; Zhao, X.; Zhang, W. VEGF-like protein from Apostichopus japonicus promotes cell proliferation and migration. Dev. Comp. Immunol. 2019, 92, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Claesson-Welsh, L.; Welsh, M. VEGFA and tumour angiogenesis. J. Intern. Med. 2013, 273, 114–127. [Google Scholar] [CrossRef]
- Ling, M.; Quan, L.; Lai, X.; Lang, L.; Li, F.; Yang, X.; Fu, Y.; Feng, S.; Yi, X.; Zhu, C.; et al. VEGFB Promotes Myoblasts Proliferation and Differentiation through VEGFR1-PI3K/Akt Signaling Pathway. Int. J. Mol. Sci. 2021, 22, 13352. [Google Scholar] [CrossRef]
- Glinton, K.E.; Ma, W.; Lantz, C.; Grigoryeva, L.S.; DeBerge, M.; Liu, X.; Febbraio, M.; Kahn, M.; Oliver, G.; Thorp, E.B. Macrophage-produced VEGFC is induced by efferocytosis to ameliorate cardiac injury and inflammation. J. Clin. Investig. 2022, 132, e140685. [Google Scholar] [CrossRef]
- Bower, N.I.; Vogrin, A.J.; Le Guen, L.; Chen, H.; Stacker, S.A.; Achen, M.G.; Hogan, B.M. Vegfd modulates both angiogenesis and lymphangiogenesis during zebrafish embryonic development. Development 2017, 144, 507–518. [Google Scholar] [CrossRef]
- Inder, M.K.; Wise, L.M.; Fleming, S.B.; Mercer, A.A. The C-terminus of viral vascular endothelial growth factor-E partially blocks binding to VEGF receptor-1. FEBS J. 2008, 275, 207–217. [Google Scholar] [CrossRef]
- Song, M.; Yang, H.; Yao, S.; Ma, F.; Li, Z.; Deng, Y.; Deng, H.; Zhou, Q.; Lin, S.; Wei, Y. A critical role of vascular endothelial growth factor D in zebrafish embryonic vasculogenesis and angiogenesis. Biochem. Biophys. Res. Commun. 2007, 357, 924–930. [Google Scholar] [CrossRef]
- Han, S.; Kim, D.; Shivakumar, M.; Lee, Y.-J.; Garg, T.; Miller, J.E.; Kim, J.H.; Kim, D.; Lee, Y.; Zheng, Y. The Effects of Alternative Splicing on MiRNA Binding Sites in Bladder Cancer. PLoS ONE 2018, 13, e0190708. [Google Scholar] [CrossRef]
- Shibuya, M. Vascular endothelial growth factor (VEGF) and its receptor (VEGFR) signaling in angiogenesis: A crucial target for anti- and pro-angiogenic therapies. Genes Cancer 2011, 2, 1097–1105. [Google Scholar] [CrossRef]
- Olsson, A.K.; Dimberg, A. VEGF receptor signaling—In control of vascular function. Nat. Rev. Mol. Cell Biol. 2006, 7, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Koch, S.; Tugues, S.; Li, X.; Gualandi, L.; Claesson-Welsh, L. Signal transduction by vascular endothelial growth factor receptors. Biochem. J. 2011, 437, 169–183. [Google Scholar] [CrossRef] [PubMed]
- P17948. Available online: https://www.uniprot.org/uniprotkb/P17948/entry (accessed on 5 April 2025).
- Warren, C.M.; Iruela-Arispe, M.L. Signaling Circuitry in Vascular Morphogenesis. Curr. Opin. Hematol. 2010, 17, 213–218. [Google Scholar] [CrossRef] [PubMed]
- White, A.L.; Bix, G.J. VEGFA Isoforms as Pro-angiogenic Therapeutics for Cerebrovascular Diseases. Biomolecules 2023, 13, 702. [Google Scholar] [CrossRef] [PubMed]
- Cudmore, M.J.; Hewett, P.W.; Ahmad, S.; Wang, K.-Q.; Cai, M.; Al-Ani, B.; Fujisawa, T.; Ma, B.; Sissaoui, S.; Ramma, W.; et al. The role of heterodimerization between VEGFR-1 and VEGFR-2 in the regulation of endothelial cell homeostasis. Nat. Commun. 2012, 3, 972. [Google Scholar] [CrossRef]
- Uemura, A.; Fruttiger, M.; D’AMore, P.A.; De Falco, S.; Joussen, A.M.; Sennlaub, F.; Brunck, L.R.; Johnson, K.T.; Lambrou, G.N.; Rittenhouse, K.D.; et al. VEGFR1 signaling in retinal angiogenesis and microinflammation. Prog. Retin. Eye Res. 2021, 84, 100954. [Google Scholar] [CrossRef]
- Jones, M.C.; Caswell, P.T.; Moran-Jones, K.; Roberts, M.; Barry, S.T.; Gampel, A.; Mellor, H.; Norman, J.C. VEGFR1 (Flt1) regulates Rab4 recycling to control fibronectin polymerization and endothelial vessel branching. Traffic 2009, 10, 754–766. [Google Scholar] [CrossRef]
- Dutta, D.; Ray, S.; Vivian, J.L.; Paul, S. Activation of the VEGFR1 chromatin domain: An angiogenic signal-ETS1/HIF-2alpha regulatory axis. J. Biol. Chem. 2008, 283, 25404–25413. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, B. Accelerated coronary angiogenesis by vegfr1-knockout endocardial cells. PLoS ONE 2013, 8, e70570. [Google Scholar] [CrossRef]
- Terman, B.I.; Dougher-Vermazen, M.; Carrion, M.E.; Dimitrov, D.; Armellino, D.C.; Gospodarowicz, D.; Böhlen, P. Identification of the KDR tyrosine kinase as a vascular endothelial cell growth factor receptor. Biochem. Biophys. Res. Commun. 1992, 187, 1579–1586. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, M. Vascular endothelial growth factor receptor-1 (VEGFR-1/Flt-1): A dual regulator for angiogenesis. Angiogenesis 2006, 9, 225–230. [Google Scholar] [CrossRef]
- Ferrara, N. Vascular endothelial growth factor: Basic science and clinical progress. Endocr. Rev. 2004, 25, 581–611. [Google Scholar] [CrossRef]
- Kearney, J.B.; Kappas, N.C. Vascular endothelial growth factor receptor Flt-1 negatively regulates developmental blood vessel formation by modulating endothelial cell division. Blood 1999, 94, 2287–2296. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, M.; Claesson-Welsh, L. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp. Cell Res. 2006, 312, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Bagherzadeh, A. Characterization of a bicyclic peptide neuropilin-1 (NP-1) antagonist (EG3287) reveals importance of vascular endothelial growth factor exon 8 for NP-1 binding and role of NP-1 in K.D.R. signaling. J. Biol. Chem. 2006, 281, 13493–13502. [Google Scholar] [CrossRef]
- Fischer, C.; Jonckx, B. Anti-PlGF inhibits growth of VEGF(R)-inhibitor-resistant tumors without affecting healthy vessels. Cell 2008, 131, 463–475. [Google Scholar] [CrossRef]
- Kendall, R.L.; Thomas, K.A. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc. Natl. Acad. Sci. USA 1993, 90, 10705–10709. [Google Scholar] [CrossRef] [PubMed]
- Villaruz, L.C.; Socinski, M.A. The role of anti-angiogenesis in non-small-cell lung cancer: An update. Current Oncology Reports. 2015, 17, 26. [Google Scholar] [CrossRef] [PubMed]
- Soker, S.; Takashima, S. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell 1998, 92, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, W.; Jiang, K.; Chen, B.; Wang, K.; Lao, L.; Hou, C.; Wang, F.; Zhang, C.; Shen, H. MicroRNA-300 regulates the ubiquitination of PTEN through the CRL4B(DCAF13) E3 ligase in osteosarcoma cells. Mol. Ther. Nucleic Acids 2018, 10, 254–268. [Google Scholar] [CrossRef]
- Storkebaum, E.; Lambrechts, D.; Dewerchin, M.; Moreno-Murciano, M.-P.; Appelmans, S.; Oh, H.; Van Damme, P.; Rutten, B.; Man, W.Y.; De Mol, M.; et al. Treatment of motoneuron degeneration by intracerebroventricular delivery of VEGF in a rat model of A.L.S. Nat. Neurosci. 2005, 8, 85–92. [Google Scholar] [CrossRef]
- Cudmore, M.; Ahmad, S.; Al-Ani, B.; Fujisawa, T.; Coxall, H.; Chudasama, K.; Devey, L.R.; Wigmore, S.J.; Abbas, A.; Hewett, P.W.; et al. Negative regulation of soluble Flt-1 and soluble endoglin release by heme oxygenase-1. Circulation 2007, 115, 1789–1797. [Google Scholar] [CrossRef]
- Tai, Y.; Chow, A.; Han, S.; Coker, C.; Ma, W.; Gu, Y.; Navarro, V.E.; Kandpal, M.; Hibshoosh, H.; Kalinsky, K.; et al. FLT1 activation in cancer cells promotes PARP-inhibitor resistance in breast cancer. EMBO Mol. Med. 2024, 16, 1957–1980. [Google Scholar] [CrossRef]
- Huang, H.; Shen, J.; Vinores, S.A. Blockade of VEGFR1 and 2 suppresses pathological angiogenesis and vascular leakage in the eye. PLoS ONE 2011, 6, e21411. [Google Scholar] [CrossRef]
- Sekiguchi, K.; Ito, Y.; Hattori, K.; Inoue, T.; Hosono, K.; Honda, M.; Numao, A.; Amano, H.; Shibuya, M.; Unno, N.; et al. VEGF Receptor 1-Expressing Macrophages Recruited from Bone Marrow Enhances Angiogenesis in Endometrial Tissues. Sci. Rep. 2019, 9, 7037. [Google Scholar] [CrossRef]
- Bordei, P.; Surdu, L.S.; Serbanescu, L.; Tudorache, I.S. Morphometry of the Placental Vessels. Rev. Chim. 2019, 70, 4119–4122. [Google Scholar] [CrossRef]
- Failla, C.M.; Carbo, M.; Morea, V. Positive and Negative Regulation of Angiogenesis by Soluble Vascular Endothelial Growth Factor Receptor-1. Int. J. Mol. Sci. 2018, 19, 1306. [Google Scholar] [CrossRef] [PubMed]
- Dougher, M.; Terman, B.I. Autophosphorylation of K.D.R. in the kinase domain is required for maximal VEGF-stimulated kinase activity and receptor internalization. Oncogene 1999, 18, 1619–1627. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.-H.; Lee, E.; Bader, J.S.; Popel, A.S. Angiogenesis interactome and time course microarray data reveal the distinct activation patterns in endothelial cells. PLoS ONE 2014, 9, e110871. [Google Scholar] [CrossRef]
- Ferrara, N. Role of vascular endothelial growth factor in regulation of physiological angiogenesis. Am. J. Physiol. Cell Physiol. 2001, 280, C1358–C1366. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, F.; Radi, M.; Brullo, C.; Schenone, S. Vascular Endothelial Growth Factor (VEGF) Receptors: Drugs and new inhibitors. J. Med. Chem. 2012, 55, 10797–10822. [Google Scholar] [CrossRef]
- Li, C.; Ai, J.; Zhang, D.; Peng, X.; Chen, X.; Gao, Z.; Su, Y.; Zhu, W.; Ji, Y.; Chen, X.; et al. Design, Synthesis, and Biological Evaluation of Novel Imidazo[1,2-alpha]pyridine Derivatives as Potent c-Met Inhibitors. ACS Med. Chem. Lett. J. 2015, 6, 507–512. [Google Scholar] [CrossRef]
- P35968. Available online: https://www.uniprot.org/uniprotkb/P35968/entry (accessed on 5 April 2025).
- Peng, F.-W.; Liu, D.-K.; Zhang, Q.-W.; Xu, Y.-G.; Shi, L. VEGFR-2 inhibitors and the therapeutic applications thereof: A patent review (2012–2016). Expert Opin. Ther. Patents 2017, 27, 987–1004. [Google Scholar] [CrossRef]
- Available online: https://www.genome.jp/kegg-bin/show_pathway?hsa04370 (accessed on 5 April 2025).
- Available online: https://www.genome.jp/dbget-bin/www_bget?pathway+hsa04330 (accessed on 5 April 2025).
- Available online: https://www.wikipathways.org/pathways/WP3888.html (accessed on 5 April 2025).
- Siveen, K.S.; Prabhu, K.; Krishnankutty, R.; Kuttikrishnan, S.; Tsakou, M.; Alali, F.Q.; Dermime, S.; Mohammad, R.M.; Uddin, S. Vascular Endothelial Growth Factor (VEGF) Signaling in Tumour Vascularization: Potential and Challenges. Curr. Vasc. Pharmacol. 2017, 15, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.J.; Xu, Z.; Wang, Z.; Zhang, H.; Zhuang, Z.W.; Simons, M.; Min, W. SUMOylation of VEGFR2 regulates its intracellular trafficking and pathological angiogenesis. Nat. Commun. 2018, 9, 3303, Correction in Nat. Commun. 2019, 10, 3679. [Google Scholar] [CrossRef]
- Peach, C.J.; Mignone, V.W.; Arruda, M.A.; Alcobia, D.C.; Hill, S.J.; Kilpatrick, L.E.; Woolard, J. Molecular Pharmacology of VEGF-A Isoforms: Binding and Signalling at VEGFR2. Int. J. Mol. Sci. 2018, 19, 1264. [Google Scholar] [CrossRef]
- Pulkkinen, H.H.; Kiema, M.; Lappalainen, J.P.; Toropainen, A.; Beter, M.; Tirronen, A.; Holappa, L.; Niskanen, H.; Kaikkonen, M.U.; Laakkonen, J.P. BMP6/TAZ-Hippo signaling modulates angiogenesis and endothelial cell response to VEGF. Angiogenesis 2020, 24, 129–144. [Google Scholar] [CrossRef]
- Abhinand, C.S.; Raju, R.; Soumya, S.J.; Arya, P.S.; Sudhakaran, P.R. VEGF-A/VEGFR2 signaling network in endothelial cells relevant to angiogenesis. J. Cell Commun. Signal. 2016, 10, 347–354. [Google Scholar] [CrossRef]
- Sunitha, P.; Raju, R.; Sajil, C.K.; Abhinand, C.S.; Nair, A.S.; Oommen, O.V.; Sugunan, V.S.; Sudhakaran, P.R. Temporal VEGFA responsive genes in HUVECs: Gene signatures and potential ligands/receptors fine-tuning angiogenesis. J. Cell Commun. Signal. 2019, 13, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Diaconu, A.; Coculescu, B.-I.; Manole, G.; Coculescu, E.C.; Stocheci, C.M.; Tudorache, I.-S.; Dinca, A.-L.; Dincă, V.G. Lipoprotein-associated phospholipase A2 (Lp-PLA2)—Possible diagnostic and risk biomarker in chronic ischaemic heart disease. J. Enzym. Inhib. Med. Chem. 2021, 36, 68–73. [Google Scholar] [CrossRef]
- Chatterjee, S.; Heukamp, L.C.; Siobal, M.; Schöttle, J.; Wieczorek, C.; Peifer, M.; Frasca, D.; Koker, M.; König, K.; Meder, L.; et al. Tumor VEGF: VEGFR2 autocrine feed-forward loop triggers angiogenesis in lung cancer. J. Clin. Investig. 2013, 123, 1732–1740. [Google Scholar] [CrossRef]
- Shibuya, M. VEGF-VEGFR Signals in Health and Disease. Biomol. Ther. 2014, 22, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.B.; Binu, S.; Soumya, S.J.; Haritha, K.; Sudhakaran, P.R. Regulation of vascular endothelial growth factor by metabolic context of the cell. Glycoconj. J. 2014, 31, 427–434. [Google Scholar] [CrossRef]
- Kitazume, S.; Imamaki, R.; Ogawa, K.; Taniguchi, N. Sweet role of platelet endothelial cell adhesion molecule in understanding angiogenesis. Glycobiology 2014, 24, 1260–1264. [Google Scholar] [CrossRef] [PubMed]
- Eichmann, A.; Simons, M. VEGF signaling inside vascular endothelial cells and beyond. Curr. Opin. Cell Biol. 2012, 24, 188–193. [Google Scholar] [CrossRef]
- Félétou, M. The Endothelium: Part 1: Multiple Functions of the Endothelial Cells—Focus on Endothelium-Derived Vasoactive Mediators. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2011; Volume 200, pp. 135–156. [Google Scholar]
- Ellis, L.M.; Hicklin, D.J. VEGF-targeted therapy: Mechanisms of anti-tumor activity. Nat. Rev. Cancer 2008, 8, 579–591. [Google Scholar] [CrossRef]
- Gavard, J.; Gutkind, J.S. VEGF controls endothelial-cell permeability by promoting the β-arrestin-dependent endocytosis of VE-cadherin. Nat. Cell Biol. 2006, 8, 1223–1234. [Google Scholar] [CrossRef] [PubMed]
- Karkkainen, M.J.; Haiko, P.; Sainio, K.; Partanen, J.; Taipale, J.; Petrova, T.V.; Jeltsch, M.; Jackson, D.G.; Talikka, M.; Rauvala, H.; et al. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat. Immunol. 2004, 5, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Poliseno, L.; Tuccoli, A. MicroRNAs modulate the angiogenic properties of HUVECs. Blood 2006, 108, 3068–3071. [Google Scholar] [CrossRef]
- Dumont, D.J.; Gradwohl, G. Dominant-negative and targeted null mutations in the endothelial receptor tyrosine kinase, tek, reveal a critical role in vasculogenesis of the embryo. Genes Dev. 1994, 8, 1897–1909. [Google Scholar] [CrossRef]
- Lee, T.H.; Sengupta, S. Vascular endothelial growth factor mediates intracrine survival in human breast carcinoma cells through internally expressed VEGFR1/FLT1. PLoS Med. 2007, 4, e186. [Google Scholar] [CrossRef]
- Kunhiraman, H.; Edatt, L.; Thekkeveedu, S.; Poyyakkara, A.; Raveendran, V.; Kiran, M.S.; Sudhakaran, P.; Kumar, S.V. 2-Deoxy Glucose Modulates Expression and Biological Activity of VEGF in a SIRT-1 Dependent Mechanism. J. Cell. Biochem. 2016, 118, 252–262. [Google Scholar] [CrossRef]
- Lohela, M.; Bry, M.; Tammela, T.; Alitalo, K. VEGFs and receptors involved in angiogenesis versus lymphangiogenesis. Curr. Opin. Cell Biol. 2009, 21, 154–165. [Google Scholar] [CrossRef]
- Simons, M.; Gordon, E.; Claesson-Welsh, L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat. Rev. Mol. Cell Biol. 2016, 17, 611–625. [Google Scholar] [CrossRef]
- Das, A.; Ash, D.; Fouda, A.Y.; Sudhahar, V.; Kim, Y.-M.; Hou, Y.; Hudson, F.Z.; Stansfield, B.K.; Caldwell, R.B.; McMenamin, M.; et al. Cysteine oxidation of copper transporter CTR1 drives VEGFR2 signalling and angiogenesis. Nat. Cell Biol. 2022, 24, 35–50. [Google Scholar] [CrossRef]
- Shiau, J.-P.; Wu, C.-C.; Chang, S.-J.; Pan, M.-R.; Liu, W.; Ou-Yang, F.; Chen, F.-M.; Hou, M.-F.; Shih, S.-L.; Luo, C.-W. FAK Regulates VEGFR2 Expression and Promotes Angiogenesis in Triple-Negative Breast Cancer. Biomedicines 2021, 9, 1789. [Google Scholar] [CrossRef]
- Huwaimel, B.I.; Jonnalagadda, S.; Jonnalagadda, S.; Zahra, F.T.; Nocentini, A.; Supuran, C.T.; Mikelis, C.M.; Trippier, P.C. Chlorinated benzothiadiazines inhibit angiogenesis through suppression of VEGFR2 phosphorylation. Bioorganic Med. Chem. 2022, 67, 116805. [Google Scholar] [CrossRef]
- Arpino, J.-M.; Yin, H.; Prescott, E.K.; Staples, S.C.R.; Nong, Z.; Li, F.; Chevalier, J.; Balint, B.; O’Neil, C.; Mortuza, R.; et al. Low-flow intussusception and metastable VEGFR2 signaling launch angiogenesis in ischemic muscle. Sci. Adv. 2021, 7, eabg9509. [Google Scholar] [CrossRef]
- Nitzsche, A.; Pietilä, R.; Love, D.T.; Testini, C.; Ninchoji, T.; Smith, R.O.; Ekvärn, E.; Larsson, J.; Roche, F.P.; Egaña, I.; et al. Paladin is a phosphoinositide phosphatase regulating endosomal VEGFR2 signalling and angiogenesis. EMBO Rep. 2021, 22, e50218. [Google Scholar] [CrossRef] [PubMed]
- Lakshmikanthan, S.; Sobczak, M.; Chun, C.; Henschel, A.; Dargatz, J.; Ramchandran, R.; Chrzanowska-Wodnicka, M. Rap1 promotes VEGFR2 activation and angiogenesis by a mechanism involving integrin αvβ3. Blood 2011, 118, 2015–2026. [Google Scholar] [CrossRef]
- P35916. Available online: https://www.uniprot.org/uniprotkb/P35916/entry (accessed on 5 April 2025).
- Vitali, H.E.; Kuschel, B.; Sherpa, C.; Jones, B.W.; Jacob, N.; Madiha, S.A.; Elliott, S.; Dziennik, E.; Kreun, L.; Conatser, C.; et al. Hypoxia regulate developmental coronary angiogenesis potentially through VEGFR2- and SOX17-mediated signaling. bioRxiv 2023. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Lai, X.; Zhu, M.-H.; Long, M.; Liu, X.-L.; Wang, Z.-X.; Zhang, Y.; Guo, R.-J.; Dong, J.; Lu, Q.; et al. Saikosaponin A, a Triterpene Saponin, Suppresses Angiogenesis and Tumor Growth by Blocking VEGFR2-Mediated Signaling Pathway. Front. Pharmacol. 2021, 12, 713200. [Google Scholar] [CrossRef] [PubMed]
- Kofler, N.M.; Simons, M. Angiogenesis versus arteriogenesis: Neuropilin 1 modulation of VEGF signaling. F1000Prime Rep. 2015, 7, 26. [Google Scholar] [CrossRef]
- Zoeller, J.J.; Whitelock, J.M.; Iozzo, R.V. Perlecan regulates developmental angiogenesis by modulating the VEGF-VEGFR2 axis. Matrix Biol. 2009, 28, 284–291. [Google Scholar] [CrossRef]
- Sun, S.; Wu, H.-J.; Guan, J.-L. Nuclear FAK and its kinase activity regulate VEGFR2 transcription in angiogenesis of adult mice. Sci. Rep. 2018, 8, 2550. [Google Scholar] [CrossRef]
- Available online: https://web.expasy.org/protparam/ (accessed on 5 April 2025).
- Gelfand, M.V.; Hagan, N.; Tata, A.; Oh, W.J.; Lacoste, B.; Kang, K.T.; Kopycinska, J.; Bischoff, J.; Wang, J.H.; Gu, C. Neuropilin-1 functions as a VEGFR2 co-receptor to guide developmental angiogenesis independent of ligand binding. eLife 2014, 3, e03720. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miller, B.; Sewell-Loftin, M.K. Mechanoregulation of Vascular Endothelial Growth Factor Receptor 2 in Angiogenesis. Front. Cardiovasc. Med. 2022, 8, 804934. [Google Scholar] [CrossRef]
- Xie, Y.; Mansouri, M.; Rizk, A.; Berger, P. Regulation of VEGFR2 trafficking and signaling by Rab GTPase-activating proteins. Sci. Rep. 2019, 9, 13342. [Google Scholar] [CrossRef]
- Gao, S.; Griffin, C.T. RIPK3 modulates growth factor receptor expression in endothelial cells to support angiogenesis. Angiogenesis 2021, 24, 519–531. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, X.; Simons, M. Molecular controls of lymphatic VEGFR3 signaling. Arter. Thromb. Vasc. Biol. 2015, 35, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Mäkinen, T.; Veikkola, T.; Mustjoki, S.; Karpanen, T.; Catimel, B.; Nice, E.C.; Wise, L.; Mercer, A.; Kowalski, H.; Kerjaschki, D.; et al. Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the VEGF-C/D receptor VEGFR-3. EMBO J. 2001, 20, 4762–4773. [Google Scholar] [CrossRef] [PubMed]
- Dixelius, J.; Makinen, T.; Wirzenius, M.; Karkkainen, M.J.; Wernstedt, C.; Alitalo, K.; Claesson-Welsh, L. Ligand-induced vascular endothelial growth factor receptor-3 (VEGFR-3) heterodimerization with VEGFR-2 in primary lymphatic endothelial cells regulates tyrosine phosphorylation sites. J. Biol. Chem. 2003, 278, 40973–40979. [Google Scholar] [CrossRef]
- Heinolainen, K.; Karaman, S.; D’Amico, G.; Tammela, T.; Sormunen, R.; Eklund, L.; Alitalo, K.; Zarkada, G. VEGFR3 Modulates Vascular Permeability by Controlling VEGF/VEGFR2 Signaling. Circ. Res. 2017, 120, 1414–1425. [Google Scholar] [CrossRef] [PubMed]
- Jannaway, M.; Iyer, D.; Mastrogiacomo, D.M.; Li, K.; Sung, D.C.; Yang, Y.; Kahn, M.L.; Scallan, J.P. VEGFR3 is required for button junction formation in lymphatic vessels. Cell Rep. 2023, 42, 112777. [Google Scholar] [CrossRef]
- Wang, J.; Huang, Y.; Zhang, J.; Wei, Y.; Mahoud, S.; Bakheet, A.M.H.; Wang, L.; Zhou, S.; Tang, J. Pathway-related molecules of VEGFC/D-VEGFR3/NRP2 axis in tumor lymphangiogenesis and lymphatic metastasis. Clin. Chim. Acta 2016, 461, 165–171. [Google Scholar] [CrossRef]
- Cioffi, S.; Flore, G.; Martucciello, S.; Bilio, M.; Turturo, M.G.; Illingworth, E. VEGFR3 modulates brain microvessel branching in a mouse model of 22q11.2 deletion syndrome. Life Sci. Alliance 2022, 5, e202101308. [Google Scholar] [CrossRef]
- Iljin, K.; Karkkainen, M.J.; Lawrence, E.C.; Kimak, M.A.; Uutela, M.; Taipale, J.; Pajusola, K.; Alhonen, L.; Halmekytö, M.; Finegold, D.N.; et al. VEGFR3 gene structure, regulatory region, and sequence polymorphisms. FASEB J. 2021, 15, 1028–1036. [Google Scholar] [CrossRef]
- Kaipainen, A.; Korhonen, J. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc. Natl. Acad. Sci. USA 1995, 92, 3566–3570. [Google Scholar] [CrossRef]
- Joukov, V.; Pajusola, K. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and K.D.R. (VEGFR-2) receptor tyrosine kinases. EMBO J. 1996, 15, 1751–1761. [Google Scholar] [CrossRef]
- Alitalo, K.; Tammela, T. Lymphangiogenesis: In vitro and in vivo models. In Tumor Angiogenesis; Humana Press: Totowa, NJ, USA, 2002; pp. 97–120. [Google Scholar]
- Karkkainen, M.J.; Saaristo, A. A model for gene therapy of human hereditary lymphedema. Proc. Natl. Acad. Sci. USA 2004, 101, 936–941. [Google Scholar] [CrossRef]
- Makinen, T.; Jussila, L. Inhibition of lymphangiogenesis with resulting lymphedema in transgenic mice expressing soluble VEGF receptor-3. Nat. Med. 2001, 7, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Karpanen, T.; Alitalo, K. Molecular biology and pathology of lymphangiogenesis. Annu. Rev. Pathol. Mech. Dis. 2008, 3, 367–397. [Google Scholar] [CrossRef] [PubMed]
- Achen, M.G.; Mann, G.B.; Stacker, S.A. Targeting lymphangiogenesis to prevent tumour metastasis. Br. J. Cancer 2006, 94, 1355–1360. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Favier, B.; Alam, A. Neuropilin-2 interacts with VEGFR-2 and VEGFR-3, promoting the survival and migration of human endothelial cells. Blood 2006, 108, 1243–1250. [Google Scholar] [CrossRef]
- Xu, L.; Xu, Q.; Li, X.; Zhang, X. MicroRNA-21 regulates the proliferation and apoptosis of cervical cancer cells via tumor necrosis factor-α. Mol. Med. Rep. 2017, 16, 4659–4663. [Google Scholar] [CrossRef] [PubMed]
- Cursiefen, C.; Chen, L.; Saint-Geniez, M.; Hamrah, P.; Jin, Y.; Rashid, S.; Pytowski, B.; Persaud, K.; Wu, Y.; Streilein, J.W.; et al. Nonvascular VEGF receptor-3 expression by corneal epithelium maintains avascularity and vision. Proc. Natl. Acad. Sci. USA 2006, 103, 11405–11410. [Google Scholar] [CrossRef] [PubMed]
- Sosne, G.; Szliter, E.A.; Barrett, R.; Kernacki, K.A.; Kleinman, H.; Hazlett, L.D. Thymosin beta 4 promotes corneal wound healing and decreases inflammation in vivo following alkali injury. Exp. Eye Res. 2004, 78, 483–492. [Google Scholar] [CrossRef]
- Askari, A.T.; Unzek, S.; Popović, Z.B.; Goldman, C.K.; Forudi, F.; Kiedrowski, M.; Rovner, A.; Ellis, S.G.; Thomas, J.D.; DiCorleto, P.E.; et al. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet 2003, 362, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Connors, S.L.; Macklin, E.A.; Smith, K.D.; Fahey, J.W.; Talalay, P.; Zimmerman, A.W. Sulforaphane treatment of autism spectrum disorder (ASD). Proc. Natl. Acad. Sci. USA 2014, 111, 15550–15555. [Google Scholar] [CrossRef]
- Guan, F.-Y.; Yang, S.-J.; Liu, J.; Yang, S.-R. Effect of astragaloside IV against rat myocardial cell apoptosis induced by oxidative stress via mitochondrial ATP-sensitive potassium channels. Mol. Med. Rep. 2015, 12, 371–376. [Google Scholar] [CrossRef]
- Gene Cards. Available online: https://www.genecards.org/cgi-bin/carddisp.pl?gene=HIF1A (accessed on 5 April 2025).
- Breen, E.; Tang, K.; Olfert, M.; Knapp, A.; Wagner, P. Skeletal muscle capillarity during hypoxia: VEGF and its activation. High Alt. Med. Biol. 2008, 9, 158–166. [Google Scholar] [CrossRef]
- Cardoso, R.; Love, R.; Nilsson, C.L.; Bergqvist, S.; Nowlin, D.; Yan, J.; Liu, K.K.; Zhu, J.; Chen, P.; Deng, Y.L.; et al. Identification of Cys255 in HIF-1 alpha as a novel site for development of covalent inhibitors of HIF-1 alpha /ARNT PasB domain protein-protein interaction. Protein Sci. 2012, 21, 1885–1896. [Google Scholar] [CrossRef]
- UniProt. Available online: https://www.uniprot.org/uniprotkb/Q16665/entry (accessed on 5 April 2025).
- Jin, X.; Dai, L.; Ma, Y.; Wang, J. Implications of HIF-1α in the tumorigenesis and progression of pancreatic cancer. Cancer Cell Int. 2020, 20, 273. [Google Scholar] [CrossRef]
- Brum, P.O.; Viola, G.D.; Saibro-Girardi, C.; Tiefensee-Ribeiro, C.; Brum, M.O.; Gasparotto, J.; Krolow, R.; Moreira, J.C.F. Hypoxia-Inducible Factor-1α (HIF-1α) Inhibition Impairs Retinoic Acid-Induced Differentiation in SH-SY5Y Neuroblastoma Cells, Leading to Reduced Neurite Length and Diminished Gene Expression Related to Cell Differentiation. Neurochem. Res. 2022, 47, 409–421. [Google Scholar] [CrossRef]
- Takikawa, A.; Mahmood, A.; Nawaz, A.; Kado, T.; Okabe, K.; Yamamoto, S.; Aminuddin, A.; Senda, S.; Tsuneyama, K.; Ikutani, M.; et al. HIF-1α in Myeloid Cells Promotes Adipose Tissue Remodeling Toward Insulin Resistance. Diabetes 2016, 65, 3649–3659. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Sinha, S.; Shrivastava, N. Therapeutic Targeting Hypoxia-Inducible Factor (HIF-1) in Cancer: Cutting Gordian Knot of Cancer Cell Metabolism. Front. Genet. 2022, 13, 849040. [Google Scholar] [CrossRef]
- Steiner, S.; Hammer, A. Gene therapy in peripheral arterial disease: A systematic review and meta-analysis of randomized, controlled trials. Eur. Hear J. 2013, 34, P351. [Google Scholar] [CrossRef]
- Heikal, L.; Ghezzi, P.; Mengozzi, M.; Ferns, G. Assessment of HIF-1α expression and release following endothelial injury in-vitro and in-vivo. Mol. Med. 2018, 24, 22, Correction in Mol. Med. 2020, 26, 61. [Google Scholar] [CrossRef]
- Patel, T.H.; Kimura, H.; Weiss, C.R.; Semenza, G.L.; Hofmann, L.V. Constitutively active HIF-1α improves perfusion and arterial remodeling in an endovascular model of limb ischemia. Cardiovasc. Res. 2005, 68, 144–154. [Google Scholar] [CrossRef]
- Bates, D.O.; Harper, S.J. Regulation of vascular permeability by vascular endothelial growth factors. Vasc. Pharmacol. 2002, 39, 225–237. [Google Scholar] [CrossRef]
- Bergers, G.; Hanahan, D. Modes of resistance to antiangiogenic therapy. Nat. Rev. Cancer 2008, 8, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Adamis, A.P. Ten years of anti-vascular endothelial growth factor therapy. Nat. Rev. Drug Discov. 2016, 15, 385–403. [Google Scholar] [CrossRef] [PubMed]
- Goel, H.L.; Mercurio, A.M. VEGF targets the tumor cell. Nat. Rev. Cancer 2013, 13, 871–882. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef]
- Pàez-Ribes, M.; Allen, E. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell 2009, 15, 220–231. [Google Scholar] [CrossRef]
- Voron, T.; Colussi, O. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J. Exp. Med. 2015, 212, 139–148. [Google Scholar] [CrossRef]
- Yang, J.; Yan, J.; Liu, B. Targeting VEGF/VEGFR to modulate antitumor immunity. Front. Immunol. 2018, 9, 978. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Potente, M.; Gerhardt, H.; Carmeliet, P. Basic and therapeutic aspects of angiogenesis. Cell 2011, 146, 873–887. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, D.A.; Jin, K. From angiogenesis to neuropathology. Nature 2005, 438, 954–959. [Google Scholar] [CrossRef]
- Ding, B.S.; Nolan, D.J. Endothelial-derived angiocrine signals induce and sustain regenerative lung alveolarization. Cell 2010, 147, 539–553. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Lai, E.C. Adult-specific functions of animal miRNAs. Nat. Rev. Genet. 2013, 14, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Chen, P.; Zhong, J.; Cheng, Y.; Chen, H.; He, Y.; Chen, C. HIF-1α in myocardial ischemia-reperfusion injury. Mol. Med. Rep. 2021, 23, 352. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.; Zadeh, L.R.; Baradaran, B.; Molavi, O.; Ghesmati, Z.; Sabzichi, M.; Ramezani, F. Up-down regulation of HIF-1α in cancer progression. Gene 2021, 798, 145796. [Google Scholar] [CrossRef]
- Chen, W.; Wu, P.; Yu, F.; Luo, G.; Qing, L.; Tang, J. HIF-1α Regulates Bone Homeostasis and Angiogenesis, Participating in the Occurrence of Bone Metabolic Diseases. Cells 2022, 11, 3552. [Google Scholar] [CrossRef]
- Harki, J.; Sana, A.; van Noord, D.; van Diest, P.J.; van der Groep, P.; Kuipers, E.J.; Moons, L.M.G.; Biermann, K.; Tjwa, E.T.T.L. Hypoxia-inducible factor 1-α in chronic gastrointestinal ischemia. Virchows Arch. 2014, 466, 125–132. [Google Scholar] [CrossRef]
- Szuba, A.; Skobe, M. Therapeutic lymphangiogenesis with human recombinant VEGF-C. FASEB J. 2002, 16, 1985–1987. [Google Scholar] [CrossRef]
- Karkkainen, M.J.; Ferrell, R.E.; Lawrence, E.C.; Kimak, M.A.; Levinson, K.L.; McTigue, M.A.; Alitalo, K.; Finegold, D.N. Missense mutations interfere with VEGFR-3 signalling in primary lymphoedema. Nat. Genet. 2000, 25, 153–159. [Google Scholar] [CrossRef]
- Kaux, J.-F.; Janssen, L.; Drion, P.; Nusgens, B.; Libertiaux, V.; Pascon, F.; Heyeres, A.; Hoffmann, A.; Lambert, C.; Le Goff, C.; et al. Vascular Endothelial Growth Factor-111 (VEGF-111) and tendon healing: Preliminary results in a rat model of tendon injury. Muscle Ligaments Tendons J. 2019, 4, 24–28. [Google Scholar] [CrossRef]
- Nakatsu, M.N.; Sainson, R.C.A.; Pérez-del-Pulgar, S.; Aoto, J.N.; Aitkenhead, M.; Taylor, K.L.; Carpenter, P.M.; Hughes, C.C.W. VEGF(121) and VEGF(165) regulate blood vessel diameter through vascular endothelial growth factor receptor 2 in an in vitro angiogenesis model. Lab. Investig. 2003, 83, 1873–1885. [Google Scholar] [CrossRef]
- Poltorak, Z.; Cohen, T.; Sivan, R.; Kandelis, Y.; Spira, G.; Vlodavskyi, I.; Keshet, E.; Neufeld, G. VEGF145, a Secreted Vascular Endothelial Growth Factor Isoform That Binds to Extracellular Matrix. J. Biol. Chem. 1997, 272, 7151–7158. [Google Scholar] [CrossRef] [PubMed]
- Lange, T.; Guttmann-Raviv, N.; Baruch, L.; Machluf, M.; Neufeld, G. VEGF162, A New Heparin-binding Vascular Endothelial Growth Factor Splice Form That Is Expressed in Transformed Human Cells. J. Biol. Chem. 2003, 278, 17164–17169. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Xu, J.; Wang, X.; Ge, C.; Li, S.; Wanga, L.; Xu, Y. Enrichment and detection of VEGF165 in blood samples on a microfluidic chip integrated with multifunctional units†. Lab Chip 2023, 23, 2469–2476. [Google Scholar] [CrossRef]
- Oosthuyse, B.; Moons, L.; Storkebaum, E.; Beck, H.; Nuyens, D.; Brusselmans, K.; Van Dorpe, J.; Hellings, P.; Gorselink, M.; Heymans, S.; et al. Deletion of the hypoxia-response element in the vascular endothelial growth factor promoter causes motor neuron degeneration. Nat. Genet. 2001, 28, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Tugues, S.; Honjo, S.; König, C.; Padhan, N.; Kroon, J.; Gualandi, L.; Li, X.; Barkefors, I.; Thijssen, V.L.J.L.; Griffioen, A.W.; et al. Tetraspanin CD63 promotes vascular endothelial growth factor receptor 2–β1 integrin complex formation, thereby regulating activation and downstream signaling in endothelial cells in vitro and in vivo. J. Biol. Chem. 2013, 288, 19060–19071. [Google Scholar] [CrossRef] [PubMed]
- Houck, K.A.; Ferrara, N.; Winer, J.; Cachianes, G.; Li, B.; Leung, D.W. The vascular endothelial growth factor family: Identification of a fourth molecular species and characterization of alternative splicing of RNA. Mol. Endocrinol. 1991, 5, 1806–1814. [Google Scholar] [CrossRef] [PubMed]
- Reddy, G.R.; Bhojani, M.S.; McConville, P.; Moody, J.; Moffat, B.; Hallagan, B.; Rehemtulla, A. Vascular targeted nanoparticles for imaging and treatment of brain tumors. Clin. Cancer Res. 2006, 12, 6677–6686. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, L.D.; Del Gatto, A.; Pedone, C.; Benedetti, E. Targeting angiogenesis: Structural characterization and biological properties of a de novo engineered VEGF mimicking peptide. Proc. Natl. Acad. Sci. USA 2005, 102, 14215–14220. [Google Scholar] [CrossRef]
- Yang, X.; Jin, H.; Liu, K.; Gu, Q.; Xu, X. A novel peptide derived from human pancreatitis-associated protein inhibits inflammation in vivo and in vitro and blocks NF-κB signaling pathway. PLoS ONE 2011, 6, e29155. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, M.; Li, S.; Erasquin, U.J.; Wang, H.; Ren, L.; Chen, C.; Wang, Y.; Cai, C. “Click” immobilization of a VEGF-mimetic peptide on decellularized endothelial extracellular matrix to enhance angiogenesis. ACS Appl. Mater. Interfaces 2014, 6, 8401–8406. [Google Scholar] [CrossRef]
- Xu, X.; Cai, Y.; Wei, Y.; Donate, F.; Juarez, J.; Parry, G.; Meehan, E.J.; Ahn, R.W.; Ugolkov, A.; Mazar, A.P.; et al. Identification of a new epitope in uPAR as a target for the cancer therapeutic monoclonal antibody ATN-658, a structural homolog of the uPAR binding integrin CD11b (αM). PLoS ONE 2014, 9, e85349, Corrrection in: PLoS ONE 2014, 9, e99911. [Google Scholar] [CrossRef]
- Smyth, A.P.; Rook, S.L.; Detmar, M.; Robinson, G.S. Antisense oligonucleotides inhibit vascular endothelial growth factor expression in normal human epidermal keratinocytes. J. Investig. Dermatol. 1997, 108, 523–526. [Google Scholar] [CrossRef]
- Martin, A.C.B.; Campos, A.C.F.; Ferreira, M.O.; Angelo, C.S.; de Lima, C.H.L.; Castilho, R.O.; Castro, M.Á.A. Recombinant disintegrin domain of ADAM9 inhibits migration and invasion of DU145 prostate tumor cells. Cell Adhes. Migr. 2015, 9, 293–299. [Google Scholar] [CrossRef]
- Lyabin, D.N.; Eliseeva, I.A.; Ovchinnikov, L.P. YB-1 protein: Functions and regulation. Wiley Interdiscip. Rev. RNA 2014, 5, 95–110. [Google Scholar] [CrossRef] [PubMed]
- Hauser, A.K.; Anderson, K.W.; Hilt, J.Z. Peptide conjugated magnetic nanoparticles for magnetically mediated energy delivery to lung cancer cells. Nanomedicine 2016, 11, 1769–1785. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Losordo, D.W.; Vale, P.R.; Hendel, R.C.; Milliken, C.E.; Fortuin, F.D.; DeYoung, M.B.; Stewart, D.J.; Lee, J.S.; Mendelsohn, J. Gene therapy for myocardial angiogenesis: Initial clinical results with direct myocardial injection of phVEGF165 as sole therapy for myocardial ischemia. Circulation 2002, 105, 724–729. [Google Scholar] [CrossRef]
- Thurston, G.; Rudge, J.S.; Ioffe, E.; Zhou, H.; Ross, L.; Croll, S.D.; Glazer, N.; Holash, J.; McDonald, D.M.; Yancopoulos, G.D. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat. Med. 2000, 6, 460–463. [Google Scholar] [CrossRef]
- Shah, A.; Lindquist, J.A.; Rosendahl, L.; Schmitz, I.; Mertens, P.R. Novel Insights into YB-1 Signaling and Cell Death Decisions. Cancers 2021, 13, 3306. [Google Scholar] [CrossRef]
- Farzin, A.; Etesami, S.A.; Quint, J.; Memic, A.; Tamayol, A. Magnetic nanoparticles in cancer therapy and diagnosis. Adv. Healthc. Mater. 2020, 9, e1901058. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ploug, M. Structure-function relationships in the interaction between the urokinase-type plasminogen activator and its receptor. Curr. Pharm. Des. 2003, 9, 1499–1528. [Google Scholar] [CrossRef]
- Masaki, I.; Yonemitsu, Y.; Yamashita, A.; Sata, S.; Tanii, M.; Komori, K.; Nakagawa, K.; Hou, X.; Nagai, Y.; Hasegawa, M.; et al. Angiogenic gene therapy for experimental critical limb ischemia: Acceleration of limb loss by overexpression of vascular endothelial growth factor 165 but not of fibroblast growth factor-2. Circ. Res. 2002, 90, 966–973. [Google Scholar] [CrossRef]
- Sarkar, K.; Cai, Z.; Gupta, R.; Parajuli, N.; Fox-Talbot, K.; Darshan, M.S.; Gonzalez, F.J.; Semenza, G.L. Hypoxia-inducible factor 1 transcriptional activity in endothelial cells is required for acute phase cardioprotection induced by ischemic preconditioning. Proc. Natl. Acad. Sci. USA 2012, 109, 10504–10509. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, J.I.; Shields, D.J.; Barillas, S.G.; Acevedo, L.M.; Murphy, E.; Huang, J.; Scheppke, L.; Stockmann, C.; Johnson, R.S.; Angle, N.; et al. A role for VEGF as a negative regulator of pericyte function and vessel maturation. Nature 2008, 456, 809–813. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Patijn, G.; Terpstra, O.T.; Kay, M.A. Stimulation of liver growth by exogenous human hepatocyte growth factor in normal and partially hepatectomized rats. Hepatology 1993, 18, 1443–1449. [Google Scholar]
- Marsano, A.; Maidhof, R. Scaffold stiffness affects the contractile function of three-dimensional engineered cardiac constructs. Biotechnol. Prog. 2016, 28, 737–747. [Google Scholar] [CrossRef]
- Bird, A.C.; Bressler, N.M.; Bressler, S.B.; Chisholm, I.H.; Hyman, L.; Johnson, L.V.; Kaiser-Kupfer, M.I.; Klein, R.; Miller, D.; Munoz, B.; et al. An international classification and grading system for age-related maculopathy and age-related macular degeneration. Surv. Ophthalmol. 1995, 39, 367–374. [Google Scholar] [PubMed]
- Etienne-Manneville, S. Actin and microtubules in cell motility: Which one is in control? Traffic 2004, 5, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Jain, R.K. Angiogenesis in cancer and other diseases. Nature 2000, 407, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, H.; Betsholtz, C. Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res. 2003, 314, 15–23. [Google Scholar] [CrossRef]
- Vion, A.-C.; Alt, S.; Klaus-Bergmann, A.; Szymborska, A.; Zheng, T.; Perović, T.; Hammoutene, A.; Bartels-Klein, E.; Hollfinger, I.; Rautou, P.-E.; et al. Primary cilia sensitize endothelial cells to BMP and prevent excessive vascular regression. J. Cell Biol. 2018, 217, 1651–1665. [Google Scholar] [CrossRef]
- Nonaka, S.; Shiratori, H.; Saijoh, Y.; Hamada, H. Determination of left–right patterning in mice by ciliary motion in the node. Nature 2002, 418, 96–99. [Google Scholar] [CrossRef]
- Lamalice, L.; Le Boeuf, F.; Huot, J. Endothelial cell migration during angiogenesis. Circ. Res. 2007, 100, 782–794. [Google Scholar] [CrossRef]
- Gerhardt, H.; Golding, M.; Fruttiger, M.; Ruhrberg, C.; Lundkvist, A.; Abramsson, A.; Jeltsch, M.; Mitchell, C.; Alitalo, K.; Shima, D.; et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J. Cell Biol. 2003, 161, 1163–1177. [Google Scholar] [CrossRef]
- Simons, M.; Mlodzik, M. Planar cell polarity signaling: From fly development to human disease. Annu. Rev. Genet. 2008, 42, 517–540. [Google Scholar] [CrossRef]
- Hynes, R.O. Integrins: Bidirectional, allosteric signaling machines. Cell 2002, 110, 673–687. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.D.; Li, Y.S.; Kim, M.; Li, S.; Yuan, S.; Chien, S.; Shyy, J.Y. Mechanotransduction in response to shear stress: Roles of receptor tyrosine kinases, integrins, and Shc. J. Biol. Chem. 1999, 274, 18393–18400. [Google Scholar] [CrossRef]
- Carmeliet, P. Mechanisms of angiogenesis and arteriogenesis. Nat. Med. 2000, 6, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Angelini, T.E.; Hannezo, E.; Trepat, X.; Marquez, M.; Fredberg, J.J.; Weitz, D.A. Glass-like dynamics of collective cell migration. Proc. Natl. Acad. Sci. USA 2011, 108, 4714–4719. [Google Scholar] [CrossRef]
- Avraamides, C.J.; Garmy-Susini, B.; Varner, J.A. Integrins in angiogenesis and lymphangiogenesis. Nat. Rev. Cancer 2008, 8, 604–617. [Google Scholar] [CrossRef] [PubMed]
- Phng, L.-K.; Gerhardt, H. Angiogenesis: A team effort coordinated by Notch. Dev. Cell 2009, 16, 196–208. [Google Scholar] [CrossRef]
- Etienne-Manneville, S.; Hall, A. Rho GTPases in cell biology. Nature 2002, 420, 629–635. [Google Scholar] [CrossRef]
- Trimm, E.; Red-Horse, K. Vascular endothelial cell development and diversity. Nat. Rev. Cardiol. 2022, 20, 197–210. [Google Scholar] [CrossRef]
- Glaviano, A.; Foo, A.S.C.; Lam, H.Y.; Yap, K.C.H.; Jacot, W.; Jones, R.H.; Eng, H.; Nair, M.G.; Makvandi, P.; Geoerger, B.; et al. PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Mol. Cancer 2023, 22, 138. [Google Scholar] [CrossRef]
- Liu, C.-L.; Chen, S.-F.; Wu, M.-Z.; Jao, S.-W.; Lin, Y.-S.; Yang, C.-Y.; Lee, T.-Y.; Wen, L.-W.; Lan, G.-L.; Nieh, S. The molecular and clinical verification of therapeutic resistance via the p38 MAPK-Hsp27 axis in lung cancer. Oncotarget 2016, 7, 14279–14290. [Google Scholar] [CrossRef]
- Xu, X.; Wu, S.; Zhang, Y.; Fan, W.; Lin, X.; Chen, K.; Lin, X. m6A modification of VEGFA mRNA by RBM15/YTHDF2/IGF2BP3 contributes to angiogenesis of hepatocellular carcinoma. Mol. Carcinog. 2024, 63, 2174–2189. [Google Scholar] [CrossRef]
- Payaningal, R.; Somanath, P.; Malinin, N.L.; Byzova, T.V. Cooperation between integrin αvβ3 and VEGFR2 in angiogenesis. Angiogenesis 2009, 12, 177–185. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Blanco, R.; Gerhardt, H. VEGF and Notch in tip and stalk cell selection. Cold Spring Harb. Perspect. Med. 2012, 3, a006569. [Google Scholar] [CrossRef] [PubMed]
- Ellertsdóttir, E.; Lenard, A.; Blum, Y.; Krudewig, A.; Herwig, L.; Affolter, M.; Belting, G.-G. Vascular morphogenesis in the zebrafish embryo. Dev. Biol. 2010, 341, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Thiele, W.; Krishnan, J.; Rothley, M.; Weih, D.; Plaumann, D.; Kuch, V.; Quagliata, L.; Weich, H.A.; Sleeman, J.P. VEGFR-3 is expressed on megakaryocyte precursors in the murine bone marrow and plays a regulatory role in megakaryopoiesis. Blood 2012, 120, 1899–1907. [Google Scholar] [CrossRef]
- Wan, L.Q.; Vunjak-Novakovic, G. Micropatterning chiral morphogenesis. Commun. Integr. Biol. 2011, 4, 745–748. [Google Scholar] [CrossRef]
- Park, G.B.; Kim, D. PI3K catalytic isoform alteration promotes the LIMK1-related metastasis through the PAK1 or ROCK1/2 activation in cigarette smoke-exposed ovarian cancer cells. Anticancer. Res. 2017, 37, 1805–1818. [Google Scholar] [CrossRef] [PubMed]
- Soffe, R.; Tang, S.-Y.; Baratchi, S.; Nahavandi, S.; Nasabi, M.; Cooper, J.M.; Mitchell, A.; Khoshmanesh, K. Controlled rotation and vibration of patterned cell clusters using dielectrophoresis. Anal. Chem. 2015, 87, 2389–2395. [Google Scholar] [CrossRef]
- Graham, J.A.; Dumont, J.R.; Winter, S.S.; Brown, J.E.; LaChance, P.A.; Amon, C.C.; Farnes, K.B.; Morris, A.J.; Streltzov, N.A.; Taube, J.S. Angular Head Velocity Cells within Brainstem Nuclei Projecting to the Head Direction Circuit. J. Neurosci. 2023, 43, 8403–8424. [Google Scholar] [CrossRef]
- Romano, D.J.; Gomez-Salinero, J.M.; Šunić, Z.; Checco, A.; Rabbany, S.Y. Tracking of Endothelial Cell Migration and Stiffness Measurements Reveal the Role of Cytoskeletal Dynamics. Int. J. Mol. Sci. 2022, 23, 568. [Google Scholar] [CrossRef]
- Linville, R.M.; Arevalo, D.; Maressa, J.C.; Zhao, N.; Searson, P.C. Three-dimensional induced pluripotent stem-cell models of human brain angiogenesis. Microvasc. Res. 2020, 132, 104042. [Google Scholar] [CrossRef] [PubMed]
- Zambuto, S.G.; Jain, I.; Theriault, H.S.; Underhill, G.H.; Harley, B.A.C. Cell Chirality of Micropatterned Endometrial Microvascular Endothelial Cells. Adv. Healthc. Mater. 2024, 13, e2303928. [Google Scholar] [CrossRef]
- Suarez, R.K.; Darveau, C.A. Multi-level regulation and metabolic scaling. J. Exp. Biol. 2005, 208 Pt 9, 1627–1634. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gan, H.; Zhang, M.; Sun, T. Modulating cell behaviors on chiral polymer brush films with different hydrophobic side groups. Langmuir 2012, 28, 2791–2798. [Google Scholar] [CrossRef]
- Levesque, M.J.; Nerem, R.M. The elongation and orientation of cultured endothelial cells in response to shear stress. J. Biomech. Eng. 1985, 107, 341–347. [Google Scholar] [CrossRef]
- Siekmann, A.F.; Lawson, N.D. Notch signalling limits angiogenic cell behaviour in developing zebrafish arteries. Nature 2007, 445, 781–784. [Google Scholar] [CrossRef]
- Bentley, T.E.; Franco, C.A.; Philippides, A.; Blanco, R.; Dierkes, M.; Gebala, V.; Stanchi, F.; Jones, M.L.; Aspalter, I.M.; Cagna, G.; et al. The role of differential VE-cadherin dynamics in cell rearrangement during angiogenesis. Nat. Cell Biol. 2014, 16, 309–321. [Google Scholar] [CrossRef]
- Ferrara, N.; Carver-Moore, K.; Chen, H.; Dowd, M.; Lu, L.; O’Shea, K.S.; Powell-Braxton, L.; Hillan, K.J.; Moore, M.W. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 1996, 380, 439–442. [Google Scholar] [CrossRef]
- Carmeliet, P.; Ferreira, V.; Breier, G.; Pollefeyt, S.; Kieckens, L.; Gertsenstein, M.; Fahrig, M.; Vandenhoeck, A.; Harpal, K.; Eberhardt, C.; et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 1996, 380, 435–439. [Google Scholar] [CrossRef]
- Shalaby, F.; Rossant, J.; Yamaguchi, T.P.; Gertsenstein, M.; Wu, X.F.; Breitman, M.L.; Schuh, G.G. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature 1995, 376, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Hellström, M.; Phng, L.-K.; Hofmann, J.J.; Wallgard, E.; Coultas, L.; Lindblom, P.; Alva, J.; Nilsson, A.K.; Karlsson, L.; Gaiano, N.; et al. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature 2007, 445, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Forsythe, J.A.; Jiang, B.H.; Iyer, N.V.; Agani, F.; Leung, S.W.; Koos, R.D.; Semenza, G.L. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol. Cell. Biol. 1996, 16, 4604–4613. [Google Scholar] [CrossRef]
- Semenza, G.L. HIF-1 and mechanisms of hypoxia sensing. Curr. Opin. Cell Biol. 2001, 13, 167–171. [Google Scholar] [CrossRef]
- Wan, L.Q.; Ronaldson, K.; Park, M.; Taylor, G.; Zhang, Y.; Gimble, J.M.; Vunjak-Novakovic, G.; Chen, W. Micropatterned mammalian cells exhibit phenotype-specific left-right asymmetry. Proc. Natl. Acad. Sci. USA 2011, 108, 12295–12300. [Google Scholar] [CrossRef]
- Tee, Y.H.; Shemesh, T.; Thiagarajan, V.; Hariadi, R.F.; Anderson, K.L.; Page, C.; Volkmann, N.; Hanein, D.; Sivaramakrishnan, S.; Kozlov, M.M. Cellular chirality arising from the self-organization of the actin cytoskeleton. Nat Cell Biol. 2015, 17, 445–457. [Google Scholar] [CrossRef]
- Wan, L.Q.; Chin, A.S.; Worley, K.E.; Ray, P. Cell chirality: Emergence of asymmetry from cell culture. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150413. [Google Scholar] [CrossRef]
- Honn, K.V.; Tang, D.G.; Grossi, I.; Duniec, Z.M.; Timar, J.; Renaud, C.; Leithauser, M.; Blair, I.; Johnson, C.R.; Diglio, C.A.; et al. Tumor cell-derived 12(S)-hydroxyeicosatetraenoic acid induces microvascular endothelial cell retraction. Cancer Res. 1994, 54, 565–574. [Google Scholar]
- Cuenca, M.V.; Cochrane, A.; van den Hil, F.E.; de Vries, A.A.F.; Oberstein, S.A.J.L.; Mummery, C.L.; Orlova, V.V. Engineered 3D vessel-on-chip using hiPSC-derived endothelial- and vascular smooth muscle cells. Stem Cell Rep. 2021, 16, 2159–2168. [Google Scholar] [CrossRef]
- Chin, A.J.; Tsang, M.; Weinberg, E.S. Heart and gut chiralities are controlled independently from initial heart position in the developing zebrafish. Dev. Biol. 2000, 227, 403–421. [Google Scholar] [CrossRef] [PubMed]
- Witte, K.; Strickland, D.; Glotzer, M. Cell cycle entry triggers a switch between two modes of Cdc42 activation during yeast polarization. eLife. 2017, 6, e26722. [Google Scholar] [CrossRef] [PubMed]
- Haddad, J.J.; Land, S.C. Redox/ROS regulation of lipopolysaccharide-induced mitogen-activated protein kinase (MAPK) activation and MAPK-mediated TNF-alpha biosynthesis. Br. J. Pharmacol. 2002, 135, 520–536. [Google Scholar] [CrossRef]
- Estrada, C.C.; Maldonado, A.; Mallipattu, S.K. Therapeutic Inhibition of VEGF Signaling and Associated Nephrotoxicities. J. Am. Soc. Nephrol. 2019, 30, 187–200. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, J.; He, X.; Chen, X.; Dong, L.; Lin, J.; Wang, H.; Weng, W.; Cheng, K. Regulation of Macrophage Polarization on Chiral Potential Distribution of CFO/P(VDF-TrFE) Films. ACS Biomater. Sci. Eng. 2023, 9, 2524–2533. [Google Scholar] [CrossRef]
- Singh, A.V.; Mehta, K.K.; Worley, K.; Dordick, J.S.; Kane, R.S.; Wan, L.Q. Carbon nanotube-induced loss of multicellular chirality on micropatterned substrate is mediated by oxidative stress. ACS Nano 2014, 8, 2196–2205. [Google Scholar] [CrossRef]
- Rode, S.; Elgeti, J.; Gompper, G. Chiral-filament self-assembly on curved manifolds. Soft Matter 2020, 16, 10548–10557. [Google Scholar] [CrossRef]
- Yin, Q.; Zheng, M.; Luo, Q.; Jiang, D.; Zhang, H.; Chen, C. YB-1 as an Oncoprotein: Functions, Regulation, Post-Translational Modifications, and Targeted Therapy. Cells 2022, 11, 1217. [Google Scholar] [CrossRef] [PubMed]
- Gaurav, I.; Wang, X.; Thakur, A.; Iyaswamy, A.; Thakur, S.; Chen, X.; Kumar, G.; Li, M.; Yang, Z. Peptide-Conjugated Nano Delivery Systems for Therapy and Diagnosis of Cancer. Pharmaceutics 2021, 13, 1433. [Google Scholar] [CrossRef]
- Nakamura, T.; Nishizawa, T.; Hagiya, M.; Seki, T.; Shimonishi, M.; Sugimura, A.; Tashiro, K.; Shimizu, S. Molecular cloning and expression of human hepatocyte growth factor. Nature 1989, 342, 440–443. [Google Scholar] [CrossRef] [PubMed]
- Ibukiyama, C. Angiogenic therapy using fibroblast growth factor and vascular endothelial growth factor for ischemic vascular lesions. Jpn. Heart J. 1996, 37, 285–300. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Ingber, D.E. Control of cytoskeletal mechanics by extracellular matrix, cell shape, and mechanical tension. Biophys. J. 1994, 66, 2181–2189. [Google Scholar] [CrossRef]
- Wirth, M.; Sauer, W.H.B. Bioactive Molecules: Perfectly Shaped for Their Target? Mol. Inform. 2011, 30, 677–688. [Google Scholar] [CrossRef]
- Folkman, J. Isolation of a tumor factor responsible for angiogenesis. J. Exp. Med. 1971, 133, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.W.; Cachianes, G.; Kuang, W.J.; Goeddel, D.V.; Ferrara, N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 1989, 246, 1306–1309. [Google Scholar] [CrossRef] [PubMed]
- Plate, K.H.; Breier, G.; Weich, H.A.; Risau, W. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature. 1992, 359, 845–848. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.F.; Berse, B.; Jackman, R.W.; Tognazzi, K.; Manseau, E.J.; Senger, D.R.; Dvorak, H.F. Expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in adenocarcinomas of the gastrointestinal tract. Cancer Res. 1993, 53, 4727–4735. [Google Scholar]
- De Vries, C.; Escobedo, J.A.; Ueno, H.; Houck, K.A.; Ferrara, N.; Williams, L.T. The fms-like tyrosine kinase 1 is a receptor for VEGF. Science 1992, 255, 989–991. [Google Scholar] [CrossRef]
- Ferrara, N.; Gerber, H.-P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef]
- Carmeliet, P.; Moons, L.; Luttun, A.; Vincenti, V.; Compernolle, V.; De Mol, M.; Wu, Y.; Bono, F.; Devy, L.; Beck, H. Synergism between VEGF and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat. Med. 2001, 7, 575–583. [Google Scholar] [CrossRef]
- Ren, S.; Yang, Y. The proliferation and angiogenesis in hemangioma-derived endothelial cells is affected by STC2 medicated VEGFR2/Akt/eNOS pathway. Pak. J. Med. Sci. 2023, 39, 1119–1123. [Google Scholar] [CrossRef]
- Marsh, D.J.; Postnov, D.D.; Sosnovtseva, O.V.; Holstein-Rathlou, N.-H. The nephron-arterial network and its interactions. Am. J. Physiol. Ren. Physiol. 2019, 316, F769–F784. [Google Scholar] [CrossRef] [PubMed]
- Miquerol, L.; Langille, B.L.; Nagy, A. Embryonic development is disrupted by modest increases in vascular endothelial growth factor gene expression. Development 2000, 127, 3941–3946. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Folkman, J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 1996, 86, 353–364. [Google Scholar] [CrossRef]
- Soldi, R.; Mitola, P.; Strasly, L.; Defilippi, L.; Tarone, G.; Bussolino, F. Role of αvβ3 integrin in the activation of vascular endothelial growth factor receptor-2. EMBO J. 1999, 18, 882–892. [Google Scholar] [CrossRef]
- Claesson-Welsh, L. Vascular permeability—The essentials. Upsala J. Med. Sci. 2015, 120, 135–143. [Google Scholar] [CrossRef]
- Ferrara, N.; Davis-Smyth, T. The biology of vascular endothelial growth factor. Endocr. Rev. 1997, 18, 4–25. [Google Scholar] [CrossRef]
- Ringvall, M.; Thulin, Å.; Zhang, L.; Cedervall, J.; Tsuchida-Straeten, N.; Jahnen-Dechent, W.; Siegbahn, A.; Olsson, A.-K. Enhanced Platelet Activation Mediates the Accelerated Angiogenic Switch in Mice Lacking Histidine-Rich Glycoprotein. PLoS ONE 2011, 6, e14526. [Google Scholar] [CrossRef] [PubMed]
- Codutti, A.; Charsooghi, M.A.; Cerdá-Doñate, E.; Taïeb, H.M.; Robinson, T.; Faivre, D.; Klumpp, S. Interplay of surface interaction and magnetic torque in single-cell motion of magnetotactic bacteria in microfluidic confinement. Elife 2022, 11, e71527. [Google Scholar] [CrossRef] [PubMed]
- Villari, G.; Gioelli, N.; Valdembri, D.; Serini, G. Vesicle choreographies keep up cell-to-extracellular matrix adhesion dynamics in polarized epithelial and endothelial cells. Matrix Biol. 2022, 112, 62–71. [Google Scholar] [PubMed]
- Binny, R.N.; Plank, M.J.; James, A. Spatial moment dynamics for collective cell movement incorporating a neighbour-dependent directional bias. J. R. Soc. Interface 2015, 12, 20150228. [Google Scholar] [CrossRef]
- Piercy, C.; Adam, J.; Harrison, J.N.; Heiss, C.; Campagnolo, P.; Creagh-Brown, B. Endovascular endothelial cell biopsy—A systematic review. Vasa 2025, 54, 305–313. [Google Scholar]
- Etchegaray, C.; Meunier, N. A stochastic model for cell adhesion to the vascular wall. J. Math. Biol. 2019, 79, 1665–1697. [Google Scholar] [CrossRef] [PubMed]
- Loy, N.; Preziosi, L. A Statistical Mechanics Approach to Describe Cell Reorientation Under Stretch. Bull. Math. Biol. 2023, 85, 60. [Google Scholar] [CrossRef]
- Mahaworasilpa, T.L.; Coster, H.G.; George, E.P. Forces on biological cells due to applied alternating (AC) electric fields. II. Electro-rotation. Biochim. Biophys. Acta 1996, 1281, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.H.; Bray, M.G.; Holzbaur, E.L.F. Methods for assessing nuclear rotation and nuclear positioning in developing skeletal muscle cells. Methods Mol. Biol. 2016, 1411, 269–290. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Woods, B.L.; Cannon, K.S.; Vogt, E.J.D.; Crutchley, J.M.; Gladfelter, A.S. Interplay of septin amphipathic helices in sensing membrane-curvature and filament bundling. Mol. Biol. Cell 2021, 32, br5. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, G.; Guo, M.; Wang, J. Predicting protein-protein interactions using high-quality non-interacting pairs. BMC Bioinform. 2018, 19 (Suppl. 19), 525. [Google Scholar] [CrossRef]
- Natarajan, R.; Basak, S.C.; Balaban, A.T.; Klun, J.A.; Schmidt, W.F. Chirality index, molecular overlay and biological activity of diastereoisomeric mosquito repellents. Pest Manag. Sci. 2005, 61, 1193–1201. [Google Scholar] [CrossRef]
- Quesenberry, P.; Borgovan, T.; Nwizu, C.; Dooner, M.; Goldberg, L. Heuristic bias in stem cell biology. Stem Cell Res. Ther. 2019, 10, 241. [Google Scholar] [CrossRef]
- Mak, M.; Spill, F.; Kamm, R.D.; Zaman, M.H. Single-Cell Migration in Complex Microenvironments: Mechanics and Signaling Dynamics. J. Biomech. Eng. 2016, 138, 021004. [Google Scholar] [CrossRef]
- Park, P.J.; Lee, K.-J.-B. A modified active Brownian dynamics model using asymmetric energy conversion and its application to the molecular motor system. J. Biol. Phys. 2013, 39, 439–452. [Google Scholar] [CrossRef]
- Gambarini, G.; Testarelli, L.; Milana, V.; Pecci, R.; Bedini, R.; Pongione, G.; Gerosa, R.; De Luca, M. Angular deflection of rotary nickel titanium files: A comparative study. Ann. Ist. Super. Sanita 2009, 45, 423–426. [Google Scholar] [CrossRef]
- Wu, J.; Lee, K.C.; Dickinson, R.B.; Lele, T.P. How dynein and microtubules rotate the nucleus. J. Cell. Physiol. 2010, 226, 2666–2674. [Google Scholar] [CrossRef] [PubMed]
- Stokes, C.L.; Lauffenburger, D.A.; Williams, S.K. Migration of individual microvessel endothelial cells: Stochastic model and parameter measurement. J. Cell Sci. 1991, 99 Pt 2, 419–430. [Google Scholar] [CrossRef]
- Rahman, T.; Peters, F.; Wan, L.Q. Cell jamming regulates epithelial chiral morphogenesis. J. Biomech. 2023, 147, 111435. [Google Scholar] [CrossRef]
- Im, S.W.; Ahn, H.-Y.; Kim, R.M.; Cho, N.H.; Kim, H.; Lim, Y.-C.; Lee, H.-E.; Nam, K.T. Chiral Surface and Geometry of Metal Nanocrystals. Adv. Mater. 2019, 32, e1905758. [Google Scholar] [CrossRef]
- Abraham, E.; Nitzan, A. Quantifying the Chirality of Vibrational Modes in Helical Molecular Chains. Phys. Rev. Lett. 2024, 133, 268001. [Google Scholar] [CrossRef]
- Takano, N.; Hiramoto, M.; Yamada, Y.; Kokuba, H.; Tokuhisa, M.; Hino, H.; Miyazawa, K. Azithromycin, a potent autophagy inhibitor for cancer therapy, perturbs cytoskeletal protein dynamics. Br. J. Cancer 2023, 128, 1838–1849. [Google Scholar] [CrossRef]
- Lavelle, C.; Recouvreux, P.; Wong, H.; Bancaud, A.; Viovy, J.-L.; Prunell, A.; Victor, J.-M. Right-handed nucleosome: Myth or reality? Cell 2009, 139, 1216–1217. [Google Scholar] [CrossRef]
- Khor, E.-S.; Noor, S.M.; Wong, P.-F. MiR-107 inhibits the sprouting of intersegmental vessels of zebrafish embryos. Protoplasma 2021, 259, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Song, H.-H.G.; Rumma, R.T.; Ozaki, C.K.; Edelman, E.R.; Chen, C.S. Vascular Tissue Engineering: Progress, Challenges, and Clinical Promise. Cell Stem Cell 2018, 22, 340–354. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Chápuli, R. Evolution of angiogenesis. Int. J. Dev. Biol. 2011, 55, 345–351. [Google Scholar] [CrossRef]
- Zhang, H.; Rahman, T.; Lu, S.; Adam, A.P.; Wan, L.Q. Helical vasculogenesis driven by cell chirality. Sci Adv. 2024, 10, eadj3582. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.; Wan, L.Q. Cell chirality regulates intercellular junctions and endothelial permeability. Sci. Adv. 2018, 4, eaat2111. [Google Scholar] [CrossRef] [PubMed]
- Vergroesen, T.M.; Vermeulen, V.; Merks, R.M.H. Falsifying computational models of endothelial cell network formation through quantitative comparison with in vitro models. PLoS Comput. Biol. 2025, 21, e1012965. [Google Scholar]
- Guo, Y.; Zhang, S.; Wang, D.; Heng, B.C.; Deng, X. Role of cell rearrangement and related signaling pathways in the dynamic process of tip cell selection. Cell Commun. Signal. 2024, 22, 24. [Google Scholar] [CrossRef]
- Wang, X.; Bove, A.M.; Simone, G.; Ma, B. Molecular Bases of VEGFR-2-Mediated Physiological Function and Pathological Role. Front. Cell Dev. Biol. 2020, 8, 599281. [Google Scholar] [CrossRef]
- Matuszewska, K.; Pereira, M.; Petrik, D.; Lawler, J.; Petrik, J. Normalizing Tumor Vasculature to Reduce Hypoxia, Enhance Perfusion, and Optimize Therapy Uptake. Cancers 2021, 13, 4444. [Google Scholar] [CrossRef]
- Loosley, A.J.; O’Brien, X.M.; Reichner, J.S.; Tang, J.X. Describing Directional Cell Migration with a Characteristic Directionality Time. PLoS ONE 2015, 10, e0127425. [Google Scholar] [CrossRef]
- Vion, A.-C.; Perovic, T.; Petit, C.; Hollfinger, I.; Bartels-Klein, E.; Frampton, E.; Gordon, E.; Claesson-Welsh, L.; Gerhardt, H. Endothelial Cell Orientation and Polarity Are Controlled by Shear Stress and VEGF Through Distinct Signaling Pathways. Front. Physiol. 2021, 11, 623769. [Google Scholar] [CrossRef]
- Jakka, V.V.S.V.; Bursa, J. Finite Element Simulations of Mechanical Behaviour of Endothelial Cells. BioMed Res. Int. 2021, 2021, 8847372. [Google Scholar] [CrossRef]
- Yamamoto, T.; Hiraiwa, T.; Shibata, T. Collective cell migration of epithelial cells driven by chiral torque generation. Phys. Rev. Res. 2020, 2, 043326. [Google Scholar] [CrossRef]

| # | Property | Description | References |
|---|---|---|---|
| 1 | Structure | VEGFR1 is a transmembrane protein belonging to the receptor tyrosine kinase (R.T.K.) family. | [29] |
| 2 | Function | VEGFR1 primarily functions as a receptor for VEGF-A. | [30] |
| 3 | Binding Affinity | VEGFR1 has a high affinity for VEGF-A. | [31] |
| 4 | Role in Development | VEGFR1 plays a critical role in the formation of the vascular system during embryonic development. | [32] |
| 5 | Signal Transduction | Upon activation, VEGFR1 undergoes autophosphorylation and activates downstream signaling pathways. | [33] |
| 6 | Angiogenesis Regulation | VEGFR1 is involved in the negative regulation of angiogenesis. | [34] |
| 7 | Therapeutic Target | VEGFR1 has been explored as a therapeutic target for antiangiogenic drugs. | [35] |
| 8 | Soluble Form | Due to alternative splicing, VEGFR1 can exist in a soluble form (sVEGFR1) and act as a decoy receptor for VEGF-A. | [36] |
| 9 | Expression in Cancer | VEGFR1 expression is observed in various cancers and is associated with tumor angiogenesis, progression, and poor prognosis. | [37] |
| 10 | Interaction with Neuropilin-1 | VEGFR1 can form a complex with neuropilin-1, enhancing VEGF-A binding and signaling. | [38] |
| 11 | Regulation by miRNAs | VEGFR1 expression can be regulated by microRNAs (miRNAs) in various physiological and pathological conditions. | [39] |
| 12 | Role in Neuroprotection | VEGFR1 has been implicated in neuroprotection and neuronal survival in addition to its role in angiogenesis. | [40] |
| # | Property | Description | References |
|---|---|---|---|
| 1 | Structure | VEGFR2 is a transmembrane protein belonging to the receptor tyrosine kinase (R.T.K.) family. | [67] |
| 2 | Function | VEGFR2 primarily functions as a receptor for VEGF-A, mediating most—VEGF-induced angiogenic responses. | [26] |
| 3 | Signal Transduction | Upon activation by VEGF-A binding, VEGFR2 undergoes autophosphorylation and activates downstream signaling pathways, including the phosphoinositide 3-kinase (PI3K)/Akt pathway and the mitogen-activated protein kinase (MAPK) pathway. | [19] |
| 4 | Angiogenic Response | VEGFR2 activation leads to endothelial cell proliferation, migration, and survival, contributing to angiogenesis. | [20] |
| 5 | Role in Development: | VEGFR2 plays a crucial role in embryonic vascular development and angiogenesis. | [31] |
| 6 | Regulation of Blood Pressure | VEGFR2 signaling plays a crucial role in regulating blood pressure and vascular tone. | [68] |
| 7 | Therapeutic Target: | VEGFR2 targets antiangiogenic therapy in cancer and other diseases characterized by abnormal angiogenesis. | [69] |
| 8 | Endothelial Barrier Function | VEGFR2 signaling is involved in regulating endothelial barrier function, influencing vascular permeability. | [70] |
| 9 | Lymphangiogenesis | VEGFR2 plays a crucial role in lymphangiogenesis, the process by which new lymphatic vessels are formed. | [71] |
| 10 | Regulation by miRNAs | VEGFR2 expression can be regulated by microRNAs (miRNAs) in various physiological and pathological conditions | [72] |
| 11 | Tie-2 Interaction: | VEGFR2 can form a complex with the Tie-2 receptor, influencing vascular development and stability. | [73] |
| 12 | Metastasis Promotion | VEGFR2 signaling has been implicated in promoting tumor metastasis through its effects on tumor vasculature and the migration of cancer cells. | [74] |
| # | Property | Description | References |
|---|---|---|---|
| 1 | Structure | VEGFR3 is a transmembrane protein belonging to the receptor tyrosine kinase (R.T.K.) family. | [102] |
| 2 | Function | VEGFR3 primarily functions as a receptor for Vascular Endothelial Growth Factor C (VEGF-C) and Vascular Endothelial Growth Factor D (VEGF-D), regulating lymphangiogenesis. | [103] |
| 3 | Lymphangiogenesis: | VEGFR3 is a crucial regulator of lymphangiogenesis, the process by which new lymphatic vessels form. | [104] |
| 4 | Developmental Role | VEGFR3 plays a crucial role in the development of the lymphatic system, including the sprouting and patterning of lymphatic vessels. | [105] |
| 5 | Signal Transduction | Activation of VEGFR3 by its ligands triggers downstream signaling cascades, including the phosphoinositide 3-kinase (PI3K)/Akt pathway and the mitogen-activated protein kinase (MAPK) pathway, which regulate the function of lymphatic endothelial cells. | [106] |
| 6 | Role in Cancer Metastasis: | VEGFR3 signaling has been implicated in tumor metastasis by promoting lymphangiogenesis and facilitating the dissemination of cancer cells through lymphatic vessels. | [107] |
| 7 | Therapeutic Target | Targeting VEGFR3 has been explored as a potential therapeutic strategy for inhibiting lymphangiogenesis and metastasis in cancer. | [108] |
| 8 | Interactions with Neuropilins | VEGFR3 can form complexes with neuropilin receptors, modulating its signaling and function in lymphatic endothelial cells. | [109] |
| 9 | Regulation by miRNAs: | VEGFR3 expression can be regulated by microRNAs (miRNAs), which in turn influence lymphangiogenesis and cancer progression. | [110] |
| 10 | Angiogenesis in Corneal Lymphatics | VEGFR3 plays a role in angiogenesis in corneal lymphatic vessels, influencing corneal inflammation and wound healing. | [111] |
| 11 | Role in Lymphedema: | VEGFR3 signaling is implicated in the pathogenesis of lymphedema, providing potential therapeutic targets for its treatment. | [112] |
| 12 | Developmental Disorders | Mutations in VEGFR3 are associated with primary lymphedema and other developmental disorders affecting the lymphatic system. | [113] |
| # | Property | Description | References |
|---|---|---|---|
| 1 | Targeting Alternative Isoforms | Alternative VEGF and VEGFR isoforms and cancer and angiogenesis are being studied more. VEGF-A isoforms like VEGF-A165b have been studied for their effects on vascular function and tumor growth. | [128] |
| 2 | Therapeutic Resistance Mechanisms | Cancer patients’ resistance to VEGF–VEGFR-targeted anti-angiogenic treatments is attracting attention. Current study suggests activating alternate pro-angiogenic pathways and adapting tumor cells and the microenvironment. | [129] |
| 3 | Development of Novel Therapeutics | New VEGF–VEGFR pathway therapies include monoclonal antibodies, small-molecule inhibitors, and gene-based methods are being developed. Combination therapies—targeting various angiogenic pathway components or mixing anti-angiogenic drugs with other treatments—are also being studied to improve efficacy and overcome resistance. | [130] |
| 4 | Role of VEGFRs in Non-Canonical Signaling | Recent studies have revealed that VEGFRs participate in non-canonical signaling pathways extending beyond angiogenesis, including roles in immune modulation, neuroprotection, and metabolic regulation. A deeper understanding of these non-angiogenic functions could open new avenues for therapeutic strategies across a range of diseases. | [131] |
| 5 | Emerging Biomarkers | VEGF–VEGFR pathway biomarkers are being studied for cancer and other illness prognoses. VEGF concentrations, VEGFR expression patterns, and VEGF/VEGFR gene variants may assist guide treatment and predict patient outcomes. | [20] |
| 6 | Role in Neurovascular Diseases | The VEGF–VEGFR pathway has been increasingly implicated in neurovascular disorders such as stroke, Alzheimer’s disease, and diabetic retinopathy. Ongoing research is exploring how VEGF signaling influences neurovascular function and assessing its potential as a therapeutic target in these conditions. | [132] |
| 7 | Engineering VEGF Mimetics | Researchers are developing VEGF mimetics and engineered VEGF variants designed to provide improved pharmacokinetics and reduced toxicity. These synthetic ligands aim to enhance the efficacy of VEGF-based therapies while minimizing adverse effects. | [133] |
| 8 | Role of VEGFRs in Immune Modulation | Recent studies have demonstrated that VEGF receptors (VEGFRs) play a role in modulating immune responses, particularly within the tumor microenvironment. VEGFR signaling has been shown to influence the expression of inhibitory checkpoints on CD8+ T cells, suggesting a close interplay between angiogenic regulation and immune control in cancer. | [134] |
| 9 | Exploring Antiangiogenic Therapies in Combination with Immunotherapy | There is increasing interest in combining anti-angiogenic therapies targeting the VEGF–VEGFR pathway with immunotherapeutic approaches in cancer treatment. Both preclinical and clinical studies have reported encouraging outcomes, underscoring the potential synergistic benefit of simultaneously targeting angiogenesis and immune checkpoints. | [135] |
| 10 | Role of VEGF-VEGFR Signaling in Metabolic Regulation: | Recent studies have revealed that VEGF–VEGFR signaling extends beyond angiogenesis to the regulation of cellular metabolism. In endothelial and other cell types, VEGFR signaling influences key metabolic processes, pointing to important implications for metabolic diseases and potential therapeutic strategies. | [136] |
| 11 | Therapeutic Targeting of VEGF-VEGFR Pathway in Neurodegenerative Diseases | Recent studies have demonstrated that VEGF–VEGFR signaling extends beyond its classical role in angiogenesis to include the regulation of cellular metabolism. Through VEGFR signaling, endothelial and other cell types can modulate key metabolic processes, suggesting potential implications for metabolic disorders as well as opportunities for novel therapeutic interventions. | [137] |
| 12 | Role of VEGF-VEGFR Signaling in Organ Development and Regeneration: | Research has shown that VEGF–VEGFR signaling is critical for both organ development and regeneration. Endothelial-derived endocrine signals mediated by VEGFRs play a central role in initiating and sustaining regenerative processes, highlighting the therapeutic potential of this pathway in tissue engineering and regenerative medicine. | [138] |
| 13 | Mechanisms of VEGF-VEGFR Axis in Cancer Metastasis: | Recent studies have highlighted the role of the VEGF–VEGFR pathway in cancer metastasis. Beyond its classical function in angiogenesis, VEGF–VEGFR signaling can directly influence tumor cells and the tumor microenvironment, facilitating tumor cell dissemination and metastatic progression. | [139] |
| 14 | Exploring VEGF-VEGFR Signaling in Tissue Engineering and Regenerative Medicine: | To enhance vascularization and tissue repair, research in tissue engineering and regenerative medicine has extensively investigated the VEGF–VEGFR pathway. Both VEGF-based therapeutics and engineered biomaterial constructs have been explored as strategies to improve vascular integration and functional outcomes in regenerative applications. | [140] |
| 15 | Role of VEGF-VEGFR Pathway in Age-Related Macular Degeneration (AMD | The VEGF–VEGFR pathway is a key contributor to the pathogenesis of age-related macular degeneration (AMD), one of the leading causes of vision loss in the elderly. The introduction of anti-VEGF therapies has transformed AMD management by suppressing abnormal neovascularization and helping to preserve visual function. | [141] |
| # | Name of VEGF Mimetic Peptide | Aa Sequence | Reference |
|---|---|---|---|
| 1 | VEGF-Mimetic Peptide (CBO-P11): | CGGSNH2 | [159] |
| 2 | VEGF-Mimetic Peptide VEGF-A (86–92) | YKHKGFFQ | [160] |
| 3 | VEGF-Mimetic Peptide Vintafolide (EC145) | Ac-SGGR-amino deoxyglucose-folic acid | [161] |
| 4 | VEGF-Mimetic Peptide QK-B: | QK-B | [166] |
| 5 | VEGF-Mimetic Peptide QK-F11: | QK-F11 | [167] |
| 6 | VEGF-Mimetic Peptide (YP15): | YP15 | [168] |
| 7 | VEGF-Mimetic Peptide (AV-3): | EELRYYNKNR | [164] |
| 8 | Vascular Endothelial Growth Factor Peptide (VEGF-31): | TNPNRKTKGKE | [169] |
| 9 | VEGF-Mimetic Peptide (ZGDHu-1): | YPDKHLRGD | [170] |
| 10 | VEGF-Mimetic Peptide (VGX-1000): | YTRKYKFKIR | [171] |
| 11 | VEGF-Mimetic Peptide (LXY30): | LTTSHLLYHLNTKHCFYGG | [165] |
| 12 | VEGF-Mimetic Peptide (PRWTEKT) | PRWTEKT | [172] |
| 13 | VEGF-Mimetic Peptide (C7): | C7 | [173] |
| 14 | VEGF-Mimetic Peptide (ZG29) | AGKHLMFGYWKERGRKG | [174] |
| 15 | VEGF-Mimetic Peptide (V1): | CTTGRTPR | [175] |
| 16 | VEGF-Mimetic Peptide (MF1): | MFYSYFPSD | [176] |
| 17 | VEGF-Mimetic Peptide (YLL3): | YLLDVDTKVTP | [177] |
| 18 | VEGF-Mimetic Peptide (YLL9) | YLLGLVITGT | [178] |
| 19 | VEGF-Mimetic Peptide (RGD-4C) | CRRETAWAC | [179] |
| 20 | VEGF-Mimetic Peptide (UPARANT): | AE105-NH2 | [180] |
| # | Name | Description | Reference |
|---|---|---|---|
| 1 | Vascular Endothelial Growth Factor (VEGF) | The introduction of the VEGF gene aims to stimulate the production of vascular endothelial growth factor, a key factor in angiogenesis. | [181] |
| 2 | Fibroblast Growth Factor (FGF) | FGFs, particularly FGF-2, are involved in angiogenesis. Gene therapy delivering FGF genes can enhance blood vessel formation. | [182] |
| 3 | Hypoxia-Inducible Factor-1 (HIF-1) | HIF-1 is a transcription factor that regulates responses to low oxygen levels (hypoxia). HIF-1 gene therapy aims to induce angiogenesis under hypoxic conditions. | [183] |
| 4 | Platelet-Derived Growth Factor (PDGF) | PDGF plays a role in cell growth and division, including vascular smooth muscle cells. Gene therapy with PDGF aims to promote vessel formation. | [184] |
| 5 | Angiopoietin-1 (Ang-1) Gene Therapy | Ang-1 is involved in stabilizing blood vessels. Gene therapy with Ang-1 aims to enhance vessel maturation and stability | [177] |
| 6 | Hepatocyte Growth Factor (H.G.F.) | H.G.F. is known for its angiogenic and tissue regeneration properties. Gene therapy with H.G.F. may promote angiogenesis | [185] |
| 7 | hymosin Beta-4 (Tβ4) | Tβ4 is a peptide involved in cell migration, angiogenesis, and tissue repair. Gene therapy with Tβ4 may enhance these processes. | [186] |
| 8 | Stromal Cell-Derived Factor-1 (SDF-1) | SDF-1 is involved in recruiting stem cells and promoting angiogenesis. Gene therapy with SDF-1 aims to enhance tissue repair. | [187] |
| 9 | Granulocyte-Colony Stimulating Factor (G-CSF) | G-CSF stimulates the production of granulocytes and stem cells and has been explored for its angiogenic potential | [188] |
| 10 | Notch-1 Gene | Notch signaling is involved in vascular development. Gene therapy targeting Notch-1 may influence angiogenesis. | [189] |
| # | Property | Description | References |
|---|---|---|---|
| 1 | VEGF and Directional Migration: | To create directed blood arteries, VEGF gradients induce endothelial cell migration via VEGFR signaling. Chiral directed movement indicates bias or rotation. Under VEGF gradients, intrinsically chiral cells move or align. VEGF signaling and chiral cues regulate cell migration, which shapes vascular architecture throughout development and wound healing. | [218] |
| 2 | VEGFR and Actin Cytoskeleton in Chiral Migration: | Actin cytoskeleton, which maintains cell polarity and chirality, is influenced by VEGFR activity. One-directional lamellipodia or filopodia improve asymmetric cell migration via VEGF-driven actin rearrangement. In certain studies, VEGF promotes endothelial cell chirality, coordinating polarity and boosting chiral migration during angiogenesis. | [161] |
| 3 | Cell Chirality and Tissue-Level Organization: | VEGF/VEGFR signaling coordinates tissue topologies via regulating cellular chirality. Chiral migration by VEGF/VEGFR helps endothelial cells generate spiraling or coiling structures for vascular patterning and function. Organ asymmetry and blood circulation require precise matching of vascular and cellular chirality during heart and brain development. VEGF/VEGFR signaling coordinates tissue topologies via regulating cellular chirality. Chiral migration by VEGF/VEGFR helps endothelial cells generate spiraling or coiling structures for vascular patterning and function. During cardiac and brain development, vascular and cellular chirality must be balanced for organ asymmetry and blood circulation. | [228] |
| 4 | VEGF in Developmental Left-Right Asymmetry: | According to developmental biology studies, VEGF signaling may interact with pathways that cause left-right (LR) asymmetry in embryonic development, where cellular chirality is critical. VEGF/VEGFR expression by mesodermal and endothelial cells during organ development causes the asymmetric architecture of organs like the heart and lungs, which require coordinated cellular chirality and directed vascularization. | [229] |
| 5 | Cytoskeletal Dynamics and VEGFR Interactions | VEGFR activation promotes lamellipodia and filopodia production by reorganizing the cytoskeleton. These structures are necessary for directed cell migration and can move chirally. Cytoskeletal remodeling by VEGF/VEGFR and downstream effectors such Rho GTPases helps cells migrate in certain directions during angiogenesis by chirality. | [164] |
| 6 | VEGF Gradient Formation and Directional Chirality | LAMP and filopodia production is generally promoted by VEGFR activation, which reorganizes the cytoskeleton. They are necessary for directed cell migration and can migrate chirally. VEGF/VEGFR and downstream effectors like Rho GTPases govern cytoskeletal reorganization to help cells migrate in certain directions during angiogenesis. | [165] |
| 7 | VEGF-Mediated Chiral Cell Polarity and Planar Cell Polarity (PCP) Pathway | Planar cell polarity (PCP) governs tissue cell orientation and alignment, and VEGF signaling interacts with it. PCP components assist endothelial cells produce coordinated chiral patterns in blood vessel development. This route orients cells appropriately in response to VEGF stimulation, encouraging polar cells. | [230] |
| 8 | Interaction with Integrins for Coordinated Chiral Migration | Cell alignment and polarity are improved by integrins and VEGFRs strengthening cell adherence to the ECM. Integrin-VEGFR crosstalk enhances cell-ECM connections needed for chiral migratory patterns during angiogenesis, especially where cells need stable adhesion to travel directionally. | [168] |
| 9 | Role of Mechanical Forces and Shear Stress on VEGF Signaling and Chirality | Blood flow-induced shear stress affects VEGF/VEGFR expression and chiral endothelial cell alignment. Mechanotransduction favors chiral polarity and migration in vascular tissues, where cells respond to fluid dynamics. | [171] |
| # | Cellular Indices | Tissue Indices |
|---|---|---|
| 1 | Endothelial Cell Density: Measures the number of endothelial cells in a given area. Higher density typically indicates active angiogenesis. | Oxygen Tension (pO2): Hypoxia is a potent stimulus for angiogenesis. Measuring tissue oxygen levels can guide interventions in ischemic tissues. |
| 2 | Proliferation Markers: Proteins like Ki-67 or PCNA can indicate cell proliferation rates. Increased expression correlates with angiogenic activity. | pH Levels: The acidity of the microenvironment can influence cellular behavior and angiogenic responses. Analyzing tissue pH can help in understanding disease states. |
| 3 | Migration Assays: Evaluating the ability of endothelial cells to migrate towards a gradient of angiogenic factors helps understand their responsiveness to stimuli. | Extracellular Matrix (ECM) Composition: The types and organization of ECM components (like collagen and fibronectin) affect angiogenesis. Changes in ECM composition can be indicative of disease progression. |
| 4 | Tube Formation Assays: Assessing the ability of endothelial cells to form capillary-like structures in vitro is a direct measure of angiogenic potential. | Vascular Density: Quantifying the number of blood vessels per unit area in a tissue sample provides a direct measure of angiogenic activity. |
| 5 | Gene Expression Profiles: Analyzing the expression of genes involved in angiogenesis (like VEGF, FGF) provides insight into cellular responses to various conditions. | Inflammatory Markers: Assessing levels of pro-inflammatory cytokines (like IL-1, TNF-α) can give insight into the tissue’s angiogenic response, as inflammation often drives angiogenesis. |
| 6 | Endothelial Cell Senescence Markers: Markers like p16INK4a and telomerase activity can indicate the aging status of endothelial cells, influencing their angiogenic potential. | Mechanical Properties: The stiffness or elasticity of tissue can influence angiogenesis. Stiffer matrices may promote vascularization, while softer ones may inhibit it. |
| 7 | Endothelial Cell Senescence Markers: Markers like p16INK4a and telomerase activity can indicate the aging status of endothelial cells, influencing their angiogenic potential. | Vascular Endothelial Growth Factor (VEGF) Levels: Measuring VEGF concentrations in tissues can indicate angiogenic activity, as it is a key regulator of blood vessel formation. |
| 8 | Angiogenic Factor Expression: Levels of factors such as VEGF, FGF, and angiopoietins are crucial for assessing the pro-angiogenic state of cells. | Vascular Density (VD): Quantifying the number of blood vessels per unit area in a tissue sample, often assessed through histological techniques. |
| 9 | Adhesion Molecule Expression: The presence of molecules like ICAM-1, VCAM-1, and E-selectin on endothelial cells can indicate their readiness to interact with leukocytes and other cells, influencing angiogenesis. | Microvessel Density (MVD): A specific measure of the density of small blood vessels, typically used in tumor studies to assess angiogenesis. |
| 10 | Signal Transduction Pathway Activity: Assessing the activation of pathways like PI3K/Akt, MAPK/ERK, and Notch signaling can provide insights into the cellular mechanisms driving angiogenesis. | Fibrosis Index: Evaluating the extent of fibrosis in tissues can indicate chronic conditions that may affect angiogenesis, as fibrotic tissues may have altered blood supply. |
| 11 | Nitric Oxide Production: Measurement of nitric oxide levels can indicate endothelial cell function, as it plays a key role in vascular relaxation and angiogenesis. | Lactate Levels: Elevated lactate in tissues can indicate anaerobic metabolism due to insufficient blood supply, which can influence angiogenic processes. |
| 12 | Migration and Invasion Assays: Evaluating the ability of endothelial cells to migrate and invade through a Matrigel matrix can indicate their potential for angiogenesis. | Histological Scoring of Inflammation: Assessing the presence of inflammatory cells and associated markers can help gauge the inflammatory microenvironment that often drives angiogenesis. |
| 13 | Expression of Matrix Metalloproteinases (MMPs): The expression levels of MMPs (like MMP-2 and MMP-9) are critical for assessing the ability of cells to remodel the extracellular matrix during angiogenesis. | Proteoglycan and Glycosaminoglycan Levels: The presence and composition of these ECM components can influence endothelial cell behavior and angiogenesis. |
| 14 | Cytokine Production: The levels of pro-inflammatory cytokines (like IL-6 and IL-8) produced by endothelial cells can influence angiogenesis and the recruitment of other cells. | Collagen Organization: Analyzing the alignment and type of collagen in the extracellular matrix can provide insights into the structural support for angiogenesis. |
| 15 | Apoptotic Markers: Assessing markers of apoptosis (like caspases or Annexin V) can help determine the survival of endothelial cells in the context of angiogenesis. | Tissue Growth Factor Levels: The levels of transforming growth factor-beta (TGF-β) and other growth factors can influence angiogenic processes and tissue remodeling. |
| 16 | Staining for Endothelial Cell Markers: Immunostaining for specific markers such as CD31 or VE-cadherin can confirm endothelial cell identity and help quantify their presence. | Mechanical Stiffness: Measuring the stiffness of tissues can provide information on how mechanical properties influence endothelial cell behavior and angiogenesis. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lungu, C.N.; Gurau, G.; Mehedinti, M.C. Pro-Angiogenic Bioactive Molecules in Vascular Morphogenesis: Integrating Endothelial Cell Dynamics. Curr. Issues Mol. Biol. 2025, 47, 851. https://doi.org/10.3390/cimb47100851
Lungu CN, Gurau G, Mehedinti MC. Pro-Angiogenic Bioactive Molecules in Vascular Morphogenesis: Integrating Endothelial Cell Dynamics. Current Issues in Molecular Biology. 2025; 47(10):851. https://doi.org/10.3390/cimb47100851
Chicago/Turabian StyleLungu, Claudiu N., Gabriela Gurau, and Mihaela C. Mehedinti. 2025. "Pro-Angiogenic Bioactive Molecules in Vascular Morphogenesis: Integrating Endothelial Cell Dynamics" Current Issues in Molecular Biology 47, no. 10: 851. https://doi.org/10.3390/cimb47100851
APA StyleLungu, C. N., Gurau, G., & Mehedinti, M. C. (2025). Pro-Angiogenic Bioactive Molecules in Vascular Morphogenesis: Integrating Endothelial Cell Dynamics. Current Issues in Molecular Biology, 47(10), 851. https://doi.org/10.3390/cimb47100851







