Immunomodulatory Effects of Ganoderma lucidum Bioactive Compounds on Gut–Brain and Gut–Liver Axis Disorders
Abstract
1. Introduction
2. Current Research Status on the Pharmacological Effects and Immunomodulatory Mechanisms of Ganoderma lucidum
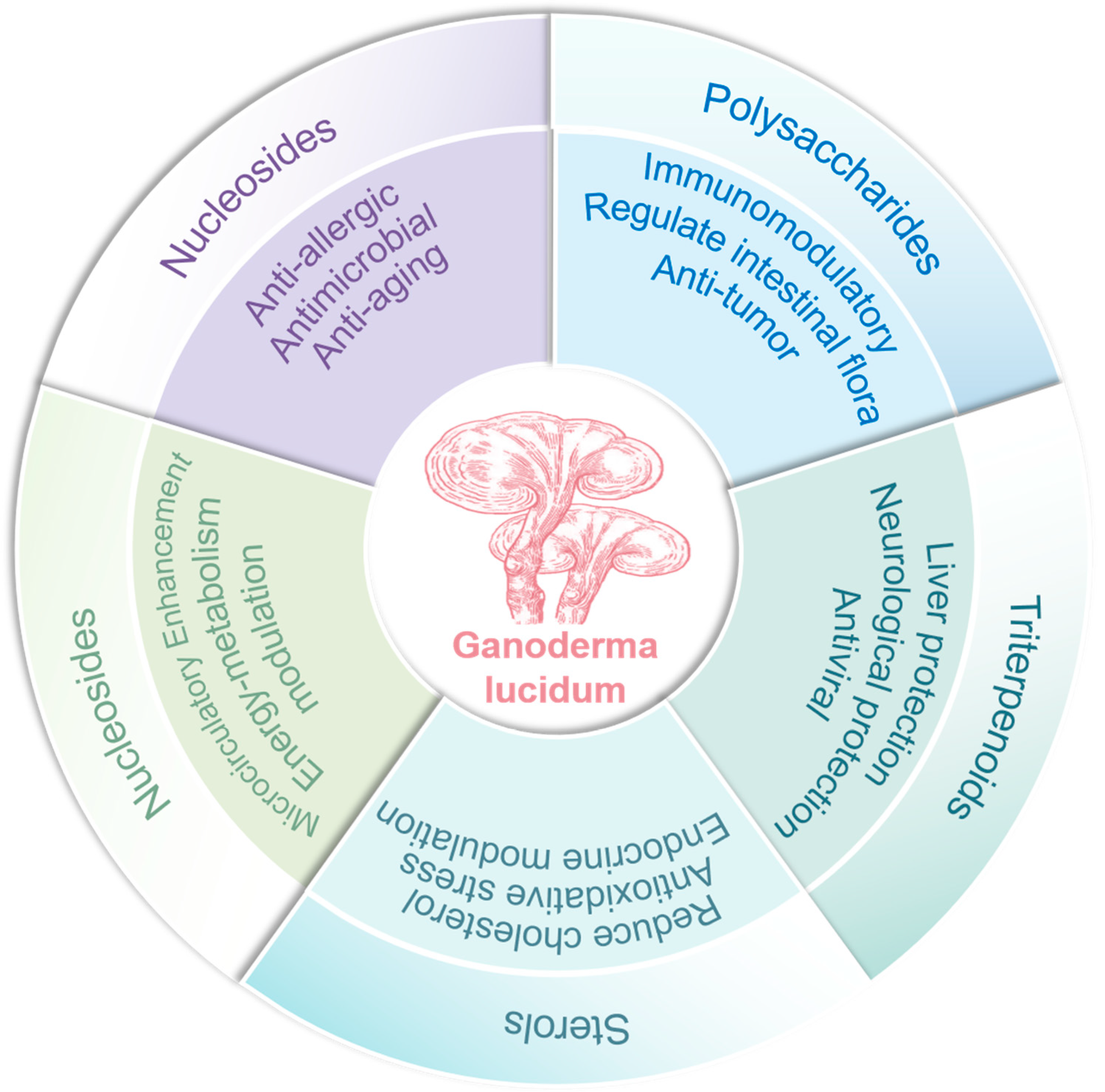
2.1. Chemical Structural Diversity and Bioactivities of Major Bioactive Constituents in Ganoderma lucidum
2.2. Current Research Status on the Pharmacological Effects of Ganoderma lucidum
2.3. Immunomodulation and the Mechanism of Immunomodulatory Effects of Ganoderma lucidum
2.3.1. The Role of Immunomodulation in Disease
2.3.2. Direct Immune Effects
2.3.3. Regulating Gut Microbiota
3. Mechanisms of Immunomodulation in Brain–Gut–Liver Axis Disorders and Advances in Their Treatment and Prevention
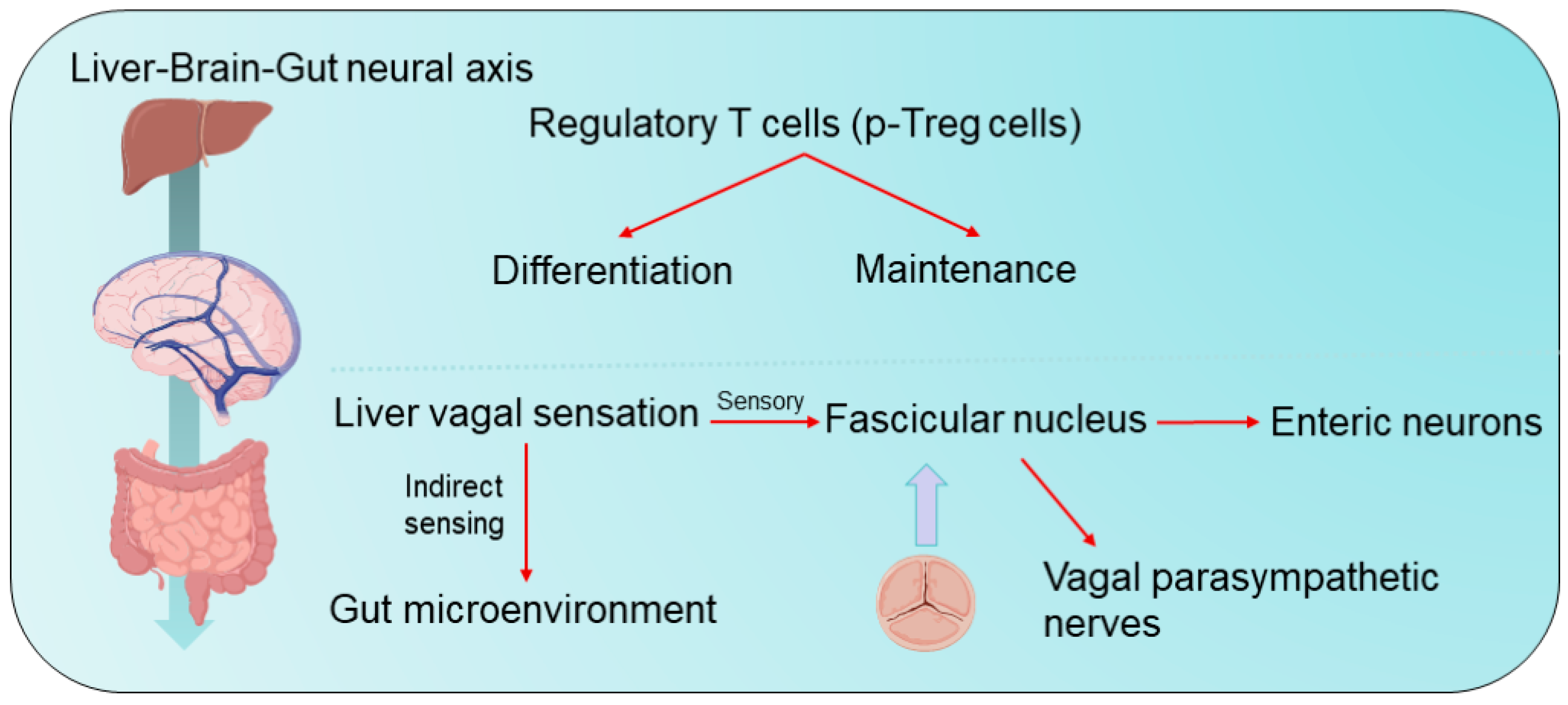
3.1. Mechanisms of Immunomodulation in Hepato-Intestinal Axis-Like Diseases
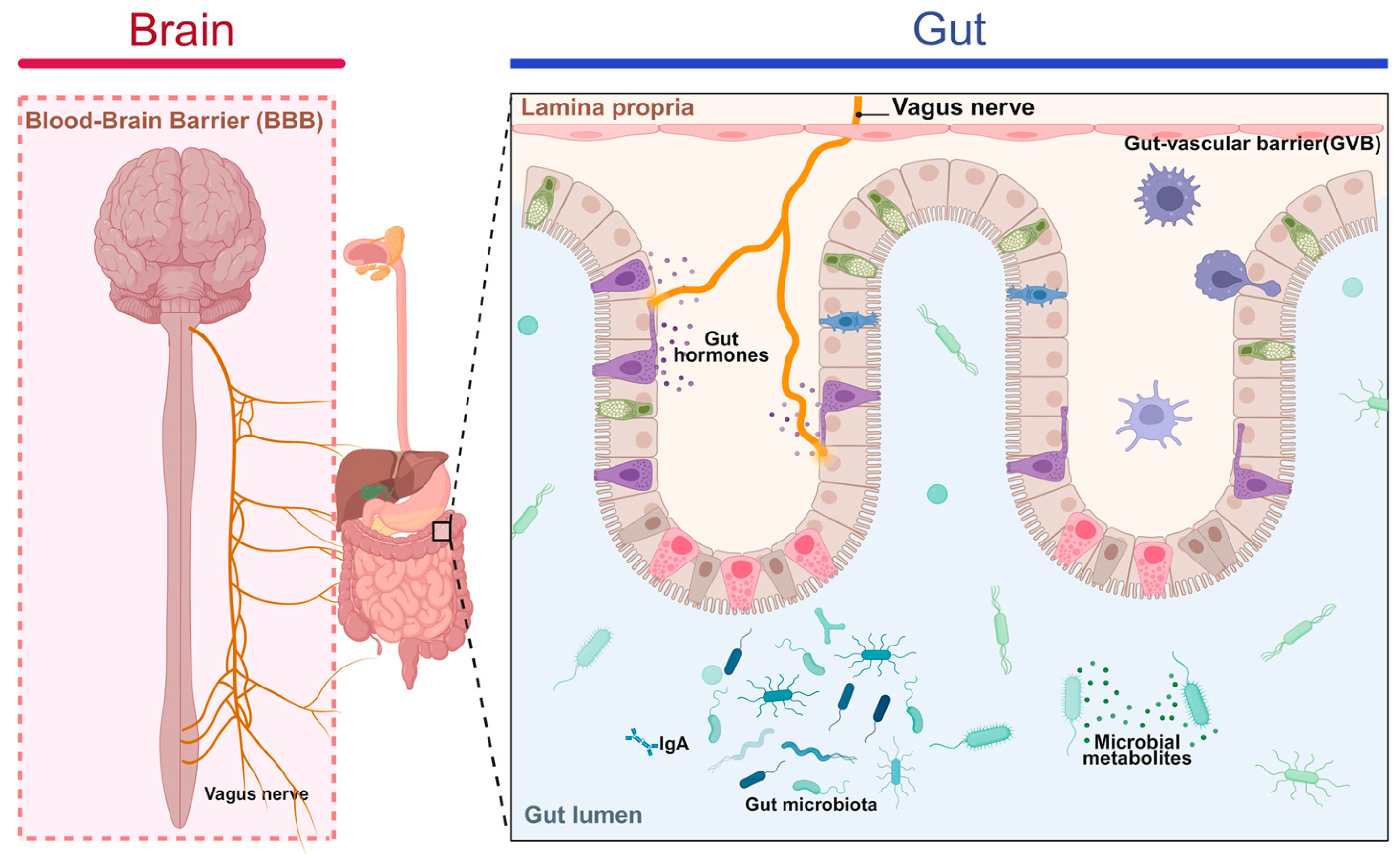
3.2. Mechanisms of Immunomodulation in Gut–Brain Axis Disorders
3.3. Treatment and Prevention of Gut–Brain Axis and Gut–Liver Axis Disorders
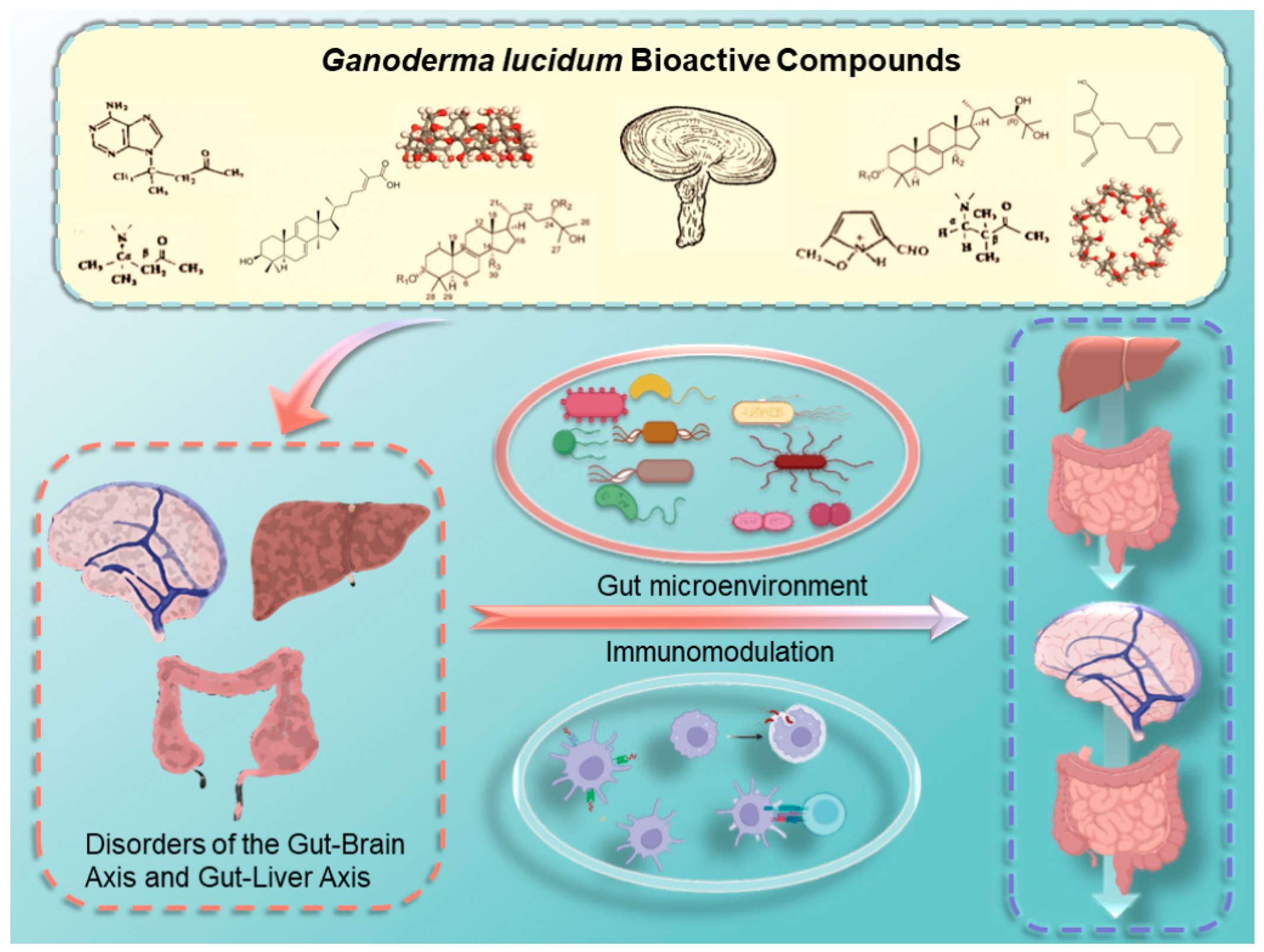
4. Ganoderma lucidum: The Immune Guardian of the Gut–Liver and Gut–Brain Axes
4.1. Ganoderma lucidum Prevents and Treats Diseases by Regulating the Immune System
4.2. Ganoderma lucidum Prevents and Treats Diseases by Regulating Intestinal Flora
5. Prospects and Challenges
5.1. Potential to Alleviate Mood and Improve Mental Health
5.2. Prospects for Application in Irritable Bowel Syndrome (IBS) and Other Functional Gastrointestinal Disorders
5.3. Potential for Prevention and Management of Non-Alcoholic Fatty Liver Disease (NAFLD)
5.4. Potential to Alleviate Liver Fibrosis and Cirrhosis
5.5. Multifunctional Mechanism of Action of Ganoderma lucidum in Combination with Precision Medicine
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmad, R.; Riaz, M.; Khan, A.; Aljamea, A.; Algheryafi, M.; Sewaket, D.; Alqathama, A. Ganoderma lucidum (Reishi) an edible mushroom; a comprehensive and critical review of its nutritional, cosmeceutical, mycochemical, pharmacological, clinical, and toxicological properties. Phytother. Res. 2021, 35, 6030–6062. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhang, C.C.; Xing, Z.; Ahmad, Z.; Li, J.S.; Chang, M.W. Pharmacological effects of natural and its extracts on neurological diseases: A comprehensive review. Int. J. Biol. Macromol. 2019, 121, 1160–1178. [Google Scholar] [CrossRef]
- Swallah, M.S.; Bondzie-Quaye, P.; Yu, X.; Fetisoa, M.R.; Shao, C.S.; Huang, Q. Elucidating the protective mechanism of ganoderic acid DM on breast cancer based on network pharmacology and in vitro experimental validation. Biotechnol. Appl. Biochem. 2024, 72, 415–436. [Google Scholar] [CrossRef] [PubMed]
- Raza, S.H.A.; Zhong, R.M.; Li, X.M.; Pant, S.D.; Shen, X.; BinMowyna, M.N.; Luo, L.; Lei, H.T. Ganoderma triterpenoids investigating their role in medicinal applications and genomic protection. J. Pharm. Pharmacol. 2024, 76, 1535–1551. [Google Scholar] [CrossRef] [PubMed]
- Sanodiya, B.S.; Thakur, G.S.; Baghel, R.K.; Prasad, G.B.K.S.; Bisen, P.S. Ganoderma lucidum: A Potent Pharmacological Macrofungus. Curr. Pharm. Biotechnol. 2009, 10, 717–742. [Google Scholar] [CrossRef]
- Quan, Y.-Z.; Ma, A.; Ren, C.-Q.; An, Y.-P.; Qiao, P.-S.; Gao, C.; Zhang, Y.-K.; Li, X.-W.; Lin, S.-M.; Li, N.-N.; et al. Ganoderic acids alleviate atherosclerosis by inhibiting macrophage M1 polarization via TLR4/MyD88/NF-κB signaling pathway. Atherosclerosis 2024, 391, 117478. [Google Scholar] [CrossRef]
- Viroel, F.J.M.; Laurino, L.F.; Caetano, É.L.A.; Jozala, A.F.; Spim, S.R.V.; Pickler, T.B.; Sercundes, M.K.; Gomes, M.C.; Hataka, A.; Grotto, D.; et al. Ganoderma lucidum Modulates Glucose, Lipid Peroxidation and Hepatic Metabolism in Streptozotocin-Induced Diabetic Pregnant Rats. Antioxidants 2022, 11, 1035. [Google Scholar] [CrossRef]
- Cao, Y.J.; Yang, Y.H.; Liang, Z.H.; Guo, W.L.; Lv, X.C.; Ni, L.; Chen, Y.T. Synthesis of Ganoderic Acids Loaded Zein-Chitosan Nanoparticles and Evaluation of Their Hepatoprotective Effect on Mice Given Excessive Alcohol. Foods 2024, 13, 2760. [Google Scholar] [CrossRef]
- Ling, T.T.; Arroyo-Cruz, L.V.; Smither, W.R.; Seighman, E.K.; Martínez-Montemayor, M.M.; Rivas, F. Early Preclinical Studies of Ergosterol Peroxide and Biological Evaluation of Its Derivatives. ACS Omega 2024, 9, 37117–37127. [Google Scholar] [CrossRef]
- Shen, W.P.; Wu, J.M.; Shi, L.Y.; Feng, H.S.; Yang, X.D.; Zhang, Y. Explore the mechanisms of triterpenoids from in the protection against Alzheimer’s disease via microbiota-gut-brain axis with the aid of network pharmacology. Fitoterapia 2024, 178, 106150. [Google Scholar] [CrossRef]
- Ren, L.; Zhang, J.; Zhang, T. Immunomodulatory activities of polysaccharides from Ganoderma on immune effector cells. Food Chem. 2021, 340, 127933. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Qin, X.; Liu, X. A review of polysaccharides from Ganoderma lucidum: Preparation methods, structural characteristics, bioactivities, structure-activity relationships and potential applications. Int. J. Biol. Macromol. 2025, 303, 140645. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, H.; Shen, Y.; Zhao, X.; Wang, X.; Wang, J.; Fan, K.; Zhan, X. Characterization of a novel polysaccharide from Ganoderma lucidum and its absorption mechanism in Caco-2 cells and mice model. Int. J. Biol. Macromol. 2018, 118, 320–326. [Google Scholar] [CrossRef]
- Galappaththi, M.C.A.; Patabendige, N.M.; Premarathne, B.M.; Hapuarachchi, K.K.; Tibpromma, S.; Dai, D.-Q.; Suwannarach, N.; Rapior, S.; Karunarathna, S.C. A Review of Ganoderma Triterpenoids and Their Bioactivities. Biomolecules 2022, 13, 24. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Zhang, C.; Tang, C.; Wu, X.; Hu, S.; Luo, Q.; Jia, N.; Fan, L.; Wang, Y.; Jiang, W.; et al. Triterpenes from Ganoderma lucidum inhibit hepatocellular carcinoma by regulating enhancer-associated lncRNA in vivo. J. Ethnopharmacol. 2025, 336, 118706. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, T.; Jiang, C.; Hu, H.; Fei, Y.; Yang, Y.; An, H.; Qin, H.; Zhu, Z.; Yang, Y.; et al. Ganoderic acid A regulates CSF1R to reprogram tumor-associated macrophages for immune therapy of hepatocellular carcinoma. Int. Immunopharmacol. 2025, 161, 114989. [Google Scholar] [CrossRef]
- Kou, R.-W.; Li, Z.-Q.; Wang, J.-L.; Jiang, S.-Q.; Zhang, R.-J.; He, Y.-Q.; Xia, B.; Gao, J.-M. Ganoderic Acid A Mitigates Inflammatory Bowel Disease through Modulation of AhR Activity by Microbial Tryptophan Metabolism. J. Agric. Food Chem. 2024, 72, 17912–17923. [Google Scholar] [CrossRef]
- Xiao, C.; Chen, J.; Zhou, X.; Liu, H. Ganoderic acid A alleviate psoriasis by inhibiting GSDMD-mediated pyroptosis. Tissue Cell 2025, 97, 103078. [Google Scholar] [CrossRef]
- Zahmoul, S.H.; Chaabouni, R.L.; Srih, A.; Dogan, H.H.; Varicioglu, E.; Sbissi, I.; Kües, U.; Toumi, L.; Tlili, A.; Peron, G.; et al. Nutritional and pharmacological potentials of the medicinal mushroom Ganoderma adspersum (Schulz.) Donk. S. Afr. J. Bot. 2024, 166, 360–374. [Google Scholar] [CrossRef]
- Cheng, M.T.; Zhang, L.Y.; Wang, J.; Sun, X.M.; Qi, Y.T.; Chen, L.J.; Han, C.C. The Artist’s Conk Medicinal Mushroom (Agaricomycetes): Mycological Mycochemical, and Pharmacological Properties: A Review. Int. J. Med. Mushrooms 2024, 26, 13–66. [Google Scholar] [CrossRef]
- Zhang, H.; Li, N.N.; Zhang, Y.K.; Xu, Y.; Lu, F.; Lin, D.M.; Lin, S.Q.; Li, M.; Yang, B.X. Polysaccharide Peptide Alleviates Cyclophosphamide-Induced Male Reproductive Injury by Reducing Oxidative Stress and Apoptosis. Biomedicines 2024, 12, 1632. [Google Scholar] [CrossRef]
- Chen, C.; Xu, R.X.; Guo, C.X.; Li, X.K.; Zhao, Y.X.; Luo, D.Q. Lanostane triterpenoids from Ganoderma calidophilum exhibit potent anti-tumor activity by inhibiting PTP1B. Chem-Biol. Interact. 2024, 403, 111253. [Google Scholar] [CrossRef]
- Paterska, M.; Czerny, B.; Cielecka-Piontek, J. Macrofungal Extracts as a Source of Bioactive Compounds for Cosmetical Anti-Aging Therapy: A Comprehensive Review. Nutrients 2024, 16, 2810. [Google Scholar] [CrossRef]
- Kubota, A.; Kobayashi, M.; Sarashina, S.; Takeno, R.; Okamoto, K.; Narumi, K.; Furugen, A.; Suzuki, Y.; Takahashi, N.; Iseki, K. Reishi mushroom Ganoderma lucidum Modulates IgA production and alpha-defensin expression in the rat small intestine. J. Ethnopharmacol. 2018, 214, 240–243. [Google Scholar] [CrossRef]
- Lin, Y.-L.; Liang, Y.-C.; Lee, S.-S.; Chiang, B.-L. Polysaccharide purified fromGanoderma luciduminduced activation and maturation of human monocyte-derived dendritic cells by the NF-κB and p38 mitogen-activated protein kinase pathways. J. Leukoc. Biol. 2005, 78, 533–543. [Google Scholar] [CrossRef]
- Lian, W.H.; Yang, X.; Duan, Q.D.; Li, J.; Zhao, Y.T.; Yu, C.H.; He, T.Z.; Sun, T.X.; Zhao, Y.; Wang, W.A. The Biological Activity of on Neurodegenerative Diseases: The Interplay between Different Active Compounds and the Pathological Hallmarks. Molecules 2024, 29, 2516. [Google Scholar] [CrossRef] [PubMed]
- Meng, M.; Wang, L.F.; Yao, Y.; Lin, D.M.; Wang, C.Y.; Yao, J.L.; Sun, H.J.; Liu, M.Z. polysaccharide peptide (GLPP) attenuates rheumatic arthritis in rats through inactivating NF-κB and MAPK signaling pathways. Phytomedicine 2023, 119, 155010. [Google Scholar] [CrossRef]
- Chanphen, R.; Pruksatrakul, T.; Choowong, W.; Choeyklin, R.; Surawatanawong, P.; Isaka, M. Ganopyrone A, a highly rearranged lanostane triterpenoid with antimalarial activity from artificially cultivated fruiting bodies of Ganoderma colossus. Phytochemistry 2024, 224, 114168. [Google Scholar] [CrossRef]
- Liu, Y.; Wen, H.K.; Xu, R.X.; Liu, C.; Li, X.H.; Qin, X.D.; Zhao, Y.X.; Jia, Y.X.; Luo, D.Q. Semisynthesis and antitumor activity of endertiin B and related triterpenoids from. Org. Biomol. Chem. 2024, 22, 4978–4986. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.D.; Li, G.F.; Zhai, Y.F.; Tong, L.G.; Ru, Y.L.; Wu, M.Y.; Hu, J.M.; Wang, M.Y.; Meng, Y.X.; Sun, B.; et al. Immunomodulatory effects and multi-omics analysis of Codonopsis Pilosula Extract in septic rats. J. Ethnopharmacol. 2025, 337, 118847. [Google Scholar] [CrossRef] [PubMed]
- Morse, B.A.; Motovilov, K.; Brode, W.M.; Tee, F.M.; Melamed, E. A review of intravenous immunoglobulin in the treatment of neuroimmune conditions, acute COVID-19 infection, and post-acute sequelae of COVID-19 Syndrome. Brain Behav. Immun. 2025, 123, 725–738. [Google Scholar] [CrossRef]
- Karunarathna, S.C.; Ediriweera, A.; Prasannath, K.; Yang, M.F.; Hapuarachchi, K.K. Exploring the health benefits of bioactive compounds and mechanisms of action; immunomodulatory, and anti-tumour activities. New Zealand J. Bot. 2024, 63, 2325–2409. [Google Scholar] [CrossRef]
- Zheng, Y.X.; Ren, Z.L.; Liu, Y.; Yan, J.T.; Chen, C.A.; He, Y.H.; Shi, Y.Y.; Cheng, F.F.; Wang, Q.G.; Li, C.X.; et al. T cell interactions with microglia in immune-inflammatory processes of ischemic stroke. Neural Regen. Res. 2025, 20, 1277–1292. [Google Scholar] [CrossRef]
- Araújo-Rodrigues, H.; Sousa, A.S.; Relvas, J.B.; Tavaria, F.K.; Pintado, M. An Overview on Mushroom Polysaccharides: Health-promoting Properties, Prebiotic and Gut Microbiota Modulation Effects and Structure-function Correlation. Carbohydr. Polym. 2024, 333, 121978. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, J.W.; Hao, H.Q. Antitumor immunostimulatory activity of the traditional Chinese medicine polysaccharide on hepatocellular carcinoma. Front. Immunol. 2024, 15, 1369110. [Google Scholar] [CrossRef]
- Cadar, E.; Negreanu-Pirjol, T.; Pascale, C.; Sirbu, R.; Prasacu, I.; Negreanu-Pirjol, B.S.; Tomescu, C.L.; Ionescu, A.M. Natural Bio-Compounds from and Their Beneficial Biological Actions for Anticancer Application: A Review. Antioxidants 2023, 12, 1907. [Google Scholar] [CrossRef]
- Liu, C.W.; Song, X.M.; Li, Y.Z.; Ding, C.; Li, X.; Dan, L.W.; Xu, H.N.; Zhang, D.D. A Comprehensive Review on the Chemical Composition, Pharmacology and Clinical Applications of. Am. J. Chin. Med. 2023, 51, 1983–2040. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.F.; Song, T.T.; Lv, G.Y.; Liu, J.; Jin, Q.L. Structural properties and immunomodulatory activity of an α-glucan purified from the fruiting body of Stropharia rugosoannulata. Chem. Biol. Technol. Ag. 2023, 10, 100. [Google Scholar] [CrossRef]
- Pasdaran, A.; Hassani, B.; Tavakoli, A.; Kozuharova, E.; Hamedi, A. A Review of the Potential Benefits of Herbal Medicines, Small Molecules of Natural Sources, and Supplements for Health Promotion in Lupus Conditions. Life 2023, 13, 1589. [Google Scholar] [CrossRef]
- Xie, J.; Lin, D.M.; Li, J.; Zhou, T.H.; Lin, S.Q.; Lin, Z.X. Effects of polysaccharide peptide ameliorating cyclophosphamide-induced immune dysfunctions based on metabolomics analysis. Front. Nutr. 2023, 10, 1179749. [Google Scholar] [CrossRef] [PubMed]
- Li, J.P.; Chu, C.L.; Chao, W.R.; Yeh, C.S.; Lee, Y.J.; Chen, D.C.; Yang, S.F.; Chao, Y.H. Ling Zhi-8, a fungal immunomodulatory protein in alleviates CPT-11-induced intestinal injury via restoring claudin-1 expression. Aging 2023, 15, 3621–3634. [Google Scholar] [CrossRef]
- Ekiz, E.; Oz, E.; Abd El-Aty, A.M.; Proestos, C.; Brennan, C.; Zeng, M.M.; Tomasevic, I.; Elobeid, T.; Çadirci, K.; Bayrak, M.; et al. Exploring the Potential Medicinal Benefits of From Metabolic Disorders to Coronavirus Infections. Foods 2023, 12, 1512. [Google Scholar] [CrossRef]
- Feng, T.; Ma, Z.C.; Li, J.Y.; Sun, L.L.; Wang, M.; Chen, M.L.; Ren, X.L. The Current Status and Trends of Chinese Medicine Polysaccharides: A Bibliometric Review [1998–2021]. Starch-Starke 2023, 75, 2200221. [Google Scholar] [CrossRef]
- Hu, N.; Qu, Y.; Liu, T.Y.; Zhou, Y.; Liu, C.; Wang, J.H.; Yang, B.F.; Li, C.L. Immunomodulatory effects and mechanisms of Tiepishihu Xiyangshen granules on cyclophosphamide induced immuno-suppression via TLR4/MAPKs and PI3K/AKT/FOXO3a signal pathways. J. Ethnopharmacol. 2023, 307, 116192. [Google Scholar] [CrossRef]
- Wang, C.; Shi, S.; Chen, Q.; Lin, S.; Wang, R.; Wang, S.; Chen, C. Antitumor and Immunomodulatory Activities of Ganoderma lucidum Polysaccharides in Glioma-Bearing Rats. Integr. Cancer Ther. 2018, 17, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.T.; Ma, X.M.; Li, C.H.; Meng, H.; Han, C.C. A review: Structure-activity relationship between saponins and cellular immunity. Mol. Biol. Rep. 2023, 50, 2779–2793. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.E.N.; Yuan, Z.H.; Wang, Y.; Du, Z.H.; Guo, J.J.; Xia, L.L.; Shan, Y. New Mechanistic Insight into the Protective Effects of Polysaccharides Against Palmitic Acid-Induced Cell Damage in Porcine Intestinal Epithelial Cell Line IPEC-J2. Nat. Prod. Commun. 2022, 17, 1934578x221128103. [Google Scholar] [CrossRef]
- Lai, Y.; Fang, Q.; Guo, X.R.; Lei, H.; Zhou, Q.; Wu, N.N.; Song, C. Effect of polysaccharides from on regulating gut microbiota and short-chain fatty acids in mice. J. Food Meas. Charact. 2023, 17, 1354. [Google Scholar] [CrossRef]
- Wang, G.L.; Li, J.Y.; Wang, Y.; Chen, Y.; Wen, Q.L. Extraction, Structure and Bioactivity of Polysaccharides from Tricholoma matsutake (S. Ito et Imai) Singer (Review). Appl. Biochem. Microbiol. 2022, 58, 375–381. [Google Scholar] [CrossRef]
- Liu, L.P.; Feng, J.; Gao, K.; Zhou, S.; Yan, M.Q.; Tang, C.H.; Zhou, J.; Liu, Y.F.; Zhang, J.S. Influence of carbon and nitrogen sources on structural features and immunomodulatory activity of exopolysaccharides from. Process Biochem. 2022, 119, 96–105. [Google Scholar] [CrossRef]
- Fontes, A.; Ramalho-Santos, J.; Zischka, H.; Azul, A.M. Mushrooms on the plate: Trends towards NAFLD treatment, health improvement and sustainable diets. Eur. J. Clin. Invest. 2022, 52, e13667. [Google Scholar] [CrossRef]
- Ahmad, M.F.; Ahmad, F.A.; Khan, M.I.; Alsayegh, A.A.; Wahab, S.; Alam, M.I.; Ahmed, F. Ganoderma lucidum: A potential source to surmount viral infections through β-glucans immunomodulatory and triterpenoids antiviral properties. Int. J. Biol. Macromol. 2021, 187, 769–779. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Yang, X.; Yang, X.; Xue, J.; Yang, Y. Ganoderic Acid A To Alleviate Neuroinflammation of Alzheimer’s Disease in Mice by Regulating the Imbalance of the Th17/Tregs Axis. J. Agric. Food Chem. 2021, 69, 14204–14214. [Google Scholar] [CrossRef]
- Zhao, R.; Chen, Q.; He, Y.-m. The effect of Ganoderma lucidum extract on immunological function and identify its anti-tumor immunostimulatory activity based on the biological network. Sci. Rep. 2018, 8, 12680. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.K.; Wang, W.L.; Bian, J.Y.; Gao, Y.C.; Hao, Z.T.; Tan, J.Q. Recent advances in medicinal and edible homologous polysaccharides: Extraction, purification, structure, modification, and biological activities. Int. J. Biol. Macromol. 2022, 222, 1110–1126. [Google Scholar] [CrossRef]
- Sipping, M.T.K.; Mediesse, F.K.; Kenmogne, L.V.; Kanemoto, J.E.N.; Njamen, D.; Boudjeko, T. Polysaccharide-Rich Fractions from (Ganodermataceae) as Chemopreventive Agents in N-Diethylnitrosamine-Induced Hepatocellular Carcinoma in Wistar Rats. Evidence-Based Complement. Altern. Med. 2022, 2022, 8198859. [Google Scholar] [CrossRef]
- Monga, S.; Fares, B.; Yashaev, R.; Melamed, D.; Kahana, M.; Fares, F.; Weizman, A.; Gavish, M. The Effect of Natural-Based Formulation (NBF) on the Response of RAW264.7 Macrophages to LPS as an In Vitro Model of Inflammation. J. Fungi 2022, 8, 321. [Google Scholar] [CrossRef]
- Sheng, Z.L.; Wen, L.R.; Yang, B. Structure identification of a polysaccharide in mushroom Lingzhi spore and its immunomodulatory activity. Carbohydr. Polym. 2022, 278, 118939. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F.; Li, M.M.; Wang, R.; Wang, B.Y.; Athari, S.S.; Wang, J.L. Ganoderma modulates allergic asthma pathologic features via anti-inflammatory effects. Resp. Physiol. Neurobiol. 2022, 299, 103843. [Google Scholar] [CrossRef]
- Ahmad, M.F.; Wahab, S.; Ahmad, F.A.; Ashraf, S.A.; Abullais, S.S.; Saad, H.H. Ganoderma lucidum: A potential pleiotropic approach of ganoderic acids in health reinforcement and factors influencing their production. Fungal Biol. Rev. 2022, 39, 100–125. [Google Scholar] [CrossRef]
- Hu, Y.L.; Lin, Z.X.; Fu, H.R.; Xia, J.; Xiong, W.; Li, G.L. Immunomodulatory effect of polysaccharide extract on peritoneal macrophage function of BALB/c mice. Cell Mol. Biol. 2022, 68, 31–35. [Google Scholar] [CrossRef]
- Gao, Y.Y.; Liu, X.P.; Zhou, Y.H.; He, J.Y.; Di, B.; Zheng, X.Y.; Guo, P.T.; Zhang, J.; Wang, C.K.; Jin, L. The Addition of Hot Water Extract of Juncao-Substrate Residue to Diets Enhances Growth Performance, Immune Function, and Intestinal Health in Broilers. Animals 2024, 14, 2926. [Google Scholar] [CrossRef]
- Chen, S.Y.; Chen, K.D.; Lin, Y.; Wang, S.; Yu, H.C.; Chang, C.H.; Cheng, T.C.; Hsieh, C.Y.; Li, J.Y.; Lai, H.H.; et al. Ganoderic acid T, a triterpenoid, modulates the tumor microenvironment and enhances the chemotherapy and immunotherapy efficacy through downregulating galectin-1 levels. Toxicol. Appl. Pharm. 2024, 491, 117069. [Google Scholar] [CrossRef]
- Fang, Y.; Zhang, Q.; Yu, C.Y.; Xu, X.Q.; Lei, P.; Xu, H.; Li, S. In vitro digestion and fecal fermentation of exopolysaccharides from basidiospore-derived submerged fermentation. Food Res. Int. 2024, 196, 115019. [Google Scholar] [CrossRef] [PubMed]
- Han, X.Q.; Luo, R.F.; Ye, N.J.; Hu, Y.C.; Fu, C.M.; Gao, R.; Fu, S.; Gao, F. Research progress on natural 13-glucan in intestinal diseases. Int. J. Biol. Macromol. 2022, 219, 1244–1260. [Google Scholar] [CrossRef]
- Guo, C.; Guo, D.; Fang, L.; Sang, T.; Wu, J.; Guo, C.; Wang, Y.; Wang, Y.; Chen, C.; Chen, J.; et al. Ganoderma lucidum polysaccharide modulates gut microbiota and immune cell function to inhibit inflammation and tumorigenesis in colon. Carbohydr. Polym. 2021, 267, 118231. [Google Scholar] [CrossRef]
- Li, M.Y.; Yu, L.L.; Zhao, J.X.; Zhang, H.; Chen, W.; Zhai, Q.X.; Tian, F.W. Role of dietary edible mushrooms in the modulation of gut microbiota. J. Funct. Foods 2021, 83, 104538. [Google Scholar] [CrossRef]
- Rutitzky, L.I.; Stadecker, M.J. CD4 T cells producing pro-inflammatory interleukin-17 mediate high pathology in schistosomiasis. Mem. I Oswaldo Cruz 2006, 101, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, T.P.; Peachey, L.E.; Ajami, N.J.; MacDonald, A.S.; Hsieh, M.H.; Brindley, P.J.; Cantacessi, C.; Rinaldi, G. Schistosoma mansoni infection is associated with quantitative and qualitative modifications of the mammalian intes-tinal microbiota. Sci. Rep. 2018, 8, 12072. [Google Scholar] [CrossRef]
- Rossi, B.; Previtali, L.; Salvi, M.; Gerami, R.; Tomasoni, L.R.; Quiros-Roldan, E. Female Genital Schistosomiasis: A Neglected among the Neglected Tropical Diseases. Microorganisms 2024, 12, 458. [Google Scholar] [CrossRef]
- Zou, H.; Ali, W.; Deng, K.; Chen, Y.; Sun, J.; Wang, T.; Ma, Y.G.; Liu, Z.P. The protective effect of luteolin on cadmium induced liver intestinal toxicity in chicken by Gut-liver axis regulation. Poult. Sci. 2024, 103, 104242. [Google Scholar] [CrossRef]
- Tonetti, F.R.; Eguileor, A.; Mrdjen, M.; Pathak, V.; Travers, J.; Nagy, L.E.; Llorente, C. Gut-liver axis: Recent concepts in pathophysiology in alcohol-associated liver disease. Hepatology 2024, 80, 1342–1371. [Google Scholar] [CrossRef]
- Guo, C.Y.; Li, Q.J.; Chen, R.H.; Fan, W.H.; Zhang, X.; Zhang, Y.Q.; Guo, L.P.; Wang, X.; Qu, X.Y.; Dong, H.J. Baicalein alleviates non-alcoholic fatty liver disease in mice by ameliorating intestinal barrier dysfunction. Food Funct. 2023, 14, 2138–2148. [Google Scholar] [CrossRef]
- Durkovicova, Z.; Faktorova, X.; Jakabovicova, M.; Szantova, M. Molecular mechanisms in the pathogenesis of metabolically associated fatty liver disease. Bratisl. Med. J. 2023, 124, 427–436. [Google Scholar] [CrossRef]
- Ishizawa, S.; Nishi, A.; Kaifuchi, N.; Shimobori, C.; Nahata, M.; Yamada, C.; Iizuka, S.; Ohbuchi, K.; Nishiyama, M.; Fujitsuka, N.; et al. Integrated analysis of effect of daisaikoto, a traditional Japanese medicine, on the metabolome and gut microbiome in a mouse model of nonalcoholic fatty liver disease. Gene 2022, 846, 146856. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, H.; Hashimoto, K.; Yuan, S.Y.; Zhang, J.C. The gut-liver axis in sepsis: Interaction mechanisms and therapeutic potential. Crit. Care 2022, 26, 213. [Google Scholar] [CrossRef]
- Joyce, S.A.; O’Malley, D. Bile acids, bioactive signalling molecules in interoceptive gut-to-brain communication. J. Physiol. 2022, 600, 2565–2578. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, T.M.; Georgiev, I.P.; Ontsouka, E.; Hammon, H.M.; Pfaffl, M.W.; Blum, J.W. Abundance of message for insulin-like growth factors-I and -II and for receptors for growth hormone, insulin-like growth factors-I and -II, and insulin in the intestine and liver of pre- and full-term calves. J. Anim. Sci. 2003, 81, 2294–2300. [Google Scholar] [CrossRef]
- Gondolesi, G.E. History of clinical intestinal transplantation. Hum. Immunol. 2024, 85, 110788. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.R.; Li, C.J.; Yu, T.J.; Guo, H.M.; Sun, H.; Mao, S.Y.; Zhou, Z.H.; Jin, W.; Liu, K.Q.; Xie, L.; et al. Global analysis of qualitative and quantitative metabolism of Notoginsenoside R1 in rat liver-brain-gut axis based on LC-IT-TOF/MS combing mMDF strategy. Phytomedicine 2022, 104, 154261. [Google Scholar] [CrossRef]
- Itoh, T.; Miyazono, D.; Sugata, H.; Mori, C.; Takahata, M. Anti-inflammatory effects of heat-killed Lactiplantibacillus argentoratensis BBLB001 on a gut inflammation co-culture cell model and dextran sulfate sodium-induced colitis mouse model. Int. Immunopharmacol. 2024, 143, 113408. [Google Scholar] [CrossRef]
- Ge, L.; Liu, S.M.; Li, S.; Yang, J.; Hu, G.R.; Xu, C.Q.; Song, W.A. Psychological stress in inflammatory bowel disease: Psychoneuroimmunological insights into bidirectional gut-brain communications. Front. Immunol. 2022, 13, 1016578. [Google Scholar] [CrossRef]
- Choy, C.T.; Chan, U.K.; Siu, P.L.K.; Zhou, J.W.; Wong, C.H.; Lee, Y.W.; Chan, H.W.; Tsui, J.C.C.; Loo, S.K.F.; Tsui, S.K.W. A Novel E3 Probiotics Formula Restored Gut Dysbiosis and Remodelled Gut Microbial Network and Microbiome Dysbiosis Index (MDI) in Southern Chinese Adult Psoriasis Patients. Int. J. Mol. Sci. 2023, 24, 6571. [Google Scholar] [CrossRef] [PubMed]
- Carbia, C.; Lannoy, S.; Maurage, P.; López-Caneda, E.; O’Riordan, K.J.; Dinan, T.G.; Cryan, J.F. A biological framework for emotional dysregulation in alcohol misuse: From gut to brain. Mol. Psychiatr. 2021, 26, 1098–1118. [Google Scholar] [CrossRef]
- Leonardi, I.; Gao, I.H.; Lin, W.Y.; Allen, M.; Li, X.V.; Fiers, W.D.; De Celie, M.B.; Putzel, G.G.; Yantiss, R.K.; Johncilla, M.; et al. Mucosal fungi promote gut barrier function and social behavior via Type 17 immunity. Cell 2022, 185, 831-846.e14. [Google Scholar] [CrossRef]
- Campagnoli, L.I.M.; Marchesi, N.; Varesi, A.; Morozzi, M.; Mascione, L.; Ricevuti, G.; Esposito, C.; Galeotti, N.; Pascale, A. New therapeutic avenues in multiple sclerosis: Is there a place for gut microbiota-based treatments? Pharmacol. Res. 2024, 209, 107456. [Google Scholar] [CrossRef]
- Butler, M.I.; Cryan, J.F.; Dinan, T.G. Man and the Microbiome: A New Theory of Everything? Annu. Rev. Clin. Psycho 2019, 15, 371–398. [Google Scholar] [CrossRef]
- Vuong, H.E.; Hsiao, E.Y. Emerging Roles for the Gut Microbiome in Autism Spectrum Disorder. Biol. Psychiat 2017, 81, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.C.; Rustenhoven, J.; Nelson, C.A.; Dykstra, T.; Ferreiro, A.; Papadopoulos, Z.; Burnham, C.A.D.; Dantas, G.; Fremont, D.H.; Kipnis, J. A novel immune modulator IM33 mediates a glia-gut-neuronal axis that controls lifespan. Neuron 2023, 111, 3244–3254.e8. [Google Scholar] [CrossRef]
- Wong, M.L.; Inserra, A.; Lewis, M.D.; Mastronardi, C.A.; Leong, L.; Choo, J.; Kentish, S.; Xie, P.; Morrison, M.; Wesselingh, S.L.; et al. Inflammasome signaling affects anxiety- and depressive-like behavior and gut microbiome composition. Mol. Psychiatr. 2016, 21, 797–805. [Google Scholar] [CrossRef]
- Ford, A.C.; Staudacher, H.M.; Talley, N.J. Postprandial symptoms in disorders of gut-brain interaction and their potential as a treatment target. Gut 2024, 73, 1199–1211. [Google Scholar] [CrossRef]
- Ghezzi, L.; Cantoni, C.; Pinget, G.V.; Zhou, Y.J.; Piccio, L. Targeting the gut to treat multiple sclerosis. J. Clin. Invest. 2021, 131, e143774. [Google Scholar] [CrossRef]
- Thirion, F.; Sellebjerg, F.; Fan, Y.; Lyu, L.; Hansen, T.H.; Pons, N.; Levenez, F.; Quinquis, B.; Stankevic, E.; Sondergaard, H.B.; et al. The gut microbiota in multiple sclerosis varies with disease activity. Genome Med. 2023, 15, 1. [Google Scholar] [CrossRef] [PubMed]
- Gracie, D.J.; Guthrie, E.A.; Hamlin, P.J.; Ford, A.C. Bi-directionality of Brain-Gut Interactions in Patients with Inflammatory Bowel Disease. Gastroenterology 2018, 154, 1635–1646.e3. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Haq, R.; Schlachetzki, J.C.M.; Glass, C.K.; Mazmanian, S.K. Microbiome-microglia connections via the gut-brain axis. J. Exp. Med. 2019, 216, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Gracie, D.J.; Ford, A.C. Reply. Gastroenterology 2018, 155, 1652–1653. [Google Scholar] [CrossRef]
- Rhee, S.H.; Pothoulakis, C.; Mayer, E.A. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat. Rev. Gastro Hepat. 2009, 6, 306–314. [Google Scholar] [CrossRef]
- Fung, T.C.; Olson, C.A.; Hsiao, E.Y. Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci. 2017, 20, 145–155. [Google Scholar] [CrossRef]
- Bonaz, B.L.; Bernstein, C.N. Brain-Gut Interactions in Inflammatory Bowel Disease. Gastroenterology 2013, 144, 36–49. [Google Scholar] [CrossRef]
- Ortega, M.A.; Alvarez-Mon, M.A.; García-Montero, C.; Fraile-Martínez, O.; Monserrat, J.; Martinez-Rozas, L.; Rodríguez-Jiménez, R.; Alvarez-Mon, M.; Lahera, G. Microbiota-gut-brain axis mechanisms in the complex network of bipolar disorders: Potential clinical implications and translational opportunities. Mol. Psychiatr. 2023, 28, 2645–2673. [Google Scholar] [CrossRef]
- Yan, Y.Q.; Ramanan, D.; Rozenberg, M.; McGovern, K.; Rastelli, D.; Vijaykumar, B.; Yaghi, O.; Voisin, T.; Mosaheb, M.; Chiu, I.; et al. Interleukin-6 produced by enteric neurons regulates the number and phenotype of microbe-responsive regulatory T cells in the gut. Immunity 2021, 54, 499–513.e5. [Google Scholar] [CrossRef]
- Pinna, G. Role of PPAR-Allopregnanolone Signaling in Behavioral and Inflammatory Gut-Brain Axis Communications. Biol. Psychiat 2023, 94, 609–618. [Google Scholar] [CrossRef]
- Ritz, N.L.; Draper, L.A.; Bastiaanssen, T.F.S.; Turkington, C.J.R.; Peterson, V.L.; van de Wouw, M.; Vlckova, K.; Fülling, C.; Guzzetta, K.E.; Burokas, A.; et al. The gut virome is associated with stress-induced changes in behaviour and immune responses in mice. Nat. Microbiol. 2024, 9, 359–376. [Google Scholar] [CrossRef]
- Wallrapp, A.; Chiu, I.M. Neuroimmune Interactions in the Intestine. Annu. Rev. Immunol. 2024, 42, 489–519. [Google Scholar] [CrossRef]
- Hanscom, M.; Loane, D.J.; Shea-Donohue, T. Brain-gut axis dysfunction in the pathogenesis of traumatic brain injury. J. Clin. Invest. 2021, 131, 43777. [Google Scholar] [CrossRef]
- Agirman, G.; Hsiao, E.Y. SnapShot: The microbiota-gut-brain axis. Cell 2021, 184, 2524–2525. [Google Scholar] [CrossRef] [PubMed]
- Sudaarsan, A.S.K.; Ghosh, A.R. Appraisal of postbiotics in cancer therapy. Front. Pharmacol. 2024, 15, 1436021. [Google Scholar] [CrossRef] [PubMed]
- Lázaro, A.; Vila-Donat, P.; Manyes, L. Emerging mycotoxins and preventive strategies related to gut microbiota changes: Probiotics, prebiotics, and postbiotics—A systematic review. Food Funct. 2024, 15, 8998–9023. [Google Scholar] [CrossRef] [PubMed]
- Flori, L.; Benedetti, G.; Martelli, A.; Calderone, V. Gut-vascular axis and postbiotics: The pharmacological potential of metabolites encourages broader definitions. Pharmacol. Res. 2024, 208, 107416. [Google Scholar] [CrossRef]
- Iser-Bem, P.N.; Lobato, T.B.; Alecrim-Zeza, A.L.; de Oliveira, L.C.D.; Passos, M.E.P.; Manuel, R.; Diniz, V.L.S.; Correa, I.S.; de Oliveira, S.P.; da Silva, E.B.; et al. Ganoderma lucidum dry extract supplementation modulates T lymphocyte function in older women. Brit J. Nutr. 2024, 132, 130–140. [Google Scholar] [CrossRef]
- Chugh, R.M.; Mittal, P.; Mp, N.; Arora, T.; Bhattacharya, T.; Chopra, H.; Cavalu, S.; Gautam, R.K. Fungal Mushrooms: A Natural Compound With Therapeutic Applications. Front. Pharmacol. 2022, 13, 925387. [Google Scholar] [CrossRef]
- Seweryn, E.; Ziala, A.; Gamian, A. Health-Promoting of Polysaccharides Extracted from Ganoderma lucidum. Nutrients 2021, 13, 2725. [Google Scholar] [CrossRef]
- Gallo, A.L.; Soler, F.; Pellizas, C.; Vélez, M.L. Polysaccharide extracts from mycelia of effect on dendritic cell immunomodulation and antioxidant activity. J. Food Meas. Charact. 2022, 16, 3251–3262. [Google Scholar] [CrossRef]
- Lin, J.W.; Chen, H.; Bai, Y.D.; Li, S.K.; Liang, G.Y.; Fan, T.N.; Gao, N.Y.; Wu, X.P.; Li, H.; Chen, G.; et al. Ganoderma immunomodulatory proteins: Mushrooming functional FIPs. Appl. Microbiol. Biot. 2022, 106, 2367–2380. [Google Scholar] [CrossRef]
- Kuo, H.C.; Liu, Y.W.; Lum, C.C.; Hsu, K.D.; Lin, S.P.; Hsieh, C.W.; Lin, H.W.; Lu, T.Y.; Cheng, K.C. Ganoderma formosanum Exopolysaccharides Inhibit Tumor Growth via Immunomodulation. Int. J. Mol. Sci. 2021, 22, 11251. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, J.; Hou, T.; An, S.Y.; Guo, B.Y.; Liu, C.C.; Hu, L.Y.; Huang, Y.H.; Zhang, S.; Song, M.Y.; et al. Extraction kinetics, physicochemical properties and immunomodulatory activity of the novel continuous phase transition extraction of polysaccharides from. Food Funct. 2021, 12, 9708–9718. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.L.; Cheng, P.Y.; Chin, C.L.; Chuang, K.T.; Lin, J.Y.; Chang, N.; Pan, C.K.; Lin, C.S.; Pan, S.C.; Chiang, B.L. A novel mucosal bivalent vaccine of EV-A71/EV-D68 adjuvanted with polysaccharides from protects mice against EV-A71 and EV-D68 lethal challenge. J. Biomed. Sci. 2023, 30, 96. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Li, S.; Ding, R.; Li, Y.; Li, C.L.; Gu, R. Antitumor effects of polysaccharides from medicinal lower plants: A review. Int. J. Biol. Macromol. 2023, 252, 126313. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Meng, T.; Wang, T.; Li, J.; Zhang, W.; Chen, J.H.; Lin, Z.X.; Lin, B.S. Hot water extract from spent mushroom substrate of Ganoderma lucidu m improves immune function in immune deficient mice. Trop. J. Pharm. Res. 2023, 22, 143–150. [Google Scholar] [CrossRef]
- Yang, Y.H.; Li, C.X.; Zhang, R.B.; Shen, Y.; Xu, X.J.; Yu, Q.M. A review of the pharmacological action and mechanism of natural plant polysaccharides in depression. Front. Pharmacol. 2024, 15, 1348019. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Zhong, J.; Huang, Y.C.; Jian, J.C.; Cai, S.H. Effect of Ganoderma lucidum polysaccharides as immunostimulants against Vibrio harveyi in pearl gentian grouper (♀Epinephelus fuscoguttatus × ♂Epinephelus lanceolatus). Front. Mar. Sci. 2022, 9, 968838. [Google Scholar] [CrossRef]
- Abdelmoaty, A.A.A.; Chen, J.; Zhang, K.; Wu, C.H.; Li, Y.; Li, P.; Xu, J.H. Senolytic effect of triterpenoid complex from Ganoderma lucidum on adriamycin-induced senescent human hepato-cellular carcinoma cells model in vitro and in vivo. Front. Pharmacol. 2024, 15, 1422363. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.Y.; Zhong, L.; Cheng, L.; Chen, L.; Tong, R.S.; Shi, J.Y.; Bai, L. Ganoderma lucidum: Current advancements of characteristic components and experimental progress in anti-liver fibrosis. Front. Pharmacol. 2023, 13, 1094405. [Google Scholar] [CrossRef]
- Chen, D.L.; Guo, Y.R.; Qi, L.K.; Tang, X.C.; Yadi, L.; Feng, J.X.; Zhu, X.X.; Miao, Z.; Ou, S.; Wang, D.D.; et al. Metabolic regulation of extracts in high sugar and fat diet-induced obese mice by regulating the gut-brain axis. J. Funct. Foods 2020, 65, 103639. [Google Scholar] [CrossRef]
- Jiang, L.Y.; Kan, Y.N.; Yu, Z.P.; Jian, B.Y.; Yao, S.J.; Lv, L.Y.; Liu, J.C. Prebiotic Effects of Chinese Herbal Polysaccharides on NAFLD Amelioration: The Preclinical Progress. Nat. Prod. Commun. 2022, 17, 1934578x221124751. [Google Scholar] [CrossRef]
- Batbayar, S.; Kim, M.J.; Kim, H.W. Medicinal Mushroom Lingzhi or Reishi, (W.Curt.:Fr.) P. Karst., β-Glucan Induces Toll-like Receptors and Fails to Induce Inflammatory Cytokines in NF-κB Inhibitor-Treated Macrophages. Int. J. Med. Mushrooms 2011, 13, 213–225. [Google Scholar] [CrossRef]
- Chang, C.J.; Chen, Y.Y.M.; Lu, C.C.; Lin, C.S.; Martel, J.; Tsai, S.H.; Ko, Y.F.; Huang, T.T.; Ojcius, D.M.; Young, J.D.; et al. Ganoderma lucidum stimulates NK cell cytotoxicity by inducing NKG2D/NCR activation and secretion of perforin and granulysin. Innate Immun-Lond. 2014, 20, 301–311. [Google Scholar] [CrossRef]
- Hasnat, M.A.; Pervin, M.; Cha, K.M.; Kim, S.K.; Lim, B.O. Anti-inflammatory activity on mice of extract of grown on rice via modulation of MAPK and NF-κB pathways. Phytochemistry 2015, 114, 125–136. [Google Scholar] [CrossRef]
- Xu, J.; Xiao, C.M.; Xu, H.S.; Yang, S.X.; Chen, Z.M.; Wang, H.Z.; Zheng, B.S.; Mao, B.Z.; Wu, X.Q. Anti-inflammatory effects of sterols via attenuation of the p38 MAPK and NF-κB pathways in LPS-induced RAW 264.7 macrophages. Food Chem. Toxicol. 2021, 150, 112073. [Google Scholar] [CrossRef]
- Sun, Q.M.; Xin, X.; An, Z.M.; Hu, Y.Y.; Feng, Q. Therapeutic Potential of Natural Plants Against Non-Alcoholic Fatty Liver Disease: Targeting the Interplay Between Gut Microbiota and Bile Acids. Front. Cell. Infect. Microbiol. 2022, 12, 854879. [Google Scholar] [CrossRef]
- Li, K.; Jiang, Y.P.; Wang, N.N.; Lai, L.Y.; Xu, S.Y.; Xia, T.S.; Yue, X.Q.; Xin, H.L. Traditional Chinese Medicine in Osteoporosis Intervention and the Related Regulatory Mechanism of Gut Microbiome. Am. J. Chin. Med. 2023, 51, 1957–1981. [Google Scholar] [CrossRef]
- Wang, J.J.; Pu, J.F.; Zhang, Z.X.; Feng, Z.; Han, J.; Su, X.J.; Shi, L. Triterpenoids of inhibited S180 sarcoma and H22 hepatoma in mice by regulating gut microbiota. Heliyon 2023, 9, e16682. [Google Scholar] [CrossRef]
- Liu, X.Z.; Huang, L.L.; Shi, Y.; Wang, X.G.; Luo, Y.L.; Wei, S.Y.; Qin, Y.C.; Lu, Y.W.; Zhang, W.L.; Ju, Y.; et al. Ganoderma lingzhi culture enhance growth performance via improvement of antioxidant activity and gut probiotic proliferation in Sanhuang broilers. Front. Vet. Sci. 2023, 10, 1143649. [Google Scholar] [CrossRef]
- Meneses, M.E.; Martínez-Carrera, D.; González-Ibáñez, L.; Torres, N.; Sánchez-Tapia, M.; Márquez-Mota, C.C.; Rendón, G.; Mitzi, V.; Morales, A.; Tello-Salgado, I.; et al. Effects of Mexican extracts on liver, kidney, and the gut microbiota of Wistar rats: A repeated dose oral toxicity study. PLoS ONE 2023, 18, e0283605. [Google Scholar] [CrossRef]
- Ülger, I.; Kaliber, M.; Hizlisoy, H.; Ayasan, T. Effects of Reishi Mushroom Powder Inclusion into Quail Diets on Animal Performance, Carcass Traits, Intestinal Microflora and Serum Parameters. J. Hell. Vet. Med. Soc. 2023, 74, 5845–5852. [Google Scholar] [CrossRef]
- Zheng, M.; Pi, X.W.; Li, H.X.; Cheng, S.S.; Su, Y.; Zhang, Y.; Man, C.X.; Jiang, Y.J. Ganoderma spp. polysaccharides are potential prebiotics: A review. Crit. Rev. Food Sci. 2024, 64, 909–927. [Google Scholar] [CrossRef]
- Lv, X.C.; Wu, Q.; Yuan, Y.J.; Li, L.; Guo, W.L.; Lin, X.B.; Huang, Z.R.; Rao, P.F.; Ai, L.Z.; Ni, L. Organic chromium derived from the chelation of polysaccharide and chromium (III) alleviates metabolic syndromes and intestinal microbiota dysbiosis induced by high-fat and high-fructose diet. Int. J. Biol. Macromol. 2022, 219, 964–979. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Hou, L.M.; Cui, M.; Liu, J.N.; Wang, M.Z.; Xie, J.W. The traditional Chinese medicine and non-small cell lung cancer: From a gut microbiome perspective. Front. Cell. Infect. Microbiol. 2023, 13, 1151557. [Google Scholar] [CrossRef]
- Leng, Y.; Wang, F.; Chen, C.B.; Wan, X.L.; Li, X.Y.; Wang, H.; Wang, S.M. Protective Effect of Spore Powder on Acute Liver Injury in Mice and its Regulation of Gut Microbiota. Front. Biosci. 2023, 28, 23. [Google Scholar] [CrossRef] [PubMed]
- Li, L.Y.; Guo, Y.N.; Huang, Q.; Shi, X.J.; Liu, Q.Q.; Wang, F.; Liu, Q.F.; Yu, K.; Wang, Z. GPP (composition of Ganoderma lucidum polysaccharides and Polyporus umbellatus polysaccharides) protects against DSS-induced murine colitis by enhancing immune function and regulating intestinal flora. Food Sci. Hum. Well 2022, 11, 795–805. [Google Scholar] [CrossRef]
- Qin, X.J.; Fang, Z.N.; Zhang, J.K.; Zhao, W.B.; Zheng, N.; Wang, X.E. Regulatory effect of and its active components on gut flora in diseases. Front. Microbiol. 2024, 15, 1362479. [Google Scholar] [CrossRef]
- Chen, W.J.; Wei, Y.S.; Lo, H.I.; Chu, H.F.; Tseng, M.C.; Lee, B.H.; Shen, T.L. Liquid-State Fermented GANO99 Regulates Gut Microbiota and Concomitantly Modulates the Behavioral Deficits and Neurohistopathological Hallmarks of Alzheimer’s Disease in a Preclinical Transgenic Mouse Model of Alzheimer’s Disease. J. Food Biochem. 2024, 2024, 6676977. [Google Scholar] [CrossRef]
- Fernandes, A.; Nair, A.; Kulkarni, N.; Todewale, N.; Jobby, R. Exploring Mushroom Polysaccharides for the Development of Novel Prebiotics: A Review. Int. J. Med. Mushrooms 2023, 25, 1–10. [Google Scholar] [CrossRef]
- Huang, Q.Q.; Zhang, Y.H.; Chu, Q.; Song, H.Z. The Influence of Polysaccharides on Lipid Metabolism: Insights from Gut Microbiota. Mol. Nutr. Food Res. 2024, 68, e2300522. [Google Scholar] [CrossRef] [PubMed]
- Li, M.Y.; Yu, L.L.; Zhai, Q.X.; Chu, C.Q.; Wang, S.H.; Zhao, J.X.; Zhang, H.; Tian, F.W.; Chen, W. Combined Ganoderma lucidum polysaccharide and ciprofloxacin therapy alleviates Salmonella enterica infection, protects the intestinal barrier, and regulates gut microbiota. Food Funct. 2023, 14, 6896–6913. [Google Scholar] [CrossRef]
- Zhang, F.L.; Huang, W.Q.; Zhao, L.A. Regulatory Effects of Ganoderma lucidum, Grifola frondosa, and American ginseng Extract Formulation on Gut Microbiota and Fecal Metabolomics in Mice. Foods 2023, 12, 3804. [Google Scholar] [CrossRef]
- Zhao, J.H.; Hu, Y.X.; Qian, C.; Hussain, M.; Liu, S.Z.; Zhang, A.Q.; He, R.J.; Sun, P.L. The Interaction between Mushroom Polysaccharides and Gut Microbiota and Their Effect on Human Health: A Review. Biology 2023, 12, 122. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Yang, C.; Xia, J.; Wang, W.X.; Li, N.G. lucidum triterpenes restores intestinal flora balance in non-hepatitis B virus-related hepatocellular carcinoma: Evidence of 16S rRNA sequencing and network pharmacology analysis. Front. Pharmacol. 2023, 14, 1197418. [Google Scholar] [CrossRef]

| Active Component | Effect | Mechanism | Target | References |
|---|---|---|---|---|
| Polysaccharides | Activate immune cells (macrophages, DCs, NK cells) | Combined with TLR receptors, they promote cytokine secretion and enhance phagocytic function. | TLR4, Dectin-1, NF-κB, MAPK | [24,25,48,49,50,51] |
| Enhance humoral immunity and cellular immunity | Promote IL-2 and IFN-γ secretion; stimulate B/T cell proliferation and differentiation | IL-2/IFN-γ signaling axis | [45,46] | |
| Triterpenoids | Anti-inflammatory, antioxidant | Inhibit NF-κB/MAPK pathways, reducing the release of inflammatory factors | NF-κBPKC, MAPK | [26,52] |
| Induce immune homeostasis | Regulate Treg cell activity and inhibit excessive Th17 activation | Achieving a healthy balance between Treg and Th17 cells | [53] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, L.; Geng, Z.; Wang, Y.; Wen, J.; Liu, D. Immunomodulatory Effects of Ganoderma lucidum Bioactive Compounds on Gut–Brain and Gut–Liver Axis Disorders. Curr. Issues Mol. Biol. 2025, 47, 842. https://doi.org/10.3390/cimb47100842
Zhao L, Geng Z, Wang Y, Wen J, Liu D. Immunomodulatory Effects of Ganoderma lucidum Bioactive Compounds on Gut–Brain and Gut–Liver Axis Disorders. Current Issues in Molecular Biology. 2025; 47(10):842. https://doi.org/10.3390/cimb47100842
Chicago/Turabian StyleZhao, Liting, Zijun Geng, Ying Wang, Jiawei Wen, and Da Liu. 2025. "Immunomodulatory Effects of Ganoderma lucidum Bioactive Compounds on Gut–Brain and Gut–Liver Axis Disorders" Current Issues in Molecular Biology 47, no. 10: 842. https://doi.org/10.3390/cimb47100842
APA StyleZhao, L., Geng, Z., Wang, Y., Wen, J., & Liu, D. (2025). Immunomodulatory Effects of Ganoderma lucidum Bioactive Compounds on Gut–Brain and Gut–Liver Axis Disorders. Current Issues in Molecular Biology, 47(10), 842. https://doi.org/10.3390/cimb47100842






