To Repair a Broken Heart: Stem Cells in Ischemic Heart Disease
Abstract
1. Introduction
2. Ischemic Heart Disease (IHD)
2.1. Pathophysiology of Ischemic Heart Disease (IHD)
2.2. Current Treatment Strategies for Ischemic Heart Disease (IHD)
3. Stem and Progenitor Cell Therapies for Ischemic Heart Disease (IHD)
3.1. Cell Therapies
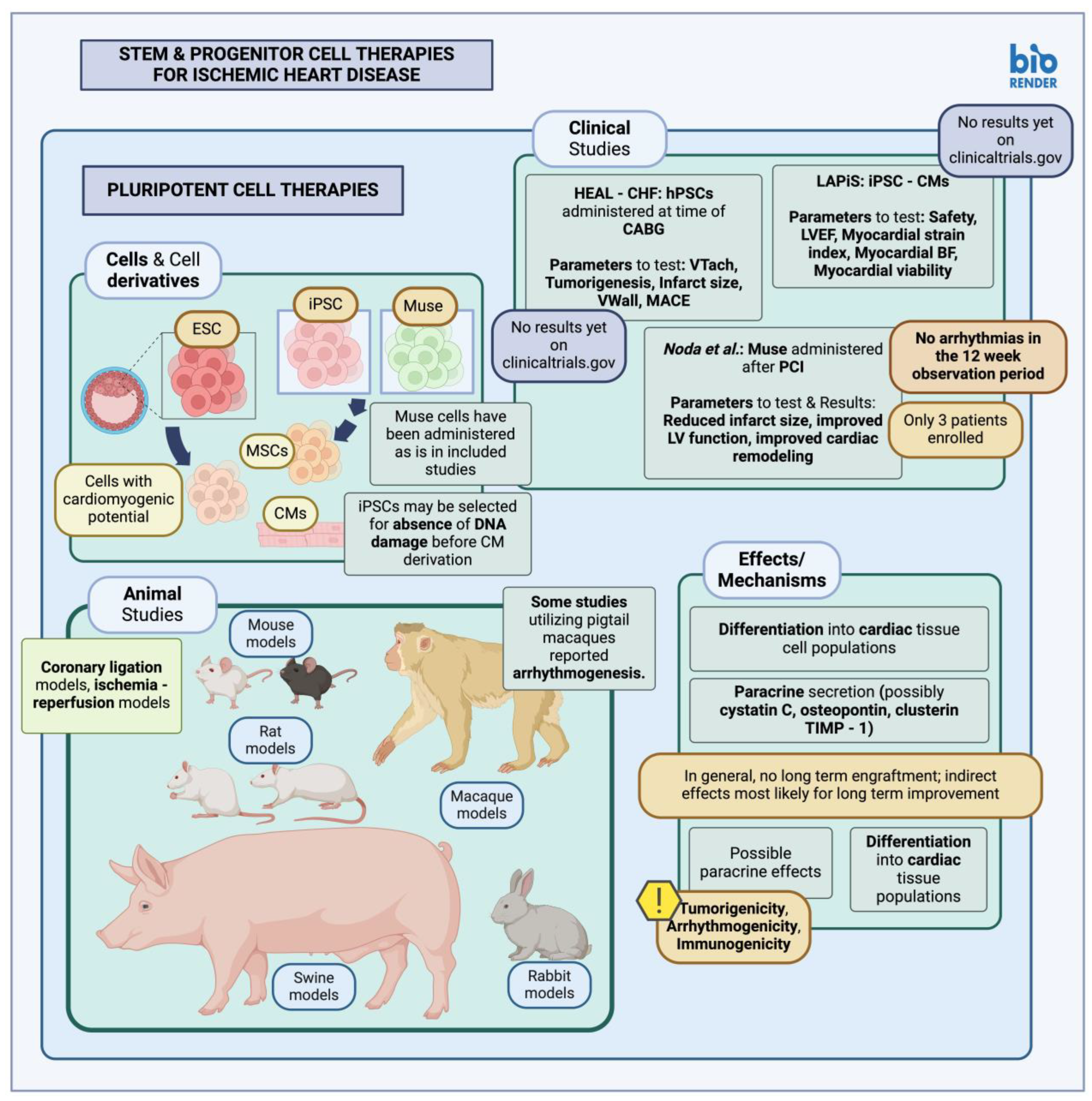
3.1.1. Pluripotent Stem Cells in Animal Studies and Clinical Trials
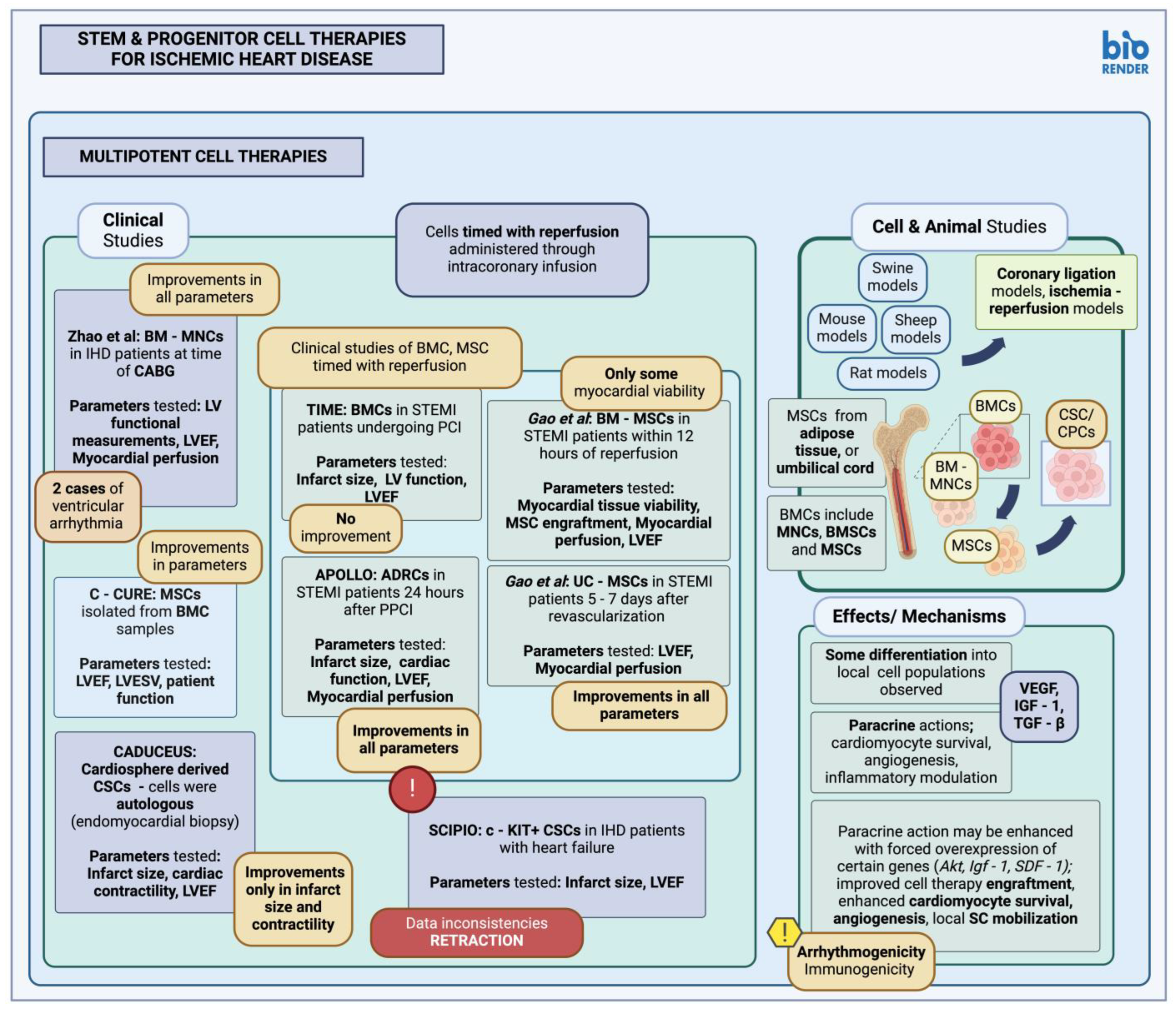
3.1.2. Multipotent Stem Cells in Animal Studies and Clinical Trials
| Study | Cell Type | Model | Method | Comments |
|---|---|---|---|---|
| Orlic et al., 2001 [111] | BMC | Mouse | Intramyocardial injection | Cells administered post-MI (coronary artery ligation); BMCs were selected based on Lin (Lin-) and c-KIT (c-KIT+) expression. New cardiomyocytes and blood vessels were observed in the infarct border 9 days after administration. |
| Kamihata et al., 2001 [112] | BM-MNC | Swine | Intramyocardial injection | Cells administered post-MI (LAD ligation); BM-MNCs were shown to contribute to endothelial cell lineages in the area, increasing capillary density, improving local blood flow and cardiac function. |
| Bel et al., 2003 [113] | BMC | Sheep | Intramyocardial injection | Cells administered post-MI (LCx ligation); no differentiation of implanted BMCs into endothelial cells, or considerable BMC engraftment was observed. The study detected no differences in LV ejection fraction, global wall motion, or LV geometric parameters between control and experimental groups. |
| de Silva et al., 2008 [114] | BMC | Swine | Intracoronary infusion | Cells administered post-MI (LAD occlusion and reperfusion); no differentiation of implanted cells was observed through subsequent immunofluorescence studies. The study detected no differences in LV ejection fraction, ventricular volume, or size of the resulting infarct between control and experimental groups. |
| Bartunek et al., 2013 [115] | BM-MSC | Clinical trial | Endomyocardial injection | C-CURE trial; BMC samples selected for MSCs, with subsequent MSCs administered in patients with chronic heart failure due to IHD. From the parameters tested results include an improvement in the LV ejection fraction, reduced LV end-systolic volume, and improvement in patient function. |
| Gao et al., 2013 [116] | BM-MSC | Clinical trial | Intracoronary infusion | BM-derived MSCs were administered in patients with STEMI undergoing reperfusion within 12 h. The study reported some improvement in cardiac tissue viability, although no significant improvement was observed in terms of MSC engraftment, cardiac tissue perfusion, function (LVEF), between experimental and control groups. |
| Traverse et al., 2014 [117] | BMC | Clinical trial | Intracoronary infusion | TIME trial; BMCs were administered in patients with STEMI, undergoing PCI, 3 or 7 days after the event. The study reported no significant differences in LV ejections fraction, local LV function, as well as infarct size reduction between the experimental and control groups. |
| Zhao et al., 2008 [118] | BM-MNC | Clinical trial | Intramyocardial injection | BM-MNCs administered in IHD patients, at time of CABG; the study reported improved LV geometric parameters compared to control (LV wall thickness), improved LV ejection fraction, improved local myocardial perfusion. The study reported two instances of ventricular arrhythmia after BM-MNC administration. |
| Berry et al., 2006 [119] | MSC | Rat | Intramyocardial injection | Cells administered post-MI (LAD ligation); improvement in cardiac function, and reduced rate of apoptosis and local fibrosis was observed, although there was no effect on local angiogenesis. No differentiation of injected MSCs into differentiated cardiomyocyte was observed. |
| Gnecchi et al., 2005 [120] | MSC-Akt medium | Rat | Intramyocardial injection | Medium conditioned with hypoxic MSC overexpressing Akt, was administered post-MI (coronary occlusion); results showed a reduction in infarct size, and cardiomyocyte apoptosis. |
| Haider et al., 2008 [121] | MSC | Rat | Intramyocardial injection | IGF-1 modified MSCs administered post-MI (coronary artery ligation); MSCs were modified to overexpress Igf-1, resulting in higher, local MSC engraftment and survival. There were improvements in LV function parameters and local angiogenesis, as well as reduction in infarct size after administration of MSC-SDF-1+ cells. The study reported increased local stem cell mobilization (CD31+, c-KIT+, MDR1+, CD34+), due to the SDF-1 secretion from transplanted MSCs. |
| Houtgraaf et al., 2012 [122] | ADRC | Clinical trial | Intracoronary infusion | APOLLO trial; adipose-derived multipotent stem cells were administered in STEMI patients, 24 h after primary PCI. ADRCs in question were patient-derived through liposuction. The study reported improvement in LV ejection fraction and cardiac function overall, reduced infarct size, and improved local myocardial perfusion. |
| Gao et al., 2015 [123] | UC-MSC | Clinical trial | Intracoronary infusion | Cells administered in STEMI patients 5–7 days after revascularization; the study reported improvements in LV ejection fraction, and myocardial perfusion. |
| the SCIENCE investigators, 2023 [124] | AD-MSC | Clinical trial | Intramyocardial injection | Cells administered in patients with heart failure due to chronic ischemic heart disease; no significant improvement in LVESV, LVEF, cardiac function. |
| Bolli et al., 2013 [125] | CSC | Swine | Intracoronary infusion | c-KIT+ CSCs administered post-MI (ischemia-reperfusion); cells expressing sarcomeric proteins, staining positive for Ki67, were identified, along with cells expressing cardiac markers alluding to local generation of differentiated cardiomyocytes. Differentiation into vascular structures was also reported. The study reported increased LV ejection fraction and improved hemodynamic measurements after CSC infusion. |
| Makkar et al., 2012 [126] | CSC | Clinical trial | Intracoronary infusion | CADUCEUS trial; cardiosphere-derived CPC/CSC were administered in patients. Cells were autologous, procured through endomyocardial biopsy. The study reported, among others, a decrease in scar size, and an increase in cardiac contractility; however, no significant improvement in LV ejection fraction was reported. |
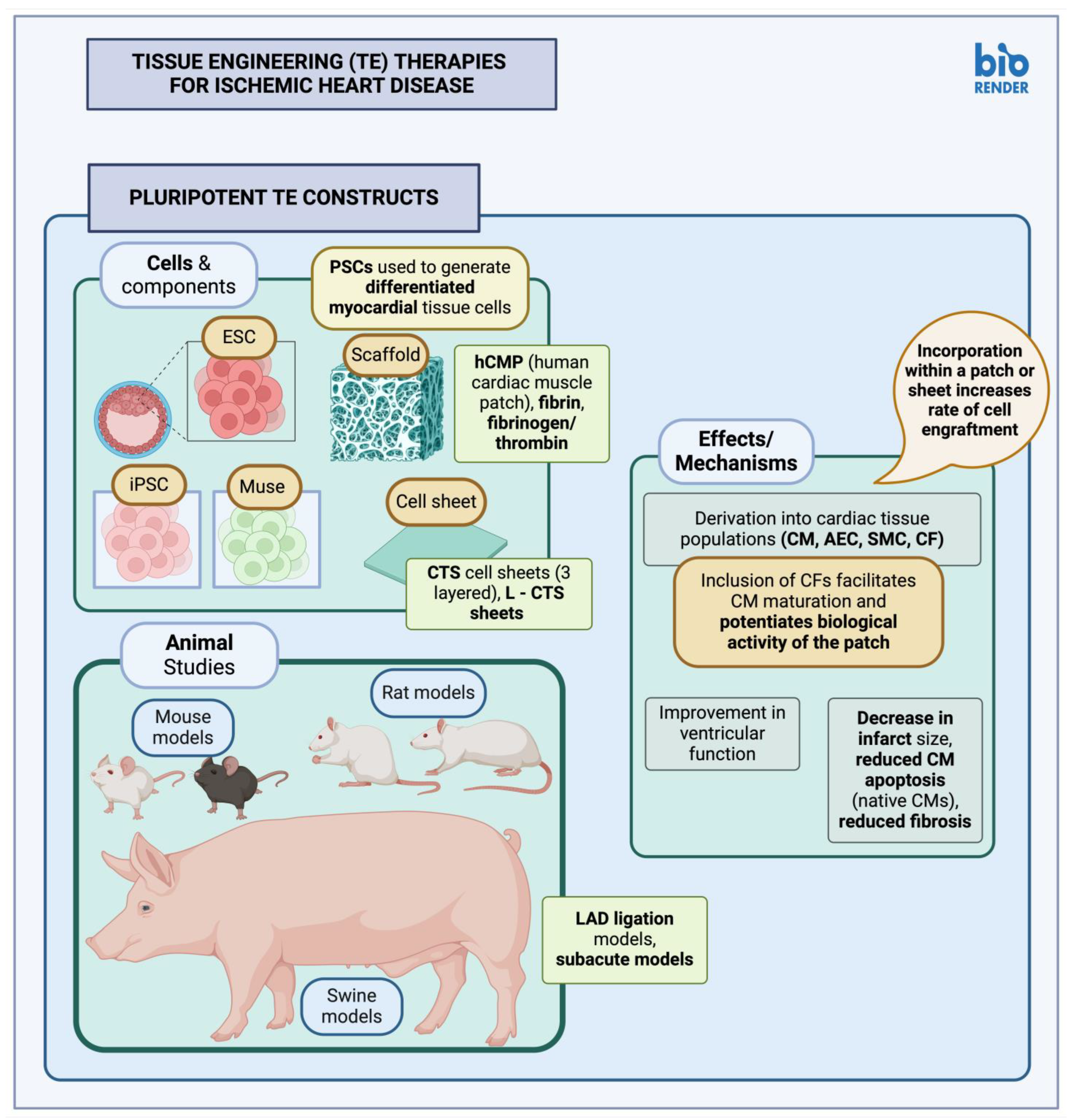
3.2. Tissue-Engineered Therapies
3.2.1. Pluripotent Stem Cell Constructs
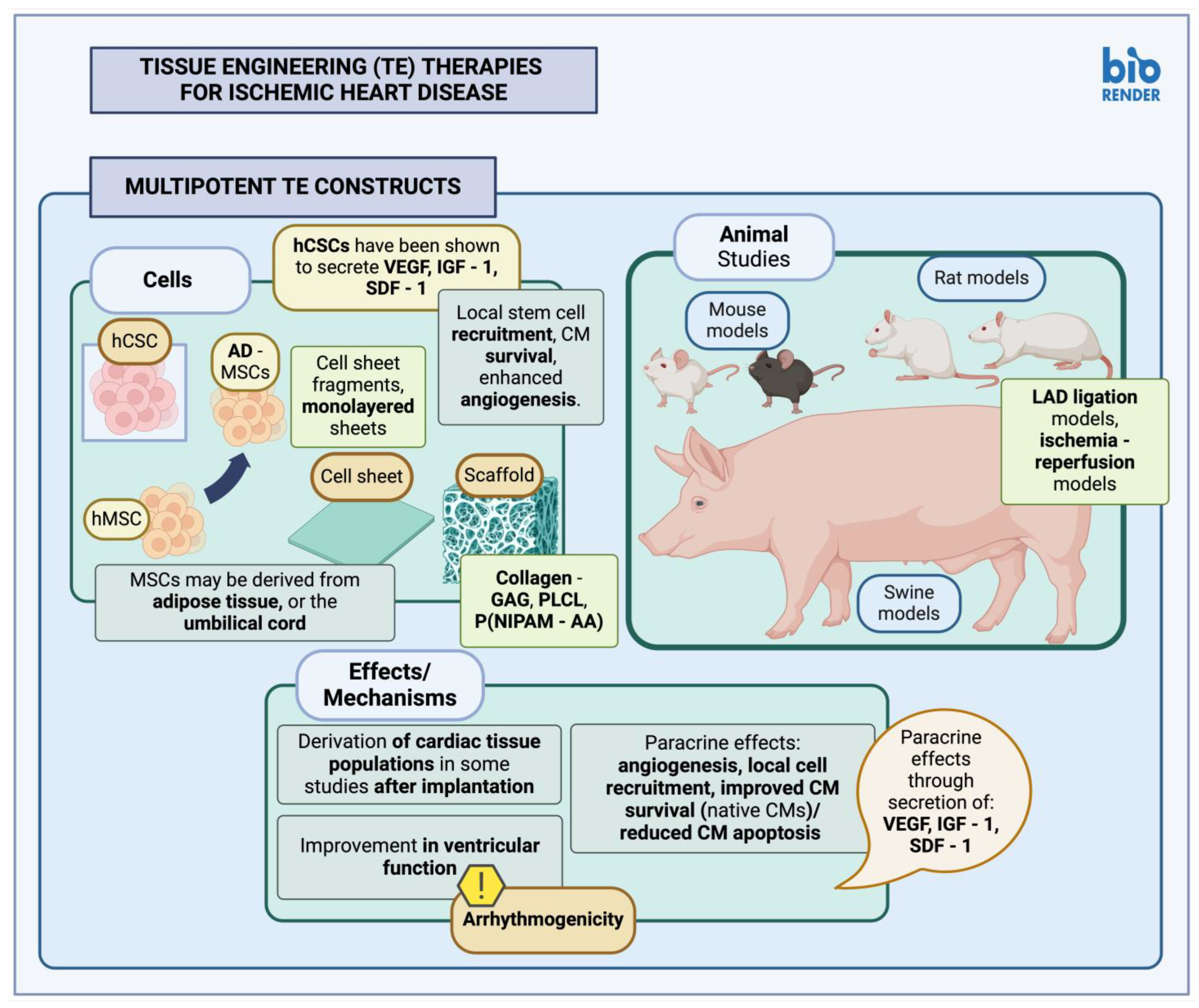
3.2.2. Multipotent Stem Cell Constructs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics—2022 Update: A Report from the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef]
- Heart Disease Facts|Cdc.Gov. Available online: https://www.cdc.gov/heartdisease/facts.htm (accessed on 6 August 2023).
- Khera, A.V.; Kathiresan, S. Genetics of Coronary Artery Disease: Discovery, Biology and Clinical Translation. Nat. Rev. Genet. 2017, 18, 331–344. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Theroux, P. Pathophysiology of Coronary Artery Disease. Circulation 2005, 111, 3481–3488. [Google Scholar] [CrossRef] [PubMed]
- Reed, S.C.; Dhir, N.; Widmer, R.J. Optimal Cardiovascular Medical Therapy: Current Guidelines and New Developments. Bayl. Univ. Med. Cent. Proc. 2022, 35, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Lorenzen, U.S.; Buggeskov, K.B.; Nielsen, E.E.; Sethi, N.J.; Carranza, C.L.; Gluud, C.; Jakobsen, J.C. Coronary Artery Bypass Surgery plus Medical Therapy versus Medical Therapy Alone for Ischaemic Heart Disease: A Protocol for a Systematic Review with Meta-Analysis and Trial Sequential Analysis. Syst. Rev. 2019, 8, 246. [Google Scholar] [CrossRef]
- Yap, J.; Chew, D.P.; Stone, G.W.; Tan, J.W.C. Pharmacotherapy in Stable Coronary Artery Disease: Historical Perspectives and New Insights from the ISCHEMIA Trial. Eur. Cardiol. 2021, 16, e04. [Google Scholar] [CrossRef]
- Jia, S.; Liu, Y.; Yuan, J. Evidence in Guidelines for Treatment of Coronary Artery Disease. Adv. Exp. Med. Biol. 2020, 1177, 37–73. [Google Scholar] [CrossRef]
- Nieuwlaat, R.; Schwalm, J.-D.; Khatib, R.; Yusuf, S. Why Are We Failing to Implement Effective Therapies in Cardiovascular Disease? Eur. Heart J. 2013, 34, 1262–1269. [Google Scholar] [CrossRef]
- Gelchu, T.; Abdela, J. Drug Therapy Problems among Patients with Cardiovascular Disease Admitted to the Medical Ward and Had a Follow-Up at the Ambulatory Clinic of Hiwot Fana Specialized University Hospital: The Case of a Tertiary Hospital in Eastern Ethiopia. SAGE Open Med. 2019, 7, 2050312119860401. [Google Scholar] [CrossRef]
- Martín, I.R. Post-operative complications in cardiac surgery patients. Chest 2021, 160, A81. [Google Scholar] [CrossRef]
- Tang, B.; Yang, H. Post Percutaneous Coronary Interventional Outcomes on Proximal vs Non-Proximal Lesions of the Left and Right Coronary Arteries. Medicine 2019, 98, e16905. [Google Scholar] [CrossRef]
- Doenst, T.; Schneider, U.; Can, T.; Caldonazo, T.; Diab, M.; Siemeni, T.; Färber, G.; Kirov, H. Cardiac Surgery 2021 Reviewed. J. Thorac. Cardiovasc. Surg. 2022, 70, 278–288. [Google Scholar] [CrossRef]
- Bianco, V.; Kilic, A.; Gleason, T.G.; Aranda-Michel, E.; Habertheuer, A.; Wang, Y.; Navid, F.; Kacin, A.; Sultan, I. Reoperative Cardiac Surgery Is a Risk Factor for Long-Term Mortality. Ann. Thorac. Surg. 2020, 110, 1235–1242. [Google Scholar] [CrossRef]
- Virani, S.S.; Newby, L.K.; Arnold, S.V.; Bittner, V.; Brewer, L.C.; Demeter, S.H.; Dixon, D.L.; Fearon, W.F.; Hess, B.; Johnson, H.M.; et al. 2023 AHA/ACC/ACCP/ASPC/NLA/PCNA Guideline for the Management of Patients with Chronic Coronary Disease. J. Am. Coll. Cardiol. 2023, 82, 833–955, Correction in J. Am. Coll. Cardiol. 2023, 82, 1808. [Google Scholar] [CrossRef] [PubMed]
- Severino, P.; D’Amato, A.; Pucci, M.; Infusino, F.; Adamo, F.; Birtolo, L.I.; Netti, L.; Montefusco, G.; Chimenti, C.; Lavalle, C.; et al. Ischemic Heart Disease Pathophysiology Paradigms Overview: From Plaque Activation to Microvascular Dysfunction. Int. J. Mol. Sci. 2020, 21, 8118. [Google Scholar] [CrossRef] [PubMed]
- Lusis, A.J. Atherosclerosis. Nature 2000, 407, 233–241. [Google Scholar] [CrossRef]
- Plasschaert, H.; Heeneman, S.; Daemen, M.J. Progression in Atherosclerosis: Histological Features and Pathophysiology of Atherosclerotic Lesions. Top. Magn. Reson. Imaging 2009, 20, 227. [Google Scholar] [CrossRef]
- Fruchart, J.-C.; Nierman, M.C.; Stroes, E.S.G.; Kastelein, J.J.P.; Duriez, P. New Risk Factors for Atherosclerosis and Patient Risk Assessment. Circulation 2004, 109, III-15–III-19. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.N.; Halushka, M.K. Blood Vessels. In Robbins & Cotran Pathologic Basis of Disease; Elsevier: Philadelphia, PA, USA, 2021; ISBN 978-0-323-53113-9. [Google Scholar]
- Rafieian-Kopaei, M.; Setorki, M.; Doudi, M.; Baradaran, A.; Nasri, H. Atherosclerosis: Process, Indicators, Risk Factors and New Hopes. Int. J. Prev. Med. 2014, 5, 927–946. [Google Scholar]
- Vilela, E.M.; Fontes-Carvalho, R. Inflammation and Ischemic Heart Disease: The next Therapeutic Target? Rev. Port. Cardiol. 2021, 40, 785–796. [Google Scholar] [CrossRef]
- Chen, M.; Masaki, T.; Sawamura, T. LOX-1, the Receptor for Oxidized Low-Density Lipoprotein Identified from Endothelial Cells: Implications in Endothelial Dysfunction and Atherosclerosis. Pharmacol. Ther. 2002, 95, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Marzilli, M.; Huqi, A. Coronary Vasospasm and Coronary Atherosclerosis. J. Am. Coll. Cardiol. 2012, 59, 663–664. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ludmer, P.L.; Selwyn, A.P.; Shook, T.L.; Wayne, R.R.; Mudge, G.H.; Alexander, R.W.; Ganz, P. Paradoxical Vasoconstriction Induced by Acetylcholine in Atherosclerotic Coronary Arteries. N. Engl. J. Med. 1986, 315, 1046–1051. [Google Scholar] [CrossRef] [PubMed]
- Beltrame, J.F. Variant Angina. Available online: https://www.escardio.org/Journals/E-Journal-of-Cardiology-Practice/Volume-11/Variant-Angina (accessed on 17 November 2023).
- Lanza, G.A.; Careri, G.; Crea, F. Mechanisms of Coronary Artery Spasm. Circulation 2011, 124, 1774–1782. [Google Scholar] [CrossRef] [PubMed]
- Saeed, S.; Flygel, M.; Løland, K.H.; Packer, E. Catheter-Induced Vasospasm of the Anatomically Normal Right Coronary Artery in a Patient with Non-ST Elevation Myocardial Infarction and Obstructive Disease in the Left Anterior Descending Artery; A Challenging Case Report and Review of the Literature. Curr. Probl. Cardiol. 2023, 48, 101432. [Google Scholar] [CrossRef] [PubMed]
- Vila, E.; Salaices, M. Cytokines and Vascular Reactivity in Resistance Arteries. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H1016–H1021. [Google Scholar] [CrossRef] [PubMed]
- Shimokawa, H.; Satoh, K. 2015 ATVB Plenary Lecture. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1756–1769. [Google Scholar] [CrossRef]
- Shimokawa, H.; Sunamura, S.; Satoh, K. RhoA/Rho-Kinase in the Cardiovascular System. Circ. Res. 2016, 118, 352–366. [Google Scholar] [CrossRef]
- Noma, K.; Oyama, N.; Liao, J.K. Physiological Role of ROCKs in the Cardiovascular System. Am. J. Physiol. Cell Physiol. 2006, 290, C661–C668. [Google Scholar] [CrossRef]
- Piccirillo, F.; Carpenito, M.; Verolino, G.; Chello, C.; Nusca, A.; Lusini, M.; Spadaccio, C.; Nappi, F.; Di Sciascio, G.; Nenna, A. Changes of the Coronary Arteries and Cardiac Microvasculature with Aging: Implications for Translational Research and Clinical Practice. Mech. Ageing Dev. 2019, 184, 111161. [Google Scholar] [CrossRef]
- Ferraro, R.; Latina, J.M.; Alfaddagh, A.; Michos, E.D.; Blaha, M.J.; Jones, S.R.; Sharma, G.; Trost, J.C.; Boden, W.E.; Weintraub, W.S.; et al. Evaluation and Management of Patients with Stable Angina: Beyond the Ischemia Paradigm: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 76, 2252–2266. [Google Scholar] [CrossRef]
- Melaku, L.; Dabi, A. The Cellular Biology of Atherosclerosis with Atherosclerotic Lesion Classification and Biomarkers. Bull. Natl. Res. Cent. 2021, 45, 225. [Google Scholar] [CrossRef]
- Virmani, R.; Kolodgie, F.D.; Burke, A.P.; Farb, A.; Schwartz, S.M. Lessons from Sudden Coronary Death. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1262–1275. [Google Scholar] [CrossRef] [PubMed]
- Tousoulis, D.; Androulakis, E.; Kontogeorgou, A.; Papageorgiou, N.; Charakida, M.; Siama, K.; Latsios, G.; Siasos, G.; Kampoli, A.-M.; Tourikis, P.; et al. Insight to the Pathophysiology of Stable Angina Pectoris. Curr. Pharm. Des. 2013, 19, 1593–1600. [Google Scholar] [PubMed]
- Badimon, L.; Padró, T.; Vilahur, G. Atherosclerosis, Platelets and Thrombosis in Acute Ischaemic Heart Disease. Eur. Heart J. Acute Cardiovasc. Care 2012, 1, 60–74. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.C.; Joffe, H.V. (Eds.) Chapter 3—Unstable Angina and Non-ST Elevation Myocardial Infarction. In The Most Common Inpatient Problems in Internal Medicine; W.B. Saunders: Philadelphia, PA, USA, 2007; pp. 51–81. ISBN 978-1-4160-3203-8. [Google Scholar]
- Ghafoor, M.; Kamal, M.; Nadeem, U.; Husain, A.N. Educational Case: Myocardial Infarction: Histopathology and Timing of Changes. Acad. Pathol. 2020, 7, 2374289520976639. [Google Scholar] [CrossRef] [PubMed]
- Lodrini, A.M.; Goumans, M.-J. Cardiomyocytes Cellular Phenotypes after Myocardial Infarction. Front. Cardiovasc. Med. 2021, 8, 750510. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, Y.; Doan, J.; Murray, J.; Molkentin, J.D.; Liu, Q. Transforming Growth Factor β–Activated Kinase 1 Signaling Pathway Critically Regulates Myocardial Survival and Remodeling. Circulation 2014, 130, 2162–2172. [Google Scholar] [CrossRef] [PubMed]
- Luedde, M.; Lutz, M.; Carter, N.; Sosna, J.; Jacoby, C.; Vucur, M.; Gautheron, J.; Roderburg, C.; Borg, N.; Reisinger, F.; et al. RIP3, a Kinase Promoting Necroptotic Cell Death, Mediates Adverse Remodelling after Myocardial Infarction. Cardiovasc. Res. 2014, 103, 206–216. [Google Scholar] [CrossRef]
- Prabhu, S.D.; Frangogiannis, N.G. The Biological Basis for Cardiac Repair After Myocardial Infarction: From Inflammation to Fibrosis. Circ. Res. 2016, 119, 91–112. [Google Scholar] [CrossRef]
- Forte, E. The Multifaceted Effect of Efferocytosis on Cardiac Repair after Infarction. Nat. Cardiovasc. Res. 2022, 1, 283. [Google Scholar] [CrossRef]
- Yutian, L.; Qianqian, L.; Fan, G.-C. Macrophage efferocytosis in cardiac pathophysiology and repair. Shock 2021, 55, 177–188. [Google Scholar] [CrossRef]
- Kain, V.; Prabhu, S.D.; Halade, G.V. Inflammation Revisited: Inflammation versus Resolution of Inflammation following Myocardial Infarction. Basic. Res. Cardiol. 2014, 109, 444. [Google Scholar] [CrossRef] [PubMed]
- Zeisberg, E.M.; Tarnavski, O.; Zeisberg, M.; Dorfman, A.L.; McMullen, J.R.; Gustafsson, E.; Chandraker, A.; Yuan, X.; Pu, W.T.; Roberts, A.B.; et al. Endothelial-to-Mesenchymal Transition Contributes to Cardiac Fibrosis. Nat. Med. 2007, 13, 952–961. [Google Scholar] [CrossRef]
- Schumacher, D.; Curaj, A.; Staudt, M.; Simsekyilmaz, S.; Kanzler, I.; Boor, P.; Klinkhammer, B.M.; Li, X.; Bucur, O.; Kaabi, A.; et al. Endogenous Modulation of Extracellular Matrix Collagen during Scar Formation after Myocardial Infarction. Int. J. Mol. Sci. 2022, 23, 14571. [Google Scholar] [CrossRef]
- Jensen, R.V.; Hjortbak, M.V.; Bøtker, H.E. Ischemic Heart Disease: An Update. Semin. Nucl. Med. 2020, 50, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.V.; Revelo, M.P. (Eds.) Ischemic Heart Disease. In Diagnostic Pathology: Cardiovascular, 2nd ed.; Diagnostic Pathology; Elsevier: Philadelphia, PA, USA, 2018; pp. 110–113. ISBN 978-0-323-59560-5. [Google Scholar]
- Lawton, J.S.; Tamis-Holland, J.E.; Bangalore, S.; Bates, E.R.; Beckie, T.M.; Bischoff, J.M.; Bittl, J.A.; Cohen, M.G.; DiMaio, J.M.; Don, C.W.; et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e18–e114. [Google Scholar] [CrossRef]
- Mishra, P.K.; Adameova, A.; Hill, J.A.; Baines, C.P.; Kang, P.M.; Downey, J.M.; Narula, J.; Takahashi, M.; Abbate, A.; Piristine, H.C.; et al. Guidelines for Evaluating Myocardial Cell Death. Am. J. Physiol. Heart Circ. Physiol. 2019, 317, H891–H922, Correction in J. Physiol. Heart Circ. Physiol. 2019, 317, H1390–H1390. [Google Scholar] [CrossRef]
- Doll, J.A.; Hira, R.S.; Kearney, K.E.; Kandzari, D.E.; Riley, R.F.; Marso, S.P.; Grantham, J.A.; Thompson, C.A.; McCabe, J.M.; Karmpaliotis, D.; et al. Management of Percutaneous Coronary Intervention Complications. Circ. Cardiovasc. Interv. 2020, 13, e008962. [Google Scholar] [CrossRef]
- Al-Azizi, K.M.; Moubarak, G.; Szerlip, M.I.; Kluis, A.; Kazem, A.; Bennett, M.M.; Foster, L.; Thomas, S.; Dib, C.; Sayfo, S.; et al. Impact of Iodinated Contrast Shortage on Contrast-Associated Acute Kidney Injury: A Single Center Experience. Bayl. Univ. Med. Cent. Proc. 2024, 37, 218–226. [Google Scholar] [CrossRef]
- Fiore, A.; Grande, A.M.; Gatti, G. Major Complications of Cardiac Surgery. In The High-Risk Surgical Patient; Aseni, P., Grande, A.M., Leppäniemi, A., Chiara, O., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 537–550. ISBN 978-3-031-17273-1. [Google Scholar]
- Isomi, M.; Sadahiro, T.; Ieda, M. Progress and Challenge of Cardiac Regeneration to Treat Heart Failure. J. Cardiol. 2019, 73, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Eng, G.; Lee, B.W.; Radisic, M.; Vunjak-Novakovic, G. Chapter 32—Cardiac Tissue Engineering. In Principles of Tissue Engineering, 5th ed.; Lanza, R., Langer, R., Vacanti, J.P., Atala, A., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 593–616. ISBN 978-0-12-818422-6. [Google Scholar]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021, Correction in J. Am. Coll. Cardiol. 2021, 77, 1958–1959. [Google Scholar] [CrossRef] [PubMed]
- Poss, K.D.; Wilson, L.G.; Keating, M.T. Heart Regeneration in Zebrafish. Science 2002, 298, 2188–2190. [Google Scholar] [CrossRef] [PubMed]
- Poss, K.D.; Nechiporuk, A.; Hillam, A.M.; Johnson, S.L.; Keating, M.T. Mps1 Defines a Proximal Blastemal Proliferative Compartment Essential for Zebrafish Fin Regeneration. Development 2002, 129, 5141–5149. [Google Scholar] [CrossRef] [PubMed]
- Bondue, A.; Lapouge, G.; Paulissen, C.; Semeraro, C.; Iacovino, M.; Kyba, M.; Blanpain, C. Mesp1 Acts as a Master Regulator of Multipotent Cardiovascular Progenitor Specification. Cell Stem Cell 2008, 3, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Siebel, C.; Lendahl, U. Notch Signaling in Development, Tissue Homeostasis, and Disease. Physiol. Rev. 2017, 97, 1235–1294. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wei, J.; Zhang, P.; Zhang, X.; Wang, Y.; Chen, W.; Zhao, Y.; Cui, X. Neuregulin-1, a Potential Therapeutic Target for Cardiac Repair. Front. Pharmacol. 2022, 13, 945206. [Google Scholar] [CrossRef]
- Fernández-Ruiz, I. Gene Therapy against Hippo Triggers Cardiomyocyte Renewal after MI. Nat. Rev. Cardiol. 2021, 18, 611. [Google Scholar] [CrossRef]
- Díaz del Moral, S.; Benaouicha, M.; Muñoz-Chápuli, R.; Carmona, R. The Insulin-like Growth Factor Signalling Pathway in Cardiac Development and Regeneration. Int. J. Mol. Sci. 2021, 23, 234. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/β-Catenin Signalling: Function, Biological Mechanisms, and Therapeutic Opportunities. Signal Transduct. Target. Ther. 2022, 7, 3. [Google Scholar] [CrossRef]
- Bass-Stringer, S.; Tai, C.M.K.; McMullen, J.R. IGF1–PI3K-Induced Physiological Cardiac Hypertrophy: Implications for New Heart Failure Therapies, Biomarkers, and Predicting Cardiotoxicity. J. Sport. Health Sci. 2021, 10, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Sala, V.; Gallo, S.; Leo, C.; Gatti, S.; Gelb, B.D.; Crepaldi, T. Signaling to Cardiac Hypertrophy: Insights from Human and Mouse RASopathies. Mol. Med. 2012, 18, 938–947. [Google Scholar] [CrossRef] [PubMed]
- Kinugawa, K.; Yonekura, K.; Ribeiro, R.C.J.; Eto, Y.; Aoyagi, T.; Baxter, J.D.; Camacho, S.A.; Bristow, M.R.; Long, C.S.; Simpson, P.C. Regulation of Thyroid Hormone Receptor Isoforms in Physiological and Pathological Cardiac Hypertrophy. Circ. Res. 2001, 89, 591–598. [Google Scholar] [CrossRef]
- Porrello, E.R.; Mahmoud, A.I.; Simpson, E.; Hill, J.A.; Richardson, J.A.; Olson, E.N.; Sadek, H.A. Transient Regenerative Potential of the Neonatal Mouse Heart. Science 2011, 331, 1078–1080. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, E.; Zhao, M.; Chong, Z.; Fan, C.; Tang, Y.; Hunter, J.D.; Borovjagin, A.V.; Walcott, G.P.; Chen, J.Y.; et al. Regenerative Potential of Neonatal Porcine Hearts. Circulation 2018, 138, 2809–2816. [Google Scholar] [CrossRef]
- Bergmann, O.; Bhardwaj, R.D.; Bernard, S.; Zdunek, S.; Barnabé-Heider, F.; Walsh, S.; Zupicich, J.; Alkass, K.; Buchholz, B.A.; Druid, H.; et al. Evidence for Cardiomyocyte Renewal in Humans. Science 2009, 324, 98–102. [Google Scholar] [CrossRef]
- Eschenhagen, T.; Bolli, R.; Braun, T.; Field, L.J.; Fleischmann, B.K.; Frisén, J.; Giacca, M.; Hare, J.M.; Houser, S.; Lee, R.T.; et al. Cardiomyocyte Regeneration. Circulation 2017, 136, 680–686. [Google Scholar] [CrossRef]
- Wang, W.E.; Li, L.; Xia, X.; Fu, W.; Liao, Q.; Lan, C.; Yang, D.; Chen, H.; Yue, R.; Zeng, C.; et al. Dedifferentiation, Proliferation, and Redifferentiation of Adult Mammalian Cardiomyocytes After Ischemic Injury. Circulation 2017, 136, 834–848. [Google Scholar] [CrossRef] [PubMed]
- Anversa, P.; Kajstura, J. Ventricular Myocytes Are Not Terminally Differentiated in the Adult Mammalian Heart. Circ. Res. 1998, 83, 1–14. [Google Scholar] [CrossRef]
- Hierlihy, A.M.; Seale, P.; Lobe, C.G.; Rudnicki, M.A.; Megeney, L.A. The Post-Natal Heart Contains a Myocardial Stem Cell Population. FEBS Lett. 2002, 530, 239–243. [Google Scholar] [CrossRef]
- Smith, A.J.; Lewis, F.C.; Aquila, I.; Waring, C.D.; Nocera, A.; Agosti, V.; Nadal-Ginard, B.; Torella, D.; Ellison, G.M. Isolation and Characterization of Resident Endogenous C-Kit+ Cardiac Stem Cells from the Adult Mouse and Rat Heart. Nat. Protoc. 2014, 9, 1662–1681. [Google Scholar] [CrossRef]
- Wu, J.M.F.; Hsueh, Y.-C.; Ch’ang, H.-J.; Luo, C.-Y.; Wu, L.-W.; Nakauchi, H.; Hsieh, P.C.H. Circulating Cells Contribute to Cardiomyocyte Regeneration After Injury. Circ. Res. 2015, 116, 633–641. [Google Scholar] [CrossRef]
- Garbern, J.C.; Lee, R.T. Heart Regeneration: 20 Years of Progress and Renewed Optimism. Dev. Cell 2022, 57, 424–439. [Google Scholar] [CrossRef]
- Yuh, D.D. Johns Hopkins Textbook of Cardiothoracic Surgery, 2nd ed.; McGraw-Hill Companies New York: New York, NY, USA, 2014; ISBN 978-0-07-166350-2. [Google Scholar]
- Johnston, P.V. Gary Gerstenblith Stem Cells for Cardiac Surgical Disease. In Johns Hopkins Textbook of Cardiothoracic Surgery; McGraw-Hill Companies New York: New York, NY, USA, 2014; p. 1443. ISBN 978-0-07-166350-2. [Google Scholar]
- Yin, X.; Yin, X.; Pan, X.; Zhang, J.; Fan, X.; Li, J.; Zhai, X.; Jiang, L.; Hao, P.; Wang, J.; et al. Post-Myocardial Infarction Fibrosis: Pathophysiology, Examination, and Intervention. Front. Pharmacol. 2023, 14, 1070973. [Google Scholar] [CrossRef] [PubMed]
- Rolland, L.; Jopling, C. The Multifaceted Nature of Endogenous Cardiac Regeneration. Front. Cardiovasc. Med. 2023, 10, 1138485. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Han, D.; Liang, P.; Li, Y.; Cao, F. The Current Dilemma and Breakthrough of Stem Cell Therapy in Ischemic Heart Disease. Front. Cell Dev. Biol. 2021, 9, 636136. [Google Scholar] [CrossRef]
- Abu-Dawud, R.; Graffmann, N.; Ferber, S.; Wruck, W.; Adjaye, J. Pluripotent Stem Cells: Induction and Self-Renewal. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20170213. [Google Scholar] [CrossRef] [PubMed]
- Singla, D.K.; Lyons, G.E.; Kamp, T.J. Transplanted Embryonic Stem Cells Following Mouse Myocardial Infarction Inhibit Apoptosis and Cardiac Remodeling. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H1308–H1314. [Google Scholar] [CrossRef]
- Min, J.-Y.; Yang, Y.; Sullivan, M.F.; Ke, Q.; Converso, K.L.; Chen, Y.; Morgan, J.P.; Xiao, Y.-F. Long-Term Improvement of Cardiac Function in Rats after Infarction by Transplantation of Embryonic Stem Cells. J. Thorac. Cardiovasc. Surg. 2003, 125, 361–369. [Google Scholar] [CrossRef]
- Help Therapeutics Epicardial Injection of Allogeneic Human Pluripotent Stem Cell-Derived Cardiomyocytes to Treat Severe Chronic Heart Failure. 2022. Available online: https://clinicaltrials.gov/study/NCT03763136#publications (accessed on 25 December 2023).
- Heartseed Inc. A Phase I/II Study of Human Induced Pluripotent Stem (iPS) Cell-Derived Cardiomyocyte Spheroids (HS-001) in Patients with Severe Heart Failure, Secondary to Ischemic Heart Disease. 2022. Available online: https://clinicaltrials.gov/study/NCT04945018 (accessed on 25 December 2023).
- Noda, T.; Nishigaki, K.; Minatoguchi, S. Safety and Efficacy of Human Muse Cell-Based Product for Acute Myocardial Infarction in a First-in-Human Trial. Circ. J. 2020, 84, 1189–1192. [Google Scholar] [CrossRef]
- Kannappan, R.; Turner, J.F.; Miller, J.M.; Fan, C.; Rushdi, A.G.; Rajasekaran, N.S.; Zhang, J. Functionally Competent DNA Damage-Free Induced Pluripotent Stem Cell–Derived Cardiomyocytes for Myocardial Repair. Circulation 2019, 140, 520–522. [Google Scholar] [CrossRef] [PubMed]
- Thavapalachandran, S.; Le, T.Y.L.; Romanazzo, S.; Rashid, F.N.; Ogawa, M.; Kilian, K.A.; Brown, P.; Pouliopoulos, J.; Barry, A.M.; Fahmy, P.; et al. Pluripotent Stem Cell-Derived Mesenchymal Stromal Cells Improve Cardiac Function and Vascularity after Myocardial Infarction. Cytotherapy 2021, 23, 1074–1084. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Wakao, S.; Kushida, Y.; Minatoguchi, S.; Mikami, A.; Higashi, K.; Baba, S.; Shigemoto, T.; Kuroda, Y.; Kanamori, H.; et al. S1P–S1PR2 Axis Mediates Homing of Muse Cells Into Damaged Heart for Long-Lasting Tissue Repair and Functional Recovery After Acute Myocardial Infarction. Circ. Res. 2018, 122, 1069–1083. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.J.H.; Yang, X.; Don, C.W.; Minami, E.; Liu, Y.-W.; Weyers, J.J.; Mahoney, W.M.; Van Biber, B.; Cook, S.M.; Palpant, N.J.; et al. Human Embryonic Stem Cell-Derived Cardiomyocytes Regenerate Non-Human Primate Hearts. Nature 2014, 510, 273–277. [Google Scholar] [CrossRef]
- Yamada, Y.; Minatoguchi, S.; Baba, S.; Shibata, S.; Takashima, S.; Wakao, S.; Okura, H.; Dezawa, M.; Minatoguchi, S. Human Muse Cells Reduce Myocardial Infarct Size and Improve Cardiac Function without Causing Arrythmias in a Swine Model of Acute Myocardial Infarction. PLoS ONE 2022, 17, e0265347. [Google Scholar] [CrossRef]
- Soma, Y.; Tani, H.; Morita-Umei, Y.; Kishino, Y.; Fukuda, K.; Tohyama, S. Pluripotent Stem Cell-Based Cardiac Regenerative Therapy for Heart Failure. J. Mol. Cell Cardiol. 2024, 187, 90–100. [Google Scholar] [CrossRef]
- Hatani, T.; Yoshida, Y. Transplantation of Human Induced Pluripotent Stem Cell-Derived CardiomyocytesHuman Induced Pluripotent Stem Cell-Derived Cardiomyocytes (iPSC-CMs) in a Mouse Myocardial InfarctionMyocardial Infarction Model. In Pluripotent Stem-Cell Derived Cardiomyocytes; Yoshida, Y., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2021; pp. 285–293. ISBN 978-1-07-161484-6. [Google Scholar]
- Kobayashi, H.; Ichimura, H.; Ohashi, N.; Shiba, Y. Transplantation of Pluripotent Stem Cell-Derived Cardiomyocytes into a Myocardial Infarction Model of Cynomolgus Monkey. Methods Mol. Biol. 2021, 2320, 295–302. [Google Scholar] [CrossRef]
- Higuchi, A.; Ku, N.-J.; Tseng, Y.-C.; Pan, C.-H.; Li, H.-F.; Kumar, S.S.; Ling, Q.-D.; Chang, Y.; Alarfaj, A.A.; Munusamy, M.A.; et al. Stem Cell Therapies for Myocardial Infarction in Clinical Trials: Bioengineering and Biomaterial Aspects. Lab. Investig. 2017, 97, 1167–1179. [Google Scholar] [CrossRef]
- Grimaldi, V.; Mancini, F.P.; Casamassimi, A.; Al-Omran, M.; Zullo, A.; Infante, T.; Napoli, C. Potential Benefits of Cell Therapy in Coronary Heart Disease. J. Cardiol. 2013, 62, 267–276. [Google Scholar] [CrossRef]
- Selvakumar, D.; Reyes, L.; Chong, J.J.H. Cardiac Cell Therapy with Pluripotent Stem Cell-Derived Cardiomyocytes: What Has Been Done and What Remains to Do? Curr. Cardiol. Rep. 2022, 24, 445–461. [Google Scholar] [CrossRef]
- Alanazi, R.F.; Alhwity, B.S.; Almahlawi, R.M.; Alatawi, B.D.; Albalawi, S.A.; Albalawi, R.A.; Albalawi, A.A.; Abdel-Maksoud, M.S.; Elsherbiny, N. Multilineage Differentiating Stress Enduring (Muse) Cells: A New Era of Stem Cell-Based Therapy. Cells 2023, 12, 1676. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, E.; Oguma, Y.; Kushida, Y.; Wakao, S.; Okawa, K.; Dezawa, M. Naïve Pluripotent-like Characteristics of Non-Tumorigenic Muse Cells Isolated from Human Amniotic Membrane. Sci. Rep. 2022, 12, 17222. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, M.Z.; Suszynska, M.; Borkowska, S.; Ratajczak, J.; Schneider, G. The Role of Sphingosine-1 Phosphate and Ceramide-1 Phosphate in Trafficking of Normal Stem Cells and Cancer Cells. Expert. Opin. Ther. Targets 2014, 18, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, Y.; Wakao, S.; Kitada, M.; Murakami, T.; Nojima, M.; Dezawa, M. Isolation, Culture and Evaluation of Multilineage-Differentiating Stress-Enduring (Muse) Cells. Nat. Protoc. 2013, 8, 1391–1415. [Google Scholar] [CrossRef] [PubMed]
- Gill, J.K.; Rehsia, S.K.; Verma, E.; Sareen, N.; Dhingra, S. Stem Cell Therapy for Cardiac Regeneration: Past, Present, and Future. Can. J. Physiol. Pharmacol. 2024, 102, 3. [Google Scholar] [CrossRef] [PubMed]
- Yanamandala, M.; Zhu, W.; Garry, D.J.; Kamp, T.J.; Hare, J.M.; Jun, H.; Yoon, Y.; Bursac, N.; Prabhu, S.D.; Dorn, G.W.; et al. Overcoming the Roadblocks to Cardiac Cell Therapy Using Tissue Engineering. J. Am. Coll. Cardiol. 2017, 70, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Bando, H.; Piazza, M.D.; Gowing, G.; Herberts, C.; Jackman, S.; Leoni, G.; Libertini, S.; MacLachlan, T.; McBlane, J.W.; et al. Tumorigenicity Assessment of Cell Therapy Products: The Need for Global Consensus and Points to Consider. Cytotherapy 2019, 21, 1095–1111. [Google Scholar] [CrossRef] [PubMed]
- Sobhani, A.; Khanlarkhani, N.; Baazm, M.; Mohammadzadeh, F.; Najafi, A.; Mehdinejadiani, S.; Sargolzaei Aval, F. Multipotent Stem Cell and Current Application. Acta Med. Iran 2017, 55, 6–23. [Google Scholar]
- Orlic, D.; Kajstura, J.; Chimenti, S.; Jakoniuk, I.; Anderson, S.M.; Li, B.; Pickel, J.; McKay, R.; Nadal-Ginard, B.; Bodine, D.M.; et al. Bone Marrow Cells Regenerate Infarcted Myocardium. Nature 2001, 410, 701–705. [Google Scholar] [CrossRef]
- Kamihata, H.; Matsubara, H.; Nishiue, T.; Fujiyama, S.; Tsutsumi, Y.; Ozono, R.; Masaki, H.; Mori, Y.; Iba, O.; Tateishi, E.; et al. Implantation of Bone Marrow Mononuclear Cells Into Ischemic Myocardium Enhances Collateral Perfusion and Regional Function via Side Supply of Angioblasts, Angiogenic Ligands, and Cytokines. Circulation 2001, 104, 1046–1052. [Google Scholar] [CrossRef]
- Bel, A.; Messas, E.; Agbulut, O.; Richard, P.; Samuel, J.-L.; Bruneval, P.; Hagège, A.A.; Menasché, P. Transplantation of Autologous Fresh Bone Marrow Into Infarcted Myocardium: A Word of Caution. Circulation 2003, 108, II-247–II-252. [Google Scholar] [CrossRef] [PubMed]
- de Silva, R.; Raval, A.N.; Hadi, M.; Gildea, K.M.; Bonifacino, A.C.; Yu, Z.-X.; Yau, Y.Y.; Leitman, S.F.; Bacharach, S.L.; Donahue, R.E.; et al. Intracoronary Infusion of Autologous Mononuclear Cells from Bone Marrow or Granulocyte Colony-Stimulating Factor-Mobilized Apheresis Product May Not Improve Remodelling, Contractile Function, Perfusion, or Infarct Size in a Swine Model of Large Myocardial Infarction. Eur. Heart J. 2008, 29, 1772–1782. [Google Scholar] [CrossRef] [PubMed]
- Bartunek, J.; Behfar, A.; Dolatabadi, D.; Vanderheyden, M.; Ostojic, M.; Dens, J.; El, N.B.; Banovic, M.; Beleslin, B.; Vrolix, M.; et al. Cardiopoietic Stem Cell Therapy in Heart Failure. J. Am. Coll. Cardiol. 2013, 61, 2329–2338. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.R.; Pei, X.T.; Ding, Q.A.; Chen, Y.; Zhang, N.K.; Chen, H.Y.; Wang, Z.G.; Wang, Y.F.; Zhu, Z.M.; Li, T.C.; et al. A Critical Challenge: Dosage-Related Efficacy and Acute Complication Intracoronary Injection of Autologous Bone Marrow Mesenchymal Stem Cells in Acute Myocardial Infarction. Int. J. Cardiol. 2013, 168, 3191–3199. [Google Scholar] [CrossRef] [PubMed]
- Traverse, J.H.; Henry, T.D.; Pepine, C.J.; Willerson, J.T.; Ellis, S.G. One-Year Follow-up of Intracoronary Stem Cell Delivery on Left Ventricular Function following ST-Elevation Myocardial Infarction. JAMA 2014, 311, 301–302. [Google Scholar] [CrossRef]
- Zhao, Q.; Sun, Y.; Xia, L.; Chen, A.; Wang, Z. Randomized Study of Mononuclear Bone Marrow Cell Transplantation in Patients with Coronary Surgery. Ann. Thorac. Surg. 2008, 86, 1833–1840. [Google Scholar] [CrossRef]
- Berry, M.F.; Engler, A.J.; Woo, Y.J.; Pirolli, T.J.; Bish, L.T.; Jayasankar, V.; Morine, K.J.; Gardner, T.J.; Discher, D.E.; Sweeney, H.L. Mesenchymal Stem Cell Injection after Myocardial Infarction Improves Myocardial Compliance. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H2196–H2203. [Google Scholar] [CrossRef]
- Gnecchi, M.; He, H.; Liang, O.D.; Melo, L.G.; Morello, F.; Mu, H.; Noiseux, N.; Zhang, L.; Pratt, R.E.; Ingwall, J.S.; et al. Paracrine Action Accounts for Marked Protection of Ischemic Heart by Akt-Modified Mesenchymal Stem Cells. Nat. Med. 2005, 11, 367–368. [Google Scholar] [CrossRef]
- Haider, H.K.; Jiang, S.; Idris, N.M.; Ashraf, M. IGF-1-Overexpressing Mesenchymal Stem Cells Accelerate Bone Marrow Stem Cell Mobilization via Paracrine Activation of SDF-1alpha/CXCR4 Signaling to Promote Myocardial Repair. Circ. Res. 2008, 103, 1300–1308. [Google Scholar] [CrossRef]
- Houtgraaf, J.H.; den Dekker, W.K.; van Dalen, B.M.; Springeling, T.; de Jong, R.; van Geuns, R.J.; Geleijnse, M.L.; Fernandez-Aviles, F.; Zijlsta, F.; Serruys, P.W.; et al. First Experience in Humans Using Adipose Tissue–Derived Regenerative Cells in the Treatment of Patients with ST-Segment Elevation Myocardial Infarction. J. Am. Coll. Cardiol. 2012, 59, 539–540. [Google Scholar] [CrossRef]
- Gao, L.R.; Chen, Y.; Zhang, N.K.; Yang, X.L.; Liu, H.L.; Wang, Z.G.; Yan, X.Y.; Wang, Y.; Zhu, Z.M.; Li, T.C.; et al. Intracoronary Infusion of Wharton’s Jelly-Derived Mesenchymal Stem Cells in Acute Myocardial Infarction: Double-Blind, Randomized Controlled Trial. BMC Med. 2015, 13, 162. [Google Scholar] [CrossRef]
- The SCIENCE Investigators. Effect of Allogeneic Adipose Tissue-Derived Mesenchymal Stromal Cell Treatment in Chronic Ischaemic Heart Failure with Reduced Ejection Fraction—The Science Trial. Eur. J. Heart Fail. 2023, 25, 576–587. [Google Scholar] [CrossRef]
- Bolli, R.; Tang, X.-L.; Sanganalmath, S.K.; Rimoldi, O.; Mosna, F.; Abdel-Latif, A.; Jneid, H.; Rota, M.; Leri, A.; Kajstura, J. Intracoronary Delivery of Autologous Cardiac Stem Cells Improves Cardiac Function in a Porcine Model of Chronic Ischemic Cardiomyopathy. Circulation 2013, 128, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Makkar, R.R.; Smith, R.R.; Cheng, K.; Malliaras, K.; Thomson, L.E.; Berman, D.; Czer, L.S.; Marbán, L.; Mendizabal, A.; Johnston, P.V.; et al. Intracoronary Cardiosphere-Derived Cells for Heart Regeneration after Myocardial Infarction (CADUCEUS): A Prospective, Randomised Phase 1 Trial. Lancet 2012, 379, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Medscape Medical News: Harvard, Brigham Call for Retraction of 31 Papers by Disgraced Cardiac Stem Cell Doc. Available online: https://www.medscape.com/viewarticle/903475 (accessed on 26 February 2024).
- The BMJ News: NEJM Retracts Article from Former Researcher Once Hailed as Heart Stem Cell Pioneer. Available online: https://www.bmj.com/content/363/bmj.k4432 (accessed on 26 February 2024).
- Afzal, M.R.; Samanta, A.; Shah, Z.I.; Jeevanantham, V.; Abdel-Latif, A.; Zuba-Surma, E.K.; Dawn, B. Adult Bone Marrow Cell Therapy for Ischemic Heart Disease. Circ. Res. 2015, 117, 558–575. [Google Scholar] [CrossRef] [PubMed]
- Ali-Hasan-Al-Saegh, S.; Mirhosseini, S.J.; Lotfaliani, M.-R.; Dehghan, H.R.; Sedaghat-Hamedani, F.; Kayvanpour, E.; Rezaeisadrabadi, M.; Ghaffari, N.; Vahabzadeh, V.; Jebran, A.F.; et al. Transplantation of Bone Marrow Stem Cells during Cardiac Surgery. Asian Cardiovasc. Thorac. Ann. 2015, 23, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Ala, M. The Beneficial Effects of Mesenchymal Stem Cells and Their Exosomes on Myocardial Infarction and Critical Considerations for Enhancing Their Efficacy. Aging Res. Rev. 2023, 89, 101980. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Minatoguchi, S.; Kanamori, H.; Mikami, A.; Okura, H.; Dezawa, M.; Minatoguchi, S. Stem Cell Therapy for Acute Myocardial Infarction—Focusing on the Comparison between Muse Cells and Mesenchymal Stem Cells. J. Cardiol. 2022, 80, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Kern, S.; Eichler, H.; Stoeve, J.; Klüter, H.; Bieback, K. Comparative Analysis of Mesenchymal Stem Cells from Bone Marrow, Umbilical Cord Blood, or Adipose Tissue. Stem Cells 2006, 24, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, L.M.; Eisenberg, C.A. Adult Stem Cells and Their Cardiac Potential. Anat. Rec. Part. A Discov. Mol. Cell. Evol. Biol. 2004, 276A, 103–112. [Google Scholar] [CrossRef]
- Mehanna, R.A.; Essawy, M.M.; Barkat, M.A.; Awaad, A.K.; Thabet, E.H.; Hamed, H.A.; Elkafrawy, H.; Khalil, N.A.; Sallam, A.; Kholief, M.A.; et al. Cardiac Stem Cells: Current Knowledge and Future Prospects. World J. Stem Cells 2022, 14, 1–40. [Google Scholar] [CrossRef]
- Tompkins, B.A.; Balkan, W.; Winkler, J.; Gyöngyösi, M.; Goliasch, G.; Fernández-Avilés, F.; Hare, J.M. Preclinical Studies of Stem Cell Therapy for Heart Disease. Circ. Res. 2018, 122, 1006–1020. [Google Scholar] [CrossRef]
- Mahmud, S.; Alam, S.; Emon, N.U.; Boby, U.H.; Kamruzzaman; Ahmed, F.; Monjur-Al-Hossain, A.S.M.; Tahamina, A.; Rudra, S.; Ajrin, M. Opportunities and Challenges in Stem Cell Therapy in Cardiovascular Diseases: Position Standing in 2022. Saudi Pharm. J. 2022, 30, 1360–1371. [Google Scholar] [CrossRef]
- Expression of Concern: The SCIPIO Trial. Lancet 2014, 383, 1279. [CrossRef]
- Xiao, W.; Shi, J. Application of Adipose-Derived Stem Cells in Ischemic Heart Disease: Theory, Potency, and Advantage. Front. Cardiovasc. Med. 2024, 11, 1324447. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; An, M.; Haubner, B.J.; Penninger, J.M. Cardiac Regeneration: Options for Repairing the Injured Heart. Front. Cardiovasc. Med. 2023, 9, 981982. [Google Scholar] [CrossRef]
- Guo, Q.-Y.; Yang, J.-Q.; Feng, X.-X.; Zhou, Y.-J. Regeneration of the Heart: From Molecular Mechanisms to Clinical Therapeutics. Mil. Med. Res. 2023, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Huang, Y.; Singh, R.; Wang, Z.Z. Arrhythmogenic Risks of Stem Cell Replacement Therapy for Cardiovascular Diseases. J. Cell Physiol. 2020, 235, 6257–6267. [Google Scholar] [CrossRef] [PubMed]
- Carbone, R.G.; Negrini, S.; Murdaca, G.; Fontana, V.; Puppo, F. Stem Cells Treatment in Chronic Ischemic Heart Disease: A Narrative Review. Am. J. Stem Cells 2023, 12, 65–72. [Google Scholar] [PubMed]
- Harland, N.; Knoll, J.; Amend, B.; Abruzzese, T.; Abele, H.; Jakubowski, P.; Stenzl, A.; Aicher, W.K. Xenogenic Application of Human Placenta-Derived Mesenchymal Stromal Cells in a Porcine Large Animal Model. Cell Transpl. 2024, 33, 9636897241226737. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-W.; Chen, B.; Yang, X.; Fugate, J.A.; Kalucki, F.A.; Futakuchi-Tsuchida, A.; Couture, L.; Vogel, K.W.; Astley, C.A.; Baldessari, A.; et al. Human Embryonic Stem Cell–Derived Cardiomyocytes Restore Function in Infarcted Hearts of Non-Human Primates. Nat. Biotechnol. 2018, 36, 597–605, Erratum in Nat. Biotechnol. 2018, 36, 899. [Google Scholar] [CrossRef] [PubMed]
- Askar, S.F.A.; Ramkisoensing, A.A.; Atsma, D.E.; Schalij, M.J.; de Vries, A.A.F.; Pijnappels, D.A. Engraftment Patterns of Human Adult Mesenchymal Stem Cells Expose Electrotonic and Paracrine Proarrhythmic Mechanisms in Myocardial Cell Cultures. Circ. Arrhythmia Electrophysiol. 2013, 6, 380–391. [Google Scholar] [CrossRef][Green Version]
- Chandrasekhar, S.K.; Thankam, F.G.; Ouseph, J.C.; Agrawal, D.K. Myocardial Tissue Engineering: Fundamentals and Future. In Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2022; pp. 33–51. ISBN 978-0-12-824064-9. [Google Scholar]
- Qasim, M.; Arunkumar, P.; Powell, H.M.; Khan, M. Current Research Trends and Challenges in Tissue Engineering for Mending Broken Hearts. Life Sci. 2019, 229, 233–250. [Google Scholar] [CrossRef]
- Lou, X.; Tang, Y.; Ye, L.; Pretorius, D.; Fast, V.G.; Kahn-Krell, A.M.; Zhang, J.; Zhang, J.; Qiao, A.; Qin, G.; et al. Cardiac Muscle Patches Containing Four Types of Cardiac Cells Derived from Human Pluripotent Stem Cells Improve Recovery from Cardiac Injury in Mice. Cardiovasc. Res. 2023, 119, 1062–1076. [Google Scholar] [CrossRef] [PubMed]
- Masumoto, H.; Ikuno, T.; Takeda, M.; Fukushima, H.; Marui, A.; Katayama, S.; Shimizu, T.; Ikeda, T.; Okano, T.; Sakata, R.; et al. Human iPS Cell-Engineered Cardiac Tissue Sheets with Cardiomyocytes and Vascular Cells for Cardiac Regeneration. Sci. Rep. 2014, 4, 6716. [Google Scholar] [CrossRef] [PubMed]
- Ishigami, M.; Masumoto, H.; Ikuno, T.; Aoki, T.; Kawatou, M.; Minakata, K.; Ikeda, T.; Sakata, R.; Yamashita, J.K.; Minatoya, K. Human iPS Cell-Derived Cardiac Tissue Sheets for Functional Restoration of Infarcted Porcine Hearts. PLoS ONE 2018, 13, e0201650. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, J.; Zhang, P.; Xiong, Q.; Wu, S.C.; Xia, L.; Roy, S.S.; Tolar, J.; O’Connell, T.D.; Kyba, M.; et al. Derivation and High Engraftment of Patient-Specific Cardiomyocyte Sheet Using Induced Pluripotent Stem Cells Generated from Adult Cardiac Fibroblast. Circ. Heart Fail. 2015, 8, 156–166. [Google Scholar] [CrossRef]
- Augustine, R.; Dan, P.; Hasan, A.; Khalaf, I.M.; Prasad, P.; Ghosal, K.; Gentile, C.; McClements, L.; Maureira, P. Stem Cell-Based Approaches in Cardiac Tissue Engineering: Controlling the Microenvironment for Autologous Cells. Biomed. Pharmacother. 2021, 138, 111425. [Google Scholar] [CrossRef]
- Gao, L.; Gregorich, Z.R.; Zhu, W.; Mattapally, S.; Oduk, Y.; Lou, X.; Kannappan, R.; Borovjagin, A.V.; Walcott, G.P.; Pollard, A.E.; et al. Large Cardiac Muscle Patches Engineered from Human Induced-Pluripotent Stem Cell–Derived Cardiac Cells Improve Recovery from Myocardial Infarction in Swine. Circulation 2018, 137, 1712–1730. [Google Scholar] [CrossRef]
- Xiang, Z.; Liao, R.; Kelly, M.S.; Spector, M. Collagen–GAG Scaffolds Grafted onto Myocardial Infarcts in a Rat Model: A Delivery Vehicle for Mesenchymal Stem Cells. Tissue Eng. 2006, 12, 2467–2478. [Google Scholar] [CrossRef]
- Simpson, D.; Liu, H.; Fan, T.-H.M.; Nerem, R.; Dudley, S.C., Jr. A Tissue Engineering Approach to Progenitor Cell Delivery Results in Significant Cell Engraftment and Improved Myocardial Remodeling. Stem Cells 2007, 25, 2350–2357. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Jeong, S.I.; Shin, Y.M.; Lim, K.S.; Shin, H.S.; Lee, Y.M.; Koh, H.C.; Kim, K.-S. Transplantation of Mesenchymal Stem Cells within a Poly(Lactide-Co-ε-Caprolactone) Scaffold Improves Cardiac Function in a Rat Myocardial Infarction Model. Eur. J. Heart Fail. 2009, 11, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Miyahara, Y.; Nagaya, N.; Kataoka, M.; Yanagawa, B.; Tanaka, K.; Hao, H.; Ishino, K.; Ishida, H.; Shimizu, T.; Kangawa, K.; et al. Monolayered Mesenchymal Stem Cells Repair Scarred Myocardium after Myocardial Infarction. Nat. Med. 2006, 12, 459–465. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.; Qu, X.; Liu, Y.; Harada, A.; Hua, Y.; Yoshida, N.; Ishida, M.; Tabata, A.; Sun, L.; et al. Development of a Thick and Functional Human Adipose-Derived Stem Cell Tissue Sheet for Myocardial Infarction Repair in Rat Hearts. Stem Cell Res. Ther. 2023, 14, 380. [Google Scholar] [CrossRef]
- Huang, C.-C.; Tsai, H.-W.; Lee, W.-Y.; Lin, W.-W.; Chen, D.-Y.; Hung, Y.-W.; Chen, J.-W.; Hwang, S.-M.; Chang, Y.; Sung, H.-W. A Translational Approach in Using Cell Sheet Fragments of Autologous Bone Marrow-Derived Mesenchymal Stem Cells for Cellular Cardiomyoplasty in a Porcine Model. Biomaterials 2013, 34, 4582–4591. [Google Scholar] [CrossRef]
- Tang, J.; Cui, X.; Caranasos, T.G.; Hensley, M.T.; Vandergriff, A.C.; Hartanto, Y.; Shen, D.; Zhang, H.; Zhang, J.; Cheng, K. Heart Repair Using Nanogel-Encapsulated Human Cardiac Stem Cells in Mice and Pigs with Myocardial Infarction. ACS Nano 2017, 11, 9738–9749. [Google Scholar] [CrossRef]
- Mei, X.; Cheng, K. Recent Development in Therapeutic Cardiac Patches. Front. Cardiovasc. Med. 2020, 7, 610364. [Google Scholar] [CrossRef] [PubMed]
| Process/ Mechanism | Key Mediators | Comments/Observations |
|---|---|---|
| Atherosclerosis | oxLDL, macrophages, VSMCs | Endothelial dysfunction, intimal lipoprotein accumulation, foam cells PLT adhesion, release of TGF-β, FGF VSMC proliferation, ECM deposition (collagen) and fibrous plaque formation. Inflammatory cell infiltrate, extracellular lipid core necrosis and fibromuscular cap composition all influence plaque stability. |
| Inflammation | oxLDL, macrophages | Endothelial oxLDL adhesion through LOX-1, expression of NF-kB and endothelial adhesion molecules MI: immunogenic CM fragments, upregulation of NF-kB, production of pro-inflammatory cytokines (TNF-α, IL-1β, IL-6, IL-18). |
| Coronary vessel reactivity | ROCK, PKC | Increased MLC sensitivity to Ca2+; direct effect of ROCK on MLC, indirectly effect through ROCK, PKC-mediated, MLCPh inhibition. |
| Microvascular dysfunction | AGE, ROS, RNS, DAG-PKC | Hyperglycemia: AGEs, ROS impair antioxidant system function and decrease NOS bioavailability (endothelial dysfunction). Intercellular junction disruption through DAG-PKC signaling. Aging: Increased ROS, RNS production |
| Study | Model | Method | Comments |
|---|---|---|---|
| Singla et al., 2007 [87] | Mouse | Intramyocardial injection | mESCs administered post-MI (LCA ligation); results in the parameters studied: decreased CM apoptosis (TUNEL staining), decreased fibrosis, reduced CM hypertrophy, possibly due to paracrine secretion of factors such as cystatin C, osteopontin, clusterin, TIMP-1. Higher proportion of viable myocardium in the peri-infarct area, in subjects receiving the mESCs. |
| Kannappan et al., 2019 [92] | Mouse | Intramyocardial injection | iPSC-CM administered post-MI; Nutlin-3a (MDM2 inhibitor) to activate p53, inducing apoptosis in populations, to select for DNA-damage-free iPSCs, before CM derivation. Increased engraftment rate for DNA-damage-free CMs, compared to control CMs. |
| Min et al., 2003 [88] | Rat | Intramyocardial injection | mESCs administered 20 min post-MI (LCA ligation); mESC populations were selected for cardiomyogenic potential and transfected with GFP to identify engrafted cells. Reduced infarct size, reduced LV mass and LV mass/body weight ratio, mESC engraftment, increased angiogenesis observed through increased capillary density (up to 32 weeks later). |
| Thavapalachandran et al., 2021 [93] | Rat | Intramyocardial injection | iPSC-MSC administered post-MI; improved LV function, enhanced angiogenesis, no effect on infarct size, no continuous engraftment. No observable arrhythmias (absence of locally generated re-entrant circuits normally produced by sustained iPSC-MSC engraftment causing scar heterogeneity). |
| Yamada et al., 2018 [94] | Rabbit | Intravenous infusion | Muse cells administered post-MI (ischemia-reperfusion model); results include reduced infarct size, increased ejection fraction, reduced left ventricle geometric values, with engrafted Muse cells identified in the target tissues (up to 6 months later). Muse migration towards target tissues through S1P-S1PR signaling. |
| Chong et al., 2014 [95] | Pigtail macaque | Intramyocardial injection | hESCs-CMs administered post-MI (percutaneous ischemia-reperfusion injury model); reduced infarct size, remuscularization, appropriate revascularization in the infarcted area, no statistically significant effect on LV function. Arrhythmias observed in subjects receiving hESC-CMs; PVC, wide QRS rhythms, ventricular tachycardia; this is contrast to previously similar experiments in small animal models. |
| Yamada et al., 2022 [96] | Swine | Intravenous infusion | Muse cells administered post-MI (ischemia-reperfusion model); results include reduced infarct size, increased ejection fraction, reduced left ventricular geometric values. No arrhythmias were observed during the study. |
| Help Therapeutics, 2022 [89] | Phase I/II clinical trial | Epicardial injection | HEAL-CHF trial; hPSC-CMs administered at the time of CABG. Parameters measured include sustained ventricular arrhythmias, tumorigenesis, infarct size, ventricular wall thickness, MACE (death, non-lethal MI, hospitalization due to the worsening of heart failure) No results posted yet on clinicaltrials.gov (accessed on 25 December 2023) |
| Heartseed Inc., 2022 [90] | Phase I/II clinical trial | Injection | LAPiS trial; iPSC-CM administered in patients with heart failure, secondary to ischemic heart disease; parameters measured include safety/tolerability, LV ejection fraction, index of myocardial strain, myocardial blood flow and viability, among others. No results posted yet on clinicaltrials.gov (accessed on 25 December 2023) |
| Noda et al., 2020 [91] | Clinical trial | Intravenous infusion | Muse cells administered post-STEMI, after PCI; only 3 patients were enrolled in the study. Resulting parameters include reduced infarct size, improved LV function and remodeling. No fatal arrhythmias reported for the duration of 12 weeks after therapy administration. |
| Study | Cell Type | Model | Constructs | Comments |
|---|---|---|---|---|
| Masumoto et al., 2014 [150] | hiPSC | Rat | hiPSC-CTS cell sheet | hiPSC used to induce cardiac and vascular tissue cells simultaneously (CMs, ECs, vascular MCs) through protocols utilizing Dkk1, VEGF and an intermediate mesodermal linage stage. A three-layered cell sheet was transplanted post-MI (subacute model); ventricular wall contraction was improved, along with other cardiac function parameters, post-MI myocardial fibrosis was reduced. Cell engraftment was also successful, with relevant area of engraftment up to 44%; there were also clusters of ECs around CMs, alluding to the possibility for local angiogenesis, although a vascular structure of graft origin could not be verified. |
| Ishigami et al., 2018 [151] | hiPSC | Swine | L-CTS sheet | Cardiac tissue populations (CMs, ECs, vascular MCs) were induced from human iPSC populations; cell sheets of clinical-grade size (L-CTS) were created. L-CTS were transplanted post-MI (LAD ligation); results showed improved systolic function and LV ejection fraction, decreased local fibrosis, improved capillary density. |
| Zhang et al., 2015 [152] | hciPSC | Mouse | CM sheet | hciPSCs were generated from human left atrial appendage tissue and differentiated into CMs; CM cell sheets were created with the Matrigel sandwich method. CM sheets were implanted post-MI (LAD ligation); results showed improvements in cardiac function, increased local vascularity, and reduced native CM apoptosis. |
| Lou et al., 2023 [149] | hESC, hiPSC | Mouse | hCMP | hPSCs were used to derive hPSC-CMs, hPSC-SMCs, hPSC-AECs, hPSC-CFs, which were then suspended in fibrinogen and thrombin within an appropriately shaped mold, to create hCMPs. Inclusion of cardiac fibroblasts aided in CM maturation (sarcomere structure and organization, CM potentials), within hCMPs, as well as hCMP engraftment. hCMPs were implanted post-MI (LAD ligation); results showed improved LV ejection fraction, decreased infarct size. |
| Gao et al., 2018 [154] | hiPSC | Swine | hCMP | hiPSCs were used to generate CM, EC, SMC, which were then suspended within fibrin on an appropriate scaffold, to generate hCMPs. In this study, hCMPs constructed were large and thick enough to test a clinically relevant product. hCMPs were implanted post-MI (LAD ligation); the study showed improved LV function, reduced infarct size, a decrease in associated myocardial hypertrophy as well as reduced apoptosis of native CMs. |
| Study | Cell Type | Model | Constructs | Comments |
|---|---|---|---|---|
| Xiang et al., 2006 [155] | MSC | Rat | Collagen-GAG scaffold | Construct administered post-MI (LAD ischemia reperfusion); the study evaluated effect of ECM cross-linking methods in contrast efficacy. Only constructs generated through DHT crosslinking and subsequent carbodiimide treatment seemed to survive engraftment, although MSCs within both scaffold types seemed to survive. Results showed improved angiogenesis in both scaffold groups. |
| Simpson et al., 2007 [156] | hMSCs | Rat | Cardiac patch | Patches composed of a rat tail type I collagen scaffold were administered post-MI (LAD ligation); although successful MSC engraftment was reported, there were no detectable hMSC populations after 4 weeks, despite persistence of improvements. The study reported improvements in cardiac geometric parameters and hemodynamic measurements; mechanism for these effects was thought to be increased local myofibroblasts (a-SMA+), both patch-derived as well as due to local recruitment. |
| Jin et al., 2009 [157] | MSC | Rat | PLCL | Constructs were administered post-MI (cryoinjury method); evidence of myogenesis was observed in injured tissue after implantation, denoted through an increase in cardiac markers (MHC, a-actin, Troponin I), as well as through detection of labeled MSCs expressing a-actin, Troponin I post-implantation (higher in the groups that received MSCs via scaffold) Results showed decreased area of infarct, improved cardiac function and LV ejection fraction. |
| Miyahara et al., 2006 [158] | AD-MSC | Rat | Monolayered MSC sheet | Cell sheets implanted post-MI (coronary ligation); cell sheets appeared thickened post-implantation, with evidence of cardiomyocyte differentiation, and angiogenesis. The study reported improved cardiac function, and reversal of myocardial wall thinning after MI. |
| Zhang et al., 2023 [159] | AD-MSC | Rat | PLGA scaffold | Construct administered post-MI (LAD ligation); histological evaluation including fibrosis, angiogenesis, and cardiac remodeling, along with echocardiography. Reduced cardiac fibrosis, cardiac hypertrophy observed post-injury, increased new blood vessel formation in the infarct border zone, functional improvement. |
| Huang et al., 2013 [160] | MSC | Swine | MSC cell sheet fragments | Cell sheet fragments generated through thermo-responsive methylcellulose hydrogel system; lack of proteolytic enzymes during generation to prevent dissociation of MSCs from their surrounding ECM. MSCs used in the study were autologous. Mechanism of action was thought to be MSC differentiation into relevant cell types (endothelial cells, smooth muscle cells), lack of MSC dissociation from their associated ECM. Cell sheet fragments implanted post-MI; results showed improved cardiac function, and infarct size. The study reported arrhythmias associated with MSC implantation. |
| Tang et al., 2017 [161] | hCSC | Mouse, Swine | Thermosensitive nanogel | hCSC encapsulation within a nanogel composed of P(NIPAM-AA), which contained hydrophilic moieties, to facilitate hCSC survival and growth; implantation post-MI (LAD ligation), two types of animal models. Results of the study included improved local cardiomyocyte survival, enhanced local angiogenesis, reduced myocardial fibrosis, and improved cardiac function. Mechanism of action was thought to be use of an appropriate hydrogel for cell delivery and survival, and hCSC-mediated secretion of VEGF, IGF-1, SDF-1, facilitating local stem cell recruitment, local cardiomyocyte survival and local angiogenesis. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stougiannou, T.M.; Christodoulou, K.C.; Dimarakis, I.; Mikroulis, D.; Karangelis, D. To Repair a Broken Heart: Stem Cells in Ischemic Heart Disease. Curr. Issues Mol. Biol. 2024, 46, 2181-2208. https://doi.org/10.3390/cimb46030141
Stougiannou TM, Christodoulou KC, Dimarakis I, Mikroulis D, Karangelis D. To Repair a Broken Heart: Stem Cells in Ischemic Heart Disease. Current Issues in Molecular Biology. 2024; 46(3):2181-2208. https://doi.org/10.3390/cimb46030141
Chicago/Turabian StyleStougiannou, Theodora M., Konstantinos C. Christodoulou, Ioannis Dimarakis, Dimitrios Mikroulis, and Dimos Karangelis. 2024. "To Repair a Broken Heart: Stem Cells in Ischemic Heart Disease" Current Issues in Molecular Biology 46, no. 3: 2181-2208. https://doi.org/10.3390/cimb46030141
APA StyleStougiannou, T. M., Christodoulou, K. C., Dimarakis, I., Mikroulis, D., & Karangelis, D. (2024). To Repair a Broken Heart: Stem Cells in Ischemic Heart Disease. Current Issues in Molecular Biology, 46(3), 2181-2208. https://doi.org/10.3390/cimb46030141







