Abstract
Morning-time heart attacks are associated with an ablation in the sleep-time dip in blood pressure, the mechanism of which is unknown. The epigenetic changes are the hallmark of sleep and circadian clock disruption and homocystinuria (HHcy). The homocystinuria causes ablation in the dip in blood pressure during sleep. Interestingly, HHcy is generated during the epigenetic gene turning off and turning on (i.e., imprinting) by methylation of the DNA promoter. The mitochondrial sulfur metabolism by 3-mercaptopyruvate sulfur transferase (3MST), ATP citrate lyase (ACYL), and epigenetic rhythmic methylation are regulated by folate 1-carbon metabolism (FOCM), i.e., the methionine (M)-SAM-SAH-Hcy, adenosine, and uric acid cycle. Epigenetic gene writer (DNMT), gene eraser (TET/FTO), and editor de-aminase (ADAR) regulate the rhythmic, i.e., reversible methylation/demethylation of H3K4, H3K9, H4K20, m6A, and m5C. The mitochondrial ATP citrate cycle and creatine kinase (CK) regulate chromatin transcription, maturation, and accessibility as well as muscle function. The transcription is regulated by methylation. The maturation and accessibility are controlled by acetylation. However, it is unclear whether a high fat dysbiotic diet (HFD) causes dysrhythmic expression of the gene writer, eraser, and editor, creating hyperuricemia and cardiac and renal dysfunction. We hypothesized that an HFD increases the gene writer (DNMT1) and editor (ADAR), decreases the eraser (TET/FTO), and increases uric acid to cause chronic diseases. This increases the levels of H3K4, H3K9, H4K20, m6A, and m5C. Interestingly, the DNMT1KO mitigates. Further, the DNMT1KO and ADAR inhibition attenuate HFD-induced NGAL/FGF23/TMPRSS2/MMP2, 9, 13, and uric acid levels and improve cardiac and renal remodeling. Although the novel role of nerve endings by the Piezo channels (i.e., the combination of ENaC, VDAC, TRPV, K+, and Mg2+ channels) in the interoception is suggested, interestingly, we and others have shown mechanisms independent of the nerve, by interoception, such as the cargo of the exosome in denervation models of heart failure. If proper and appropriate levels of these enzymes are available to covert homocysteine to hydrogen sulfide (H2S) during homocystinuria, then the H2S can potentially serve as a newer form of treatment for morning heart attacks and renal sulfur transsulfuration transport diseases.
1. Introduction
Combat soldiers, shift workers, and anxiety/depression patients face the formidable consequences of sleep deprivation and cardiovascular and renal diseases. The role of disruption in sleep and the circadian clock in chronic diseases is suggested [1,2,3,4,5,6,7,8,9,10]; however, the mechanism(s) are far from being understood. We discuss a paradigm shift mechanistic pathway that causes the circadian clock-related diseases through the activation of the superior cervical ganglion (SCG) and the excitatory neurotransmitter receptor NMDA-R1 by disrupted epigenetic folate 1-carbon metabolism (FOCM) and increased homocysteine (Hcy, i.e., homocystinuria, HHcy) [11,12,13,14]. In addition, although the inhibition of ATP citrate lyase (ACLYi) attenuates hyperlipidemia [15,16], and ACLY acetylates the histones by epigenetic mechanisms [17], their role in heart failure and the epigenetic control of homocystinuria is unknown. Here we suggest that ACYL inhibition mitigates heart failure and renal homocystinuria by FOCM.
2. Discussion
Studies have demonstrated the overactivation of the sympathetic superior cervical ganglion (SCG) by homocystinuria [18] and the disruption of the sleep–wake pattern [19,20,21]. Interestingly, sympathetic denervation has been identified as an underlying cause of the activation of the excitatory neurotransmitter receptor (NMDA-R1) in heart disease. The cardiac neuronal, interstitial, and perivascular fibrosis and ECM remodeling are the reasons for overactivation of the superior cervical ganglia and cardiac hypertrophy during clock ablation-induced heart failure [22]. The severe damage to the endocardial and coronary endothelia by chronic stresses causes overactivation of the cardiac excitatory neurotransmitter (NMDA-R1) and suppression of the cardiac inhibitory neurotransmitter (GABA-R1), suggesting dysregulation of sympathetic and parasympathetic control of the heart, i.e., paradoxical contraction to acetylcholine [23,24]. Interestingly, the association of HFpEF with extra-cardiac features [25], including disorders of the central and peripheral clock system, has been suggested [26,27]. The mechanisms are unclear. The denervation of the heart mitigated the pacing-induced heart failure [28], suggesting a role of sympathetic activation contributing to heart failure [29]. Interestingly, studies have also suggested a relationship between NMDAergic (sympathetic) and GABAergic (parasympathetic) to BMAL1 and Period (Per 2) [30,31,32,33,34], contributing to chronic neuronal overactivation and neuro-inflammation during clock ablation-induced heart failure.
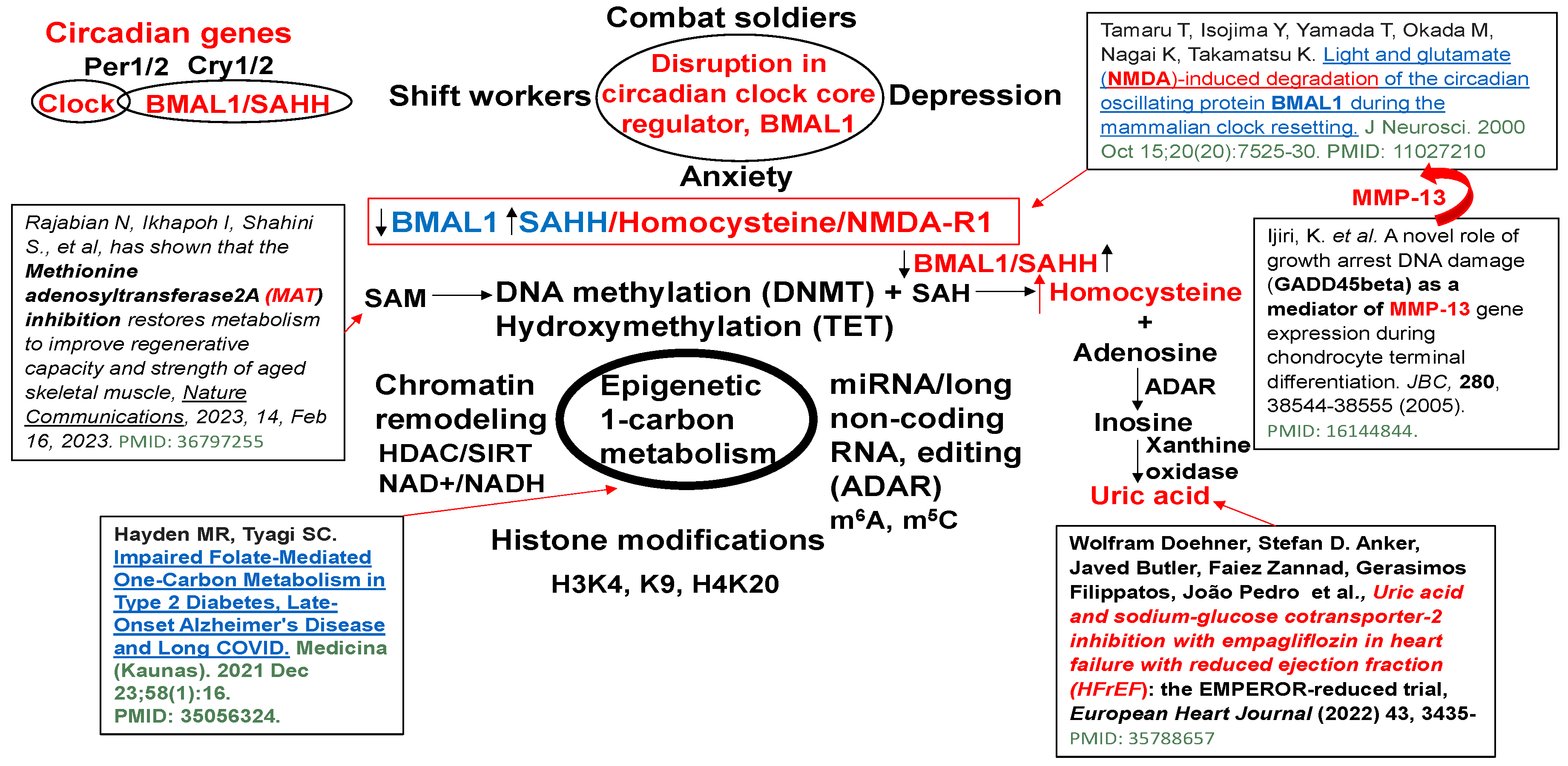
S-adenosyl homocysteine hydrolase (SAHH) controls the circadian clock gene transcription by interacting with the core clock regulator BMAL1 (Figure 1). The BMAL1–SAHH association occurs at the chromatin, promoting rhythmic H3K4 trimethylation (H3K4me3) and cyclic BMAL1 recruitment of target genes [35]. The basic mechanisms of regulation of circadian genes by the CLOCK-BMAL1 nuclear transcription factor, as well as the downstream Period (Per) and cryptochrome (Cry) genes and clock-controlled genes (CCGs), are important [36]; however, the mechanism is unclear. The trimethylates histone H3K4, on the nucleosomes near the circadian gene, promotes rhythmic methylation and generates homocysteine (Hcy, i.e., homocystinuria, HHcy). The HHcy is a product of the epigenetic folate 1-carbon metabolism (FOCM) cycle, unequivocally (Figure 2). Interestingly, the abrogation in the sleep-time dip in blood pressure is one of the causes of morning heart attacks and homocystinuria. The homocystinuria abrogates the sleep-time dip in blood pressure [19,20,21].
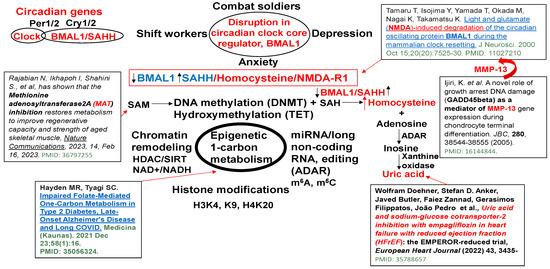
Figure 1.
Dysregulation in epigenetic 1-carbon metabolism and Hcy generation causes the disruption of the circadian clock gene core regulator, BMAL1, associated with SAHH/Hcy generation, activating NMDA-R1, and causing anxiety/depression and sleep disturbance [31,37,38,39,40].
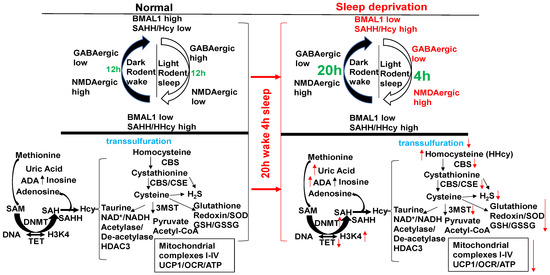
Figure 2.
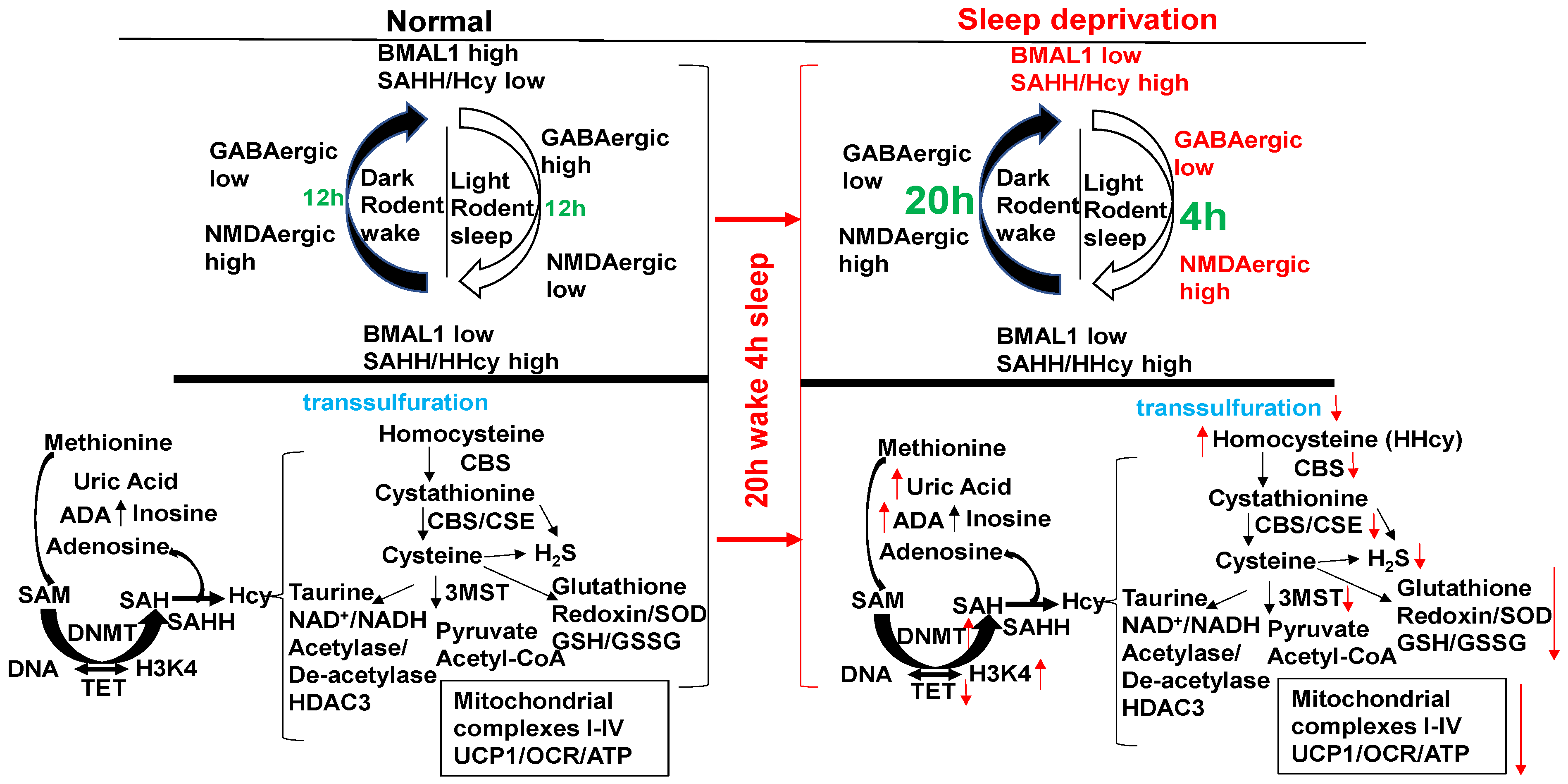
The circadian clock disruption in the sleep/wake cycle (20 h wake/4 h sleep) via BMAL1/SAHH induces epigenetic gene writer (DNMT) and decreases in gene eraser (TET), creating HHcy, activating NMDAergic, leading to HFrEF. Melatonin (NMDAR1 antagonist) mitigates HFrEF. Unlike diurnal humans, mice sleep in the day/light and wake in the night/dark (nocturnal). With a “single hit”, aorta–vena cava fistula (AVF) without injury, the heart creates HFpEF in 6–8 wks through the activity of MMP1/ADAMTS1.
The relationship between the circadian rhythms of genes and the epigenetic regulation of these genes and clock relation are characterized by the specific profile of DNA methylation in CpG-islands, which are associated with the senescence of somatic cells and stem cells [41,42]. It has been shown that circadian rhythms operate by very finely tuned regulation of transcription and are controlled by various epigenetic mechanisms, including the activation of enhancers/suppressors, acetylation/deacetylation of histones and other proteins, as well as DNA methylation [43]. Almost 20% of all genes expressed by the cell are affected by the oscillations associated with circadian rhythms [44,45,46,47]. Circadian regulators control several genes that activate the cell cycle and regulate histone modification, accessibility, maturation, and DNA methylation. Therefore, the approaches for determining the epigenetic age from methylation profiles across CpG islands in individual cells are significant.
Hcy is constitutively generated by SAHH, epigenetic gene regulation by the writer (DNMT) and eraser (TET) [48]. During off-printing and on-printing of the genes by active DNA methylation, Hcy is recycled back to methionine and vice versa by the FOCM pathway [48,49]. However, during dysbiosis and the passive DNA methylation due to TET dysfunction, and an increase in SAHH levels, the Hcy is accumulated (i.e., HHcy) [48] and inhibits the active gene expression. Also, H2S (hydrogen sulfide, an antioxidant and a potent neurotransmitter) induces TET2 during HHcy [49]. This reveals a direct link between the clock gene, BMAL1, and homocystinuria in de-activating the normal circadian cycle [35]. This elicits that homocystinuria not only causes developmental dysregulation of gene off-printing and on-printing (i.e., neuro-tube defects) but also causes circadian clock dysregulation, including vascular dementia, spasms, and arrhythmia [13,50].
BMAL1 is a constitutive suppressor of MMP-9 [51]. Conversely, a decrease in BMAL1 levels during wake activates MMPs. Because the growth arrest and DNA-damage-inducible 45 beta (GADD45beta) is an essential mediator of MMP-13 expression during terminal cell differentiation [37], the growth arrest and GADD45beta gene product has been implicated in the stress response, cell cycle arrest, and apoptosis. Therefore, the level of GADD45beta in development and disease is novel. The BMAL1/SAHH/Hcy control of the DMNT/H3K4/TET/GADD45/MMP13 is unique (Figure 1). Homocystinuria exacerbates vasospasm and arrhythmia [13,50,52,53], and these events are mostly affected by clock gene dysregulation. The chromatin transcription is regulated by methylation. The maturation and accessibility are controlled by acetylation (Figure 3). Hcy antagonizes the inhibitory (GABAergic) and agonizes the excitatory (NMDAergic) transmitters [52,54]. Interestingly, muscimol and baclofen are used as GABAergic agonists in the mitigation of anxiety and vascular-associated dementias [55,56,57,58,59,60,61,62,63,64,65,66,67]. Although both melatonin and MK801 are used as NMDAergic blockers/antagonists [68,69,70,71,72,73], their use to mitigate disruptions in the circadian clock system is unknown. Melatonin is a clinically proven antagonist of NMDA-R; therefore, it is significant to use melatonin to mitigate NMDAergic-associated disruption in the circadian clock system [74].
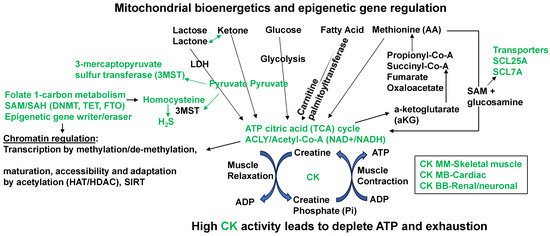
Figure 3.
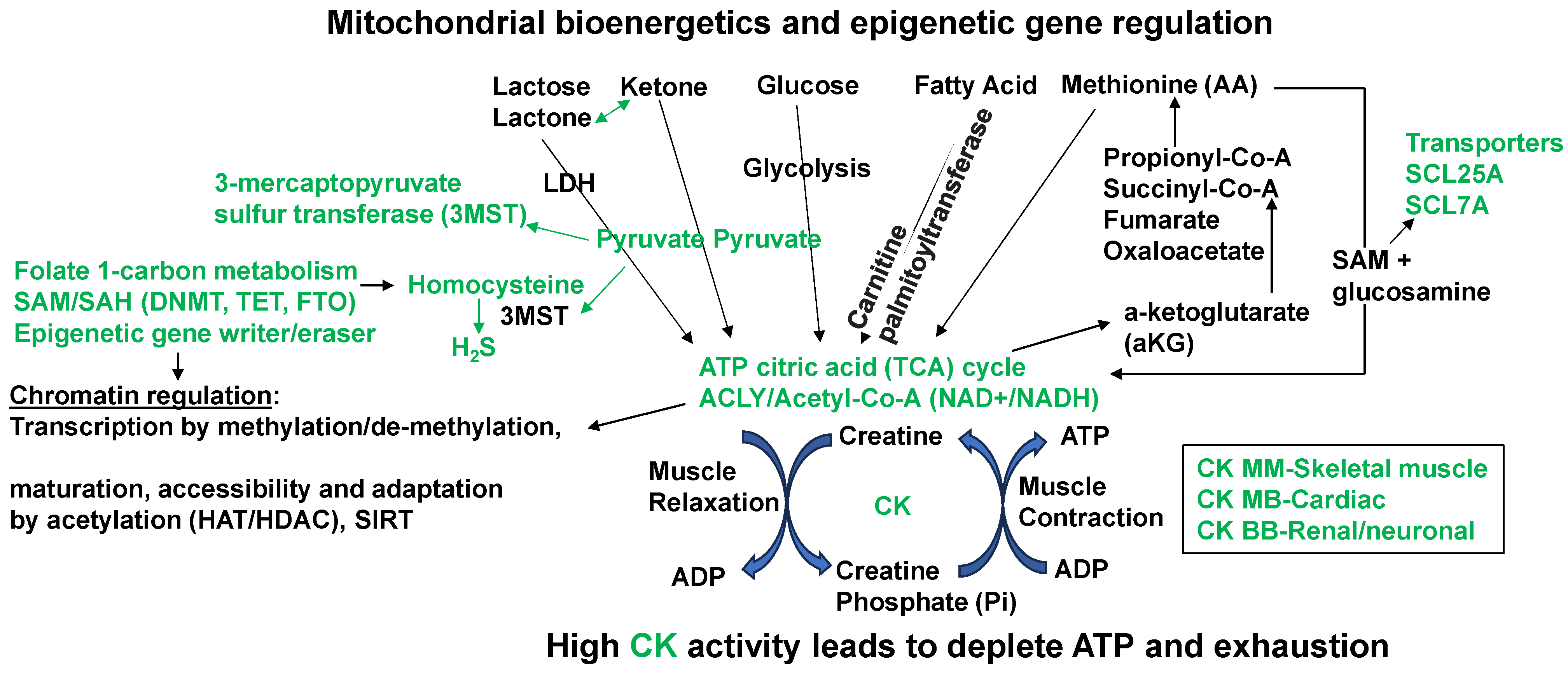
Prominent mitochondrial bioenergetics and epigenetics gene relation biochemical pathways that convert the homocysteine to hydrogen sulfide (H2S). The amino acid (AA) methionine modulation of the SAM/SAH ratio in muscle contraction/relaxation and exhaustion of ATP during heart failure. The epigenetic writer (DNMT1) and eraser (TET) via SAM/SAH pathways generate Hcy that is converted to H2S by mitochondrial 3MST. The high fat/meat/protein/methionine diet (HFD) decreases muscle contraction by exhausting ATP and increasing creatine kinase (CK) levels.
An increase in blood homocysteine, i.e., homocystinuria (HHcy), is also a comorbid condition for clock-mediated cardiovascular diseases. HHcy appears to be associated not only with chronic heart failure but also with acute myocardial infarction (MI) [75,76,77]. There are five ways by which Hcy is accumulated in the plasma and tissues: (i) by a methionine-rich protein diet; (ii) by hyper-demethylation of methionine by methyltransferase (MT) during DNA/RNA methylation reactions; (iii) by hypo-remethylation of Hcy to methionine by MTHFR/vitamin B12/folate; (iv) by heterozygous/homozygous mutation in the cystathionine β synthase (CBS), B6, and transsulfuration deficiency; and (v) by renal metabolic disease and volume retention.
Mammalian vascular cells lack the CBS enzyme [78,79]. Many studies have shown that mitochondria play a crucial role in cell survival during ischemia or ischemia–reperfusion (I/R) injury [80]. The I/R injury leads to excessive cytosolic Ca2+, mitochondrial Ca2+ overload, and a rapid increase in the overall levels of the reactive oxygen species (ROS). It is thought that mitochondrial autophagy, or mitophagy, is the major route by which mitochondria are degraded [7]. Mitochondrial Ca2+ (Ca2+m) overload and oxidative stress are the major triggers of the mitochondrial permeability transition (MPT) and loss of mitochondrial membrane potential (ΔΨm). Further, the mitochondrial permeability transition pore plays an important role in mitophagy [81,82,83]. The mitophagy may play an essential role in maintaining mitochondrial function and genetic integrity (Figure 4).
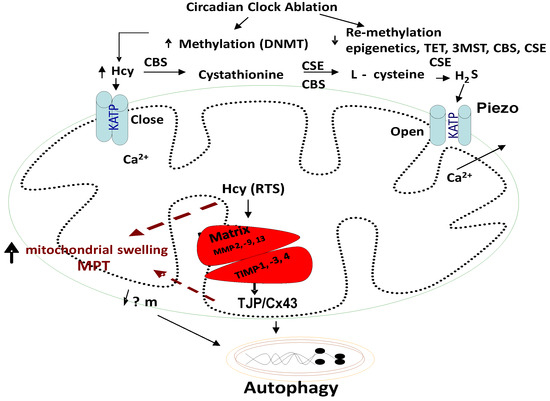
Figure 4.
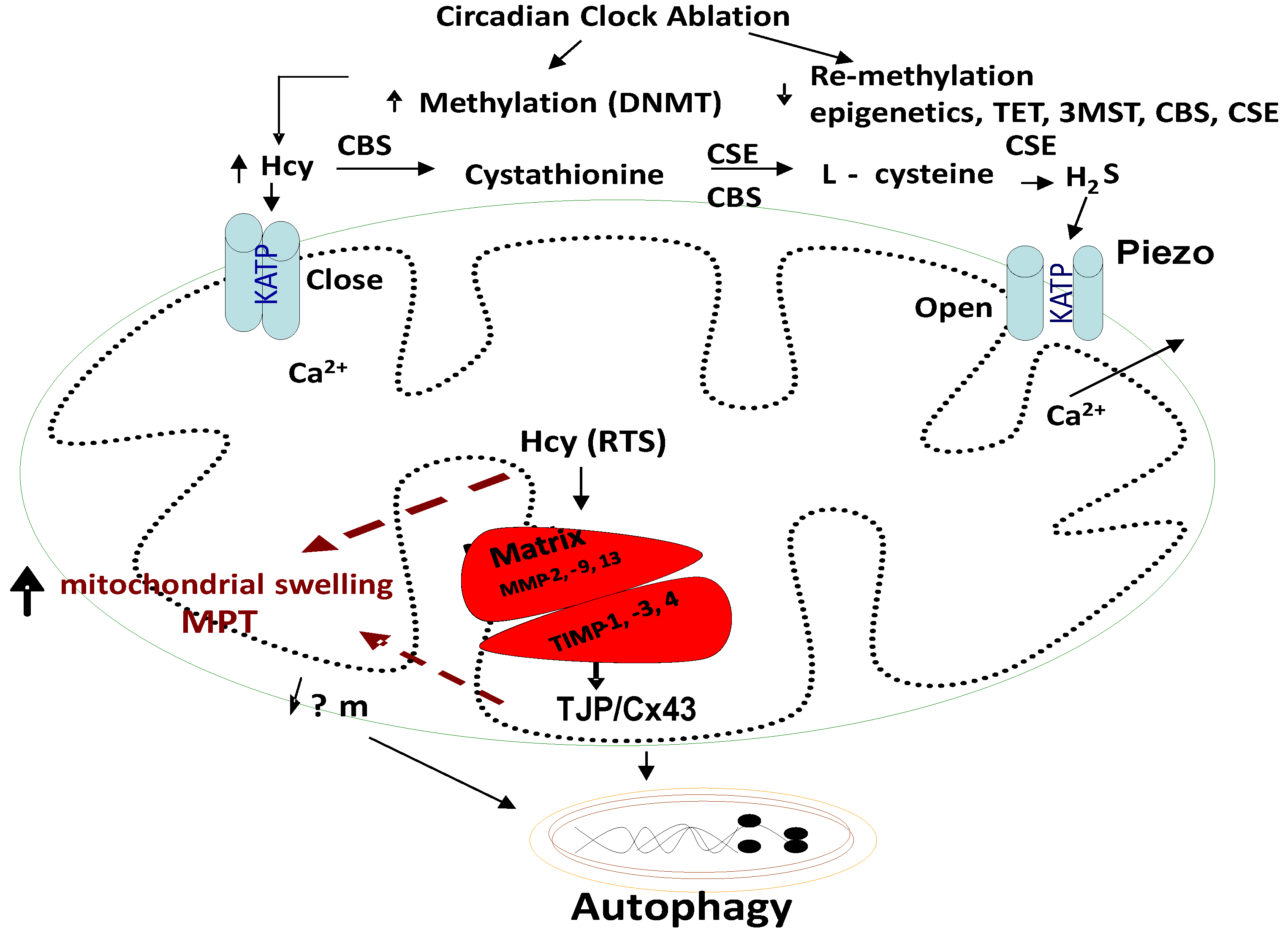
Homocysteine (Hcy) closes Piezo KATP channels, and H2S opens these Piezo channels. Further, the closed KATP channels trap the calcium ions in the mitochondria and result in arrhythmias and cell death/autophagy; however, the H2S reverses these alterations.
Homocysteine, Mitophagy, Ca2+ ion, Mitochondrial KATP Channels, and Hydrogen Sulfide (H2S): Although the novel role of nerve endings in the Piezo channels (i.e., combination of ENaC, TRPV, K+, and Mg2+ channels) in the interoception is suggested [84,85,86,87,88,89], we and others have shown mechanisms independent of the nerve by interoception, such as the cargo of exosome in denervation models of heart failure [90,91,92,93,94]. The mitochondria also have an ATP-sensitive ‘K+’ conductance, recognized as the mitochondrial KATP (mtKATP) channel [95,96,97,98]. We demonstrated that HHcy decreases myocyte contractile amplitude with the increase in calcium concentration and mitochondrial oxidative stress [77]. An increase in Ca2+ influx and oxidative stress in mitochondria leads to mtKATP channel closing [99]. KATP channels, when open, shorten the action potential duration and limit Ca2+ influx into the myocytes. Activation of mtKATP by potassium (K+) channel openers has been associated with increased survival of cardiac cells following ischemia and improved post-ischemic recovery of heart muscle [100,101]. H2S is generated endogenously as a product of the de-sulfuration (i.e., transsulfuration) pathways; however, in the past it has long been labeled a pungent cytotoxic gas, but now it is regarded as the third most endogenous produced signaling gasotransmitter molecule. Furthermore, CBS is the major enzyme that is involved in H2S production in the body, but its expression is confined to the brain, kidney, lung and is surprisingly absent in the cardiac tissue [102,103]. The other enzymes that play a role in H2S production are cystathionine gamma lyase (CSE) and 3-mercaptopyruvate sulfur transferase (3MST), which produce H2S via homocysteine to cysteine metabolism (Figure 1, Figure 2 and Figure 3). The H2S has been shown to protect the myocardium from oxidative and endoplasmic reticulum (ER) stress induced by homocystinuria [104,105]. In vivo studies have also demonstrated the efficacy of H2S in attenuating myocardial reperfusion injury by protecting mitochondrial function [80,106,107,108,109,110]. Our group has shown that H2S protects the cells from oxidative stress induced by homocystinuria [111]. H2S levels in human plasma are reported to be ~50 μM, and in vitro studies suggest that it behaves as a vasodilator, and transiently reduces blood pressure by opening KATP channels [112]. However, the physiological roles of Hcy and H2S in mitophagy are not well defined. We speculate that during chronic stress/load, the levels of Hcy are increased and cause mitochondrial calcium mishandling, in part, by closing the mtKATP channels via mitochondria dysfunction, leading to abnormal mitophagy (Figure 3 and Figure 4).
Hyperhomocysteinemia (HHcy), Oxidative Stress, Extracellular and Intracellular Matrix Metalloproteinase, Tissue Inhibitors of Metalloproteinases and Mitophagy: Matrix metalloproteinases (MMPs) and membrane-bound, zinc-dependent endoproteinases are known as collagenases (MMPs-1, -8, and 13), stromelysins (MMP-3 and 10), matrilysins (MMP-7 and -26), membrane-type MMPs (MT-MMPs, MMP-1 to MMP-8), and gelatinases (MMP-2 and 9), and the disintegrin metalloproteinase (including the ADAM). They share structural domains but differ in their substrate specificities [113,114,115,116,117,118,119]. We have shown that the basement membrane matrix of the endothelium mostly contains latent MMPs in part due to the coordination of active-site zinc ions with constitutive nitric oxide in a ternary complex (MMP/NO/TIMP) [120]. Increased oxidative stress leads to generation of nitro-tyrosine residues in the tissue inhibitor of metalloproteinase (TIMP) and release of the active MMP [121]. TIMPs are a family of enzymes that regulate the activity of MMPs, and four have been identified: TIMP-1, -2, -3, and -4 [122,123,124]. Thus, TIMPs play important roles in regulating cellular functions such as invasion, migration, differentiation, and proliferation. These functions are dependent on the cellular matrix composition. During chronic heart failure, increase in the load results in oxidative stress, leading to MMPs activation. We know that oxidative stress plays an important role in the induction of heart failure [125]. Previously, we have found that Hcy induced the generation of ROS production by upregulation of NADPH oxidase and downregulation of thioredoxin in microvascular endothelial cells (MVECs) [120]. Reactive oxygen species (ROS) subsequently induce the synthesis of matrix MMPs in the endothelial cells [126]. We have previously shown that Hcy increases mtROS production, which in turn initiates the mitochondrial membrane depolarization, cytochrome-c release, and the activation of caspase-9, thus leading to apoptosis [127]. Recently, several studies have indicated that ROS may be involved in the induction of mitophagy [128,129]. It is suggested that mitochondria are important regulators of apoptosis and mitophagy. Acute activation of MMP-2 leads to a reduction in contractile performance following the ischemia/reperfusion (I/R) injury [130]. We and others have shown the presence of MMPs in the cardiac mitochondria (mtMMP) [131,132,133]. However, the physiological consequence(s) of mtMMPs’ activation is not well understood. Although there is little information regarding the molecular mechanisms by which MMP-2 disrupts the mitochondria, it is well recognized that ROS generated by mitochondria can drive both MMP-2 expression and activation [134]. The activation of MMPs degrades the mitochondrial membrane and impairs mitochondrial function [77,135]. TIMP-1 is induced in heart failure, and TIMP-4 is highly expressed in the heart and is decreased during chronic cardiac failure [136,137,138,139]. TIMP-3 is induced by the loss of mitochondrial membrane potential and the release of cytochrome c, which might lead to mitophagy [140,141]. We speculate that in chronic stress/load, the level of Hcy is increased, causing an increase in mitochondrial oxidative stress and activation of latent resident mtMMPs, decreasing the TIMPs, and hence, inducing mitophagy (mitochondrial damage), leading to myocardium dysfunction. We also surmise the mechanism by which activated mtMMPs degrade the mitochondrial membrane and impair the mitochondrial functions, leading to a decline in the contractility of the myocardium.
Homocystinuria, Mitochondrial Gap Junctions, and the Mitophagy: Cardiomyocytes are connected cell-to-cell by the intercalated discs, which contain three types of cell junctions: gap, adherens, and desmosomes [113]. Gap junctions (GJs) contain connexin-43. Primarily three connexins are present in the heart. Connexin-37 and -43 are in the endothelium, while connexin-43 and -45 are present in the myocytes [142]. The expression of connexin-43 is reduced in ischemic heart disease [143]. Increasing evidence indicates that connexin-43 interacts with tight junction protein [144]. The downregulation of ZO-1 and claudin-5 expression are matched with the diminished expression levels of connexin-43, suggesting that the tight junction proteins play an important role in the gap junction formation [145,146,147]. Cx43 is abundantly expressed in cardiomyocytes; however, its role in modulating the myocyte mitophagy has not been well established. Cx43 was found to be present in the inner membrane of myocyte mitochondria (mtCx43), and it appears that it is cardioprotective during ischemia/reperfusion injury [148,149,150,151,152,153]. Mitochondrial Cx43 is a novel regulator of mitochondrial functions, and degradation of Cx43 may cause mitophagy [154]. Furthermore, mtCx43 participates in mtKATP-mediated ROS generation and cardioprotection. The hexameric connexin 43 protein forms a large conductance ion channel like the Bcl2 protein. Moreover, Cx channels are voltage gated and can sense mitochondrial membrane potential. The protective role of mtCx43 can be explained based on its interaction with the mitochondrial permeability transition pore, a multiprotein channel that stabilizes the mitochondrial permeability transition pore [154]. Paradoxically, Hcy increases the expression of Cx43 and nitrosylates of Cx43, which causes mitochondrial dysfunction [154,155]. Furthermore, overexpression of Cx43 is associated with the activation of MMPs such as MMP-2 and MMP-9, which increases mitochondrial oxidative stress, activates mtMMPs, and degrades mtCx43, leading to contractile and electrical dysfunction in the cardiomyocytes, in part, by opening mitochondrial permeability transition pores [156]. Mitochondrial Ca2+ overload also leads to the opening of the mitochondrial permeability transition pore and the release of inducible factors. The mitochondrial ROS causes the collapse of the mitochondrial membrane potential (ΔΨm), a drop in ATP concentration, a reduction in the cell cycle, and the loss of mtDNA. This suggests that mitophagy in the infarcted heart, in part, leads to the failure of the cellular mitochondrial network and not maintaining ΔΨm, and strongly suggests that mitochondria play a key role in the recovery of electrical activity in the post-ischemic myocardium [157,158] (Figure 4).
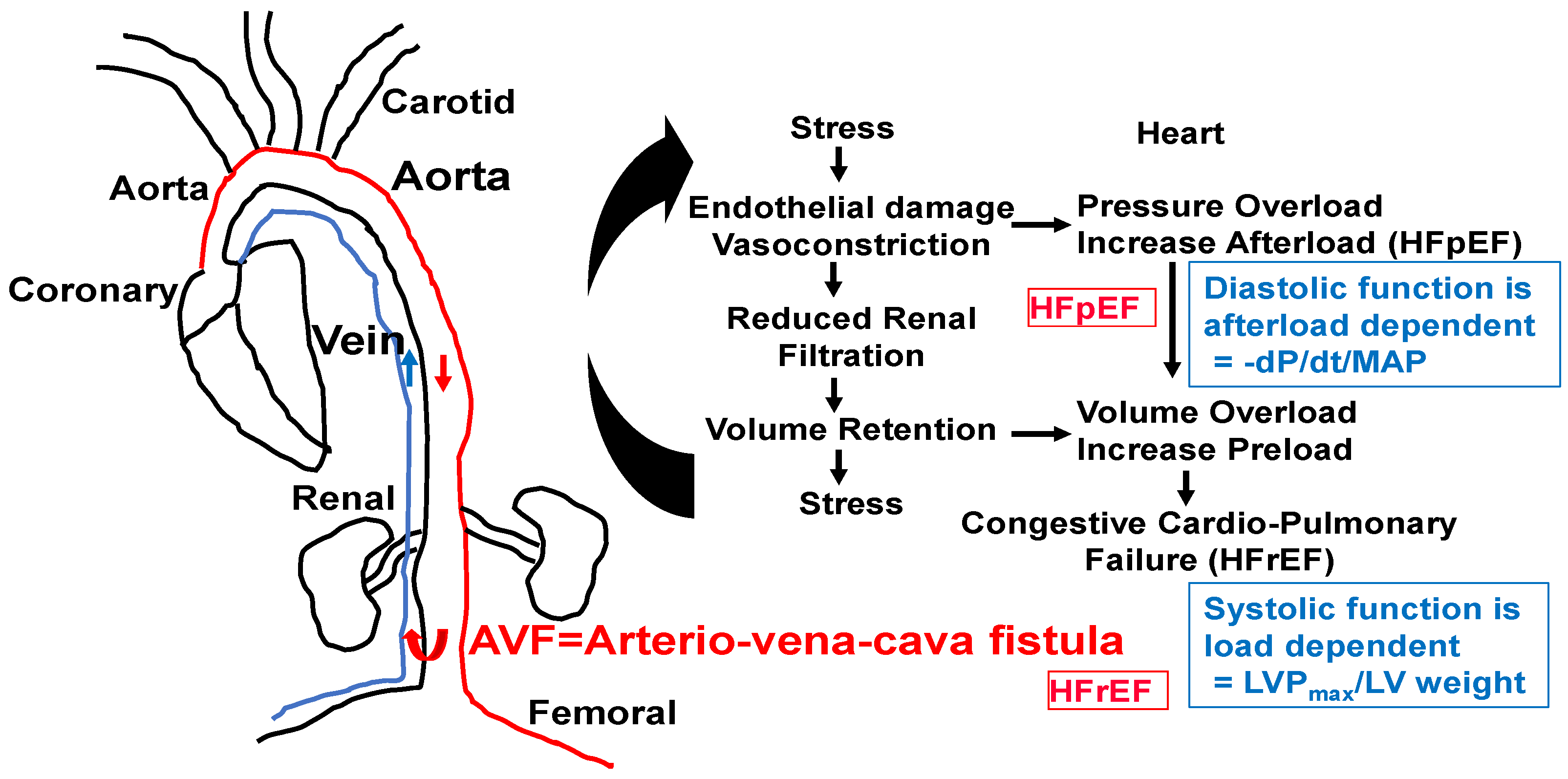
Because chronic volume overload increases with age, the increase in venous return by the aorta vena cava fistula (AVF) creates congestive cardiopulmonary heart failure that leads to transition from HFpEF to HFrEF. We suggest that homocysteine antagonizes GABAergic and agonizes NMDAergic [13,14] and contributes to the transition from HFpEF to HFrEF (Figure 5 and Figure 6).
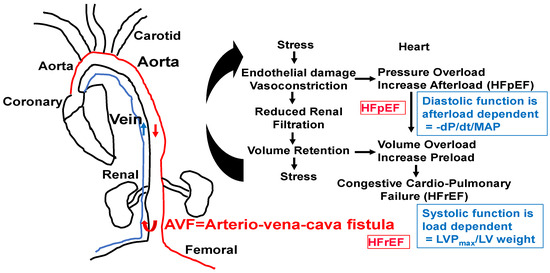
Figure 5.
AVF model of congestive HFpEF leading to HFrEF. The hypertrophy and fibrosis are increased with an increase in end-diastolic diameter (EDD), E/e’ ratio, and preserved EF. M-mode short axis ECHO, ventricular filling (E/A ratio), and flow velocity (E/e’ ratio, diastolic function) will be measured. The LVEDD and wall thickness as an index of heart failure will be measured. To determine diastolic dysfunction, LV wall contractile force and LV pressure will be measured by a Millar catheter positioned in the right common carotid artery in anesthetized mice. After measuring aortic pressure, the catheter will be advanced to the LV. Maximum systolic LV pressure (LVP); EDP; and the derivative of fall in pressure after systole, –dP/dt, will be measured. Because diastolic function is afterload-dependent, we will also measure the ratio between the rate of fall in pressure (–dP/dt) and mean arterial pressure (MAP). HFpEF and HFrEF will be distinguished by serial ECHO. HFpEF will be identified by the E/e’ ratio, cardiac fibrosis, and hypertrophy (~6–8 wks post-AVF) and HFrEF by rEF, hypertrophy, and blood–heart barrier (BHB) leakage/LV wall dilatation.
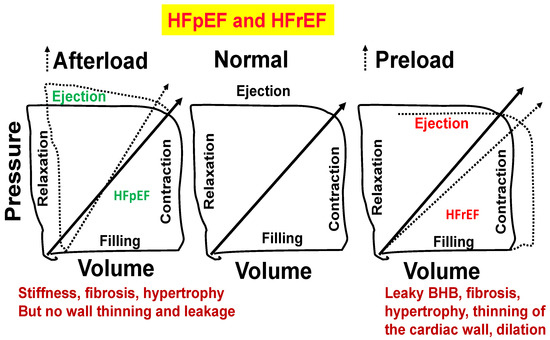
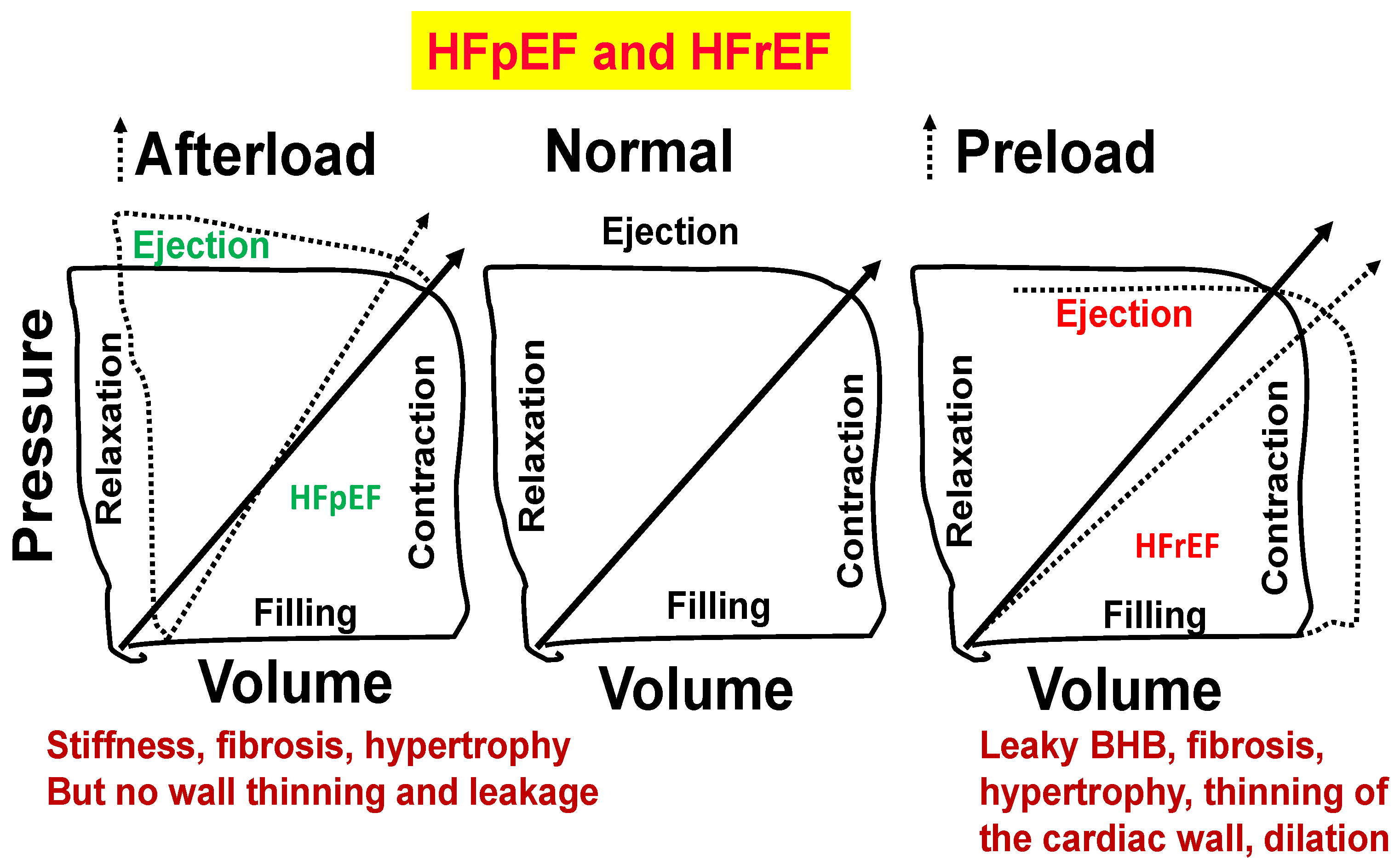
Figure 6.
Normal heart (middle panel). Stiffness, fibrosis, and hypertrophy but no wall thinning and leakage (left panel). Blood–heart barrier (BHB) leakage, myocyte slippage during systole, fibrosis, hypertrophy, thinning of the cardiac wall, and dilation (right panel).
3. Conclusions and Future Direction
It is important to investigate circadian rhythm regulation by homocystinuria and its effects on cardiovascular disorders. The circadian periodicity in cardiovascular function and reactivity in relationship to the pathogenesis of cardiovascular disease [38,159,160,161,162], arrhythmia, vascular dementia, and spasm through mechanisms such as epigenetic folate 1-carbon metabolism and DNA methylation by the gene writer and gene eraser are innovative ideas. The idea that inhibitory and excitatory neurotransmitters, respectively, are regulated by the clock gene and epigenetic modifiers during the circadian cycle is novel. The endothelial dysfunction, acetylcholinergic versus muscarinic, and paradoxical vasoconstriction [23] instead of vasodilation, causing vascular dementia, are novel. The hypothesis that the epigenetic dysregulation of clock genes by homocystinuria causes cardiovascular dysfunction, arrhythmia, and vasospasm is novel, and mitigation by an NMDAergic blocker is therapeutically innovative. Homocysteine is metabolized in the body to produce an endogenous gaseous substance, hydrogen sulfide (H2S). Despite the experimentally proven protective role of H2S in a variety of cardiovascular diseases, the potential role of H2S in mitophagy has remained untouched. In this context, we opine that to ameliorate Hcy-induced mitochondrial damage, exogenous H2S, with or without folic acid (FA; to lower Hcy levels), should also be investigated. Additionally, the cardioprotective roles of H2S and FA need to be investigated. Future research outcome(s) of this novel idea may lead us to better understand the Hcy-induced cardiac remodeling, especially in the beating myocytes. We are confident that such research endeavor will open newer avenues for future investigations regarding the therapeutic potential of this novel gaseous substance in homocystinuria-associated abnormal mitophagy and the associated cardiovascular–renal diseases.
Funding
A part of this study was supported by NIH grants AR-71789 and HL139047.
Institutional Review Board Statement
All results in animal models were obtained with the approval of the Institutional Review Board.
Conflicts of Interest
The author declares no conflict of interest.
References
- Tyagi, S.C.; Pushpakumar, S.; Sen, U.; Akinterinwa, O.E.; Zheng, Y.; Mokshagundam, S.P.L.; Kalra, D.K.; Singh, M. Role of circadian clock system in the mitochondrial trans-sulfuration pathway and tissue remodeling. Can. J. Physiol. Pharmacol. 2024, 102, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Corrigan, S.L.; Roberts, S.S.H.; Warmington, S.A.; Drain, J.R.; Tait, J.L.; Bulmer, S.; Main, L.C. Overnight heart rate variability responses to military combat engineer training. Appl. Ergon. 2023, 107, 103935. [Google Scholar] [CrossRef] [PubMed]
- Knapik, J.J.; Caldwell, J.A.; Ritland, B.M. Sleep and Injuries in Military Personnel with Suggestions for Improving Sleep and Mitigating Effects of Sleep Loss. J. Spec. Oper. Med. 2022, 22, 102–110. [Google Scholar] [CrossRef] [PubMed]
- LaGoy, A.D.; Conkright, W.R.; Proessl, F.; Sinnott, A.M.; Beckner, M.E.; Jabloner, L.; Eagle, S.R.; Sekel, N.M.; Roma, P.G.; Dretsch, M.N.; et al. Less daytime sleepiness and slow wave activity during sleep predict better physical readiness in military personnel. Sleep Health 2023, 9, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Jameson, J.T.; Markwald, R.R.; Kubala, A.G.; Roma, P.G.; Biggs, A.T.; Lai, K.; Russell, D.W. Sleep deficiency, operational fatigue and the interplay of compromising factors: Analysis to aid in fatigue management. J. Sleep Res. 2023, 32, e13788. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Sun, Y.; Zhang, H.; Wang, B.; Chen, C.; Wang, Y.; Chen, J.; Tan, X.; Zhang, J.; Xia, F.; et al. Long-term night shift work is associated with the risk of atrial fibrillation and coronary heart disease. Eur. Heart J. 2021, 42, 4180–4188. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, M.A.; Shah, A.M.; Borlaug, B.A. Heart Failure with Preserved Ejection Fraction in Perspective. Circ. Res. 2019, 124, 1598–1617. [Google Scholar] [CrossRef]
- Ingle, K.A.; Kain, V.; Goel, M.; Prabhu, S.D.; Young, M.E.; Halade, G.V. Cardiomyocyte-specific Bmal1 deletion in mice triggers diastolic dysfunction, extracellular matrix response, and impaired resolution of inflammation. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H1827–H1836. [Google Scholar] [CrossRef] [PubMed]
- Muller, J.E.; Stone, P.H.; Turi, Z.G.; Rutherford, J.D.; Czeisler, C.A.; Parker, C.; Poole, W.K.; Passamani, E.; Roberts, R.; Robertson, T.; et al. Circadian variation in the frequency of onset of acute myocardial infarction. N. Engl. J. Med. 1985, 313, 1315–1322. [Google Scholar] [CrossRef] [PubMed]
- Gumz, M.L.; Shimbo, D.; Abdalla, M.; Balijepalli, R.C.; Benedict, C.; Chen, Y.; Earnest, D.J.; Gamble, K.L.; Garrison, S.R.; Gong, M.C.; et al. Toward Precision Medicine: Circadian Rhythm of Blood Pressure and Chronotherapy for Hypertension—2021 NHLBI Workshop Report. Hypertension 2023, 80, 503–522. [Google Scholar] [CrossRef] [PubMed]
- Kaur, T.; Sharma, K.; Groban, L. Subanesthetic Ketamine Infusion Reducing Symptoms of Depression in a Patient with End-Stage Heart Failure Enrolled in Hospice Care: A Case Report. J. Palliat. Med. 2023, 26, 1435–1438. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Pushpakumar, S.; Zheng, Y.; Homme, R.P.; Smolenkova, I.; Mokshagundam, S.P.L.; Tyagi, S.C. Hydrogen sulfide mitigates skeletal muscle mitophagy-led tissue remodeling via epigenetic regulation of the gene writer and eraser function. Physiol. Rep. 2022, 10, e15422. [Google Scholar] [CrossRef] [PubMed]
- Moshal, K.S.; Kumar, M.; Tyagi, N.; Mishra, P.K.; Metreveli, N.; Rodriguez, W.E.; Tyagi, S.C. Restoration of contractility in hyperhomocysteinemia by cardiac-specific deletion of NMDA-R1. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H887–H892. [Google Scholar] [CrossRef] [PubMed]
- Lominadze, D.; Tyagi, N.; Sen, U.; Ovechkin, A.; Tyagi, S.C. Homocysteine alters cerebral microvascular integrity and causes remodeling by antagonizing GABA-A receptor. Mol. Cell. Biochem. 2012, 371, 89–96. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Meddeb, M.; Koleini, N.; Jun, S.; Keykhaei, M.; Farshidfar, F.; Zhao, L.; Kwon, S.; Lin, B.; Keceli, G.; Paolocci, N. ATP citrate lyase supports cardiac function and NAD+/NADH balance and is depressed in human heart failure. bioRxiv 2024. [Google Scholar] [CrossRef]
- Nishimoto, K.; Okahashi, N.; Maruyama, M.; Izumi, Y.; Nakatani, K.; Ito, Y.; Iida, J.; Bamba, T.; Matsuda, F. Lipidome and metabolome analyses reveal metabolic alterations associated with MCF-7 apoptosis upon 4-hydroxytamoxifen treatment. Sci. Rep. 2023, 13, 18549. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Gammon, S.T.; Tan, L.; Karlstaedt, A. ATP-dependent citrate lyase drives LV dysfunction by metabolic remodeling of the heart. bioRxiv 2024. [Google Scholar] [CrossRef]
- Miller, A.; Mujumdar, V.; Shek, E.; Guillot, J.; Angelo, M.; Palmer, L.; Tyagi, S.C. Hyperhomocyst(e)inemia induces multiorgan damage. Heart Vessel. 2000, 15, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Bremner, W.; Holmes, E.W.; Kanabrocki, E.L.; Hermida, R.C.; Ayala, D.; Garbincius, J.; Third, J.L.; Ryan, M.D.; Johnson, M.; Foley, S.; et al. Circadian rhythm of serum total homocysteine in men. Am. J. Cardiol. 2000, 86, 1153–1156. [Google Scholar] [CrossRef]
- Dong, Y.-F.; Zhan, B.-M.; Hao, Q.-Y.; Ruan, Z.-H.; Xu, Z.-X.; Deng, M.; Chen, D.-W.; Zou, Y.-Q.; Chen, J.; Li, P.; et al. Plasma Homocysteine Levels Are Associated with Circadian Blood Pressure Variation in Chinese Hypertensive Adults. Am. J. Hypertens. 2017, 30, 1203–1210. [Google Scholar] [CrossRef]
- Veerabhadrappa, P.; Schutte, A.E. Homocysteine and Nighttime Blood Pressure Dipping—Is There a Connection? Am. J. Hypertens. 2017, 30, 1151–1152. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, K.A.; Ahles, A.; Dueck, A.; Esfandyari, D.; Pichler, P.; Weber, K.; Kotschi, S.; Bartelt, A.; Sinicina, I.; Graw, M.; et al. Immune-mediated denervation of the pineal gland underlies sleep disturbance in cardiac disease. Science 2023, 381, 285–290. [Google Scholar] [CrossRef]
- Ludmer, P.L.; Selwyn, A.P.; Shook, T.L.; Wayne, R.R.; Mudge, G.H.; Alexander, R.W.; Ganz, P. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. N. Engl. J. Med. 1986, 315, 1046–1051. [Google Scholar] [CrossRef]
- Sudhakar Veeranki, Suresh C Tyagi, Defective homocysteine metabolism: Potential implications for skeletal muscle malfunction. Int. J. Mol. Sci. 2013, 14, 15074–15091. [CrossRef] [PubMed]
- Homme, R.P.; Zheng, Y.; Smolenkova, I.; Singh, M.; Tyagi, S.C. Remote Hind-Limb Ischemia Mechanism of Preserved Ejection Fraction During Heart Failure. Front. Physiol. 2021, 12, 745328. [Google Scholar] [CrossRef]
- Han, L.; Li, M.; Xie, W.; Lu, J.; Yu, L.; Liu, X.; Lv, N.; Zhang, L.; Zhang, Y.; Liu, Y.; et al. Association Between Orthostatic Hypotension with Coronary Slow Flow in Patients with Chest Pain: A Single Center Experience. Clin. Cardiol. 2024, 47, e70050. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Su, W.; Liu, S.; Zhao, G.; Esser, K.; Schroder, E.A.; Lefta, M.; Stauss, H.M.; Guo, Z.; Gong, M.C. Smooth-muscle BMAL1 participates in blood pressure circadian rhythm regulation. J. Clin. Investig. 2015, 125, 324–336. [Google Scholar] [CrossRef]
- Sato, N.; Vatner, S.F.; Shen, Y.-T.; Kudej, R.K.; Ghaleh-Marzban, B.; Uechi, M.; Asai, K.; Mirsky, I.; Patrick, T.A.; Shannon, R.P.; et al. Effects of cardiac denervation on development of heart failure and catecholamine desensitization. Circulation 1997, 95, 2130–2140. [Google Scholar] [CrossRef]
- Buchholz, B.; Donato, M.; Perez, V.; Deutsch, A.C.R.; Höcht, C.; Del Mauro, J.S.; Rodríguez, M.; Gelpi, R.J. Changes in the loading conditions induced by vagal stimulation modify the myocardial infarct size through sympathetic-parasympathetic interactions. Pflug. Arch. 2015, 467, 1509–1522. [Google Scholar] [CrossRef]
- Alhilali, M.; Hearn, J.; Rong, J.; Jain, L.; Bolam, S.; Monk, A.; Munro, J.; Dalbeth, N.; Poulsen, R. IL-1beta induces changes in expression of core circadian clock components PER2 and BMAL1 in primary human chondrocytes through the NMDA receptor/CREB and NF-kappaB signalling pathways. Cell. Signal. 2021, 87, 110143. [Google Scholar] [CrossRef]
- Tamaru, T.; Isojima, Y.; Yamada, T.; Okada, M.; Nagai, K.; Takamatsu, K. Light and glutamate-induced degradation of the circadian oscillating protein BMAL1 during the mammalian clock resetting. J. Neurosci. 2000, 20, 7525–7530. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.M.; Halter, K.A.; Prosser, R.A. Circadian rhythm and sleep-wake systems share the dynamic extracellular synaptic milieu. Neurobiol. Sleep Circadian Rhythm. 2018, 5, 15–36. [Google Scholar] [CrossRef] [PubMed]
- Hearn, J.I.; Alhilali, M.; Kim, M.; Kalev-Zylinska, M.L.; Poulsen, R.C. N-methyl-D-aspartate receptor regulates the circadian clock in megakaryocytic cells and impacts cell proliferation through BMAL1. Platelets 2023, 34, 2206918. [Google Scholar] [CrossRef] [PubMed]
- Malkiewicz, M.A.; Grzywinska, M.; Malinowski, K.S.; Partinen, E.; Partinen, M.; Cubala, W.J.; Winklewski, P.J.; Sieminski, M. Effect of series of periodic limb movements in sleep on blood pressure, heart rate and high frequency heart rate variability. Neurol. Neurochir. Polska, 2023; online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Greco, C.M.; Cervantes, M.; Fustin, J.-M.; Ito, K.; Ceglia, N.; Samad, M.; Shi, J.; Koronowski, K.B.; Forne, I.; Ranjit, S.; et al. S-adenosyl-l-homocysteine hydrolase links methionine metabolism to the circadian clock and chromatin remodeling. Sci. Adv. 2020, 6, eabc5629. [Google Scholar] [CrossRef]
- Hor, C.N.; Yeung, J.; Jan, M.; Emmenegger, Y.; Hubbard, J.; Xenarios, I.; Naef, F.; Franken, P. Sleep-wake-driven and circadian contributions to daily rhythms in gene expression and chromatin accessibility in the murine cortex. Proc. Natl. Acad. Sci. USA 2019, 116, 25773–25783. [Google Scholar] [CrossRef]
- Ijiri, K.; Zerbini, L.F.; Peng, H.; Correa, R.G.; Lu, B.; Walsh, N.; Zhao, Y.; Taniguchi, N.; Huang, X.-L.; Otu, H.; et al. A novel role for GADD45beta as a mediator of MMP-13 gene expression during chondrocyte terminal differentiation. J. Biol. Chem. 2005, 280, 38544–38555. [Google Scholar] [CrossRef]
- Rajabian, N.; Ikhapoh, I.; Shahini, S.; Choudhury, D.; Thiyagarajan, R.; Shahini, A.; Kulczyk, J.; Kulczyk, K.; Saha, S.; Mohamed, M.A.; et al. Methionine adenosyltransferase2A (MAT) inhibition restores metabolism to improve regenerative capacity and strength of aged skeletal muscle. Nat. Commun. 2023, 14, 886. [Google Scholar] [CrossRef]
- Hayden, M.R.; Tyagi, S.C. Impaired Folate-Mediated One-Carbon Metabolism in Type 2 Diabetes, Late-Onset Alzheimer’s Disease and Long COVID. Medicina 2021, 58, 16. [Google Scholar] [CrossRef]
- Doehner, W.; Anker, S.D.; Butler, J.; Zannad, F.; Filippatos, G.; Ferreira, J.P.; Salsali, A.; Kaempfer, C.; Brueckmann, M.; Pocock, S.J.; et al. Uric acid and sodium-glucose cotransporter-2 inhibition with empagliflozin in heart failure with reduced ejection fraction: The EMPEROR-reduced trial. Eur. Heart J. 2022, 43, 3435–3446. [Google Scholar] [CrossRef]
- Horvath, S. DNA methylation age of human tissues and cell types. Genome Biol. 2013, 14, R115. [Google Scholar] [CrossRef]
- Greenberg, M.V.C.; Bourc’his, D. The diverse roles of DNA methylation in mammalian development and disease. Nat. Rev. Mol. Cell Biol. 2019, 20, 590–607. [Google Scholar] [CrossRef] [PubMed]
- Gbadegesin, M.A.; Beeching, J.R. Enhancer/Suppressor mutator (En/Spm)-like transposable elements of cassava (Manihot esculenta) are transcriptionally inactive. Genet. Mol. Res. 2010, 9, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Littleton, E.S.; Childress, M.L.; Gosting, M.L.; Jackson, A.N.; Kojima, S. Genome-wide correlation analysis to identify amplitude regulators of circadian transcriptome output. Sci. Rep. 2020, 10, 21839. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rabinovich-Nikitin, I.; Kirshenbaum, E.; Kirshenbaum, L.A. Autophagy, clock genes and cardiovascular disease. Can. J. Cardiol. 2023; online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Rabinovich-Nikitin, I.; Crandall, M.; Kirshenbaum, L.A. Circadian regulation of genetic and hormonal risk factors of cardiovascular disease in women. Can. J. Physiol. Pharmacol. 2023, 101, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rabinovich-Nikitin, I.; Kirshenbaum, L.A. Circadian regulated control of myocardial ischemia-reperfusion injury. Trends Cardiovasc. Med. 2022; online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, S.C.; Stanisic, D.; Singh, M. Epigenetic memory: Gene writer, eraser and homocysteine. Mol. Cell. Biochem. 2021, 476, 507–512. [Google Scholar] [CrossRef]
- Pushpakumar, S.; Singh, M.; Sen, U.; Tyagi, N.; Tyagi, S.C. The role of the mitochondrial trans-sulfuration in cerebro-cardio renal dysfunction during trisomy down syndrome. Mol. Cell. Biochem. 2024, 479, 825–829. [Google Scholar] [CrossRef] [PubMed]
- Qipshidze, N.; Metreveli, N.; Lominadze, D.; Tyagi, S.C. Folic acid improves acetylcholine-induced vasoconstriction of coronary vessels isolated from hyperhomocysteinemic mice: An implication to coronary vasospasm. J. Cell. Physiol. 2011, 226, 2712–2720. [Google Scholar] [CrossRef]
- Gwon, D.H.; Lee, W.-Y.; Shin, N.; Kim, S.I.; Jeong, K.; Lee, W.-H.; Kim, D.W.; Hong, J.; Lee, S.Y. BMAL1 Suppresses Proliferation, Migration, and Invasion of U87MG Cells by Downregulating Cyclin B1, Phospho-AKT, and Metalloproteinase-9. Int. J. Mol. Sci. 2020, 21, 2352. [Google Scholar] [CrossRef]
- Soni, C.V.; Tyagi, S.C.; Todnem, N.D.; Givvimani, S.; Pushpakumar, S.B.; Villafane, J.; Maldonado, C. Hyperhomocysteinemia Alters Sinoatrial and Atrioventricular Nodal Function: Role of Magnesium in Attenuating These Effects. Cell Biochem. Biophys. 2016, 74, 59–65. [Google Scholar] [CrossRef]
- Nahlawi, M.; Seshadri, N.; Boparai, N.; Naso, A.; Jacobsen, D.W.; McCarthy, P.; Young, J.; Robinson, K. Usefulness of plasma vitamin B(6), B(12), folate, homocysteine, and creatinine in predicting outcomes in heart transplant recipients. Am. J. Cardiol. 2002, 89, 834–837. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.S.; Huang, T.H.; Lai, M.C.; Huang, C.W. HHcy causes anxiety and increase in glutamate/NMDA-R1 excitatory neurotransmitter. The Role of Glutamate Receptors in Epilepsy. Biomedicines 2023, 11, 783. [Google Scholar] [CrossRef] [PubMed]
- Unger, T.; Becker, H.; Dietz, R.; Ganten, D.; Lang, R.E.; Rettig, R.; Schömig, A.; Schwab, N.A. Antihypertensive effect of the GABA receptor agonist muscimol in spontaneously hypertensive rats. Role of the sympathoadrenal axis. Circ. Res. 1984, 54, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Kishi, T.; Hirooka, Y.; Sakai, K.; Shigematsu, H.; Shimokawa, H.; Takeshita, A. Overexpression of eNOS in the RVLM causes hypotension and bradycardia via GABA release. Hypertension 2001, 38, 896–901. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.R. Alcohol withdrawal seizures. Epilepsy Behav. 2009, 15, 92–97. [Google Scholar] [CrossRef]
- Rosansky, S.J.; Menachery, S.J.; Wagner, C.M.; Jackson, K. Circadian blood pressure variation versus renal function. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 1995, 26, 716–721. [Google Scholar] [CrossRef]
- Rodrigo, G.C.; Denniff, M. Time-of-day variation in vascular function. Exp. Physiol. 2016, 101, 1030–1034. [Google Scholar] [CrossRef]
- Griffiths, R.; Williams, D.C.; O’Neill, C.; Dewhurst, I.C.; Ekuwem, C.E.; Sinclair, C.D. Synergistic inhibition of [3H]muscimol binding to calf-brain synaptic membranes in the presence of L-homocysteine and pyridoxal 5′-phosphate. A possible mechanism for homocysteine-induced seizures. Eur. J. Biochem. 1983, 137, 467–478. [Google Scholar] [CrossRef]
- Loscalzo, J. Homocysteine and dementias. N. Engl. J. Med. 2002, 346, 466–468. [Google Scholar] [CrossRef]
- Folbergrova, J. NMDA and not non-NMDA receptor antagonists are protective against seizures induced by homocysteine in neonatal rats. Exp. Neurol. 1994, 130, 344–350. [Google Scholar] [CrossRef]
- Seshadri, S.; Beiser, A.; Selhub, J.; Jacques, P.F.; Rosenberg, I.H.; D’Agostino, R.B.; Wilson, P.W.; Wolf, P.A. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N. Engl. J. Med. 2002, 346, 476–483. [Google Scholar] [CrossRef]
- Sieklucka, M.; Bortolotto, Z.; Heim, C.; Block, F.; Sontag, K.H. Decreased susceptibility to seizures induced by bicuculline after transient bilateral clamping of the carotid arteries in rats. J. Neural Transm. Gen. Sect. 1991, 83, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Malinow, M.; Levenson, J.; Giral, P.; Nieto, F.; Razavian, M.; Segond, P.; Simon, A. Role of blood pressure, uric acid, and hemorheological parameters on plasma homocyst(e)ine concentration. Atherosclerosis 1995, 114, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Olney, J.W.; Price, M.T.; Salles, K.; Labruyere, J.; Ryerson, R.; Mahan, K.; Frierdich, G.; Samson, L. L-homocysteic acid: An endogenous excitotoxic ligand of the NMDA receptor. Brain Res. Bull. 1987, 19, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Kriegstein, A.R. GABA puts the brake on stem cells. Nat. Neurosci. 2005, 8, 1132–1133. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Shi, X.-Y.; Ge, W.-R.; Sun, Y.-L.; Zhang, S.; Wang, J.; Hu, L.-Y.; Zou, L.-P.; Yang, G. The Instigation of the Associations Between Melatonin, Circadian Genes, and Epileptic Spasms in Infant Rats. Front. Neurol. 2020, 11, 497225. [Google Scholar] [CrossRef]
- Hajmirzaeyian, A.; Chamanara, M.; Rashidian, A.; Shakyba, S.; Nassireslami, E.; Akhavan-Sigari, R. Melatonin attenuated the behavioral despair induced by acute neurogenic stress through blockade of N-methyl D-aspartate receptors in mice. Heliyon 2021, 7, e05900. [Google Scholar] [CrossRef]
- Furuta, T.; Nakagawa, I.; Yokoyama, S.; Morisaki, Y.; Saito, Y.; Nakase, H. Melatonin-Induced Postconditioning Suppresses NMDA Receptor through Opening of the Mitochondrial Permeability Transition Pore via Melatonin Receptor in Mouse Neurons. Int. J. Mol. Sci. 2022, 23, 3822. [Google Scholar] [CrossRef]
- Seymen, C.M.; Ilgaz, C.; Erdogan, D.; Elmas, C.; Saglam, A.S.Y.; Elmazoglu, Z.; Aral, B.S.; Kaplanoglu, G.T. Melatonin Modulates NMDA-Receptor 2B/Calpain-1/Caspase-12 Pathways in Rat Brain after Long Time Exposure to GSM Radiation. Turk. Neurosurg. 2019, 29, 887–900. [Google Scholar] [CrossRef]
- Aminzadeh, A.; Mehrzadi, S. Melatonin attenuates homocysteine-induced injury in human umbilical vein endothelial cells. Fundam. Clin. Pharmacol. 2018, 32, 261–269. [Google Scholar] [CrossRef]
- Murawska-Cialowicz, E.; Januszewska, L.; Zuwala-Jagiello, J.; Milczarska, J.; Zawadzki, M.; Paprocka-Borowicz, M.; Wierzbicka-Damska, I. Melatonin decreases homocysteine level in blood of rats. J. Physiol. Pharmacol. 2008, 59, 717–729. [Google Scholar]
- Fonken, L.K.; Nelson, R.J. The effects of light at night on circadian clocks and metabolism. Endocr. Rev. 2014, 35, 648–670. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, W.; Herrmann, M.; Joseph, J.; Tyagi, S.C. Homocysteine, brain natriuretic peptide and chronic heart failure: A critical review. Clin. Chem. Lab. Med. 2007, 45, 1633–1644. [Google Scholar] [CrossRef] [PubMed]
- Moshal, K.S.; Camel, C.K.; Kartha, G.K.; Steed, M.M.; Tyagi, N.; Sen, U.; Kang, Y.J.; Lominadze, D.; Maldonado, C.; Tyagi, S.C. Cardiac dys-synchronization and arrhythmia in hyperhomocysteinemia. Curr. Neurovascular Res. 2007, 4, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Moshal, K.S.; Tipparaju, S.M.; Vacek, T.P.; Kumar, M.; Singh, M.; Frank, I.E.; Patibandla, P.K.; Tyagi, N.; Rai, J.; Metreveli, N.; et al. Mitochondrial matrix metalloproteinase activation decreases myocyte contractility in hyperhomocysteinemia. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H890–H897. [Google Scholar] [CrossRef]
- Finkelstein, J.D. Methionine metabolism in mammals. J. Nutr. Biochem. 1990, 1, 228–237. [Google Scholar] [CrossRef]
- Finkelstein, J.D. The metabolism of homocysteine: Pathways and regulation. Eur. J. Pediatr. 1998, 157 (Suppl. S2), S40–S44. [Google Scholar] [CrossRef]
- Elrod, J.W.; Calvert, J.W.; Morrison, J.; Doeller, J.E.; Kraus, D.W.; Tao, L.; Jiao, X.; Scalia, R.; Kiss, L.; Szabo, C.; et al. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc. Natl. Acad. Sci. USA 2007, 104, 15560–15565. [Google Scholar] [CrossRef]
- Onishi, M.; Yamano, K.; Sato, M.; Matsuda, N.; Okamoto, K. Molecular mechanisms and physiological functions of mitophagy. EMBO J. 2021, 40, e104705. [Google Scholar] [CrossRef]
- Cotán, D.; Cordero, M.D.; Garrido-Maraver, J.; Oropesa-Ávila, M.; Rodríguez-Hernández, A.; Gómez Izquierdo, L.; De la Mata, M.; De Miguel, M.; Lorite, J.B.; Infante, E.R.; et al. Secondary coenzyme Q10 deficiency triggers mitochondria degradation by mitophagy in MELAS fibroblasts. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2011, 25, 2669–2687. [Google Scholar]
- Rodríguez-Hernández, A.; Cordero, M.D.; Salviati, L.; Artuch, R.; Pineda, M.; Briones, P.; Gómez Izquierdo, L.; Cotán, D.; Navas, P.; Sánchez-Alcázar, J.A. Coenzyme Q deficiency triggers mitochondria degradation by mitophagy. Autophagy 2009, 5, 19–32. [Google Scholar] [CrossRef]
- Nonomura, K.; Woo, S.-H.; Chang, R.B.; Gillich, A.; Qiu, Z.; Francisco, A.G.; Ranade, S.S.; Liberles, S.D.; Patapoutian, A. Piezo2 senses airway stretch and mediates lung inflation-induced apnoea. Nature 2017, 541, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.-Z.; Marshall, K.L.; Min, S.; Daou, I.; Chapleau, M.W.; Abboud, F.M.; Liberles, S.D.; Patapoutian, A. PIEZOs mediate neuronal sensing of blood pressure and the baroreceptor reflex. Science 2018, 362, 464–467. [Google Scholar] [CrossRef] [PubMed]
- Murthy, S.E.; Loud, M.C.; Daou, I.; Marshall, K.L.; Schwaller, F.; Kühnemund, J.; Francisco, A.G.; Keenan, W.T.; Dubin, A.E.; Lewin, G.R.; et al. The mechanosensitive ion channel Piezo2 mediates sensitivity to mechanical pain in mice. Sci. Transl. Med. 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Marshall, K.L.; Saade, D.; Ghitani, N.; Coombs, A.M.; Szczot, M.; Keller, J.; Ogata, T.; Daou, I.; Stowers, L.T.; Bönnemann, C.G.; et al. PIEZO2 in sensory neurons and urothelial cells coordinates urination. Nature 2020, 588, 290–295. [Google Scholar] [CrossRef]
- Hill, R.Z.; Loud, M.C.; Dubin, A.E.; Peet, B.; Patapoutian, A. PIEZO1 transduces mechanical itch in mice. Nature 2022, 607, 104–110. [Google Scholar] [CrossRef]
- Servin-Vences, M.R.; Lam, R.M.; Koolen, A.; Wang, Y.; Saade, D.N.; Loud, M.; Kacmaz, H.; Frausto, S.; Zhang, Y.; Beyder, A.; et al. PIEZO2 in somatosensory neurons controls gastrointestinal transit. Cell 2023, 186, 3386–3399.e15. [Google Scholar] [CrossRef]
- Majumder, A.; Singh, M.; George, A.K.; Homme, R.P.; Laha, A.; Tyagi, S.C. Remote ischemic conditioning as a cytoprotective strategy in vasculopathies during hyperhomocysteinemia: An emerging research perspective. J. Cell. Biochem. 2019, 120, 77–92. [Google Scholar] [CrossRef]
- Scalco, A.; Lee, E.N.; Johnson, M.A.; Sorensen, M.L.; Hilton, T.N.; Omonaka, R.K.; Zeimantz, S.; Aicher, S.A.; Woodward, W.R.; Habecker, B.A. Hypertension-induced heart failure disrupts cardiac sympathetic innervation. Am. J. Physiol. Heart Circ. Physiol. 2024; online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Pushpakumar, S.; Singh, M.; Zheng, Y.; Akinterinwa, O.E.; Mokshagundam, S.P.L.; Sen, U.; Kalra, D.K.; Tyagi, S.C. Renal Denervation Helps Preserve the Ejection Fraction by Preserving Endocardial-Endothelial Function during Heart Failure. Int. J. Mol. Sci. 2023, 24, 7302. [Google Scholar] [CrossRef]
- Singh, M.; Tyagi, S.C. Interoception by exosomes in transition from HFpEF to HfrEF. In Proceedings of the 2nd Annual National Institutes of Health (NIH) Investigator Meeting on Interoception Research, Bethesda, MD, USA, 11 November 2023. [Google Scholar]
- Vemuri, S.; Singh, M.; Homme, R.P.; Tyagi, S.C. Interoception, heart failure and exosomal cargo as potential biomarkers. Gene Rep. 2023, 33, 101849. [Google Scholar] [CrossRef]
- Garlid, K.D.; Paucek, P.; Yarov-Yarovoy, V.; Sun, X.; Schindler, P.A. The mitochondrial KATP channel as a receptor for potassium channel openers. J. Biol. Chem. 1996, 271, 8796–8799. [Google Scholar] [CrossRef]
- Inoue, I.; Nagase, H.; Kishi, K.; Higuti, T. ATP-sensitive K+ channel in the mitochondrial inner membrane. Nature 1991, 352, 244–247. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, B. Myocardial K(ATP) channels in preconditioning. Circ. Res. 2000, 87, 845–855. [Google Scholar] [CrossRef] [PubMed]
- Jabůrek, M.; Yarov-Yarovoy, V.; Paucek, P.; Garlid, K.D. State-dependent inhibition of the mitochondrial KATP channel by glyburide and 5-hydroxydecanoate. J. Biol. Chem. 1998, 273, 13578–13582. [Google Scholar] [CrossRef] [PubMed]
- Marinovic, J.; Ljubkovic, M.; Stadnicka, A.; Bosnjak, Z.J.; Bienengraeber, M. Role of sarcolemmal ATP-sensitive potassium channel in oxidative stress-induced apoptosis: Mitochondrial connection. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H1317–H1325. [Google Scholar] [CrossRef]
- Garlid, K.D.; Paucek, P.; Yarov-Yarovoy, V.; Murray, H.N.; Darbenzio, R.B.; D’Alonzo, A.J.; Lodge, N.J.; Smith, M.A.; Grover, G.J. Cardioprotective effect of diazoxide and its interaction with mitochondrial ATP-sensitive K+ channels. Possible mechanism of cardioprotection. Circ. Res. 1997, 81, 1072–1082. [Google Scholar] [CrossRef]
- Liu, Y.; Downey, J.M. Ischemic preconditioning protects against infarction in rat heart. Am. J. Physiol. 1992, 263, H1107–H1112. [Google Scholar] [CrossRef]
- Chen, X.; Jhee, K.H.; Kruger, W.D. Production of the neuromodulator H2S by cystathionine beta-synthase via the condensation of cysteine and homocysteine. J. Biol. Chem. 2004, 279, 52082–52086. [Google Scholar] [CrossRef]
- Singh, S.; Padovani, D.; Leslie, R.A.; Chiku, T.; Banerjee, R. Relative contributions of cystathionine beta-synthase and gamma-cystathionase to H2S biogenesis via alternative trans-sulfuration reactions. J. Biol. Chem. 2009, 284, 22457–22466. [Google Scholar] [CrossRef]
- Chang, L.; Geng, B.; Yu, F.; Zhao, J.; Jiang, H.; Du, J.; Tang, C. Hydrogen sulfide inhibits myocardial injury induced by homocysteine in rats. Amino Acids 2008, 34, 573–585. [Google Scholar] [CrossRef]
- Jiang, S.; Xu, W.; Chen, Z.; Cui, C.; Fan, X.; Cai, J.; Gong, Y.; Geng, B. Hydrogen sulphide reduces hyperhomocysteinaemia-induced endothelial ER stress by sulfhydrating protein disulphide isomerase to attenuate atherosclerosis. J. Cell. Mol. Med. 2021, 25, 3437–3448. [Google Scholar] [CrossRef]
- Kannan, S.; Boovarahan, S.R.; Rengaraju, J.; Prem, P.; Kurian, G.A. Attenuation of cardiac ischemia-reperfusion injury by sodium thiosulfate is partially dependent on the effect of cystathione beta synthase in the myocardium. Cell Biochem. Biophys. 2019, 77, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Karwi, Q.G.; Bornbaum, J.; Boengler, K.; Torregrossa, R.; Whiteman, M.; Wood, M.E.; Schulz, R.; Baxter, G.F. AP39, a mitochondria-targeting hydrogen sulfide (H2S) donor, protects against myocardial reperfusion injury independently of salvage kinase signalling. Br. J. Pharmacol. 2017, 174, 287–301. [Google Scholar] [CrossRef] [PubMed]
- Nandi, S.; Ravindran, S.; Kurian, G.A. Role of endogenous hydrogen sulfide in cardiac mitochondrial preservation during ischemia reperfusion injury. Biomed. Pharmacother. 2018, 97, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Sivarajah, A.; Collino, M.; Yasin, M.; Benetti, E.; Gallicchio, M.; Mazzon, E.; Cuzzocrea, S.; Fantozzi, R.; Thiemermann, C. Anti-apoptotic and anti-inflammatory effects of hydrogen sulfide in a rat model of regional myocardial I/R. Shock 2009, 31, 267–274. [Google Scholar] [CrossRef]
- Yao, X.; Tan, G.; He, C.; Gao, Y.; Pan, S.; Jiang, H.; Zhang, Y.; Sun, X. Hydrogen sulfide protects cardiomyocytes from myocardial ischemia-reperfusion injury by enhancing phosphorylation of apoptosis repressor with caspase recruitment domain. Tohoku J. Exp. Med. 2012, 226, 275–285. [Google Scholar] [CrossRef]
- Tyagi, N.; Moshal, K.S.; Sen, U.; Vacek, T.P.; Kumar, M.; Hughes, W.M., Jr.; Kundu, S.; Tyagi, S.C. H2S protects against methionine-induced oxidative stress in brain endothelial cells. Antioxid. Redox Signal. 2009, 11, 25–33. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, J.; Lu, Y.; Wang, R. The vasorelaxant effect of H2S as a novel endogenous gaseous K(ATP) channel opener. EMBO J. 2001, 20, 6008–6016. [Google Scholar] [CrossRef]
- Rosenberg, G.A. Matrix metalloproteinases in neuroinflammation. Glia 2002, 39, 279–291. [Google Scholar] [CrossRef]
- Azevedo, A.; Prado, A.F.; Antonio, R.C.; Issa, J.P.; Gerlach, R.F. Matrix metalloproteinases are involved in cardiovascular diseases. Basic Clin. Pharmacol. Toxicol. 2014, 115, 301–314. [Google Scholar] [CrossRef]
- Bassiouni, W.; Ali, M.A.M.; Schulz, R. Multifunctional intracellular matrix metalloproteinases: Implications in disease. FEBS J. 2021, 288, 7162–7182. [Google Scholar] [CrossRef]
- Clark, I.M.; Swingler, T.E.; Sampieri, C.L.; Edwards, D.R. The regulation of matrix metalloproteinases and their inhibitors. Int. J. Biochem. Cell Biol. 2008, 40, 1362–1378. [Google Scholar] [CrossRef] [PubMed]
- Madzharova, E.; Kastl, P.; Sabino, F.; Auf dem Keller, U. Post-Translational Modification-Dependent Activity of Matrix Metalloproteinases. Int. J. Mol. Sci. 2019, 20, 3077. [Google Scholar] [CrossRef] [PubMed]
- Parks, W.C. Matrix metalloproteinases in repair. Wound Repair Regen. Off. Publ. Wound Health Soc. Eur. Tissue Repair Soc. 1999, 7, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Van Doren, S.R.; Marcink, T.C.; Koppisetti, R.K.; Jurkevich, A.; Fulcher, Y.G. Peripheral membrane associations of matrix metalloproteinases. Biochim. Et Biophys. Acta Mol. Cell Res. 2017, 1864, 1964–1973. [Google Scholar] [CrossRef]
- Tyagi, S.C.; Ratajska, A.; Weber, K.T. Myocardial matrix metalloproteinase(s): Localization and activation. Mol. Cell. Biochem. 1993, 126, 49–59. [Google Scholar] [CrossRef]
- Tyagi, N.; Sedoris, K.C.; Steed, M.; Ovechkin, A.V.; Moshal, K.S.; Tyagi, S.C. Mechanisms of homocysteine-induced oxidative stress. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H2649–H2656. [Google Scholar] [CrossRef]
- Mannello, F.; Gazzanelli, G. Tissue inhibitors of metalloproteinases and programmed cell death: Conundrums, controversies and potential implications. Apoptosis Int. J. Program. Cell Death 2001, 6, 479–482. [Google Scholar] [CrossRef]
- Brew, K.; Dinakarpandian, D.; Nagase, H. Tissue inhibitors of metalloproteinases: Evolution, structure and function. Biochim. Et Biophys. Acta 2000, 1477, 267–283. [Google Scholar] [CrossRef]
- Brew, K.; Nagase, H. The tissue inhibitors of metalloproteinases (TIMPs): An ancient family with structural and functional diversity. Biochim. Et Biophys. Acta 2010, 1803, 55–71. [Google Scholar] [CrossRef]
- Fukuda, K.; Davies, S.S.; Nakajima, T.; Ong, B.H.; Kupershmidt, S.; Fessel, J.; Amarnath, V.; Anderson, M.E.; Boyden, P.A.; Viswanathan, P.C.; et al. Oxidative mediated lipid peroxidation recapitulates proarrhythmic effects on cardiac sodium channels. Circ. Res. 2005, 97, 1262–1269. [Google Scholar] [CrossRef]
- Tyagi, N.; Gillespie, W.; Vacek, J.C.; Sen, U.; Tyagi, S.C.; Lominadze, D. Activation of GABA-A receptor ameliorates homocysteine-induced MMP-9 activation by ERK pathway. J. Cell. Physiol. 2009, 220, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, N.; Moshal, K.S.; Ovechkin, A.V.; Rodriguez, W.; Steed, M.; Henderson, B.; Roberts, A.M.; Joshua, I.G.; Tyagi, S.C. Mitochondrial mechanism of oxidative stress and systemic hypertension in hyperhomocysteinemia. J. Cell. Biochem. 2005, 96, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Band, M.; Joel, A.; Hernandez, A.; Avivi, A. Hypoxia-induced BNIP3 expression and mitophagy: In vivo comparison of the rat and the hypoxia-tolerant mole rat, Spalax ehrenbergi. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2009, 23, 2327–2335. [Google Scholar]
- Luan, Y.; Luan, Y.; Feng, Q.; Chen, X.; Ren, K.D.; Yang, Y. Emerging Role of Mitophagy in the Heart: Therapeutic Potentials to Modulate Mitophagy in Cardiac Diseases. Oxidative Med. Cell. Longev. 2021, 2021, 3259963. [Google Scholar] [CrossRef] [PubMed]
- Bowser, D.N.; Minamikawa, T.; Nagley, P.; Williams, D.A. Role of mitochondria in calcium regulation of spontaneously contracting cardiac muscle cells. Biophys. J. 1998, 75, 2004–2014. [Google Scholar] [CrossRef]
- Limb, G.A.; Matter, K.; Murphy, G.; Cambrey, A.D.; Bishop, P.N.; Morris, G.E.; Khaw, P.T. Matrix metalloproteinase-1 associates with intracellular organelles and confers resistance to lamin A/C degradation during apoptosis. Am. J. Pathol. 2005, 166, 1555–1563. [Google Scholar] [CrossRef]
- Ma, Y.S.; Chen, Y.C.; Lu, C.Y.; Liu, C.Y.; Wei, Y.H. Upregulation of matrix metalloproteinase 1 and disruption of mitochondrial network in skin fibroblasts of patients with MERRF syndrome. Ann. N. Y. Acad. Sci. 2005, 1042, 55–63. [Google Scholar] [CrossRef]
- Hughes, B.G.; Fan, X.; Cho, W.J.; Schulz, R. MMP-2 is localized to the mitochondria-associated membrane of the heart. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H764–H770. [Google Scholar] [CrossRef]
- Nelson, K.K.; Melendez, J.A. Mitochondrial redox control of matrix metalloproteinases. Free Radic. Biol. Med. 2004, 37, 768–784. [Google Scholar] [CrossRef]
- Jobin, P.G.; Butler, G.S.; Overall, C.M. New intracellular activities of matrix metalloproteinases shine in the moonlight. Biochim. Et Biophys. Acta Mol. Cell Res. 2017, 1864, 2043–2055. [Google Scholar] [CrossRef]
- Tummalapalli, C.M.; Heath, B.J.; Tyagi, S.C. Tissue inhibitor of metalloproteinase-4 instigates apoptosis in transformed cardiac fibroblasts. J. Cell. Biochem. 2001, 80, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Khezheva, F.M.; Mazur, N.A.; Masenko, V.P. Metalloproteinase activity of the blood in patients with arterial hypertension with paroxysmal form of atrial fibrillation. Kardiologiia 2007, 47, 10–14. [Google Scholar] [PubMed]
- Lindsay, M.M.; Maxwell, P.; Dunn, F.G. TIMP-1: A marker of left ventricular diastolic dysfunction and fibrosis in hypertension. Hypertension 2002, 40, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Spoto, B.; Testa, A.; Parlongo, R.M.; Tripepi, G.; D’Arrigo, G.; Mallamaci, F.; Zoccali, C. Tissue inhibitor of metalloproteinases (TIMP-1), genetic markers of insulin resistance and cardiomyopathy in patients with kidney failure. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2012, 27, 2440–2445. [Google Scholar] [CrossRef]
- Hanaoka, K.; Tanaka, E.; Takata, T.; Miyauchi, M.; Aoyama, J.; Kawai, N.; Dalla-Bona, D.A.; Yamano, E.; Tanne, K. Platelet-derived growth factor enhances proliferation and matrix synthesis of temporomandibular joint disc-derived cells. Angle Orthod. 2006, 76, 486–492. [Google Scholar]
- Givvimani, S.; Munjal, C.; Tyagi, N.; Sen, U.; Metreveli, N.; Tyagi, S.C. Mitochondrial division/mitophagy inhibitor (Mdivi) ameliorates pressure overload induced heart failure. PLoS ONE 2012, 7, e32388. [Google Scholar] [CrossRef]
- Dupont, E.; Ko, Y.; Rothery, S.; Coppen, S.R.; Baghai, M.; Haw, M.; Severs, N.J. The gap-junctional protein connexin40 is elevated in patients susceptible to postoperative atrial fibrillation. Circulation 2001, 103, 842–849. [Google Scholar] [CrossRef]
- Peters, N.S.; Green, C.R.; Poole-Wilson, P.A.; Severs, N.J. Cardiac arrhythmogenesis and the gap junction. J. Mol. Cell. Cardiol. 1995, 27, 37–44. [Google Scholar] [CrossRef]
- Kostin, S. Zonula occludens-1 and connexin 43 expression in the failing human heart. J. Cell. Mol. Med. 2007, 11, 892–895. [Google Scholar] [CrossRef]
- Luo, T.; Liu, H.; Chen, B.; Liu, H.; Abdel-Latif, A.; Kitakaze, M.; Wang, X.; Wu, Y.; Chou, D.; Kim, J.K. A Novel Role of Claudin-5 in Prevention of Mitochondrial Fission Against Ischemic/Hypoxic Stress in Cardiomyocytes. Can. J. Cardiol. 2021, 37, 1593–1606. [Google Scholar] [CrossRef]
- Mays, T.A.; Binkley, P.F.; Lesinski, A.; Doshi, A.A.; Quaile, M.P.; Margulies, K.B.; Janssen, P.M.; Rafael-Fortney, J.A. Claudin-5 levels are reduced in human end-stage cardiomyopathy. J. Mol. Cell. Cardiol. 2008, 45, 81–87. [Google Scholar] [CrossRef] [PubMed]
- McCaffrey, G.; Staatz, W.D.; Quigley, C.A.; Nametz, N.; Seelbach, M.J.; Campos, C.R.; Brooks, T.A.; Egleton, R.D.; Davis, T.P. Tight junctions contain oligomeric protein assembly critical for maintaining blood-brain barrier integrity in vivo. J. Neurochem. 2007, 103, 2540–2555. [Google Scholar] [CrossRef] [PubMed]
- Boengler, K.; Dodoni, G.; Rodriguez-Sinovas, A.; Cabestrero, A.; Ruiz-Meana, M.; Gres, P.; Konietzka, I.; Lopez-Iglesias, C.; Garcia-Dorado, D.; Di Lisa, F.; et al. Connexin 43 in cardiomyocyte mitochondria and its increase by ischemic preconditioning. Cardiovasc. Res. 2005, 67, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Boengler, K.; Schulz, R. Connexin 43 and Mitochondria in Cardiovascular Health and Disease. Adv. Exp. Med. Biol. 2017, 982, 227–246. [Google Scholar]
- Boengler, K.; Schulz, R.; Heusch, G. Connexin 43 signalling and cardioprotection. Heart Br. Card. Soc. 2006, 92, 1724–1727. [Google Scholar] [CrossRef]
- Denuc, A.; Núñez, E.; Calvo, E.; Loureiro, M.; Miro-Casas, E.; Guarás, A.; Vázquez, J.; Garcia-Dorado, D. New protein-protein interactions of mitochondrial connexin 43 in mouse heart. J. Cell. Mol. Med. 2016, 20, 794–803. [Google Scholar] [CrossRef]
- Jeyaraman, M.M.; Srisakuldee, W.; Nickel, B.E.; Kardami, E. Connexin43 phosphorylation and cytoprotection in the heart. Biochim. Et Biophys. Acta 2012, 1818, 2009–2013. [Google Scholar] [CrossRef]
- Schulz, R.; Görge, P.M.; Görbe, A.; Ferdinandy, P.; Lampe, P.D.; Leybaert, L. Connexin 43 is an emerging therapeutic target in ischemia/reperfusion injury, cardioprotection and neuroprotection. Pharmacol. Ther. 2015, 153, 90–106. [Google Scholar] [CrossRef]
- Goubaeva, F.; Mikami, M.; Giardina, S.; Ding, B.; Abe, J.; Yang, J. Cardiac mitochondrial connexin 43 regulates apoptosis. Biochem. Biophys. Res. Commun. 2007, 352, 97–103. [Google Scholar] [CrossRef]
- Li, H.; Brodsky, S.; Kumari, S.; Valiunas, V.; Brink, P.; Kaide, J.; Nasjletti, A.; Goligorsky, M.S. Paradoxical overexpression and translocation of connexin43 in homocysteine-treated endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H2124–H2133. [Google Scholar] [CrossRef]
- Zhou, H.Z.; Ma, X.; Gray, M.O.; Zhu, B.Q.; Nguyen, A.P.; Baker, A.J.; Simonis, U.; Cecchini, G.; Lovett, D.H.; Karliner, J.S. Transgenic MMP-2 expression induces latent cardiac mitochondrial dysfunction. Biochem. Biophys. Res. Commun. 2007, 358, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Akar, F.G.; Aon, M.A.; Tomaselli, G.F.; O’Rourke, B. The mitochondrial origin of postischemic arrhythmias. J. Clin. Investig. 2005, 115, 3527–3535. [Google Scholar] [CrossRef] [PubMed]
- Gambardella, J.; Sorriento, D.; Ciccarelli, M.; Del Giudice, C.; Fiordelisi, A.; Napolitano, L.; Trimarco, B.; Iaccarino, G.; Santulli, G. Functional Role of Mitochondria in Arrhythmogenesis. Adv. Exp. Med. Biol. 2017, 982, 191–202. [Google Scholar] [PubMed]
- Durgan, D.J.; Young, M.E. The cardiomyocyte circadian clock: Emerging roles in health and disease. Circ. Res. 2010, 106, 647–658. [Google Scholar] [CrossRef]
- Sakoh, T.; Nakayama, M.; Tsuchihashi, T.; Yoshitomi, R.; Tanaka, S.; Katafuchi, E.; Fukui, A.; Shikuwa, Y.; Anzai, N.; Kitazono, T.; et al. Associations of fibroblast growth factor 23 with urate metabolism in patients with chronic kidney disease. Metabolism 2016, 65, 1498–1507. [Google Scholar] [CrossRef] [PubMed]
- Kubo, S.; Nishida, Y.; Kubota, Y.; Higashiyama, A.; Sugiyama, D.; Hirata, T.; Miyamatsu, N.; Tanabe, A.; Hirata, A.; Tatsumi, Y.; et al. Higher serum uric acid level is inversely associated with renal function assessed by cystatin C in a Japanese general population without chronic kidney disease: The KOBE study. BMC Nephrol. 2019, 20, 117. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.J.; Kim, S.S.; Bae, M.J.; Yi, Y.S.; Jeon, Y.K.; Kim, B.H.; Song, S.H.; Kim, I.J.; Kim, Y.K. High-normal serum uric acid predicts the development of chronic kidney disease in patients with type 2 diabetes mellitus and preserved kidney function. J. Diabetes Complicat. 2014, 28, 130–134. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).