Neurological Sequelae of Post-COVID-19 Fatigue: A Narrative Review of Dipeptidyl Peptidase IV-Mediated Cerebrovascular Complications
Abstract
1. Introduction
2. SARS-CoV-2, Post-COVID-19 Fatigue, and Multi-Organ Injury
Heart and Brain Crosstalk: Roles of Thrombo-Inflammation and Post-COVID-19 Fatigue
3. Current Updates on Molecular and Cellular Mechanism of Post-COVID-19 Fatigue
3.1. Microstructural Changes—Endothelial Cells (ECs)
3.2. Microstructural Changes—Neuro–Glia–Vascular Unit (NGVU)
3.3. Meso-Structural Responses—Pro-Inflammatory Cells
3.4. Molecular Mechanism of Post-COVID-19 Fatigue
4. Novel Insights into DPPIV’s Role in Metabolic Syndrome and COVID-19
4.1. Potential Role of DPPIV as Receptor for SARS-CoV-2 Infection
4.2. Emerging Research on DPPIV and COVID-19
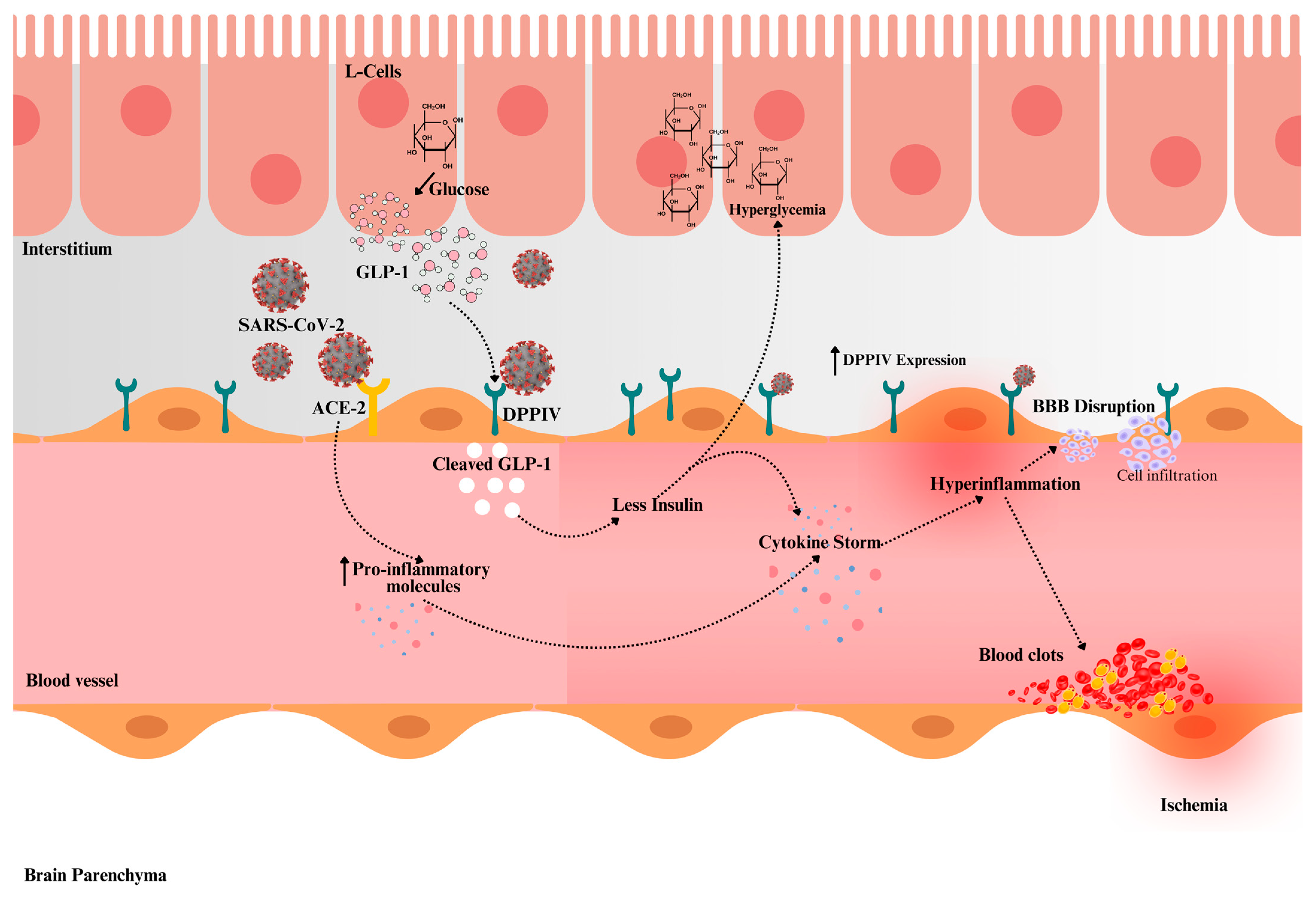
5. DDPIV and Post-COVID-19 Fatigue: Impact on Cerebrovascular Disease
5.1. Confounding Factors Influencing DPPIV-Mediated Post-COVID-19 Fatigue and Cerebrovascular Complications
5.2. Future Recommendations
Limitations of Current Narrative Review
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Centers for Disease Control and Prevention. COVID Data Tracker. 2022. Available online: https://covid.cdc.gov/covid-data-tracker/#datatracker-home (accessed on 8 November 2023).
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.; Ferrando, S.J.; Dornbush, R.; Lynch, S.; Shahar, S.; Klepacz, L.; Smiley, A. Impact of COVID-19 on employment: Sociodemographic, medical, psychiatric and neuropsychological correlates. Front. Rehabil. Sci. 2023, 4, 1150734. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Kuznik, B.I.; Shapovalov, K.G.; Chalisova, N.I. Changes in the state of vital systems with long COVID-19. Biol. Bull. Rev. 2023, 13, 112–123. [Google Scholar] [CrossRef]
- Becerra-García, J.A.; Sánchez-Gutiérrez, T. Long-COVID psychological symptoms in child and adolescent population: A standardized proposal for its exploration. Enfermedades Infecc. Microbiol. Clínica 2023, 41, 384–385. [Google Scholar] [CrossRef]
- Gupta, M.; Gupta, N.; Esang, M. Long COVID in children and adolescents. Prim. Care Companion CNS Disord. 2022, 24, 21r03218. [Google Scholar] [CrossRef]
- Guido, C.A.; Lucidi, F.; Midulla, F.; Zicari, A.M.; Bove, E.; Avenoso, F.; Amedeo, I.; Mancino, E.; Nenna, R.; De Castro, G.; et al. Neurological and psychological effects of long COVID in a young population: A cross-sectional study. Front. Neurol. 2022, 13, 925144. [Google Scholar] [CrossRef]
- Batra, A.; Clark, J.R.; Kang, A.K.; Ali, S.; Patel, T.R.; Shlobin, N.A.; Hoffman, S.C.; Lim, P.H.; Orban, Z.S.; Visvabharathy, L.; et al. Persistent viral RNA shedding of SARS-CoV-2 is associated with delirium incidence and six-month mortality in hospitalized COVID-19 patients. Geroscience 2022, 44, 1241–1254. [Google Scholar] [CrossRef]
- Natarajan, A.; Zlitni, S.; Brooks, E.F.; Vance, S.E.; Dahlen, A.; Hedlin, H.; Park, R.M.; Han, A.; Schmidtke, D.T.; Verma, R.; et al. Gastrointestinal symptoms and fecal shedding of SARS-CoV-2 RNA suggest prolonged gastrointestinal infection. Med 2022, 3, 371–387. [Google Scholar] [CrossRef]
- Herman, J.D.; Atyeo, C.; Zur, Y.; Cook, C.E.; Patel, N.J.; Vanni, K.M.; Kowalski, E.N.; Qian, G.; Srivatsan, S.; Shadick, N.A.; et al. Humoral immunity to an endemic coronavirus is associated with postacute sequelae of COVID-19 in individuals with rheumatic diseases. Sci. Transl. Med. 2023, 6, 15. [Google Scholar] [CrossRef]
- van Kessel, S.A.M.; Olde Hartman, T.C.; Lucassen, P.L.B.J.; van Jaarsveld, C.H.M. Post-acute and long-COVID-19 symptoms in patients with mild diseases: A systematic review. Fam. Pract. 2022, 39, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Rioux, P.; Chaumon, M.; Demers, A.; Fitzback-Fortin, H.; Kübel, S.L.; Lebrun, C.; Mendoza-Duran, E.; Micillo, L.; Racine, C.; Thibault, N.; et al. Psychological time during the COVID-19 lockdown: Canadian data. Timing Time Percept. 2022, 10, 326–343. [Google Scholar] [CrossRef]
- Medline, A.; Hayes, L.; Valdez, K.; Hayashi, A.; Vahedi, F.; Capell, W.; Sonnenberg, J.; Glick, Z.; Klausner, J.D. Evaluating the impact of stay-at-home orders on the time to reach the peak burden of COVID-19 cases and deaths: Does timing matter? BMC Public Health 2020, 20, 1750. [Google Scholar] [CrossRef] [PubMed]
- Elisabeth, A.L.; Karlen, S.B.; Magkos, F. The Effect of COVID-19-related Lockdowns on Diet and Physical Activity in Older Adults: A Systematic Review. Aging Dis. 2021, 12, 1935–1947. [Google Scholar] [CrossRef] [PubMed]
- Holdsworth, D.A.; Chamley, R.; Barker-Davies, R.; O’Sullivan, O.; Ladlow, P.; Mitchell, J.L.; Dewson, D.; Mills, D.; May SL, J.; Cranley, M.; et al. Comprehensive clinical assessment identifies specific neurocognitive deficits in working-age patients with long-COVID. PLoS ONE 2022, 17, e0267392. [Google Scholar] [CrossRef]
- Leng, A.; Shah, M.; Ahmad, S.A.; Premraj, L.; Wildi, K.; Li Bassi, G.; Pardo, C.A.; Choi, A.; Cho, S.M. Pathogenesis underlying neurological manifestations of long COVID syndrome and potential therapeutics. Cells 2023, 12, 816. [Google Scholar] [CrossRef]
- Avittan, H.; Kustovs, D. Cognition and mental health in pediatric patients following COVID-19. Int. J. Environ. Res. Public Health 2023, 20, 5061. [Google Scholar] [CrossRef]
- Govil-Dalela, T.; Sivaswamy, L. Neurological effects of COVID-19 in children. Pediatr. Clin. N. Am. 2021, 68, 1081–1091. [Google Scholar] [CrossRef]
- Bradley, B.T.; Bryan, A. Emerging respiratory infections: The infectious disease pathology of SARS, MERS, pandemic influenza, and Legionella. Semin. Diagn. Pathol. 2019, 36, 152–159. [Google Scholar] [CrossRef]
- Rees, A.R. Immunological challenges of the “new” infections: Corona viruses. In A New History of Vaccines for Infectious Diseases; Elsevier: Amsterdam, The Netherlands, 2022; pp. 395–450. [Google Scholar]
- Desforges, M.; Le Coupanec, A.; Dubeau, P.; Bourgouin, A.; Lajoie, L.; Dubé, M.; Talbot, P.J. Human coronaviruses and other respiratory viruses: Underestimated opportunistic pathogens of the central nervous system? Viruses 2019, 12, 14. [Google Scholar] [CrossRef]
- Chiara, M.; D’Erchia, A.M.; Gissi, C.; Manzari, C.; Parisi, A.; Resta, N.; Zambelli, F.; Picardi, E.; Pavesi, G.; Horner, D.S.; et al. Next generation sequencing of SARS-CoV-2 genomes: Challenges, applications and opportunities. Brief. Bioinform. 2021, 22, 616–630. [Google Scholar] [CrossRef] [PubMed]
- John, G.; Sahajpal, N.S.; Mondal, A.K.; Ananth, S.; Williams, C.; Chaubey, A.; Rojiani, A.M.; Kolhe, R. Next-generation sequencing (NGS) in COVID-19: A tool for SARS-CoV-2 diagnosis, monitoring new strains and phylodynamic modeling in molecular epidemiology. Curr. Issues Mol. Biol. 2021, 43, 845–867. [Google Scholar] [CrossRef] [PubMed]
- Galeotti, C.; Bayry, J. Autoimmune and inflammatory diseases following COVID-19. Nat. Rev. Rheumatol. 2020, 16, 413–414. [Google Scholar] [CrossRef] [PubMed]
- Stein, S.R.; Ramelli, S.C.; Grazioli, A.; Chung, J.Y.; Singh, M.; Yinda, C.K.; Winkler, C.W.; Sun, J.; Dickey, J.M.; Ylaya, K.; et al. SARS-CoV-2 infection and persistence throughout the human body and brain. Nature 2022, 612, 758–763. [Google Scholar] [CrossRef]
- Dennis, A.; Wamil, M.; Alberts, J.; Oben, J.; Cuthbertson, D.J.; Wootton, D.; Crooks, M.; Gabbay, M.; Brady, M.; Hishmeh, L.; et al. Multiorgan impairment in low-risk individuals with post-COVID-19 syndrome: A prospective, community-based study. BMJ Open 2021, 11, e048391. [Google Scholar] [CrossRef]
- Conway, E.M.; Mackman, N.; Warren, R.Q.; Wolberg, A.S.; Mosnier, L.O.; Campbell, R.A.; Gralinski, L.E.; Rondina, M.T.; van de Veerdonk, F.L.; Hoffmeister, K.M.; et al. Understanding COVID-19-associated coagulopathy. Nat. Rev. Immunol. 2022, 22, 639–649. [Google Scholar] [CrossRef]
- Kansagra, A.P.; Goyal, M.S.; Hamilton, S.; Albers, G.W. Collateral effect of COVID-19 on stroke evaluation in the United States. N. Engl. J. Med. 2020, 383, 400–401. [Google Scholar] [CrossRef]
- Newell, K.L.; Waickman, A.T. Inflammation, immunity, and antigen persistence in post-acute sequelae of SARS-CoV-2 infection Immunity and inflammation in post-acute sequelae of SARS-CoV-2 infection. Curr. Opin. Immunol. 2022, 77, 102228. [Google Scholar] [CrossRef]
- Toscano, G.; Palmerini, F.; Ravaglia, S.; Ruiz, L.; Invernizzi, P.; Cuzzoni, M.G.; Franciotta, D.; Baldanti, F.; Daturi, R.; Postorino, P.; et al. Guillain-Barré syndrome associated with SARS-CoV-2. N. Engl. J. Med. 2020, 382, 2574–2576. [Google Scholar] [CrossRef]
- Lao, W.P.; Imam, S.A.; Nguyen, S.A. Anosmia, hyposmia, and dysgeusia as indicators for positive SARS-CoV-2 infection. World J. Otolaryngol. Head Neck Surg. 2020, 6, S22–S25. [Google Scholar] [CrossRef]
- Spudich, S.; Nath, A. Nervous system consequences of COVID-19. Science 2022, 375, 267–269. [Google Scholar] [CrossRef] [PubMed]
- Akimoto, T.; Hara, M.; Tasaki, K.; Kurosawa, Y.; Nakamoto, T.; Hirose, S.; Mizoguchi, T.; Yokota, Y.; Ninomiya, S.; Nakajima, H. Delayed encephalopathy after COVID-19: A case series of six patients. Medicine 2022, 101, e31029. [Google Scholar] [CrossRef] [PubMed]
- Taube, M. Depression and brain fog as long-COVID mental health consequences: Difficult, complex and partially successful treatment of a 72-year-old patient-A case report. Front. Psychiatry 2023, 14, 1153512. [Google Scholar] [CrossRef] [PubMed]
- Savardashtaki, A.; Johnston, T.P.; Sahebkar, A. COVID-19 and cardiac injury: Clinical manifestations, biomarkers, mechanisms, diagnosis, treatment, and follow up. Expert. Rev. Anti Infect. Ther. 2021, 19, 345–357. [Google Scholar]
- Bowe, B.; Xie, Y.; Xu, E.; Al-Aly, Z. Kidney outcomes in Long COVID. J. Am. Soc. Nephrol. 2021, 32, 2851–2862. [Google Scholar] [CrossRef]
- Wang, S.; Farland, L.V.; Gaskins, A.J.; Mortazavi, J.; Wang, Y.X.; Tamimi, R.M.; Rich-Edwards, J.W.; Zhang, D.; Terry, K.L.; Chavarro, J.E.; et al. Association of laparoscopically confirmed endometriosis with long COVID-19: A prospective cohort study. Am. J. Obstet. Gynecol. 2023, 228, 714.e1–714.e13. [Google Scholar] [CrossRef]
- Zhang, D.; Weng, S.; Xia, C.; Ren, Y.; Liu, Z.; Xu, Y.; Yang, X.; Wu, R.; Peng, L.; Sun, L.; et al. Gastrointestinal symptoms of long COVID-19 related to the ectopic colonization of specific bacteria that move between the upper and lower alimentary tract and alterations in serum metabolites. BMC Med. 2023, 21, 264. [Google Scholar] [CrossRef]
- Levi, M.; Thachil, J.; Iba, T.; Levy, J.H. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020, 7, e438–e440. [Google Scholar] [CrossRef]
- Lukiw, W.J.; Pogue, A.; Hill, J.M. SARS-CoV-2 Infectivity and Neurological Targets in the Brain. Cell Mol. Neurobiol. 2022, 42, 217–224. [Google Scholar] [CrossRef]
- Douaud, G.; Lee, S.; Alfaro-Almagro, F.; Arthofer, C.; Wang, C.; McCarthy, P.; Lange, F.; Andersson JL, R.; Griffanti, L.; Duff, E.; et al. SARS-CoV-2 is associated with changes in brain structure in UK biobank. Nature 2022, 604, 697–707. [Google Scholar] [CrossRef]
- Ntaios, G.; Michel, P.; Georgiopoulos, G.; Guo, Y.; Li, W.; Xiong, J.; Calleja, P.; Ostos, F.; González-Ortega, G.; Fuentes, B.; et al. Characteristics and outcomes in patients with COVID-19 and acute ischemic stroke: The global COVID-19 stroke registry. Stroke 2020, 51, e254–e258. [Google Scholar] [CrossRef] [PubMed]
- Karnik, M.; Beeraka, N.M.; Uthaiah, C.A.; Nataraj, S.M.; Bettadapura AD, S.; Aliev, G.; Madhunapantula, S.V. A Review on SARS-CoV-2-induced neuroinflammation, neurodevelopmental complications, and recent updates on the vaccine development. Mol. Neurobiol. 2021, 58, 4535–4563. [Google Scholar] [CrossRef] [PubMed]
- Lyoo, K.S.; Kim, H.M.; Lee, B.; Che, Y.H.; Kim, S.J.; Song, D.; Hwang, W.; Lee, S.; Park, J.H.; Na, W.; et al. Direct neuronal infection of SARS-CoV-2 reveals cellular and molecular pathology of chemosensory impairment of COVID-19 patients. Emerg. Microbes Infect. 2022, 11, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Fahlberg, M.D.; Blair, R.V.; Doyle-Meyers, L.A.; Midkiff, C.C.; Zenere, G.; Russell-Lodrigue, K.E.; Monjure, C.J.; Haupt, E.H.; Penney, T.P.; Lehmicke, G.; et al. Cellular events of acute, resolving or progressive COVID-19 in SARS-CoV-2 infected non-human primates. Nat. Commun. 2020, 11, 6078. [Google Scholar] [CrossRef] [PubMed]
- Michiels, C. Endothelial cell functions. J. Cell Physiol. 2003, 196, 430–443. [Google Scholar] [CrossRef]
- Gavriilaki, E.; Eftychidis, I.; Papassotiriou, I. Update on endothelial dysfunction in COVID-19: Severe disease, long COVID-19 and pediatric characteristics. J. Lab. Med. 2021, 45, 293–302. [Google Scholar] [CrossRef]
- Tarnawski, A.S.; Ahluwalia, A. Endothelial cells and blood vessels are major targets for COVID-19-induced tissue injury and spreading to various organs. World J. Gastroenterol. 2022, 28, 275–289. [Google Scholar] [CrossRef]
- Bayat, A.H.; Azimi, H.; Hassani Moghaddam, M.; Ebrahimi, V.; Fathi, M.; Vakili, K.; Mahmoudiasl, G.R.; Forouzesh, M.; Boroujeni, M.E.; Nariman, Z.; et al. COVID-19 causes neuronal degeneration and reduces neurogenesis in human hippocampus. Apoptosis 2022, 27, 852–868. [Google Scholar] [CrossRef]
- Plantone, D.; Locci, S.; Bergantini, L.; Manco, C.; Cortese, R.; Meocci, M.; Cavallaro, D.; d’Alessandro, M.; Bargagli, E.; De Stefano, N. Brain neuronal and glial damage during acute COVID-19 infection in absence of clinical neurological manifestations. J. Neurol. Neurosurg. Psychiatry 2022, 93, 1343–1348. [Google Scholar] [CrossRef]
- Klein, R.S.; Garber, C.; Howard, N. Infectious immunity in the central nervous system and brain function. Nat. Immunol. 2017, 18, 132–141. [Google Scholar] [CrossRef]
- Wongchitrat, P.; Chanmee, T.; Govitrapong, P. Molecular mechanisms associated with neurodegeneration of neurotropic viral infection. Mol. Neurobiol. 2023, 61, 2881–2903. [Google Scholar] [CrossRef] [PubMed]
- Beckman, D.; Bonillas, A.; Diniz, G.B.; Ott, S.; Roh, J.W.; Elizaldi, S.R.; Schmidt, B.A.; Sammak, R.L.; Van Rompay KK, A.; Iyer, S.S.; et al. SARS-CoV-2 infects neurons and induces neuroinflammation in a non-human primate model of COVID-19. Cell Rep. 2022, 41, 111573. [Google Scholar] [CrossRef]
- Zorzo, C.; Solares, L.; Mendez, M.; Mendez-Lopez, M. Hippocampal alterations after SARS-CoV-2 infection: A systematic review. Behav. Brain Res. 2023, 455, 114662. [Google Scholar] [CrossRef]
- Klein, J.; Wood, J.; Jaycox, J.R.; Dhodapkar, R.M.; Lu, P.; Gehlhausen, J.R.; Tabachnikova, A.; Greene, K.; Tabacof, L.; Malik, A.A.; et al. Distinguishing features of Long COVID identified through immune profiling. Nature 2023, 623, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Glynne, P.; Tahmasebi, N.; Gant, V.; Gupta, R. Long COVID following mild SARS-CoV-2 infection: Characteristic T cell alterations and response to antihistamines. J. Investig. Med. 2022, 70, 61–67. [Google Scholar] [CrossRef]
- Arun, S.; Storan, A.; Myers, B. Mast cell activation syndrome and the link with long COVID. Br. J. Hosp. Med. 2022, 83, 1–10. [Google Scholar] [CrossRef]
- Peluso, M.J.; Lu, S.; Tang, A.F.; Durstenfeld, M.S.; Ho, H.E.; Goldberg, S.A.; Forman, C.A.; Munter, S.E.; Hoh, R.; Tai, V.; et al. Markers of immune activation and inflammation in individuals with postacute sequelae of severe acute respiratory syndrome coronavirus 2 infection. J. Infect. Dis. 2021, 224, 1839–1848. [Google Scholar] [CrossRef] [PubMed]
- Barnes, B.J.; Adrover, J.M.; Baxter-Stoltzfus, A.; Borczuk, A.; Cools-Lartigue, J.; Crawford, J.M.; Daßler-Plenker, J.; Guerci, P.; Huynh, C.; Knight, J.S.; et al. Targeting potential drivers of COVID-19: Neutrophil extracellular traps. J. Exp. Med. 2020, 217, e20200652. [Google Scholar] [CrossRef]
- Wang, R.; Lee, J.H.; Kim, J.; Xiong, F.; Hasani, L.A.; Shi, Y.; Simpson, E.N.; Zhu, X.; Chen, Y.T.; Shivshankar, P.; et al. SARS-CoV-2 restructures host chromatin architecture. Nat. Microbiol. 2023, 8, 679–694. [Google Scholar] [CrossRef]
- Mohamed, M.S.; Johansson, A.; Jonsson, J.; Schiöth, H.B. Review dissecting the molecular mechanisms surrounding post-COVID-19 syndrome and neurological features. Int. J. Mol. Sci. 2022, 23, 4275. [Google Scholar] [CrossRef]
- Ragab, D.; Salah Eldin, H.; Taeimah, M.; Khattab, R.; Salem, R. The COVID-19 cytokine storm; what we know so far. Front. Immunol. 2020, 11, 1446. [Google Scholar] [CrossRef] [PubMed]
- Scurati, R.; Papini, N.; Giussani, P.; Alberti, G.; Tringali, C. The challenge of Long COVID-19 management: From disease molecular hallmarks to the proposal of exercise as therapy. Int. J. Mol. Sci. 2022, 23, 12311. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.A.; Knauer, M.J.; Nicholson, M.; Daley, M.; Van Nynatten, L.R.; Martin, C.; Patterson, E.K.; Cepinskas, G.; Seney, S.L.; Dobretzberger, V.; et al. Elevated vascular transformation blood biomarkers in Long-COVID indicate angiogenesis as a key pathophysiological mechanism. Mol. Med. 2022, 28, 122. [Google Scholar] [CrossRef]
- Gomes, S.M.R.; Brito, A.C.S.; Manfro, W.F.P.; Ribeiro-Alves, M.; Ribeiro, R.S.A.; da Cal, M.S.; Lisboa, V.D.C.; Abreu, D.P.B.; Castilho, L.D.R.; Porto, L.C.M.S.; et al. High levels of pro-inflammatory SARS-CoV-2-specific biomarkers revealed by in vitro whole blood cytokine release assay (CRA) in recovered and long-COVID-19 patients. PLoS ONE 2023, 18, e0283983. [Google Scholar] [CrossRef]
- Petrella, C.; Nenna, R.; Petrarca, L.; Tarani, F.; Paparella, R.; Mancino, E.; Di Mattia, G.; Conti, M.G.; Matera, L.; Bonci, E.; et al. Serum NGF and BDNF in Long-COVID-19 adolescents: A pilot study. Diagnostics 2022, 12, 1162. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020, 181, 271–280. [Google Scholar] [CrossRef]
- Adimulam, T.; Arumugam, T.; Gokul, A.; Ramsuran, V. Genetic Variants within SARS-CoV-2 Human Receptor Genes May Contribute to Variable Disease Outcomes in Different Ethnicities. Int. J. Mol. Sci. 2023, 24, 8711. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Z.; Yang, L.; Lian, X.; Xie, Y.; Li, S.; Xin, S.; Cao, P.; Lu, J. The MERS-CoV receptor DPP4 as a candidate binding target of the SARS-CoV-2 spike. iScience 2020, 23, 101160. [Google Scholar] [CrossRef] [PubMed]
- Nag, S.; Mandal, S.; Mukherjee, O.; Mukherjee, S.; Kundu, R. DPP-4 Inhibitors as a savior for COVID-19 patients with diabetes. Future Virol. 2023, 18, 321–333. [Google Scholar] [CrossRef]
- Bassendine, M.F.; Bridge, S.H.; McCaughan, G.W.; Gorrell, M.D. COVID-19 and comorbidities: A role for dipeptidyl peptidase 4 (DPP4) in disease severity? J. Diabetes 2020, 12, 649–658. [Google Scholar] [CrossRef]
- Wu, K.C.H.; He, Q.; Bennett, A.N.; Li, J.; Chan, K.H.K. Shared genetic mechanism between type 2 diabetes and COVID-19 using pathway-based association analysis. Front. Genet. 2022, 13, 1063519. [Google Scholar] [CrossRef] [PubMed]
- Deacon, C.F. Dipeptidyl peptidase 4 inhibition with sitagliptin: A new therapy for type 2 diabetes. Expert. Opin. Investig. Drugs 2007, 16, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.N.; Gupta, A.M.; Banerjee, D.; Chakrabarti, J.; Raghavendra, P.B. Unraveling DPP4 receptor interactions with SARS-CoV-2 variants and MERS-CoV: Insights into pulmonary disorders via immunoinformatics and molecular dynamics. Viruses 2023, 15, 2056. [Google Scholar] [CrossRef]
- Sebastián-Martín, A.; Sánchez, B.G.; Mora-Rodríguez, J.M.; Bort, A.; Díaz-Laviada, I. Role of dipeptidyl peptidase-4 (DPP4) on COVID-19 physiopathology. Biomedicines 2022, 10, 2026. [Google Scholar] [CrossRef]
- Icard, P.; Lincet, H.; Wu, Z.; Coquerel, A.; Forgez, P.; Alifano, M.; Fournel, L. The key role of Warburg effect in SARS-CoV-2 replication and associated inflammatory response. Biochimie 2021, 180, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Mehdipour, A.R.; Hummer, G. Dual nature of human ACE2 glycosylation in binding to SARS-CoV-2 spike. Proc. Natl. Acad. Sci. USA 2021, 118, e2100425118. [Google Scholar] [CrossRef]
- Lambeir, A.M.; Durinx, C.; Scharpé, S.; De Meester, I. Dipeptidyl-peptidase IV from bench to bedside: An update on structural properties functions clinical aspects of the enzyme, DPP IV. Crit. Rev. Clin. Lab. Sci. 2003, 40, 209–294. [Google Scholar] [CrossRef]
- Arscott, W.T.; LaBauve, A.E.; May, V.; Wesley, U.V. Suppression of neuroblastoma growth by dipeptidyl peptidase IV: Relevance of chemokine regulation and caspase activation. Oncogene 2009, 28, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Gorrell, M.D. Dipeptidyl peptidase IV and related enzymes in cell biology and liver disorders. Clin. Sci. 2005, 108, 277–292. [Google Scholar] [CrossRef]
- Klemann, C.; Wagner, L.; Stephan, M.; von Hörsten, S. Cut to the chase: A review of CD26/dipeptidyl peptidase-4’s (DPP4) entanglement in the immune system. Clin. Exp. Immunol. 2016, 185, 1–21. [Google Scholar] [CrossRef]
- Lu, G.; Hu, Y.; Wang, Q.; Qi, J.; Gao, F.; Li, Y.; Zhang, Y.; Zhang, W.; Yuan, Y.; Bao, J.; et al. Molecular Basis of Binding between Novel Human Coronavirus MERS-CoV and Its Receptor CD26. Nature 2013, 500, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Callebaut, C.; Krust, B.; Jacotot, E.; Hovanessian, A.G. T cell activation antigen, CD26, as a cofactor for entry of HIV in CD4+ cells. Science 1993, 262, 2045–2050. [Google Scholar] [CrossRef] [PubMed]
- Raj, V.S.; Mou, H.; Smits, S.L.; Dekkers, D.H.; Müller, M.A.; Dijkman, R.; Muth, D.; Demmers, J.A.; Zaki, A.; Fouchier, R.A.; et al. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature 2013, 495, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Brooke, G.N.; Prischi, F. Structural and functional modelling of SARS-CoV-2 entry in animal models. Sci. Rep. 2020, 10, 15917. [Google Scholar] [CrossRef]
- Vankadari, N.; Wilce, J.A. Emerging WuHan (COVID-19) coronavirus: Glycan shield and structure prediction of spike glycoprotein and its interaction with human CD26. Emerg. Microbes Infect. 2020, 9, 601–604. [Google Scholar] [CrossRef]
- Radzikowska, U.; Ding, M.; Tan, G.; Zhakparov, D.; Peng, Y.; Wawrzyniak, P.; Wang, M.; Li, S.; Morita, H.; Altunbulakli, C.; et al. Distribution of ACE2, CD147, CD26, and other SARS-CoV-2 associated molecules in tissues and immune cells in health and in asthma, COPD, obesity, hypertension, and COVID-19 risk factors. Allergy 2020, 75, 2829–2845. [Google Scholar] [CrossRef]
- Gao, Q.; Zhang, W.; Li, T.; Yang, G.; Zhu, W.; Chen, N.; Jin, H. Interrelationship between 2019-nCov receptor DPP4 and diabetes mellitus targets based on protein interaction network. Sci. Rep. 2022, 12, 188. [Google Scholar] [CrossRef]
- Singh, A.; Singh, R.S.; Sarma, P.; Batra, G.; Joshi, R.; Kaur, H.; Sharma, A.R.; Prakash, A.; Medhi, B. A Comprehensive Review of Animal Models for Coronaviruses: SARS-CoV-2, SARS-CoV, and MERS-CoV. Virol. Sin. 2020, 35, 290–304. [Google Scholar] [CrossRef]
- Pitocco, D.; Tartaglione, L.; Viti, L.; Di Leo, M.; Pontecorvi, A.; Caputo, S. SARS-CoV-2 and DPP4 inhibition: Is it time to pray for Janus Bifrons? Diabetes Res. Clin. Pract. 2020, 163, 108162. [Google Scholar] [CrossRef]
- Groppa, S.A.; Ciolac, D.; Duarte, C.; Garcia, C.; Gasnaș, D.; Leahu, P.; Efremova, D.; Gasnaș, A.; Bălănuță, T.; Mîrzac, D.; et al. Molecular mechanisms of SARS-CoV-2/COVID-19 pathogenicity on the central nervous system: Bridging experimental probes to clinical evidence and therapeutic interventions. Adv. Exp. Med. Biol. 2022, 1376, 1–27. [Google Scholar]
- Mahgoub, S.; Fatahala, S.S.; Sayed, A.I.; Atya, H.B.; El-Shehry, M.F.; Afifi, H.; Awad, S.M.; El-Hameed, R.H.A.; Taha, H. Novel hit of DPP-4Is as promising antihyperglycemic agents with dual antioxidant/anti-inflammatory effects for type 2 diabetes with/without COVID-19. Bioorg. Chem. 2022, 128, 106092. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Su, X.; Cao, W.; Tan, M.; Zhu, G.; Gao, J.; Zhou, L. The discovery and characterization of a potent DPP-IV inhibitory peptide from oysters for the treatment of type 2 diabetes based on computational and experimental studies. Mar. Drugs 2024, 22, 361. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.M.; Park, J.H. Pleiotropic benefits of DPP-4 inhibitors beyond glycemic control. Clin. Med. Insights Endocrinol. Diabetes 2021, 14, 11795514211051698. [Google Scholar] [CrossRef]
- Rajpal, A.; Sayyed Kassem, L.; Aron, D.C. Management of diabetes in elderly patients during the COVID-19 pandemic: Current and future perspectives. Expert. Rev. Endocrinol. Metab. 2021, 16, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Nádasdi, Á.; Sinkovits, G.; Bobek, I.; Lakatos, B.; Förhécz, Z.; Prohászka, Z.Z.; Réti, M.; Arató, M.; Cseh, G.; Masszi, T.; et al. Decreased circulating dipeptidyl peptidase-4 enzyme activity is prognostic for severe outcomes in COVID-19 inpatients. Biomark. Med. 2022, 16, 317–330. [Google Scholar] [CrossRef]
- Wesley, U.V.; Hatcher, J.F.; Ayvaci, E.R.; Klemp, A.; Dempsey, R.J. Regulation of Dipeptidyl Peptidase IV in the post-stroke rat brain and in vitro ischemia: Implications for chemokine-mediated neural progenitor cell migration and angiogenesis. Mol. Neurobiol. 2017, 54, 4973–4985. [Google Scholar] [CrossRef]
- Rudroff, T.; Workman, C.D.; Bryant, A.D. Potential factors that contribute to post-COVID-19 fatigue in women. Brain Sci. 2022, 12, 556. [Google Scholar] [CrossRef]
- Hong, S.; Jung, C.H.; Han, S.; Park, C.Y. Increasing age associated with higher dipeptidyl peptidase-4 inhibition rate is a predictive factor for efficacy of dipeptidyl peptidase-4 inhibitors. Diabetes Metab. J. 2022, 46, 63–70. [Google Scholar] [CrossRef]
- Akinosoglou, K.; Schinas, G.; Bletsa, E.; Bristianou, M.; Lanaras, L.; Michailides, C.; Katsikas, T.; Barkas, F.; Liberopoulos, E.; Kotsis, V.; et al. COVID-19 outcomes and diabetes mellitus: A comprehensive multicenter prospective cohort study. Microorganisms 2023, 11, 1416. [Google Scholar] [CrossRef]
- Park, M.J.; Hwang, J.; Ahn, J.; Park, S.J.; Song, E.; Jang, A.; Choi, K.M.; Baik, S.H.; Yoo, H.J. Ischaemic stroke in patients with diabetes requiring urgent procedures during the COVID-19 pandemic in South Korea: A retrospective, nationwide, population-based cohort study using data from the National Emergency Department Information System. BMJ Open 2023, 13, e074381. [Google Scholar] [CrossRef]
- Malempati, M.; Patel, M.; Patel, J. Ischemic stroke and COVID-19 infection—A review of clinical case reports. Egypt J. Intern. Med. 2024, 36, 50. [Google Scholar] [CrossRef]
- Narayanan, N.; Naik, D.; Sahoo, J.; Kamalanathan, S. Dipeptidyl peptidase 4 inhibitors in COVID-19: Beyond glycemic control. World J. Virol. 2022, 11, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Posadas-Sánchez, R.; Sánchez-Muñoz, F.; Guzmán-Martín, C.A.; Hernández-Díaz Couder, A.; Rojas-Velasco, G.; Fragoso, J.M.; Vargas-Alarcón, G. Dipeptidylpeptidase-4 levels and DPP4 gene polymorphisms in patients with COVID-19. Association with disease and with severity. Life Sci. 2021, 276, 119410. [Google Scholar] [CrossRef] [PubMed]
- Bhargave, A.; Devi, K.; Ahmad, I.; Yadav, A.; Gupta, R. Genetic variation in DPP-IV gene linked to predisposition of T2DM: A case control study. J. Diabetes Metab. Disord. 2022, 21, 1709–1716. [Google Scholar] [CrossRef]
- Adli , A.; Rahimi, M.; Khodaie, R.; Hashemzaei, N.; Hosseini, S.M. Role of genetic variants and host polymorphisms on COVID-19: From viral entrance mechanisms to immunological reactions. J. Med. Virol. 2022, 94, 1846–1865. [Google Scholar] [CrossRef]
| Organ/System. | Sequel Symptoms | References |
|---|---|---|
| Nervous System | Brain fog; cognitive and memory | [31,32,33,34,35,36,37] |
| Dizziness | ||
| Headache | ||
| Stroke | ||
| Joint pain | ||
| EC dysfunction—BBB damage | ||
| Microthromboembolism | ||
| Anxiety, stress, and depression | ||
| Anosmia (loss of sense of smell) | ||
| Ageusia (loss of taste) | ||
| Insomnia (sleep disturbance) | ||
| Guillain–Barré syndrome | ||
| Chronic fatigue | ||
| Encephalopathy | ||
| Respiratory | Cough | [4,5] |
| Sore throat | ||
| Dyspnea (difficulty of breathing) | ||
| Modified diffusion capacity restricting pattern, and obstructive pattern | ||
| Cardiovascular | Myocardial damage | [38] |
| Palpitations | ||
| An irregular pulse | ||
| Chest pain | ||
| Myocarditis | ||
| Gastrointestinal | Diarrhea | [39,40,41] |
| Vomiting/nausea |
| Mechanism | DPPIV Role | COVID-19-Related Cerebrovascular Complications |
|---|---|---|
| Endothelial dysfunction | DPPIV degrades GLP-1, leading to endothelial damage and impaired vascular function | Increases risk of stroke, microthrombi, and blood–brain barrier disruption |
| Inflammatory pathways | DPPIV activates pro-inflammatory cytokines (TNF-α, IL-6, and IL-1β), enhancing immune response | Exacerbates vascular inflammation and thrombotic events |
| Glucose metabolism | DPPIV’s regulation of glucose homeostasis is disrupted, particularly in diabetic patients | Hyperglycemia worsens inflammation and thrombosis, leading to stroke |
| Therapeutic inhibition | DPPIV inhibitors (gliptins) reduce inflammation, endothelial dysfunction, and hyperglycemia | Potential to lower cerebrovascular risks in COVID-19 patients |
| Confounding Factor | Impact on DPPIV Activity | Influence on Post-COVID-19 Complications |
|---|---|---|
| Age | Increases with age, leading to elevated inflammation and endothelial dysfunction | Heightened risk of stroke, cognitive decline, and post-COVID-19 fatigue |
| Diabetes and obesity | Elevated DPPIV levels due to glucose metabolism dysregulation | Worsens hyperglycemia and inflammation, and increases cerebrovascular risks |
| Hypertension | Increased DPPIV linked to worsened endothelial dysfunction and inflammation | Greater risk of cerebrovascular complications and neurovascular damage |
| Genetic predisposition | Genetic variations may influence DPPIV expression and activity | May heighten susceptibility to cerebrovascular and inflammatory complications |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Che Mohd Nassir, C.M.N.; Che Ramli, M.D.; Jaffer, U.; Abdul Hamid, H.; Mehat, M.Z.; Mohamad Ghazali, M.; Kottakal Cheriya, E.N. Neurological Sequelae of Post-COVID-19 Fatigue: A Narrative Review of Dipeptidyl Peptidase IV-Mediated Cerebrovascular Complications. Curr. Issues Mol. Biol. 2024, 46, 13565-13582. https://doi.org/10.3390/cimb46120811
Che Mohd Nassir CMN, Che Ramli MD, Jaffer U, Abdul Hamid H, Mehat MZ, Mohamad Ghazali M, Kottakal Cheriya EN. Neurological Sequelae of Post-COVID-19 Fatigue: A Narrative Review of Dipeptidyl Peptidase IV-Mediated Cerebrovascular Complications. Current Issues in Molecular Biology. 2024; 46(12):13565-13582. https://doi.org/10.3390/cimb46120811
Chicago/Turabian StyleChe Mohd Nassir, Che Mohd Nasril, Muhammad Danial Che Ramli, Usman Jaffer, Hafizah Abdul Hamid, Muhammad Zulfadli Mehat, Mazira Mohamad Ghazali, and Ebrahim Nangarath Kottakal Cheriya. 2024. "Neurological Sequelae of Post-COVID-19 Fatigue: A Narrative Review of Dipeptidyl Peptidase IV-Mediated Cerebrovascular Complications" Current Issues in Molecular Biology 46, no. 12: 13565-13582. https://doi.org/10.3390/cimb46120811
APA StyleChe Mohd Nassir, C. M. N., Che Ramli, M. D., Jaffer, U., Abdul Hamid, H., Mehat, M. Z., Mohamad Ghazali, M., & Kottakal Cheriya, E. N. (2024). Neurological Sequelae of Post-COVID-19 Fatigue: A Narrative Review of Dipeptidyl Peptidase IV-Mediated Cerebrovascular Complications. Current Issues in Molecular Biology, 46(12), 13565-13582. https://doi.org/10.3390/cimb46120811







