Comprehensive Transcriptomic and Physiological Insights into the Response of Root Growth Dynamics During the Germination of Diverse Sesame Varieties to Heat Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material, Heat Treatment, and Sample Collection
2.2. Phenotypic and Physiological Measurements
2.3. Quantification of IAA and ABA by Enzyme-Linked Immunosorbent Assay (ELISA) Kit
2.4. RNA-Seq and Data Analysis
2.5. Validation of RNA-Seq Results by qRT-PCR
2.6. Statistical Analysis
3. Results
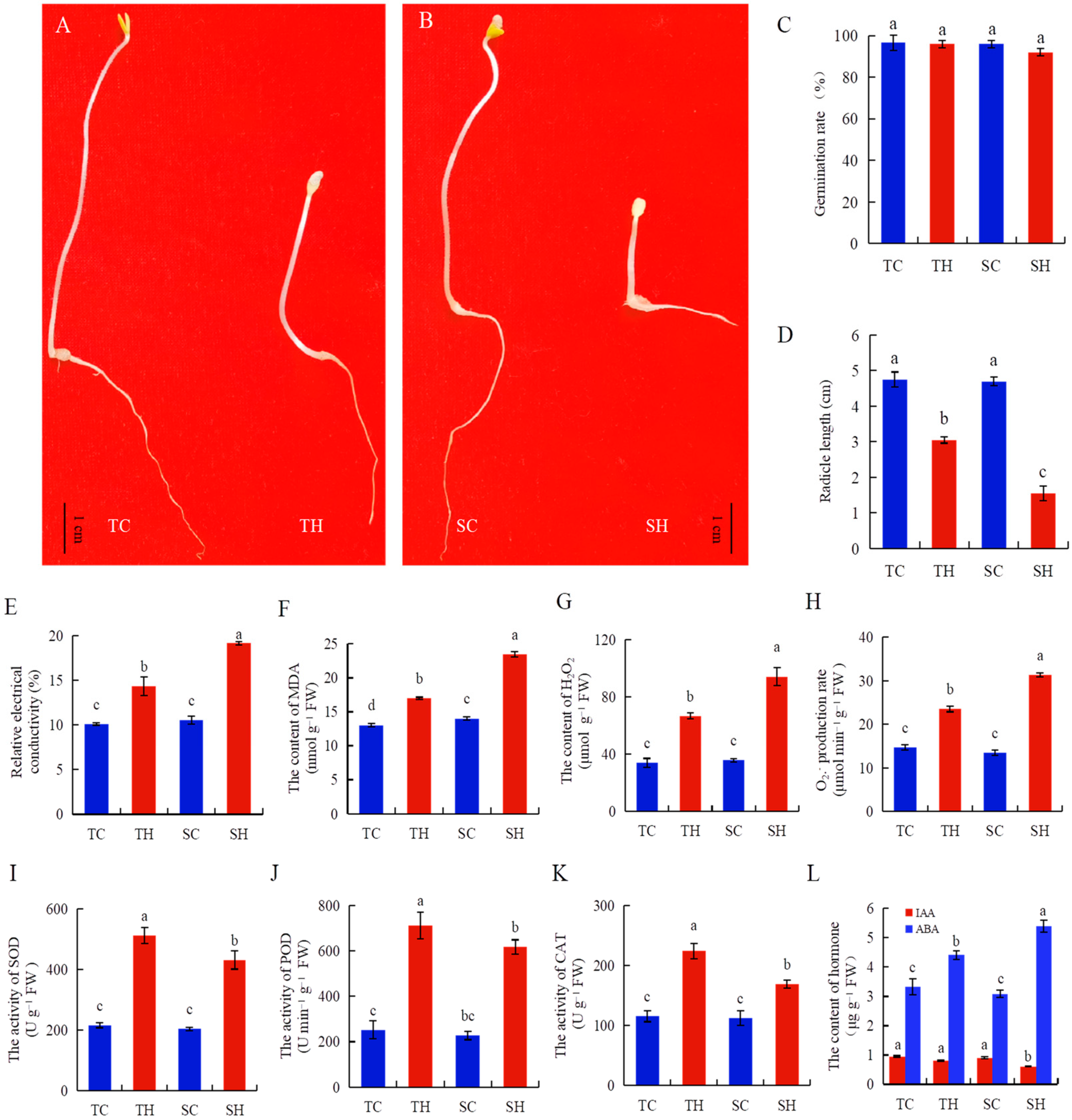
3.1. The Roots of Zheng Taizhi 3 Are More Thermal Resistant than SP19 During Germination
3.2. Overview of RNA-Seq
3.3. Identification and Analysis of Antioxidant Enzyme Related Genes
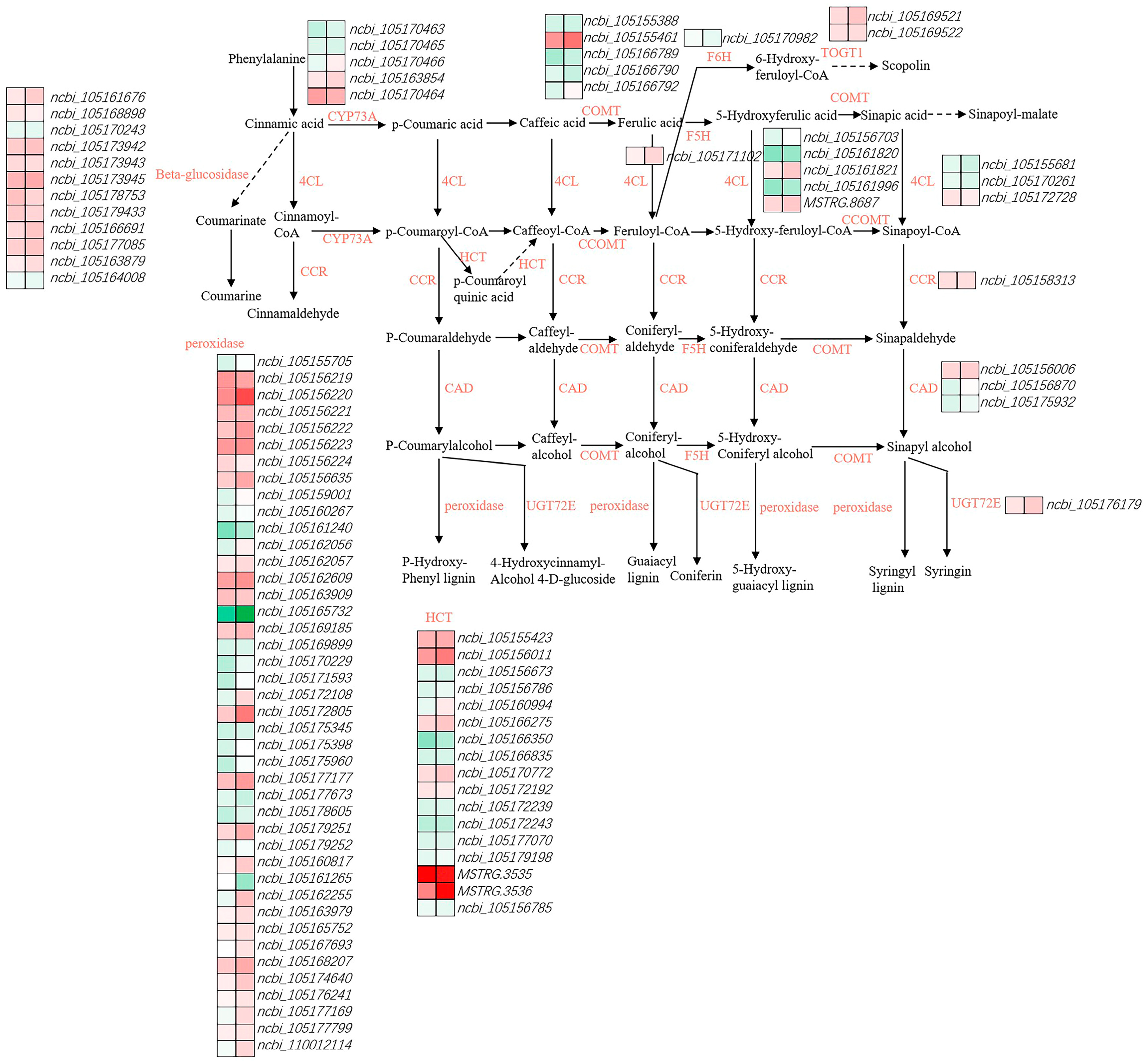
3.4. Identification and Analysis of Phenylpropanoid Biosynthesis-Related Genes
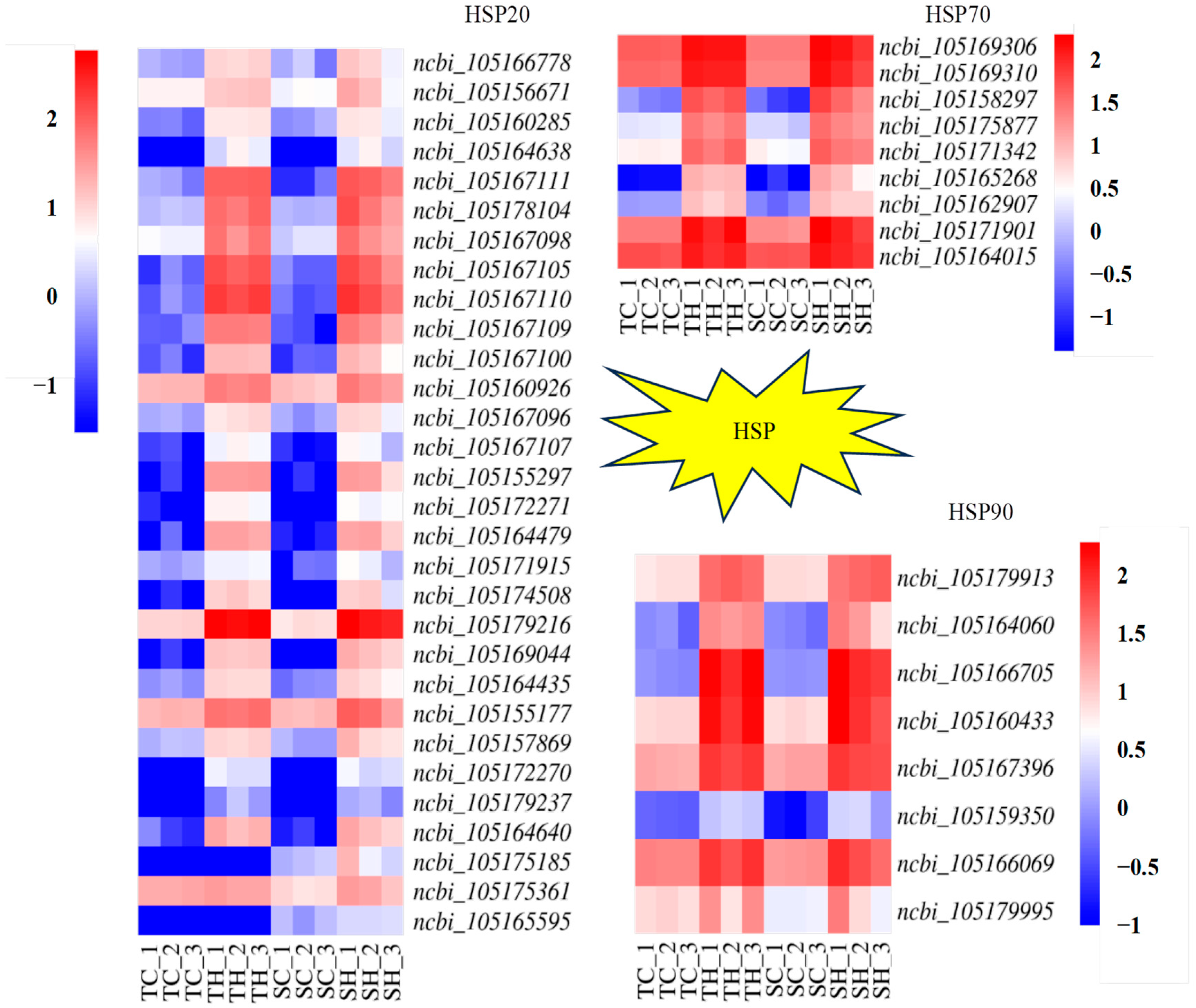
3.5. Identification and Analysis of HSP-Encoding Genes
3.6. Identification and Analysis of Signal Transduction-Related Genes
4. Discussion
4.1. Physiological Response of Root Growth Dynamics During the Germination of Diverse Sesame Varieties to Heat Stress
4.2. Phenylpropanoid Biosynthesis of the Root Growth Dynamics During the Germination of Diverse Sesame Varieties in Response to Heat Stress
4.3. HSP of the Root Growth Dynamics During the Germination of Diverse Sesame Varieties in Response to Heat Stress
4.4. Plant Hormone Signal Transduction of the Root Growth Dynamics During the Germination of Diverse Sesame Varieties in Reponse to Heat Stress
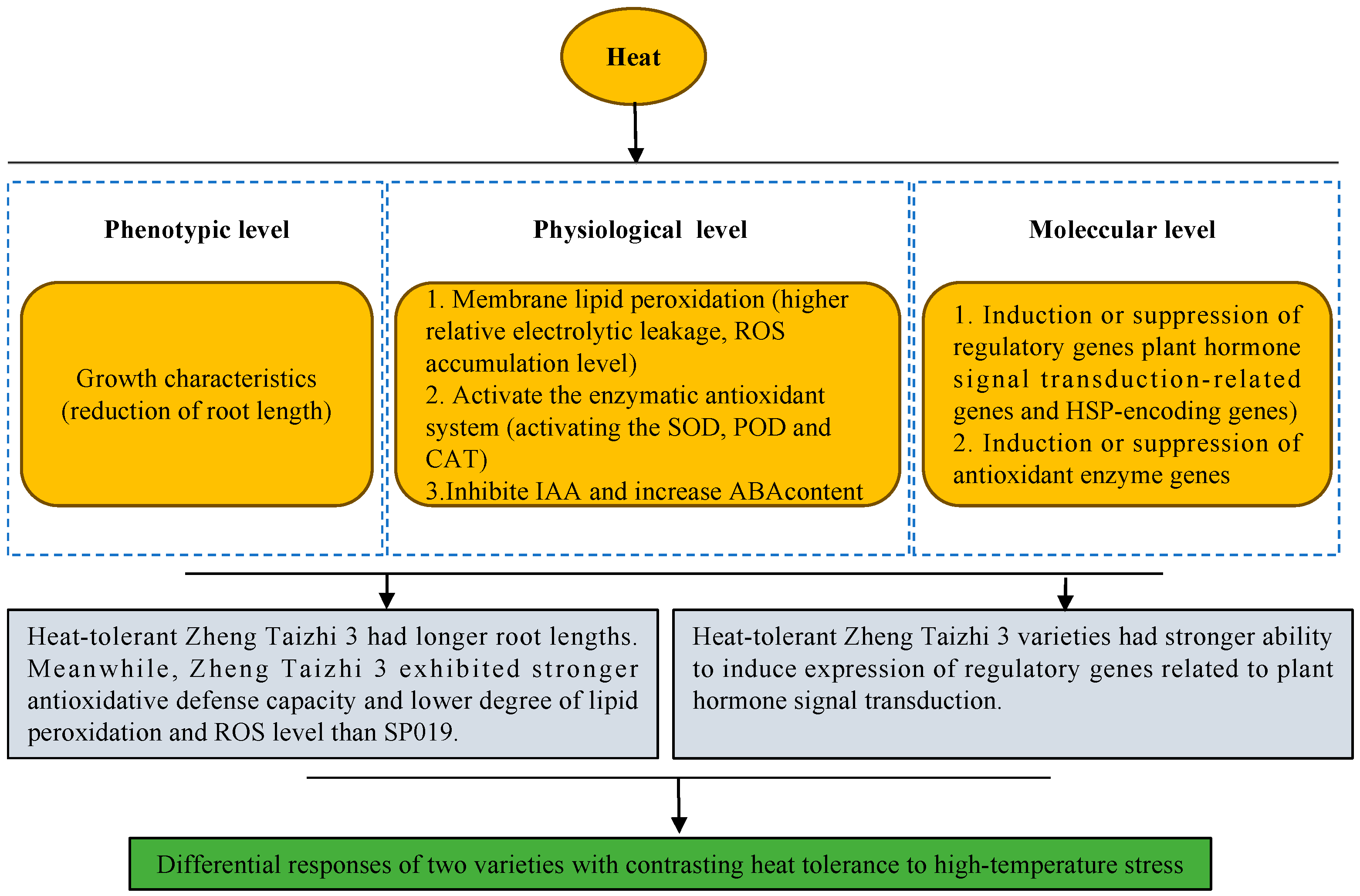
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lesk, C.; Rowhani, P.; Ramankutty, N. Influence of extreme weather disasters on global crop production. Nature 2016, 529, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Roychowdhury, R.; Fujita, M. Physiological, biochemical, and molecular mechanisms of heat Stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef] [PubMed]
- Baath, G.; Kakani, V.; Northup, B.; Gowda, P.; Rocateli, A.; Singh, H. Quantifying and modeling the influence of temperature on growth and reproductive development of sesame. J. Plant Growth Regul. 2020, 41, 143–152. [Google Scholar] [CrossRef]
- Su, X.; Gao, T.; Zhang, P.; Li, F.; Wang, D.; Tian, Y.; Lu, H.; Zhang, H.; Wei, S. Comparative physiological and transcriptomic analysis of sesame cultivars with different tolerance responses to heat stress. Physiol. Mol. Biol. Plants 2022, 28, 1131–1146. [Google Scholar] [CrossRef]
- Jacob, P.; Hirt, H.; Bendahmane, A. The heat-shock protein/chaperone network and multiple stress resistance. Plant Biotechnol. J. 2017, 15, 405–414. [Google Scholar] [CrossRef]
- Zhang, Y.; Zou, B.; Lu, S.; Ding, Y.; Liu, H.; Hua, J. Expression and promoter analysis of the OsHSP16.9C gene in rice. Biochem. Biophys. Res. Commun. 2016, 479, 60–265. [Google Scholar] [CrossRef]
- Bor, M.; Seckin, B.; Ozgur, R.; Yılmaz, Ö. Comparative effects of drought, salt, heavy metal and heat stresses on gamma-aminobutryric acid levels of sesame (Sesamum indicum L.). Acta Physiol. Plant. 2009, 31, 655–659. [Google Scholar] [CrossRef]
- Giri, A.; Heckathorn, S.; Mishra, S.; Krause, C. Heat stress decreases levels of nutrient-uptake and -assimilation proteins in tomato roots. Plants 2017, 6, 6. [Google Scholar] [CrossRef]
- Heckathorn, S.A.; Giri, A.; Mishra, S.; Bista, D. Heat stress and roots. In Climate Change and Plant Abiotic Stress Tolerance; Wiley-Blackwell: Hoboken, NJ, USA, 2013; Volume 5, pp. 109–136. [Google Scholar]
- Zhao, Y.; Liu, Y.; Ji, X.; Sun, J.; Hu, X. Physiological and proteomic analyses reveal cAMP-regulated key factors in maize root tolerance to heat stress. Food Energy Secur. 2021, 2, e309. [Google Scholar] [CrossRef]
- Xu, Q.; Huang, B. Morphological and physiological characteristics associated with heat tolerance in creeping bentgrass. Crop Sci. 2021, 41, 127–133. [Google Scholar] [CrossRef]
- Jiang, Y.; Huang, B. Physiological responses to heat stress alone or in combination with drought: A comparison between tall fescue and perennial ryegrass. Hortscience 2001, 36, 682–686. [Google Scholar] [CrossRef]
- Hong, J.; Geem, K.R.; Kim, J.; Jo, I.H.; Yang, T.J.; Shim, D.; Ryu, H. Prolonged exposure to high temperature inhibits shoot primary and root secondary growth in panax ginseng. Int. J. Mol. Sci. 2022, 23, 11647. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Yang, Z.; Xu, C.; Wang, L.; Huang, H.; Yang, S. The Interactive effects of daytime high temperature and humidity on growth and endogenous hormone concentration of tomato seedlings. HortScience 2022, 55, 1575–1583. [Google Scholar] [CrossRef]
- Su, X.; Xin, L.; Li, Z.; Zheng, H.; Mao, J.; Yang, Q. Physiology and transcriptome analyses reveal a protective effect of the radical scavenger melatonin in aging maize seeds. Free. Radic. Res. 2018, 52, 1094–1109. [Google Scholar] [CrossRef]
- Kulshreshtha, M.; Gopal, B. Allelopathic influence of Hydrilla verticillata (L.F.) Royle on the distribution of Ceratophyllum species. Aquat. Bot. 1983, 16, 207–209. [Google Scholar] [CrossRef]
- Elstner, E.F.; Heupel, A. Inhibition of nitrite formation from hydroxylammoniumchloride: A simple assay for superoxide dismutase. Anal. Biochem. 1976, 70, 616–620. [Google Scholar] [CrossRef]
- Brennan, T.; Frenkel, C. Involvement of hydrogen peroxide in the regulation of senescence in pear. Plant Physiol. 1977, 59, 411–416. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, J.H. Effect of abscisic acid on active oxygen species, antioxidative defence system and oxidative damage in leaves of maize seedlings. Plant Cell Physiol. 2001, 42, 1265–1273. [Google Scholar] [CrossRef]
- Ma, H.; Li, P.; Liu, X.; Li, C.; Zhang, S.; Wang, X.; Tao, X. Poly-γ-glutamic acid enhanced the drought resistance of maize by improving photosynthesis and affecting the rhizosphere microbial community. BMC Plant Biol. 2022, 22, 11. [Google Scholar] [CrossRef]
- Tian, Z.; Zhao, M.; Wang, J.; Yang, Q.; Ma, Y.; Yang, X.; Ma, L.; Qi, Y.; Li, J.; Quinet, M.; et al. Exogenous melatonin improves germination rate in buckwheat under high temperature stress by regulating seed physiological and biochemical characteristics. PeerJ 2024, 12, e17136. [Google Scholar] [CrossRef]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive oxygen species homeostasis and signaling during drought and salinity stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Y.; Tan, S.; Li, Z.; Yuan, Z.; Glanc, M.; Domjan, D.; Wang, K.; Xuan, W.; Guo, Y.; et al. Root growth adaptation is mediated by PYLs ABA receptor-PP2A protein phosphatase complex. Adv. Sci. 2020, 7, e1901455. [Google Scholar] [CrossRef]
- Wu, C.; Cui, K.; Wang, W.; Li, Q.; Fahad, S.; Hu, Q.; Huang, J.; Nie, L.; Peng, S. Heat-induced phytohormone changes are associated with disrupted early reproductive development and reduced yield in rice. Sci. Rep. 2016, 6, e34978. [Google Scholar] [CrossRef] [PubMed]
- Musatenko, L.; Vedenicheva, N.; Vasyuk, V.; Generalova, V.; Martyn, G.; Sytnik, K. Phytohormones in seedlings of maize hybrids differing in their tolerance to high temperatures. Russ. J. Plant Physiol. 2003, 50, 444–448. [Google Scholar] [CrossRef]
- Vogt, T. Phenylpropanoid biosynthesis. Mol. Plant 2010, 3, 2–20. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Lu, S. Biosynthesis and regulation of phenylpropanoids in plants. Crit. Rev. Plant Sci. 2017, 36, 257–290. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, R.; Yang, L.; Duan, X.; Zhang, C.; Yu, X.; Ye, X. Transcriptome analyses revealed the wax and phenylpropanoid biosynthesis pathways related to disease resistance in rootstock-grafted cucumber. Plants 2023, 12, 2963. [Google Scholar] [CrossRef]
- Hu, L.; Hao, C.; Fan, R.; Wu, B.; Tan, L.; Wu, H. De Novo assembly and characterization of fruit transcriptome in black pepper (Piper nigrum). PLoS ONE 2015, 10, e0129822. [Google Scholar] [CrossRef]
- Ul Haq, S.; Khan, A.; Ali, M.; Khattak, A.M.; Gai, W.-X.; Zhang, H.-X.; Wei, A.-M.; Gong, Z.-H. Heat shock proteins: Dynamic biomolecules to counter plant biotic and abiotic stresses. Int. J. Mol. Sci. 2019, 20, 5321. [Google Scholar] [CrossRef]
- Cho, E.-K.; Hong, C.-B. Over-expression of NtHSP70-1 protects chlorophyll from high temperature in plants. J. Life Sci. 2008, 18, 99–103. [Google Scholar] [CrossRef]
- Sedaghatmehr, M.; Mueller-Roeber, B.; Balazadeh, S. The plastid metalloprotease FtsH6 and small heat shock protein HSP21 jointly regulate thermomemory in Arabidopsis. Nat. Commun. 2016, 7, e12439. [Google Scholar] [CrossRef] [PubMed]
- Kotak, S.; Larkindale, J.; Lee, U.; von Koskull-Döring, P.; Vierling, E.; Scharf, K.D. Complexity of the heat stress response in plants. Curr. Opin. Plant Biol. 2007, 10, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Fragkostefanakis, S.; Simm, S.; Paul, P.; Bublak, D.; Scharf, K.D.; Schleiff, E. Chaperone network composition in Solanum lycopersicum explored by transcriptome profiling and microarray meta-analysis. Plant Cell Environ. 2015, 38, 693–709. [Google Scholar] [CrossRef] [PubMed]
- Teale, W.D.; Paponov, I.A.; Palme, K. Auxin in action: Signalling, transport and the control of plant growth and development. Nat. Rev. Mol. Cell Biol. 2006, 7, 847–859. [Google Scholar] [CrossRef]
- De Wit, M.; Lorrain, S.; Fankhauser, C. Auxin-mediated plant architectural changes in response to shade and high temperature. Physiol. Plant 2014, 151, 13–24. [Google Scholar] [CrossRef]
- Sharma, L.; Dalal, M.; Verma, R.K.; Kumar, S.V.V.; Yadav, S.K.; Pushkar, S.; Kushwaha, S.R.; Bhowmik, A.; Chinnusamy, V. Auxin protects spikelet fertility and grain yield under drought and heat stresses in rice. Environ. Exp. Bot. 2018, 150, 9–24. [Google Scholar] [CrossRef]
- Mo, X.; Qian, J.; Liu, P.; Zeng, H.; Chen, G.; Wang, Y. Exogenous betaine enhances the protrusion vigor of rice seeds under heat stress by regulating plant hormone signal transduction and its interaction network. Antioxidants 2022, 11, 1792. [Google Scholar] [CrossRef]
- Swarup, R.; Kargul, J.; Marchant, A.; Zadik, D.; Rahman, A.; Mills, R.; Yemm, A.; May, S.; Williams, L.; Millner, P.; et al. Structure-function analysis of the presumptive arabidopsis auxin permease AUX1. Plant Cell 2004, 16, 3069–3083. [Google Scholar] [CrossRef]
- Wang, J.; An, C.; Guo, H.; Yang, X.; Chen, J.; Zong, J.; Li, J.; Liu, J. Physiological and transcriptomic analyses reveal the mechanisms underlying the salt tolerance of Zoysia japonica Steud. BMC Plant Biol. 2020, 20, 114. [Google Scholar] [CrossRef]
- Lee, S.C.; Luan, S. ABA signal transduction at the crossroad of biotic and abiotic stress responses. Plant Cell Environ. 2012, 35, 53–60. [Google Scholar] [CrossRef]
- Zhang, Q.; Kong, X.; Yu, Q.; Ding, Y.; Li, X.; Yang, Y. Responses of PYR/PYL/RCAR ABA receptors to contrasting stresses, heat and cold in arabidopsis. Plant Signal. Behav. 2019, 14, e1670596. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, X.; Li, C.; Yu, Y.; Li, L.; Wang, L.; Lu, D.; Zhao, Y.; Sun, Y.; Tan, Z.; Liang, H. Comprehensive Transcriptomic and Physiological Insights into the Response of Root Growth Dynamics During the Germination of Diverse Sesame Varieties to Heat Stress. Curr. Issues Mol. Biol. 2024, 46, 13311-13327. https://doi.org/10.3390/cimb46120794
Su X, Li C, Yu Y, Li L, Wang L, Lu D, Zhao Y, Sun Y, Tan Z, Liang H. Comprehensive Transcriptomic and Physiological Insights into the Response of Root Growth Dynamics During the Germination of Diverse Sesame Varieties to Heat Stress. Current Issues in Molecular Biology. 2024; 46(12):13311-13327. https://doi.org/10.3390/cimb46120794
Chicago/Turabian StyleSu, Xiaoyu, Chunming Li, Yongliang Yu, Lei Li, Lina Wang, Dandan Lu, Yulong Zhao, Yao Sun, Zhengwei Tan, and Huizhen Liang. 2024. "Comprehensive Transcriptomic and Physiological Insights into the Response of Root Growth Dynamics During the Germination of Diverse Sesame Varieties to Heat Stress" Current Issues in Molecular Biology 46, no. 12: 13311-13327. https://doi.org/10.3390/cimb46120794
APA StyleSu, X., Li, C., Yu, Y., Li, L., Wang, L., Lu, D., Zhao, Y., Sun, Y., Tan, Z., & Liang, H. (2024). Comprehensive Transcriptomic and Physiological Insights into the Response of Root Growth Dynamics During the Germination of Diverse Sesame Varieties to Heat Stress. Current Issues in Molecular Biology, 46(12), 13311-13327. https://doi.org/10.3390/cimb46120794





