Desialylation and Apoptosis in Immune Thrombocytopenia: Implications for Pathogenesis and Treatment
Abstract
1. Introduction
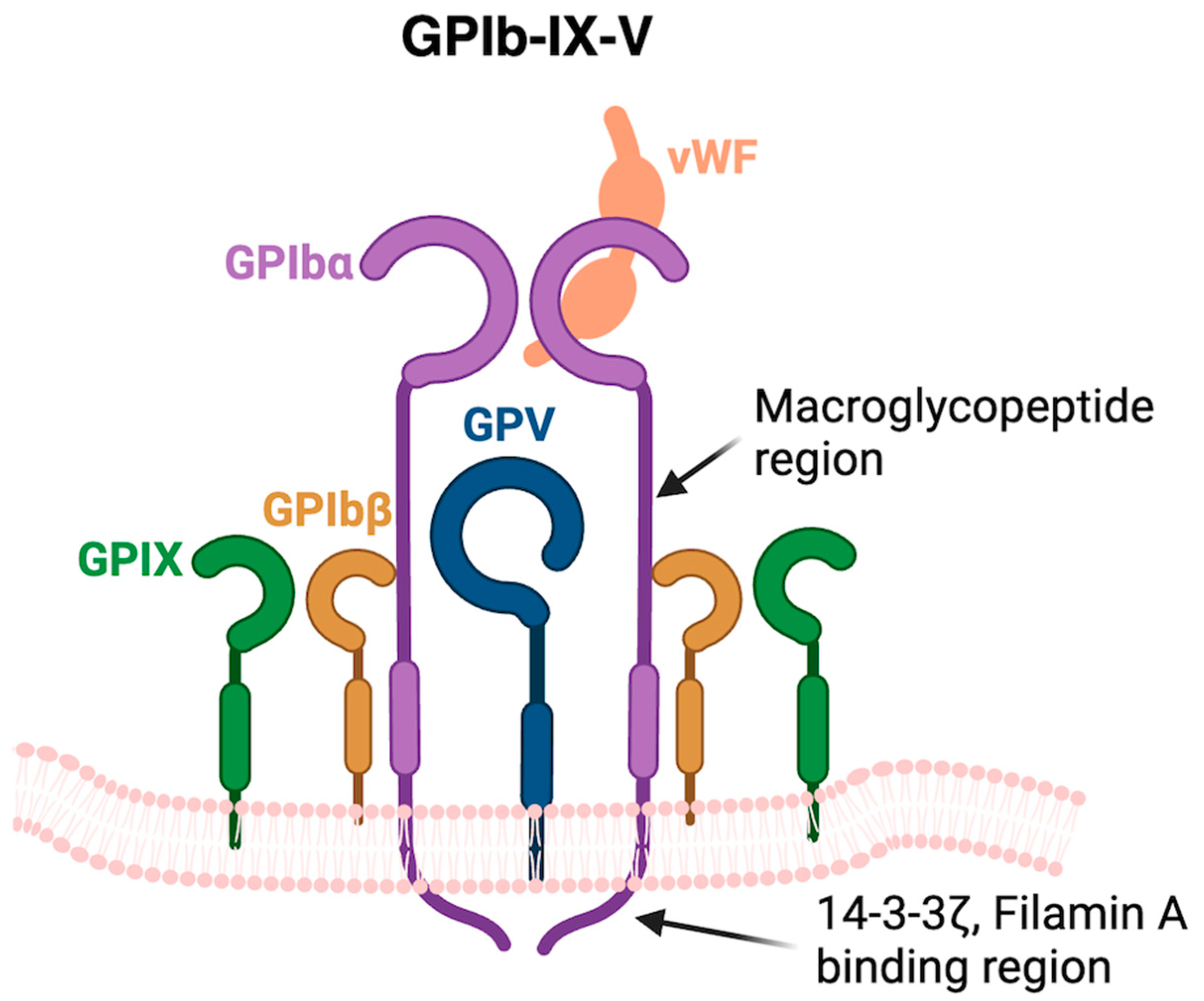
2. Platelet Biogenesis and the Regulation of Lifespan
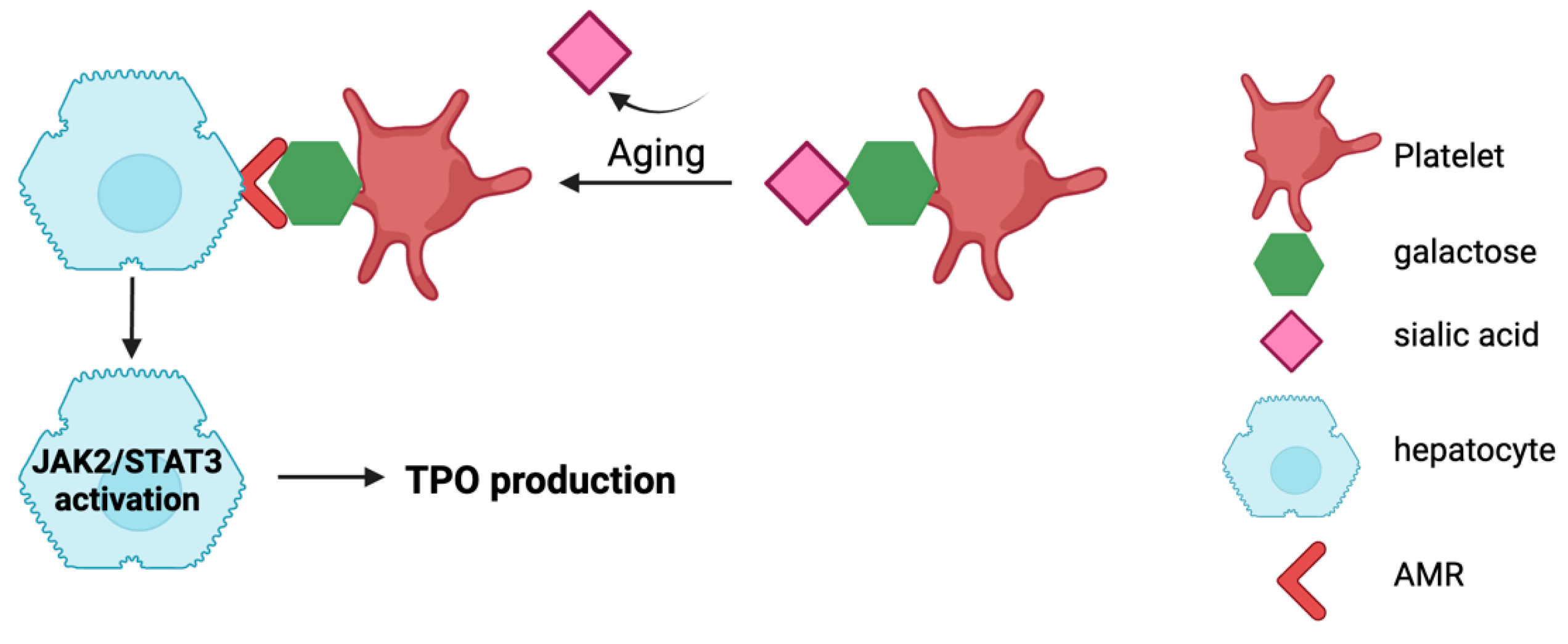
2.1. Platelet Desialylation and the Regulation of Lifespan
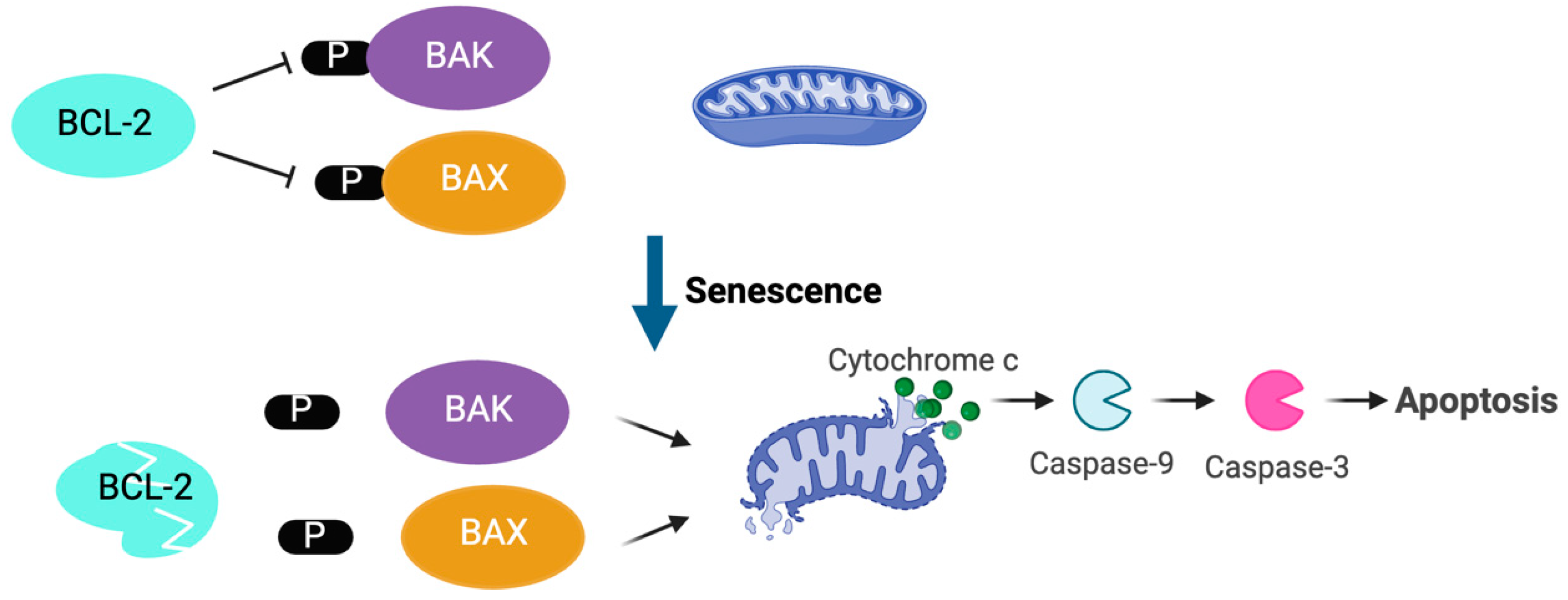
2.2. Platelet Apoptosis and Platelet Lifespan
| Comment | Reference; Year |
|---|---|
| Nucleus is not required for apoptosis | [47]; 1994 |
| Apoptosis-like events associated with platelet activation | [48]; 1999 |
| Anti-platelet antibodies modulate caspase activity and regulate platelet lifespan in mice | [49]; 2002 |
| Apoptosis associated with shortened platelet survival in rabbits | [50]; 2004 |
| Anti GPIIb antibody induces platelet apoptosis in mice | [51]; 2006 |
| Thrombin induces platelet apoptosis | [52,53]; 2007, 2006 |
| Apoptosis program controls platelet lifespan | [42]; 2007 |
| Cold storage leads to platelet apoptosis | [54]; 2010 |
| Apoptotic platelets observed in paediatric patients with ITP | [55]; 2012 |
| Platelet apoptosis in adult ITP | [56,57]; 2018, 2016 |
| The presence of anti-platelet antibodies predicts apoptosis in ITP | [38]; 2022 |
3. Immune Thrombocytopenia
3.1. Presentation
3.2. Pathogenesis
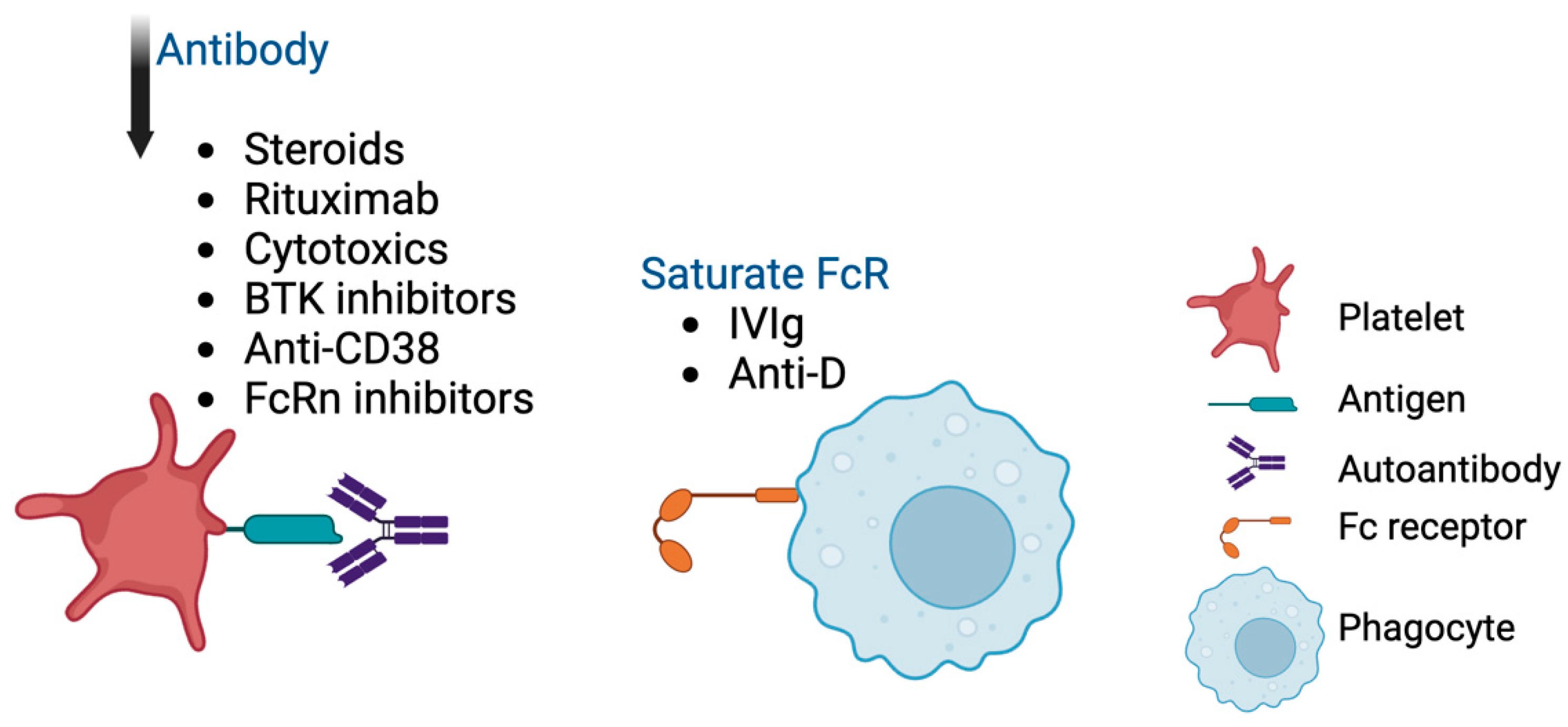
3.2.1. Fc-Dependent Pathway
3.2.2. Fc-Independent Pathway
4. Therapeutic Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cines, D.B.; Bussel, J.B.; Liebman, H.A.; Luning Prak, E.T. The ITP syndrome: Pathogenic and clinical diversity. Blood 2009, 113, 6511–6521. [Google Scholar] [CrossRef] [PubMed]
- Chong, B.H.; Choi, P.Y.; Khachigian, L.; Perdomo, J. Drug-induced immune thrombocytopenia. Hematol. Oncol. Clin. N. Am. 2013, 27, 521–540. [Google Scholar] [CrossRef]
- Provan, D.; Stasi, R.; Newland, A.C.; Blanchette, V.S.; Bolton-Maggs, P.; Bussel, J.B.; Chong, B.H.; Cines, D.B.; Gernsheimer, T.B.; Godeau, B.; et al. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood 2010, 115, 168–186. [Google Scholar] [CrossRef]
- Lambert, M.P.; Gernsheimer, T.B. Clinical updates in adult immune thrombocytopenia. Blood 2017, 129, 2829–2835. [Google Scholar] [CrossRef]
- Verissimo, V.; Carter, T.; Wright, H.; Robertson, J.; Osborn, M.; Bradbeer, P.; Sabesan, V.; Saxon, B.; Barbaro, P.; Crighton, G.; et al. Australian and New Zealand consensus guideline for paediatric newly diagnosed immune thrombocytopaenia endorsed by Australian New Zealand Children’s Haematology and Oncology Group. J. Paediatr. Child Health 2023, 59, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Harrington, W.J.; Minnich, V.; Hollingsworth, J.W.; Moore, C.V. Demonstration of a thrombocytopenic factor in the blood of patients with thrombocytopenic purpura. J. Lab. Clin. Med. 1951, 38, 1–10. [Google Scholar]
- Schwartz, R.S. Immune thrombocytopenic purpura—From agony to agonist. N. Engl. J. Med. 2007, 357, 2299–2301. [Google Scholar] [CrossRef] [PubMed]
- Shulman, N.R.; Marder, V.J.; Weinrach, R.S. Similarities between known antiplatelet antibodies and the factor responsible for thrombocytopenia in idiopathic purpura. Physiologic, serologic and isotopic studies. Ann. N. Y. Acad. Sci. 1965, 124, 499–542. [Google Scholar] [CrossRef] [PubMed]
- Hollenhorst, M.A.; Al-Samkari, H.; Kuter, D.J. Markers of autoimmunity in immune thrombocytopenia: Prevalence and prognostic significance. Blood Adv. 2019, 3, 3515–3521. [Google Scholar] [CrossRef]
- Grozovsky, R.; Giannini, S.; Falet, H.; Hoffmeister, K.M. Regulating billions of blood platelets: Glycans and beyond. Blood 2015, 126, 1877–1884. [Google Scholar] [CrossRef]
- Cines, D.B.; Cuker, A.; Semple, J.W. Pathogenesis of immune thrombocytopenia. Presse Med. 2014, 43, e49–e59. [Google Scholar] [CrossRef] [PubMed]
- Broudy, V.C.; Lin, N.L.; Kaushansky, K. Thrombopoietin (c-mpl ligand) acts synergistically with erythropoietin, stem cell factor, and interleukin-11 to enhance murine megakaryocyte colony growth and increases megakaryocyte ploidy in vitro. Blood 1995, 85, 1719–1726. [Google Scholar] [CrossRef] [PubMed]
- Debili, N.; Wendling, F.; Katz, A.; Guichard, J.; Breton-Gorius, J.; Hunt, P.; Vainchenker, W. The Mpl-ligand or thrombopoietin or megakaryocyte growth and differentiative factor has both direct proliferative and differentiative activities on human megakaryocyte progenitors. Blood 1995, 86, 2516–2525. [Google Scholar] [CrossRef] [PubMed]
- Broudy, V.C.; Lin, N.L.; Sabath, D.F.; Papayannopoulou, T.; Kaushansky, K. Human platelets display high-affinity receptors for thrombopoietin. Blood 1997, 89, 1896–1904. [Google Scholar] [CrossRef]
- Sungaran, R.; Markovic, B.; Chong, B.H. Localization and regulation of thrombopoietin mRNa expression in human kidney, liver, bone marrow, and spleen using in situ hybridization. Blood 1997, 89, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xia, Y.; Kuter, D.J. Interaction of thrombopoietin with the platelet c-mpl receptor in plasma: Binding, internalization, stability and pharmacokinetics. Br. J. Haematol. 1999, 106, 345–356. [Google Scholar] [CrossRef]
- Deutsch, V.R.; Tomer, A. Megakaryocyte development and platelet production. Br. J. Haematol. 2006, 134, 453–466. [Google Scholar] [CrossRef]
- Kuter, D.J.; Rosenberg, R.D. The reciprocal relationship of thrombopoietin (c-Mpl ligand) to changes in the platelet mass during busulfan-induced thrombocytopenia in the rabbit. Blood 1995, 85, 2720–2730. [Google Scholar] [CrossRef] [PubMed]
- Kuter, D.J.; Gernsheimer, T.B. Thrombopoietin and platelet production in chronic immune thrombocytopenia. Hematol. Oncol. Clin. N. Am. 2009, 23, 1193–1211. [Google Scholar] [CrossRef]
- Grozovsky, R.; Begonja, A.J.; Liu, K.; Visner, G.; Hartwig, J.H.; Falet, H.; Hoffmeister, K.M. The Ashwell-Morell receptor regulates hepatic thrombopoietin production via JAK2-STAT3 signaling. Nat. Med. 2015, 21, 47–54. [Google Scholar] [CrossRef]
- Berndt, M.C.; Gregory, C.; Kabral, A.; Zola, H.; Fournier, D.; Castaldi, P.A. Purification and preliminary characterization of the glycoprotein Ib complex in the human platelet membrane. Eur. J. Biochem. 1985, 151, 637–649. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Deng, W.; Zhou, L.; Xu, Y.; Yang, W.; Liang, X.; Wang, Y.; Kulman, J.D.; Zhang, X.F.; Li, R. Identification of a juxtamembrane mechanosensitive domain in the platelet mechanosensor glycoprotein Ib-IX complex. Blood 2015, 125, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, J.; Packham, M.A.; Cazenave, J.P.; Reimers, H.J.; Mustard, J.F. Effects on platelet function of removal of platelet sialic acid by neuraminidase. Lab. Investig. 1975, 32, 476–484. [Google Scholar] [PubMed]
- Eto, K.; Kunishima, S. Linkage between the mechanisms of thrombocytopenia and thrombopoiesis. Blood 2016, 127, 1234–1241. [Google Scholar] [CrossRef]
- Judson, P.A.; Anstee, D.J.; Clamp, J.R. Isolation and characterization of the major oligosaccharide of human platelet membrane glycoprotein GPIb. Biochem. J. 1982, 205, 81–90. [Google Scholar] [CrossRef]
- Madoff, M.A.; Ebbe, S.; Baldini, M. Sialic acid of human blood platelets. J. Clin. Investig. 1964, 43, 870–877. [Google Scholar] [CrossRef]
- Karpatkin, S.; Shulman, S. Asialo platelets enhance thrombopoiesis. Trans. Assoc. Am. Physicians 1980, 93, 244–250. [Google Scholar]
- Kotzé, H.F.; van Wyk, V.; Badenhorst, P.N.; Heyns, A.D.; Roodt, J.P.; Lötter, M.G. Influence of platelet membrane sialic acid and platelet-associated IgG on ageing and sequestration of blood platelets in baboons. Thromb. Haemost. 1993, 70, 676–680. [Google Scholar] [CrossRef]
- Sorensen, A.L.; Rumjantseva, V.; Nayeb-Hashemi, S.; Clausen, H.; Hartwig, J.H.; Wandall, H.H.; Hoffmeister, K.M. Role of sialic acid for platelet life span: Exposure of beta-galactose results in the rapid clearance of platelets from the circulation by asialoglycoprotein receptor-expressing liver macrophages and hepatocytes. Blood 2009, 114, 1645–1654. [Google Scholar] [CrossRef]
- Jansen, A.J.; Josefsson, E.C.; Rumjantseva, V.; Liu, Q.P.; Falet, H.; Bergmeier, W.; Cifuni, S.M.; Sackstein, R.; von Andrian, U.H.; Wagner, D.D.; et al. Desialylation accelerates platelet clearance after refrigeration and initiates GPIbalpha metalloproteinase-mediated cleavage in mice. Blood 2012, 119, 1263–1273. [Google Scholar] [CrossRef]
- Li, J.; Callum, J.L.; Lin, Y.; Zhou, Y.; Zhu, G.; Ni, H. Severe platelet desialylation in a patient with glycoprotein Ib/IX antibody-mediated immune thrombocytopenia and fatal pulmonary hemorrhage. Haematologica 2014, 99, e61–e63. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; van der Wal, D.E.; Zhu, G.; Xu, M.; Yougbare, I.; Ma, L.; Vadasz, B.; Carrim, N.; Grozovsky, R.; Ruan, M.; et al. Desialylation is a mechanism of Fc-independent platelet clearance and a therapeutic target in immune thrombocytopenia. Nat. Commun. 2015, 6, 7737. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Wu, Y.; Zhou, H.; Qin, P.; Ni, H.; Peng, J.; Hou, M. Successful treatment with oseltamivir phosphate in a patient with chronic immune thrombocytopenia positive for anti-GPIb/IX autoantibody. Platelets 2015, 26, 495–497. [Google Scholar] [CrossRef]
- Revilla, N.; Corral, J.; Miñano, A.; Mingot-Castellano, M.E.; Campos, R.M.; Velasco, F.; Gonzalez, N.; Galvez, E.; Berrueco, R.; Fuentes, I.; et al. Multirefractory primary immune thrombocytopenia; targeting the decreased sialic acid content. Platelets 2019, 30, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Grodzielski, M.; Goette, N.P.; Glembotsky, A.C.; Constanza Baroni Pietto, M.; Mendez-Huergo, S.P.; Pierdominici, M.S.; Montero, V.S.; Rabinovich, G.A.; Molinas, F.C.; Heller, P.G.; et al. Multiple concomitant mechanisms contribute to low platelet count in patients with immune thrombocytopenia. Sci. Rep. 2019, 9, 2208. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.S.; Perdomo, J.S.; Leung, H.H.L.; Yan, F.; Chong, B.H. Acquired Glanzmann thrombasthenia associated with platelet desialylation. J. Thromb. Haemost. 2020, 18, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Marini, I.; Zlamal, J.; Faul, C.; Holzer, U.; Hammer, S.; Pelzl, L.; Bethge, W.; Althaus, K.; Bakchoul, T. Autoantibody-mediated desialylation impairs human thrombopoiesis and platelet lifespan. Haematologica 2021, 106, 196–207. [Google Scholar] [CrossRef]
- Zheng, S.S.; Ahmadi, Z.; Leung, H.H.L.; Wong, R.; Yan, F.; Perdomo, J.S.; Chong, B.H. Antiplatelet antibody predicts platelet desialylation and apoptosis in immune thrombocytopenia. Haematologica 2022, 107, 2195–2205. [Google Scholar] [CrossRef]
- Leeksma, C.H.W.; Cohen, J.A. Determination of the Life of Human Blood Platelets using Labelled Diisopropylfluorophosphonate. Nature 1955, 175, 552–553. [Google Scholar] [CrossRef]
- Josefsson, E.C.; Dowling, M.R.; Lebois, M.; Kile, B.T. Chapter 3—The Regulation of Platelet Life Span. In Platelets, 3rd ed.; Michelson, A.D., Ed.; Academic Press: Cambridge, MA, USA, 2013; pp. 51–65. [Google Scholar]
- Kile, B.T. The role of apoptosis in megakaryocytes and platelets. Br. J. Haematol. 2014, 165, 217–226. [Google Scholar] [CrossRef]
- Mason, K.D.; Carpinelli, M.R.; Fletcher, J.I.; Collinge, J.E.; Hilton, A.A.; Ellis, S.; Kelly, P.N.; Ekert, P.G.; Metcalf, D.; Roberts, A.W.; et al. Programmed anuclear cell death delimits platelet life span. Cell 2007, 128, 1173–1186. [Google Scholar] [CrossRef] [PubMed]
- McArthur, K.; Chappaz, S.; Kile, B.T. Apoptosis in megakaryocytes and platelets: The life and death of a lineage. Blood 2018, 131, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Nimmer, P.M.; Tahir, S.K.; Chen, J.; Fryer, R.M.; Hahn, K.R.; Iciek, L.A.; Morgan, S.J.; Nasarre, M.C.; Nelson, R.; et al. Bcl-2 family proteins are essential for platelet survival. Cell Death Differ. 2007, 14, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Gardino, A.K.; Yaffe, M.B. 14-3-3 proteins as signaling integration points for cell cycle control and apoptosis. Semin. Cell Dev. Biol. 2011, 22, 688–695. [Google Scholar] [CrossRef]
- Grozovsky, R.; Fraser, C.; Hoffmeister, K.M.; Sarosiek, K.; Giannini, S. Desialylation and Apoptosis Crosstalk to Modulate Platelet Clearance. Blood 2019, 134, 1055. [Google Scholar] [CrossRef]
- Jacobson, M.D.; Burne, J.F.; Raff, M.C. Programmed cell death and Bcl-2 protection in the absence of a nucleus. EMBO J. 1994, 13, 1899–1910. [Google Scholar] [CrossRef]
- Wolf, B.B.; Goldstein, J.C.; Stennicke, H.R.; Beere, H.; Amarante-Mendes, G.P.; Salvesen, G.S.; Green, D.R. Calpain functions in a caspase-independent manner to promote apoptosis-like events during platelet activation. Blood 1999, 94, 1683–1692. [Google Scholar] [CrossRef]
- Piguet, P.F.; Vesin, C. Modulation of platelet caspases and life-span by anti-platelet antibodies in mice. Eur. J. Haematol. 2002, 68, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Rand, M.L.; Wang, H.; Bang, K.W.A.; Poon, K.S.V.; Packham, M.A.; Freedman, J. Procoagulant surface exposure and apoptosis in rabbit platelets: Association with shortened survival and steady-state senescence. J. Thromb. Haemost. 2004, 2, 651–659. [Google Scholar] [CrossRef]
- Leytin, V.; Mykhaylov, S.; Starkey, A.F.; Allen, D.J.; Lau, H.; Ni, H.; Semple, J.W.; Lazarus, A.H.; Freedman, J. Intravenous immunoglobulin inhibits anti-glycoprotein IIb-induced platelet apoptosis in a murine model of immune thrombocytopenia. Br. J. Haematol. 2006, 133, 78–82. [Google Scholar] [CrossRef]
- Leytin, V.; Allen, D.J.; Mykhaylov, S.; Lyubimov, E.; Freedman, J. Thrombin-triggered platelet apoptosis. J. Thromb. Haemost. 2006, 4, 2656–2663. [Google Scholar] [CrossRef] [PubMed]
- Lopez, J.J.; Salido, G.M.; Gomez-Arteta, E.; Rosado, J.A.; Pariente, J.A. Thrombin induces apoptotic events through the generation of reactive oxygen species in human platelets. J. Thromb. Haemost. 2007, 5, 1283–1291. [Google Scholar] [CrossRef]
- van der Wal, D.E.; Du, V.X.; Lo, K.S.; Rasmussen, J.T.; Verhoef, S.; Akkerman, J.W. Platelet apoptosis by cold-induced glycoprotein Ibalpha clustering. J. Thromb. Haemost. 2010, 8, 2554–2562. [Google Scholar] [CrossRef] [PubMed]
- Winkler, J.; Kroiss, S.; Rand, M.L.; Azzouzi, I.; Annie Bang, K.W.; Speer, O.; Schmugge, M. Platelet apoptosis in paediatric immune thrombocytopenia is ameliorated by intravenous immunoglobulin. Br. J. Haematol. 2012, 156, 508–515. [Google Scholar] [CrossRef]
- Goette, N.P.; Glembotsky, A.C.; Lev, P.R.; Grodzielski, M.; Contrufo, G.; Pierdominici, M.S.; Espasandin, Y.R.; Riveros, D.; García, A.J.; Molinas, F.C.; et al. Platelet Apoptosis in Adult Immune Thrombocytopenia: Insights into the Mechanism of Damage Triggered by Auto-Antibodies. PLoS ONE 2016, 11, e0160563. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Yan, R.; Zhou, K.; Li, X.; Zhang, Y.; Liu, C.; Jiang, M.; Ye, H.; Meng, X.; Pang, N.; et al. Akt-mediated platelet apoptosis and its therapeutic implications in immune thrombocytopenia. Proc. Natl. Acad. Sci. USA 2018, 115, E10682-e10691. [Google Scholar] [CrossRef]
- Cohen, Y.C.; Djulbegovic, B.; Shamai-Lubovitz, O.; Mozes, B. The bleeding risk and natural history of idiopathic thrombocytopenic purpura in patients with persistent low platelet counts. Arch. Intern. Med. 2000, 160, 1630–1638. [Google Scholar] [CrossRef]
- Deckmyn, H.; De Reys, S. Functional effects of human antiplatelet antibodies. Semin. Thromb. Hemost. 1995, 21, 46–59. [Google Scholar] [CrossRef]
- Doobaree, I.U.; Nandigam, R.; Bennett, D.; Newland, A.; Provan, D. Thromboembolism in adults with primary immune thrombocytopenia: A systematic literature review and meta-analysis. Eur. J. Haematol. 2016, 97, 321–330. [Google Scholar] [CrossRef]
- Hill, Q.A.; Newland, A.C. Fatigue in immune thrombocytopenia. Br. J. Haematol. 2015, 170, 141–149. [Google Scholar] [CrossRef]
- Portielje, J.E.; Westendorp, R.G.; Kluin-Nelemans, H.C.; Brand, A. Morbidity and mortality in adults with idiopathic thrombocytopenic purpura. Blood 2001, 97, 2549–2554. [Google Scholar] [CrossRef] [PubMed]
- Frederiksen, H.; Maegbaek, M.L.; Norgaard, M. Twenty-year mortality of adult patients with primary immune thrombocytopenia: A Danish population-based cohort study. Br. J. Haematol. 2014, 166, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Norgaard, M.; Jensen, A.O.; Engebjerg, M.C.; Farkas, D.K.; Thomsen, R.W.; Cha, S.; Zhao, S.; Sorensen, H.T. Long-term clinical outcomes of patients with primary chronic immune thrombocytopenia: A Danish population-based cohort study. Blood 2011, 117, 3514–3520. [Google Scholar] [CrossRef] [PubMed]
- Schoonen, W.M.; Kucera, G.; Coalson, J.; Li, L.; Rutstein, M.; Mowat, F.; Fryzek, J.; Kaye, J.A. Epidemiology of immune thrombocytopenic purpura in the General Practice Research Database. Br. J. Haematol. 2009, 145, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, D.E.; Heitink-Polle, K.M.J.; Porcelijn, L.; van der Schoot, C.E.; Vidarsson, G.; Bruin, M.C.A.; de Haas, M. Anti-platelet antibodies in childhood immune thrombocytopenia: Prevalence and prognostic implications. J. Thromb. Haemost. 2020, 18, 1210–1220. [Google Scholar] [CrossRef]
- Brighton, T.A.; Evans, S.; Castaldi, P.A.; Chesterman, C.N.; Chong, B.H. Prospective evaluation of the clinical usefulness of an antigen-specific assay (MAIPA) in idiopathic thrombocytopenic purpura and other immune thrombocytopenias. Blood 1996, 88, 194–201. [Google Scholar] [CrossRef]
- Al-Samkari, H.; Rosovsky, R.P.; Karp Leaf, R.S.; Smith, D.B.; Goodarzi, K.; Fogerty, A.E.; Sykes, D.B.; Kuter, D.J. A modern reassessment of glycoprotein-specific direct platelet autoantibody testing in immune thrombocytopenia. Blood Adv. 2019, 4, 9–18. [Google Scholar] [CrossRef]
- He, R.; Reid, D.M.; Jones, C.E.; Shulman, N.R. Spectrum of Ig classes, specificities, and titers of serum antiglycoproteins in chronic idiopathic thrombocytopenic purpura. Blood 1994, 83, 1024–1032. [Google Scholar] [CrossRef]
- Kiefel, V.; Santoso, S.; Kaufmann, E.; Mueller-Eckhardt, C. Autoantibodies against platelet glycoprotein Ib/IX: A frequent finding in autoimmune thrombocytopenic purpura. Br. J. Haematol. 1991, 79, 256–262. [Google Scholar] [CrossRef]
- Vollenberg, R.; Jouni, R.; Norris, P.A.A.; Burg-Roderfeld, M.; Cooper, N.; Rummel, M.J.; Bein, G.; Marini, I.; Bayat, B.; Burack, R.; et al. Glycoprotein V is a relevant immune target in patients with immune thrombocytopenia. Haematologica 2019, 104, 1237–1243. [Google Scholar] [CrossRef]
- Garner, S.F.; Campbell, K.; Metcalfe, P.; Keidan, J.; Huiskes, E.; Dong, J.F.; Lopez, J.A.; Ouwehand, W.H. Glycoprotein V: The predominant target antigen in gold-induced autoimmune thrombocytopenia. Blood 2002, 100, 344–346. [Google Scholar] [CrossRef] [PubMed]
- Porcelijn, L.; Huiskes, E.; Oldert, G.; Schipperus, M.; Zwaginga, J.J.; de Haas, M. Detection of platelet autoantibodies to identify immune thrombocytopenia: State of the art. Br. J. Haematol. 2018, 182, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, J. Pathogenesis in immune thrombocytopenia: New insights. Hematol. Am. Soc. Hematol. Educ. Program 2012, 2012, 306–312. [Google Scholar] [CrossRef]
- Stasi, R.; Cooper, N.; Del Poeta, G.; Stipa, E.; Laura Evangelista, M.; Abruzzese, E.; Amadori, S. Analysis of regulatory T-cell changes in patients with idiopathic thrombocytopenic purpura receiving B cell-depleting therapy with rituximab. Blood 2008, 112, 1147–1150. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhao, H.; Poon, M.C.; Han, Z.; Gu, D.; Xu, M.; Jia, H.; Yang, R.; Han, Z.C. Abnormality of CD4+CD25+ regulatory T cells in idiopathic thrombocytopenic purpura. Eur. J. Haematol. 2007, 78, 139–143. [Google Scholar] [CrossRef]
- Ling, Y.; Cao, X.; Yu, Z.; Ruan, C. Circulating dendritic cells subsets and CD4+Foxp3+ regulatory T cells in adult patients with chronic ITP before and after treatment with high-dose dexamethasome. Eur. J. Haematol. 2007, 79, 310–316. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, D.; Zhu, X.; Qu, X.; Ji, C.; Hou, M. Elevated profile of Th17, Th1 and Tc1 cells in patients with immune thrombocytopenic purpura. Haematologica 2009, 94, 1326–1329. [Google Scholar] [CrossRef]
- Stasi, R.; Del Poeta, G.; Stipa, E.; Evangelista, M.L.; Trawinska, M.M.; Cooper, N.; Amadori, S. Response to B-cell-depleting therapy with rituximab reverts the abnormalities of T-cell subsets in patients with idiopathic thrombocytopenic purpura. Blood 2007, 110, 2924–2930. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, B.M.; Guo, X.; Xu, L.; You, X.; West, R.B.; Bussel, J.B.; Zehnder, J.L. Blood transcriptome and clonal T-cell correlates of response and non-response to eltrombopag therapy in a cohort of patients with chronic immune thrombocytopenia. Haematologica 2020, 105, e129–e132. [Google Scholar] [CrossRef]
- Fogarty, P.F.; Rick, M.E.; Zeng, W.; Risitano, A.M.; Dunbar, C.E.; Bussel, J.B. T cell receptor VB repertoire diversity in patients with immune thrombocytopenia following splenectomy. Clin. Exp. Immunol. 2003, 133, 461–466. [Google Scholar] [CrossRef]
- Nieswandt, B.; Bergmeier, W.; Rackebrandt, K.; Gessner, J.E.; Zirngibl, H. Identification of critical antigen-specific mechanisms in the development of immune thrombocytopenic purpura in mice. Blood 2000, 96, 2520–2527. [Google Scholar] [CrossRef] [PubMed]
- Hoffmeister, K.M.; Felbinger, T.W.; Falet, H.; Denis, C.V.; Bergmeier, W.; Mayadas, T.N.; von Andrian, U.H.; Wagner, D.D.; Stossel, T.P.; Hartwig, J.H. The clearance mechanism of chilled blood platelets. Cell 2003, 112, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Hoffmeister, K.M.; Josefsson, E.C.; Isaac, N.A.; Clausen, H.; Hartwig, J.H.; Stossel, T.P. Glycosylation restores survival of chilled blood platelets. Science 2003, 301, 1531–1534. [Google Scholar] [CrossRef]
- Rumjantseva, V.; Grewal, P.K.; Wandall, H.H.; Josefsson, E.C.; Sorensen, A.L.; Larson, G.; Marth, J.D.; Hartwig, J.H.; Hoffmeister, K.M. Dual roles for hepatic lectin receptors in the clearance of chilled platelets. Nat. Med. 2009, 15, 1273–1280. [Google Scholar] [CrossRef]
- Qiao, J.; Al-Tamimi, M.; Baker, R.I.; Andrews, R.K.; Gardiner, E.E. The platelet Fc receptor, FcgammaRIIa. Immunol. Rev. 2015, 268, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.; Moore, J.C.; Finch, C.N.; Warkentin, T.E.; Kelton, J.G. The IgG subclasses of platelet-associated autoantibodies directed against platelet glycoproteins IIb/IIIa in patients with idiopathic thrombocytopenic purpura. Br. J. Haematol. 2003, 122, 818–824. [Google Scholar] [CrossRef]
- Cantoni, S.; Carpenedo, M.; Nichelatti, M.; Sica, L.; Rossini, S.; Milella, M.; Popescu, C.; Cairoli, R. Clinical relevance of antiplatelet antibodies and the hepatic clearance of platelets in patients with immune thrombocytopenia. Blood 2016, 128, 2183–2185. [Google Scholar] [CrossRef]
- Amini, S.N.; Nelson, V.S.; Porcelijn, L.; Netelenbos, T.; Zwaginga, J.J.; de Haas, M.; Schipperus, M.R.; Kapur, R. The interplay between GPIb/IX antibodies, platelet hepatic sequestration, and TPO levels in patients with chronic ITP. Blood Adv. 2023, 7, 1066–1069. [Google Scholar] [CrossRef]
- Morodomi, Y.; Kanaji, S.; Won, E.; Ruggeri, Z.M.; Kanaji, T. Mechanisms of anti-GPIbα antibody–induced thrombocytopenia in mice. Blood 2020, 135, 2292–2301. [Google Scholar] [CrossRef]
- Qiao, J.; Liu, Y.; Li, D.; Wu, Y.; Li, X.; Yao, Y.; Niu, M.; Fu, C.; Li, H.; Ma, P.; et al. Imbalanced expression of Bcl-xL and Bax in platelets treated with plasma from immune thrombocytopenia. Immunol. Res. 2016, 64, 604–609. [Google Scholar] [CrossRef]
- Nassa, G.; Giurato, G.; Cimmino, G.; Rizzo, F.; Ravo, M.; Salvati, A.; Nyman, T.A.; Zhu, Y.; Vesterlund, M.; Lehtiö, J.; et al. Splicing of platelet resident pre-mRNAs upon activation by physiological stimuli results in functionally relevant proteome modifications. Sci. Rep. 2018, 8, 498. [Google Scholar] [CrossRef] [PubMed]
- Mills, E.W.; Green, R.; Ingolia, N.T. Slowed decay of mRNAs enhances platelet specific translation. Blood 2017, 129, e38–e48. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Christodoulides, N.; Resendiz, J.C.; Berndt, M.C.; Kroll, M.H. Cytoplasmic domains of GpIbalpha and GpIbbeta regulate 14-3-3zeta binding to GpIb/IX/V. Blood 2000, 95, 551–557. [Google Scholar] [CrossRef]
- George, N.M.; Targy, N.; Evans, J.J.; Zhang, L.; Luo, X. Bax contains two functional mitochondrial targeting sequences and translocates to mitochondria in a conformational change- and homo-oligomerization-driven process. J. Biol. Chem. 2010, 285, 1384–1392. [Google Scholar] [CrossRef]
- Li, S.; Wang, Z.; Liao, Y.; Zhang, W.; Shi, Q.; Yan, R.; Ruan, C.; Dai, K. The glycoprotein Ibalpha-von Willebrand factor interaction induces platelet apoptosis. J. Thromb. Haemost. 2010, 8, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhang, W.; Yan, R.; Liao, Y.; Zhao, L.; Ruan, C.; Du, X.; Dai, K. Identification of a novel 14-3-3zeta binding site within the cytoplasmic domain of platelet glycoprotein Ibalpha that plays a key role in regulating the von Willebrand factor binding function of glycoprotein Ib-IX. Circ. Res. 2009, 105, 1177–1185. [Google Scholar] [CrossRef]
- Vrbensky, J.R.; Moore, J.E.; Arnold, D.M.; Smith, J.W.; Kelton, J.G.; Nazy, I. The sensitivity and specificity of platelet autoantibody testing in immune thrombocytopenia: A systematic review and meta-analysis of a diagnostic test. J. Thromb. Haemost. 2019, 17, 787–794. [Google Scholar] [CrossRef]
- Olsson, B.; Andersson, P.O.; Jernas, M.; Jacobsson, S.; Carlsson, B.; Carlsson, L.M.; Wadenvik, H. T-cell-mediated cytotoxicity toward platelets in chronic idiopathic thrombocytopenic purpura. Nat. Med. 2003, 9, 1123–1124. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Chu, X.; Wang, L.; Zhu, Y.; Li, L.; Ma, D.; Peng, J.; Hou, M. Cell-mediated lysis of autologous platelets in chronic idiopathic thrombocytopenic purpura. Eur. J. Haematol. 2006, 76, 427–431. [Google Scholar] [CrossRef]
- Zhao, C.; Li, X.; Zhang, F.; Wang, L.; Peng, J.; Hou, M. Increased cytotoxic T-lymphocyte-mediated cytotoxicity predominant in patients with idiopathic thrombocytopenic purpura without platelet autoantibodies. Haematologica 2008, 93, 1428–1430. [Google Scholar] [CrossRef]
- Qiu, J.; Liu, X.; Li, X.; Zhang, X.; Han, P.; Zhou, H.; Shao, L.; Hou, Y.; Min, Y.; Kong, Z.; et al. CD8+ T cells induce platelet clearance in the liver via platelet desialylation in immune thrombocytopenia. Sci. Rep. 2016, 6, 27445. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.; Sayed, A.A.; Han, P.; Tan, M.M.H.; Watt, E.; Constantinescu-Bercu, A.; Cocker, A.T.H.; Khoder, A.; Saputil, R.C.; Thorley, E.V.; et al. The role of CD8+ T cell clones in immune thrombocytopenia. Blood 2023, 141, 2417–2429. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, L.; Zhao, C.; Li, L.; Peng, J.; Hou, M. CD8+ T cells suppress autologous megakaryocyte apoptosis in idiopathic thrombocytopenic purpura. Br. J. Haematol. 2007, 139, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Vrbensky, J.R.; Nazy, I.; Toltl, L.J.; Ross, C.; Ivetic, N.; Smith, J.W.; Kelton, J.G.; Arnold, D.M. Megakaryocyte apoptosis in immune thrombocytopenia. Platelets 2018, 29, 729–732. [Google Scholar] [CrossRef]
- Choi, P.Y.; Merriman, E.; Bennett, A.; Enjeti, A.K.; Tan, C.W.; Goncalves, I.; Hsu, D.; Bird, R. Consensus guidelines for the management of adult immune thrombocytopenia in Australia and New Zealand. Med. J. Aust. 2022, 216, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, K.; Tani, P.; Piro, L.; McMillan, R. The effect of therapy on platelet-associated autoantibody in chronic immune thrombocytopenic purpura. Blood 1993, 81, 2872–2877. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, L.; Hao, H.; Jansen, A.J.G.; Liu, G.; Li, H.; Liu, X.; Zhao, Y.; Peng, J.; Hou, M. First line treatment of adult patients with primary immune thrombocytopenia: A real-world study. Platelets 2020, 31, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Cragg, M.S.; Walshe, C.A.; Ivanov, A.O.; Glennie, M.J. The biology of CD20 and its potential as a target for mAb therapy. Curr. Dir. Autoimmun. 2005, 8, 140–174. [Google Scholar] [CrossRef] [PubMed]
- Arnold, D.M.; Vrbensky, J.R.; Karim, N.; Smith, J.W.; Liu, Y.; Ivetic, N.; Kelton, J.G.; Nazy, I. The effect of rituximab on anti-platelet autoantibody levels in patients with immune thrombocytopenia. Br. J. Haematol. 2017, 178, 302–307. [Google Scholar] [CrossRef]
- Szczepanik, A.B.; Sikorska, A.; Slomkowski, M.; Konopka, L. The use of vinca alkaloids in preparation for splenectomy of corticosteroid refractory chronic immune thrombocytopenic purpura patients. Int. J. Lab. Hematol. 2007, 29, 347–351. [Google Scholar] [CrossRef]
- Quiquandon, I.; Fenaux, P.; Caulier, M.T.; Pagniez, D.; Huart, J.J.; Bauters, F. Re-evaluation of the role of azathioprine in the treatment of adult chronic idiopathic thrombocytopenic purpura: A report on 53 cases. Br. J. Haematol. 1990, 74, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Kuter, D.J.; Efraim, M.; Mayer, J.; Trněný, M.; McDonald, V.; Bird, R.; Regenbogen, T.; Garg, M.; Kaplan, Z.; Tzvetkov, N.; et al. Rilzabrutinib, an Oral BTK Inhibitor, in Immune Thrombocytopenia. N. Engl. J. Med. 2022, 386, 1421–1431. [Google Scholar] [CrossRef] [PubMed]
- Ware, R.E.; Zimmerman, S.A. Anti-D: Mechanisms of action. Semin. Hematol. 1998, 35, 14–22. [Google Scholar] [PubMed]
- Bradbury, C.A.; Pell, J.; Hill, Q.; Bagot, C.; Cooper, N.; Ingram, J.; Breheny, K.; Kandiyali, R.; Rayment, R.; Evans, G.; et al. Mycophenolate Mofetil for First-Line Treatment of Immune Thrombocytopenia. N. Engl. J. Med. 2021, 385, 885–895. [Google Scholar] [CrossRef]
- Choi, P.Y.; Roncolato, F.; Badoux, X.; Ramanathan, S.; Ho, S.J.; Chong, B.H. A novel triple therapy for ITP using high-dose dexamethasone, low-dose rituximab, and cyclosporine (TT4). Blood 2015, 126, 500–503. [Google Scholar] [CrossRef]
- Kuter, D.J. Novel therapies for immune thrombocytopenia. Br. J. Haematol. 2022, 196, 1311–1328. [Google Scholar] [CrossRef]
- Semple, J.W.; Provan, D. The immunopathogenesis of immune thrombocytopenia: T cells still take center-stage. Curr. Opin. Hematol. 2012, 19, 357–362. [Google Scholar] [CrossRef]
- Dameshek, W.; Miller, E.B. The megakaryocytes in idiopathic thrombocytopenic purpura, a form of hypersplenism. Blood 1946, 1, 27–50. [Google Scholar] [CrossRef]
- Stahl, C.P.; Zucker-Franklin, D.; McDonald, T.P. Incomplete antigenic cross-reactivity between platelets and megakaryocytes: Relevance to ITP. Blood 1986, 67, 421–428. [Google Scholar] [CrossRef]
- Houwerzijl, E.J.; Blom, N.R.; van der Want, J.J.; Esselink, M.T.; Koornstra, J.J.; Smit, J.W.; Louwes, H.; Vellenga, E.; de Wolf, J.T. Ultrastructural study shows morphologic features of apoptosis and para-apoptosis in megakaryocytes from patients with idiopathic thrombocytopenic purpura. Blood 2004, 103, 500–506. [Google Scholar] [CrossRef]
- Iraqi, M.; Perdomo, J.; Yan, F.; Choi, P.Y.; Chong, B.H. Immune thrombocytopenia: Antiplatelet autoantibodies inhibit proplatelet formation by megakaryocytes and impair platelet production in vitro. Haematologica 2015, 100, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Lev, P.R.; Grodzielski, M.; Goette, N.P.; Glembotsky, A.C.; Espasandin, Y.R.; Pierdominici, M.S.; Contrufo, G.; Montero, V.S.; Ferrari, L.; Molinas, F.C.; et al. Impaired proplatelet formation in immune thrombocytopenia: A novel mechanism contributing to decreased platelet count. Br. J. Haematol. 2014, 165, 854–864. [Google Scholar] [CrossRef]
- Peerschke, E.I.; Andemariam, B.; Yin, W.; Bussel, J.B. Complement activation on platelets correlates with a decrease in circulating immature platelets in patients with immune thrombocytopenic purpura. Br. J. Haematol. 2010, 148, 638–645. [Google Scholar] [CrossRef]
- Najaoui, A.; Bakchoul, T.; Stoy, J.; Bein, G.; Rummel, M.J.; Santoso, S.; Sachs, U.J. Autoantibody-mediated complement activation on platelets is a common finding in patients with immune thrombocytopenic purpura (ITP). Eur. J. Haematol. 2012, 88, 167–174. [Google Scholar] [CrossRef]
- Peerschke, E.I.; Panicker, S.; Bussel, J. Classical complement pathway activation in immune thrombocytopenia purpura: Inhibition by a novel C1s inhibitor. Br. J. Haematol. 2016, 173, 942–945. [Google Scholar] [CrossRef]
- Stasi, R. Eltrombopag for the treatment of idiopathic thrombocytopenic purpura. Expert. Rev. Hematol. 2008, 1, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Kuter, D.J. New thrombopoietic growth factors. Blood 2007, 109, 4607–4616. [Google Scholar] [CrossRef]
- Cines, D.B.; Gernsheimer, T.; Wasser, J.; Godeau, B.; Provan, D.; Lyons, R.; Altomare, I.; Wang, X.; Lopez, A. Integrated analysis of long-term safety in patients with chronic immune thrombocytopaenia (ITP) treated with the thrombopoietin (TPO) receptor agonist romiplostim. Int. J. Hematol. 2015, 102, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Bussel, J.B.; Kuter, D.J.; Aledort, L.M.; Kessler, C.M.; Cuker, A.; Pendergrass, K.B.; Tang, S.; McIntosh, J. A randomized trial of avatrombopag, an investigational thrombopoietin-receptor agonist, in persistent and chronic immune thrombocytopenia. Blood 2014, 123, 3887–3894. [Google Scholar] [CrossRef]
- Broome, C.M.; Roth, A.; Kuter, D.J.; Scully, M.; Smith, R.; Wang, J.; Reuter, C.; Hobbs, W.E.; Daak, A.A.A. Safety and Efficacy of Classical Complement Pathway Inhibition with Sutimlimab in Chronic Immune Thrombocytopenia. Blood Adv. 2023, 7, 987–996. [Google Scholar] [CrossRef]
- Davies, B.E. Pharmacokinetics of oseltamivir: An oral antiviral for the treatment and prophylaxis of influenza in diverse populations. J. Antimicrob. Chemother. 2010, 65 (Suppl. 2), ii5–ii10. [Google Scholar] [CrossRef] [PubMed]
- Moscona, A. Neuraminidase inhibitors for influenza. N. Engl. J. Med. 2005, 353, 1363–1373. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Yu, Q.; Ye, Z.Q.; Sun, Y.; He, Q.; Li, X.M.; Zhang, W.; Luo, J.; Gu, X.; Zheng, X.; et al. A nonsynonymous SNP in human cytosolic sialidase in a small Asian population results in reduced enzyme activity: Potential link with severe adverse reactions to oseltamivir. Cell Res. 2007, 17, 357–362. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Alioglu, B.; Tasar, A.; Ozen, C.; Selver, B.; Dallar, Y. An experience of oseltamivir phosphate (tamiflu) in a pediatric patient with chronic idiopathic thrombocytopenic purpura: A case report. Pathophysiol. Haemost. Thromb. 2010, 37, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Bigot, P.; Auffret, M.; Gautier, S.; Weinborn, M.; Ettahar, N.K.; Coupe, P. Unexpected platelets elevation in a patient with idiopathic thrombocytopenia treated with oseltamivir for influenza infection. Fundam. Clin. Pharmacol. 2016, 30, 483–485. [Google Scholar] [CrossRef]
- Álvarez-Román, M.T.; Rivas Pollmar, M.I.; Bernardino, J.I.; Lozano, M.L.; Martín-Salces, M.; Fernández-Bello, I.; Jiménez-Yuste, V.; Butta, N.V. Thrombopoietin receptor agonists in conjunction with oseltamivir for immune thrombocytopenia. AIDS 2016, 30, 1141–1142. [Google Scholar] [CrossRef]
- Sun, L.; Wang, J.; Shao, L.; Yuan, C.; Zhao, H.; Li, D.; Wang, Z.; Han, P.; Yu, Y.; Xu, M.; et al. Dexamethasone plus oseltamivir versus dexamethasone in treatment-naive primary immune thrombocytopenia: A multicentre, randomised, open-label, phase 2 trial. Lancet Haematol. 2021, 8, e289–e298. [Google Scholar] [CrossRef]
- Stasi, R.; Newland, A.C. ITP: A historical perspective. Br. J. Haematol. 2011, 153, 437–450. [Google Scholar] [CrossRef]
| Comment | Reference; Year |
|---|---|
| Sialic acid is present on platelets; can be cleaved with neuraminidase | [26]; 1964 |
| Desialylated rabbit platelets are rapidly cleared in vivo; no significant changes to platelet function in vitro | [23]; 1975 |
| Injection of desialylated platelets induced platelet production. Authors suggested that thrombopoiesis may be regulated by uptake of desialylated platelets | [27]; 1980 |
| Sialic acid removal shortens platelet lifespan in primates | [28]; 1993 |
| Platelets lacking sialic acid are recognised by asialoglycoprotein receptors | [29]; 2009 |
| Cold storage leads to platelet desialylation | [30]; 2012 |
| Platelet desialylation by anti GPIb/IX antibody | [31]; 2014 |
| Hepatic Ashwell–Morell receptor binds and removes desialylated platelets | [20]; 2015 |
| Anti GPIbα, not anti GPIIb/IIIa antibodies, induced desialylation and hepatic platelet uptake in mice | [32]; 2015 |
| ITP patient with anti GPIb/IX antibodies successfully treated with oseltamivir | [33]; 2015 |
| TPO-RAs in combination with oseltamivir induced sustained platelet production in patients with anti GPIb antibodies | [34]; 2019 |
| Plasma from patients with ITP affected the sialylation pattern of control platelets | [35]; 2019 |
| Desialylation induced by anti GPIIb/IIIa antibodies and is FcγRIIa-dependent | [36,37]; 2020, 2021 |
| Destruction of human platelets induced by anti-GPIIb/IIIa antibodies was prevented with oseltamivir in a humanised mouse model of ITP | [37,38]; 2021, 2022 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, S.S.; Perdomo, J.S. Desialylation and Apoptosis in Immune Thrombocytopenia: Implications for Pathogenesis and Treatment. Curr. Issues Mol. Biol. 2024, 46, 11942-11956. https://doi.org/10.3390/cimb46110709
Zheng SS, Perdomo JS. Desialylation and Apoptosis in Immune Thrombocytopenia: Implications for Pathogenesis and Treatment. Current Issues in Molecular Biology. 2024; 46(11):11942-11956. https://doi.org/10.3390/cimb46110709
Chicago/Turabian StyleZheng, Shiying Silvia, and José Sail Perdomo. 2024. "Desialylation and Apoptosis in Immune Thrombocytopenia: Implications for Pathogenesis and Treatment" Current Issues in Molecular Biology 46, no. 11: 11942-11956. https://doi.org/10.3390/cimb46110709
APA StyleZheng, S. S., & Perdomo, J. S. (2024). Desialylation and Apoptosis in Immune Thrombocytopenia: Implications for Pathogenesis and Treatment. Current Issues in Molecular Biology, 46(11), 11942-11956. https://doi.org/10.3390/cimb46110709






