Unraveling the Potential of Isorhamnetin as an Adjuvant in Depression Treatment with Escitalopram
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Study Design and Treatments
2.3. Behavioral Paradigms
2.3.1. Forced Swim Test
2.3.2. Open Field Test
2.4. Biochemical Assays
2.5. Data Analysis
3. Results
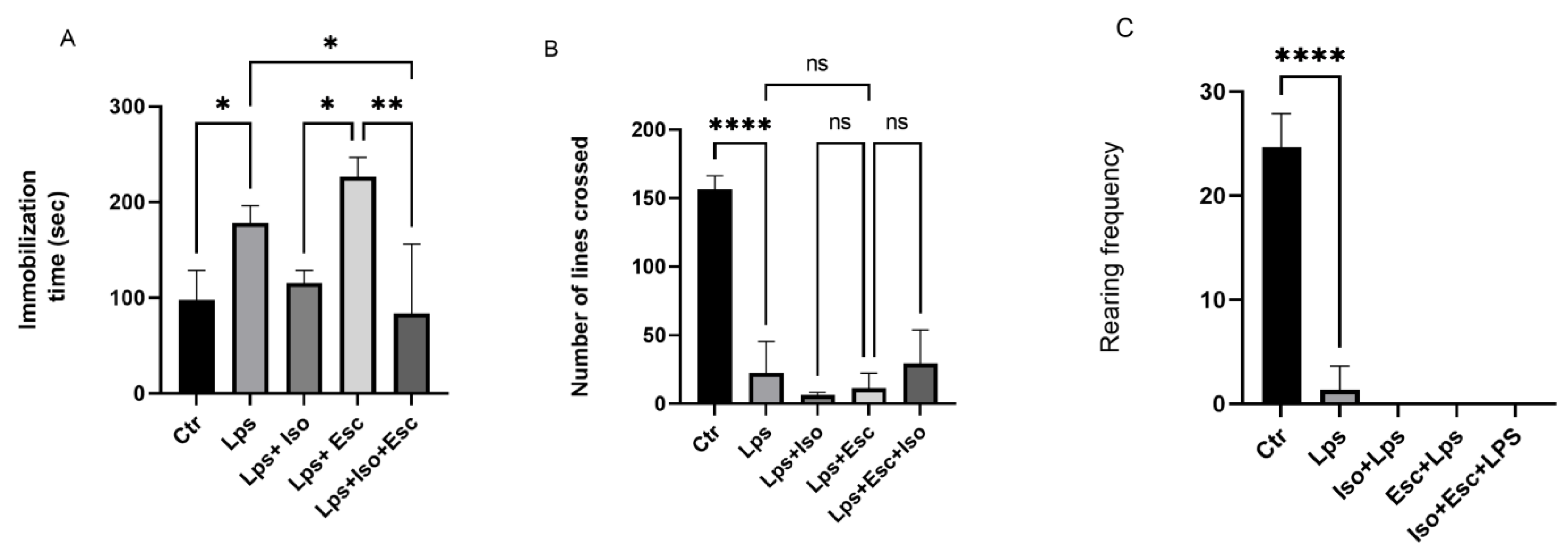
3.1. Forced Swim Test
3.2. Open Field Test
3.3. Nrf2 Expression
3.4. BDNF Expression
3.5. HO-1 Expression
3.6. NO Levels
3.7. IL-6 Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kessler, R.C.; Bromet, E.J. The Epidemiology of Depression Across Cultures. Annu. Rev. Public Health 2013, 34, 119–138. [Google Scholar] [CrossRef] [PubMed]
- Gammoh, O.S.; Bashatwah, R. Potential Strategies to Optimize the Efficacy of Antidepressants: Beyond the Monoamine Theory. Electron. J. Gen. Med. 2023, 20, em513. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, L.; Zhang, J. Curcumin in Antidepressant Treatments: An Overview of Potential Mechanisms, Pre-Clinical/Clinical Trials and Ongoing Challenges. Basic Clin. Pharmacol. Toxicol. 2020, 127, 243–253. [Google Scholar] [CrossRef]
- Liu, Y.; Ho, R.C.; Mak, A. Interleukin (IL)-6, Tumour Necrosis Factor Alpha (TNF- α) and Soluble Interleukin-2 Receptors (SIL-2R) Are Elevated in Patients with Major Depressive Disorder: A Meta-Analysis and Meta-Regression. J. Affect. Disord. 2012, 139, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.J.C.; Spiers, J.G.; Sernia, C.; Lavidis, N.A. Response of the Nitrergic System to Activation of the Neuroendocrine Stress Axis. Front. Neurosci. 2015, 9, 3. [Google Scholar] [CrossRef]
- Suzuki, E.; Yagi, G.; Nakaki, T.; Kanba, S.; Asai, M. Elevated Plasma Nitrate Levels in Depressive States. J. Affect. Disord. 2001, 63, 221–224. [Google Scholar] [CrossRef]
- Wegener, G.; Volke, V. Nitric Oxide Synthase Inhibitors as Antidepressants. Pharmaceuticals 2010, 3, 273–299. [Google Scholar] [CrossRef]
- Cao, Q.; Zou, Q.; Zhao, X.; Zhang, Y.; Qu, Y.; Wang, N.; Murayama, S.; Qi, Q.; Hashimoto, K.; Lin, S.; et al. Regulation of BDNF Transcription by Nrf2 and MeCP2 Ameliorates MPTP-Induced Neurotoxicity. Cell Death Discov. 2022, 8, 267. [Google Scholar] [CrossRef]
- Robledinos-Antón, N.; Fernández-Ginés, R.; Manda, G.; Cuadrado, A. Activators and Inhibitors of NRF2: A Review of Their Potential for Clinical Development. Oxidative Med. Cell. Longev. 2019, 2019, 9372182. [Google Scholar] [CrossRef]
- Balogun, E.; Hoque, M.; Gong, P.; Killeen, E.; Green, C.J.; Foresti, R.; Alam, J.; Motterlini, R. Curcumin Activates the Haem Oxygenase-1 Gene via Regulation of Nrf2 and the Antioxidant-Responsive Element. Biochem. J. 2003, 371, 887–895. [Google Scholar] [CrossRef]
- Fusar-poli, L.; Vozza, L.; Gabbiadini, A.; Vanella, A.; Tinacci, S.; Petralia, A.; Signorelli, M.S.; Vozza, L.; Gabbiadini, A.; Vanella, A.; et al. Curcumin for Depression: A Meta-Analysis. Crit. Rev. Food Sci. Nutr. 2019, 60, 2643. [Google Scholar] [CrossRef] [PubMed]
- Saki, K.; Bahmani, M.; Rafieian-Kopaei, M. The Effect of Most Important Medicinal Plants on Two Importnt Psychiatric Disorders (Anxiety and Depression)—A Review. Asian Pac. J. Trop. Med. 2014, 7, S34–S42. [Google Scholar] [CrossRef]
- Jia, W.; Wang, X.; Xu, D.; Zhao, A.; Zhang, Y. Common Traditional Chinese Medicinal Herbs for Dysmenorrhea. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2006, 20, 819–824. [Google Scholar] [CrossRef]
- Ferreres, F.; Grosso, C.; Gil-Izquierdo, A.; Valentão, P.; Andrade, P.B. Phenolic Compounds from Jacaranda Caroba (Vell.) A. DC.: Approaches to Neurodegenerative Disorders. Food Chem. Toxicol. 2013, 57, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, J.; Lin, Z.; Liang, W.; Qin, L.; Ding, J.; Chen, S.; Zhou, L. Isorhamnetin Alleviates Airway Inflammation by Regulating the Nrf2/Keap1 Pathway in a Mouse Model of COPD. Front. Pharmacol. 2022, 13, 860362. [Google Scholar] [CrossRef] [PubMed]
- Gong, G.; Guan, Y.Y.; Zhang, Z.L.; Rahman, K.; Wang, S.J.; Zhou, S.; Luan, X.; Zhang, H. Isorhamnetin: A Review of Pharmacological Effects. Biomed. Pharmacother. 2020, 128, 110301. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.-J.; Song, J.-Q.; Pan, S.-Y.; Wang, K. Treatment with Isorhamnetin Protects the Brain against Ischemic Injury in Mice. Neurochem. Res. 2016, 41, 1939–1948. [Google Scholar] [CrossRef]
- Ishola, I.O.; Osele, M.O.; Chijioke, M.C.; Adeyemi, O.O. Isorhamnetin Enhanced Cortico-Hippocampal Learning and Memory Capability in Mice with Scopolamine-Induced Amnesia: Role of Antioxidant Defense, Cholinergic and BDNF Signaling. Brain Res. 2019, 1712, 188–196. [Google Scholar] [CrossRef]
- Ekici, M.; Gungor, H.; Mert, D.G. Kaempferol and Isorhamnetin Alleviate Lipopolysaccharide-Induced Anxiety and Depression-Like Behavioral in Balb/C Mice. J. Hell. Vet. Med. Soc. 2023, 74, 5753–5764. [Google Scholar] [CrossRef]
- Mendez-David, I.; Tritschler, L.; El Ali, Z.; Damiens, M.H.; Pallardy, M.; David, D.J.; Kerdine-Römer, S.; Gardier, A.M. Nrf2-Signaling and BDNF: A New Target for the Antidepressant-like Activity of Chronic Fluoxetine Treatment in a Mouse Model of Anxiety/Depression. Neurosci. Lett. 2015, 597, 121–126. [Google Scholar] [CrossRef]
- Yeh, Y.H.; Kuo, C.T.; Chang, G.J.; Chen, Y.H.; Lai, Y.J.; Cheng, M.L.; Chen, W.J. Rosuvastatin Suppresses Atrial Tachycardia-Induced Cellular Remodeling via Akt/Nrf2/Heme Oxygenase-1 Pathway. J. Mol. Cell. Cardiol. 2015, 82, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, G.; Tang, H.; Pan, R.; Wang, H.; Jin, F.; Yan, X.; Xing, Y.; Chen, G.; Fu, Y.; et al. Madecassoside Ameliorates Lipopolysaccharide-Induced Neurotoxicity in Rats by Activating the Nrf2-HO-1 Pathway. Neurosci. Lett. 2019, 709, 134386. [Google Scholar] [CrossRef] [PubMed]
- Hämäläinen, M.; Nieminen, R.; Vuorela, P.; Heinonen, M.; Moilanen, E. Anti-Inflammatory Effects of Flavonoids: Genistein, Kaempferol, Quercetin, and Daidzein Inhibit STAT-1 and NF-ΚB Activations, Whereas Flavone, Isorhamnetin, Naringenin, and Pelargonidin Inhibit Only NF-ΚB Activation along with Their Inhibitory Effect on I. Mediators Inflamm. 2007, 2007, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Shin, B.Y.; Han, J.Y.; Kim, M.G.; Wi, J.E.; Kim, Y.W.; Cho, I.J.; Kim, S.C.; Shin, S.M.; Ki, S.H. Isorhamnetin Protects against Oxidative Stress by Activating Nrf2 and Inducing the Expression of Its Target Genes. Toxicol. Appl. Pharmacol. 2014, 274, 293–301. [Google Scholar] [CrossRef]
- Gammoh, O.; Ibrahim, A.; Qnais, E.; Alqudah, A.; Altaber, S.; Aljabali, A.A.A.; Tambuwala, M.M. Vitamins C and D Exhibit Similar Antidepressant Effects to Escitalopram Mediated by NOx and FKBPL in a Stress-Induced Mice Model. Nutrients 2023, 15, 2692. [Google Scholar] [CrossRef]
- Zhu, Y.; Sun, L.; Zhang, H.; Li, Y.; Lai, S. Effects of Isorhamnetin on Protein Expression of VEGF, MMP-2 and Endostatin in Lewis Lung Cancer Mouse. Int. J. Clin. Exp. Med. 2017, 10, 11488–11495. [Google Scholar]
- Kinra, M.; Ranadive, N.; Mudgal, J.; Zhang, Y.; Govindula, A.; Anoopkumar-Dukie, S.; Davey, A.K.; Grant, G.D.; Nampoothiri, M.; Arora, D. Putative Involvement of Sirtuin Modulators in LPS-Induced Sickness Behaviour in Mice. Metab. Brain Dis. 2022, 37, 1969–1976. [Google Scholar] [CrossRef]
- Porsolt, R.D.; Bertin, A.; Blavet, N.; Deniel, M.; Jalfre, M. Immobility Induced by Forced Swimming in Rats: Effects of Agents Which Modify Central Catecholamine and Serotonin Activity. Eur. J. Pharmacol. 1979, 57, 201–210. [Google Scholar] [CrossRef]
- Dishman, R.K.; Armstrong, R.B.; Delp, M.D.; Graham, R.E.; Dunn, A.L. Open-Field Behavior Is Not Related to Treadmill Performance in Exercising Rats. Physiol. Behav. 1988, 43, 541–546. [Google Scholar] [CrossRef]
- Qi, F.; Sun, J.-H.; Yan, J.-Q.; Li, C.-M.; Lv, X.-C. Anti-Inflammatory Effects of Isorhamnetin on LPS-Stimulated Human Gingival Fibroblasts by Activating Nrf2 Signaling Pathway. Microb. Pathog. 2018, 120, 37–41. [Google Scholar] [CrossRef]
- Yang, B.; Li, X.-P.; Ni, Y.-F.; Du, H.-Y.; Wang, R.; Li, M.-J.; Wang, W.-C.; Li, M.-M.; Wang, X.-H.; Li, L.; et al. Protective Effect of Isorhamnetin on Lipopolysaccharide-Induced Acute Lung Injury in Mice. Inflammation 2016, 39, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Jamali-Raeufy, N.; Baluchnejadmojarad, T.; Roghani, M. Isorhamnetin Exerts Neuroprotective Effects in STZ-Induced Diabetic Rats via Attenuation of Oxidative Stress, Inflammation and Apoptosis. J. Chem. Neuroanat. 2019, 102, 101709. [Google Scholar] [CrossRef] [PubMed]
- Jangra, A.; Lukhi, M.M.; Sulakhiya, K.; Baruah, C.C.; Lahkar, M. Protective Effect of Mangiferin against Lipopolysaccharide-Induced Depressive and Anxiety-like Behaviour in Mice. Eur. J. Pharmacol. 2014, 740, 337–345. [Google Scholar] [CrossRef]
- Yao, W.; Lin, S.; Su, J.; Cao, Q.; Chen, Y.; Chen, J.; Zhang, Z.; Hashimoto, K.; Qi, Q.; Zhang, J.-C. Activation of BDNF by Transcription Factor Nrf2 Contributes to Antidepressant-like Actions in Rodents. Transl. Psychiatry 2021, 11, 140. [Google Scholar] [CrossRef]
- Paudel, P.; Jung, H.A.; Choi, J.S. Anthraquinone and Naphthopyrone Glycosides from Cassia Obtusifolia Seeds Mediate Hepatoprotection via Nrf2-Mediated HO-1 Activation and MAPK Modulation. Arch. Pharm. Res. 2018, 41, 677–689. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, G.; Cui, L.; Wang, Q. Myricetin Attenuates Depressant-like Behavior in Mice Subjected to Repeated Restraint Stress. Int. J. Mol. Sci. 2015, 16, 28377–28385. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Kim, S.C.; Shin, B.Y.; Jin, S.H.; Jo, M.J.; Jegal, K.H.; Kim, Y.W.; Lee, J.R.; Ku, S.K.; Cho, I.J.; et al. O-Methylated Flavonol Isorhamnetin Prevents Acute Inflammation through Blocking of NF-ΚB Activation. Food Chem. Toxicol. 2013, 59, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Jiang, C.; Li, D.; Yao, L.; Lin, Y.; Wang, B.; Qiu, J.; Wang, W.; Wang, W. Isorhamnetin Alleviates Esophageal Mucosal Injury in a Chronic Model of Reflux Esophagitis. Eur. J. Pharmacol. 2019, 864, 172720. [Google Scholar] [CrossRef]
- Maes, M.; Bosmans, E.; De Jongh, R.; Kenis, G.; Vandoolaeghe, E.; Neels, H. Increased serum IL-6 and IL-1 receptor antagonist concentrations in major depression and treatment resistant depression. Cytokine 1997, 9, 853–858. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gammoh, O.; Qnais, E.Y.; Athamneh, R.Y.; Al-Jaidi, B.; Al-Tawalbeh, D.; Altaber, S.; Alqudah, A.; Aljabali, A.A.A.; Tambuwala, M.M. Unraveling the Potential of Isorhamnetin as an Adjuvant in Depression Treatment with Escitalopram. Curr. Issues Mol. Biol. 2023, 45, 7668-7679. https://doi.org/10.3390/cimb45090484
Gammoh O, Qnais EY, Athamneh RY, Al-Jaidi B, Al-Tawalbeh D, Altaber S, Alqudah A, Aljabali AAA, Tambuwala MM. Unraveling the Potential of Isorhamnetin as an Adjuvant in Depression Treatment with Escitalopram. Current Issues in Molecular Biology. 2023; 45(9):7668-7679. https://doi.org/10.3390/cimb45090484
Chicago/Turabian StyleGammoh, Omar, Esam Y. Qnais, Rabaa Y. Athamneh, Bilal Al-Jaidi, Deniz Al-Tawalbeh, Sara Altaber, Abdelrahim Alqudah, Alaa A. A. Aljabali, and Murtaza M. Tambuwala. 2023. "Unraveling the Potential of Isorhamnetin as an Adjuvant in Depression Treatment with Escitalopram" Current Issues in Molecular Biology 45, no. 9: 7668-7679. https://doi.org/10.3390/cimb45090484
APA StyleGammoh, O., Qnais, E. Y., Athamneh, R. Y., Al-Jaidi, B., Al-Tawalbeh, D., Altaber, S., Alqudah, A., Aljabali, A. A. A., & Tambuwala, M. M. (2023). Unraveling the Potential of Isorhamnetin as an Adjuvant in Depression Treatment with Escitalopram. Current Issues in Molecular Biology, 45(9), 7668-7679. https://doi.org/10.3390/cimb45090484








