1. Introduction
During the prenatal and postnatal period, exposure to factors such as stress, food deprivation, insecticides, chemicals, and disease can affect fetal development and lead to birth defects in neonatals [
1,
2,
3,
4].
Phytotherapy is used worldwide as a therapeutic resource by various populations, including pregnant women. However, most plants with phytotherapeutic potential do not receive adequate attention related to their toxicity, i.e., for most of them, there are no data about their safety of use, especially during pregnancy [
5,
6].
Several tests have been used to evaluate the potential toxicity of substances since the 16th century. However, in the 1990s, the use of animals in toxicological tests increased exponentially, especially after the establishment of the lethal dose protocol of 50% (DL
50). After that, regression analyses were developed to adjust the dose–response effects in animals, aiming at the calculation of the LD
50. During the 20th century, several other methods of toxicological tests using laboratory animals were developed, many of which are still used today, such as embryotoxicity tests. For more than 40 years of development, the methodologies of toxicological evaluations have remained relatively unchanged and cover many well-known and widely used models in the scientific community [
7].
Chrysobalanus icaco L., belonging to the Chrysobalanaceae family and popularly known as “Abajuru”, “Guajuru”, and “Ajuru”, among others, is native to both America and Africa [
8]. In the Americas,
Chrysobalanus icaco L. is distributed mainly along coastal areas from Florida (USA) to southern Brazil [
9]. It is a medium-sized bushy plant, with alternate and simple leaves; pentamerous flowers and usually white or purple; and dry fruit or fleshy drupe and planoconvex cotyledons [
10]. Riverside populations consumed its fruit in natura [
11], while its roots and leaves are used in traditional medicine in the form of tea, especially for the treatment of diseases such as hemorrhage and chronic diarrhea [
12].
Tests in rodents have reported that the aqueous extract of the bark and leaves of
C. icaco have analgesic properties [
13,
14]. Other pharmacological studies have also been reported, for example, on the anti-inflammatory activity of the aqueous extract of its bark [
13], the antimicrobial activity of the methanolic leaf extract, and the hypoglycemic activity of the aqueous extract of its leaves [
15,
16]. Additionally, the aqueous extract of
C. icaco leaves can prevent weight gain and liver fat accumulation in hypercaloric diet-induced obese mice [
17,
18].
Despite the already published pharmacological effects and traditional use of
C. icaco, to our knowledge, there are no commercial products based on this plant; in addition, the reports of its toxicological potential are limited, especially on the interferences in the animal reproductive period. Some studies have shown that the administration of the aqueous extract of leaves and bark of
C. icaco does not induce signs of toxicity at a dose of 2000 mg/kg [
13,
19]. However, Ribeiro et al. [
19] (2020) demonstrated that the administration of the aqueous extract of
C. icaco leaves at doses of 100, 200, and 400 mg/kg, over 28 days, caused mild hepatic and renal toxicity.
The search for new products of natural origin that benefit the health and well-being of the population has grown in recent years. Furthermore, the development of novel forms of treatment not only contributes to strengthening national science through the accumulation of know-how, but also to building a sustainable economy based on native ecological balance and the local income.
Considering that maternal–fetal toxicological studies play a fundamental role in understanding the risks associated with pregnancy and the neonatal consequences, studies dealing with these issues are highly relevant. Thus, the evaluation of the toxicological safety of C. icaco can guide on its use during pregnancy and depict this natural product as a new form of treatment by combining environmental, socioeconomic, and innovation aspects. Given this, this study was developed to analyze the embryotoxic and teratogenic effects of the oral administration of the aqueous extract of C. icaco during the pre-implantation and organogenesis periods in Wistar rats.
4. Discussion
The use of natural products for different disease treatments has increased over the years; furthermore, studies on the effects of these products on the reproductive performance of females is of fundamental importance [
29]. The
C. icaco species is widely used in traditional medicine as an alternative and natural treatment of diseases [
30,
31]; however, to the best of our knowledge, no studies have been found reporting on the safety of its species during the gestational period. Therefore, several reproductive parameters were evaluated after the administration of the aqueous extract of
C. icaco leaves to obtain more information about its reproductive toxicity.
In previous studies carried out by our research group, the phytochemical analysis performed by UPLC-DAD-ESI-QTOF-MS/MS identified the presence of terpenes, aglycones, and glycosylated flavonoids derived from myricetin and quercetin in the aqueous extract of
C. icaco leaves (AECi) [
19]. Phenolic compounds are substances included in the group of phytoestrogens capable of performing estrogen-like activities. Some phytoestrogens can cross the placental barrier and promote harmful effects on neonates [
32,
33]. At high concentrations, phytoestrogens have anti-estrogenic activity, and decreased estrogen levels can affect the development of placental cells [
34,
35,
36].
Rodents are excellent models for the study of reproductive physiology due to their small size, high reproduction rate, and the ease of obtaining inbred lines [
37]. The general health of females in the gestational period provides relevant information about the reproductive toxic effects of a substance [
38]. Behavioral changes, variations in food consumption, and changes in body and organ weights during this phase can be used as an important indicator of systemic toxicity [
39]. Our results showed that there was no behavioral change in rats treated with AECi when compared to the control group. Furthermore, no changes were observed in food consumption, water consumption, and body or organ weights, indicating that the administration of different doses of AECi (100, 200, and 400 mg/kg) does not cause systemic maternal toxicity. Ribeiro et al. [
19] (2020) showed that the aqueous extract of
C. icaco presented low toxicity as the single administration of a 2000 mg/Kg dose did not affect the intake of water and food or body weight of the treated animals. These authors also found that animals treated with AECi at doses of 100, 200, and 400 mg/kg for 28 consecutive days showed no death or signs of toxicity (piloeration, diarrhea, or changes in locomotor activity).
In female rats, the process of implantation of the blastocyst in the endometrium occurs from the 1st to the 5th day of gestation and, during this period, the embryo is quite susceptible to lethality [
40]. The success of pregnancy depends on the maintenance of progesterone and estrogen levels. Estrogens produce a suitable environment for fertilization, implantation, and nutrition, while progesterone is responsible for maintaining the conditions of the endometrium for a possible pregnancy. The corpus luteum is the primary source of these hormones, which are very important in the process of preparing the uterine mucosa to receive the embryo [
37,
41].
Successful implantation is closely related to endometrial cell proliferation, decidualization, and increased blood flow [
42,
43]. Various xenobiotics can interfere with mitotic division, thus interrupting the pregnancy. These substances can interfere before and after the implantation process, resulting in pre- and post-implantation embryo loss, developmental delay, malformations, and even fetal death [
44,
45]. In the literature, it is possible to find several toxic plants that affect the fertilization and implantation process, such as
Coleus barbatus,
Jatropha curcas, and
Indigofera suffruticosa [
46,
47,
48]. In the present study, it is possible to affirm that the treatment with AECi did not change the number of corpora lutea and the number of implantation sites when compared to the control group. These data revealed the absence of embryotoxic effects of AECi, suggesting a normal developmental capacity of implanted blastocysts.
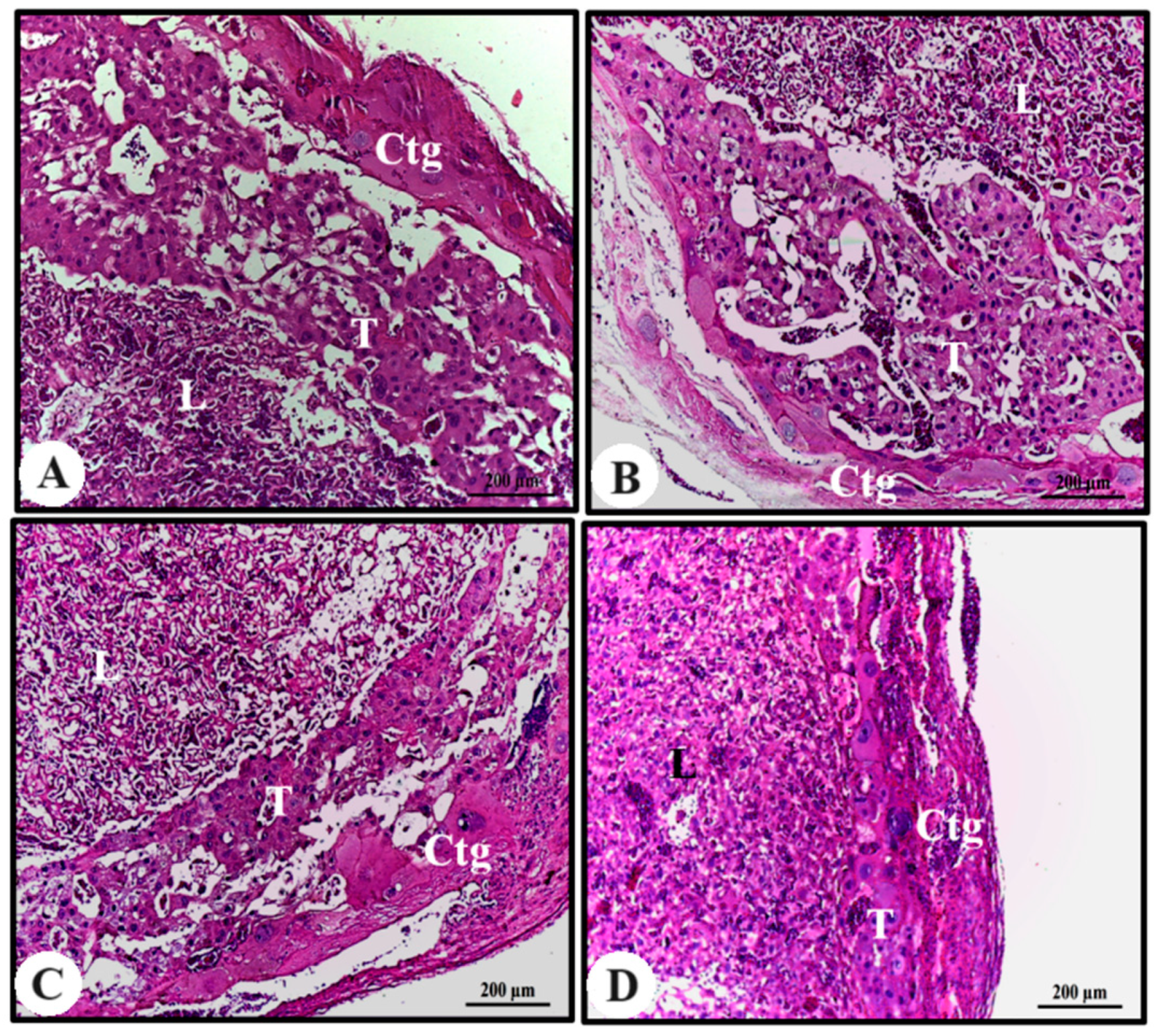
The study of the placenta is an essential tool to assess placental toxicity and its subsequent effects on the fetus. The adequate supply of nutrients through the placenta is directly linked to fetal development in the uterine environment, as any placental dysfunction promotes a higher incidence of fetal distress [
49,
50]; given this, parameters such as fetal weight, placental weight, and placental index feature in the evaluation of embryofetotoxicity. In this present study, it was observed that fetal body weight, placental weight, and placental index were not affected by the administration of AECi, thus confirming the extract safety for the fetus.
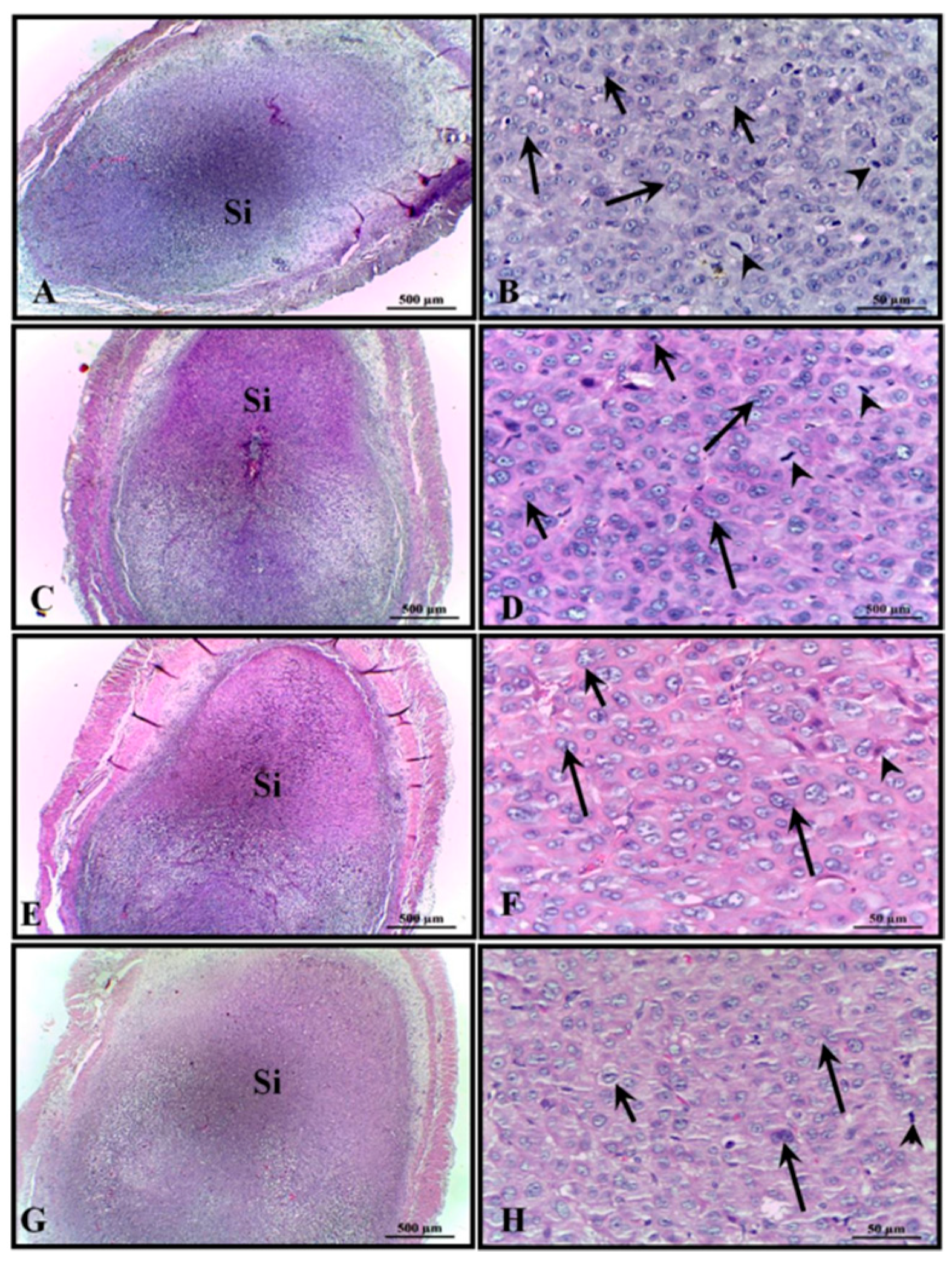
Rat placenta is hemochorial (discoidal) and divided into a fetal part composed of the labyrinth and junctional zone (basal zone), and a maternal part, in this case composed of the decidua and metrial gland. Maternal–fetal exchanges of nutrients, metabolites, and gases occur in the labyrinth and, in this region, a layer of trophoblasts separates the maternal from the fetal blood. The basal zone, which forms below the labyrinth, is composed of cells containing glycogen, spongiotrophoblasts, and giant trophoblastic cells; it plays an important role in the metabolism of chemicals. The decidua, the maternal portion, consists of decidual cells of the mesometrium and the metrial gland, formed by fibroblasts, trophoblasts, fibroblasts, and natural killer cells [
51,
52,
53].
Despite the reduction in the total area of the placental disc of the animals treated with 400 mg/kg of AECi and that observed in their layers, no difference was observed in its constituents. It is possible that flavonoids exert an antiestrogenic role and thereby interfered with the total area of the placenta and placental constituents.
The absence of changes in both the ossification points and the viscera of animals treated with AECi suggests that the extract does not possess teratogenic effects. As a future perspective, we will consider the elucidation of the results reported in the topic “Placental morphometric analysis” of this work.
5. Conclusions
Based on the results, we can affirm that the oral administration of the aqueous extract of C. icaco (AECi) at different doses (100, 200, and 400 mg/kg) did not affect body weight and water and feed consumption of pregnant rats during the period of embryogenesis and organogenesis. Additionally, no clinical signs of toxicity (piloerection, diarrhea, salivation, bleeding, and death) were observed. After euthanasia, the absolute and relative organ weights of the treated rats were like the control ones; neither presented macroscopic signs of toxicity in the organs. Results were also similar for treated and control animals, considering the periods of pre-implantation and organogenesis. Finally, the administration of AECi did not cause malformations or anomalies in the viscera of neonates from treated rats. There is a lack of research on studies reporting C. icaco toxicity, during the gestational period or any other. Thus, it is possible to affirm that AECi did not induce any changes in maternal toxicity and reproductive performance, highlighting the importance of this investigation and suggesting that, under the experimental conditions used in this study, the extract does not cause reproductive toxicity.