Ouabain Induces Transcript Changes and Activation of RhoA/ROCK Signaling in Cultured Epithelial Cells (MDCK)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Massive RNA Sequencing (mRNA-Seq)
2.2.1. Treatment of MDCK Cells in Mature Monolayers with or without Ouabain
2.2.2. RNA Extraction
2.2.3. Library Preparation and Sequencing for Massive RNA Sequencing (RNA-Seq)
2.2.4. Bioinformatic Analysis
2.3. Quantitative Reverse Transcription-Polymerase Chain Reaction (RT-qPCR)
2.4. RhoA Protein Activation Assay
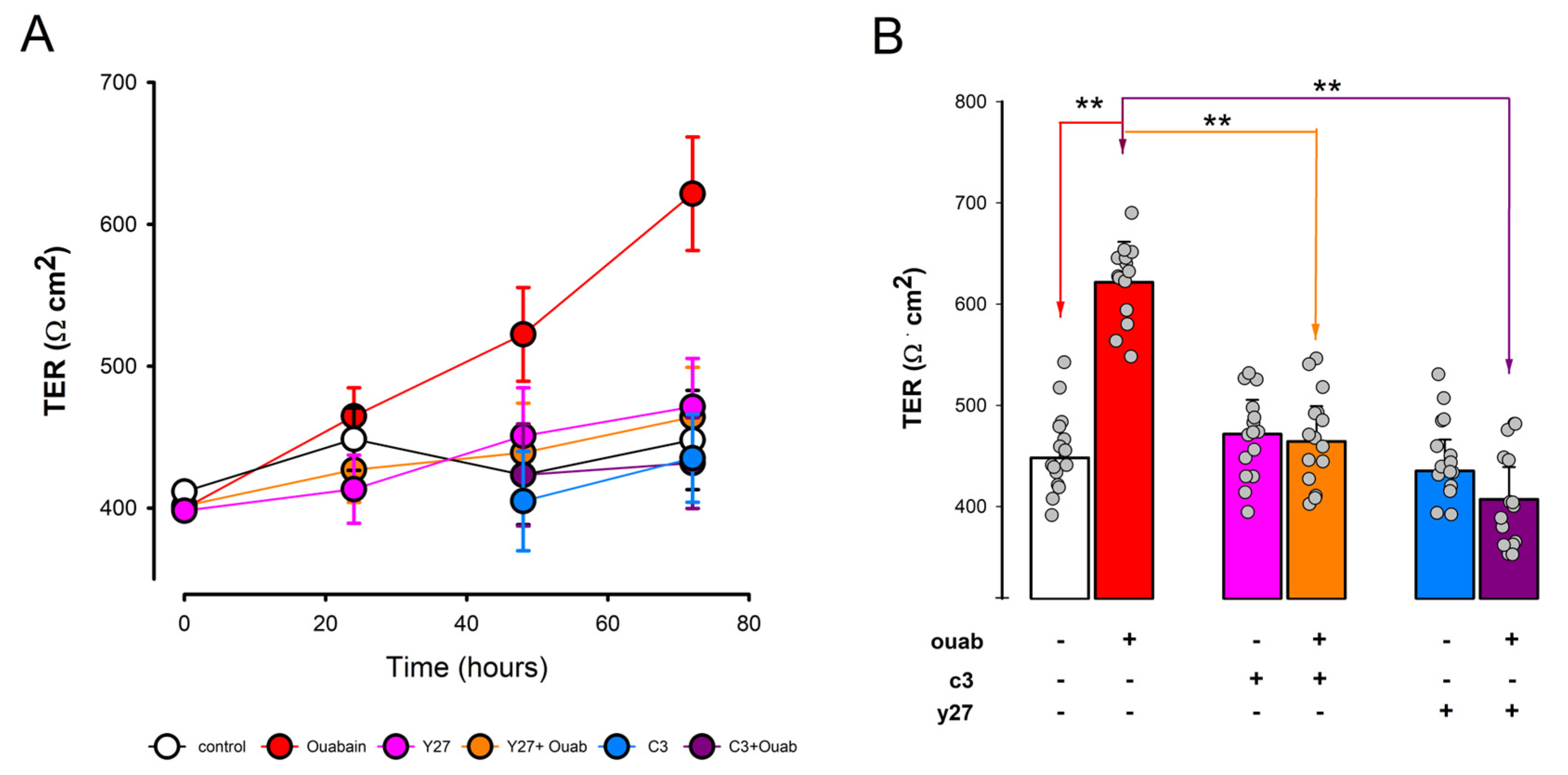
2.5. Transepithelial Electrical Resistance (TER)
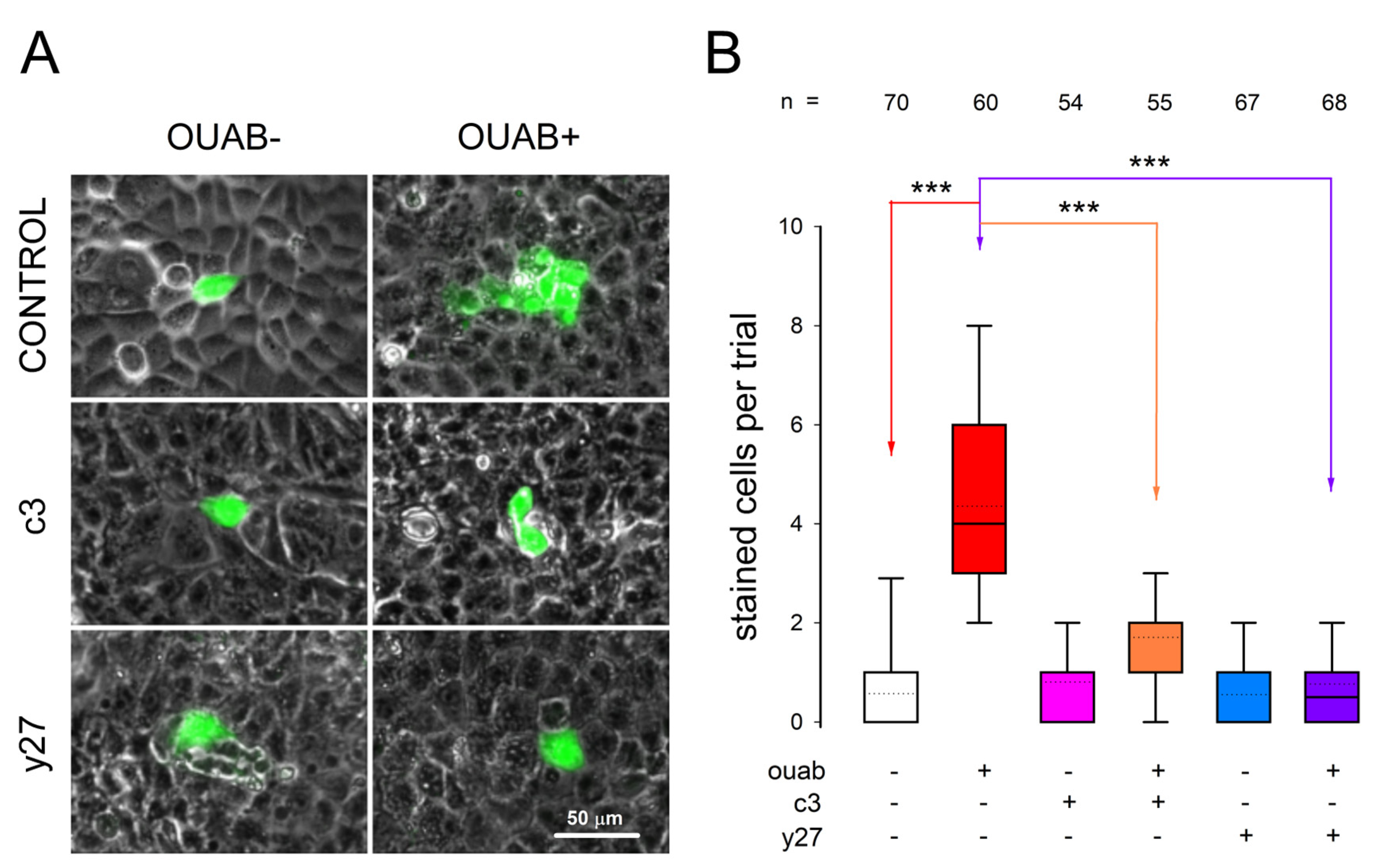
2.6. Measurement of Gap Junctional Intercellular Communication by Dye Transfer Assays
2.7. Materials and Chemicals
2.8. Statistical Analysis
3. Results
3.1. Ouabain Modifies Gene Expression in Epithelial Cells
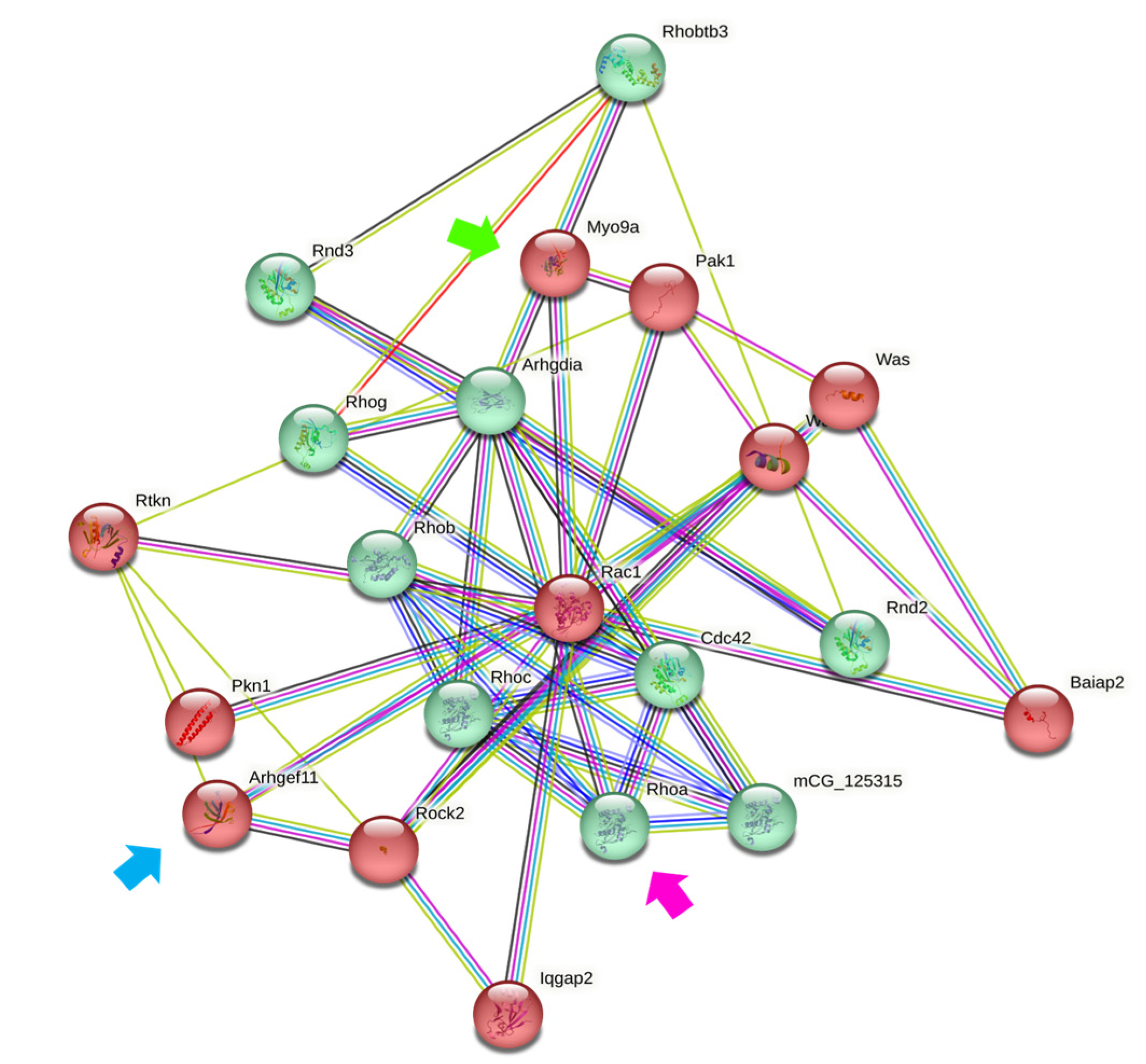
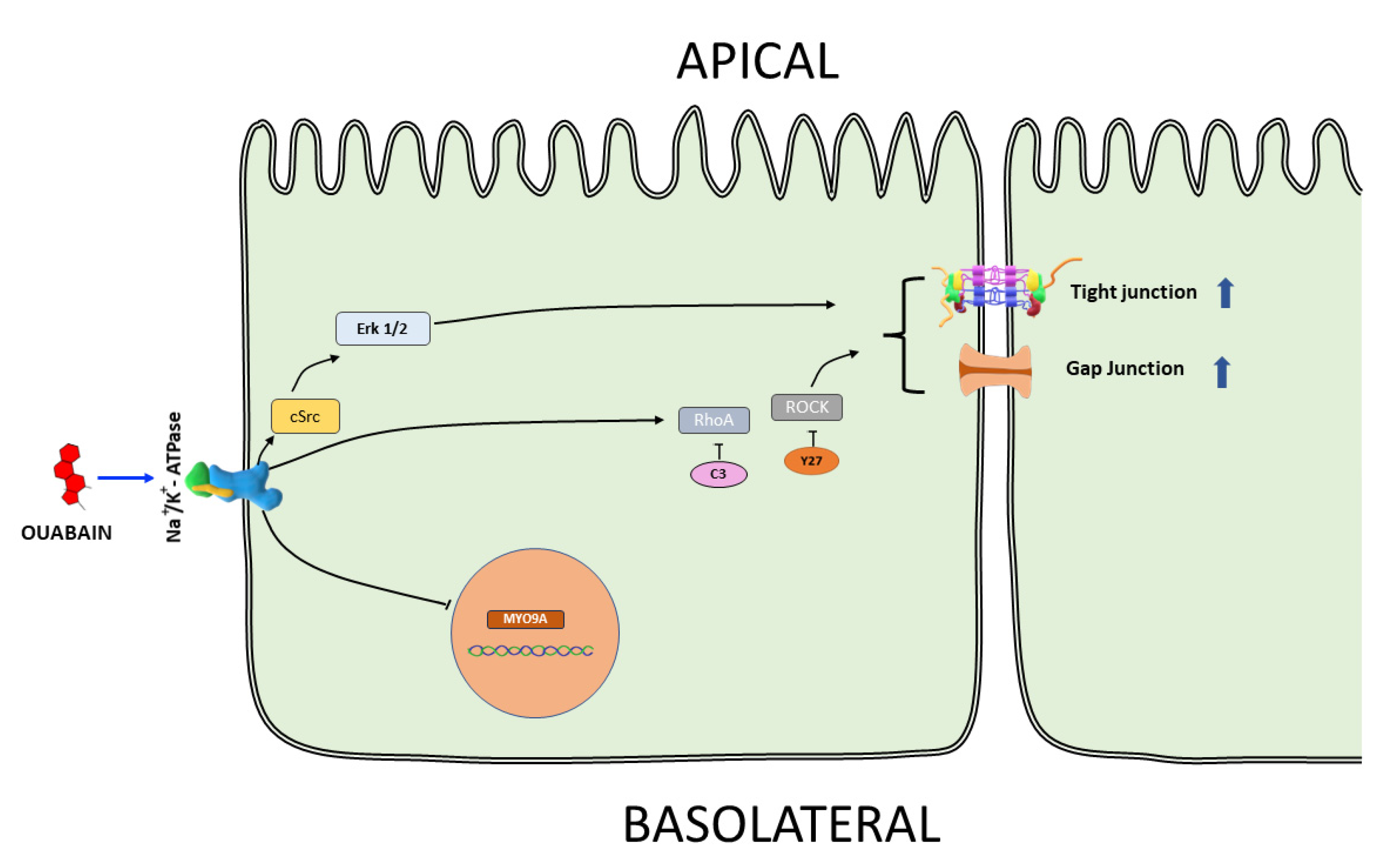
3.2. Downregulation of MYO9A Suggests the Participation of Rho/ROCK Signaling in the Modulation of Epithelial Properties by Ouabain
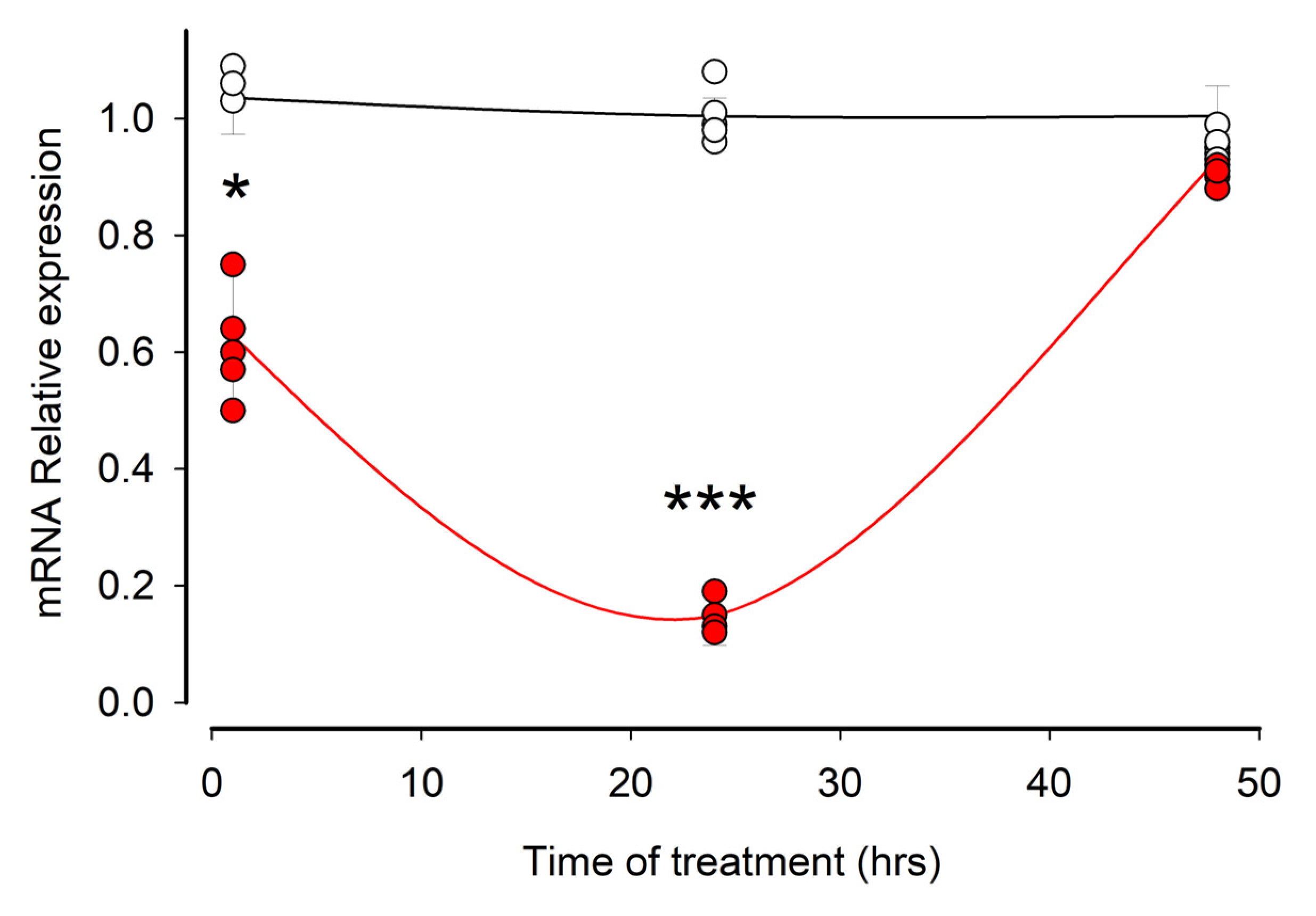
3.3. Ouabain Induces a Temporary Reduction in the Amount of mRNA of MYO9A
3.4. Ouabain Enhances the Amount of GTP-Loaded RhoA
3.5. Involvement of RhoA-ROCK on Ouabain-Promoted Enhancement of Tight Junction Sealness
3.6. Involvement of RhoA-ROCK on Ouabain-Promoted Enhancement of Gap Junctional Intercellular Communication
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Center for Biotechnology Information. PubChem Compound Summary for CID 439501, Ouabain. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Ouabain (accessed on 9 May 2023).
- Deepak, D.; Srivastava, S.; Khare, N.K.; Khare, A. Cardiac glycosides. In Fortschritte der Chemie Organischer Naturstoffe; Springer: Vienna, Austria, 1996; Volume 69, pp. 71–155. [Google Scholar] [CrossRef]
- Botelho, A.F.M.; Pierezan, F.; Soto-Blanco, B.; Melo, M.M. A review of cardiac glycosides: Structure, toxicokinetics, clinical signs, diagnosis, and antineoplastic potential. Toxicon 2019, 158, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Hollman, A. Plants and cardiac glycosides. Br. Heart J. 1985, 54, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Valente, R.C.; Capella, L.S.; Monteiro, R.Q.; Rumjanek, V.M.; Lopes, A.G.; Capella, M.A. Mechanisms of ouabain toxicity. FASEB J. 2003, 17, 1700–1702. [Google Scholar] [CrossRef] [PubMed]
- Haustein, K.O. Therapeutic range of cardiac glycosides. In Cardiac Glycoside Receptors and Positive Inotropy; Erdmann, E., Ed.; Steinkopff: Heidelberg, Germany, 1984. [Google Scholar]
- García-Ramos, J.; Peralta, B. The actions of digitalis and ouabain on some properties of the cardiac muscle. Arch. Inst. Cardiol. Mex. 1946, 16, 93–108. (In Spanish) [Google Scholar]
- Brody, T.M. Ouabain-induced inhibition of cardiac (Na+ + K+)-Atpase and the positive inotropic response. Ann. N. Y. Acad. Sci. 1974, 242, 684–687. [Google Scholar] [CrossRef]
- Allen, J.C.; Schwartz, A. Na+, K+-ATPase, the transport enzyme: Evidence for its proposed role as pharmacologic receptor for cardiac glycosides. Ann. N. Y. Acad. Sci. 1974, 242, 646–656. [Google Scholar] [CrossRef]
- Askari, A. The other functions of the sodium pump. Cell Calcium 2019, 84, 102105. [Google Scholar] [CrossRef]
- Hamlyn, J.M.; Blaustein, M.P.; Bova, S.; DuCharme, D.W.; Harris, D.W.; Mandel, F.; Mathews, W.R.; Ludens, J.H. Identification and characterization of a ouabain-like compound from human plasma. Proc. Natl. Acad. Sci. USA 1991, 88, 6259–6263. [Google Scholar] [CrossRef]
- Fishman, M.C. Endogenous digitalis-like activity in mammalian brain. Proc. Natl. Acad. Sci. USA 1979, 76, 4661–4663. [Google Scholar] [CrossRef] [PubMed]
- Schoner, W.; Bauer, N.; Müller-Ehmsen, J.; Krämer, U.; Hambarchian, N.; Schwinger, R.; Moeller, H.; Kost, H.; Weitkamp, C.; Schweitzer, T.; et al. Ouabain as a Mammalian Hormone. Ann. N. Y. Acad. Sci. 2003, 986, 678–684. [Google Scholar] [CrossRef]
- Hamlyn, J.M. Natriuretic Hormones, Endogenous Ouabain, and Related Sodium Transport Inhibitors. Front. Endocrinol. 2014, 5, 199. [Google Scholar] [CrossRef] [PubMed]
- Blaustein, M.P.; Hamlyn, J.M. Ouabain, endogenous ouabain and ouabain-like factors: The Na+ pump/ouabain receptor, its linkage to NCX, and its myriad functions. Cell Calcium 2020, 86, 102159. [Google Scholar] [CrossRef] [PubMed]
- Cereijido, M.; Robbins, E.S.; Dolan, W.J.; Rotunno, C.A.; Sabatini, D.D. Polarized monolayers formed by epithelial cells on a permeable and translucent support. J. Cell Biol. 1978, 77, 853–880. [Google Scholar] [CrossRef] [PubMed]
- Simmons, N. Cultured monolayers of MDCK cells: A novel model system for the study of epithelial development and function. Gen. Pharmacol. Vasc. Syst. 1982, 13, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Larre, I.; Lazaro, A.; Contreras, R.G.; Balda, M.S.; Matter, K.; Flores-Maldonado, C.; Ponce, A.; Flores-Benitez, D.; Rincon-Heredia, R.; Padilla-Benavides, T.; et al. Ouabain modulates epithelial cell tight junction. Proc. Natl. Acad. Sci. USA 2010, 107, 11387–11392. [Google Scholar] [CrossRef] [PubMed]
- Castillo, A.; Ortuño-Pineda, C.; Flores-Maldonado, C.; Larre, I.; Martínez Rendón, J.; Hinojosa, L.; Ponce, A.; Ogazón, A.; Serrano, M.; Valdes, J.; et al. Ouabain Modulates the Adherens Junction in Renal Epithelial Cells. Cell. Physiol. Biochem. 2019, 52, 1381–1397. [Google Scholar] [CrossRef]
- Ponce, A.; Larre, I.; Castillo, A.; Garcia-Villegas, R.; Romero, A.; Flores-Maldonado, C.; Martinez-Rendón, J.; Contreras, R.G.; Cereijido, M. Ouabain Increases Gap Junctional Communication in Epithelial Cells. Cell. Physiol. Biochem. 2014, 34, 2081–2090. [Google Scholar] [CrossRef]
- Ponce, A.; Larre, I.; Castillo, A.; Flores-Maldonado, C.; Verdejo-Torres, O.; Contreras, R.G.; Cereijido, M. Ouabain Modulates the Distribution of Connexin 43 in Epithelial Cells. Cell. Physiol. Biochem. 2016, 39, 1329–1338. [Google Scholar] [CrossRef]
- Larre, I.; Castillo, A.; Flores-Maldonado, C.; Contreras, R.G.; Galvan, I.; Muñoz-Estrada, J.; Cereijido, M. Ouabain modulates ciliogenesis in epithelial cells. Proc. Natl. Acad. Sci. USA 2011, 108, 20591–20596. [Google Scholar] [CrossRef]
- Larre, I.; Ponce, A.; Franco, M.; Cereijido, M. The emergence of the concept of tight junctions and physiological regulation by ouabain. Semin. Cell Dev. Biol. 2014, 36, 149–156. [Google Scholar] [CrossRef]
- Larre, I.; Ponce, A.; Fiorentino, R.; Shoshani, L.; Contreras, R.G.; Cereijido, M. Contacts and cooperation between cells depend on the hormone ouabain. Proc. Natl. Acad. Sci. USA 2006, 103, 10911–10916. [Google Scholar] [CrossRef]
- Contreras, R.G.; Flores-Maldonado, C.; Lázaro, A.; Shoshani, L.; Flores-Benitez, D.; Larré, I.; Cereijido, M. Ouabain binding to Na+, K+-ATPase relaxes cell attachment and sends a specific signal (NACos) to the nucleus. J. Membr. Biol. 2004, 198, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Corey, D.R. RNA sequencing: Platform selection, experimental design, and data interpretation. Nucleic Acid Ther. 2012, 22, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gerstein, M.; Snyder, M. RNA-Seq: A revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009, 10, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-Length Transcriptome Assembly from RNA-Seq Data without a Reference Genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef]
- Schmieder, R.; Edwards, R. Fast Identification and Removal of Sequence Contamination from Genomic and Metagenomic Datasets. PLoS ONE 2011, 6, e17288. [Google Scholar] [CrossRef]
- Evans, T.; Loose, M. AlignWise: A Tool for Identifying Protein-Coding Sequence and Correcting Frameshifts. BMC Bioinform. 2015, 16, 376. [Google Scholar] [CrossRef] [PubMed]
- Alva, V.; Nam, S.-Z.; Söding, J.; Lupas, A.N. The MPI Bioinformatics Toolkit as an Integrative Platform for Advanced Protein Sequence and Structure Analysis. Nucleic Acids Res. 2016, 44, W410–W415. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate Transcript Quantification from RNA-Seq Data with or without a Reference Genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef]
- Ebenhoch, R.; Akhdar, A.; Reboll, M.R.; Korf-Klingebiel, M.; Gupta, P.; Armstrong, J.; Huang, Y.; Frego, L.; Rybina, I.; Miglietta, J.; et al. Crystal structure and receptor-interacting residues of MYDGF—A protein mediating ischemic tissue repair. Nat. Commun. 2019, 10, 5379. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Y.; Feng, J.; Liu, W.; Li, Y.; Liu, J.; Yin, Q.; Lian, H.; Liu, L.; Nie, Y. Mydgf promotes Cardiomyocyte proliferation and Neonatal Heart regeneration. Theranostics 2020, 10, 9100–9112. [Google Scholar] [CrossRef] [PubMed]
- Kajkowski, E.M.; Lo, C.F.; Ning, X.; Walker, S.; Sofia, H.J.; Wang, W.; Edris, W.; Chanda, P.; Wagner, E.; Vile, S.; et al. Beta-Amyloid peptide-induced apoptosis regulated by a novel protein containing a g protein activation module. J. Biol. Chem. 2001, 276, 18748–18756. [Google Scholar] [CrossRef] [PubMed]
- Odh, G.; Hindemith, A.; Rosengren, A.M.; Rosengren, E.; Rorsman, H. Isolation of a new tautomerase monitored by the conversion of D-dopachrome to 5,6-dihydroxyindole. Biochem. Biophys. Res. Commun. 1993, 197, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Aroca, P.; Solano, F.; Salinas, C.; García-Borrón, J.C.; Lozano, J.A. Regulation of the final phase of mammalian melanogenesis. The role of dopachrome tautomerase and the ratio between 5,6-dihydroxyindole-2-carboxylic acid and 5,6-dihydroxyindole. Eur. J. Biochem. 1992, 208, 155–163. [Google Scholar] [CrossRef]
- Xu, F.; Li, M.Y.; Chen, J. D-dopachrome tautomerase from Japanese sea bass (Lateolabrax japonicus) is a chemokine-like cytokine and functional homolog of macrophage migration inhibitory factor. Zool. Res. 2020, 41, 39–50. [Google Scholar] [CrossRef]
- Kong, Y.Z.; Chen, Q.; Lan, H.Y. Macrophage Migration Inhibitory Factor (MIF) as a Stress Molecule in Renal Inflammation. Int. J. Mol. Sci. 2022, 23, 4908. [Google Scholar] [CrossRef]
- Nishihira, J.; Fujinaga, M.; Kuriyama, T.; Suzuki, M.; Sugimoto, H.; Nakagawa, A.; Tanaka, I.; Sakai, M. Molecular cloning of human D-dopachrome tautomerase cDNA: N-terminal proline is essential for enzyme activation. Biochem. Biophys. Res. Commun. 1998, 243, 538–544. [Google Scholar] [CrossRef]
- Lee, S.K.; Son le, T.; Choi, H.J.; Ahnn, J. Dicarbonyl/l-xylulose reductase (DCXR): The multifunctional pentosuria enzyme. Int. J. Biochem. Cell Biol. 2013, 45, 2563–2567. [Google Scholar] [CrossRef]
- Ebert, B.; Kisiela, M.; Maser, E. Human DCXR—Another ‘moonlighting protein’ involved in sugar metabolism, carbonyl detoxification, cell adhesion and male fertility? Biol. Rev. Camb. Philos. Soc. 2015, 90, 254–278. [Google Scholar] [CrossRef]
- Yogev, O.; Yogev, O.; Singer, E.; Shaulian, E.; Goldberg, M.; Fox, T.D.; Pines, O. Fumarase: A mitochondrial metabolic enzyme and a cytosolic/nuclear component of the DNA damage response. PLoS Biol. 2010, 8, e1000328. [Google Scholar] [CrossRef] [PubMed]
- Leshets, M.; Silas, Y.B.H.; Lehming, N.; Pines, O. Fumarase: From the TCA Cycle to DNA Damage Response and Tumor Suppression. Front. Mol. Biosci. 2018, 5, 68. [Google Scholar] [CrossRef] [PubMed]
- Alam, N.A.; Rowan, A.J.; Wortham, N.C.; Pollard, P.J.; Mitchell, M.; Tyrer, J.P.; Barclay, E.; Calonje, E.; Manek, S.; Adams, S.J.; et al. Genetic and functional analyses of FH mutations in multiple cutaneous and uterine leiomyomatosis, hereditary leiomyomatosis and renal cancer, and fumarate hydratase deficiency. Hum. Mol. Genet. 2003, 12, 1241–1252. [Google Scholar] [CrossRef] [PubMed]
- Goldschmidt-Reisin, S.; Kitakawa, M.; Herfurth, E.; Wittmann-Liebold, B.; Grohmann, L.; Graack, H.R. Mammalian mitochondrial ribosomal proteins. N-terminal amino acid sequencing, characterization, and identification of corresponding gene sequences. J. Biol. Chem. 1998, 273, 34828–34836. [Google Scholar] [CrossRef]
- Kenmochi, N.; Suzuki, T.; Uechi, T.; Magoori, M.; Kuniba, M.; Higa, S.; Watanabe, K.; Tanaka, T. The human mitochondrial ribosomal protein genes: Mapping of 54 genes to the chromosomes and implications for human disorders. Genomics 2001, 77, 65–70. [Google Scholar] [CrossRef]
- Antoniou, N.; Lagopati, N.; Balourdas, D.I.; Nikolaou, M.; Papalampros, A.; Vasileiou, P.V.S.; Myrianthopoulos, V.; Kotsinas, A.; Shiloh, Y.; Liontos, M.; et al. The Role of E3, E4 Ubiquitin Ligase (UBE4B) in Human Pathologies. Cancers 2019, 12, 62. [Google Scholar] [CrossRef]
- Sirisaengtaksin, N.; Gireud, M.; Yan, Q.; Kubota, Y.; Liontos, M.; Meza, D.; Waymire, J.C.; Zage, P.E.; Bean, A.J. UBE4B protein couples ubiquitination and sorting machineries to enable epidermal growth factor receptor (EGFR) degradation. J. Biol. Chem. 2014, 289, 3026–3039. [Google Scholar] [CrossRef]
- Woodfield, S.E.; Guo, R.J.; Liu, Y.; Major, A.M.; Hollingsworth, E.F.; Indiviglio, S.; Whittle, S.B.; Mo, Q.; Bean, A.J.; Ittmann, M.; et al. Neuroblastoma patient outcomes, tumor differentiation, and ERK activation are correlated with expression levels of the ubiquitin ligase UBE4B. Genes Cancer 2016, 7, 13–26. [Google Scholar] [CrossRef]
- Memarzadeh, K.; Savage, D.J.; Bean, A.J. Low UBE4B expression increases sensitivity of chemoresistant neuroblastoma cells to EGFR and STAT5 inhibition. Cancer Biol. Ther. 2019, 20, 1416–1429. [Google Scholar] [CrossRef]
- Zhao, R.; Qi, Y.; Chen, J.; Zhao, Z.J. FYVE-DSP2, a FYVE domain-containing dual specificity protein phosphatase that dephosphorylates phosphotidylinositol 3-phosphate. Exp. Cell Res. 2001, 265, 329–338. [Google Scholar] [CrossRef]
- Blondeau, F.; Laporte, J.; Bodin, S.; Superti-Furga, G.; Payrastre, B.; Mandel, J.-L. Myotubularin, a phosphatase deficient in myotubular myopathy, acts on phosphatidylinositol 3-kinase and phosphatidylinositol 3-phosphate pathway. Hum. Mol. Genet. 2000, 9, 2223–2229. [Google Scholar] [CrossRef] [PubMed]
- Naughtin, M.J.; Sheffield, D.A.; Rahman, P.; Hughes, W.E.; Gurung, R.; Stow, J.L.; Nandurkar, H.H.; Dyson, J.M.; Mitchell, C.A. The myotubularin phosphatase MTMR4 regulates sorting from early endosomes. J. Cell Sci. 2010, 123, 3071–3083. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.; Green, M.R. The RNA polymerase I transcription factor, upstream binding factor, interacts directly with the TATA box-binding protein. J. Biol. Chem. 1994, 269, 30140–30146. [Google Scholar] [CrossRef]
- Russell, J.; Zomerdijk, J.C. The RNA polymerase I transcription machinery. Biochem. Soc. Symp. 2006, 73, 203–216. [Google Scholar] [CrossRef]
- Reinhard, J.; Scheel, A.A.; Diekmann, D.; Hall, A.; Ruppert, C.; Bähler, M. A novel type of myosin implicated in signaling by rho family GTPases. EMBO J. 1995, 14, 697–704. [Google Scholar] [CrossRef]
- Gorman, S.W.; Haider, N.B.; Grieshammer, U.; Swiderski, R.E.; Kim, E.; Welch, J.W.; Searby, C.; Leng, S.; Carmi, R.; Sheffield, V.C.; et al. The cloning and developmental expression of unconventional myosin IXA (MYO9A) a gene in the Bardet-Biedl syndrome (BBS4) region at chromosome 15q22-q23. Genomics 1999, 59, 150–160. [Google Scholar] [CrossRef]
- Chieregatti, E.; Gärtner, A.; Stöffler, H.-E.; Bähler, M. Myr 7 is a novel myosin IX-RhoGAP expressed in rat brain. J. Cell Sci. 1998, 111, 3597–3608. [Google Scholar] [CrossRef]
- Bähler, M. Are class III and class IX myosins motorized signalling molecules? Biochim. Biophys. Acta. 2000, 1496, 52–59. [Google Scholar] [CrossRef]
- Jaffe, A.B.; Hall, A. Rho GTPases: Biochemistry and biology. Annu. Rev. Cell Dev. Biol. 2005, 21, 247–269. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Gene Ontology Consortium. The Gene Ontology resource: Enriching a Gold mine. Nucleic Acids Res. 2021, 49, D325–D334. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [PubMed]
- Wilde, C.; Aktories, K. The Rho-ADP-ribosylating C3 exoenzyme from Clostridium botulinum and related C3-like transferases. Toxicon 2001, 39, 1647–1660. [Google Scholar] [CrossRef]
- Aktories, K.; Hall, A. Botulinum ADP-ribosyltransferase C3: A new tool to study low molecular weight GTP-binding proteins. Trends Pharmacol. Sci. 1989, 10, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Ishizaki, T.; Uehata, M.; Tamechika, I.; Keel, J.; Nonomura, K.; Maekawa, M.; Narumiya, S. Pharmacological properties of Y-27632, a specific inhibitor of rho-associated kinases. Mol. Pharmacol. 2000, 57, 976–983. [Google Scholar] [PubMed]
- Davies, S.P.; Reddy, H.; Caivano, M.; Cohen, P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 2000, 351, 95–105. [Google Scholar] [CrossRef]
- Silva, E.; Soares-da-Silva, P. New insights into the regulation of Na+,K+-ATPase by ouabain. Int. Rev. Cell Mol. Biol. 2012, 294, 99–132. [Google Scholar] [CrossRef]
- Xie, Z. Ouabain interaction with cardiac Na/K-ATPase reveals that the enzyme can act as a pump and as a signal transducer. Cell. Mol. Biol. 2001, 47, 383–390. [Google Scholar]
- Taurin, S.; Dulin, N.O.; Pchejetski, D.; Grygorczyk, R.; Tremblay, J.; Hamet, P.; Orlov, S.N. c-Fos expression in ouabain-treated vascular smooth muscle cells from rat aorta: Evidence for an intracellular-sodium-mediated, calcium-independent mechanism. J. Physiol. 2002, 543, 835–847. [Google Scholar] [CrossRef]
- Muto, S.; Nemoto, J.; Okada, K.; Miyata, Y.; Kawakami, K.; Saito, T.; Asano, Y. Intracellular Na+ directly modulates Na+,K+-ATPase gene expression in normal rat kidney epithelial cells. Kidney Int. 2000, 57, 1617–1635. [Google Scholar] [CrossRef]
- Smolyaninova, L.V.; Shiyan, A.A.; Kapilevich, L.V.; Lopachev, A.V.; Fedorova, T.N.; Klementieva, T.S.; Moskovtsev, A.A.; Kubatiev, A.A.; Orlov, S.N. Transcriptomic changes triggered by ouabain in rat cerebellum granule cells: Role of α3- and α1-Na+, K+-ATPase-mediated signaling. PLoS ONE 2019, 14, e0222767. [Google Scholar] [CrossRef] [PubMed]
- Ando, K.; Omi, N.; Shimosawa, T.; Takahashi, K.; Fujita, T. Effects of ouabain on the growth and DNA synthesis of PC12 cells. J. Cardiovasc. Pharmacol. 2001, 37, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Huang, L.; Xie, Z.; Huang, W.H.; Askari, A. Partial inhibition of Na+/K+-ATPase by ouabain induces the Ca2+-dependent expressions of early-response genes in cardiac myocytes. J. Biol. Chem. 1996, 271, 10372–10378. [Google Scholar] [CrossRef] [PubMed]
- Tverskoi, A.M.; Sidorenko, S.V.; Klimanova, E.A.; Akimova, O.A.; Smolyaninova, L.V.; Lopina, O.D.; Orlov, S.N. Effects of Ouabain on Proliferation of Human Endothelial Cells Correlate with Na+,K+-ATPase Activity and Intracellular Ratio of Na+ and K. Biochemistry 2016, 81, 876–883. [Google Scholar] [CrossRef]
- Valente, R.C.; Capella, L.S.; Oliveira, M.M.; Nunes-Lima, L.T.; Cruz, F.C.; Palmieri, R.R.; Lopes, A.G.; Capella, M.A. Diverse actions of ouabain and its aglycone ouabagenin in renal cells. Cell Biol. Toxicol. 2010, 26, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Klimanova, E.A.; Tverskoi, A.M.; Koltsova, S.V.; Sidorenko, S.V.; Lopina, O.D.; Tremblay, J.; Hamet, P.; Kapilevich, L.V.; Orlov, S.N. Time- and dose dependent actions of cardiotonic steroids on transcriptome and intracellular content of Na+ and K+: A comparative analysis. Sci. Rep. 2017, 7, 45403. [Google Scholar] [CrossRef]
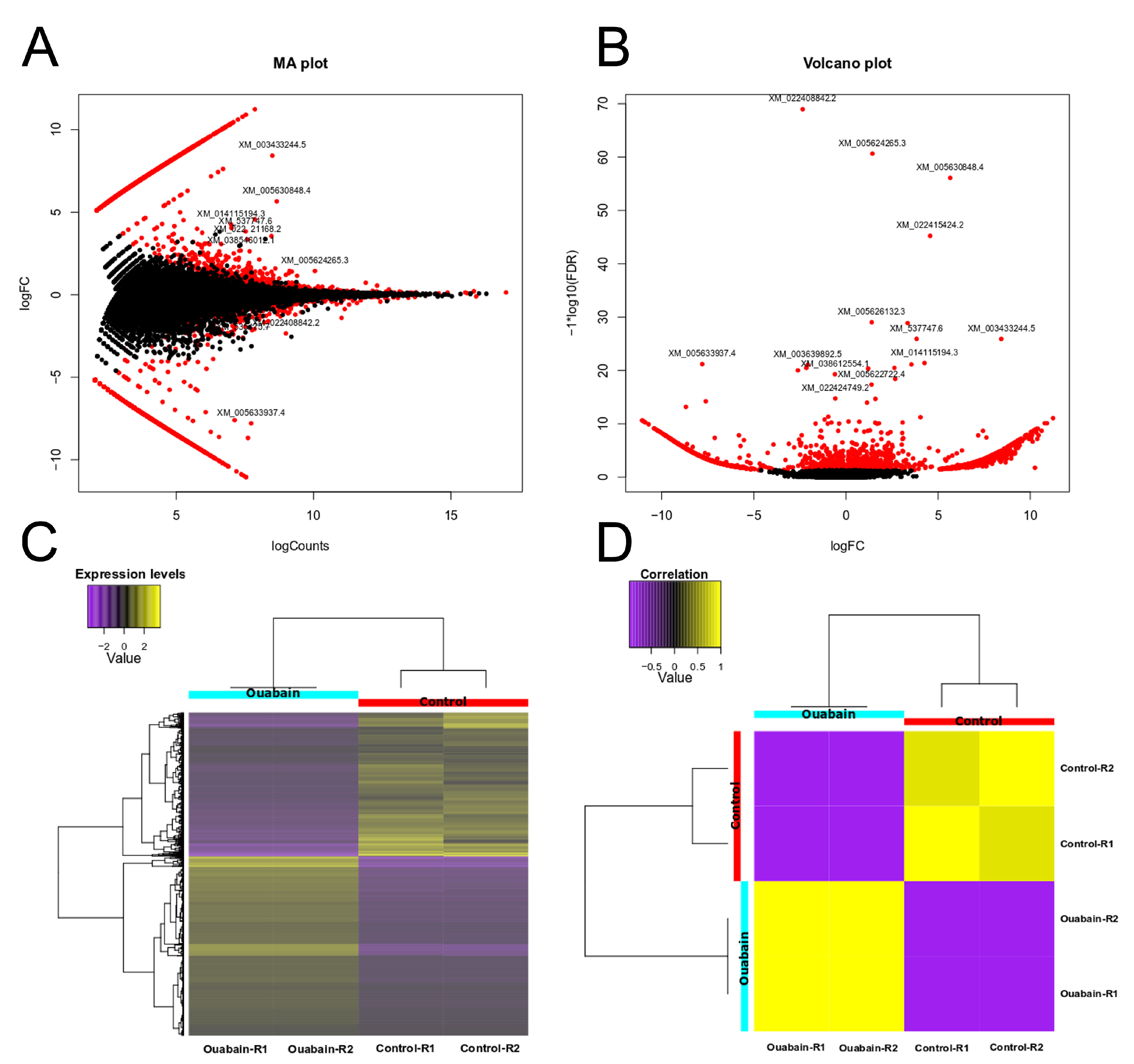
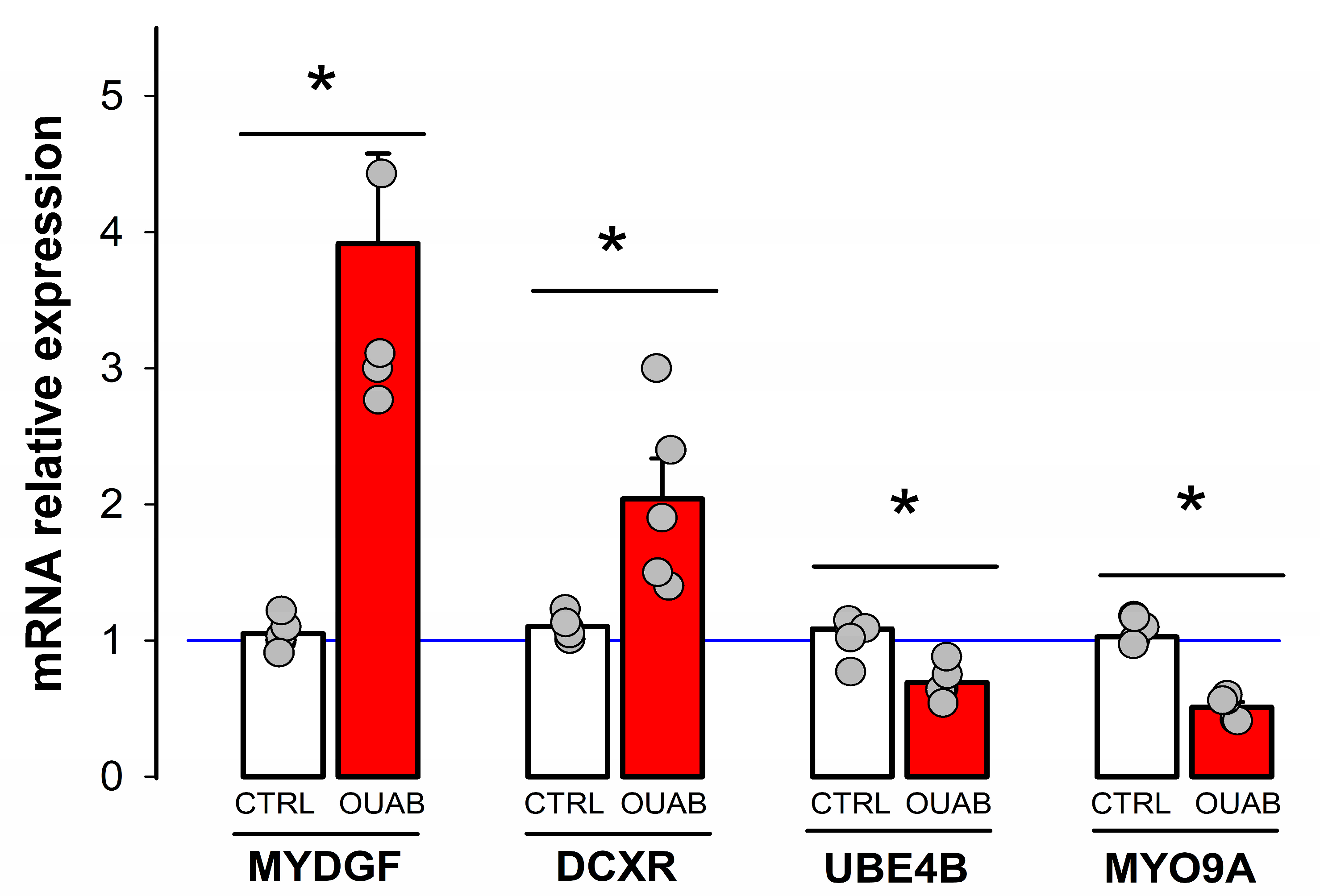

| MYDGF | forward | 5′-CTTGTGGGCTGCGTTGCTGCTG-3′ |
| reverse | 5′-GACTCATCTGCCACTTCTCGTTGG-3′ | |
| MYO9A | forward | 5′-CTTCGAGCTATGGGCCTTCAGGAG-3′ |
| reverse | 5′-GTAGTGGGTCAATGGTGTCAGGGC-3′ | |
| DCXR | forward | 5′-GCGTCTACTGCTCCACCAAGGGTG-3′ |
| reverse | 5′-GGTCACTCAGCAGGAAGAGGATGG-3′ | |
| UBE4B | forward | 5′-CATGTCCCAGGTGGATGTGGATTC-3′ |
| reverse | 5′-CTGTCTGGTGACCGAGCTGCTGC-3′ | |
| PRP0 | forward | 5′-GCAGGTGTTTGACAATGGCAGC-3′ |
| reverse | 5′-GCCTTGACCTTTTCAGCAAGTGG-3′ |
| Number | Gene | Description | Acc. No. | Fold Change |
|---|---|---|---|---|
| 1 | MYDGF | Myeloid derived growth factor | XM_849589 | 4.53 |
| 2 | TM2D1 | TM2 domain-containing protein 1-like, transcript variant X2 | XM_003639021 | 3.61 |
| 3 | DCDX | D-dopachrome decarboxylase-like | XM_003639927.2 | 2.99 |
| 4 | DCXR | Dicarbonyl/L-xylulose reductase | XM_038675393.1 | 2.62 |
| 5 | FH | Fumarate hydratase transcript variant 1 | XM_537215.4 | 2.60 |
| 6 | MRPL39 | Mitochondrial ribosomal protein L39 transcript variant X2 | XM_005638779.1 | 2.07 |
| 7 | UBE4B | Ubiquitination factor E4B transcript variant X1 | XM_844731.3 | 0.43 |
| 8 | MTMR4 | Myotubularin related protein 4 transcript variant X5 | XM_005624734.1 | 0.32 |
| 9 | UBTF | Upstream binding transcription factor, RNA polymerase I | XM_537622.5 | 0.19 |
| 10 | MYO9A | Myosin IXA | XM_544755.4 | 0.16 |
| Biological Process | GO-Term |
|---|---|
| Apical junction assembly | GO:0043297 |
| Cell adhesion | GO:0007155 |
| Cell junction assembly | GO:0034329 |
| Cell–cell junction organization | GO:0045216 |
| Epithelial cell differentiation | GO:0030855 |
| Epithelium development | GO:0060429 |
| Establishment of epithelial cell apical/basal polarity | GO:0045198 |
| Establishment or maintenance of cell polarity | GO:0007163 |
| Morphogenesis of an epithelium | GO:0002009 |
| Rac protein signal transduction | GO:0016601 |
| Regulation of cell-substrate adhesion | GO:0010810 |
| Rho protein signal transduction | GO:0007266 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Rendón, J.; Hinojosa, L.; Xoconostle-Cázares, B.; Ramírez-Pool, J.A.; Castillo, A.; Cereijido, M.; Ponce, A. Ouabain Induces Transcript Changes and Activation of RhoA/ROCK Signaling in Cultured Epithelial Cells (MDCK). Curr. Issues Mol. Biol. 2023, 45, 7538-7556. https://doi.org/10.3390/cimb45090475
Martínez-Rendón J, Hinojosa L, Xoconostle-Cázares B, Ramírez-Pool JA, Castillo A, Cereijido M, Ponce A. Ouabain Induces Transcript Changes and Activation of RhoA/ROCK Signaling in Cultured Epithelial Cells (MDCK). Current Issues in Molecular Biology. 2023; 45(9):7538-7556. https://doi.org/10.3390/cimb45090475
Chicago/Turabian StyleMartínez-Rendón, Jacqueline, Lorena Hinojosa, Beatriz Xoconostle-Cázares, José Abrahán Ramírez-Pool, Aída Castillo, Marcelino Cereijido, and Arturo Ponce. 2023. "Ouabain Induces Transcript Changes and Activation of RhoA/ROCK Signaling in Cultured Epithelial Cells (MDCK)" Current Issues in Molecular Biology 45, no. 9: 7538-7556. https://doi.org/10.3390/cimb45090475
APA StyleMartínez-Rendón, J., Hinojosa, L., Xoconostle-Cázares, B., Ramírez-Pool, J. A., Castillo, A., Cereijido, M., & Ponce, A. (2023). Ouabain Induces Transcript Changes and Activation of RhoA/ROCK Signaling in Cultured Epithelial Cells (MDCK). Current Issues in Molecular Biology, 45(9), 7538-7556. https://doi.org/10.3390/cimb45090475







